Abstract
Food allergy is one of the major causes that promote EoE; therefore, we tested the hypothesis that IL-18 is involved in food allergen-induced EoE pathogenesis. Accordingly, we examined normal SPT+ and SPT− EoE patient blood and biopsy samples for IL-18, IL-18Rα, ICAM and VCAM expression Herein, we show increased IL-18 level is highly significant in food allergen SPT+ compared to SPT− EoE patients. We also report that IL-18Rα+ cells and mRNA levels are induced in the esophageal biopsies of EoE patients and blood IL-18 levels correlate with esophageal eosinophilia (p<0.01). Additionally, we report that the levels of esophageal eosinophil and mast cells correlate with ICAM expression in human EoE. Mechanistically, we show that IL-18 in vitro stimulates iNKT cells and endothelial cells and induce eosinophil active cytokines IL-5, IL-13. We provide the evidence that IL-18 is critical cytokine involved in activation of iNKT cells and ICAM in promoting human EoE..
Keywords: EoE, ICAM, interleukin-IL-18, IL-18Rα, iNKT cells
1. Introduction
In recent years, major advances have occurred in understanding eosinophil and mast cells (MCs) accumulation (1-3) and remodeling in EoE (4, 5); however, the mechanisms of EoE induction, progression, diagnosis and treatment are still not clearly understood. EoE patients that have <15 eosinophils/hpf in esophageal biopsies are hard to differentiate from gastroesophageal reflux disease (GERD), as some EoE patients may have PPI-responsiveness (6). Experimental modeling in has established that Th2 cytokine signaling is required for induction of EoE. Considerable evidence supports a critical role for the Th2 cytokines interleukin (IL)-5, IL-13 and IL-15 in EoE pathogenesis (7, 8). The gene exhibiting the highest increase of expression in the esophagus of EoE patients is eotaxin-3, an eosinophil chemoattractant and activator produced by esophageal epithelial cells (2). Additionally, we, and others, reported that IL-15 responsive iNKT cells are induced in EoE and that neutralization of iNKT cells ameliorates the severity of in pediatric EoE (9-13). Earlier, our microarray analyses showed increased levels of the IL-18Rα transcript in EoE patients (2). IL-18 activates B cells and natural killer (NK) T cells in an antigen-independent manner (14, 15) and this process has been shown to contribute in a number of intestinal allergic responses including celiac disease, a disease that shares features with EoE (16-19). IL-18 is a pleiotropic cytokine that is elevated in a number of eosinophilic allergic diseases such as food allergy, dermatitis, asthma, and colitis (20-22). Inflammatory cells involved in innate immunity secrete IL-18 (16, 23) and it has been reported that IL-18 stimulates iNKT cells without T cell receptor (TCR) engagement (24, 25). The present study indicates that IL-18 may be involved in the pathogenesis of EoE. Herein, we demonstrate that IL-18 and its specific receptor IL-18Rα are increased in the blood and esophagus of EoE patients, respectively. Notably, IL-18Rα transcript levels correlate significantly with esophageal eosinophilia in active and treated EoE patients. Mechanistically, IL-18 primes iNKT cells to produce eosinophil active Th2 cytokines (e.g. IL-5, IL-13). Taken together, our current findings define a novel role for IL-18 in Th2 responses and provide evidence that IL-18 may have an important role in EoE pathogenesis by activating iNKT cells. Therefore, in the current study we tested the hypothesis that the induction of IL-18 is associated with the esophageal eosinophilia in human EoE. Herein, we report that indeed IL-18 may have a significant role in food allergic (SPT+) EoE patients.
2. Materials and Methods
2.1 Selection of EoE and non-EoE patients and tissue collection
Formalin-fixed, paraffin-embedded biopsy samples were obtained from the esophagus of normal individuals or EoE patients as per an Institutional Review Board (IRB)-approved protocol. The comparison control normal subjects (non-EoE), chronic esophagitis (CE) and EoE patients were selected without regard to age, atopic status or gender. Diagnosis was established based on the maximum eosinophil count per high power field (hpf) (×400). Control individuals (non-EoE or CE) were defined as having 0-2 esophageal eosinophils/hpf and no basal layer expansion. The normal biopsies were obtained from patients who showed symptoms typical of gastroesophageal reflux disease (GERD) and EoE but were found normal upon microscopic analyses of esophageal biopsies. Typically, these patients had abdominal pain, and some had allergic diseases including asthma or rhinitis. Patients with EoE were defined as having ≥ 15-esophageal eosinophils/hpf and included other allergic diseases such as asthma or atopic dermatitis. The CE (chronic esophagitis) patients define, as the group of patient does not qualify with the definition of EoE or normal. They have esophageal eosinophilia <15eosinophil/hpf with basal cell hyperplasia. The hospital pathologist as consistent with reflux esophagitis evaluated the eosinophils and mast cells level in the esophageal biopsies of non-EoE and EoE patients. The fresh biopsy samples were collected in RNA latter buffer for RNA isolation and blood was drawn from each normal, EoE and non-EoE patients at the time of scheduled biopsy (including before and after treatment of EoE patients) at Cincinnati Children’s and Tulane University School of Medicine in a citrate-coated tubes. The blood plasma was isolated by centrifugation and stored at −20C for cytokines analysis. All samples were used according to the patients’ consent and IRB approved protocol of CCHMC, Cincinnati, OH (Year 2011-12) and TUSM, New Orleans, LA (Year 2013-14). The pool EoE and non-EoE patient data generated at both hospital centers is presented and the patient’s detailed clinical characteristics are shown in Table 1. Our current study was performed on heterogeneous patients population, as PPI trial was not performed on all EoE patients, and is our limitation.
