Abstract
Aims: This study examined the role of endoplasmic reticulum (ER) stress in mediating chronic intermittent hypoxia (IH)-induced neurocognitive deficits. We designed experiments to demonstrate that ER stress is initiated in the hippocampus under chronic IH and determined its role in apoptotic cell death, impaired synaptic structure and plasticity, and memory deficits. Results: Two weeks of IH disrupted ER fine structure and upregulated ER stress markers, glucose-regulated protein 78, caspase-12, and C/EBP homologous protein, in the hippocampus, which could be suppressed by ER stress inhibitors, tauroursodeoxycholic acid (TUDCA) and 4-phenylbutyric acid. Meanwhile, ER stress induced apoptosis via decreased Bcl-2, promoted reactive oxygen species production, and increased malondialdehyde formation and protein carbonyl, as well as suppressed mitochondrial function. These effects were largely prevented by ER stress inhibitors. On the other hand, suppression of oxidative stress could reduce ER stress. In addition, the length of the synaptic active zone and number of mature spines were reduced by IH. Long-term recognition memory and spatial memory were also impaired, which was accompanied by reduced long-term potentiation in the Schaffer collateral pathway. These effects were prevented by coadministration of the TUDCA. Innovation and Conclusion: These results show that ER stress plays a critical role in underlying memory deficits in obstructive sleep apnea (OSA)-associated IH. Attenuators of ER stress may serve as novel adjunct therapeutic agents for ameliorating OSA-induced neurocognitive impairment. Antioxid. Redox Signal. 23, 695–710.
Introduction
Obstructive sleep apnea (OSA) is a very common breathing and sleep disorder characterized by intermittent hypoxia (IH) (14), which is mainly caused by the inspiratory collapse of the pharyngeal airway during sleep. OSA is a common disorder irrespective of age (22, 36) and is often associated with behavioral and neuropsychological deficits, including impaired learning and memory function (3, 38, 50, 63).
Although deficiencies in long-term synaptic plasticity have been reported based on animal models (63), which could help explain neurocognitive dysfunction in patients, the specific mechanisms underlying the chain of events from IH to cognitive impairment are still elusive. In fact, a large number of factors have been proposed to be involved, such as apoptosis, increased reactive oxygen species (ROS) production, excitotoxicity, decreased cAMP-responsive element-binding protein phosphorylation, nitric oxide production, inflammation, and reduced brain-derived neurotrophic factor (BDNF) production (13, 19, 20, 23, 35, 45, 70). Among these factors, increased levels of ROS and apoptotic neuronal cell death are strongly believed to contribute to brain damage underlying IH-induced cognitive impairment. There is evidence that the levels of ROS become elevated in repeated hypoxia and reoxygenation cycles (32, 62). Since one severe consequence of increased oxidative stress is the activation of the endoplasmic reticulum (ER) stress response, which could lead to various forms of cellular malfunction and even cell death via apoptosis (29, 53), ER stress may play a critical role in underlying chronic IH-induced impairment in neuroplasticity and memory function.
All secretory and integral membrane proteins are folded in the ER, which is also the site where proteins are post-translationally modified in ATP-dependent chaperone-mediated processes (28). Accumulation of unfolded or misfolded proteins in the ER affects cellular functions and will induce the unfolded protein response (UPR) to minimize the proteotoxicity caused by the defective proteins. The activation of the UPR sensors is controlled by the ER chaperone, glucose-regulated protein 78 (Grp78) (47).
Innovation.
Our study is the first comprehensive demonstration that endoplasmic reticulum (ER) stress induces cognitive impairment after intermittent hypoxia exposure in mice. Our findings identified that ER stress-induced apoptosis in neurons was increased by upregulation of C/EBP homologous protein and caspase-12, oxidative stress, and mitochondrial dysfunction. Furthermore, the morphology of synapses and spines was also altered likely due to ER stress-induced protein degradation, resulting in the weakening of synaptic connections. Both effects contribute to the impairment of long-term synaptic plasticity and memory impairment, which could be rescued by tauroursodeoxycholic acid, an inhibitor of ER stress. Our results suggest that suppression of ER stress activation may represent a novel treatment strategy for neuronal protection in obstructive sleep apnea.
Many studies in recent years have shown that ER stress contributes to a variety of disease conditions, including cancer, diabetes, and inflammation (27, 61). Some studies also link hypoxia/reoxygenation exposure to ER stress. For example, it has been shown that hypoxia/reoxygenation injury could induce cardiomyocytic apoptosis via activation of ER stress (66). Caspase-12 and C/EBP homologous protein (CHOP), two marker proteins of ER stress, were also upregulated under hypoxia/reoxygenation conditions (43). In the brain, cellular damage via ER stress is also implied in neurodegeneration and cerebral ischemic attack (48). However, despite these observations, the role and mechanism of ER stress in the development of cognitive impairment in OSA are not clear.
In this study, based on a mouse model mimicking OSA-induced IH, we searched for evidence for IH-induced ER stress in the brain and its role in underlying synaptic plasticity and neurocognitive dysfunctions. Since synapse damage and degeneration are associated with elevating CHOP expression and could be prevented by ER stress inhibitors (41, 44), we also studied the changes in the ultrastructure of synapses in the hippocampus under IH and the roles played by ER stress. We made use of tauroursodeoxycholic acid (TUDCA) and 4-phenylbutyric acid (PBA), two chemical chaperones that have been shown to reduce ER stress by facilitating proper protein folding (42, 64). Multiple reports have demonstrated that TUDCA is an effective inhibitor of ER stress-induced apoptosis (26) and can also facilitate spine formation (45). Thus, in this study, the effects of TUDCA under IH conditions were examined systematically.
Results
IH induces ER stress in hippocampal CA1 neurons
Experiments were performed on young adult mice (6–8 weeks old) that exhibited robust hippocampal synaptic plasticity and memory behavior. To investigate the role of ER stress in the hippocampus under IH treatment, we first examined the expression of three ER stress markers, Grp78, caspase-12, and CHOP. Under conditions of ER stress, increased Grp78 is dissociated from unfolded proteins and activates ER stress receptors triggering the UPR, while caspase-12 and CHOP are known to be specifically related to apoptosis (21, 33). As shown in Figure 1A and B, these three proteins in the hippocampus were upregulated after 14 days of IH treatment (p<0.01 compared with the control group, four mice per group, Fig. 1B). Interestingly, the levels of these three proteins were significantly reduced when the animals were treated with the ER stress inhibitor, TUDCA, 2 days before and during the IH treatment (p<0.05 compared with the IH-treated group, Fig. 1B). PBA, another commonly employed ER stress inhibitor (42, 54), also had a similar effect in suppressing ER stress activation (Supplementary Fig. S1A, B; Supplementary Data are available online at www.liebertpub.com/ars). However, TUDCA and PBA themselves did not alter the basal levels of these proteins (p>0.05, Fig. 1B; Supplementary Fig. S1B). Similar effects were also evident after 7 days of IH treatment (Supplementary Fig. S2).
FIG. 1.
Activation of ER stress after chronic IH. (A) Upregulation of Grp78, caspase-12, and CHOP after 14 days of IH treatment, which was prevented by the injection of TUDCA. (B) Shows the pooled data from four animals. One-way ANOVA [F(3, 12)=6.62, p<0.01 for Grp78; F(3, 12)=6.05, p<0.01 for caspase-12; F(3, 12)=4.53, p=0.02 for CHOP], followed by post hoc analysis with Newman–Keuls's test. (C) Significant alterations in ER ultrastructure were observed after 14 days of hypoxia/reoxygenation exposure. Most of the perinuclear ER (indicated by arrowheads) formed parallel sheets in CA1 pyramidal neurons in the control group (i) and TUDCA treatment (ii). In contrast, ERs in the IH group (iii) were swollen, distorted, and frequently misoriented, suggesting protein aggregation in the lumen. The inset in each picture is enlarged and displayed on the right. Treatment with TUDCA prevented the alteration in ER structure (iv). *p<0.05; **p<0.01; ns, not significant. Scale bars: 0.5 μm. ANOVA, analysis of variance; CHOP, C/EBP homologous protein; ER, endoplasmic reticulum; Grp78, glucose-regulated protein 78; IH, intermittent hypoxia; TUDCA, tauroursodeoxycholic acid.
