Abstract
Background:
Despite major advances in the management of metastatic colorectal cancer (mCRC) with liver-only involvement, relapse rates are high and reliable prognostic markers are needed.
Methods:
To assess the prognostic impact of BRAF and RAS mutations in a large series of liver-resected patients, medical records of 3024 mCRC patients were reviewed. Eligible cases undergoing potentially curative liver resection were selected. BRAF and RAS mutational status was tested on primary and/or metastases by means of pyrosequencing and mass spectrometry genotyping assay. Primary endpoint was relapse-free survival (RFS).
Results:
In the final study population (N=309) BRAF mutant, RAS mutant and all wild-type (wt) patients were 12(4%), 160(52%) and 137(44%), respectively. Median RFS was 5.7, 11.0 and 14.4 months respectively and differed significantly (Log-rank, P=0.043). At multivariate analyses, BRAF mutant had a higher risk of relapse in comparison to all wt (multivariate hazard ratio (HR)=2.31; 95% CI, 1.09–4.87; P=0.029) and to RAS mutant (multivariate HR=2.06; 95% CI, 1.02–4.14; P=0.044). Similar results were obtained in terms of overall survival. Compared with all wt patients, RAS mutant showed a higher risk of death (HR=1.47; 95% CI, 1.05–2.07; P=0.025), but such effect was lost at multivariate analyses.
Conclusions:
BRAF mutation is associated with an extremely poor median RFS after liver resection and with higher probability of relapse and death. Knowledge of BRAF mutational status may optimise clinical decision making in mCRC patients potentially candidate to hepatic surgery. RAS status as useful marker in this setting might require further studies.
Keywords: BRAF, RAS, metastatic colorectal cancer, liver, prognostic
Resection of liver metastases (CLM) represents a possibility of cure in metastatic colorectal cancer (mCRC) (Tomlinson et al, 2007). Currently, the number of patients candidate to hepatic resection has dramatically increased thanks to the integration of new surgical techniques with more effective therapies (Kopetz et al, 2009; Primrose, 2010). Consequently, overall survival (OS) rates progressively increased, exceeding 50% at 5 years in resected patients (Hayashi et al, 2010).
However, liver resection is a complex and costly procedure and tumour relapse occurs in almost two-thirds of patients after a potentially curative resection (de Jong et al, 2009). Thus, it is evident that the need for prompt identification of patients at higher risk of recurrence. Several studies examined prognostic markers for recurrence after CLM resection but only common clinico-pathological characteristics are included in risk estimating scoring systems (Nordlinger et al, 1996; Fong et al, 1999; Rees et al, 2008; Primrose, 2010). Available scores are not sensitive enough to definitely exclude patients from a potentially useless surgery, which, at the same time, may stand as the only chance for cure.
During the last decade, the assessment of RAS and BRAF mutational status gained increasing importance for an optimal management of CRC (Schmoll et al, 2012; Douillard et al, 2013). BRAF V600E mutation, occurring in 6–10% of mCRC, defines a subgroup with low probability of long-term survival, specific clinico-biological features with high rate of nodal and peritoneal metastases (Richman et al, 2009; Saridaki et al, 2010; Tran et al, 2011; Yokota et al, 2011). RAS mutations, occurring in about 50% of mCRC(Peeters et al, 2013; Morris et al, 2014; Schirripa et al, 2014), are determinants of resistance to anti-EGFR monoclonal antibodies (Lievre et al, 2006; Amado et al, 2008; Karapetis et al, 2008; Van Cutsem et al, 2011; Douillard et al, 2013) and are linked to higher incidence of lung and brain metastases (Cejas et al, 2009; Tie et al, 2011; Kim et al, 2012). The prognostic role of RAS mutations is controversial and a mild negative effect is reported both in the adjuvant and in the metastatic setting (Andreyev et al, 2001; Richman et al, 2009; Van Cutsem et al, 2011).
In this complex scenario, BRAF and RAS mutations might increase the chance of selecting appropriate candidates for liver resection. Some authors suggested a possible negative prognostic role for RAS mutations in patients undergoing CLM resection, while the extremely small number of BRAF mutant patients identified in the published series did not allow to draw definitive conclusions (Teng et al, 2012; Karagkounis et al, 2013; Umeda et al, 2013; Vauthey et al, 2013).
Moving from the above-mentioned considerations, we carried out the present work to investigate BRAF and RAS mutations as prognostic biomarkers in a wide population of patients who underwent liver resection with curative intent.
Materials and Methods
Patients' selection and clinical data collection
Clinical records from three Italian Oncology Units with a high volume of mCRC patients were reviewed. Data from consecutive mCRC patients referred to the Units of Pisa (2005–2012), Padova (1995–2012) and Udine (2000–2012) were evaluated for inclusion.
Patients with histological diagnosis of colorectal adenocarcinoma who underwent liver resection with curative intent defined by a multidisciplinary team were selected according to the following eligibility criteria:
Availability of tumour tissue for mutational status evaluation;
Adequate follow-up defined as ‘clinical visits, including evaluation of CEA level and a chest/abdomen CT scan performed within 3 months from liver resection and then repeated at least once every 4 months for 3 years after resection'.
Baseline characteristics collected are reported in Table 1. Data concerning systemic therapies before and/or after liver resection and sites of first relapse were also collected.
