Abstract
Background:
Metastatic colorectal cancer (mCRC) that harbours a BRAF V600E mutation (BRAF MT) is associated with poorer outcomes. However, whether this mutation is predictive of treatment benefit from anti-epidermal growth factor receptor (EGFR) monoclonal antibodies (mAbs) is uncertain.
Methods:
We conducted a systematic review and meta-analysis of randomised controlled trials (RCTs) published up to July 2014 that evaluated the effect of BRAF MT on the treatment benefit from anti-EGFR mAbs for mCRC.
Results:
Seven RCTs met the inclusion criteria for assessment of overall survival (OS), whereas eight RCTs met the inclusion criteria for assessment of progression-free survival (PFS). For RAS WT/BRAF MT tumours, the hazard ratio for OS benefit with anti-EGFR mAbs was 0.97 (95% CI; 0.67–1.41), whereas the hazard ratio was 0.81 (95% CI; 0.70–0.95) for RAS WT/BRAF WT tumours. However, the test of interaction (P=0.43) was not statistically significant, highlighting that the observed differences in the effect of anti-EGFR mAbs on OS according to the BRAF mutation status may be due to chance alone. Regarding PFS benefit with anti-EGFR mAbs, the hazard ratio was 0.86 (95% CI; 0.61–1.21) for RAS WT/BRAF MT tumours as compared with 0.62 (95% CI; 0.50–0.77) for RAS WT/BRAF WT tumours (test of interaction, P=0.07).
Interpretation:
This meta-analysis demonstrates that there is insufficient evidence to definitively state that RAS WT/BRAF MT individuals attain a different treatment benefit from anti-EGFR mAbs for mCRC compared with RAS WT/BRAF WT individuals. As such, there are insufficient data to justify the exclusion of anti-EGFR mAb therapy for patients with RAS WT/BRAF MT mCRC.
Keywords: BRAF V600E mutation, metastatic colorectal cancer, anti-EGFR monoclonal antibodies, predictive biomarkers
Elucidation of the genetic underpinnings of metastatic colorectal cancer (mCRC) has identified an important role for the epidermal growth factor receptor (EGFR) and the downstream mitogen-activated protein kinase (MAPK) pathways in disease progression leading to the development of multiple targeted therapies for this malignancy. In this regard, the anti-EGFR monoclonal antibodies (mAbs), cetuximab and panitumumab, are important therapeutics in the treatment of mCRC that block MAPK pathway activation by targeting the extracellular domain of EGFR. It is well established that mutations in exons 2, 3, and 4 of the KRAS and NRAS oncogenes (collectively present in ∼50% of mCRC tumours) are predictive of resistance to anti-EGFR mAb therapy (Sorich et al, 2015). On this basis, the use of cetuximab and panitumumab is limited to individuals with RAS wild-type (WT) tumours in many treatment guidelines (NCCN, 2014). However, not all RAS WT tumours respond to anti-EGFR mAbs, and as the cost of antineoplastic mAb therapy is high and treatment-related toxicity can be considerable, there remains significant scope to identify additional predictive markers of treatment benefit.
Like RAS, the serine/threonine-protein kinase BRAF is a downstream signalling protein in the EGFR-mediated MAPK pathway. The BRAF mutant colon cancers appear to be a distinct subset with recognisable clinicopathological characteristics. They often arise from serrated adenomas, occur in the right side of the colon more commonly in women, are high grade in nature, and are strongly associated with defective mismatch repair (Lochhead et al, 2013; Gonsalves et al, 2014). As with RAS mutations, mutation of codon 600 in the activation segment of the BRAF gene (BRAF MT) causes constitutive activation of the MAPK pathway, and is implicated as a source of impaired response to anti-EGFR mAbs in patients with mCRC (Benvenuti et al, 2007; Cappuzzo et al, 2008; Di Nicolantonio et al, 2008; Freeman et al, 2008; Laurent-Puig et al, 2009; Loupakis et al, 2009; Molinari et al, 2009; Perrone et al, 2009; Sartore-Bianchi et al, 2009; Tol et al, 2009). Notably, a meta-analysis of data from observational studies has provided evidence that BRAF MT is associated with a poor prognosis (i.e., negative prognostic biomarker) in mCRC (Yuan et al, 2013). Based on preclinical studies (Prahallad et al, 2012) that demonstrated synergistic activities between EGFR mAb and BRAF inhibitors/MEK inhibitors, clinical trials are ongoing that evaluate alternate approaches such as the addition of the triple chemotherapy regimen (oxaliplatin+irinotecan+5-Fluorouracil), BRAF inhibitors, and MEK inhibitors to anti-EGFR mAb therapy regimens (www.clinicaltrials.gov—NCT01902173, NCT02164916). However, whether BRAF MT also causes resistance to anti-EGFR mAb therapy (i.e., is a predictive biomarker) is currently uncertain. This study undertook a systematic review and meta-analysis of randomised controlled trial (RCT) data to quantitatively evaluate the evidence for BRAF MT as a negative predictive biomarker for efficacy of anti-EGFR mAb therapy in mCRC.
