Abstract
Responding to real or potential threats in the environment requires the coordination of autonomic, neuroendocrine, and behavioral processes to promote adaptation and survival. These diverging systems necessitate input from the limbic forebrain to integrate and modulate functional output in accordance with contextual demand. In the current review, we discuss the potential role of the medial prefrontal cortex (mPFC) as a coordinator of behavioral and physiological stress responses across multiple temporal and contextual domains. Further, we highlight converging evidence from rodent and human research indicating the necessity of the mPFC for modulating physiological energetic systems to mobilize or limit energetic resources as needed to ultimately promote behavioral adaptation in the face of stress. We review literature that indicates that glucocorticoids act as one of the primary messengers in the reallocation of energetic resources having profound effects locally within the mPFC, as well as shaping how the mPFC acts within a network of brain structures to modulate responses to stress. Finally, we discuss how rodent, as well as human studies point toward a critical role of the mPFC in the coordination of anticipatory responses to stress and why this distinction is an important one to make in stress neurobiology.
Keywords: Prefrontal Cortex, Stress, HPA axis, Autonomic Nervous System, Executive Function, Glucocorticoids
Introduction
Stress responsiveness is a highly conserved process that promotes survival despite uncontrollable and often unpredictable changes in the environment (i.e. context). The physiological stress response engages autonomic and neuroendocrine systems to mobilize energy needed to meet the challenge at hand. Accordingly, these responses are both tightly regulated throughout the central nervous system and adaptable to the energetic needs of the individual. Thus, stress can be considered a stimulus that mobilizes energetic systems to respond to an ongoing or anticipated challenge [1].
Energy mobilization following stress occurs mainly through activity of the autonomic and neuroendocrine systems. The sympathetic arm of the autonomic nervous system (ANS) is engaged within seconds of stressor presentation, in order to prepare the individual to respond immediately, and rapidly subsides as the result of reflex activation of the parasympathetic arm of the ANS [2]. Sympathetic activation is driven by preganglionic neurons in the interomediolateral cell column (IML), which in turn innervate sympathetic ganglionic neurons and participate in specific somato- and viscero-sympathetic reflexes. Sympathetic reflexes are controlled by descending afferent inputs from regions such as the rostral ventrolateral medulla (RVLM), paraventricular nucleus of the hypothalamus (PVN), medullary raphe nuclei, locus coeruleus (LC), and lateral hypothalamus (LH) [2,3]. Stress also stimulates sympathetic innervation of the adrenal medulla, which triggers the release of epinephrine (along with norepinephrine) into the bloodstream, promoting hepatic gluconeogenesis and contributing to increased heart rate, cardiac output, and blood pressure [4]. These changes, along with others, promote energy mobilization and enable the individual to cope with environmental demand. Parasympathetic output is controlled by descending preganglionic neurons in the dorsal motor nucleus of the vagus (DMV), medullary nucleus ambiguous (NAmb), and sacral parasympathetic nucleus [2]. Importantly, IML and medullary nuclei coordinate appropriate autonomic responses based on descending information from the limbic forebrain and hypothalamus, via inputs from autonomic integrative sites in the hindbrain (e.g. raphe pallidus, the lateral parabrachial nucleus, and the Kolliker-Fuse nucleus), midbrain (e.g. periaqueductal grey), and forebrain [e.g. dorsomedial hypothalamus (DMH)] [2].
The primary neuroendocrine stress response is generated by the hypothalamic–pituitary–adrenocortical (HPA) axis, and occurs on a slower timescale than the autonomic response, allowing for prolonged and amplified responses to stress [2]. Upon stressor initiation, corticotropin-releasing hormone (CRH) is released from neurosecretory neurons in the medial parvocellular PVN into the external zone of the median eminence and travels to the anterior pituitary via the hypophysial portal system [5]. In turn, CRH and co-secretagogues such as arginine vasopressin (AVP) synergistically trigger the release of adrenocorticotropic hormone (ACTH) from anterior pituitary corticotropes [5,6]. By way of systemic circulation, ACTH acts at the adrenal cortex to induce the release of glucocorticoids (e.g., cortisol in humans, non-human primates and corticosterone in rats, mice) [7]. Glucocorticoids are then able to travel via systematic circulation to peripheral as well as central targets, whereby they exert a multitude of effects. In the periphery, glucocorticoids induce gluconeogenesis and promote glycogen breakdown, lipolysis, and proteolysis [8]. Glucocorticoids exert their effects in the brain and periphery through binding to mineralocorticoid (MR) and glucocorticoid receptors (GR) localized to neurons and/or glia. Classically, circulating glucocorticoids are thought to diffuse through cell membranes, where they bind to intracellular MR and GR. Activated MR and GR dissociate from heat shock proteins and dimerize with other activated MR and GR receptors, respectively [9,10]. The MR or GR dimers translocate to the nucleus, where they modify gene expression via binding of liganded complexes to glucocorticoid response elements [11]. Both GR and MR may also affect gene expression by transrepression, mediated by binding to other transcription factor complexes and interfering with their transcriptional effects (e.g., AP1). Glucocorticoids also bind to membrane-associated MR and GR, though the molecular underpinnings of this process are still being uncovered (see [10]). The MR has a much higher binding-affinity for glucocorticoids, and is thought to be partially occupied at basal levels [12]. In contrast, the GR has a much lower binding-affinity for glucocorticoids, and is thought to be largely unoccupied at basal levels. Accordingly, MR is thought to sense resting levels of glucocorticoids important for circadian regulation of the HPA axis and mnemonic function, whereas the GR is thought to be important for sensing stress-induced levels of glucocorticoids [13,14]. However, the membrane MR has a lower binding-affinity for glucocorticoids than the cytosolic variant, and plays an important role in the rapid effects of glucocorticoids, particularly in the hippocampus [15]. The MR and GR are expressed in key stress-regulatory regions, including the medial prefrontal cortex (mPFC), hippocampus, amygdala, hypothalamus, and hindbrain, with MR expression being more restricted than that of GR [16,17].
Responding to stress can be anticipatory or reflexive, and requires the involvement of multiple stress-regulatory brain circuits [1,18]. Anticipatory responses occur whenever the stressor is expected and requires the organism to reference prior experiences to predict the need for energy mobilization. Reflexive responses tend to be more ‘systemic’ in nature and may result from physical stressors such as blood loss, pain, or hypoxia, which require immediate responses. Anticipatory responses often recruit forebrain and limbic circuits that project to the PVN via multi-synaptic projections, whereas reflexive responses tend to activate hindbrain pathways that project directly to the PVN or autonomic motor centers [2].
The mPFC is a critical regulator of autonomic and neuroendocrine stress responses, in addition to its prominent role in executive function [19–21]. The focus of this review will be to discuss the role of the mPFC in stress regulation as it relates to neuroendocrine, autonomic, and behavioral responses to stress. Further, we propose that the mPFC is a coordinator of brain circuits that mediate responses to stress, particularly (albeit not exclusively) anticipatory in nature.