Table 1. Final study population characteristics according to mutational status.
| Characteristics | BRAF mut (N=12) | RAS mut (N=160) | All wt (N=137) | P |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
|
Sex | ||||
| Male | 7 (58) | 94 (59) | 91 (66) | 0.38 |
| Female | 5 (42) | 66 (41) | 46 (34) | — |
|
Age | ||||
| <65 years | 8 (67) | 89 (56) | 80 (58) | 0.71 |
| ⩾65 years | 4 (33) | 71 (44) | 57 (42) | — |
|
ECOG PS | ||||
| 0 | 8 (67) | 139 (87) | 120 (88) | 0.12 |
| 1–2 | 4 (33) | 21 (13) | 17 (12) | — |
|
Primary tumour site | ||||
| Right colon | 7 (58) | 61 (38) | 19 (14) | <0.0001 |
| Left colon | 3 (25) | 55 (35) | 80 (59) | — |
| Rectum | 2 (17) | 43 (27) | 37 (27) | — |
|
Liver only | ||||
| Yes | 10 (83) | 142 (89) | 129 (94) | 0.17 |
| No | 2 (17) | 18 (11) | 8 (6) | — |
|
Unilobar mts | ||||
| Yes | 9 (75) | 102 (64) | 87 (64) | 0.72 |
| No | 3 (25) | 58 (36) | 50 (36) | — |
|
Time to mts | ||||
| Synchronous | 10 (83) | 110 (69) | 88 (64) | 0.34 |
| Metachronous | 2 (17) | 50 (31) | 49 (36) | — |
|
Resection outcome | ||||
| R0 | 10 (83) | 129 (81) | 119 (87) | 0.34 |
| R1/R2 Expl. Lapar. | 2 (17) | 31 (19) | 18 (13) | — |
|
Primary lymph nodes | ||||
| No | 2 (17) | 45 (29) | 49 (36) | 0.21 |
| Yes | 10 (83) | 111 (71) | 86 (64) | — |
| NA | 0 | 4 | 2 | — |
|
DFI <12 months | ||||
| Yes | 11 (92) | 128 (80) | 103 (75) | 0.31 |
| No | 1 (8) | 32 (20) | 34 (25) | — |
|
>1 liver mts | ||||
| No | 5 (42) | 63 (40) | 64 (48) | 0.37 |
| Yes | 7 (58) | 96 (60) | 70 (52) | — |
| NA | 0 | 1 | 3 | — |
|
Mts diameter >5 cm | ||||
| No | 8 (67) | 127 (82) | 96 (76) | 0.29 |
| Yes | 4 (33) | 28 (18) | 30 (24) | — |
| NA | 0 | 5 | 11 | — |
|
CEA>200 ng ml−1 | ||||
| No | 12 (100) | 117 (98) | 84 (93) | 0.24 |
| Yes | 0 (0) | 3 (2) | 6 (7) | — |
| NA | 0 | 40 | 47 | — |
|
Clinical risk scorea | ||||
| Low | 5 (42) | 67 (47) | 64 (54) | 0.42 |
| High | 7 (58) | 76 (53) | 54 (46) | — |
| NA | 0 | 17 | 19 | |
Abbreviations: CEA=carcinoembryonic antigen; DFI=disease-free interval; ECOG=Eastern Cooperative Oncology Group; Expl. Lapar.=exploratory laparotomy; mts=metastasis; mut=mutant; N=number; NA=not available; PS=performance status; wt=wild-type.
Clinical risk score is defined as previously described by Fong et al (1999): patients with 0 to 2 risk features were categorised as ‘low risk', while those with 3 to 5 features as ‘high risk'. Bold entries indicate significant results.
Patients who met these selection criteria were included in the ‘eligible patients' population'.
Molecular analyses
Primary and/or corresponding liver metastasis were retrieved from the archives of Pathology Departments of the three collaborating Institutions.
A screening genotyping for KRAS (exon 2) and BRAF V600E mutation was run by means of Pyrosequencing on the PyroMark Q96 ID instrument (Qiagen, Hilden, Germany) with commercially available kits (Diatech Pharmacogenetics, Ancona, Italy).
KRAS (exon 2) wild-type (wt), BRAF wt and patients with discordant results on primary and corresponding liver metastasis were centrally re-evaluated by means of MassARRAY (Sequenom Inc., San Diego, CA, USA) with the CE-IVD marked kit Myriapod Colon Status (Diatech Pharmacogenetics) on primary or corresponding liver metastasis. The assay allows simultaneous analyses of KRAS, BRAF and NRAS, tested mutations are listed in Supplementary Table 1.
Patients with informative mutational status results were defined as ‘final study population', and based on their mutational status were categorised as: BRAF mut, RAS mut and all wt (BRAF and RAS wt).
Methods for microsatellite instability determination are described in the Supplementary Appendix 1.
Statistical considerations
Results of BRAF and RAS mutational analyses were used as categorical variables. Fisher's exact test or χ2-test was used to compare clinical and biological features according to mutational status.
The primary endpoint was relapse-free survival (RFS) according to BRAF and RAS status in the final study population. All other analyses were exploratory and aimed to assess secondary endpoints. RFS was defined as the time from liver resection to first disease recurrence or death due to any cause; OS was defined as the time from liver resection to death due to any cause. Overall survival and RFS analyses were determined according to the Kaplan–Meier method and survival curves were compared using the log-rank test. Statistical significance was set at P<0.05 for a bilateral test.
The correlation of mutational status and clinico-pathological characteristics with survival was firstly assessed in the univariate analyses. Cox proportional hazard model was adopted in the multivariate analysis, including as covariates variables correlated with survival in the univariate analyses (P<0.1). An exploratory recursive partitioning analysis was performed.
