Abstract
Background:
Ipilimumab improves the survival of metastatic melanoma patients. Despite documented, durable objective responses, a significant number of patients fails to benefit from treatment. The aim of this study was to identify an upfront marker for treatment benefit.
Methods:
A total of 187 metastatic melanoma patients treated in three Italian Institutions with 3 mg kg−1 ipilimumab, and 27 patients treated with 10 mg kg−1 ipilimumab, were evaluated. Neutrophil-to-lymphocyte ratio (NLR) was calculated from pre-therapy full blood counts. Progression-free survival (PFS) and overall survival (OS) were assessed using the Kaplan–Meier method, and multivariate Cox models were applied, adjusting for confounders and other prognostic factors.
Results:
In the training cohort of 69 patients treated at European Institute of Oncology, pre-therapy NLR was identified as the strongest and independent marker for treatment benefit in multivariate analyses. Patients with baseline NLR<5 had a significantly improved PFS (HR=0.38; 95% CI: 0.22–0.66; P=0.0006) and OS (HR=0.24; 95% CI: 0.13–0.46; P<0.0001) compared with those with a NLR⩾5. Associations of low NLR with improved survival were confirmed in three validation cohorts of patients.
Conclusion:
Our findings show that baseline NLR is strongly and independently associated with outcome of patients treated with ipilimumab, and may serve to identify patients most likely to benefit from this therapy.
Keywords: neutrophils, lymphocytes, ipilimumab, melanoma, biomarker
Even though the mortality associated with metastatic melanoma is still high, novel therapeutic strategies that improve overall survival (OS) have been recently introduced in the clinical practice.
Ipilimumab, a fully human monoclonal antibody that blocks cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), was the first agent that improved survival of advanced melanoma patients in a randomized, controlled phase III trial (Hodi et al, 2010). Objective responses were observed in 10.9% of patients, and disease control was obtained in 28.5% of patients, with 1- and 2-year survival rates of 45.6% and 23.5%, respectively (Hodi et al, 2010). Importantly, ipilimumab showed a pattern of responses that extended beyond those of cytotoxic agents, and are captured by immune-related response criteria (irRC (Wolchok et al, 2009)). Long-term survival data of patients who received ipilimumab in phase II clinical trials showed that durable responses were achieved in a significant proportion of patients (Prieto et al, 2012; Wolchok et al, 2013; Maio et al, 2015; Schadendorf et al, 2015). The immunomodulatory activity of ipilimumab also induced an unusual safety profile, with autoinflammatory side effects such as colitis, dermatitis, hepatitis and hypophisitis, termed immune-related adverse events (irAEs). Grade 3 or 4 irAEs were observed in 15% of ipilimumab-treated patients (Hodi et al, 2010).
Given the possible delayed yet durable responses and the potential toxicity of ipilimumab, it is pivotal to find biomarkers that allow the identification of patients who are most likely or unlikely to benefit from therapy. A number of parameters have been explored in different studies, including CTLA-4 gene polymorphisms, the number and functionality of innate and adaptive immune cells, and soluble factors such as lactate dehydrogenase (LDH) or C-reactive protein (Breunis et al, 2008; Ku et al, 2010; Di Giacomo et al, 2011; Hamid et al, 2011; Delyon et al, 2013; Di Giacomo et al, 2013; Queirolo et al, 2013; Wilgenhof et al, 2013; Berrocal et al, 2014; Kelderman et al, 2014; Simeone et al, 2014). However, in most cases, these potential biomarkers may be expensive and time-consuming, or only become evident during the course of therapy, and cannot be used for upfront selection of patients.
In the last decade, it has become evident that cancer-related inflammation is a key determinant of disease progression and survival in most cancers (Hanahan and Weinberg, 2011). Systemic inflammation is associated with alterations in peripheral blood leukocytes that can be captured by the ratio between neutrophils and lymphocytes (NLR) (Zahorec, 2001). Clinical utility of NLR estimation to predict patient outcomes in a variety of cancers has been demonstrated (Guthrie et al, 2013), including melanoma patients treated with ipilimumab combined with fotemustine (Di Giacomo et al, 2014).
In this study, by evaluating the associations of NLR with the clinical outcome of metastatic melanoma patients treated with ipilimumab, we report on a simple, cost-free and effective marker that might help the identification of patients most likely to benefit from this therapy.