Overview of Prefrontal Cytoarchitecture, Connectivity, and Neurochemical Makeup
The rodent mPFC is classified into three distinct neuroanatomical subregions based on connectivity and cytoarchitecture: the anterior cingulate (ACC), prelimbic (plPFC), and infralimbic (ilPFC) cortices [22] (see Figure 1). These three regions have functional and connectional homology to human Brodmann areas 24b, 32, and 25, respectively (Gabbott et al., 2005). The mPFC is divided into medial-lateral layers: layer I (mostly axons), layers II/III, and layers V/VI. The mPFC is agranular and output to subcortical structures arises from both deep layers (V/VI) as well as layers II/III [23]. The mPFC is further divided into dorsal and ventral components, including the ACC and dorsal plPFC and the ventral plPFC and ilPFC, respectively. The ACC in rats has mostly been implicated in motor behaviors, as it sends projections to oculomotor sites and regions involved in spatial navigation [22,24]. The ACC is also implicated in pain reactivity [24]. The plPFC projections [dorsal raphe, dorsal striatum, nucleus accumbens, basolateral complex of the amygdala (BLA), anterior bed nucleus of the stria terminalis (aBST)] are consistent with a role for the more dorsal mPFC in limbic and cognitive functions. The projections of the ilPFC projections [nucleus of the solitary tract (NTS), posterior hypothalamus (PH), central nucleus of the amygdala, parabrachial nucleus, rostral ventrolateral medulla (RVLM)] suggest a role in visceral/autonomic responses (see[22,23,25,26]).
Figure 1.
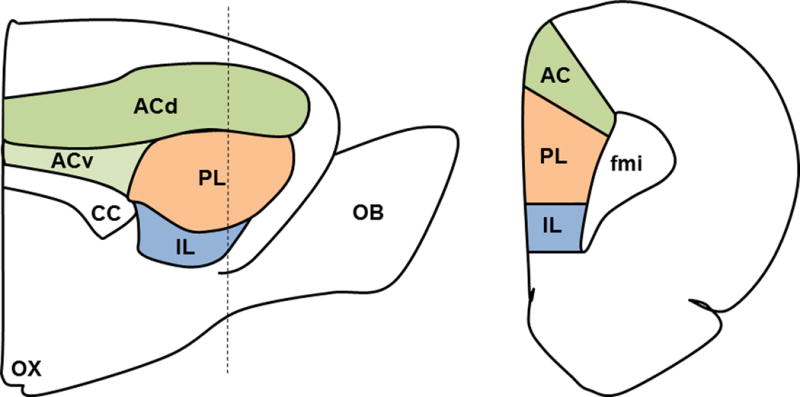
mPFC Neuroanatomy. Sagittal (left) and coronal (right) sections of the medial prefrontal cortex (mPFC) showing the anterior cingulate (AC), prelimbic (PL), and infralimbic (IL) cortices. The dotted line on the sagittal view indicates the location of the coronal section. Other abbreviations as follows: olfactory bulb (OB), forceps minor of the corpus callosum (FMI), corpus callosum (CC), dorsal AC (dAC), ventral AC (vAC), optic chiasm (OX).
The mPFC is predominantly comprised of glutamatergic pyramidal neurons (~80–90%) and an array of local interneuron populations (~10–20%). There are several different populations of interneurons that can be defined by phenotypic expression of parvalbumin (PV), cholecystokinin (CCK), vasoactive intestinal polypeptide (VIP), calretinin, calbindin, and/or somatostatin [23,27]. The majority of GABAergic interneurons express parvalbumin (~52%), and are thought to regulate glutamatergic output and regulate theta oscillations via perisomatic contacts onto pyramidal neurons [28]. Somatostatin neurons also are thought to regulate glutamatergic output via synaptic contacts onto the dendritic trees of glutamatergic pyramidal neurons [29]. These two interneuronal populations may be under the control of another interneuronal subtype, the VIP neurons [29]. Thus, the prefrontal glutamatergic neurons are under stringent regulatory control by local interneuronal circuits (see Figure 2).
Figure 2.
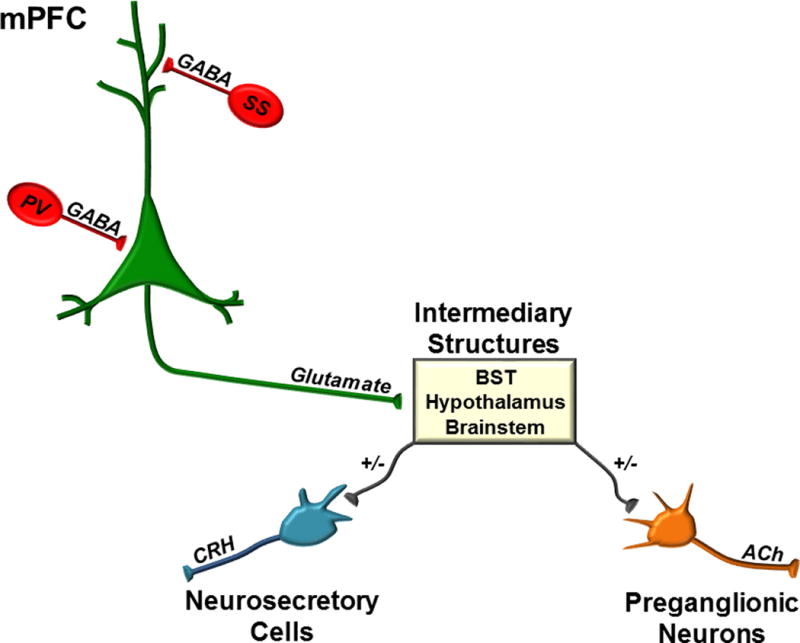
Intrinsic Circuitry and Efferents of the mPFC. The mPFC is under tight regulatory control of local interneuron populations that limit the predominantly glutamatergic output of the mPFC. Somatostatin (SS) and parvalbumin (PV) are two of the primary interneuron subtypes within the mPFC that provide dendritic and perisomatic inhibition, respectively. The mPFC projects to numerous subcortical and hindbrain targets, e.g. the bed nucleus of the stria terminalis (BST), hypothalamic subnuclei, and brainstem that mediate its effects on neuroendocrine and autonomic responses to stress. Other abbreviations as follows: corticotropin-releasing hormone (CRH) and acetylcholine (ACh).
Stress at the Synaptic Level in the mPFC
We are only now just beginning to understand how stress affects neurotransmission in the mPFC. There is substantial evidence that glucocorticoids have profound effects on prefrontal-mediated learning and memory, which begin with changes in neurotransmission at the synapse. Most studies of neurotransmission in the mPFC indicate that acute stress increases excitation of glutamatergic output neurons [30]. Acute stress increases extracellular glutamate and increases depolarization-evoked glutamate release via a GR-dependent mechanism [31]. In adolescent rats, acute stress increases NMDA- and AMPA-mediated excitatory currents [32], also adding support to the notion that stress acutely increases excitatory neurotransmission.