Results
Patient populations and mutational analyses
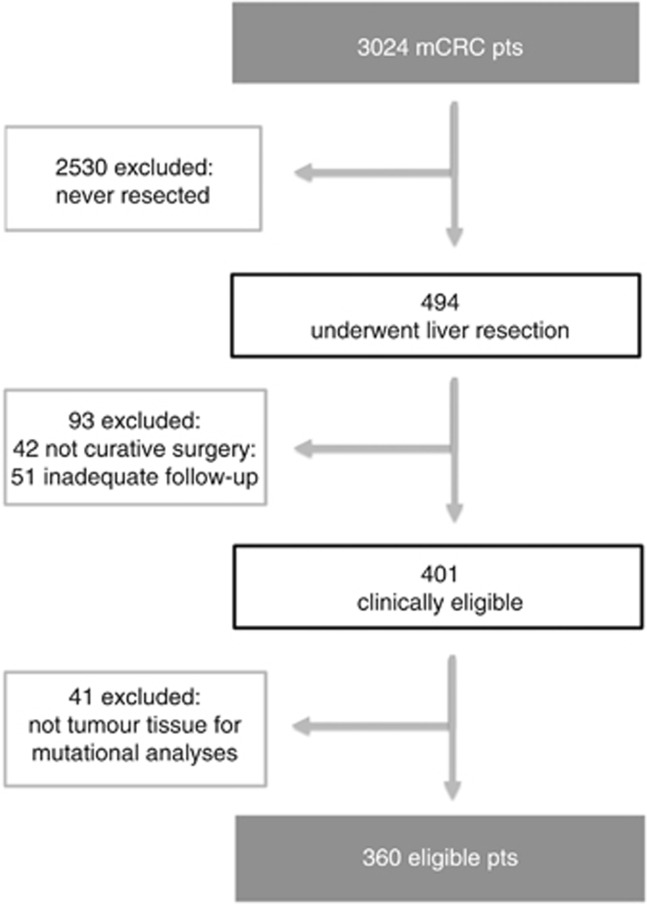
A total of 3024 mCRC patients were referred to the three institutions during the specified time frame. Case-by-case revision of medical records allowed to identify 494 subjects who underwent liver resection. Among them, 360 patients met eligibility criteria and were included in the ‘eligible patients' population' (Figure 1). Baseline characteristics are reported in Supplementary Table 2.
Figure 1.
Diagram of eligible patients population selection.
Pyrosequencing analyses
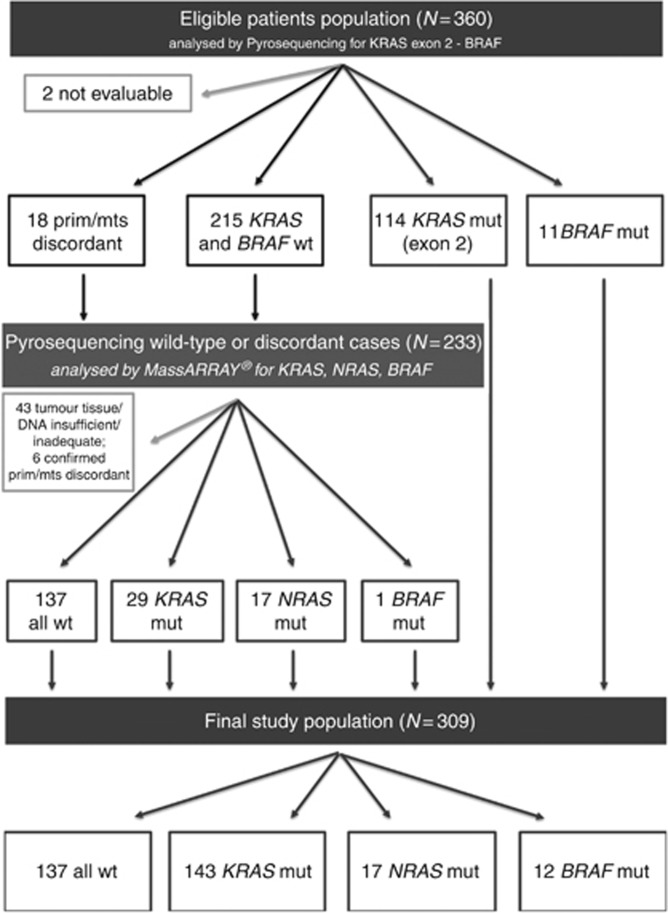
BRAF V600E mutational status was performed in the primary tumour, in a liver metastasis or both in 63 (17.5%), 59 (16.5%) and 238 (66%) cases, respectively. Eleven cases (3%) resulted BRAF mut. No discordance between primaries and related metastases was observed.
KRAS exon 2 mutational status was performed in the primary tumour, in a liver metastasis or both in 63 (17.5%), 61 (17%) and 234 (65%) cases, respectively; 2 (0.5%) samples were not evaluable. One-hundred-fourteen cases (32%) resulted KRAS mut. A discordant result between the primary tumour and related liver metastasis was found in 18 cases (8%), 12 primaries were mut with wt metastases and 6 metastases were mut with wt primaries. BRAF and KRAS exon 2 wt patients were 215 (60%).