Materials and methods
Patients
This was a retrospective analysis of data collected from melanoma patients who received ipilimumab at the European Institute of Oncology (EIO), at the Istituto Dermopatico dell'Immacolata, and at the University Hospital of Siena through the European Expanded Access Programme (EAP), or after drug commercialisation (CD). Ipilimumab (Yervoy, Bristol-Myers Squibb, New York City, NY, USA) was available upon physician request for patients with life-threatening, unresectable stage III or IV melanoma, who failed or did not tolerate previous treatments. The treatment was approved by the local ethics committees, and all participating patients signed informed consent that comprised a data privacy clause for data collection and analysis for research purpose. Data acquisition and analyses on safety and efficacy mentioned hereafter apply only to the EIO training cohort of patients. Findings on the association of NLR and survival probabilities were then validated in patients who received 3 mg kg−1 ipilimumab in EAP or CD. Data from the cohort patients treated with 10 mg kg−1 ipilimumab at the University Hospital of Siena were analysed to further validate findings on baseline NLR. Patients included in this cohort have already been described (Di Giacomo et al, 2011, 2013).
Treatment and assessments
Ipilimumab was administered 3 mg kg−1 every 3 weeks for four infusions. Patients who progressed after an initial response to treatment, could be reinduced. Complete blood cell counts with differential count and serum chemistry were performed at baseline and before each drug infusion. Tumour assessment was performed at baseline, at week 12, and every 12 weeks thereafter, and clinical responses were classified as immune-related complete response (irCR), partial response (irPR), stable disease (irSD), or progressive disease (irPD), according to irRC (Wolchok et al, 2009). Tumour responses were also evaluated according to response evaluation criteria in solid tumors (RECIST). Deterioration of PS was attributed to disease progression. Clinical benefit was represented by durable immune-related disease control rate (irDCR, defined as the percentage of patients with irSD lasting at least 24 weeks from the first drug infusion, or irCR, or irPR) and immune-related best overall response rate (irBORR, defined as the percentage of patients with irCR or irPR). Patients were continuously monitored for safety, and adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events, version 4.0. AEs were managed using protocol-specific guidelines.
Statistical analysis
Descriptive statistics were used to present patients' characteristics, safety and efficacy of treatment. Data were presented as relative frequencies (percentage).
Baseline, pre-therapy white blood cell count (WBC), absolute neutrophil count (ANC), absolute lymphocyte count (ALC) and serum LDH were analysed. NLR was calculated as the ratio of ANC to ALC (Zahorec, 2001; Walsh et al, 2005). Mann–Whitney U-test was used to evaluate the association of continuous baseline variables with disease control. Data were presented as median and interquartile range (IQR). Baseline blood cell counts and LDH were also categorised according to the upper WBC, ANC, LDH, or lower ALC, limits of normal. NLR was categorised using a threshold of 5 (Guthrie et al, 2013). The receiver operating characteristic (ROC) curve analysis was used to confirm the optimal cutoff for NLR in the training cohort of patients.
Progression-free survival (PFS) was calculated from first treatment to immune-related disease progression or death (event), or last follow-up (censored). Overall survival was calculated from first treatment to death (event) or last follow-up (censored). PFS and OS curves were estimated with the Kaplan–Meier method, and survival distributions according to the indicated parameters were compared using the Wilcoxon test. Multivariate Cox proportional hazard models were used to investigate associations of NLR with survival, adjusting for confounders and other prognostic factors such as age, sex, LDH and ECOG PS in the training cohort of patients. Similar Cox proportional hazard models were applied to the EAP and CD cohorts, adjusting also for a factor indicating the centre. Results were presented as hazard ratios (HRs) with 95% confidence intervals (95% CIs).
For all analyses, two-tailed P<0.05 was considered statistically significant. The statistical analyses were performed with the Statistical Analysis System Version 9.2 (SAS Institute, Cary, NC, USA).
Results
Patients and treatment
From August 2010 to October 2013, 69 patients received ipilimumab at EIO and were included in the training cohort of patients. As of April 2014, with a median follow-up of 10.6 months, 16 (23%) patients were still alive. Baseline patient characteristics are summarised in Table 1. The median age at first treatment was 62 years (range 33–87 years), and 61% of patients were men. Most patients (78%) had a primary cutaneous melanoma; 10% had mucosal and 7% had ocular primary melanoma. All patients had AJCC stage IV disease, with 87% M1c disease. Forty-eight patients (70%) had three or more metastatic sites, 27 patients (39%) had liver metastasis, and 16 (23%) had brain metastasis. LDH was elevated in 56% of patients, and 8 (12%) had ECOG PS 2. Twenty patients (29%) had received two or more previous lines of systemic therapy. Thirty-eight patients (55%) received all 4 doses of ipilimumab, and 2 patients were reinduced. Ten patients received 3 doses, 11 received 2 doses, and 10 received 1 dose. The most common reason for drug discontinuation was rapid disease progression.