Further evidence of ER stress was obtained by ultrastructural analysis of the ER in the hippocampus. As shown in Figure 1C, electron microscopy revealed significant alterations in ER ultrastructure in CA1 neurons from mice exposed to 14 days of IH. In contrast to the control group, in which intact and parallel rows of rough ER were found (Fig. 1C[i]), the ER in the hypoxia-treated group was not only swollen but also distorted and lost the typical parallel arrangement (Fig. 1C[iii]), suggesting the accumulation of unfolded proteins within the ER after chronic hypoxia/reoxygenation exposure. Consistent with the Western blot data on the expression of ER stress markers, TUDCA treatment could prevent the appearance of the distorted ER in the hippocampal CA1 neurons (Fig. 1C[iv]), while TUDCA itself did not have any effect on the ER structure (Fig. 1C[ii]). These data suggest that the chemical chaperone, TUDCA, could suppress ER stress by reducing the amount of unfolded proteins and therefore the expression of proapoptotic caspase-12 and CHOP.
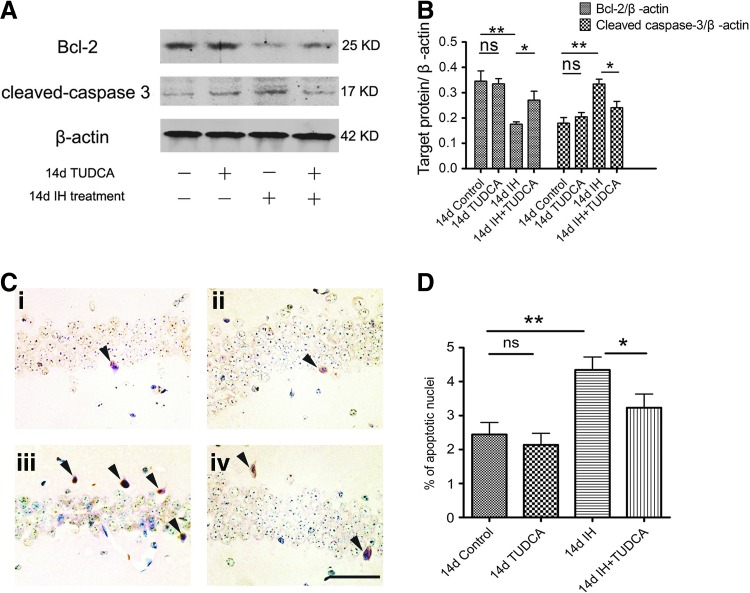
ER stress inhibitors reduce the activation of caspase-3 through modulating Bcl-2 expression
To search for a possible link between ER stress and apoptosis in the hippocampus, we examined the expression of caspase-3 and Bcl-2 after 14 days of IH and also the effects of TUDCA injection. As shown in Figure 2A and B, cleaved caspase-3 was upregulated, while Bcl-2 was downregulated in the hypoxia-treated group (p<0.01 compared with the control group, four mice per group, Fig. 2B). Simultaneous treatment with TUDCA during hypoxia could suppress the expression of cleaved caspase-3, which was accompanied by the increased expression of Bcl-2 (p<0.05 compared with the hypoxia-treated group, Fig. 2B). Similar phenomena were also observed in the treatment with PBA (Supplementary Fig. S1C, D). These data suggest that inhibition of ER stress response could reduce apoptosis induced by chronic hypoxia/reoxygenation exposure by upregulation of the antiapoptotic protein, Bcl-2. Consistent with this hypothesis, terminal transferase-mediated dUTP nick end labeling (TUNEL) staining revealed that apoptotic neurons were less commonly encountered in the hippocampal CA1 neurons in the IH+TUDCA group than in the 14-day IH-treated group (p<0.05, Fig. 2C, D).
FIG. 2.
Increased apoptosis in the hippocampus under IH was alleviated by ER stress inhibitor, TUDCA. (A) Bcl-2 was downregulated and cleaved caspase-3 was upregulated after chronic IH treatment. These effects were prevented by administration of TUDCA. Mean data from four animals are summarized in (B). One-way ANOVA [F(3, 12)=7.21, p<0.01 for Bcl-2; F(3, 12)=10.37, p<0.01 for cleaved caspase-3], followed by post hoc analysis with Newman–Keuls's test. (C) Representative TUNEL reaction on apoptotic cells in the CA1 region of the hippocampus; (i) control group; (ii) TUDCA group; (iii) IH group; (iv) IH+TUDCA group. There were more apoptotic cells (indicated by arrowheads) in the IH group than in control and TUDCA groups. However, apoptotic cells were fewer in the IH+TUDCA group when compared with the IH group. The pooled data from nine sections for each group are summarized in (D). One-way ANOVA [F(3, 32)=7.10, p<0.01], followed by post hoc Newman–Keuls's test. *p<0.05; **p<0.01; ns, not significant. Scale bar: 50 μm. TUNEL, terminal transferase-mediated dUTP nick end labeling.
Impaired mitochondrial function rescued by ER stress inhibitor
It is well known that Bcl-2 is a major antiapoptotic protein involved in the mitochondria-mediated apoptotic pathway and confers protection against lethal ER stress (55). Therefore, to investigate whether the function of mitochondria was altered after IH exposure, we analyzed both the morphology and some functional parameters of the mitochondria in the hippocampus. As shown in the electron micrographs in Figure 3, the mitochondria found in CA1 neurons in the control group (Fig. 3A[i]) and TUDCA group (Fig. 3A[ii]) displayed an intact structure with clear cristae. However, in the IH group, although the mitochondrial membranes were relatively intact, many cristae within the mitochondria disappeared (Fig. 3A[iii]). After TUDCA treatment, the cristae were more intact and easier to be observed (Fig. 3A[iv]). Consistent with these results, IH led to a clear reduction in the ATP level (Fig. 3B) and complex I activity (Fig. 3C) in the mitochondria isolated from the hippocampus when compared with the control group (p<0.01, four mice per group), which were partially rescued by TUDCA treatment (p<0.05). At the same time, we found that the impaired mitochondrial function was associated with a rise of ROS in the mitochondria (p<0.05, four mice per group) measured by a fluorometric assay with 2′,7′-dichlorofluorescein diacetate, which could also be attenuated by TUDCA treatment (p<0.05, four mice per group, Fig. 3D). These results suggest that the ER stress observed in chronic IH could induce the mitochondria-mediated apoptotic pathway that is associated with increased ROS production in the mitochondria.
FIG. 3.
Impaired mitochondrial morphology and function after IH treatment. (A) The morphology of mitochondria in the hippocampus CA1 region; (i) control group; (ii) TUDCA group; (iii) IH group; (iv) IH+TUDCA group. In the control and TUDCA groups, intact mitochondria with clear cristae were found. Much fewer cristae were found in the mitochondria from the IH group. TUDCA treatment preserved more intact mitochondria with discernable cristae. Scale bar: 0.5 μm. Mitochondrial ATP production (B) and complex I activity (C) were reduced after IH treatment and partially restored by TUDCA. One-way ANOVA [F(2, 9)=20.66 p<0.01 for ATP level]; complex I activity [F(2, 9)=7.26, p=0.01 for complex I activity], followed by post hoc Newman–Keuls's test. (D) ROS production in the mitochondria was increased after IH exposure, and they were also sensitive to TUDCA treatment. One-way ANOVA [F(2, 9)=7.26, p=0.01], followed by the post hoc Newman-Keuls's test. *p<0.05; **p<0.01; (four animals per group). ROS, reactive oxygen species.
Interaction between ROS production and ER stress in chronic IH
There is mounting evidence demonstrating a direct link between the production of ROS and ER stress (29, 51). To probe the relationship between ER stress and ROS in our model, we performed several sets of experiments. First, as shown in Figure 4A, we demonstrated that ROS in the hippocampal region was clearly elevated after 14 days of IH treatment (p<0.01, four mice per group), which could be suppressed by the cotreatment with N-acetylcysteine (NAC; p<0.01), a commonly employed antioxidant drug that serves as a prodrug for the synthesis of the endogenous glutathione (1). At the same time, the expression levels of the three ER stress markers, Grp-78, caspase-12, and CHOP, and cleaved caspase-3 were all reduced by NAC, indicating that limiting ROS production could suppress ER stress activation and apoptosis (p<0.05, four mice per group, Fig. 4B, statistics shown in Supplementary Fig. S3).
FIG. 4.
Reciprocal activation between ROS production and ER stress during IH. (A) Daily injection of NAC (100 mg/kg body weight) reduced the increased intensity of DCF fluorescence from the hippocampus in the IH group. One-way ANOVA [F(3, 12)=7.20, p<0.01], followed by post hoc Newman-Keuls's test (four animals per group). (B) ER stress markers were suppressed by NAC treatment (pooled data and statistics in Supplementary Fig. S3). (C) The intensity of DCF fluorescence of hippocampal tissues was significantly increased in the IH group, which was suppressed by TUDCA injection. One-way ANOVA [F(3, 16)=6.32, p<0.01], followed by post hoc Newman-Keuls's test (four animals per group). (D) Upregulations of MDA and protein carbonyl after 14 days of IH treatment, which were reduced by TUDCA application. One-way ANOVA [F(2, 9)=21.70, p<0.01 for MDA detection; F(2, 9)=6.74, p=0.02 for protein carbonyl detection], followed by post hoc Newman-Keuls's test (four animals per group). (E) The induction of Hsp70 and HO-1 after 14 days of IH treatment was prevented by the injection of TUDCA. One-way ANOVA [F(2, 9)=8.89, p<0.01 for Hsp70; F(2, 9)=6.85, p=0.02 for OH-1], followed by post hoc Newman-Keuls's test (four animals per group). *p<0.05; **p<0.01; ns, not significant. DCF, dichlorofluorescein; HO-1, heme oxygenase-1; Hsp70, heat shock protein 70; MDA, malondialdehyde; NAC, N-acetylcysteine.