Materials and Methods
Study eligibility criteria
Studies were eligible if they were RCTs in which treatment with an anti-EGFR antibody, either alone or combined with standard therapy, had been compared with the same standard therapy for patients with mCRC. In addition, tumours must have been assessed for BRAF mutation status (BRAF WT or BRAF MT) as a subset of the RAS (minimally KRAS exon 2 and 3) WT subgroup, and studies had to have follow-up data on overall survival (OS) or progression-free survival (PFS) outcomes. Studies were excluded if they did not provide sufficient quantitative data of the anti-EGFR treatment effect according to BRAF and RAS mutation status.
Search strategy and identification of studies
Embase, Medline, and Web of Science were searched until 25 July 2014 for the following terms: (colon cancer or colorectal cancer or colon carcinoma or metastatic colorectal cancer or mCRC) and (BRAF or B-RAF or B RAF) and (anti-EGFR or EGF or epidermal growth factor receptor or monoclonal antibody/ies or MoAb or mAb or cetuximab or panitumumab). Relevant MeSH (Medline) or Emtree (Embase) terms were used where possible. Differences in truncation symbols and wildcards between databases were considered. No restrictions were placed on the searches. Duplicate citations were removed. The titles and abstracts of all remaining citations were reviewed and irrelevant citations were discarded. Potentially relevant studies were retrieved in full text and assessed to determine whether they matched the study eligibility criteria. Hand searches of the reference lists of the relevant reports were carried out to identify any relevant studies that were missed with the search strategy. If multiple reports referred to the same data, the report containing the (largest and) most recent data was included in the review, and these data were cross-checked against the other reports. Review of papers for inclusion was undertaken independently by two investigators (MMD and MDW) with any discrepancies resolved by the other investigators (MJS and AR).
Assessment of study risk of bias
An assessment of the methodological quality of the studies included in meta-analyses was based on guidance for the evaluation of the conduct of biomarker studies that use archived tumour specimens (Simon et al, 2009; Patterson et al, 2011). For each included pharmacogenomic substudy of a RCT, four domains were used to assess the risk of bias (high, moderate, or low): (1) biomarker sample ascertainment, (2) assay analytical performance, (3) prespecified analysis plan, and (4) parent RCT. Studies were assigned a low risk of bias for each respective domain if the biomarker status was ascertained in a sufficiently large proportion of original study participants and/or the sample population used for the biomarker analysis was demonstrated to be sufficiently representative of the originally enrolled study population, the assay had been analytically validated for use with archived tissue and the assay was performed blinded to the clinical data, an analysis plan for the biomarker study was prepared before biomarker testing or analysis of the biomarker results, and the parent RCT was of low risk of bias.
Statistical analysis
The hazard ratio was used to represent the comparative treatment effect on survival outcomes for anti-EGFR mAb therapy compared with no anti-EGFR mAb therapy. Included studies generally reported hazard ratios derived from Cox proportional-hazards models stratified according to randomisation factors (e.g., Eastern Cooperative Oncology Group (ECOG) performance status). If the hazard ratio for a BRAF subgroup was not reported, the value was estimated where possible by combining smaller subgroups with a fixed-effect meta-analysis. Summary estimates of anti-EGFR mAb treatment effect hazard ratios for BRAF MT and BRAF WT tumours were pooled separately using a random-effects model based on the inverse variance method. Evidence for treatment effect modification (i.e., a predictive biomarker) by BRAF mutation status was evaluated by a test of interaction. Specifically, a random-effects meta-analysis of the interaction hazard ratio (hazard ratio for BRAF MT tumours divided by the hazard ratio for BRAF WT tumours) was calculated for each study. Exploratory analyses were undertaken to evaluate whether the line of therapy (i.e., first or subsequent line) for use of anti-EGFR mAb was associated with a differential impact of BRAF mutations on anti-EGFR mAb efficacy. The primary analyses were repeated by stratifying the trials according to first-line vs non-first-line anti-EGFR mAb use.