Table 1. Baseline characteristics of patients included in the training cohort (N=69).
| N | % | |
|---|---|---|
|
Age, years | ||
| Median, range | 62 | 33–87 |
|
Gender | ||
| Female | 27 | 39 |
| Male | 42 | 61 |
|
Primary site | ||
| Cutaneous | 54 | 78 |
| Mucosal | 7 | 10 |
| Ocular | 5 | 7 |
| Unknown | 3 | 5 |
|
M stage (LDH included) | ||
| M1a | 4 | 6 |
| M1b | 5 | 7 |
| M1c | 60 | 87 |
|
Serum LDH | ||
| <ULN | 28 | 41 |
| ⩾ ULN | 39 | 56 |
| NA | 2 | 3 |
|
ECOG PS | ||
| 0–1 | 61 | 88 |
| 2 | 8 | 12 |
|
Metastatic sites | ||
| 1 | 7 | 10 |
| 2 | 14 | 20 |
| ⩾3 | 48 | 70 |
| Patients with liver metastases | 27 | 39 |
| Patients with brain metastases | 16 | 23 |
|
Previous lines of systemic therapy | ||
| 1 | 49 | 71 |
| ⩾2 | 20 | 29 |
Abbreviations: ECOG PS=European Cooperative Oncology Group performance status; LDH=lactate dehydrogenase; NA=not available; ULN=upper limit of normal.
Safety
In general, AEs in the training cohort were predominantly immune related, manageable, and reversible (Supplementary Table 1). Eight patients had rapid disease progression and they could not be monitored for ipilimumab safety. Of the 61 evaluable patients, 50 (82%) experienced AEs, including eight grade (G) 3/4 AEs in 7 (11%) patients.
The most commonly reported irAEs were dermatologic reactions (18 G1/2 AEs in 13 patients) and gastrointestinal toxicities (31 AEs in 21 patients, including 2 G3/4 AEs). G3 diarrhoea was managed through the use of supportive medications including steroids, and required drug discontinuation. G4 colon perforation was successfully managed with surgical intervention and drug discontinuation.
Three haematologic G3 AEs (two anaemia and one thrombocytopenia), one G3 asthenia and one G3 mood alteration were apparently drug related and recovered spontaneously. Finally, one of the patients with G3 anaemia also had G3 pneumonia, which was not considered drug related.
Efficacy and markers of response
According to RECIST, 1 CR, 3 PR, 15 SD and 50 PD, were recorded in the training cohort. The DCR was 27.5% and the response rate was 6%. Two patients with SD later developed PR. According to irRC, the irDCR was 27.5% and irBORR was 8.5%. At the time of manuscript preparation, 1 irCR, 1 irPR and 2 irSD were still ongoing (26+ 12+ 30+ and 9+ months duration, respectively).
All patients performed blood tests before drug infusions, and markers of response were evaluated in pre-therapy peripheral blood.
Median WBC were 8220 (IQR: 6720–11 470 cells per μl) in patients with irPD, and 5800 (IQR: 4700–7490 cells per μl) in patients that achieved irDC. ANC were also elevated in PD patients (median 5980, IQR: 4420–8310 cells per μl) when compared with non-PD patients (median 3700, IQR: 2910–4800 cells per μl). Conversely, ALC were comparable in PD patients (median 1270, IQR: 890–1920 cells per μl) and in patients with irDC (median 1590, IQR: 1170–2000 cells per μl). Baseline WBC and ANC, but not ALC, were significantly associated with irDC (P=0.0006, P<0.0001 and P=0.2, respectively, Supplementary Figure 1A–C).
When ANC and ALC were incorporated in the NLR, we found that NLR was also associated with irDC (P=0.0002, Supplementary Figure 1D). NLR was significantly higher (median 4.28; IQR: 3.11–6.59) in patients that experienced irPD than in patients that achieved irDC (median 2.4; IQR: 1.64–3.77). NLR was considered to be elevated above a value of 5 (Guthrie et al, 2013). The ROC curve analysis was used to confirm the optimal discrimination given by this cutoff point. The area under the curve was 0.7937 (95% CI=0.68–0.91). With a threshold of 5, specificity of NLR for disease progression was 95%, and sensitivity was 44% (Supplementary Table 2). In the training cohort, 24 (35%) patients had elevated NLR, and 23 of them (96%) experienced irPD as best response.