Interestingly, treatment with TUDCA also suppressed the rise of the ROS level in the IH group (p<0.05 compared with IH treatment alone, five mice per group, Fig. 4C). Furthermore, the production of malondialdehyde (MDA), a marker for lipid peroxidation, and carbonylated protein, a marker for protein oxidation, was increased after IH exposure (p<0.001, p<0.05, respectively, four mice per group, Fig. 4D), which was reduced by TUDCA (p<0.01, p<0.05, respectively, four mice per group, Fig. 4D). At the same time, increased heat shock protein 70 (Hsp70) and heme oxygenase-1 (HO-1), two proteins responding to oxidative stress (7), could also be decreased by TUDCA treatment (p<0.01, p<0.05, respectively, four mice for each group, Fig. 4E). All these data strongly implicate that ER stress and ROS could reciprocally activate each other under the condition of chronic IH.
IH-induced ER stress impairs hippocampal synaptic plasticity
We and other investigators had previously demonstrated that hippocampal long-term synaptic plasticity is impaired after 7–14 days of IH treatments (60, 63), a phenomenon that may underlie memory impairment in OSA subjects. To test whether ER stress plays a direct role in this process, we performed two sets of experiments. First, we tested if tunicamycin, a specific ER stress inducer, can affect long-term potentiation (LTP) in the hippocampus. We found that preincubation with 10 μM of tunicamycin (80 min) reduced the magnitude of tetanus (100 Hz, 1 s)-induced LTP in the Schaffer collateral-CA1 pathway. In 11 tunicamycin-treated hippocampal slices from four mice, the magnitude of the field excitatory postsynaptic potential (fEPSP) was 128.5±3.2% of the baseline, which was significantly smaller compared with the control group (159.3±9.9%, nine slices from four mice; p<0.01, two-tailed unpaired Student's t-test, Fig. 5A, B).
FIG. 5.
Chronic IH-induced ER stress inhibited hippocampal LTP. (A, B) Tetanic stimulation (100 Hz for 1 s) was used to induce LTP in the Schaffer collateral-CA1 pathway in mouse hippocampal slices. Bath application of the ER stress inducer, tunicamycin, impaired the magnitude of LTP, reflected as a decrease in the average potentiation of fEPSPs in 50–60 min after LTP induction. **p<0.01, two-tailed unpaired Student's t-test. (C, D) Hippocampal slices obtained from mice after 14 days of IH exhibited a decrease in LTP. While TUDCA treatment alone did not have any effect on LTP, administration of TUDCA during IH treatment rescued the impaired LTP induced by IH. One-way ANOVA [F(3, 46)=6.63, p<0.01], followed by post hoc Newman-Keuls's test. *p<0.05; **p<0.01; ns, not significant. fEPSP, field excitatory postsynaptic potential; LTP, long-term potentiation.
In the second set of experiments, we investigated whether the ER stress inhibitors, TUDCA and PBA, could prevent chronic IH-induced impairment in hippocampal LTP. As shown in Figure 5C and D, the magnitude of LTP was 156.5±4.3% in the 14-day control group (13 slices, six mice). The potentiation of fEPSP induced by the same induction protocol was reduced to 131.1±7.6% (p<0.01, 11 slices from five mice). However, incubation with TUDCA significantly restored the LTP (150.5±2.9%; 13 slices from four mice; p<0.05 compared with IH alone; p>0.05 compared with the normoxia group, Fig. 5C, D). The other ER stress inhibitor, PBA, could also rescue the LTP (145.6±3.6%, eight slices from three mice; p<0.05 compared with IH alone, in Supplementary Fig. S1E, F). That the effects of TUDCA and PBA are specific to IH-induced impairment in LTP was indicated by the fact that TUDCA and PBA injection alone did not alter the magnitude of LTP.
Together, these results strongly imply that IH-induced ER stress per se could impair hippocampal LTP, which could be rescued by inhibition of the ER stress response. Similar results were obtained from mice treated with 7 days of IH (data not shown).
IH-induced ER stress alters fine synaptic structure and spine morphology
In addition to our finding on the effect of ER stress on hippocampal long-term synaptic plasticity, it has been reported that ER stress may cause synaptic damage and degeneration, which could be prevented by ER stress inhibitors (41, 44). Thus, we performed quantitative analysis to investigate the effect of IH on the fine structure of excitatory synapses in the CA1 region of the hippocampus (Supplementary Fig. S4A) and the role that might be played by ER stress. Excitatory synapses were identified by the presence of round synaptic vesicles and the asymmetric pre- and postdensity material (Supplementary Fig. S4B). Samples from four groups were studied: the normoxia group, 14-day IH group, TUDCA-injected normoxia group, and TUDCA-injected hypoxia group (Fig. 6A). As shown in Figure 6B and Supplementary Figure S4C, one-way ANOVA showed that there was no difference in the number of excitatory synapses among these four groups. To probe the fine structure of the synapse, three morphological parameters, namely the thickness of the postsynaptic density (PSD), the width of the synaptic cleft, and the length of the active zone, were quantified. There were no significant differences in the PSD thickness and synaptic cleft among the four groups. However, the length of the synaptic active zone in the IH group was significantly smaller than those found in the normoxia group (p<0.001, Fig. 6B). In addition, this shortening effect was partially rescued by injection with TUDCA when compared with the IH group (p<0.01, Fig. 6B). These results reveal that chronic IH leads to subtle changes in synaptic organization, which may underlie impaired synaptic transmission, membrane excitability, and synaptic plasticity (2, 63).
FIG. 6.
Chronic IH treatment reduced the length of the synaptic active zone in hippocampal neurons. (A) High-magnification electron photomicrograph allowed measurement of the thickness of PSD, width of synaptic cleft, and length of the active synaptic zone. Arrowheads indicate the synapses. Shown are typical micrographs from (i) the control group; (ii) TUDCA group; (iii) IH group; and (iv) IH+TUDCA group. Scale bars: 0.25 μm. (B) Data pooled from 40 sections, one-way ANOVA showed that there was no difference in the number of excitatory synapses [F(3, 156)=0.41, p=0.74, top left], thickness of PSD of excitatory synapses [F(3, 515)=0.145, p=0.93, top right], and width of synaptic cleft [F(3, 516)=2.14, p=0.09, bottom left] among these four groups. However, there was a significant difference in the length of the synaptic active zone among the treatment groups [F=(3, 536)=20.06, p<0.01, one-way ANOVA]. Post hoc Newman-Keuls's test indicated significant shortening of the length of the active zones of these synapses in the IH group, which was partially prevented by TUDCA treatment (bottom right). **p<0.01; ***p<0.001; ns, not significant. PSD, postsynaptic density.
We also analyzed the spine density and morphology of the hippocampal CA1 neurons by Golgi staining (Supplementary Fig. S5). As summarized in Figure 7A, there was a small but significant reduction in the overall spine density after 14 days of IH (p<0.01 compared with the control group), which was partially restored by TUDCA treatment (p<0.05 compared with the IH group). Analysis of the subtypes of spines revealed that only the density of the mushroom spines, but not of stubby spines and the thin spines, was altered by chronic IH treatment. The mushroom spine density decreased significantly in IH treatment (1.65±0.10 per 10 μm, n=100) when compared with the control group (2.58±0.11 per 10 μm, n=101; p<0.001). The effect of IH was partially rescued by TUDCA treatment (2.05±0.11 per 10 μm, n=86; p<0.01 compared with 14 days of IH alone). Furthermore, we examined the expression of PSD-95, a protein that is essential in the formation of mature synapse and organization of mushroom-shaped spines (11, 69). The expression of PSD-95 in the hippocampus was downregulated after 14 days of IH treatment (p<0.05, Fig. 7B). The level of this protein was partially but significantly restored when the animals were treated with TUDCA (p<0.05, Fig. 7B).
FIG. 7.