Heterogeneity between studies was assessed using the Cochrane's Q statistic and I2 statistic. Small-study effects (and risk of publication bias) were assessed by visual inspection of funnel plots and Egger's linear regression test. All reported P-values are two sided. Analyses were carried out with R 3.0.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Overview of included studies and assessment of study quality
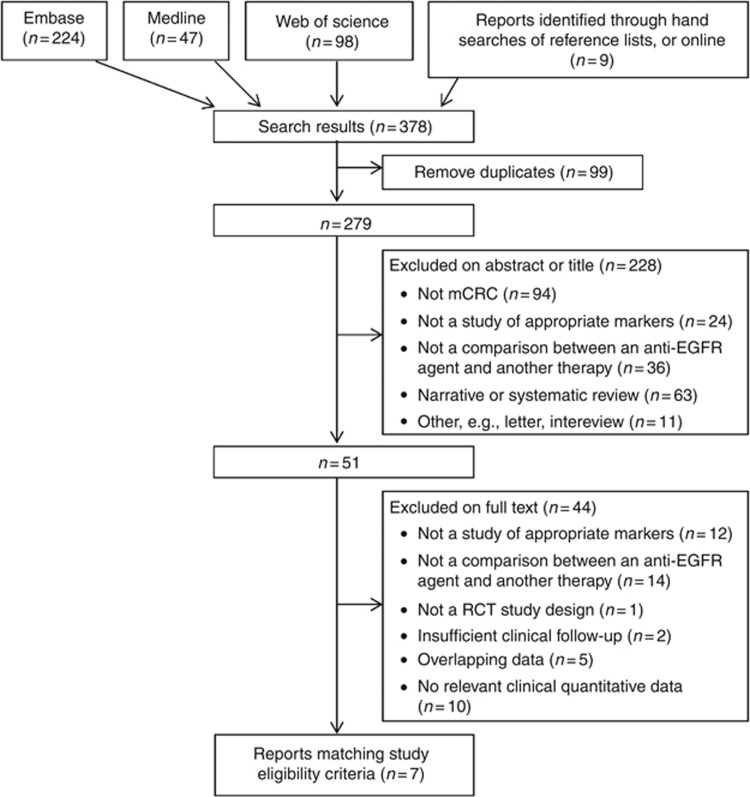
Biomarker analyses of eight RCTs published in seven study reports were included in the systematic review (Figure 1 and Table 1) (Bokemeyer et al, 2012; Douillard et al, 2013; Peeters et al, 2013; Seymour et al, 2013; Smith et al, 2013; Karapetis et al, 2014; Peeters et al, 2014). Of the 3168 participants with RAS WT tumours across the 8 RCT substudies, 2817 harboured BRAF WT and 351 (11.1%) BRAF MT tumours. All studies compared the addition of an anti-EGFR agent with background therapy, four evaluated cetuximab and four assessed panitumumab. Five studies restricted the analysis to KRAS WT tumours and two restricted analyses to KRAS WT and NRAS WT tumours. For the COIN study, analysis of PFS according to RAS status was defined based on both KRAS and NRAS mutations, whereas RAS status for OS was based on KRAS mutations only. Table 1 summarises details of background therapy, lines of treatment, RAS WT, and BRAF mutation status. Hazard ratios were typically adjusted for performance status, and the substudies of the CO.17 and PICCOLO trials adjusted the hazard ratio for a broader range of baseline characteristics. The risk of bias was generally similar between studies included in meta-analyses with respect to each of the four domains (Supplementary Table 1).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews) diagram for the systematic review.
Table 1. Summary of studies included in meta-analyses.
|
Proportion of study participants |
||||||||
|---|---|---|---|---|---|---|---|---|
| Anti-EGFR agent vs comparator | Trial (report) | Background therapy, lines of treatment | ITT, N | RAS status, N (%) of ITTa | RASWT, N (%) of ITTb | BRAF subgroup, N (%) of RAS WTc | BRAFMT, N (%) of RASWTd | Unadjusted or adjusted HR valuese |
| Cetuximab vs no cetuximab | CO.17 (Karapetis et al, 2014) | BSC, ⩾second line | 572 | NR | 208 (36) | 208 (100) | 10 (5) | Adjusted |
| CRYSTAL and OPUS (Bokemeyer et al, 2012) | FOLFIRI (CRYSTAL) or FOLFOX-4 (OPUS), first line | 1535 | 1378 (90) | 845 (55) | 800 (95) | 70 (8) | Adjusted | |
| COIN (Smith et al, 2013) | Oxaliplatin and fluoropyrimidine chemotherapy, first line | 2445 | 1949 (80) | 729 (30) | 671 (92) | 90 (12) | NR | |
| Panitumumab vs no panitumumab | 20020408 (Peeters et al, 2013) | BSC, ⩾third line | 463 | 288 (62) | 153 (33) | 130 (85) | 15 (10) | Adjusted |
| 20050181 (Peeters et al, 2014) | FOLFIRI, second line | 1186 | 1014 (85) | 421 (35) | 421 (100) | 45 (11) | NR | |
| PICCOLO (Seymour et al, 2013) | Irinotecan, ⩾second line | 696 | NR | 460 (66) | 439 (95) | 68 (15) | Adjusted | |
| PRIME (Douillard et al, 2013) | FOLFOX-4, first line | 1183 | 1060 (90) | 512 (43) | 499 (97) | 53 (10) | Adjusted | |
Abbreviations: BRAF MT=BRAF mutant; BSC=best supportive care; EGFR=epidermal growth factor receptor; FOLFIRI=folinic acid, fluorouracil, irinotecan; FOLFOX-4=folinic acid, fluorouracil, oxaliplatin; HR=hazard ratio; ITT=overall intention-to-treat population; NR=not reported in the publication; RAS WT=RAS wild type; ≥second: second or higher line treatment, ≥third: third or higher line treatment.