Repeated restraint stress, chronic unpredictable stress, or chronic corticosterone treatment decrease the dendritic complexity of pyramidal neurons [33,34]. Therefore, it is thought that under chronic stress the excitability of glutamatergic pyramidal neurons is diminished. This appears to be the case in adolescent rats, as chronic stress decreases NMDA- and AMPA-mediated currents in the mPFC through increased degradation of postsynaptic glutamate receptors [35]. Notably, the mPFC is still developing during adolescence, which is a period marked with pruning of prefrontal glutamatergic synapses, as well as increasing GABA [36]. Thus, at this point it is unclear whether increased degradation of glutamate receptors is due to chronic stress alone or stress/development interactions (see [37]). In line with this work, however, chronic corticosterone administration in adult animals decreases expression of NMDA subunit NR2B and AMPA subunits GluR2/GluR3 in the ventral mPFC [38]. Moreover, recent work correlates stress-induced decreases in glutamatergic pyramidal complexity in deep layer (V) of the mPFC with reduced excitatory neural responses to serotonin [39,40]. Thus, overall it appears that acute stress increases excitatory neurotransmission in the mPFC, while chronic stress adaptation may promote a decrease in excitability of the prefrontal glutamatergic output neurons.
Complementing the effects of acute stress on excitatory neurotransmission, it appears that glucocorticoids acutely decrease inhibitory neurotransmission via an endocannabinoid-dependent mechanism, leading to disinhibition of prefrontal glutamatergic neurons [41]. Conversely, repeated restraint stress increases the complexity of prefrontal GABAergic interneurons [42], suggesting that chronic stress enhances the ability of GABAergic neurons to inhibit glutamatergic neurons. Thus, by altering the balance between excitatory and inhibitory neurotransmission, glucocorticoids are able to induce a shift toward excitation acutely and inhibition chronically, setting the gain of prefrontal influence on downstream targets depending on context.
The actions of glucocorticoids at the level of the synapse have been extensively studied in other regions, including the hippocampus, amygdala, and hypothalamus. Through all of these studies, a common pattern generally emerges: glucocorticoids rapidly increase excitatory neurotransmission via the MR and suppress transiently increased excitatory transmission via the GR [1,43]. The latter may occur to protect information acquired during the stressful experience and promote anticipatory responses to future stressors [43]. Whether this same pattern can be generalized to the mPFC still remains to be determined, as the actions of glucocorticoids are very spatially and temporarily distinct. For instance, though responses to glucocorticoids in the BLA and hippocampus follow the same general abovementioned pattern, responses to glucocorticoids in the amygdala are much more prolonged in the BLA, a GR-mediated effect [44]. Similarly, in adolescent rats, acute stress induces sustained glutamatergic currents in the mPFC and enhances working memory via GR activation [45]. Thus, the exact mechanisms by which glucocorticoids exert their synaptic effects in the adult prefrontal cortex have yet to be elucidated. Given the high GR:MR ratio in the mPFC, however, it stands to reason that the prefrontal GR is vital to anticipatory responses to stress.
Prefrontal Regulation of ANS
The mPFC is well-positioned to modulate the ANS. The mPFC indirectly regulates the ANS through projections to pre-autonomic nuclei in the brainstem, including the NTS [23,46,47]. Indeed, excitotoxic and acute chemical lesions indicate that the mPFC can modify the parasympathetic component of the baroreflex [46,47]. Moreover, Correa and colleagues demonstrated that glutamate in the mPFC modulates baroreflex function [48]. Additionally, the ventral mPFC (including ilPFC) sends some projections to the RVLM, and electrical stimulation of the ventral mPFC in anesthetized rats initiates depressor responses accompanied by inhibition of neurons in the RVLM [49]. Less is known about specific dorsal mPFC circuits that regulate autonomic activity; however, plPFC innervates a population of neurons in the aBST that are activated by stress and thought to mediate inhibitory responses [26] (see Figure 2). Specific studies of mPFC subregional regulation of autonomic stress responses reveal interesting differences between ilPFC and plPFC. Distinct lesions of the plPFC vs. ilPFC during acute restraint indicate that the plPFC is inhibitory and ilPFC excitatory for the tachycardiac response to stress [50]. Moreover, stimulation of the plPFC increases parasympathetic activity, whereas stimulation of ilPFC promotes sympathetic activation [51]. Lesions of the ilPFC also attenuate conditioned tachycardia and pressor responses in unanesthetized, freely-moving rats [52,53]. Thus, the lesion and stimulation literature suggests that the plPFC appears to inhibit the sympathetic nervous system, while the ilPFC may drive sympathetic activation in response to acute stress.
Prefrontal Regulation of HPA axis
Prefrontal regulation of the HPA axis was first uncovered using lesion and corticosterone implant studies. Meaney and colleagues showed that lesions of the dorsal mPFC (prelimbic) attenuate negative feedback of the HPA axis, whereas corticosterone implants into the same region decrease corticosterone levels [20]. Since then, others have reproduced these effects suggesting that the plPFC acutely regulates negative feedback of the HPA axis [19,54]. Our lab recently demonstrated that glucocorticoids act at the plPFC GR to regulate negative feedback acutely. However, we also found that glucocorticoids act at the ilPFC GR to decrease corticosterone secretion, in contrast to prior lesion studies (McKlveen et al., 2013; Radley et al., 2006). Moreover, we found that glucocorticoid inhibition during chronic stress specifically involves the infralimbic, but not the prelimbic, GR [21]. Thus, acutely, glucocorticoids act via the plPFC and ilPFC GR to inhibit the HPA axis, whereas feedback under chronic stress is mediated primarily by the ilPFC.
The mPFC does not have direct projections to the PVN. Instead, the mPFC projects to intermediary regions, which in turn project to the PVN to regulate the HPA axis (see Figure 2). The plPFC sends glutamatergic projections to the aBST, which in turn sends GABAergic projections to the PVN to decrease corticosterone responses to acute stress [26]. The intermediary synaptic connections between the ilPFC and PVN has yet to be discovered. The ilPFC projects directly to the NTS (and perhaps to the NTS indirectly through the CeA), which in turn provides excitatory input to the PVN [22]. Whether the ilPFC initiates inhibition directly within this region to brake its excitatory drive to the PVN remains to be determined [55]. Additionally, the ilPFC activates aBST neurons that project to the ventral tegmental area [56], although the relevance of this circuit for stress responding is unknown. Studies from our group suggest additional ilPFC targets, such as the posterior hypothalamus (PH), may also play a role in infralimbic regulation of the HPA axis response to stress [25].
Prefrontal Regulation of Behavioral Responses to Stress
A number of signature behavioral functions are attributed to the mPFC, including working memory, behavioral flexibility, decision-making, and planning [57]. All of these processes require the ability to reference past experiences to prospectively plan an appropriate response for the given context. Working memory, in particular, requires the ability to transiently maintain a ‘mental sketch pad’ of recent information to direct subsequent actions [58]. Stress has a profound effect on each of these processes, inducing changes that are important for adaptation in the face of ongoing stress.