Chronic IH reduced the overall spine density in hippocampal neurons. (A) (Top) Microscopic observation under a higher magnification allowed quantification of spine density and classification of individual spines as stubby (white arrowheads), thin (black arrowheads), or mushroom (arrows) from (i) the control group; (ii) TUDCA group; (iii) IH group; and (iv) IH+TUDCA group. Scale bars: 6 μm. (Bottom). One-way ANOVA [F=(3, 373), p<0.01], followed by post hoc Newman-Keuls's test, revealed significant reduction in the overall spine density after 14 days of IH (p<0.01 compared with the control group), partially restored by TUDCA (p<0.05). Analysis of subtypes of spines revealed no differences in the density of stubby spines [F(3, 367)=0.14, p=0.93, data not shown] and thin spines [F(3, 367)=2.63, p=0.06, data not shown] among the groups, but the mushroom spines were selectively altered [F(3, 367)=10.10, p<0.01]. Post hoc Newman-Keuls's test revealed significantly reduced mushroom spine density in the IH group, rescued by TUDCA treatment. (B) Downregulation of PSD-95 after 14 days of IH treatment, which was prevented by the injection of TUDCA, as shown by the representative Western blot and the pooled data from four animals. One-way ANOVA [F(3, 16)=5.07, p=0.01], followed by post hoc Newman-Keuls's test. *p<0.05; **p<0.01; ***p<0.001; ns, not significant.
ER stress inhibitor rescues IH-induced long-term memory deficit
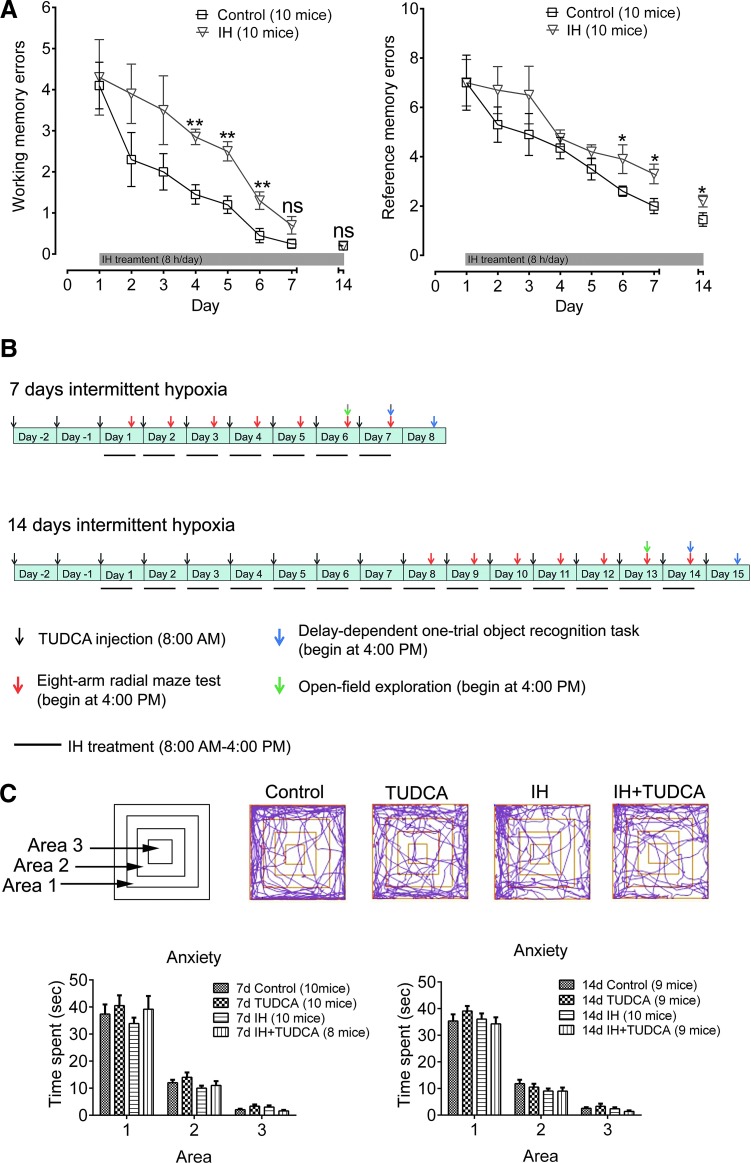
If ER stress is critical in memory impairment observed in OSA animal models or OSA patients, it should be possible to restore IH-induced memory dysfunction by suppressing ER stress. We first tested the effect of chronic IH on working memory, an essential component of executive functions, as well as long-term reference memory by means of the radial arm maze test. As shown in Figure 8A, two-way ANOVA disclosed that daily training had a significant effect on the number of both working [F(7, 144)=18.37, p<0.01] and reference [F=(7, 144)=14.63, p<0.01] memory errors, and IH also produced significant effects [F(1, 144)=15.90, p<0.01 for working memory errors, and F(1, 144)=7.953, p<0.01 for reference memory errors]. Two-tailed unpaired Student's t-test showed increased numbers of working memory errors on some days (fourth to sixth) during the course of maze training in the IH group (p<0.01, Fig. 8A). However, on day 7, the errors were reduced and no significant differences were found between the normoxia and hypoxia groups (p>0.05, Fig. 8A). The lack of long-lasting effect on working memory function was confirmed by the insignificant difference between the two groups after 14 days of IH treatment (p>0.05, Fig. 8A). In contrast, 7 days of IH increased the reference memory errors (p<0.05, Fig. 8A), which persisted after 14 days of treatment. These findings suggest that under our experimental paradigm, the long-term memory was more sensitive to IH treatment.
FIG. 8.
Effects of chronic IH on memory functions and experimental design of effects of ER stress inhibition. (A) Executive function exemplified by working memory (left) and long-term memory represented by reference memory (right) were tested by the radial arm maze test. No significant difference in working memory was found after 7 and 14 days of IH. In contrast, significantly increased reference memory errors were induced after 7 days of IH treatment and persisted after 14 days. Analyses by two-way ANOVA, see text for details. (B) TUDCA (100 mg/kg body weight) was injected 2 days before and during the entire 7 or 14 days of IH treatment, which lasted from 8:00 A.M. to 4:00 P.M. After IH exposure, behavior tests were used to evaluate the anxiety level and long-term memory (4:00 P.M. to 9:00 P.M.). (C) In some animals, the anxiety level after prolonged IH treatment was assessed by the open-field test. Top, three concentric areas of the open arena were defined, and the paths of the animals from typical experiments in different groups are shown. Bottom, one-way ANOVA showed that the time spent by the animals in different areas had no differences among all groups after 7 days of IH treatment [F(3, 34)=0.66, p=0.58 for Area 1; F(3, 34)=1.58, p=0.21 for Area 2; F(3, 34)=2.28, p=0.10 for Area 3] and 14 days of IH treatment [F(3, 33)=0.84, p=0.48 for Area 1; F(3, 33)=1.15, p=0.35 for Area 2; F(3, 33)=1.15, p=0.35 for Area 3].
To further probe the impact of IH on memory function and the potential rescue effect of suppressing ER stress, we tested the effects of TUDCA on two types of long-term memory: the one-trial object recognition task for recognition memory and the radial arm maze task for long-term spatial memory. Since the emotional status of the animals may affect their performance in the memory test, we first examined if there was any sign of anxiety in the animals undergoing IH based on their explorative behavior in the open field (39). The overall design and schedule of the behavioral tests for both 7- and 14-day IH groups are summarized in Figure 8B. In the open field, the level of anxiety is gauged by the free behavior of the animals, which normally spend more time near the edge of the field than the central area (Fig. 8C). We found that in both 7- and 14-day IH groups, the time spent in the three concentric areas by the mice in different groups did not differ significantly (p>0.05, one-way ANOVA, Fig. 8C), reflecting that the level of anxiety was not affected by IH or drug treatments.