The proportion of the original clinical trial participants that were evaluable for KRAS (CO.17, CRYSTAL, OPUS, COIN, 20020408, and PICCOLO) or RAS (20050181 and PRIME) mutation analysis.
The proportion of the original clinical trial participants for which KRAS (CO.17, CRYSTAL, OPUS, COIN, 20020408, and PICCOLO) or RAS (20050181 and PRIME) WT status was ascertained.
The proportion of the KRAS (CO.17, CRYSTAL, OPUS, COIN, 20020408, and PICCOLO) or RAS (20050181 and PRIME) WT group for which a BRAF – WT or MT – mutation status was ascertained.
The proportion of the KRAS (CO.17, CRYSTAL, OPUS, COIN, 20020408, and PICCOLO) or RAS (20050181 and PRIME) WT group for which a BRAF MT status was ascertained.
Whether the predictive analysis HR values presented in the publication for overall survival (OS) and progression-free survival (PFS), in the RAS WT subgroup according to BRAF mutation status (WT or MT), were adjusted or unadjusted (variables adjusted for: CO.17 trial: Eastern Cooperative Oncology Group (ECOG) performance status, gender, age, baseline lactate dehydrogenase level, baseline alkaline phosphatase, baseline haemoglobin, number of disease sites, number of previous chemotherapy drug classes, primary tumour site, and presence of liver metastases; CRYSTAL and OPUS trials: ECOG performance status; 20020408 trial: ECOG performance status and geographic region; PICCOLO trial: centre, World Health Organisation (WHO) performance status, previous oxaliplatin, previous bevacizumab, previous dose modifications, and best previous response to therapy; PRIME trial: ECOG performance status and geographic region).
Effect of BRAF mutation on OS benefit with anti-EGFR mAbs
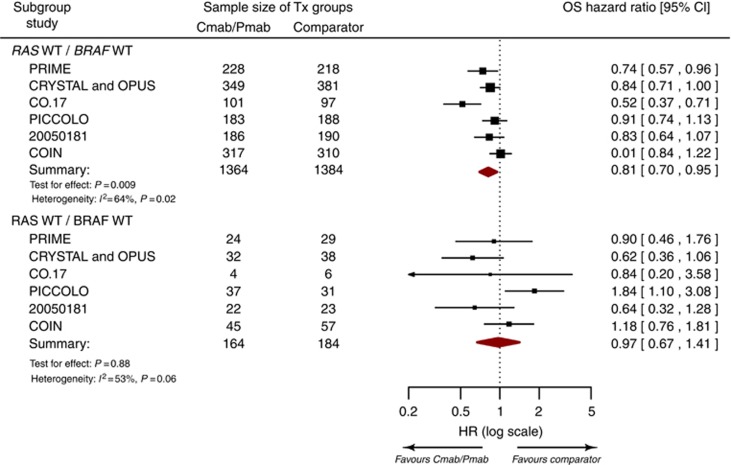
Based on the pharmacogenomic substudies of 7 RCTs, the hazard ratio for OS benefit with anti-EGFR mAb therapy was 0.97 (95% CI; 0.67–1.41) for RAS WT/BRAF MT tumours as compared with 0.81 (95% CI; 0.70–0.95) for RAS WT/BRAF WT tumours (Figure 2). However, the difference between RAS WT/BRAF MT and RAS WT/BRAF WT tumours with respect to the OS benefit of anti-EGFR mAb therapy was not statistically significant (test of interaction; P=0.43). Visual inspection and regression tests (P=0.97) did not indicate any significant relationship between study size and the test of interaction (i.e., publication bias).
Figure 2.
Forest plot of the overall survival benefit with anti-EGFR mAb therapy for subgroups defined by tumour RAS and BRAF mutations. Cmab=cetuximab; MT=mutant; Pmab=panitumumab; WT=wild type.
Effect of BRAF mutation on PFS benefit with anti-EGFR mAbs
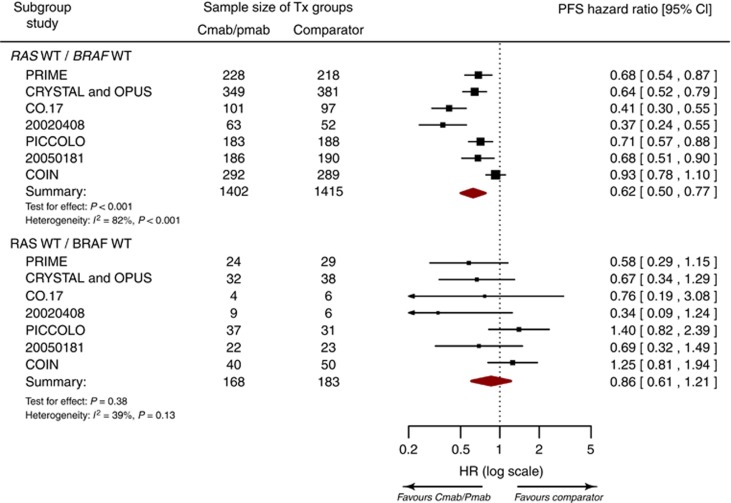
Based on the pharmacogenomic substudies of 8 RCTs, the hazard ratio for PFS benefit with anti-EGFR mAb therapy was 0.86 (95% CI; 0.61–1.21) for RAS WT/BRAF MT tumours as compared with 0.62 (95% CI; 0.50–0.77) for RAS WT/BRAF WT tumours (Figure 3). The difference between RAS WT/BRAF MT and RAS WT/BRAF WT tumours with respect to the PFS benefit of anti-EGFR mAb therapy was not statistically significant (test of interaction; P=0.07). Visual inspection and regression tests (P=0.63) did not indicate any significant relationship between study size and the test of interaction (i.e., publication bias).