Table 1. Patients clinical and pathological characteristics.
| Patients | Age (Year) |
Gender | Esophageal Disease |
Allergic Disease |
Eosinophil (Maximum/ hp |
SPT+ | SPT− | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | 11 | F | NL | Rhinitis | 0 | LTRAa | ||
| 2 | 9 | M | NL | Asthma | 0 | + | None | |
| 3 | 8 | M | NL | Unknown | 0 | None | ||
| 4 | 13 | M | NL | None | 0 | LTRAa | ||
| 5 | 7 | M | NL | Unknown | 0 | LTRA | ||
| 6 | 12 | F | NL | None | 0 | None | ||
| 7 | 6 | M | NL | Unknown | 0 | Miralax, | ||
| 8 | 4 | M | NL | Unknown | 0 | LTRA, B2RA | ||
| 9 | 1 | F | NL | Unknown | 0 | − | None | |
| 10 | 3 | M | NL | Unknown | 0 | LTRAa | ||
| 11 | 9 | F | NL | Unknown | 0 | PPI | ||
| 12 | 3 | F | NL | Unknown | 0 | None | ||
| 13 | 11 | M | NL | Rhinitis | 0 | PPI | ||
| 14 | 1 | M | NL | Unknown | 0 | mesalamine | ||
| 15 | 10 | M | NL | Unknown | 0 | LTRAa | ||
| 16 | 13 | F | EoE | Asthma | 30 | − | None | |
| 17 | 12 | M | EoE | Rhinitis/Asthma/Food Allergy |
31 | + | Elementary diet | |
| 18 | 2 | M | EoE | None | 56 | − | PPIb | |
| 19 | 3 | M | EoE | None | 79 | + | Elementary diet | |
| 20 | 5 | F | EoE | Rhinitis | 21 | − | PPI | |
| 21 | 10 | M | EoE | Asthma/ Food Allergy | 205 | + | Elementary diet | |
| 22 | 8 | F | EoE | Asthma, Atopic dermatitis |
43 | − (SNS) |
PPI | |
| 23 | 9 | M | EoE | Unknown | 35 | None | ||
| 24 | 12 | M | EoE | Eczema /Rhinitis/Food Allergy |
25 | + | Elementary diet | |
| 25 | 11 | F | EoE | Asthma | 48 | + | None | |
| 26 | 11 | M | EoE | Asthma /Rhinitis | 71 | INHGC, B2ARA | ||
| 27 | 8 | M | EoE | Asthma / Eczema | 58 | + | Elementary diet | |
| 28 | 13 | M | EoE | Asthma / Eczema | 44 | INHGC, LTRA, | ||
| 29 | 4 | F | EoE | Rhinitis | 52 | − | PPI | |
| 30 | 12 | M | EoE | Asthma /Rhinitis | 198 | − | INHGC, PPI | |
| 31 | 15 | M | EoE | Unknown | 48 | + | PPI | |
| 32 | 14 | F | EoE | Asthma / Eczema | 69 | INHGC, PPI, | ||
| 33 | 5 | M | EoE | Eczema | 53 | + | Elementary diet | |
| 34 | 12 | F | EoE | Asthma /Rhinitis | 28 | − | B2ARA | |
| 35 | 13 | M | EoE | Unknown | 34 | + | Elementary diet | |
| 36 | 10 | M | EoE | Asthma/Food Allergy/Rhinitis/Anaph ylaxis |
236 | + | Elementary diet | |
| Patients | Age (Year) |
Gender | Esophageal Disease |
Allergic Disease |
Eosinophil (Maximum/ hp |
SPT+ | SPT− | Tretment |
| 37 | 10 | F | R | Asthma/ Eczema /Rhinitis |
0 | + | SWGC, Elementary diet |
|
| 38 | 6 | M | R | Unknown | 0 | SWGC | ||
| 39 | 12 | M | R | Asthma/ Eczema | 0 | SWGC, PPI | ||
| 40 | 14 | M | R | Eczema | 0 | − | SWGC | |
| 41 | 8 | M | R | Asthma/ Eczema /Rhinitis/Food Allergy |
1 | SWGC | ||
| 42 | 4 | M | R | Unknown | 0 | SWGC | ||
| 43 | 6 | F | R | Eczema | 2 | − | SWGC | |
| 44 | 3 | M | R | Asthma/ Eczema /Rhinitis |
0 | SWGC | ||
| 50 | 14 | M | R | Rhinitis | 8 (SNS) | + | Elementary diet | |
| 45 | 5 | M | NR | Rhinitis | 24 | SWGC, PPI | ||
| 46 | 4 | F | NR | Rhinitis | 31 | − | SWGC, PPI | |
| 47 | 7 | M | NR | Asthma/ Eczema /Rhinitis/Food Allergy |
19 | + | Elementary diet | |
| 48 | 7 | M | NR | Asthma/ Eczema /Rhinitis/Food Allergy |
43 | SWGC, antibiotic | ||
| 49 | 10 | F | NR | Unknown | 24 | SWGC | ||
| 50 | 4 | M | NR | Asthma | 28 | + | Elementary diet | |
| 51 | 11 | M | NR | Food Allergy/ Anaphylaxis |
128 | − | SWGC | |
| 52 | 9 | F | NR | Unknown | 43 | SWGC | ||
| 53 | 8 | M | NR | Rhinitis | 16 | + | Elementary diet | |
| 54 | 15 | M | NR | SWGC, OGC | 61 | SWGC, OGC | ||
| 55 | 6 | M | CE | Unknown | 5 | None | ||
| 56 | 11 | M | CE | Rhinitis/Food allergy | 3 | + | SWGC, Elementary diet |
|
| 57 | 5 | F | CE | Rhinitis/ Asthma | 8 | SWGC, antibiotic | ||
| 58 | 8 | M | CE | Unknown | 4 | SWGC, PPI | ||
| 59 | 12 | F | CE | Asthma | 11 | PPI/SWGC | ||
| 60 | 9 | F | CE | Asthma/ Eczema | 7 | SWGC |
Abbreviations: B2ARA=beta 2 adrenergic receptor antagonist, EoE=eosinophilic esophagitis, F=female, H1RA=H1 receptor antagonist, INHGC=inhaled glucocorticoid, LTRA=leukotriene receptor antagonist, M=male, NL=normal, NR=FP Non-responder, OGC=oral glucocorticoid (prednisone), PPI=proton pump inhibitor, R=FP responder, SWGC=swallowed glucocorticoid (FP), SNS=Samples not sufficient to use.
2.2 Quantification of serum IL-18
IL-18 protein concentrations in the serum of normal and EoE patient samples were quantified by using a DuoSet Enzyme-Linked Immunosorbent Assay (ELISA) Development kit (R & D Systems). The detection limit was 0.9 pg/ml.
2.3 Quantitative PCR
The RNA samples (500 ng) were subjected to reverse transcription using Bioscript reverse transcriptase (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. IL-18Rα, ICAM, VCAM, IL-5 and IL-13 were quantified by real-time PCR using IQ5 (Bio-Rad). Results were then normalized to human or mouse GAPDH amplified from the same cDNA mix and expressed as relative gene expression. cDNAs were amplified using the primers listed in Table 2.