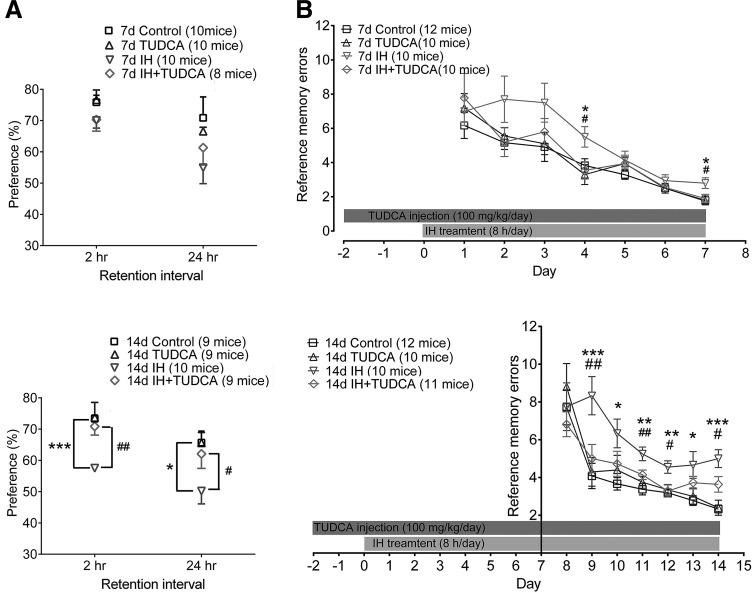
In the one-trial object recognition task, the amount of time spent with the novel object compared with the total time spent exploring both familiar and novel objects represents an index of long-term recognition memory. During the training phase, animals showed no preference for one object over another identical object in different groups, suggesting that the experimental groups were equally motivated to explore objects (data not shown). In the batch of animals exposed to 7 days of IH, the preference rate tested at 2 h after training did not reveal any difference among different treatment groups [F(3, 34)=1.493, p=0.23, one-way ANOVA]. A similar result was obtained when the test was conducted at 24 h [F(3, 34)=1.93, p=0.14]. However, in the batch of 14-day IH animals, there was a significant difference in object preference among different groups after 2 h [F(3, 33)=8.08, p<0.01] or 24 h [F(3, 33)=3.48, p=0.02]. Post hoc Newman–Keuls's test indicated a significantly lower preference to explore the novel object after 2 and 24 h of retention (p<0.001 in 2 h retention; p<0.05 in 24 h retention, compared with the control group, Fig. 9A). The impairment was partially restored by TUDCA treatment (p<0.01 in 2 h retention; p<0.05 in 24 h retention, compared with the IH group, Fig. 9A).
FIG. 9.
TUDCA rescued defects in recognition memory and spatial long-term memory in mice undergoing chronic IH treatment. (A) Hippocampus-dependent recognition memory measured by the one-trial object recognition task. The percentage of preference for the novel object was significantly reduced in the trial phase, 2 and 24 h after training, in the 14-day IH treatment group (***p<0.001; *p<0.05, compared with control, respectively). The higher preference for the novel object was restored by TUDCA application (##p<0.01; #p<0.05, compared with the IH group, respectively). One-way ANOVA, followed by Newman-Keuls's test. See text for details. (B) Acquisition of long-term spatial memory was tested by the eight-arm radial arm maze. Top, in the 7-day group, IH experiment and training were conducted at the same time. Significantly increased errors were evident only on days 4 and 7 in the IH group (*p<0.05, compared with control), which could be reduced by TUDCA treatment (#p<0.01, compared with the IH group). Bottom, in the 14 days of IH experiments, the training of radial arm maze started on day 8 until day 14 for 1 week. One-way ANOVA revealed differences in different treatment groups from day 9 onward [F(3, 39)=0.58, p=0.63 for day 8; F(3, 39)=5.70, p<0.01 for day 9; F(3, 39)=3.07, p=0.04 for day 10; F(3, 39)=6.51, p<0.01 for day 11; F(3, 39)=4.87, p<0.01 for day 12; F(3, 39)=3.49, p<0.01 for day 13; F(3, 39)=10.74, p<0.01 for day 14]. Post hoc Newman-Keuls's test showed that the average number of errors made by the IH animals was significantly higher than the normoxia group starting from day 9 (*p<0.05, **p<0,01, ***p<0.001 compared with control). On the other hand, TUDCA treatment in the IH group significantly improved the memory ability of the animals (#p<0.05, ##p<0.01, compared with the IH group).
A 7-day radial arm maze task was used to assess the acquisition of long-term spatial memory. We found that when the memory task was initiated at the same time with the IH treatment (i.e., 7 days of hypoxia treatment), two-way ANOVA disclosed that daily training (time) significantly decreased the number of reference memory errors [F=(6, 266)=31.85, p<0.01], and different treatments also produced a significant effect [F(3, 266)=7.95, p<0.01]. One-way ANOVA showed that there were significant differences in memory errors among different groups on day 4 [F(3, 38)=3.55, p=0.02] and day 7 only [F(3, 38)=3.84, p=0.02]. Post hoc analysis revealed that TUDCA reverted IH-induced memory deficits on these 2 days (p<0.05, Fig. 9B, top).
In the other set of the experiment, we exposed the animals to 14 days of IH and started the radial arm maze test on the second week, that is, on the eighth day. As can be seen in Figure 9B (bottom), longer-term exposure to IH had a strong effect on the rate of acquisition of long-term memory and the retention of the memory at the end of the week. Two-way ANOVA confirmed that daily training and treatments had significant effects on the numbers of reference memory errors [time: F(6, 287)=26.44, p<0.01; treatment: F(3, 287=16.01, p<0.01]. Significantly, higher numbers of reference memory errors were made throughout the entire week of training. On the last day of the task (day 14), the average number of errors made by the hypoxia group was 5.00±0.50 (n=9), significantly higher compared with the normoxia group (2.33±0.22; n=12; p<0.01, Fig. 9B), as shown by one-way ANOVA with Newman–Keuls's test. Daily injection of TUDCA before and throughout the hypoxia paradigm significantly improved the performance of the subjects. Thus, on day 14, the average number of errors made was 3.64±0.41 (n=11; p<0.05 compared with the IH group, Fig. 9B).
Discussion
Previous studies hinted that that ER stress could be activated in the central nervous system under chronic IH. For example, Zhu et al. (71) showed that IH -induced ER stress may cause selective damage of upper airway motoneurons in the brainstem that could explain the impaired hypoglossal nerve affecting respiratory function found in OSA. In addition, a recent study demonstrated that the CHOP level in the hippocampus was increased in mice models of OSA and it plays a role in neural injury (12).
In the present study, we performed a systematic and comprehensive investigation that pinpoints the critical role of ER stress in underlying memory impairment imposed by chronic IH. We provided evidence at multiple levels to support this conclusion. First, prolonged daily exposure to IH environment in mice resulted in the appearance of dilated and distorted ER in the hippocampus and was accompanied by upregulation of Grp78, a sensitive indicator of ER stress (21), and caspase-12 and CHOP, two key mediators of ER stress-induced apoptosis (55, 68). Mitochondrial ROS was also elevated. The ER stress inhibitors, TUDCA and PBA, specifically suppressed these phenomena. TUDCA and PBA also suppressed the proapoptotic cleaved caspase-3 and upregulated Bcl-2. It is well established that Bcl-2 is a major protein against the mitochondria-mediated apoptotic pathway and confers protection against lethal ER stress (55). Second, chronic IH induced apoptosis and fine structural changes in the synapses in the hippocampal CA1 subfield. These effects were prevented by inhibition of ER stress. Third, at the neuronal circuit level, suppression of ER stress by TUDCA as well as PBA could partially rescue the impairment in hippocampal long-term synaptic plasticity of the mice induced by repeated exposure to hypoxia/reoxygenation. In support of this, acute induction of ER stress chemically by tunicamycin could directly impair LTP. Finally, at the behavioral level, administration of TUDCA before and during IH treatment improved long-term recognition memory and the acquisition of long-term spatial memory that were impaired by prolonged IH. Taken together, these data suggest that a primary event in chronic IH-induced memory dysfunction is the activation of ER stress in the hippocampus, which results in apoptosis as well as alteration of synaptic structure and plasticity. Suppression of ER stress can rectify these changes and is therefore beneficial in restoring memory function.
What is the mechanism by which IH causes ER stress? Although we are yet to provide a comprehensive answer to this question, the cascade of events is likely to be complicated and involves multiple processes. We believe that ROS production could play a major role. It is clear that ROS levels are increased by IH treatment in experimental animals (e.g., 34) and contribute to memory deficits observed after IH treatment (e.g., 65). There is a good amount of evidence demonstrating a direct link between the production of ROS and protein folding (51, 58). In this study, limiting ROS production by NAC could suppress ER stress activation, which is consistent with other studies showing that altered redox homeostasis is sufficient to cause ER stress (9). On the other hand, as accumulation of unfolded/misfolded protein could increase ROS production (29) and therefore exacerbate ER stress, ROS generation should be considered as an integral component of ER stress. Specifically, this notion is in line with our own measurements that TUDCA could also suppress increased levels of ROS, MDA, and protein carbonyls after chronic IH and also reduce the elevated levels of Hsp70 and HO-1. The heme oxygenase pathway and heat shock proteins, two major components of the vitagene system, are induced in a variety of human diseases to adapt to oxidative stress, including ischemia and reperfusion damage, and inflammation, as well as metabolic and neurodegenerative disorders (7). Therefore, ER stress and oxidative stress accentuate each other in a positive feedback manner, interfering with cellular functions and activating proapoptotic signaling (6, 37, 40, 49).