Figure 3.
Forest plot of the progression-free survival benefit with anti-EGFR mAb therapy for subgroups defined by tumour RAS and BRAF mutations. Cmab=cetuximab; MT=mutant; Pmab=panitumumab; WT=wild type.
Impact of line of therapy
In an exploratory analysis restricted to first-line anti-EGFR mAb therapy, little difference was observed between RAS WT/BRAF WT and RAS WT/BRAF MT tumours with respect to either OS efficacy (hazard ratio 0.87 vs 0.89, P=0.96, Supplementary Figure 1) or PFS efficacy (hazard ratio 0.75 vs 0.83, P=0.45, Supplementary Figure 2). For non-first-line anti-EGFR mAb therapy there were nonstatistically significant trends towards a difference in efficacy between RAS WT/BRAF WT and RAS WT/BRAF MT tumours with respect to OS (hazard ratio 0.74 vs 1.06, P=0.38, Supplementary Figure 3) and PFS (hazard ratio 0.53 vs 0.84, P=0.05, Supplementary Figure 4).
Discussion
This meta-analysis of pharmacogenomic substudies from eight RCTs highlights that based on the standard approach for assessing predictive markers there is insufficient evidence to conclusively demonstrate that the presence of BRAF mutation is a negative predictive biomarker of benefit from the use of anti-EGFR mAbs in RAS WT mCRC.
We believe that this is the first meta-analysis to systematically and quantitatively summarise the evidence from RCTs in line with current methodological guidelines (Rothwell, 2005; Kent et al, 2010; Sun et al, 2010) with respect to the predictive value of BRAF MT for survival benefit of anti-EGFR mAb therapy in RAS WT tumours. Previous meta-analyses of observational studies have demonstrated that BRAF MT is a negative prognostic biomarker (Safaee Ardekani et al, 2012; Xu et al, 2013; Yang et al, 2013; Yuan et al, 2013; Therkildsen et al, 2014), but this is distinct from assessing whether BRAF MT modifies the treatment effect of anti-EGFR mAbs (i.e., predictive biomarker). A prior study based on pooled data from the CRYSTAL and OPUS trials (n=800) evaluated the impact of BRAF MT on the survival benefit associated with cetuximab use for RAS WT mCRC tumours (Bokemeyer et al, 2012), and concluded that there were no significant differences in outcome between the treatment groups. The inclusion of data from six additional RCTs in the current review provides a greater representation of the heterogeneity in the uncertainty regarding the impact of BRAF MT on treatment effect. The current meta-analysis includes studies where participants were administered panitumumab and cetuximab, different lines of therapy, and a range of background chemotherapy.
Recently, a meta-analysis reported the efficacy of anti-EGFR mAb treatment of mCRC with a BRAF mutation, and concluded that anti-EGFR mAb therapy did not provide benefit in this subgroup (Pietrantonio et al, 2015). The current study differs from this recent meta-analysis in terms of the statistical methods of analysis and inclusion criteria. Instead of simply estimating anti-EGFR mAb efficacy in the BRAF MT subgroup, the current study focussed on assessing whether anti-EGFR mAb efficacy differs based on BRAF mutation status (i.e., consideration of whether subgroup differences may be caused by chance alone). Guidelines for undertaking subgroup analysis of RCTs (i.e., identifying a predictive marker) clearly indicate that it is the treatment effect interaction between subgroups, rather than the treatment effect of within an individual subgroup, that should primarily be interpreted when deriving a conclusion as to whether the factor/marker influences the treatment effect (Rothwell, 2005; Kent et al, 2010; Sun et al, 2010). As Pietrantonio et al, (2015) only evaluated a single subgroup (BRAF MT), the conclusions of this study regarding the predictive value of BRAF mutation status are not valid based on guideline recommendatons. An additional contrast to the recent meta-analysis is that the current meta-analysis excluded trials comparing anti-EGFR mAb therapy with bevacizumab on the basis that they are not sufficiently comparable to the other included trials. For example, a hazard ratio of 1 in a trial of standard therapy±anti-EGFR mAb indicates a lack of efficacy, but the same hazard ratio in a trial of standard therapy+anti-EGFR mAb or bevacizumab indicates a significant benefit as bevacizumab use is associated with a significant benefit.