Sequenom analyses
About 233 cases (from 215 BRAF and KRAS exon 2 wt patients and from 18 patients showing discordant KRAS mutational status results) were tested. Forty-three cases were excluded due to tumour tissue and/or DNA insufficient and/or inadequate. Six out of 18 primary-metastases couples discordant at pyrosequencing had the previous results confirmed at MassARRAY (Sequenom Inc.) testing. Ten out of 18 discordant couples were found mut both on the primary tumour and on the corresponding liver metastasis. A BRAF, NRAS or KRAS mutation was found in 1, 17 and 29 cases, respectively, out of 190 cases.
The final study population included 309 patients with informative results: 12 (4%) BRAF mut, 160 (52%) RAS mut and 137 (44%) all wt patients. A diagram showing the selection process is shown in Figure 2. A detailed mutational status description of the final study population is shown in Supplementary Table 3.
Figure 2.
Diagram of final study population selection. N=number; prim=primary; mts=metastasis; wt=wild-type; mut=mutant.
Clinical characteristics and their association with mutational status
No differences were observed between the eligible patients population and the final study population (Supplementary Table 2).
Among patients included in the final study population: 67% had synchronous disease, 91% had liver limited disease, 57% had more than one liver metastasis and 36% had bilobar liver involvement. With regard to medical treatments administered: 50% of patients received a systemic treatment before and after liver resection (including bevacizumab or anti-EGFR monoclonal antibodies in 31% and 5% of cases, respectively), 36% received a systemic treatment only after liver resection; 14% were not treated neither before nor after liver resection; 26% received an anti-EGFR in subsequent lines; 21% received an adjuvant treatment after primary tumour resection.
Only 51 patients (17%) underwent R1/R2-exploratory laparotomy instead of curative surgery due to unexpected metastatic spread observed during surgery.
No differences in clinical or pathological characteristics were observed according to mutational status, except for primary tumour location: all wt tumours were right-, left-sided or rectal in 14%, 59% and 27% of cases, respectively. All wt tumours showed different primary tumour location in comparison to BRAF mut tumours (right-, left-sided or rectal in 58%, 25% and 17% of cases, respectively, P<0.0001) and to RAS mut tumours (right-, left- sided or rectal in 38%, 35% and 27% of cases, respectively, P<0.0001) (Table 1).
Survival analyses
At a median follow-up of 45.6 months, 236 (76%) patients showed disease recurrence and 144 (47%) patients had died.
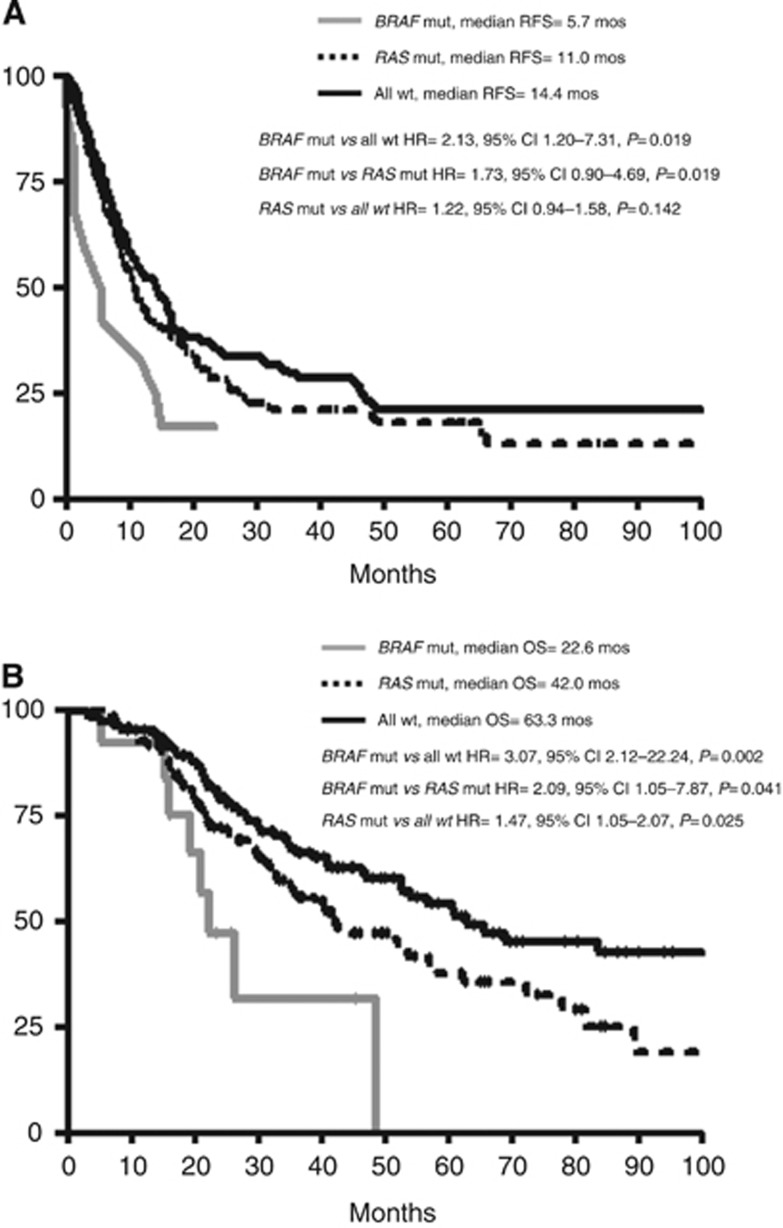
Relapse-free survival outcomes differed significantly according to mutational status (P=0.043) and median RFS were 5.7 months, 11.0 months and 14.4 months in BRAF mut, RAS mut and all wt patients, respectively. BRAF mut patients showed a significantly higher risk of relapse in comparison to all wt patients (hazard ratio (HR), 2.13; 95% CI, 1.20–7.31; P=0.019). RAS mut compared with all wt patients showed no difference in terms of RFS (HR, 1.22; 95% CI, 0.94–1.58; P=0.142) (Figure 3A). Other clinical and pathological covariates that significantly associated with inferior RFS were: presence of extra-hepatic disease (HR, 1.92; 95% CI, 1.41–4.20; P=0.001); bilobar liver involvement (HR, 1.55; 95% CI, 1.22–2.13; P=0.0009), synchronous disease (HR, 1.48; 95% CI, 1.11–1.89; P=0.006) and not R0 liver resection (HR, 2.39; 95% CI, 2.27–5.44; P<0.0001). Patients with high clinical risk score (CRS) had shorter RFS in comparison to low CRS (HR, 1.75; 95% CI, 1.35–2.35; P<0.0001) (Table 2).