In relation to this, we observed increased ROS production in the mitochondria from the hippocampus, which was associated with defective structure and impaired function, consistent with the notion that the mitochondria could be a major source of increased ROS in IH. It can be envisioned that disturbed mitochondrial calcium homeostasis due to energy depletion, as in cerebral ischemia, could be a possible cause (17, 59). In addition, our team has shown previously that BDNF, which is known to prevent ROS production as well as ER stress activation (4), was suppressed after chronic IH treatment leading to LTP impairment (63). Therefore, reduction in BDNF production could be another factor that leads to ER stress under our chronic IH paradigm.
Although apoptotic neuronal death in the hippocampus is an obvious detrimental consequence of ER stress that contributes to the memory impairment observed, our study also revealed fine sublethal changes in the structure of synapses, namely shortening of active zones of excitatory synapses and reduction in the density of mushroom spines. These findings are consistent with studies reporting that activation of ER stress could promote synaptic degeneration and disrupt neurotransmission associated with changes in synaptic morphology and submicroscopic structures (41, 44). Shortening of the active zone may reflect a condition of impaired synaptic transmission and plasticity (57, 67) and is consistent with our finding that TUDCA could restore the length of the active zone and rescue LTP. In parallel, mushroom spines, or memory spines, are related to strong synaptic connections and neuronal plasticity (18). The fact that their number is significantly reduced under chronic IH treatment further strengthens the idea that sublethal functional and structural changes in neurons contribute to neurocognitive malfunctions in sleep apnea. The exact cause of these changes is not clear. However, our finding of reduced expression of PSD-95, a key protein in mature synapse formation and reconstruction of a mushroom-shaped spine (11, 69), could be critical. This may be related to increased misfolded or unfolded proteins occurring in ER stress. Alternatively, a direct link can be suggested in that the ER stress-activated ubiquitin–proteasome system that induces protein degradation is responsible for regulating the levels and localization of several proteins, including PSD-95 (8).
In conclusion, the present study reveals that the activation of ER stress and associated increase in ROS production play an important role in cognitive deficits caused by chronic IH. Both accumulation of misfolded proteins and neuronal death result in degraded cellular functions and synaptic plasticity in key structures such as the hippocampus that underlie memory formation and consolidation. Suppression of the ER stress by chemical chaperones such as TUDCA is effective in rectifying the defects at the cellular and behavioral levels. Our study also highlights that although OSA-associated chronic IH, when compared with acute cerebral ischemia, is far milder, this form of recurrent hypoxia could also induce ER stress that leads to significant impairment in brain functions. Given the current effort in pharmaceutical industry and research laboratories to search for therapeutic agents that could treat ER stress-related diseases (27), amelioration of OSA-associated neurocognitive impairment by safe ER stress inhibitors could be a novel adjunct therapeutic treatment in the near future in this common disorder.
Materials and Methods
Animals
Six-week-old male C57BL/6J mice, weighing 20–22 g, were housed under standard conditions: 22°C, a 12-h light/12-h dark cycle, and standard food and water ad libitum. The procedures of experimentation were approved by the Animal Experimentation and Ethics Committee of the Chinese University of Hong Kong.
Mouse model of chronic IH
The chronic IH model mimics the repeated episodes of airway obstruction in OSA without the complications and interference that arise from sleep deprivation and fragmentation. The protocol was based on well-reported rodent models of sleep apnea as described in previous reports (20, 23, 63). Control animals were exposed to alternating periods of room air in identical chambers. The IH treatment lasted for 7 or 14 days.
Drug treatment
TUDCA (Sodium salt; Sigma-Aldrich) was dissolved in phosphate-buffered saline (PBS) of pH 7.4 at a concentration of 7.8 mg/ml (100 mg/kg). Based on an in vivo protocol previously published (10), TUDCA was intraperitoneally injected daily at a dosage of 100 mg/kg body weight starting 2 days before and throughout the IH treatment. The mice were randomly assigned into four groups: the PBS-treated control group (Control), TUDCA-treated control group (TUDCA), PBS-treated IH group (IH), and TUDCA-treated IH group (IH+TUDCA). PBA (Sigma-Aldrich) was prepared in PBS and sodium hydroxide was used to adjust pH to 7.4, administered at a concentration of 15.6 mg/ml (200 mg/kg) according to previous reports (46). NAC (Sigma-Aldrich), a common antioxidant, was dissolved in PBS of pH 7.4 at a concentration of 7.8 mg/ml (100 mg/kg) based on a previous study (1). The groupings and injection schedules of PBA and NAC were the same as that of TUDCA.
Behavioral studies
Mice were behaviorally tested after IH exposure on each day (8:00 A.M. and 4:00 P.M). Experiments were performed between 4:00 P.M. and 9:00 P.M (Fig. 8A). Exploratory behavior was captured by a high-definition digital camera coupled with an online video tracking system (ANY-maze). Three behavior tests were conducted. Open-field exploration was used to measure the anxiety level according to other studies (30, 31). Delay-dependent one-trial object recognition task and eight-arm radial maze test were employed to assess the hippocampus-dependent long-term recognition memory and working memory, as well as the hippocampus-dependent spatial memory (15, 39, 56). Detailed procedure could be found in the Supplementary Data.
LTP measurements
The mice were sacrificed by decapitation and the brains were immediately removed and cut into two halves in the sagittal plane. They were then immersed in ice-cold artificial cerebrospinal fluid. Three hundred-micrometer-thick parasagittal sections were cut using a vibrating microtome (Integraslice 7550MM; Campden Instruments Ltd.). A planar multielectrode array recording system (MED64 system; Alpha Med Sciences Co., Ltd.) was employed to record fEPSP for LTP measurement. fEPSPs were then recorded from the dendritic layer of CA1 neurons while providing single-pulsed electrical stimulation (0.017 Hz) to the Schaffer collateral pathway. After allowing a stable baseline of 30 min, a single 1-s train of 100 Hz stimulation (tetanus) was applied to induce LTP, which was quantified as the percentage increase of the average fEPSP amplitude recorded in the 50- to 60-min interval after tetanus compared with the baseline value.
Western blot
Expression levels of Grp78, CHOP, Caspase-12, Bcl-2, activated Caspase-3, PSD-95, Hsp70, and HO-1 were determined by first lysing freshly dissected hippocampus using an ice-cold radioimmunoprecipitation assay buffer. The total protein concentration was measured by using a BCA Protein Assay Reagent Kit (Pierce Biotechnology). Twenty micrograms of total protein of each sample was transferred to a polyvinylidene fluoride membrane (Millipore Corporation) at 18 V for 1 h by the semidry transfer method. The membranes were blocked with 5% nonfat dry milk at 37°C for 1 h and incubated with the relevant primary antibody at 4°C overnight. The blocked membranes were then incubated with the primary antibody (monoclonal anti-CHOP, polyclonal activated caspase-3, polyclonal Bcl-2, polyclonal GRP-78, polyclonal PSD-95; Cell Signaling Technology; or polyclonal anti-caspase-12, monoclonal anti-HO-1, polyclonal anti-Hsp70; Abcam), and the immunoreactive bands were visualized using a chemiluminescent reagent as recommended by the Supersignal West Dura Extended Duration Substrate kit (Pierce Chemical). The signals of the bands were quantified using the VICTOR-Z 1420 multilabel counter (EG&G WALLAC). The results were expressed as a relative density. Equal protein loading in each lane was confirmed by hybridization with a 1:10,000 dilution of β-actin antibody (Bio-Rad Laboratories).
Isolation of mitochondria from the hippocampus and measurement of mitochondrial function
Mitochondria were isolated from hippocampal tissue using the discontinuous Percoll density gradient method (52). Isolated mitochondria suspensions were used immediately to detect enzyme activities. The rate of ATP production was determined by a bioluminescence assay kit (Sigma-Aldrich; Stock No. FL-AA) as described (24). The activity of respiratory chain complex I was measured by NADH-ubiquinone oxidoreductase methods (16). More detailed information is included in the Supplementary Data.
Dichlorofluorescein oxidation assays of ROS production
To quantify the ROS level in the hippocampus, acutely dissected hippocampus was weighed and homogenized in 0.01 M PBS. The tissue homogenate was then centrifuged at 6000 rpm for 15 min (4°C) and 5 μl supernatant was mixed with 55 μl HEPES (0.02 M) and 90 μl fresh dichlorofluorescein (DCF) diacetate (20 μM; Sigma-Aldrich) in a black, flat-bottomed 96-well plate and incubated at 37°C for 30 min. Fluorescence intensity was determined with a plate reader (SynergyMx; BioTek) at an excitation wavelength of 485 nm. For detection of mitochondrial ROS, the isolated mitochondria were incubated in ROS reaction buffer (0.32 M sucrose, 10 mM Tris, 20 mM 3-(N-morpholino)propanesulfonic acid, 50 mM ethylene glycol tetraacetic acid, 0.5 mM MgCl2, 0.1 mM Pi [K+], 5 mM sodium succinate, and 10 μM H2-DCFDA. Fluorescence intensity was determined with a plate reader (SynergyMx; BioTek) at an excitation wavelength of 485 nm after incubating at 37°C for 30 min.