Survival and markers of outcome
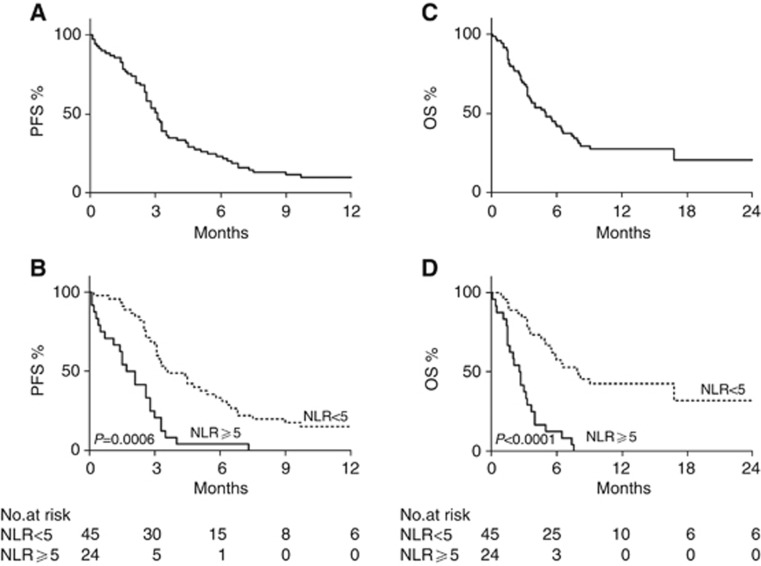
The median PFS for patients included in the training cohort was 3.1 months (Figure 1A). Univariate analysis of pre-treatment patient characteristics revealed that LDH<upper limit of normal (ULN) (P=0.03), ECOG PS 0–1 (P<0.0001) and NLR<5 (P<0.0001), but not age or gender (P=0.8 and P=0.22, respectively), were significantly associated with improved PFS. Among haematological parameters, WBC and ANC (P<0.0001 and P=0.0004) were also significantly associated with PFS (Supplementary Table 3), but not ALC (P=0.07). Median PFS was 3.6 months for patients with NLR<5, and 1.9 months for patients with NLR⩾ 5 (Figure 1B). Patients with a NLR<5 had a five-fold significantly reduced risk of progression when compared with those with an elevated NLR (HR=0.20; 95% CI: 0.10–0.41, Supplementary Table 3). In a multivariate analysis including age, sex, LDH and ECOG PS, NLR was identified as a significant and independent factor for disease progression (HR=0.38; 95% CI: 0.22–0.66; P=0.0006, Table 2).
Figure 1.
Survival of patients included in the training cohort. (A, B) Kaplan–Meier curves of progression-free survival (PFS) of the whole training population (A), and according to baseline neutrophil-to-lymphocyte ratio (NLR; B). (C, D) Kaplan–Meier curves of overall survival (OS) of the whole training population (C), and according to baseline NLR (D). (B, D) Dotted line: NLR<5; continuous line: NLR⩾5. P-values are from multivariate Cox models.
Table 2. Multivariate analysis of the associations of baseline patient characteristics and survival of patients included in the training cohort (N=69).
|
Disease progression |
Overall survival |
|||||
|---|---|---|---|---|---|---|
| Parameter | HR | 95% CI | P-valuea | HR | 95% CI | P-valuea |
| Age | 0.99 | 0.97–1.01 | 0.49 | 0.99 | 0.97–1.02 | 0.68 |
| Sex (F vs M) | 0.92 | 0.55–1.53 | 0.75 | 0.74 | 0.42–1.31 | 0.31 |
| LDH (<ULN vs ⩾ULN) | 0.56 | 0.32–0.97 | 0.04 | 0.78 | 0.43–1.43 | 0.42 |
| ECOG PS (0–1 vs 2) | 0.34 | 0.14–0.79 | 0.01 | 0.27 | 0.11–0.63 | 0.002 |
| NLR (<5 vs ⩾5) | 0.38 | 0.22–0.66 | 0.0006 | 0.24 | 0.13–0.46 | <0.0001 |
Abbreviations: CI=confidence interval; ECOG PS=European Cooperative Oncology Group performance status; F=female; HR=hazard ratio; LDH=lactate dehydrogenase; M=male; NLR=neutrophil-to-lymphocyte ratio; ULN=upper limit of normal.
P-values are from the Cox model that included age, gender, LDH and ECOG PS.
The median OS for patients included in the training cohort was 5 months (Figure 1C). As reported in Supplementary Table 3, univariate analysis showed that factors significantly associated with prolonged OS were ECOG PS 0–1 (P<0.0001), NLR<5 (P<0.0001), WBC<UNL (P<0.0001), ANC<ULN (P<0.0001) and ALC>LLN (P=0.04). Age (P=0.52), gender (P=0.22) and LDH (P=0.11) were not significantly associated with OS (Supplementary Table 3). Median OS was 8 months for patients with NLR<5 and only 2.6 months for patients with NLR⩾5 (Figure 1D). Patients with a baseline NLR<5 had a eight-fold reduced risk of death when compared with those with an elevated NLR (HR=0.12; 95% CI: 0.05–0.25, Supplementary Table 3). In a multivariate analysis, NLR was significantly and independently associated with OS (HR=0.24; 95% CI: 0.13–0.46; P<0.0001, Table 2), adjusting for age, sex, LDH and ECOG PS. Importantly, NLR remained significantly and independently associated with both PFS (P=0.004) and OS (P<0.0001) when analysed as a continuous variable.