Figure 3.
Relapse-free survival and overall survival according to mutational status. (A) Relapse-free survival; (B) overall survival. Mut=mutant; wt=wild-type; HR=hazard ratio; CI=confidence interval.
Table 2. Univariate analyses for relapse-free survival and overall survival.
|
Relapse-free survival |
Overall survival |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | N | Median (months) | HR | 95% CI | P | Median (months) | HR | 95% CI | P |
|
Mutational status | |||||||||
| All wt | 137 | 14.4 | 1 | — | — | 63.3 | 1 | — | — |
| BRAF mut | 12 | 5.7 | 2.13 | 1.20–7.31 | 0.019 | 22.6 | 3.07 | 2.12–22.94 | 0.002 |
| RAS mut | 160 | 11.0 | 1.22 | 0.94–1.58 | 0.142 | 42.0 | 1.47 | 1.05–2.07 | 0.025 |
|
Sex | |||||||||
| Male | 192 | 12.2 | 1 | — | — | 53.8 | 1 | — | — |
| Female | 117 | 10.7 | 0.98 | 0.75–1.28 | 0.878 | 40.7 | 1.19 | 0.85–1.68 | 0.313 |
|
Age | |||||||||
| <65 years | 177 | 11.7 | 1 | — | — | 52.8 | 1 | — | — |
| ⩾65 years | 132 | 11.4 | 1.09 | 0.84–1.41 | 0.526 | 46.6 | 1.17 | 0.84–1.64 | 0.349 |
|
ECOG PS | |||||||||
| 0 | 267 | 12.2 | 1 | — | — | 54.0 | 1 | — | — |
| 1–2 | 42 | 9.4 | 1.38 | 0.96–2.16 | 0.076 | 26.5 | 1.95 | 1.42–3.95 | 0.001 |
|
Primary tumour site | |||||||||
| Left colon | 138 | 12.0 | 1 | — | — | 57.3 | 1 | — | — |
| Right colon | 87 | 10.7 | 1.23 | 0.91–1.69 | 0.179 | 35.5 | 1.59 | 1.10–2.45 | 0.017 |
| Rectum | 82 | 12.6 | 1.04 | 0.76–1.43 | 0.794 | 61.1 | 0.95 | 0.63–1.43 | 0.804 |
|
Liver only | |||||||||
| Yes | 281 | 12.6 | 1 | — | — | 53.8 | 1 | — | — |
| No | 28 | 7.5 | 1.92 | 1.41–4.20 | 0.001 | 25.5 | 2.22 | 1.57–6.43 | 0.001 |
|
Unilobar mts | |||||||||
| Yes | 198 | 15.1 | 1 | — | — | 61.1 | 1 | — | — |
| No | 111 | 7.9 | 1.55 | 1.22–2.13 | 0.0009 | 34.8 | 1.59 | 1.16–2.33 | 0.006 |
|
Time to mts | |||||||||
| Metachronous | 101 | 15.1 | 1 | — | — | 56.8 | 1 | — | — |
| Synchronous | 208 | 10.4 | 1.48 | 1.11–1.89 | 0.006 | 48.7 | 1.30 | 0.91–1.82 | 0.158 |
|
Resection outcome | |||||||||
| R0 | 258 | 14.2 | 1 | — | — | 61.1 | 1 | — | — |
| R1/R2-Expl. Lapar. | 51 | 6.3 | 2.39 | 2.27–5.44 | <0.0001 | 21.6 | 3.21 | 3.66–10.85 | <0.0001 |
|
Primary lymph nodes | |||||||||
| No | 96 | 17.7 | 1 | — | — | 56.8 | 1 | — | — |
| Yes | 206 | 10.4 | 1.58 | 1.17–2.00 | 0.002 | 51.9 | 1.35 | 0.94–1.89 | 0.107 |
|
DFI <12 months | |||||||||
| Yes | 242 | 10.7 | 1 | — | — | 47.0 | 1 | — | — |
| No | 67 | 17.1 | 0.67 | 0.52–0.93 | 0.015 | 61.1 | 0.80 | 0.54–1.21 | 0.312 |
|
>1 liver mts | |||||||||
| No | 132 | 16.8 | 1 | — | — | 69.3 | 1 | — | — |
| Yes | 173 | 8.8 | 1.76 | 1.36–2.27 | <0.0001 | 36.0 | 1.84 | 1.30–2.49 | 0.0005 |
|
Mts diameter >5 cm | |||||||||
| No | 231 | 11.7 | 1 | — | — | 56.8 | 1 | — | — |
| Yes | 62 | 11.2 | 1.13 | 0.82–1.58 | 0.437 | 52.7 | 1.19 | 0.79–1.85 | 0.396 |
|
CEA>200 ng ml−1 | |||||||||
| No | 212 | 11.0 | 1 | — | — | 46.6 | 1 | — | — |
| Yes | 9 | 9.0 | 1.21 | 0.54–2.82 | 0.620 | 17.5 | 2.40 | 1.24–12.11 | 0.021 |
|
Clinical risk scorea | |||||||||
| Low | 138 | 16.6 | 1 | — | — | 58.6 | 1 | — | — |
| High | 137 | 8.6 | 1.75 | 1.35–2.35 | <0.0001 | 35.5 | 1.65 | 1.16–2.35 | 0.005 |
Abbreviations: CI=confidence interval; DFI=disease-free interval; Expl. Lapar.=explorative laparotomy; HR=hazard ratio; mts=metastasis; mut=mutant; N=number; PS=performance status; wt=wild-type.