Acute as well as chronic stress impairs working memory [59–61]. It is thought that this occurs to minimize competing information, as acute stress increases memory consolidation of the salient event at the expense of working memory via the same mechanism [60]. The dopamine system has also been implicated in impairments of spatial working memory, including both hyper- and hypo-dopaminergic function, suggesting that optimal dopamine function is essential for prefrontal regulation of working memory [59,62] . Moreover, chronic stress is thought to impair spatial working memory through a dopamine receptor-1-mediated hypo-dopaminergic mechanism in the mPFC [59].
Lesions of the mPFC demonstrate the importance of the mPFC for flexibility and attentional set-shifting [63,64]. Both acute and chronic stress impair performance in behavioral flexibility and set-shifting paradigms, which require the ability to inhibit a previously learned response [33,34,65]. Decision-making and planning are also primary executive functions of the mPFC that are negatively impacted by stress [66,67]. Acute stress impairs performance of the delayed spatial win-shift task, a prefrontal-mediated task, which requires animals to use information acquired prior to a delay to prospectively plan movement to obtain a reward [68]. Acute administration of corticosterone systemically or directly into the ilPFC rapidly impairs the decision-making ability of rats in a rodent version of the Iowa Gambling Task, a prototypic tool used in humans to assess decision-making [69,70]. Chronic stress also promotes habitual behavior in rats exposed to two operant tasks requiring them to make goal-directed decisions. This shift to habitual behavior is accompanied by atrophy of the mPFC [71]. Moreover, both chronic corticosterone and GR blockade impair decision-making based on action-outcome contingencies [72,73].
The mPFC is also important for the regulation of affect, commonly described in rodents as depression-like behavior (i.e. immobility) in the forced swim test (FST). The FST was first developed with the notion that animals placed in a container of water with no escape will continue to swim as long as possible to avoid the aversive situation. Eventually, animals will display ‘helplessness’ by ceasing to swim (floating or sinking). The general interpretation for this assay is that animals who ‘give up’ sooner in the test are more ‘helpless’ or exhibit depression-like behavior. There are two versions of this test: the classic Porsolt version, as well as the modified FST. The Porsolt FST involves two days of testing to ensure a high degree of immobility during the second session (generally 24 hours after the first session) (for an excellent review of the Porsolt FST procedure, see; [74]), whereas the modified FST involves a single exposure to obviate the potential confound of memory effects [75]. This test is widely used as an assay of despair, as chronic stress increases immobility in the FST and antidepressants reverse this effect. However, it is important to consider that immobility in the FST may be an adaptive response. Thus, increased immobility may not necessarily indicate ‘helplessness’, but rather may be a way for the animal to conserve energy. Moreover, animals undergoing chronic stress frequently lose significant weight, suggesting changes in energy balance that may make an animal more likely to become immobile to conserve energy. Therefore, it is important to temper interpretations of immobility behavior in this assay in light of potential adaptive mechanisms, as we have suggested previously [1,76].
Nevertheless, a wide body of literature has suggested that the mPFC may be involved in modulating behavior in this task. Knockdown of GR confined to the ilPFC increases immobility in the FST [21]. Notably, a homologous region is implicated in major depressive disorder (MDD) in humans, and deep brain stimulation (DBS) of this region in rats has an ‘antidepressant’-like effect in the FST [77]. Further, DBS of the ventral mPFC ameliorates stress-induced anhedonia-like behavior in the sucrose preference test [78]. Ibotenate lesions of the ventral mPFC did not block the effects of DBS [77]. Thus, spared fibers of passage may be involved in the effects of ventral mPFC DBS. However, optogenetic stimulation of mPFC cell bodies prevents social avoidance engendered by repeated social defeat and specific prefrontal glutamatergic synapses modulate coping style in the FST [79,80],supporting the importance of ventral mPFC cells in behavioral regulation under normal physiological conditions. In addition to mood and affective regulation, a substantial body of evidence [40,66] indicates that mPFC subregions are involved in distinct aspects of fear memory [40]. In particular, the ilPFC seems to be critical for extinction of conditioned fear [81].
The aggregate data suggest that the mPFC is particularly important for coordinating appropriate behavior for context-specific adaptation through sustained attention and flexible inhibition of inappropriate responses [1]. Chronic stress in particular seems to impair this process, taking the prefrontal cortex ‘offline,’ and induces a shift toward more habitual, less goal-oriented responses in rodents [82].
Clinical Evidence of Prefrontal Involvement in Stress-related Neuropsychiatric Disorders
Perhaps one of the best and probably most well-known representations of prefrontal cortical dysfunction lies in the case of Phineas Gage [83]. Indeed, damage to or lesions of the PFC cause working memory impairment [84,85], cognitive inflexibility [86,87], impairments in decision making [88,89], poor planning ability [90,91], and disrupted emotional processing [89,92]. Phineas Gage displayed deficits in many of these mPFC-mediated functions, as documented by Harlow shortly after Gage’s accident, including decision-making, working memory, and planning. He also particularly had difficulty with emotional processing to the point that it was said that he was “no longer Gage.” Thus, Gage represents a prime example of how damage to the mPFC can lead to deficits in these signature mPFC-mediated tasks. As discussed previously, these are all executive functions also affected by stress in rodents. Numerous studies have demonstrated that these behaviors are affected by stress in humans and are disrupted in patients with stress-related disorders, such as MDD [92].
As discussed previously, the dopaminergic system has been implicated in working memory deficits following stress. Likewise, the dopaminergic system is thought to potentially be involved in the pathogenesis of MDD, as patients with Parkinson’s Disease have a 2–4 fold increase in risk for developing MDD [92,93]. Further, patients with MDD tend to have significant impairments in working memory [94,95]. Thus, stress leads to disturbances in the dopaminergic system that may contribute to symptoms of stress-related neuropsychiatric disorders.
The Wisconsin-card-sorting test (WCST) is the prototypic test for measuring cognitive flexibility in humans. Subjects must be able to flexibly alter their behavior by sorting a deck of cards according to rules that change randomly throughout the task [96]. Thus, they must be able to flexibly switch their behavior and inhibit a previously correct response. Patients with lesions of the mPFC are unable to complete this task successfully. Likewise, patients with MDD have significant impairment (i.e. increased perseveration) in the WCST [97]. Acute stress also impairs cognitive flexibility, as individuals exposed to the Trier Social Stress Test (TSST) show impairments in ability to inhibit a previously learned response [98].