Multivariate analyses adjusting for age, sex, LDH and ECOG PS, showed that ANC>ULN was significantly and independently associated with poor PFS and OS (P=0.007 and P=0.0003, respectively). However, when both NLR and ANC were entered together in the model, NLR but not ANC remained significantly associated with OS (P=0.009 for NLR and P=0.32 for ANC). Similar results were obtained with PFS.
Ipilimumab therapy would require four drug infusions 3 weeks apart. In the training cohort, 10 patients (all with elevated LDH, 50% of whom with an ECOG PS of 2) experienced so rapid disease progression that they were not able to receive half of the recommended doses. To rule out the possibility that the associations of NLR with the outcome were limited to patients with a very advanced disease, a subgroup analysis was performed in EIO patients who received at least two doses of ipilimumab (N=59, Supplementary Table 4). In a multivariate Cox model, NLR remained the single and strongest independent factor for disease progression (HR=0.46; 95% CI: 0.24–0.87; P=0.02) and survival (HR=0.26; 95% CI: 0.13–0.53; P=0.0002), adjusting for age, sex, LDH and ECOG PS.
Validation of NLR association with OS
The association of NLR with OS was validated in patients treated with ipilimumab 3 mg kg−1 within the EAP (N=115) or after drug commercialisation (N=72) in three Italian Institutions. Patient characteristics are summarised in Table 3. The two groups of patients were well balanced for age, gender and baseline LDH. A major difference was noted in the number of previous lines of systemic therapies, which was higher in the EAP group. Median follow-up were 16.4 and 10.6 months, respectively, for EAP and CD groups, whereas the median OS were 7.8 and 6.5 months, respectively.
Table 3. Baseline characteristics of patients included in the validation cohorts.
| EAP (N=115) | CD (N=72) | |
|---|---|---|
|
Age, years | ||
| Median, range | 63 (23–88) | 62 (21–82) |
|
Gender,
N
(%) | ||
| Female | 49 (43) | 28 (39) |
| Male | 66 (57) | 44 (41) |
|
Primary site,
N
(%) | ||
| Cutaneous | 85 (74) | 60 (83) |
| Mucosal | 14 (12) | 4 (6) |
| Ocular | 7 (6) | 6 (8) |
| Unknown | 9 (8) | 2 (3) |
|
Serum LDH,
N
(%) | ||
| <ULN | 51 (46) | 28 (39) |
| ⩾ ULN | 61 (54) | 43 (61) |
| NA | 3 | 1 |
|
ECOG PS,
N
(%) | ||
| 0–1 | 107 (93) | 67 (93) |
| 2 | 8 (7) | 5 (7) |
|
Previous lines of systemic therapy | ||
| 1 | 65 (57) | 61 (85) |
| ⩾2 | 50 (43) | 11 (15) |
|
Institution,
N
(%) | ||
| European Institute of Oncology | 38 (33) | 39 (54) |
| Istituto Dermopatico dell'Immacolata | 47 (41) | 33 (46) |
| University Hospital of Siena | 30 (26) | 0 |
Abbreviations: CD=commercial drug; EAP=Expanded Access Programme; ECOG PS=European Cooperative Oncology Group performance status; LDH=lactate dehydrogenase; NA=not available; ULN=upper limit of normal.
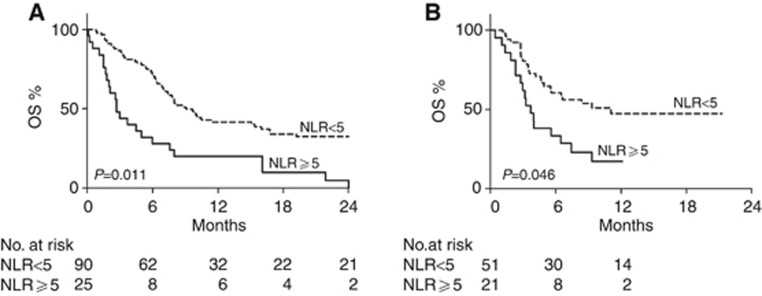
In the EAP group, when stratified according to baseline NLR, median OS were 9.1 and 2.7 months, respectively, for patients with NLR<5 or higher (Figure 2A, P<0.0001). Multivariate analysis showed that patients with NLR<5 had a significantly reduced risk of death with similar hazard ratio (HR=0.51; 95% CI: 0.30–0.86; P=0.011) when compared with patients with NLR⩾5 (Table 4). Similarly, in the CD group, patients with NLR<5 had a significantly improved survival (11.1 vs 3.7 months, Figure 2B, P=0.005). Multivariate analysis confirmed significant reduced risk of death for patients with lower NLR (HR=0.49; 95% CI: 0.25–0.99; P=0.049) when compared with patients with NLR⩾5 (Table 4). Since the proportion of patients with ECOG PS of 2 was very low (7%) and ECOG PS was not significantly associated with OS, this factor was not included in the multivariate models.