Protein carbonyl and MDA level assay
The detection of protein carbonyl and MDA was measured using a protein carbonyl colorimetric assay kit (Cayman Chemical) and MDA kit (NanJin JCSWYJS) following the procedures recommended by the manufacturer. Detailed information is included in the Supplementary Data.
TUNEL evaluation
Apoptosis was detected by the in situ TUNEL technique, using an ApopTag1 kit (Millipore Corporation) and following the procedures recommended by the manufacturer. Standard transverse paraffin sections (5 μm thick) containing the hippocampus were prepared (three mice from each group). TUNEL staining was performed on one section of five consecutive sections. After processing and dehydration, the slides were mounted with Permount and observed under a light microscope (Zeiss Microscope Axiophot 2).
Transmission electron microscopy
Transmission electron microscopy was used to investigate the ultrastructural change of the synapses in the hippocampus following the method described previously (5). The entire hippocampal region was first dissected out from the brain. Sections from parts of the hippocampal CA1 area were diced into 1–2-mm pieces and further fixated for 4 h. The pieces were postfixed in 1–2% osmium tetroxide for 30 min, dehydrated in graded alcohol, then transferred to propylene oxide, and embedded gradually in blocks of Epon 812 resin for 2 days at 60°C. Eighty nanometer sections were collected using a diamond histoknife (Diatome) on an Ultracut E microtome (Leica), and then mounted on a copper mesh and stained with uranyl acetate and lead nitrate. One section was randomly chosen from five consecutive sections, and four pictures were taken from every section. Ten sections were counted for each group. Excitatory synapses with the presence of at least three vesicles in the presynaptic bouton and a PSD were identified by a transmission electron microscope (Hitachi H-7700). PSD thickness, synaptic cleft, and the length of the active zone, key parameters of synapse morphology, were examined with UTHSCSA Image Tools 3.0 (University of Texas Medical School at San Antonio).
Golgi–Cox staining
Golgi–Cox staining was performed using the FD Rapid GolgiStain kit (FD NeuroTechnologies) according to the instructions of the manufacturer. Pyramidal neurons from the CA1 region of the hippocampus were observed under a light microscope (Zeiss Microscope Axiophot 2) by an investigator blind to the treatment. To calculate spine density, apical dendrites with spines from 20 neurons for each group were traced using a 60× lens, and the number of spines along the length was counted to give a measure of spines/10 μm length by Metamorph 7.5 software (Molecular Devices). The criteria for spine subtypes were mainly based on the relative proportion of the spine length, spine head diameter, and spine neck diameter based on well-accepted criteria (25).
Data analysis
Data are expressed as means±standard error of means. A two-tailed unpaired Student's t-test was used to compare the two groups. Significant difference was determined by one-way ANOVA, followed by Newman–Keuls's test as the post hoc test for multiple group comparisons, and two-way ANOVA factoring time and treatment for behavior tests. A p value of <0.05 was regarded as statistically significant.
Supplementary Material
Abbreviations Used
- ANOVA
analysis of variance
- BDNF
brain-derived neurotrophic factor
- CHOP
C/EBP homologous protein
- DCF
dichlorofluorescein
- EDTA
ethylenediaminetetraacetic acid
- ER
endoplasmic reticulum
- fEPSP
field excitatory postsynaptic potential
- Grp78
glucose-regulated protein 78
- HO-1
heme oxygenase-1
- Hsp70
heat shock protein 70
- IH
intermittent hypoxia
- LTP
long-term potentiation
- MDA
malondialdehyde
- NAC
N-acetylcysteine
- OSA
obstructive sleep apnea
- PBA
4-phenylbutyric acid
- PBS
phosphate-buffered saline
- PSD-95
postsynaptic density-95
- ROS
reactive oxygen species
- TUDCA
tauroursodeoxycholic acid
- TUNEL
terminal transferase-mediated dUTP nick end labeling
- UPR
unfolded protein response
Acknowledgments
This work was supported by a Hong Kong Research Grants Council grant, GRF2140848, and the Theme-based Research Scheme, T13-607/12R.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Achat-Mendes C, Anderson KL, and Itzhak Y. Impairment in consolidation of learned place preference following dopaminergic neurotoxicity in mice is ameliorated by N-acetylcysteine but not D1 and D2 dopamine receptor agonists. Neuropsychopharmacology 32: 531–541, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Alma do CE, Machado BH, and Leao RM. Chronic intermittent hypoxia depresses afferent neurotransmission in NTS neurons by a reduction in the number of active synapses. J Neurosci 32: 16736–16746, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beebe DW. and Gozal D. Obstructive sleep apnea and the prefrontal cortex: toward a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res 11: 1–16, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Boyadjieva NI. and Sarkar DK. Cyclic adenosine monophosphate and brain-derived neurotrophic factor decreased oxidative stress and apoptosis in developing hypothalamic neuronal cells: role of microglia. Alcohol Clin Exp Res 37: 1370–1379, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briones TL, Suh E, Jozsa L, Hattar H, Chai J, and Wadowska M. Behaviorally-induced ultrastructural plasticity in the hippocampal region after cerebral ischemia. Brain Res 997: 137–146, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Cabello CM, Lamore SD, Bair WB, 3rd, Davis AL, Azimian SM, and Wondrak GT. DCPIP (2,6-dichlorophenolindophenol) as a genotype-directed redox chemotherapeutic targeting NQO1*2 breast carcinoma. Free Radic Res 45: 276–292, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calabrese V, Stella AM, Butterfield DA, and Scapagnini G. Redox regulation in neurodegeneration and longevity: role of the heme oxygenase and HSP70 systems in brainstress tolerance. Antioxid Redox Signal 6: 895–913, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Caldeira MV, Salazar IL, Curcio M, Canzoniero LM, and Duarte CB. Role of the ubiquitin-proteasome system in brain ischemia: friend or foe? Prog Neurobiol 112: 50–69, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Cao SS. and Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 21: 396–413, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castro-Caldas M, Carvalho AN, Rodrigues E, Henderson CJ, Wolf CR, Rodrigues CM, and Gama MJ. Tauroursodeoxycholic acid prevents MPTP-induced dopaminergic cell death in a mouse model of Parkinson's disease. Mol Neurobiol 46: 475–486, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Winters C, Azzam R, Li X, Galbraith JA, Leapman RD, and Reese TS. Organization of the core structure of the postsynaptic density. Proc Natl Acad Sci U S A 105: 4453–4458, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou YT, Zhan G, Zhu Y, Fenik P, Panossian L, Li Y, Zhang J, and Veasey S. C/EBP homologous binding protein (CHOP) underlies neural injury in sleep apnea model. Sleep 36: 481–492, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dayyat EA, Zhang SX, Wang Y, Cheng ZJ, and Gozal D. Exogenous erythropoietin administration attenuates intermittent hypoxia-induced cognitive deficits in a murine model of sleep apnea. BMC Neurosci 13: 77, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dempsey JA, Veasey SC, Morgan BJ, and O'Connell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dere E, Huston JP, and De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev 31: 673–704, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Estornell E, Fato R, Pallotti F, and Lenaz G. Assay conditions for the mitochondrial NADH:coenzyme Q oxidoreductase. FEBS Lett 332: 127–131, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Friberg H, Wieloch T, and Castilho RF. Mitochondrial oxidative stress after global brain ischemia in rats. Neurosci Lett 334: 111–114, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Fuentes F, Zimmer D, Atienza M, Schottenfeld J, Penkala I, Bale T, Bence KK, and Arrequi CO. Protein tyrosine phosphatase PTP1B is involved in hippocampal synapse formation and learning. PloS One 7: e41536, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung SJ, Xi MC, Zhang JH, Sampogna S, Yamuy J, Morales FR, and Chase MH. Apnea promotes glutamate-induced excitotoxicity in hippocampal neurons. Brain Res 1179: 42–50, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldbart A, Row BW, Kheirandish L, Schurr A, Gozal E, Guo SZ, Payne RS, Cheng Z, Brittian KR, and Gozal D. Intermittent hypoxic exposure during light phase induces changes in cAMP response element binding protein activity in the rat CA1 hippocampal region: water maze performance correlates. Neuroscience 122: 585–590, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Gorman AM, Healy SJ, Jäger R, and Samali A. Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacol Ther 134: 306–316, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Gozal D. Obstructive sleep apnea in children: implications for the developing central nervous system. Semin Pediatr Neurol 15: 100–106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gozal D, Row BW, Kheirandish L, Liu R, Guo SZ, Qiang F, and Brittian KR. Increased susceptibility to intermittent hypoxia in aging rats: changes in proteasomal activity, neuronal apoptosis and spatial function. Neurochem 86: 1545–1552, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Hamada K, Takuwa N, Yokoyama K, and Takuwa Y. Stretch activates Jun N-terminal kinase/stress-activated protein kinase in vascular smooth muscle cells through mechanisms involving autocrine ATP stimulation of purinoceptors. J Biol Chem 273: 6334–6340, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Harris KM, Jensen FE, and Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci 2: 2685–2705, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henkel AS, Dewey AM, Anderson KA, Olivares S, and Green RM. Reducing endoplasmic reticulum stress does not improve steatohepatitis in mice fed a methionine-and choline-deficient diet. Am J Physiol Gastrointest Liver Physiol 303: 54–59, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hetz C, Chevet E, and Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov 12: 703–719, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Higa A. and Chevet E. Redox signaling loops in the unfolded protein response. Cell Signal 24: 1548–1555, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Inoue T. and Suzuki-Karasaki Y. Mitochondrial superoxide mediates mitochondrial and endoplasmic reticulum dysfunctions in TRAIL-inducedapoptosis in Jurkat cells. Free Radic Biol Med 61C: 273–284, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Kassed CA. and Herkenham M. NF-kappaB p50-deficient mice show reduced anxiety-like behaviors in tests of exploratory drive and anxiety. Behav Brain Res 154: 577–584, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Kazlauckas V, Schuh J, Dall'Igna OP, Pereira GS, Bonan CD, and Lara DR. Behavioral and cognitive profile of mice with high and low exploratory phenotypes. Behav Brain Res 162: 272–278, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Kim J, Lee S, Bhattacharjee R, Khalyfa A, Kheirandish-Gozal L, and Gozal D. Leukocyte telomere length and plasma catestatin and myeloid-related protein 8/14 concentrations in children with obstructive sleep apnea. Chest 138: 91–99, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim R, Emi M, Tanabe K, and Murakami S. Role of the unfolded protein response in cell death. Apoptosis 11: 5–13, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, and Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol 575: 229–239, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li RC, Row BW, Gozal E, Kheirandish L, Fan Q, Brittian KR, Guo SZ, Sachleben Je, and Gozal D. Cyclooxygenase 2 and intermittent hypoxia-induced spatial deficits in the rat. Am J Respir Crit Care Med 168: 469–475, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Lumeng JC. and Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 5: 242–252, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malhotra JD. and Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9: 2277–2293, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Mathieu A, Mazza S, De'cary A, Massicotte-Marquez J, Petit D, Gosselin N, Malo J, and Montplaisir J. Effects of obstructive sleep apnea on cognitive function: a comparison between younger and older OSAS patient. Sleep Med 9: 112–120, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Molinaro P, Viggiano D, Nisticò R, Sirabella R, Secondo A, Boscia F, Pannaccione A, Scorziello A, Mehdawy B, Sokolow S, Herchuelz A, Di Renzo GF, and Annunziato L. Na+-Ca2+ exchanger (NCX3) knock-out mice display an impairment in hippocampal long term potentiation and spatial learning and memory. J Neurosci 31: 7312–7321, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller C, Bandemer J, Vindis C, Camaré C, Mucher E, Guéraud F, Larroque-Cardoso P, Bernis C, Auge N, Salvayre R, and Negre-Salvayre A. Protein disulfide isomerase modification and inhibition contribute to ER stress and apoptosis induced by oxidized low density lipoproteins. Antioxid Redox Signal 18: 731–742, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Nosyreva E. and Kavalali ET. Activity-dependent augmentation of spontaneous neurotransmission during endoplasmic reticulum stress. J Neurosci 30: 7358–7368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, and Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan C, Prentice H, Price AL, and Wu JY. Beneficial effect of taurine on hypoxia- and glutamate induced endoplasmic reticulum stress pathways in primary neuronal culture. Amino Acids 43: 845–855, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Quiroz-Baez R, Ferrera P, Rosendo-Gutiérrez R, Morán J, Bermúdez-Rattoni F, and Arias C. Caspase-12 activation is involved in amyloid-β protein-induced synaptic toxicity. J Alzheimers Dis 26: 467–476, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Ramalho RM, Nunes AF, Dias RB, Amaral JD, Lo AC, D'Hooge R, Sebastião AM, and Rodrigues CM. Tauroursodeoxycholic acid suppresses amyloid β-induce synaptic toxicity in vitro and in APP/PS1 mice. Neurobiol Aging 34: 551–561, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Ricobaraza A, Cuadrado-Tejedor M, Pérez-Mediavilla A, Frechilla D, Del Río J, and García-Osta A. Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer's disease mouse model. Neuropsychopharmacology 34: 1721–1732, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Ron D. and Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, and Marciniak SJ. Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol 112: 105–118, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Santos CX, Tanaka LY, Wosniak J, and Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal 11: 2409–2427, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Sateia MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med 24: 249–259, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Schroder M. and Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem 74: 739–789, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Sims NR. and Anderson MF. Isolation of mitochondria from rat brain using Percoll density gradient centrifugation. Nat Protoc 3: 1228–1239, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Sinha K, Das J, Pal PB, and Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 87: 1157–1180, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Spitler KM, Matsumoto T, and Webb RC. Suppression of endoplasmic reticulum stress improves endothelium-dependent contractile responses in aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 305: 344–353, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szegezdi E, Fitzgerald U, and Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci 1010: 186–194, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Toumane A, Durkin T, Marighetto A, Galey D, and Jaffard R. Differential hippocampal and cortical cholinergic activation during the acquisition, retention, reversal and extinction of a spatial discrimination in an 8-arm radial maze by mice. Behav Brain Res 30: 225–234, 1988 [DOI] [PubMed] [Google Scholar]
- 57.Upreti C, Otero R, Partida C, Skinner F, Thakker R, Pacheco LF, Zhou ZY, Maqlakelidze G, Velíšková J, Velíšek L, Romanovicz D, Jones T, Stanton PK, and Garrido-Sanabria ER. Altered neurotransmitter release, vesicle recycling and presynaptic structure in the pilocarpine model of temporal lobe epilepsy. Brain 135: 869–885, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van der Vlies D, Makkinje M, Jansens A, Braakman I, Verkleij AJ, Wirtz KW, and Post JA. Oxidation of ER resident protein upon oxidative stress: effects of altering cellular redox/antioxidant status and implications for protein maturation. Antioxid Redox Signal 5: 381–387, 2003 [DOI] [PubMed] [Google Scholar]
- 59.van Vliet AR, Verfaillie T, and Agostinis P. New functions of mitochondria associated membranes in cellular signaling. Biochim Biophys Acta 1843: 2253–2262, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Wall AM, Corcoran AE, O'Halloran KD, and O'Connor JJ. Effects of proly-hydroxylase inhibition and chronic intermittent hypoxia on synaptic transmission and plasticity in the rat CA1 and dentate gyrus. Neurobiol Dis 62: 8–17, 2014 [DOI] [PubMed] [Google Scholar]
- 61.Wang S. and Kaufman RJ. The impact of unfolded protein response on human disease. J Cell Biol 197: 857–867, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Zhang SX, and Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol 174: 307–316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie H, Leung KL, Chen L, Chan YS, Ng PC, Fok TF, Wing YK, Ke Ya, Li AM, and Yung WH. Brain-derived neurotrophic factor rescues and prevents chro nic intermittent hypoxia-induced impairment of hippocampal long-term synaptic plasticity. Neurobiol Dis 40: 155–162, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, and Yoffe B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology 36: 592–601, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Gozal D, and Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience 126: 313–323, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Yeh CH, Chen TP, Wang YC, Lin YM, and Fang SW. AMP-activated protein kinase activation during cardioplegia-induced hypoxia/reoxygenation injury attenuates cardiomyocytic apoptosis via reduction of endoplasmic reticulum stress. Mediators Inflamm 2010: 130636, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yong Z, Yan L, Dong Z, Wang X, Su R, and Gong Z. The effect of chronic thienorphine administration on long-term potentiation and synaptic structure in rat hippocampus. Synapse 67: 779–785, 2013 [DOI] [PubMed] [Google Scholar]
- 68.Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, and Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol 20: 6755–6767, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshida J, Kubo T, and Yamashita T. Inhibition of branching and spine maturation by repulsive guidance molecule in cultured cortical neurons. Biochem Biophys Res Commun 372: 725–729, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Zhan G, Fenik P, Pratico D, and Veasey SC. Inducible nitric oxide synthase in long-term intermittent hypoxia: hypersomnolence and brain injury. Am J Respir Crit Care Med 171: 1414–1420, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu Y, Fenik P, Zhan G, Sanfillipo-Cohn B, Naidoo N, and Veasey SC. Eif-2a protects brainstem motoneurons in a murine model of sleep apnea. J Neurosci 28: 2168–2178, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.