Table 2.
List of primer sequences
| Genes | Sense and anti-sense primer sequence |
|---|---|
| hIL-5 | 5′-GCTTTGCATTTGAGTTTGCTAGCT3′ 5′-TGGCCGTCAATGTATTTCTTTATTAAG3′ |
| hL-13 | 5′-ACAGCCCTCAGGGAGCTCAT3′ 5′-TCAGGTTGATGCTCCATACCAT3′ |
| hIL-18Rα | 5′CGCTGCTTCTCACCTACTTT3′ 5′AAAGGTTCCCCTTCAACCAC3′ |
| hVCAM | 5′ACCACATCTACGCTGACAAT3′ 5′AACACTTGACTGTGATCGGC3′ |
| hICAM | 5′AGCGCTATAAAGGATCACGC3′ 5′ GGATGACTTTTGAGGGGGAC3′ |
| hGADPH | 5′TGGAAATCCCATCACCATCT3′ 5′GTCTTCTGGGTGGCAGTGA T3′ |
2.4 Immunofluorescence staining of biopsy samples for IL-18Rα+ cells detection
Cryostat sections from frozen esophageal biopsies of normal subjects (non-EoE) and EoE patients were fixed, blocked with normal goat serum to reduce non-specific binding, and incubated with anti-IL-18Rα+ antibody (eBioscience). The IL-18Rα+ immunostained sections were mounted with DAPI mounting material. The Images were captured using an Olympus BX51 microscope with appropriate filters. IL-18Rα+ positive cells were counted on each stained tissue sections per high power field (400×) and expressed as “IL-18Rα+cells/hpf.” Total 4-5 high power fields in each esophageal sections were evaluated in EoE or normal (non-EoE) control individual biopsies sections.
2.5 Analysis of ICAM and VCAM expression on HMVEC cells by flowcytometer
The primary microvascular endothelial cells (HMVEC, Cat # CC-2527, Lonza Group, Ltd, USA) were obtained and were exposed to 0, 5 and 50 nM IL-18 for 24 hrs. These cells were stained with cell surface molecule-specific antibodies for flowcytometer analysis. The following reagents were used for specific antigen analysis: anti-ICAM-1, anti-VCAM-1 and anti-TNFα antibodies (eBiosciences). The cells were incubated for specific antigens with the required combination of antibodies at 4°C for 45 minutes followed by two washes. The flowcytometric analysis was performed using a FACSCalibur (BD Biosciences) and analyzed using Flow J software.
2.6 IL-18 treatment to human iNKT cell line
The non-immortalized human iNKT cell line (26) was provided by Dr. Mattner (CCHMC, Cincinnati, OH) and was grown in RPMI-1640 (HyClone Laboratories, Logan, UT) and Click’s Medium (Sigma Aldrich, St. Louis, MO) containing 10% fetal calf serum (FCS, Gibco BRL), 1% 200mM L-Glutamine (Invitrogen, Grand Island, NY) and penicillin-streptomycin (Gibco BRL). The cells were grown and split into 6-well plates starting with a concentration of 1×106 cells in 2ml media per well. Subsequently, different concentrations of IL-18 (0, 100, 500, 100 ng/ml) were added to the culture protein levels using a DuoSet ELISA set (R&D Systems) as per the manufacturer’s protocol.
2.7 Statistical analysis
For all cell counts, stained slides were analyzed randomly and in a blinded fashion. The nonparametric Mann–Whitney U-test was employed for comparison of data between two groups, and Krustal–Wallis for comparison of more than two groups. Parametric data were compared using t - tests or analysis of variance. Values are reported as mean ± S.D. P -values < 0.05 were considered statistically significant.
3. Results
3.1 IL-18 is induced and correlates with esophageal eosinophilia in EoE
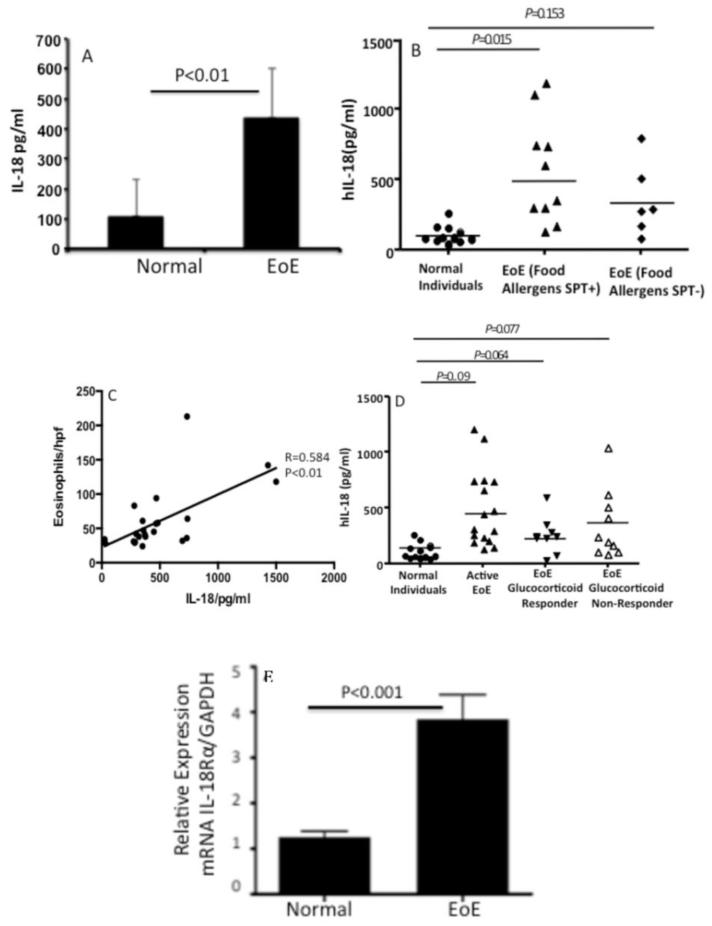
We examined the blood levels of IL-18 in normal (non-EoE) and EoE patients. The analysis revealed that indeed IL-18 levels are > 3-fold higher in active EoE patients compared to the normal (non-EoE) patients (Fig. 1 A). Second, we tested whether food allergy is responsible for the elevated IL-18 in EoE patients by examining the levels of IL-18 in food allergen skin positive (SPT+) EoE patients. We found that SPT+ EoE patients have higher levels of IL-18 compared to the food allergen SPT- EoE patients (Fig. 1B). Notably, the differences of IL-18 levels in between SPT+ and SPT− is significant but due to the small sample size these data may not be very conclusive and needs more work. Currently the sample size is our limitation. Interestingly, the blood IL-18 levels correlated to the eosinophil counts in the esophageal biopsies of EoE patients (Fig. 1 C). Further, we also found that the patients with improved EoE following oral glucocorticoid treatment had a significantly reduced IL-18 levels (Fig. 1D).