Figure 2.
Overall survival of patients included in the validation cohorts. (A, B) Kaplan–Meier curves of overall survival (OS) of patients that received ipilimumab within the EAP (A) or as a commercial drug (B), according to baseline NLR. Dotted line: NLR<5; continuous line: NLR⩾5. P-values are from multivariate Cox models.
Table 4. Multivariate analysis of the associations of baseline characteristics and survival of patients included in the validation cohorts.
|
Ipilimumab in EAP (N=115) |
Ipilimumab as CD (N=72) |
|||||
|---|---|---|---|---|---|---|
| Parameter | HR | 95% CI | P-valuea | HR | 95% CI | P-valuea |
| Age | 0.99 | 0.97–1.01 | 0.249 | 0.57 | 0.27–1.21 | 0.145 |
| Sex (F vs M) | 0.88 | 0.56–1.38 | 0.576 | 0.98 | 0.95–1.02 | 0.296 |
| LDH (<ULN vs ⩾ULN) | 0.45 | 0.27–0.74 | 0.002 | 0.44 | 0.19–1.04 | 0.062 |
| NLR (<5 vs ⩾5) | 0.51 | 0.30–0.86 | 0.011 | 0.49 | 0.25–0.99 | 0.046 |
Abbreviations: CD=commercial drug; CI=confidence interval; EAP=Expanded Access Programme; ECOG PS=European Cooperative Oncology Group performance status; F=female; HR=hazard ratio; LDH=lactate dehydrogenase; M=male; NA=not available; NLR=neutrophil-to-lymphocyte ratio; ULN=upper limit of normal.
P-values are from the Cox model that included age, gender, LDH and Institution.
Furthermore, findings on the association of baseline NLR with survival were also independently validated on a cohort of 27 patients treated with 10 mg kg−1 ipilimumab (Di Giacomo et al, 2011, 2013). The median OS for these patients was 9.6 months. When stratified according to NLR, median OS was 11.3 months for patients with NLR<5 and 6.7 months for those with NLR⩾5. In a multivariate Cox model, NLR<5 was found to be associated with improved OS (HR=0.26; 95% CI: 0.08–0.88, P=0.03, Supplementary Table 5), adjusting for age, sex and LDH.
Discussion
In this study, by analysing pre-therapy haematological parameters of a large group of metastatic melanoma patients treated with ipilimumab in three Italian Institutions, we showed that NLR was significantly associated with survival. We therefore suggest that NLR might allow the identification of patients that are more likely to benefit from this therapy, in advance of its initiation.
In the last decade, it has become evident that immunotherapy does extend survival in a subset of melanoma patients. Monoclonal antibodies that block the CTLA-4 or programmed death-1 receptor, in fact, are able to release T cells from immune checkpoints and promote their activation, proliferation and effector functions, leading to substantial immunologic and clinical antitumour activity (Ott et al, 2013). Durable responses observed with ipilimumab are encouraging, but restricted to only a limited number of patients (Hodi et al, 2010). Further, consistent with the proposed drug mechanism of action (Peggs et al, 2006), ipilimumab safety profile includes irAEs that can be life threatening and treatment limiting (Hodi et al, 2010).
Safety and efficacy data presented here are in line with the clinical experience of ipilimumab 3 mg kg−1 given through the EAP in Italy (Ascierto et al, 2014). AEs were reported by the majority of patients, but were predominantly mild, manageable and reversible. One-third of patients achieved irDC, and most of the responses were long lasting.
The median OS in the training cohort of patients was 5 months, which is lower than that reported in other studies and that of the validation cohorts. This could be due to the poor baseline patient characteristics (70% had three or more metastatic sites and 12% had ECOG PS 2). Further, only 55% of patients were able to complete the four-dose ipilimumab therapy. In the absence of an upfront selection criterion, and due to the lack of alternative treatments, also patients with a dismal life expectancy and poor conditions were treated. Identification of patients that are unlikely to respond to therapy would allow to spare them from undesirable side effects and, whenever possible, to switch them to alternative treatments.