Clinical risk score is defined as previously described by Fong et al (1999): patients with 0 to 2 risk features were categorised as ‘low risk', while those with 3 to 5 features as ‘high risk'. Bold entries indicate significant results.
At the RFS multivariate models, BRAF mutation retained its prognostic impact in terms of RFS compared with all wt patients (HR, 2.31; 95% CI, 1.09–4.87; P=0.029) and to RAS mut patients (HR, 2.06; 95% CI, 1.02–4.14; P=0.044) (Table 3).
Table 3. Multivariate analyses for relapse-free survival and overall survival.
| Characteristics | HR | 95% CI | P |
|---|---|---|---|
|
Relapse-free survival | |||
| BRAF mut vs all wt | |||
| Mutational status (BRAF mut vs all wt) | 2.31 | 1.09–4.87 | 0.029 |
| ECOG PS (1–2 vs 0) | 1.89 | 0.97–3.33 | 0.063 |
| Liver-only metastases (No vs Yes) | 0.78 | 0.35–1.70 | 0.528 |
| Unilobar mts (No vs Yes) | 2.11 | 1.32–3.37 | 0.002 |
| Time to mts (synchronous vs metachronous) | 1.03 | 0.56–1.88 | 0.930 |
| Resection outcome (R1/R2-Expl. Lapar. vs R0) | 2.28 | 1.27–4.07 | 0.006 |
| Clinical risk score (High vs Low) | 1.51 | 0.87–2.62 | 0.149 |
| BRAF mut vs RAS mut | |||
| Mutational status (BRAF mut vs RAS mut ) | 2.06 | 1.02–4.14 | 0.044 |
| ECOG PS (1–2 vs 0) | 0.95 | 0.58–1.55 | 0.833 |
| Liver-only metastases (No vs Yes) | 1.08 | 0.62–1.89 | 0.789 |
| Unilobar mts (No vs Yes) | 0.97 | 0.65–1.43 | 0.864 |
| Time to mts (synchronous vs metachronous) | 1.15 | 0.74–1.79 | 0.548 |
| Resection outcome (R1/R2-Expl. Lapar. vs R0) | 3.22 | 2.05–5.06 | <0.0001 |
| Clinical risk score (High vs Low) | 1.53 | 1.01–2.33 | 0.046 |
|
Overall survival | |||
| BRAF mut vs all wt | |||
| Mutational status (BRAF mut vs all wt ) | 2.76 | 1.12–6.81 | 0.029 |
| ECOG PS (1–2 vs 0) | 2.81 | 1.37–5.78 | 0.005 |
| Tumour site (Right vs Left and Rectum) | 1.25 | 0.60–2.59 | 0.549 |
| Liver-only metastases (No vs Yes) | 1.40 | 0.60–3.28 | 0.437 |
| Unilobar mts (No vs Yes) | 2.19 | 1.17–4.08 | 0.014 |
| Resection outcome (R1/R2-Expl. Lapar. vs R0) | 4.54 | 2.32–8.89 | <0.0001 |
| Clinical risk score (High vs Low) | 0.98 | 0.55–1.57 | 0.952 |
| BRAF mut vs RAS mut | |||
| Mutational status (BRAF mut vs RAS mut ) | 2.73 | 1.25–5.92 | 0.012 |
| ECOG PS (1–2 vs 0) | 1.07 | 0.59–1.93 | 0.833 |
| Tumour site (right vs left and rectum) | 1.22 | 0.76–1.95 | 0.415 |
| Liver-only metastases (No vs Yes) | 0.95 | 0.45–2.03 | 0.903 |
| Unilobar mts (No vs Yes) | 1.13 | 0.69–1.85 | 0.618 |
| Resection outcome (R1/R2-Expl. Lapar. vs R0) | 3.98 | 2.34–6.76 | <0.0001 |
| Clinical risk score (High vs Low) | 2.17 | 1..33–3.54 | 0.002 |
| RAS mut vs all wt | |||
| Mutational status (RAS mut vs all wt ) | 1.08 | 0.73–1.59 | 0.712 |
| ECOG PS (1–2 vs 0) | 1.79 | 1.12–2.84 | 0.015 |
| Tumour site (right vs left and rectum) | 1.34 | 0.89–2.02 | 0.165 |
| Liver-only metastases (No vs Yes) | 1.68 | 0.95–2.98 | 0.078 |
| Unilobar mts (No vs Yes) | 1.37 | 0.93–2.04 | 0.115 |
| Resection outcome (R1/R2-Expl. Lapar. vs R0) | 3.24 | 2.07–5.08 | <0.0001 |
| Clinical risk score (High vs Low) | 1.50 | 0.10–2.24 | 0.053 |
Abbreviations: HR=hazard ratio; CI=confidence interval; mut=mutant; wt=wild-type; PS=performance status; mts=metastasis; Expl. Lapar.=explorative laparotomy. Bold entries indicate significant results.