Figure 1. Induced expression of blood IL-18 and esophageal IL-18Rα+ cells in non-EoE, and active EoE patients.
Blood IL-18 was measured in non-EoE (normal) individuals and EoE patient samples by performing ELISA analysis. Additionally the patients are divided to skin test positive (SPT+) and (SPT-) for food allergens. Their blood IL-18 levels are also analyzed and shown (A, B). A correlation between the maximum eosinophil number/hpf of EoE patients with blood IL-18 levels is statistically analyzed in human EoE and is found r=0.58 and p<0.01 (C). The blood IL-18 levels in non-EoE (normal) individuals, active EoE patients (> 24 eosinophils/hpf); patients responsive to glucocorticoid treatment or non-responsive to the treatment groups are analyzed and are shown (D). The relative expression of IL-18Rα mRNA of normal (non-EoE) and EoE patients biopsies are analyzed by real time PCR analysis and is shown (E). Each data point represents an individual patient The data is expressed as mean ± SD. p-values were in between each groups are calculated using Kreskas–Wallis test followed by Dunn’s multiple comparison tests.
3.2 IL-18 Rα expressing cells increase in human EoE
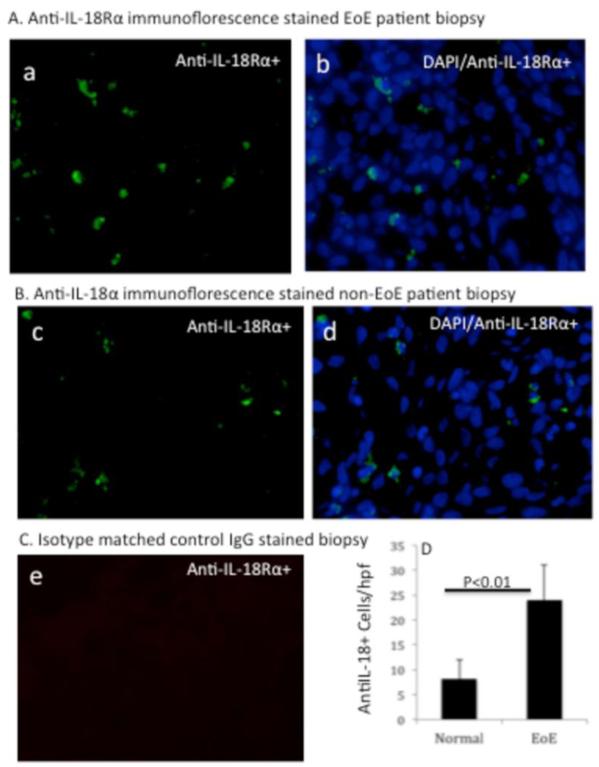
Notably, our previously reported microarray data showed an increase IL-18Rα transcript levels in EoE patients compared to the normal individuals (2). Therefore, we further validated these preliminary findings by quantifying IL-18Rα mRNA expression in the esophagus of EoE patients compared to control individuals. A ~ 4-fold increase in levels of IL-18Rα mRNA in esophageal biopsies of EoE patients was found compared to the normal (non-EoE) patients (Fig. 1 E). Next, our interest was to examine and quantitate the IL-18Rα+ cells in the esophageal biopsies of normal individual (non-EoE patient) and EoE patients. Therefore, we examined IL-18Rα positive cells by performing anti-IL-18Rα immunofluorescence staining in the frozen proximal and distal esophageal biopsies. Our analysis detected a high level of anti-IL-18Rα positive cells in the epithelial mucosa of EoE patient esophageal biopsies [Figure 2A (a-b)]. In contrast, a few numbers of anti-IL-18Rα positive cells were detected in the epithelial mucosa of non-EoE patients. [Figure 2B (c-d)]. The isotype matched negative control IgG did not detect immunoreactive cells in the esophageal mucosa of EoE patient biopsies [Figure 2 C (e)]. Quantification of the IL-18Rα-positive cells in esophageal biopsies indicated that ~ 24 ± 7 IL-18Rα+ cells/hpf, n=8 accumulated in the esophageal biopsies of EoE patients compared to ~ 8 ± 4 IL-18Rα+ cells/hpf, n=7 in the non-EoE control group (Figure 2 D).
Figure 2. Immunofluorescence analysis of esophageal IL-18Rα+ cells in active EoE patients and non-EoE individual.
A representative photomicrograph of anti-IL-18Rα FITC conjugated and DAPI mounted esophageal biopsy section of EoE patient and non-EoE individual is shown (A, B). The analysis detected IL-18Rα+ cells (stained green) in EoE patient esophageal biopsy. [A (a)], the overlapped merged figure of DAPI/IL-18Rα+ cells is shown [A, (b)]. A similar analysis was performed in the biopsy of non-EoE individual and shown [B, (c-d)]. The isotype matched control antibody has not detected any staining of IL-18Rα+ cells in EoE patient biopsy [C, (e)]. All photomicrograph shown has original magnification in 400×. The quantitation of IL-18Rα+ cells in normal (comparison control) individuals and EoE patients are shown in [D]. The level of significance was calculated by using the Mann-Whitney test, P <0.01, n= 7-8.