We observed that pre-therapy normal WBC and ANC (i.e., below the upper limit of normal) were significantly associated with improved PFS and OS, whereas normal ALC (i.e., above the lower limit of normal) were associated with improved OS, but not with PFS. Although lymphocytes are the target of ipilimumab, it is still not clear whether baseline ALC is associated with outcome. In fact, although association of baseline ALC and OS were observed in two recent studies (Berrocal et al, 2014; Kelderman et al, 2014), changes in lymphocyte counts, or on-therapy ALC, but not baseline ALC, were found to be associated with irDC and OS in other studies (Ku et al, 2010; Di Giacomo et al, 2011; Delyon et al, 2013; Di Giacomo et al, 2013; Wilgenhof et al, 2013). The finding that normal ANCs were associated with improved PFS and OS would indicate that not only lymphocytes, but also neutrophils may influence outcome under ipilimumab therapy, as recently observed also for eosinophils (Delyon et al, 2013). Strikingly, when baseline ANC and ALC were incorporated in the NLR, we observed that NLR was strongly associated with disease control and clinical outcome. In multivariate analyses, NLR<5 remained associated with a 2.6-fold reduced risk of disease progression and a four-fold reduced risk of death. These associations were confirmed when we analysed patients that received ipilimumab 3 mg kg−1 within the EAP or after drug commercialisation (two-fold reduced risk of death for both), as well as patients that received ipilimumab 10 mg kg−1 (3.8-fold reduced risk of death). Taken together, our results would suggest that benefit from ipilimumab depends not only on lymphocyte activation by ipilimumab, but also on the context in which T cells are stimulated, as illustrated here by the ratio of neutrophils to lymphocytes.
Recently, the NLR was investigated in 27 melanoma patients receiving ipilimumab 10 mg kg−1. Notably, when the median NLR was used as a cutoff, on-therapy but not baseline NLR was found to be associated with survival (Di Giacomo et al, 2013). When compared with the training cohort of patients of this study, this patient population included less patients with M1c disease (78% vs 87%), elevated LDH (41% vs 56%) and poor performance status (0% vs 12% of patients with ECOG PS of 2). However, despite differences in patient characteristics, ipilimumab dose, and outcome, when 5 was used as a cutoff for NLR in this cohort of patients, we observed that NLR was still associated with OS.
The strength of this study is that it is based on ‘real world' populations of metastatic melanoma patients treated within routine clinical practice, with one training cohort and three validation cohorts of patients. On the other hand, the major limitation of this analysis stems from the same characteristics of these populations, which are heterogeneous and not part of a controlled, randomized clinical trial, and lacks a control arm. Further, this is a retrospective study, with consequent possibility of bias, and the sample size is relatively small. These limitations did not allow to establish whether NLR is either a predictive or a prognostic marker. Association of NLR and prognosis of patients with a variety of cancer types and receiving different treatments (including surgery, chemotherapy, radiotherapy or adjuvant therapy (Guthrie et al, 2013)) would suggest that NLR is a general prognostic marker, rather than a predictive marker for ipilimumab. However, given its possible ability in identifying patients that are unlikely to benefit from this therapy in advance of its initiation, we would strongly encourage prospective studies with adequately powered sample size and clinical trials to validate our findings. The NLR would represent an ideal marker as it is derived from routine diagnostic procedures, it is does not require additional expenditure, and it is easily and promptly computable.
Acknowledgments
CM was supported by Fondazione Grazia Focacci Onlus, Milan, Italy, a no-profit Organization.
PFF has participated in advisory boards and expert panels for Bristol-Myers Squibb, Roche-Genentech and GlaxoSmithKline. AMDG has served as a speaker in Bristol-Myers Squibb, Roche-Genentech, and GlaxoSmithKline. PM has served as a consultant or in an advisory role for Bristol-Myers Squibb, Roche-Genentech, GlaxoSmithKline, Pfizer and Novartis. EC has served as a consultant for Bristol-Myers Squibb and GlaxoSmithKline. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Ascierto PA, Simeone E, Sileni VC, Pigozzo J, Maio M, Altomonte M, Del Vecchio M, Di Guardo L, Marchetti P, Ridolfi R, Cognetti F, Testori A, Bernengo MG, Guida M, Marconcini R, Mandala M, Cimminiello C, Rinaldi G, Aglietta M, Queirolo P. Clinical experience with ipilimumab 3 mg/kg: real-world efficacy and safety data from an expanded access programme cohort. J Transl Med. 2014;12 (1:116. doi: 10.1186/1479-5876-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocal A, Arance A, Lopez Martin JA, Soriano V, Munoz E, Alonso L, Espinosa E, Lopez Criado P, Valdivia J, Martin Algarra S, Spanish Melanoma Group Ipilimumab for advanced melanoma: experience from the Spanish Expanded Access Program. Melanoma Res. 2014;24 (6:577–583. doi: 10.1097/CMR.