There is a significant amount of data suggesting that stress-induced deficits in rodent prefrontal function may be similar to that of humans with MDD. Dendritic atrophy of glutamatergic neurons, as well as glial loss, are commonly observed in the PFC of chronically stressed rats, as discussed previously [33,34,99]. Similarly, in humans, MDD is associated with reductions of gray matter volume in the left subgenual cingulate cortex using both MRI [92,100] and histological measures [92]. These changes in rodents following stress and in humans with MDD are thought to share a common mechanism [92,101]. Moreover, the subgenual cingulate cortex (Brodmann area 25) shows hyper- or hypo-activation, depending on the population of patients tested [100,102]. In addition, chronic stress is associated with atrophy and decreased activation of the mPFC that are accompanied by deficits in decision-making and goal-directed behavioral flexibility [103]. Many cortical functions are lateralized and human studies of MDD tend to identify altered activity specific to the right hemisphere of the mPFC [104]. Moreover, in human, the right hemisphere is dominant in the regulation of negative emotional states and the regulation of neuroendocrine and autonomic responses to stress [105]. Interestingly, lesion studies in rodents indicate that prefrontal regulation of physiological and behavioral stress responses may be lateralized to the right hemisphere [105]. The lateralized differences in prefrontal function become especially relevant in the treatment of MDD. For instance, transcranial magnetic resonance (TMS) can be used to stimulate (high frequencies) or suppress (low frequencies) cerebral metabolism. Stimulation of the left PFC or suppression of the right PFC improves depressive symptoms, thus highlighting the importance of PFC lateralization [105]. Thus, hemisphere-specific alterations in morphology, volume, and activation of mPFC in response to stress may underlie the changes observed in patients with MDD. Further, these alterations likely account for deficits in behavioral flexibility, decision-making, planning, and mood observed in chronically stressed individuals and patients with MDD.
The Prefrontal Cortex as a Coordinator of Stress Adaptation
Herein, we propose that the mPFC acts as a coordinator of stress adaptation planning, developing, and integrating the efforts of multiple brain regions and energetic systems to generate a context-specific, appropriate behavioral response (Figure 3). The mPFC is well-positioned to act as a coordinator of autonomic and neuroendocrine responses, supporting the energetic mobilization needed for behavioral adaptation. We recently discussed the importance of context-specific energetics and how chronic stress may lead to pathology exceeding the adaptive capacity of the individual [1]. Here, we propose that prolonged activation of energetic systems or misappraisal of energetic need represent two adaptive costs that could underlie pathology. Further, stress-related illnesses, including MDD, are characterized by physiological, cognitive, and affective responses that are inappropriate to environmental context, indicating that adaptive costs may contribute to MDD. Given the role of the mPFC in appraisal (i.e. decision-making), the area may serve as an important integrator of energetic systems (i.e. HPA axis and ANS) that support appropriate behavioral responses to context.
Figure 3.
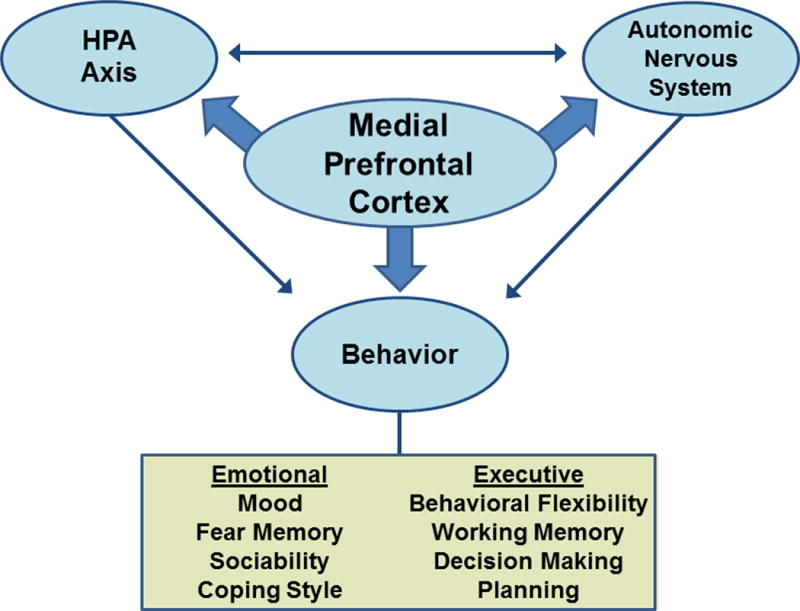
Prefrontal Coordination of Energetic Systems. A schematic representation of the potential role that the medial prefrontal cortex could play in coordinating activity between the autonomic and hypothalamic-pituitary-adrenocortical (HPA) axis to regulate both emotional/reactive and executive functions in response to environmental stimuli.
An example of prefrontal coordination of responses to stress comes from groups of men and women with mPFC damage. These individuals report more subjective ‘stress’ in the TSST. Despite reporting more stress, men with greater mPFC damage had decreased cortisol accompanied by increased heart rate. Women, in contrast, had an increased cortisol response to the TSST, with no effect on autonomic regulation [106]. Thus, damage to the mPFC results in a disconnect between subjective experience (i.e. behavior), neuroendocrine responses, and autonomic regulation. Thus, misappraisal of contextual circumstance severely compromises the ability of these individuals to appropriately coordinate behavior with the physiological systems driving energy mobilization.
Similarly, patients with mPFC damage are severely impaired in a gambling task, making the wrong decision long after they know the correct strategy [107]. Individuals without mPFC damage generate a sympathetically-mediated skin conductance response when they are considering a risky decision, whereas patients with mPFC damage do not generate this anticipatory response [107]. In spite of knowing the strategy that they should adopt, patients with mPFC damage are unable to execute effective behavioral responses, perhaps related to an inability to mobilize energetic resources to generate behavioral flexibility.
In summary, the mPFC is an important integrator of the neuroendocrine and autonomic energetic systems that drive appropriate responding in specific contexts. Dysregulated activity of the mPFC prevents appropriate responses to stress, and the inability to correctly coordinate these responses may lie at the heart of neuropsychiatric disorders linked to stress and prefrontal dysfunction.
The Prefrontal Cortex, Glucocorticoids, and Cellular Energetics
On the systems level, it has been proposed that the brain reallocates energetic resources to one network by suspending energetic resources in another [108]. For instance, acute stress may funnel energetic resources toward a ‘salience network’ (comprised of the amygdala and striatum) at the expense of energetic resources in the ‘executive network’ (e.g. the mPFC) [108]. The underlying mechanisms governing these shifts are largely unknown, but may involve glucocorticoid effects on astrocytes, neurotropic factors, neurotransmitters, mitochrondral function, and/or hormones. Further, reallocation or suppression of energetic resources in the mPFC may underlie deficits in prefrontal-mediated function associated with chronic stress or neuropsychiatric disorders, e.g. MDD.