3.3 Intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 is induced in human EoE
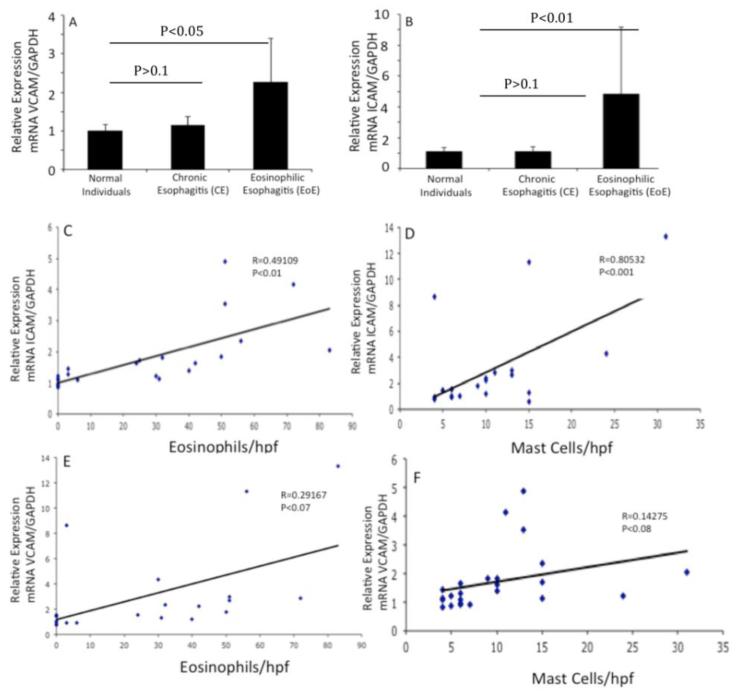
Eosinophils and mast cells express intracellular adhesion molecule (ICAM-1) and vascular cell adhesion molecule (VCAM-1) and their induction has been implicated in the adherence of these cells in a number of allergic diseases.(27, 28) Therefore, we next examined the expression of ICAM-1 and VCAM-1 in the esophageal biopsies of normal individuals, chronic esophagitis (CE) and EoE patients. Our analyses revealed that the expression of both ICAM-1 and VCAM-1 are induced in EoE compared to CE or normal individuals (Fig 3 A, B). Further, we tested the hypothesis that ICAM-1 and VCAM-1 expression correlated with the number of esophageal eosinophils and mast cells in EoE patients. Interestingly, our analysis indicated that the expression of ICAM-1 correlated well with the esophageal eosinophils and mast cells (Fig 3 C, D); however a very weak correlation of esophageal eosinophils and mast cells with VCAM-1 levels was observed in EoE patients (Fig 3, E, F).
Figure 3. Analysis of IL-18-induced ICAM-1 and VCAM-1 genes expression in human esophageal biopsies.
The mRNA levels of ICAM-1 and VCAM-1 were examined by performing quantitative real-time PCR analysis on the biopsy samples of normal individuals, CE and EoE patients. The mRNA expression was normalized to GAPDH mRNA and expressed as relative expression. Statistical significance was calculated using the Mann-Whitney test. p values for each experiment are provided in the figure (A-B). Correlations between the peak eosinophil and mast cell numbers/hpf vs. ICAM-1 and VCAM-1 mRNA expression normalized with GADPH mRNA in human EoE is shown (C and F). The r-value was calculated using the Spearman correlation test (n=17-20). Statistical significance was calculated using both the Mann–Whitney and Kruskal–Wallis test.
3.4 IL-18 Induces ICAM-1 and VCAM-1 expression in endothelial cells (HMVEC)
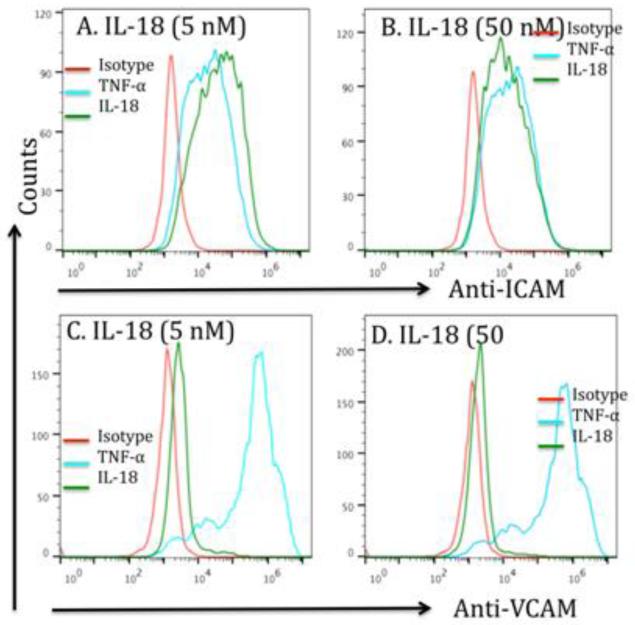
Next, we tested the hypothesis that IL-18 is responsible for the induction of ICAM-1 and VCAM-1 expression. Accordingly, we performed in vitro experimentation using rIL-18 and HMVEC cells. Our experimentation indicated that rIL-18 induces ICAM-1 and VCAM-1 expression in primary microvascular endothelial cells (HMVEC, Cat # CC-2527, Lonza Group, Ltd, USA) were exposed to 0, 5 and 50 nM IL-18 for 24 hrs. Induction of ICAM-1 and VCAM-1 to a lesser extent by both 5 and 50 nM IL-18 was demonstrated by flowcytometer, TNFα was used as a positive control that induces both ICAM-1 and VCAM-1 expression on endothelial cells (Figure 4 A-D).
Figure 4. IL-18 induces ICAM-1 and VCAM-1 on endothelial cells; but VCAM-1-deficient mice are not protected from allergen-induced EoE.
A flowcytometric analysis was performed to examine ICAM-1 and VCAM-1 expression on IL-18 treated (5 and 50 nM) and a positive control TNF-α (10 nM) on endothelial cells. The histogram showed that both doses of IL-18 treatment of endothelial cells show a significant induction in the expression of ICAM-1 (A, B) and also induces some expression of VCAM-1 (C, D). The positive control TNF-α also confirms the induced expression of both adhesion molecules on endothelial cells. The histogram is representative of three independent experiments.