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunis WB, Tarazona-Santos E, Chen R, Kiley M, Rosenberg SA, Chanock SJ. Influence of cytotoxic T lymphocyte-associated antigen 4 (CTLA4) common polymorphisms on outcome in treatment of melanoma patients with CTLA-4 blockade. J Immunother. 2008;31 (6:586–590. doi: 10.1097/CJI.0b013e31817fd8f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delyon J, Mateus C, Lefeuvre D, Lanoy E, Zitvogel L, Chaput N, Roy S, Eggermont AM, Routier E, Robert C. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol. 2013;24 (6:1697–1703. doi: 10.1093/annonc/mdt027. [DOI] [PubMed] [Google Scholar]
- Di Giacomo AM, Ascierto PA, Queirolo P, Pilla L, Ridolfi R, Santinami M, Testori A, Simeone E, Guidoboni M, Maurichi A, Orgiano L, Spadola G, Del Vecchio M, Danielli R, Calabro L, Annesi D, Giannarelli D, Maccalli C, Fonsatti E, Parmiani G, Maio M. Three-year follow-up of advanced melanoma patients who received ipilimumab plus fotemustine in the Italian Network for Tumor Biotherapy (NIBIT)-M1 phase II study. Ann Oncol. 2014;26 (4:798–803. doi: 10.1093/annonc/mdu577. [DOI] [PubMed] [Google Scholar]
- Di Giacomo AM, Calabro L, Danielli R, Fonsatti E, Bertocci E, Pesce I, Fazio C, Cutaia O, Giannarelli D, Miracco C, Biagioli M, Altomonte M, Maio M. Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother. 2013;62 (6:1021–1028. doi: 10.1007/s00262-013-1418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giacomo AM, Danielli R, Calabro L, Bertocci E, Nannicini C, Giannarelli D, Balestrazzi A, Vigni F, Riversi V, Miracco C, Biagioli M, Altomonte M, Maio M. Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy) Cancer Immunol Immunother. 2011;60 (4:467–477. doi: 10.1007/s00262-010-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88 (1:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, Guida M, Hyams DM, Gomez H, Bastholt L, Chasalow SD, Berman D. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144 (5:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363 (8:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelderman S, Heemskerk B, van Tinteren H, van den Brom RR, Hospers GA, van den Eertwegh AJ, Kapiteijn EW, de Groot JW, Soetekouw P, Jansen RL, Fiets E, Furness AJ, Renn A, Krzystanek M, Szallasi Z, Lorigan P, Gore ME, Schumacher TN, Haanen JB, Larkin JM, Blank CU. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother. 2014;63 (5:449–458. doi: 10.1007/s00262-014-1528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, Chapman PB, Schwartz GK, Allison JP, Wolchok JD. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116 (7:1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maio M, Grob JJ, Aamdal S, Bondarenko I, Robert C, Thomas L, Garbe C, Chiarion-Sileni V, Testori A, Chen TT, Tschaika M, Wolchok JD. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol. 2015;33 (10:1191–1196. doi: 10.1200/JCO.2014.56.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19 (19:5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18 (2:206–213. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18 (7:2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queirolo P, Morabito A, Laurent S, Lastraioli S, Piccioli P, Ascierto PA, Gentilcore G, Serra M, Marasco A, Tornari E, Dozin B, Pistillo MP. Association of CTLA-4 polymorphisms with improved overall survival in melanoma patients treated with CTLA-4 blockade: a pilot study. Cancer Invest. 2013;31 (5:336–345. doi: 10.3109/07357907.2013.793699. [DOI] [PubMed] [Google Scholar]
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD.2015Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma J Clin Oncole-pub ahead of print 9 February 2015doi: 10.1200/JCO.2014.56.2736 [DOI] [PMC free article] [PubMed]
- Simeone E, Gentilcore G, Giannarelli D, Grimaldi AM, Caraco C, Curvietto M, Esposito A, Paone M, Palla M, Cavalcanti E, Sandomenico F, Petrillo A, Botti G, Fulciniti F, Palmieri G, Queirolo P, Marchetti P, Ferraresi V, Rinaldi G, Pistillo MP, Ciliberto G, Mozzillo N, Ascierto PA. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother. 2014;63 (7:675–683. doi: 10.1007/s00262-014-1545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91 (3:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- Wilgenhof S, Du Four S, Vandenbroucke F, Everaert H, Salmon I, Lienard D, Marmol VD, Neyns B. Single-center experience with ipilimumab in an expanded access program for patients with pretreated advanced melanoma. J Immunother. 2013;36 (3:215–222. doi: 10.1097/CJI.0b013e31828eed39. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15 (23:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Weber JS, Maio M, Neyns B, Harmankaya K, Chin K, Cykowski L, de Pril V, Humphrey R, Lebbe C. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann Oncol. 2013;24 (8:2174–2180. doi: 10.1093/annonc/mdt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102 (1:5–14. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.