The contrasting conclusions of these meta-analyses highlight important ongoing challenges with respect to undertaking subgroup analysis to identify predictive markers of treatment effect. The approach specified in current guidelines (as undertaken in the present analysis) aim to control the risk of falsely concluding a difference in treatment effect between subgroups (i.e., to minimise risk of false positive results) by undertaking a test of interaction and controlling for multiplicity of tests. In this paradigm, a marker will only be considered predictive when there is relatively strong evidence that the differences in treatment effect observed for the subgroups are unlikely to be due to chance alone. Hence, such an approach is often poorly powered to detect real subgroup differences and the risk of false negative results is high. The current analysis shows that even with pooled data from substudies of seven to eight RCTs, analyses may still be insufficiently powered to detect predictive biomarkers. In particular, it is likely to be difficult to conclusively demonstrate the validity of predictive markers with low prevalence and markers that predict partially attenuated response to therapy (compared with markers that predict either no or reversal of effect).
Although finding predictive biomarkers that identify individuals who have no (or deleterious) response to therapy is particularly important, for high cost and/or toxic drugs, such as anti-EGFR mAbs, identification of individuals with reduced efficacy may also be of value. For example, if it was confirmed that individuals with RAS WT/BRAF MT tumours receive some, but significantly reduced, benefit from anti-EGFR mAbs compared with RAS WT/BRAF WT tumours, clinician and patient decisions regarding the use of anti-EGFR mAbs in mCRC may be influenced. It could also substantially impact the cost effectiveness of the anti-EGFR mAb therapy for RAS WT/BRAF MT tumours, and hence the subsidy decisions in some jurisdictions.
The current study highlights that there is currently insufficient evidence to mandate the clinical application of BRAF MT status in RAS WT mCRC to determine eligibility for access to anti-EGFR mAb therapy. In the absence of additional data providing a conclusive outcome, consideration of BRAF mutation status when evaluating the role of anti-EGFR mAbs as a therapeutic option in patients with mCRC should remain at the discretion of the treating physician and patient, and be considered in the context of each patient's circumstance (e.g., access to alternate therapeutic options, predisposition to toxicity, frailty), recognising that there remains a reasonable possibility that chance alone may explain the differences in anti-EGFR mAb efficacy observed between BRAF mutation subgroups.
Because of the small sample size of the RAS WT/BRAF MT tumours in all trials (n<75 for each), there is the risk of a chance imbalance in important prognostic factors despite randomisation of participants to the treatment arms. For this reason, it may be of value to adjust for potential differences in important baseline characteristics between treatment groups. Most of the substudies included adjustment for only performance status. In contrast, the substudy of the CO.17 trial adjusted for a range of baseline characteristics including baseline lactate dehydrogenase level, number of disease sites, number of previous chemotherapy drug classes, primary tumour site, and presence of liver metastases. Whether the hazard ratios reported for the other studies would differ significantly if adjusted for a wider range of prognostic factors is unknown.
The current study focussed specifically on survival outcomes. However, it may be worthwhile to evaluate Response Evaluation Criteria in Solid Tumours (RECIST response) as an additional outcome. Although response rate is not as clinically meaningful as are survival outcomes, such an analysis may be better powered to detect the impact of BRAF MT on anti-EGFR mAb efficacy (i.e., the interaction between BRAF mutation status and the odds ratio of response due to anti-EGFR mAb therapy for RAS WT tumours). It will be useful to assess in future studies whether attenuation in efficacy relates primarily to a reduced likelihood of achieving and/or a more modest depth/duration of response. It was not possible to evaluate response rate in the current study as response data were only reported according to BRAF and RAS mutation status in the publications of the CRYSTAL and OPUS trials. An important direction for future research will be to undertake a patient-level meta-analysis of these studies that includes a more consistent and extensive assessment of the value of adjusting for potential baseline imbalances between treatments in the RAS WT/BRAF MT subgroup.
In conclusion, based on the data from the pharmacogenomic substudies of eight RCTs, there is currently insufficient evidence to definitively consider BRAF MT a negative predictive biomarker of survival benefit from anti-EGFR mAb therapy for mCRC. The benefit in OS and PFS for BRAF MT tumours treated with anti-EGFR mAb therapy may be smaller or less likely, but further data are required to clarify this observation. This systematic review highlights that current evidence does not support mandatory clinical application of BRAF MT status of RAS WT mCRC to determine eligibility for access to anti-EGFR mAb therapy.