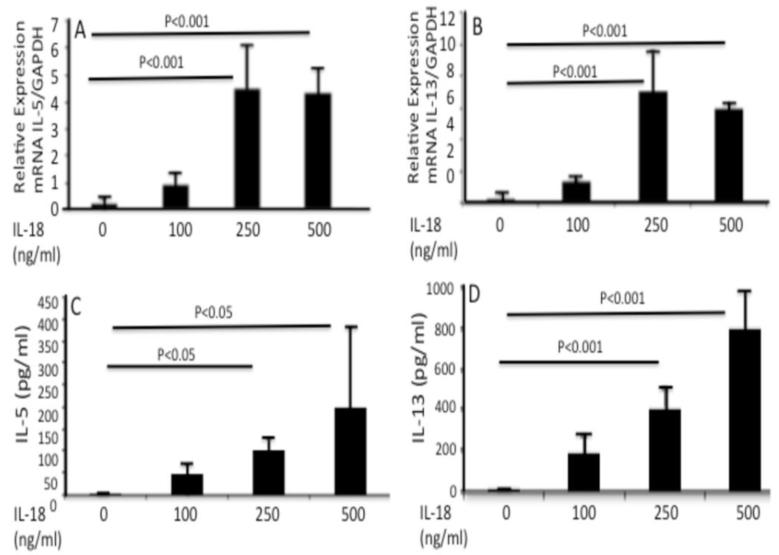
3.5 IL-18 stimulates iNKT cells to produce eosinophil active Th2 cytokines
Lastly, we focused our studies on the mechanism by which IL-18 promotes EoE in humans. We tested the hypothesis that IL-18 activates iNKT cells, as induced iNKT cells in EoE patients were earlier reported by us and others (9-13) and neutralization of iNKT cells protects against EoE in mice.(10) Accordingly, we tested the hypothesis that IL-18 stimulated a human iNKT cell line (26) to express eosinophil active Th2-type cytokines. iNKT cells (5×105) exposed to 0, 100, 250, and 500 ng/ml recombinant IL-18 for 48 hrs increased IL-5 and IL-13 transcripts (Fig 5 A, C) and proteins (Fig 5 B and D). Notably, addition of IL-18 to conventional CD4+ T cells (isolated from mouse spleen) did not produce IL-5 or IL-13 following 24, 48 and 72 hrs in vitro incubation (data not shown). All cytokine transcript data obtained was normalized with GAPDH and expressed as relative expression, the cytokine protein data is expressed as pg/ml of media. The data is expressed as mean ± SD; n= 3 independent experiments.
Figure 5. iNKT cells express IL-18Rα in experimental EoE and generate Th2 cytokine from iNKT cell line following exposure with IL-18.
iNKT cell line was exposed to IL-18 (0, 50 and 100, 250 and 500 ng/ml) for 48 hrs. IL-18 activates iNKT cells and induces eosinophil active cytokines IL-5 and IL-13 mRNA (A, C), and protein (B, D). The cytokines mRNA expression was normalized with GAPDH and shown as a relative expression of each cytokine.
4. Discussion
Esophageal eosinophilia occurs in a variety of clinical disorders including GERD, eosinophilic gastroenteritis and EoE (29, 30). A number of clinical studies suggest that these disorders (especially EoE) are occurring with increasing frequency (31-33). Our current study was designed to understand the molecular processes involved in iNKT cell-mediated EoE pathogenesis. A number of studies implicated iNKT cell accumulation in human EoE (9-13); however, it is not yet clear what mechanism accounts for activation of iNKT cells in human EoE. Our previous microarray analyses indicated that the IL-18Rα transcript was induced in EoE patients (2). In the present study, we show that the blood IL-18 and IL-18Rα+-cells in esophageal biopsies are greatly induced in EoE patients. Interestingly, the level of blood IL-18 is found significantly increased in food allergen SPT+ EoE patients while SPT- EoE patients does not have elevated IL-18 in the blood. Furthermore, the levels of IL-18 strongly correlate with esophageal eosinophils in active EoE patients and the levels of blood IL-18 significantly decrease in resolving EoE patients compared to active EoE patients. Of note, our analysis is on the smaller sample size, therefore, we recommend to establish that elevated serum levels of IL-18 as a non-invasive biomarker for the diagnosis of SPT+ EoE patients, more samples has to be analyzed for confirmed conclusion.
Additionally, our studies also found that EoE patients have increased expression ICAM-1 and VCAM-1 and that the levels of ICAM-1 significantly correlate with esophageal eosinophil and mast cell numbers. Notably, ICAM-1 is found induced in a number of allergic diseases (34) and implicated in the tissue recruitment of eosinophils and mast cells (27, 28, 35, 36). The ICAM-1 and VCAM-1 are expressed by both eosinophils and mast cells (37, 38) and our data indicates that both are induced in EoE patients. Therefore, we further tested induction of ICAM-1 and VCAM-1 expression by IL-18. Treatment of human endothelial cells with IL-18 induced both ICAM-1 and VCAM-1, with the former more significantly induced that is in accordance with EoE patient data that ICAM-1 significantly correlates with tissue eosinophils and mast cells in human EoE. Another mechanistic possibility is that induced IL-18 activates iNKT cells to release eosinophil active cytokines that promote EoE in humans. Earlier, others and we reported that iNKT cells are induced and critical in EoE (9-13). Of note, the mechanism by which iNKT cells promote EoE is not well understood. Herein, we provide the data that show IL-18 stimulates iNKT cells to produce eosinophil active cytokines like IL-5 and IL-13. This observation is in accordance with earlier reports that mature and activated iNKT cells are a prominent source of Th1 and Th2 cytokines (15, 39, 40). The present and prior studies now provide evidence that IL-18 activation causes IL-15-responsive iNKT cells to promote EoE in humans. In summary, we demonstrate that EoE patients have increased levels of IL-18 and IL-18Rα. Induction of IL-18 significantly correlates with esophageal eosinophilia in humans and, following treatment, IL-18 levels decline in resolving EoE patients. IL-18 induces ICAM-1 in esophageal endothelial cells to facilitate eosinophil and mast cell recruitment to the esophagus. More importantly, we show that IL-18 stimulates iNKT cells to produce abundant eosinophil active cytokines, IL-5 and IL-13 that are shown to be critical in EoE pathogenesis (8, 41, 42).
Taken together, the current study provides a rationale and a better understanding on the significance of the earlier findings on the significance of IL15 (15, 43) and CD1d-restricted iNKT cells in human EoE (9-13). Most importantly, the current study identifies a novel role for IL-18 in stimulating IL-15 responsive iNKT cells and B cells leading to induction of Th2 cytokines and IgE-mediated food allergen-induced EoE. Most Importantly, our current study highlights the significance of IL-18 as an future diagnostic and therapeutic target molecule for human EoE.
HIGHLIGHTS.
IL-18 is elevated in a number of eosinophilic allergic diseases including EoE.
IL-18 stimulates iNKT cells without T cell receptor (TCR) engagement.
Study provides a rationale for CD1d-restricted iNKT cells activation in human EoE.