Overall survival outcomes differed significantly according to mutational status (P=0.003), and median OS were 22.6, 42.0 and 63.3 months in BRAF mut, RAS mut and all wt patients, respectively. BRAF mut patients showed a significantly higher risk of death in comparison to all wt patients (HR, 3.07; 95% CI, 2.12–22.94; P=0.002) and to RAS mut patients (HR, 2.09; 95% CI, 1.05–7.87; P=0.041). A significant difference was also observed comparing RAS mut and all wt patients (HR, 1.47; 95% CI, 1.05–2.07; P=0.025) (Figure 3B). Other covariates associated with inferior OS were ECOG PS>0 (HR, 1.95; 95% CI, 1.42–3.95; P=0.001); extra-hepatic disease (HR, 2.22; 95% CI, 1.57–6.43; P=0.001); bilobar liver metastases (HR, 1.59; 95% CI, 1.16–2.33; P=0.006), right-sided primary tumour (HR, 1.59; 95% CI, 1.10–2.45; P=0.017) and not R0 liver resection (HR, 3.21; 95% CI, 3.66–10.85; P<0.0001). Patients with high CRS showed worse OS compared with low CRS (HR, 1.65; 95% CI, 1.16–2.35; P=0.005) (Table 2).
At the OS multivariate model, BRAF mutation was independently associated with worse outcome compared with all wt patients (HR, 2.76; 95% CI, 1.12–6.81; P=0.029) and with RAS mut patients (HR, 2.73; 95% CI, 1.25–5.92; P=0.012). RAS mutation lost its association with worse OS (HR, 1.08; 95% CI, 0.73–1.59; P=0.712) (Table 3).
Recursive partitioning analyses showed that not R0 liver resection was the most important factor in the prediction of RFS and OS. Other characteristics affecting RFS and OS were time from date of metastatic disease diagnosis to liver resection, age, bilobar liver metastases (for RFS only) and primary nodal involvement (for OS only) (Supplementary Figure 1).
Sites of first relapse
At the time of analyses, relapsed BRAF mut, RAS mut and all wt patients were 10 (83%), 127 (79%) and 99 (72%), respectively. Liver-only relapse was not associated with mutational status and was observed in 60, 43 and 49% of patients in the three groups (P=0.45). No differences were observed in terms of peritoneal (P=0.89), nodal (P=0.10) and liver relapse (P=0.61). Lung relapse was more frequently observed in RAS mut (35%) patients in comparison to all wt (21%) and BRAF mut (0%) patients (P=0.008; all wt vs RAS mut P=0.027; RAS mut vs BRAF mut P=0.030) (Supplementary Table 4).
MSI status and BRAF mutation
All BRAF mut cases were analysed for MSI status. Two cases resulted MSI-H and 1 MSI-L. Interestingly, of the 2 BRAF mut patients free of relapse at the time of the analyses 1 had a MSI-H tumour and the other a MSI-L tumour; on the other hand, 9 out of 10 relapsed patients had a MSS tumour (P=0.046).
Discussion
Extensive molecular characterisation of CRC has gained more and more importance both with predictive and prognostic intent. In the present work, starting from the revision of 3024 medical records of mCRC patients, after a careful clinical selection, we identified 360 eligible patients and collected as much data as possible on markers potentially affecting prognosis after liver resection. Finally, we performed a comprehensive RAS and BRAF molecular characterisation that lead us to identify a final study population of 309 cases.
The major and clinically relevant finding is that BRAF mutation emerges as an independent and strong negative prognostic factor also in this specific setting. BRAF mut patients had an extremely poor median RFS of 5.7 months and a significantly higher risk of relapse, as compared with both RAS mut (HR, 2.06; P=0.044) and all wt patients (HR, 2.31; P=0.029).
Other studies tried to address the same issue, but as admitted by their authors, were limited in sample size to catch the independent prognostic effect of BRAF status (Stremitzer et al, 2012; Karagkounis et al, 2013; Kemeny et al, 2013; Umeda et al, 2013; Vauthey and Kopetz, 2013; Vauthey et al, 2013). A previous study showed a significantly shorter OS for BRAF mut patients undergoing liver resection, but no data on RFS were available, while two out of six BRAF mut patients had a mutation different from the V600E, thus limiting possible conclusions (Teng et al, 2012). A recent retrospective analysis conducted at Memorial Sloan Kettering Cancer Centre on the prognostic impact of BRAF mutation in mCRC, confirmed a shorter OS for BRAF mutant patients in the subgroup undergoing resection of metastases with radical intent (Yaeger et al, 2014).
All previous experiences, as well as ours, are in line in reporting a very low incidence of BRAF mutation in patients undergoing liver resection, ranging from 2 to 4%. These data find a possible explanation in the specific clinical features and the peculiar metastatic spread usually observed in BRAF mut patients, rarely presenting with liver limited metastatic disease and just in a few cases achieve favourable clinical conditions leading to consider a radical liver resection. The low mutation rate of BRAF in this setting dilutes the clinical impact of its prognostic value possibly raising some concerns about the cost-effectiveness of its routinary use, but the implications and consequences at the ‘single-patient' level could be extremely relevant.