CSK is an advisory board member for Amgen and Merck Serono. GK is an honorary advisory board member for Bayer. The other authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, Veronese S, Siena S, Bardelli A. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti–epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–2648. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Cutsem EV, Rougier P, Ciardiello F, Heeger S, Schlichting M, Celik I, Köhne C-H. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48:1466–1475. doi: 10.1016/j.ejca.2012.02.057. [DOI] [PubMed] [Google Scholar]
- Cappuzzo F, Varella-Garcia M, Finocchiaro G, Skokan M, Gajapathy S, Carnaghi C, Rimassa L, Rossi E, Ligorio C, Di Tommaso L, Holmes AJ, Toschi L, Tallini G, Destro A, Roncalli M, Santoro A, Janne PA. Primary resistance to cetuximab therapy in EGFR FISH-positive colorectal cancer patients. Br J Cancer. 2008;99:83–89. doi: 10.1038/sj.bjc.6604439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S, Bardelli A. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- Douillard J-Y, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD. Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- Freeman DJ, Juan T, Reiner M, Hecht JR, Meropol NJ, Berlin J, Mitchell E, Sarosi I, Radinsky R, Amado RG. Association of K-ras mutational status and clinical outcomes in patients with metastatic colorectal cancer receiving panitumumab alone. Clin Colorectal Cancer. 2008;7:184–190. doi: 10.3816/CCC.2008.n.024. [DOI] [PubMed] [Google Scholar]
- Gonsalves WI, Mahoney MR, Sargent DJ, Nelson GD, Alberts SR, Sinicrope FA, Goldberg RM, Limburg PJ, Thibodeau SN, Grothey A, Hubbard JM, Chan E, Nair S, Berenberg JL, McWilliams RR, Alliance for Clinical Trials in Oncology 2014Patient and tumor characteristics and BRAF and KRAS mutations in colon cancer, NCCTG/Alliance N0147 J Natl Cancer Inst 106doi: 10.1093/jnci/dju106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karapetis CS, Jonker D, Daneshmand M, Hanson JE, O'Callaghan CJ, Marginean C, Zalcberg JR, Simes J, Moore MJ, Tebbutt NC, Price TJ, Shapiro JD, Pavlakis N, Gibbs P, Van Hazel GA, Lee U, Haq R, Virk S, Tu D, Lorimer IA, NCIC Clinical Trials Group and the Australasian Gastro-Intestinal Trials Group PIK3CA, BRAF, and PTEN status and benefit from cetuximab in the treatment of advanced colorectal cancer—results from NCIC CTG/AGITG CO.17. Clin Cancer Res. 2014;20:744–753. doi: 10.1158/1078-0432.CCR-13-0606. [DOI] [PubMed] [Google Scholar]
- Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials. 2010;11:85. doi: 10.1186/1745-6215-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent-Puig P, Cayre A, Manceau G, Buc E, Bachet J-B, Lecomte T, Rougier P, Lievre A, Landi B, Boige V, Ducreux M, Ychou M, Bibeau F, Bouché O, Reid J, Stone S, Penault-Llorca F. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924–5930. doi: 10.1200/JCO.2008.21.6796. [DOI] [PubMed] [Google Scholar]
- Lochhead P, Kuchiba A, Imamura Y, Liao X, Yamauchi M, Nishihara R, Qian ZR, Morikawa T, Shen J, Meyerhardt JA, Fuchs CS, Ogino S. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst. 2013;105:1151–1156. doi: 10.1093/jnci/djt173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loupakis F, Ruzzo A, Cremolini C, Vincenzi B, Salvatore L, Santini D, Masi G, Stasi I, Canestrari E, Rulli E, Floriani I, Bencardino K, Galluccio N, Catalano V, Tonini G, Magnani M, Fontanini G, Basolo F, Falcone A, Graziano F. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer. 2009;101:715–721. doi: 10.1038/sj.bjc.6605177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari F, Martin V, Saletti P, De Dosso S, Spitale A, Camponovo A, Bordoni A, Crippa S, Mazzucchelli L, Frattini M. Differing deregulation of EGFR and downstream proteins in primary colorectal cancer and related metastatic sites may be clinically relevant. Br J Cancer. 2009;100:1087–1094. doi: 10.1038/sj.bjc.6604848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCCN . NCCN Clinical Practice Guidelines in Oncology. Colon Cancer Version 2.2015. National Comprehensive Cancer Network; 2014. [Google Scholar]
- Patterson SD, Cohen N, Karnoub M, Truter SL, Emison E, Khambata-Ford S, Spear B, Ibia E, Sproule R, Barnes D, Bhathena A, Bristow MR, Russell C, Wang D, Warner A, Westelinck A, Brian W, Snapir A, Franc MA, Wong P, Shaw PM. Prospective–retrospective biomarker analysis for regulatory consideration: white paper from the industry pharmacogenomics working group. Pharmacogenomics. 2011;12:939–951. doi: 10.2217/pgs.11.52. [DOI] [PubMed] [Google Scholar]