One of the primary functions of glucocorticoids is to mobilize energy. The name ‘glucocorticoid’ itself is comprised of ‘gluco,’ cort,’ and ‘oid’, reflecting that it is a steroid produced in the adrenal cortex that mobilizes glucose. A very-thorough, recent review by Hermans et al. highlights the potential role of neuromodulatory system processes that may be involved in active reallocation of energetic resources to the ‘salience network’ [108]. Herein, we will focus on how glucocorticoids might funnel energetic resources away from the ‘executive network’ to the ‘salience network’ (under acute stress and perhaps in a sustained manner under chronic stress) by active suppression within the mPFC. In brain, high levels of glucocorticoids can stunt glucose mobilization through inhibition of glial glucose transport [109] and impair neuronal viability [110]. Glucocorticoids can also inhibit neurotrophic molecules such as brain-derived neurotrophic factor (BDNF), which may underlie chronic stress effects on glutamatergic neuronal plasticity in the mPFC [111–113]. Mitochondrial function (which provides ATP for neurotransmitter synthesis, ion transport, and endocytosis) is also impacted by glucocorticoids, as the GR can translocate into mitochondria and has dose-dependent effects on calcium uptake and reactive oxygen species (ROS) production [114,115]. High or chronic levels of glucocorticoids impair mitochondrial function (increasing calcium levels and ROS production), which may lead to decreased neuronal viability [115]. Mitochondrial stress is also associated with impairments in working memory, demonstrating how suppression of energetic resources locally in the mPFC can ultimately affect behavior [116]. Further, glucocorticoids downregulate the prefrontal GR, limiting the ability of the system to inhibit glucocorticoid secretion [117,118]. Thus, though not an exhaustive list, high or chronic levels of glucocorticoids profoundly suppress a number of local energetic resources that may compromise prefrontal function and ultimately the ability of the prefrontal cortex to coordinate stress adaptation.
Conclusion
The primary task for any coordinator is to plan, develop, and integrate resources to achieve a goal. We propose that the prefrontal cortex is an important coordinator of behavioral, neuroendocrine, and autonomic responses particularly to anticipatory stressors. The ability to reference past experiences and generate a context-specific response is appropriate for the mPFC given its role in executive functions, including: planning, decision-making, and response inhibition, as we have discussed throughout this review. While the mPFC can be involved in reflexive responses, it is poised to play a greater role in the coordination of anticipatory responses to stress that do not necessarily necessitate a response on the same, rapid time-scale. Thus, future studies aimed at testing stress effects on prefrontal function should make the distinction between anticipatory and reflexive responses, perhaps focusing on the former as more indicative of prefrontal participation.
The distinction between anticipatory and reflexive stress is an important one to make in stress neurobiology, because much of modern day stress is internally driven or psychogenic in nature [119]. For instance, one may become ‘stressed’ by financial, familial, or work-place issues, leading to activation of neuroendocrine and autonomic systems. These potent modern life stressors mobilize energetic systems in the absence of a direct threat to survival and can ultimately affect behavior, e.g. mood. Given the strong link between stress and neuropsychiatric disorders, understanding how anticipatory stressors might lead to alterations in stress adaptation is of paramount importance in the development of effective treatments for stress-related neuropsychiatric disorders.
Acknowledgments
J.M. McKlveen is supported by National Institutes of Health (NIH) Grant MH097430. J.P. Herman is supported by NIH Grants MH069860 and MH049698.B. Myers is supported by American Heart Association Grant 17070152 and NIH Grant HL122454.
Bibliography
- 1.Myers B, McKlveen JM, Herman JP. Glucocorticoid actions on synapses, circuits, and behavior: Implications for the energetics of stress. Front Neuroendocrinol. 2014;35(2):180–196. doi: 10.1016/j.yfrne.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benarroch EE. Central Autonomic Control. In: Robertson D, Biaggioni I, Burnstock G, Low PS, Paton JFR, editors. Primer on the Autonomic Nervous System. 3rd. San Diego, CA: Academic Press Inc; 2012. pp. 9–12. [Google Scholar]
- 4.Ziegler DR, Edwards MR, Ulrich-Lai YM, Herman JP, Cullinan WE. Brainstem origins of glutamatergic innervation of the rat hypothalamic paraventricular nucleus. J Comp Neurol. 2012;520(11):2369–2394. doi: 10.1002/cne.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 6.Rivier C, Vale W. Interaction of corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo. Endocrinology. 1983;113(3):939–942. doi: 10.1210/endo-113-3-939. [DOI] [PubMed] [Google Scholar]
- 7.Dallman MF, Jones MT. Corticosteroid feedback control of ACTH secretion: effect of stress-induced corticosterone ssecretion on subsequent stress responses in the rat. Endocrinology. 1973;92(5):1367–1375. doi: 10.1210/endo-92-5-1367. [DOI] [PubMed] [Google Scholar]
- 8.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 9.Almawi WY, Abou Jaoude MM, Li XC. Transcriptional and post-transcriptional mechanisms of glucocorticoid antiproliferative effects. Hematol Oncol. 2002;20(1):17–32. doi: 10.1002/hon.684. [DOI] [PubMed] [Google Scholar]
- 10.Prager EM, Johnson LR. Stress at the synapse: signal transduction mechanisms of adrenal steroids at neuronal membranes. Sci Signal. 2009;2(86):re5. doi: 10.1126/scisignal.286re5. [DOI] [PubMed] [Google Scholar]
- 11.Beato M, Sanchez-Pacheco A. Interaction of steroid hormone receptors with the transcription initiation complex. Endocr Rev. 1996;17(6):587–609. doi: 10.1210/edrv-17-6-587. [DOI] [PubMed] [Google Scholar]
- 12.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19(3):269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 13.De Kloet ER, Reul JM. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12(2):83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- 14.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117(6):2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 15.Joels M, Karst H, Kruegers HJ, de Kloet ER. Corticosteroid actins on electrical activity in the brain. In: Etgen AM, Pfaff DW, editors. Molecular Mechanisms of Hormone Actions on Behavior. San Diego, CA: Academic Press Inc; 2009. pp. 306–323. [Google Scholar]
- 16.Fuxe K, Wikstrom AC, Okret S, Agnati LF, Harfstrand A, Yu ZY, et al. Mapping of glucocorticoid receptor immunoreactive neurons in the rat tel- and diencephalon using a monoclonal antibody against rat liver glucocorticoid receptor. Endocrinology. 1985;117(5):1803–1812. doi: 10.1210/endo-117-5-1803. [DOI] [PubMed] [Google Scholar]
- 17.Reul JM, de Kloet ER. Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. J Steroid Biochem. 1986;24(1):269–272. doi: 10.1016/0022-4731(86)90063-4. [DOI] [PubMed] [Google Scholar]
- 18.Myers B, McKlveen JM, Herman JP. Neural regulation of the stress response: The many faces of feedback. Cell Mol Neurobiol. 2012;32(5):683–694. doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akana SF, Chu A, Soriano L, Dallman MF. Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. J Neuroendocrinol. 2001;13(7):625–637. doi: 10.1046/j.1365-2826.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 20.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13(9):3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKlveen JM, Myers B, Flak JN, Bundzikova J, Solomon MB, Seroogy KB, et al. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry. 2013;74(9):672–679. doi: 10.1016/j.biopsych.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 23.Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492(2):145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 24.Vogt BA, Vogt JL, Farber NB. Cingulate Cortex and models of disease. In: Paxinos G, editor. The Rat Nervous System. 3rd. New York, NY: Oxford Univeristy Press; 2004. pp. 705–727. [Google Scholar]