Importantly, IL-18 may be a diagnostic and therapeutic target molecule for human EoE
Acknowledgements
This work was supported in part by the grants NIH RO1 DK067255, (AM), NIH R01 AI080581 (AM). The authors also thank Drs. Jack Elias, MD. PhD (Yale University) for providing IL-18 transgenic mice, James and Nancy Lee, PhD (Mayo Clinic) for the generous supply of anti-MBP and Marc Rothenberg, MD. PhD, (CCED, Cincinnati Children’s, Cincinnati, OH) for the continuous support and encouragement and providing EoE and non-EoE patient samples for the initial study. We also thank the National Institute of Allergy and Infectious Diseases (NIAID) tetramer core facility for providing CD1d-αGalCer-tetramer. We further thank Lee L. Hamm, MD and Joseph A. Lasky, MD for providing the facility to continue eosinophil associated disorders research work at Tulane University School of Medicine. Kristen Lingle, RN, Jaya Mishra, PhD and Christine Glynn, RN, research coordinators, for their efforts in IRB approval and enrolling the patients, collecting the samples, recording and providing the patients characteristics.
Abbreviations
- CE
Chronic esophagitis
- DAPI
4′,6-diamidino-2-phenylindole fluorescent mounting material
- EoE
Eosinophilic esophagitis
- ELISA
Enzyme-Linked Immunosorbent Assay
- GERD
Gastroesophageal reflux disease
- ICAM
Intracellular adhesion molecule
- VCAM
vascular cell adhesion molecule
- IL
Interleukin
- iNKT
invariant natural killet T cell
- PCR
Polymerase chain reaction
- HMVEC
Primary human microvascular endothelial cells
- SPT+
Skin allergen positive test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rothenberg ME, Mishra A, Collins MH, Putnam PE. Pathogenesis and clinical features of eosinophilic esophagitis. The Journal of allergy and clinical immunology. 2001;108:891–894. doi: 10.1067/mai.2001.120095. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, Jameson SC, Kirby C, Konikoff MR, Collins MH, Cohen MB, Akers R, Hogan SP, Assa’ad AH, Putnam PE, Aronow BJ, Rothenberg ME. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. The Journal of clinical investigation. 2006;116:536–547. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abonia JP, Blanchard C, Butz BB, Rainey HF, Collins MH, Stringer K, Putnam PE, Rothenberg ME. Involvement of mast cells in eosinophilic esophagitis. The Journal of allergy and clinical immunology. 2010;126:140–149. doi: 10.1016/j.jaci.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. The Journal of allergy and clinical immunology. 2007;119:206–212. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Mishra A, Wang M, Pemmaraju VR, Collins MH, Fulkerson PC, Abonia JP, Blanchard C, Putnam PE, Rothenberg ME. Esophageal Remodeling Develops as a Consequence of Tissue Specific IL-5-Induced Eosinophilia. Gastroenterology. 2008;134:204–214. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES, Dohil R, Falk GW, Gonsalves N, Gupta SK, Katzka DA, Lucendo AJ, Markowitz JE, Noel RJ, Odze RD, Putnam PE, Richter JE, Romero Y, Ruchelli E, Sampson HA, Schoepfer A, Shaheen NJ, Sicherer SH, Spechler S, Spergel JM, Straumann A, Wershil BK, Rothenberg ME, Aceves SS. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. The Journal of allergy and clinical immunology. 2011;128:3–20. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 7.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. The Journal of clinical investigation. 2001;107:83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. IL-5 promotes eosinophil trafficking to the esophagus. Journal of immunology. 2002;168:2464–2469. doi: 10.4049/jimmunol.168.5.2464. [DOI] [PubMed] [Google Scholar]
- 9.Rajavelu P, Rayapudi M, Moffitt M, Mishra A, Mishra A. Significance of para-esophageal lymph nodes in food or aeroallergen-induced iNKT cell-mediated experimental eosinophilic esophagitis. American journal of physiology. Gastrointestinal and liver physiology. 2012;302:G645–654. doi: 10.1152/ajpgi.00223.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rayapudi MR,P, Zhu X, Kaul A, Nirnjan R, Dynda S, Mishra A, Mattner J, Zaidi A, Dutt P, Mishra A. Invariant natural killer T-cell neutralization is apossible novel therapy for human eosinophilicesophagitis. Clinical & Translational Immunology. 2014;3:1–11. doi: 10.1038/cti.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lexmond WS, Neves JF, Nurko S, Olszak T, Exley MA, Blumberg RS, Fiebiger E. Involvement of the iNKT cell pathway is associated with early-onset eosinophilic esophagitis and response to allergen avoidance therapy. The American journal of gastroenterology. 2014;109:646–657. doi: 10.1038/ajg.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jyonouchi S, Smith CL, Saretta F, Abraham V, Ruymann KR, Modayur-Chandramouleeswaran P, Wang ML, Spergel JM, Cianferoni A. Invariant natural killer T cells in children with eosinophilic esophagitis. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2014;44:58–68. doi: 10.1111/cea.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaidi AKM,A, Mishra A. Diagnostic and therapeutic strategies for eosinophilic esophagitis. Clin. Pract (Lond) 2014;11:351–367. doi: 10.2217/cpr.14.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reilly EC, Wands JR, Brossay L. Cytokine dependent and independent iNKT cell activation. Cytokine. 2010;51:227–231. doi: 10.1016/j.cyto.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.French AR, Holroyd EB, Yang L, Kim S, Yokoyama WM. IL-18 acts synergistically with IL-15 in stimulating natural killer cell proliferation. Cytokine. 2006;35:229–234. doi: 10.1016/j.cyto.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Boraschi D, Dinarello CA. IL-18 in autoimmunity: review. European cytokine network. 2006;17:224–252. [PubMed] [Google Scholar]
- 17.Helmby H, Takeda K, Akira S, Grencis RK. Interleukin (IL)-18 promotes the development of chronic gastrointestinal helminth infection by downregulating IL-13. The Journal of experimental medicine. 2001;194:355–364. doi: 10.1084/jem.194.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garrote JA, Gomez-Gonzalez E, Bernardo D, Arranz E, Chirdo F. Celiac disease pathogenesis: the proinflammatory cytokine network. Journal of pediatric gastroenterology and nutrition. 2008;47(Suppl 1):S27–32. doi: 10.1097/MPG.0b013e3181818fb9. [DOI] [PubMed] [Google Scholar]