- Peeters M, Oliner KS, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, Andre T, Chan E, Lordick F, Punt CJA, Strickland A, Wilson G, Ciuleanu TE, Roman L, Van Cutsem E, Yu H, Jung AS, Sidhu R, SD Patterson.2014Updated analysis of KRAS/NRAS and BRAF mutations in study 20050181 of panitumumab (pmab) plus FOLFIRI for second-line treatment (tx) of metastatic colorectal cancer (mCRC) J Clin Oncol 32356825273035 [Google Scholar]
- Peeters M, Oliner KS, Parker A, Siena S, Van Cutsem E, Huang J, Humblet Y, Van Laethem J-L, André T, Wiezorek J, Reese D, Patterson SD. Massively parallel tumor multigene sequencing to evaluate response to panitumumab in a randomized phase III study of metastatic colorectal cancer. Clin Cancer Res. 2013;19:1902–1912. doi: 10.1158/1078-0432.CCR-12-1913. [DOI] [PubMed] [Google Scholar]
- Perrone F, Lampis A, Orsenigo M, Di Bartolomeo M, Gevorgyan A, Losa M, Frattini M, Riva C, Andreola S, Bajetta E, Bertario L, Leo E, Pierotti MA, Pilotti S. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann Oncol. 2009;20:84–90. doi: 10.1093/annonc/mdn541. [DOI] [PubMed] [Google Scholar]
- Pietrantonio F, Petrelli F, Coinu A, Di Bartolomeo M, Borgonovo K, Maggi C, Cabiddu M, Iacovelli R, Bossi I, Lonati V, Ghilardi M, de Braud F, Barni S. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51:587–594. doi: 10.1016/j.ejca.2015.01.054. [DOI] [PubMed] [Google Scholar]
- Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, Beijersbergen RL, Bardelli A, Bernards R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–186. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- Safaee Ardekani G, Jafarnejad SM, Tan L, Saeedi A, Li G. The prognostic value of BRAF mutation in colorectal cancer and melanoma: a systematic review and meta-analysis. PLoS One. 2012;7:e47054. doi: 10.1371/journal.pone.0047054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartore-Bianchi A, Di Nicolantonio F, Nichelatti M, Molinari F, De Dosso S, Saletti P, Martini M, Cipani T, Marrapese G, Mazzucchelli L, Lamba S, Veronese S, Frattini M, Bardelli A, Siena S. Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer. PLoS One. 2009;4:e7287. doi: 10.1371/journal.pone.0007287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour MT, Brown SR, Middleton G, Maughan T, Richman S, Gwyther S, Lowe C, Seligmann JF, Wadsley J, Maisey N, Chau I, Hill M, Dawson L, Falk S, O'Callaghan A, Benstead K, Chambers P, Oliver A, Marshall H, Napp V, Quirke P. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol. 2013;14:749–759. doi: 10.1016/S1470-2045(13)70163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446–1452. doi: 10.1093/jnci/djp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CG, Fisher D, Claes B, Maughan TS, Idziaszczyk S, Peuteman G, Harris R, James MD, Meade A, Jasani B, Adams RA, Kenny S, Kaplan R, Lambrechts D, Cheadle JP. Somatic profiling of the epidermal growth factor receptor pathway in tumors from patients with advanced colorectal cancer treated with chemotherapy +/- cetuximab. Clin Cancer Res. 2013;19:4104–4113. doi: 10.1158/1078-0432.CCR-12-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorich MJ, Wiese MD, Rowland A, Kichenadasse G, McKinnon RA, Karapetis CS. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized controlled trials. Ann Oncol. 2015;26:13–21. doi: 10.1093/annonc/mdu378. [DOI] [PubMed] [Google Scholar]
- Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ. 2010;340:c117. doi: 10.1136/bmj.c117. [DOI] [PubMed] [Google Scholar]
- Therkildsen C, Bergmann TK, Henrichsen-Schnack T, Ladelund S, Nilbert M. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: a systematic review and meta-analysis. Acta Oncol. 2014;53:852–864. doi: 10.3109/0284186X.2014.895036. [DOI] [PubMed] [Google Scholar]
- Tol J, Nagtegaal ID, Punt CJA. BRAF mutation in metastatic colorectal cancer. N Engl J Med. 2009;361:98–99. doi: 10.1056/NEJMc0904160. [DOI] [PubMed] [Google Scholar]
- Xu Q, Xu AT, Zhu MM, Tong JL, Xu XT, Ran ZH. Predictive and prognostic roles of BRAF mutation in patients with metastatic colorectal cancer treated with anti-epidermal growth factor receptor monoclonal antibodies: a meta-analysis. J Digest Dis. 2013;14:409–416. doi: 10.1111/1751-2980.12063. [DOI] [PubMed] [Google Scholar]
- Yang Z-Y, Wu X-Y, Huang Y-F, Di M-Y, Zheng D-Y, Chen J-Z, Ding H, Mao C, Tang J-L. Promising biomarkers for predicting the outcomes of patients with KRAS wild-type metastatic colorectal cancer treated with anti-epidermal growth factor receptor monoclonal antibodies: a systematic review with meta-analysis. Int J Cancer. 2013;133:1914–1925. doi: 10.1002/ijc.28153. [DOI] [PubMed] [Google Scholar]
- Yuan ZX, Wang XY, Qin QY, Chen DF, Zhong QH, Wang L, Wang JP. The prognostic role of BRAF mutation in metastatic colorectal cancer receiving anti-EGFR monoclonal antibodies: a meta-analysis. PLoS One. 2013;8:e65995. doi: 10.1371/journal.pone.0065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.