- 25.Myers B, Carvalho-Netto EF, Wu C, Wick D, Solomon MB, Ulrich-Lai YM, Herman JP. Stress-activated corticolimbic efferents innervate the posterior hypothalamic nucleus: a novel circuit for stimulating neuroendocrine stress responses. Soc Neurosci Abstract. 2012 [Google Scholar]
- 26.Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29(22):7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubota Y, Hattori R, Yui Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 1994;649(1–2):159–173. doi: 10.1016/0006-8993(94)91060-x. [DOI] [PubMed] [Google Scholar]
- 28.Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505(7481):92–96. doi: 10.1038/nature12755. [DOI] [PubMed] [Google Scholar]
- 29.Pi H-J, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503(7477):521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13(1):22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, et al. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One. 2010;5(1):e8566. doi: 10.1371/journal.pone.0008566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, et al. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16(2):156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OFX, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25(34):7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26(30):7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73(5):962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, et al. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol. 2010;518(14):2693–2709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry. 2013;3:e238. doi: 10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34(3):707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu R-J, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA. 2008;105(1):359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33(6):773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT-Y, et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci. 2011;31(29):10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilabert-Juan J, Castillo-Gomez E, Guirado R, Molto MD, Nacher J. Chronic stress alters inhibitory networks in the medial prefrontal cortex of adult mice. Brain Struct Funct. 2013;218(6):1591–1605. doi: 10.1007/s00429-012-0479-1. [DOI] [PubMed] [Google Scholar]
- 43.Joels M, Sarabdjitsingh RA, Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol Rev. 2012;64(4):901–938. doi: 10.1124/pr.112.005892. [DOI] [PubMed] [Google Scholar]
- 44.Karst H, Berger S, Erdmann G, Schutz G, Joels M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci USA. 2010;107(32):14449–14454. doi: 10.1073/pnas.0914381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci USA. 2009;106(33):14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Resstel LBM, Fernandes KBP, Correa FMA. Medial prefrontal cortex modulation of the baroreflex parasympathetic component in the rat. Brain Res. 2004;1015(1–2):136–144. doi: 10.1016/j.brainres.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 47.Verberne AJ, Lam W, Owens NC, Sartor D. Supramedullary modulation of sympathetic vasomotor function. Clin Exp Pharmacol Physiol. 1997;24(9–10):748–754. doi: 10.1111/j.1440-1681.1997.tb02126.x. [DOI] [PubMed] [Google Scholar]
- 48.Resstel LBM, Correa FMA. Medial prefrontal cortex NMDA receptors and nitric oxide modulate the parasympathetic component of the baroreflex. Eur J Neurosci. 2006;23(2):481–488. doi: 10.1111/j.1460-9568.2005.04566.x. [DOI] [PubMed] [Google Scholar]
- 49.Verberne AJ. Medullary sympathoexcitatory neurons are inhibited by activation of the medial prefrontal cortex in the rat. Am J Physiol. 1996;270(4 Pt 2):R713–R719. doi: 10.1152/ajpregu.1996.270.4.R713. [DOI] [PubMed] [Google Scholar]
- 50.Tavares RF, Correa FMA, Resstel LBM. Opposite role of infralimbic and prelimbic cortex in the tachycardiac response evoked by acute restraint stress in rats. J Neurosci Res. 2009;87(11):2601–2607. doi: 10.1002/jnr.22070. [DOI] [PubMed] [Google Scholar]
- 51.Powell DA, Maxwell B, Penney J. Neuronal activity in the medial prefrontal cortex during Pavlovian eyeblink and nictitating membrane conditioning. J Neurosci. 1996;16(19):6296–6306. doi: 10.1523/JNEUROSCI.16-19-06296.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on respiration, “freezing,” and ultrasonic vocalizations during conditioned emotional responses in rats. Cereb Cortex. 1991;1(5):418–425. doi: 10.1093/cercor/1.5.418. [DOI] [PubMed] [Google Scholar]
- 53.Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Res. 1994;643(1–2):181–193. doi: 10.1016/0006-8993(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 54.Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26(50):12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Jalabert M, Aston-Jones G, Herzog E, Manzoni O, Georges F. Role of the bed nucleus of the stria terminalis in the control of ventral tegmental area dopamine neurons. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1336–1346. doi: 10.1016/j.pnpbp.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Broersen LM. Attentional processes and learning and memory in rats: the prefrontal cortex and hippocampus compared. Prog Brain Res. 2000;126:79–94. doi: 10.1016/S0079-6123(00)26008-1. [DOI] [PubMed] [Google Scholar]
- 58.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 59.Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20(4):1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B. Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proc Natl Acad Sci USA. 2010;107(38):16655–16660. doi: 10.1073/pnas.1011975107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arnsten AFT, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76(1):223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55(4):362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- 63.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20(11):4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17(5):1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33(2):320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- 66.Graybeal C, Kiselycznyk C, Holmes A. Stress-induced impairments in prefrontal-mediated behaviors and the role of the N-methyl-D-aspartate receptor. Neuroscience. 2012;211:28–38. doi: 10.1016/j.neuroscience.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Graham LK, Yoon T, Kim JJ. Stress impairs optimal behavior in a water foraging choice task in rats. Learn Mem. 2010;17(1):1–4. doi: 10.1101/lm.1605510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Butts KA, Weinberg J, Young AH, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proc Natl Acad Sci USA. 2011;108(45):18459–18464. doi: 10.1073/pnas.1111746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koot S, Baars A, Hesseling P, van den Bos R, Joels M. Time-dependent effects of corticosterone on reward-based decision-making in a rodent model of the Iowa Gambling Task. Neuropharmacology. 2013;70:306–315. doi: 10.1016/j.neuropharm.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 70.Koot S, Koukou M, Baars A, Hesseling P, van ’t Klooster J, Joels M, et al. Corticosterone and decision-making in male Wistar rats: the effect of corticosterone application in the infralimbic and orbitofrontal cortex. Front Behav Neurosci. 2014;8:127. doi: 10.3389/fnbeh.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325(5940):621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 72.Gourley SL, Swanson AM, Jacobs AM, Howell JL, Mo M, Dileone RJ, et al. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc Natl Acad Sci USA. 2012;109(50):20714–20719. doi: 10.1073/pnas.1208342109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swanson AM, Shapiro LP, Whyte AJ, Gourley SL. Glucocorticoid receptor regulation of action selection and prefrontal cortical dendritic spines. Commun Integr Biol. 2013;6(6):e26068. doi: 10.4161/cib.26068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castagne Vincent, Moser Paul, Porsalt Roger D. Behavioral Assessment of Antidepressant Activity in Rodents. In: JJ B, editor. Methods Behav Anal Neurosci. 2nd. Boca Raton, Florida: CRC Press; 2009. [PubMed] [Google Scholar]