- 19.Leon AJ, Garrote JA, Blanco-Quiros A, Calvo C, Fernandez-Salazar L, Del Villar A, Barrera A, Arranz E. Interleukin 18 maintains a long-standing inflammation in coeliac disease patients. Clinical and experimental immunology. 2006;146:479–485. doi: 10.1111/j.1365-2249.2006.03239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bala G, Swincow G, Odrowaz-Sypniewska G, Czerwionka-Szaflarska M. Interleukin 13 and 18 serum concentration in children with cow milk hypersensitivity. Med Wieku Rozwoj. 2004;8:33–42. [PubMed] [Google Scholar]
- 21.Campbell E, Kunkel SL, Strieter RM, Lukacs NW. Differential roles of IL-18 in allergic airway disease: induction of eotaxin by resident cell populations exacerbates eosinophil accumulation. Journal of immunology. 2000;164:1096–1102. doi: 10.4049/jimmunol.164.2.1096. [DOI] [PubMed] [Google Scholar]
- 22.Ishikura T, Kanai T, Uraushihara K, Iiyama R, Makita S, Totsuka T, Yamazaki M, Sawada T, Nakamura T, Miyata T, Kitahora T, Hibi T, Hoshino T, Watanabe M. Interleukin-18 overproduction exacerbates the development of colitis with markedly infiltrated macrophages in interleukin-18 transgenic mice. Journal of gastroenterology and hepatology. 2003;18:960–969. doi: 10.1046/j.1440-1746.2003.03097.x. [DOI] [PubMed] [Google Scholar]
- 23.Morelli AE, Zahorchak AF, Larregina AT, Colvin BL, Logar AJ, Takayama T, Falo LD, Thomson AW. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood. 2001;98:1512–1523. doi: 10.1182/blood.v98.5.1512. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, Akira S, Nakanishi K. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. Journal of immunology. 1998;161:3400–3407. [PubMed] [Google Scholar]
- 25.Tominaga K, Yoshimoto T, Torigoe K, Kurimoto M, Matsui K, Hada T, Okamura H, Nakanishi K. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. International immunology. 2000;12:151–160. doi: 10.1093/intimm/12.2.151. [DOI] [PubMed] [Google Scholar]
- 26.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 27.Schleimer RP, Sterbinsky SA, Kaiser J, Bickel CA, Klunk DA, Tomioka K, Newman W, Luscinskas FW, Gimbrone MA, Jr., McIntyre BW, et al. IL-4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium. Association with expression of VCAM-1. Journal of immunology. 1992;148:1086–1092. [PubMed] [Google Scholar]
- 28.Chihara J, Kurachi D, Hayashi N, Yamamoto T, Higashimoto I, Kakazu T, Nakajima S. Induction of expression of adhesion molecules on an eosinophilic cell line (EoL-1) by the supernatant of lymphocytes stimulated with specific allergen from asthmatic patients. International archives of allergy and immunology. 1994;104(Suppl 1):54–56. doi: 10.1159/000236753. [DOI] [PubMed] [Google Scholar]
- 29.Dahms BB. Reflux esophagitis: sequelae and differential diagnosis in infants and children including eosinophilic esophagitis. Pediatr Dev Pathol. 2004;7:5–16. doi: 10.1007/s10024-003-0203-5. [DOI] [PubMed] [Google Scholar]
- 30.Kirsch R, Bokhary R, Marcon MA, Cutz E. Activated mucosal mast cells differentiate eosinophilic (allergic) esophagitis from gastroesophageal reflux disease. Journal of pediatric gastroenterology and nutrition. 2007;44:20–26. doi: 10.1097/MPG.0b013e31802c0d06. [DOI] [PubMed] [Google Scholar]
- 31.Mishra A. Mechanism of eosinophilic esophagitis. Immunology and allergy clinics of North America. 2009;29:29–40. doi: 10.1016/j.iac.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanchard C, Wang N, Rothenberg ME. Eosinophilic esophagitis: pathogenesis, genetics, and therapy. The Journal of allergy and clinical immunology. 2006;118:1054–1059. doi: 10.1016/j.jaci.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 33.Pentiuk S, Putnam PE, Collins MH, Rothenberg ME. Dissociation between symptoms and histological severity in pediatric eosinophilic esophagitis. Journal of pediatric gastroenterology and nutrition. 2009;48:152–160. doi: 10.1097/MPG.0b013e31817f0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacon AS, McGill JI, Anderson DF, Baddeley S, Lightman SL, Holgate ST. Adhesion molecules and relationship to leukocyte levels in allergic eye disease. Investigative ophthalmology & visual science. 1998;39:322–330. [PubMed] [Google Scholar]
- 35.Nakajima S, Look DC, Roswit WT, Bragdon MJ, Holtzman MJ. Selective differences in vascular endothelial- vs. airway epithelial-T cell adhesion mechanisms. The American journal of physiology. 1994;267:L422–432. doi: 10.1152/ajplung.1994.267.4.L422. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. The Journal of experimental medicine. 1994;179:1145–1154. doi: 10.1084/jem.179.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chai OH, Han EH, Lee HK, Song CH. Mast cells play a key role in Th2 cytokine-dependent asthma model through production of adhesion molecules by liberation of TNF-alpha. Experimental & molecular medicine. 2011;43:35–43. doi: 10.3858/emm.2011.43.1.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng H, Tonnesen MG, Marchese MJ, Clark RA, Bahou WF, Gruber BL. Mast cells are potent regulators of endothelial cell adhesion molecule ICAM-1 and VCAM-1 expression. Journal of cellular physiology. 1995;165:40–53. doi: 10.1002/jcp.1041650106. [DOI] [PubMed] [Google Scholar]
- 39.Gadue P, Stein PL. NK T cell precursors exhibit differential cytokine regulation and require Itk for efficient maturation. Journal of immunology. 2002;169:2397–2406. doi: 10.4049/jimmunol.169.5.2397. [DOI] [PubMed] [Google Scholar]
- 40.Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(−)CD4(+) CD1d-dependent precursor stage. The Journal of experimental medicine. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, Foster PS, Rothenberg ME. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. The Journal of allergy and clinical immunology. 2001;108:594–601. doi: 10.1067/mai.2001.118600. [DOI] [PubMed] [Google Scholar]
- 42.Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003;125:1419–1427. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Zhu X, Wang M, Mavi P, Rayapudi M, Pandey AK, Kaul A, Putnam PE, Rothenberg ME, Mishra A. Interleukin-15 expression is increased in human eosinophilic esophagitis and mediates pathogenesis in mice. Gastroenterology. 139:182–193. e187. doi: 10.1053/j.gastro.2010.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]