- 75.Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005;182(3):335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- 76.Herman JP. Neural control of chronic stress adaptation. Front Behav Neurosci. 2013;7:61. doi: 10.3389/fnbeh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hamani C, Nobrega JN, Lozano AM. Deep brain stimulation in clinical practice and in animal models. Clin Pharmacol Ther. 2010;88(4):559–562. doi: 10.1038/clpt.2010.133. [DOI] [PubMed] [Google Scholar]
- 78.Hamani C, Machado DC, Hipolide DC, Dubiela FP, Suchecki D, Macedo CE, et al. Deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: role of serotonin and brain derived neurotrophic factor. Biol Psychiatry. 2012;71(1):30–35. doi: 10.1016/j.biopsych.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Covington HE, 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30(48):16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim S-Y, et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492(7429):428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36(2):529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harlow JM. Passage of an Iron Road Through the Head. Bost Med Surg J. 1868;39:389–393. [PMC free article] [PubMed] [Google Scholar]
- 84.Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex. 2013;49(5):1195–1205. doi: 10.1016/j.cortex.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baier B, Karnath H-O, Dieterich M, Birklein F, Heinze C, Muller NG. Keeping memory clear and stable–the contribution of human basal ganglia and prefrontal cortex to working memory. J Neurosci. 2010;30(29):9788–9792. doi: 10.1523/JNEUROSCI.1513-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Milner B, Petrides M, Smith ML. Frontal lobes and the temporal organization of memory. Hum Neurobiol. 1985;4(3):137–142. [PubMed] [Google Scholar]
- 87.Colvin MK, Dunbar K, Grafman J. The effects of frontal lobe lesions on goal achievement in the water jug task. J Cogn Neurosci. 2001;13(8):1129–1147. doi: 10.1162/089892901753294419. [DOI] [PubMed] [Google Scholar]
- 88.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 89.Levens SM, Larsen JT, Bruss J, Tranel D, Bechara A, Mellers BA. What might have been? The role of the ventromedial prefrontal cortex and lateral orbitofrontal cortex in counterfactual emotions and choice. Neuropsychologia. 2014;54:77–86. doi: 10.1016/j.neuropsychologia.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex. 1996;6(2):215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- 91.Muller NG, Machado L, Knight RT. Contributions of subregions of the prefrontal cortex to working memory: evidence from brain lesions in humans. J Cogn Neurosci. 2002;14(5):673–686. doi: 10.1162/08989290260138582. [DOI] [PubMed] [Google Scholar]
- 92.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Santamaria J, Tolosa E, Valles A. Parkinson’s disease with depression: a possible subgroup of idiopathic parkinsonism. Neurology. 1986;36(8):1130–1133. doi: 10.1212/wnl.36.8.1130. [DOI] [PubMed] [Google Scholar]
- 94.Landro NI, Rund BR, Lund A, Sundet K, Mjellem N, Asbjornsen A, et al. Honig’s model of working memory and brain activation: an fMRI study. Neuroreport. 2001;12(18):4047–4054. doi: 10.1097/00001756-200112210-00038. [DOI] [PubMed] [Google Scholar]
- 95.Rose EJ, Ebmeier KP. Pattern of impaired working memory during major depression. J Affect Disord. 2006;90(2–3):149–161. doi: 10.1016/j.jad.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 96.Goldberg E, Bougakov D. Neuropsychologic assessment of frontal lobe dysfunction. Psychiatr Clin North Am. 2005;28(3):567–580. 578–9. doi: 10.1016/j.psc.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 97.Merriam EP, Thase ME, Haas GL, Keshavan MS, Sweeney JA. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. Am J Psychiatry. 1999;156(5):780–782. doi: 10.1176/ajp.156.5.780. [DOI] [PubMed] [Google Scholar]
- 98.Plessow F, Fischer R, Kirschbaum C, Goschke T. Inflexibly focused under stress: acute psychosocial stress increases shielding of action goals at the expense of reduced cognitive flexibility with increasing time lag to the stressor. J Cogn Neurosci. 2011;23(11):3218–3227. doi: 10.1162/jocn_a_00024. [DOI] [PubMed] [Google Scholar]
- 99.Banasr M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol Disord Drug Targets. 2007;6(5):311–320. doi: 10.2174/187152707783220929. [DOI] [PubMed] [Google Scholar]
- 100.Drevets WC, Price JL, Simpson JRJ, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 101.McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16(S1):S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- 102.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 103.Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques F, Palha JA, et al. Stress-induced changes in human decision-making are reversible. Transl Psychiatry. 2012;2:e131. doi: 10.1038/tp.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27(1–2):99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- 106.Buchanan TW, Driscoll D, Mowrer SM, Sollers JJ, 3rd, Thayer JF, Kirschbaum C, et al. Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. Psychoneuroendocrinology. 2010;35(1):56–66. doi: 10.1016/j.psyneuen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- 108.Hermans EJ, Henckens MJAG, Joels M, Fernandez G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37(6):304–314. doi: 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 109.Virgin CEJ, Ha TP, Packan DR, Tombaugh GC, Yang SH, Horner HC, et al. Glucocorticoids inhibit glucose transport and glutamate uptake in hippocampal astrocytes: implications for glucocorticoid neurotoxicity. J Neurochem. 1991;57(4):1422–1428. doi: 10.1111/j.1471-4159.1991.tb08309.x. [DOI] [PubMed] [Google Scholar]
- 110.Tombaugh GC, Yang SH, Swanson RA, Sapolsky RM. Glucocorticoids exacerbate hypoxic and hypoglycemic hippocampal injury in vitro: biochemical correlates and a role for astrocytes. J Neurochem. 1992;59(1):137–146. doi: 10.1111/j.1471-4159.1992.tb08884.x. [DOI] [PubMed] [Google Scholar]
- 111.Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15(3 Pt 1):1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813(1):112–120. doi: 10.1016/s0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- 113.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 114.Picard M, McEwen BS. Mitochondria impact brain function and cognition. Proc Natl Acad Sci USA. 2014;111(1):7–8. doi: 10.1073/pnas.1321881111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci USA. 2009;106(9):3543–3548. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hara Y, Yuk F, Puri R, Janssen WGM, Rapp PR, Morrison JH. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci USA. 2014;111(1):486–491. doi: 10.1073/pnas.1311310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience. 2003;119(3):887–897. doi: 10.1016/s0306-4522(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 118.Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(1):112–119. doi: 10.1016/j.pnpbp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 119.Karatsoreos IN, McEwen BS. Annual Research Review: The neurobiology and physiology of resilience and adaptation across the life course. J Child Psychol Psychiatry. 2013;54(4):337–347. doi: 10.1111/jcpp.12054. [DOI] [PubMed] [Google Scholar]