The high rate of nodal relapse observed in our series, although not reaching the statistical significance, possibly due to the small number of BRAF mut patients, might allow to assume that BRAF mut patients could more frequently recur in a shorter time and in extra-hepatic locations due to the presence of occult micrometastatic disease. As a consequence, an intensive preoperative work-up in BRAF mut patients, potentially candidates for liver resection, could be proposed. In particular, MRI with liver-specific contrast, ultrasound scans with contrast medium and PET-CT have recently been shown to have a higher sensitivity in comparison to CT scan (Schmidt et al, 2009).
Whether the prognostic impact of BRAF mutation is independent or not from MSI status, a condition to which it is significantly associated, is still a matter of debate, also because of the extremely low frequency of the concomitant presence of these features in the metastatic setting (Goldstein et al, 2014). An interesting finding coming out from our experience is that both the 2 BRAF mutant patients free of relapse at the time of the analyses (16.3 months and 23.6 months after resection, respectively) were not MSS.
The prognostic role of RAS mutations is not confirmed in our multivariate models and this is apparently inconsistent with results by other groups. However, some explanations can be hypothesised: first of all, different inclusion criteria were adopted and this is reflected also by the relatively higher incidence of RAS mutations in our patients; second, available data come from major surgical referral centres, while our patients' selection moved from oncologic units, thus leading to a slightly different study population; third, different covariates were included in the multivariable models as a result of different selection rules for these variables. As compared with the experience by Karagkounis et al (2013) our results at the univariate analyses are very similar and not statistically significant in terms of RFS. Our pre-specified analytical criteria did not allow variables with statistical significance ⩾0.1 to enter the multivariate model. As a consequence, the models differed and this may have affected the results. Similar constraints apply to the comparison with data by Vauthey et al (2013) that were reported as 3-year survival rates and again included different variables in the multivariate models.
Interestingly, our results confirm the association of RAS mutations with an higher risk of lung relapse, as previously reported by Kemeny et al (2013) These data enforce the role of an adequate thoracic staging in preoperative work-up as well as the mandatory inclusion of intensive chest follow-up of RAS mut patients after liver resection (Maithel et al, 2010).
No specific analyses were carried out on the basis of received treatment due to the wide heterogeneity of received treatments and to the specific objective of the present study.
At recursive partitioning analyses, traditional clinico-pathological prognostic factors (such as resection margins, time to resection, extension of liver involvement, age and primary tumour nodal involvement) emerged as primary determinants (Supplementary Figure 1). This underlines the importance of coupling old and new markers to optimise future prognostication skills and clinical decision making.
Taken together, all the available data support the implementation of molecular testing in defining the risk of relapse of candidates to curative liver resection. Although data on BRAF refer to a rather small group of patients, their significance is relevant to balance pros and contra of the indication for a major surgery, with a non-negligible risk of post-operative morbidity and mortality, high costs and a great clinical commitment for patients and for health care facilities. Ultimately, many innovative therapeutic strategies are under investigation for targeting RAS and BRAF mutant CRCs. The extensive knowledge of their clinical behaviour might be crucial for the development of new therapeutic approaches in specific settings, such as the perioperative and adjuvant treatment of liver limited disease.
Acknowledgments
A special acknowledgement goes to the effort made by Mrs Alessandra Pasqualini and her friends in loving memory of her mother Mrs Donatella Servolini. This work was supported by ARCO No-profit Foundation.
The authors have declared no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- National Comprehensive Cancer Network. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf .
- Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26 (10:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, Young J, Walsh T, Ward R, Hawkins N, Beranek M, Jandik P, Benamouzig R, Jullian E, Laurent-Puig P, Olschwang S, Muller O, Hoffmann I, Rabes HM, Zietz C, Troungos C, Valavanis C, Yuen ST, Ho JW, Croke CT, O'Donoghue DP, Giaretti W, Rapallo A, Russo A, Bazan V, Tanaka M, Omura K, Azuma T, Ohkusa T, Fujimori T, Ono Y, Pauly M, Faber C, Glaesener R, de Goeij AF, Arends JW, Andersen SN, Lovig T, Breivik J, Gaudernack G, Clausen OP, De Angelis PD, Meling GI, Rognum TO, Smith R, Goh HS, Font A, Rosell R, Sun XF, Zhang H, Benhattar J, Losi L, Lee JQ, Wang ST, Clarke PA, Bell S, Quirke P, Bubb VJ, Piris J, Cruickshank NR, Morton D, Fox JC, Al-Mulla F, Lees N, Hall CN, Snary D, Wilkinson K, Dillon D, Costa J, Pricolo VE, Finkelstein SD, Thebo JS, Senagore AJ, Halter SA, Wadler S, Malik S, Krtolica K, Urosevic N. Kirsten ras mutations in patients with colorectal cancer: the 'RASCAL II' study. Br J Cancer. 2001;85 (5:692–696. doi: 10.1054/bjoc.2001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejas P, Lopez-Gomez M, Aguayo C, Madero R, de Castro Carpeno J, Belda-Iniesta C, Barriuso J, Moreno Garcia V, Larrauri J, Lopez R, Casado E, Gonzalez-Baron M, Feliu J. KRAS mutations in primary colorectal cancer tumors and related metastases: a potential role in prediction of lung metastasis. PLoS One. 2009;4 (12:e8199. doi: 10.1371/journal.pone.0008199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250 (3:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocakova I, Ruff P, Blasinska-Morawiec M, Smakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369 (11:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH.1999Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases Ann Surg 230(3309–318.discussion 318-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J, Tran B, Ensor J, Gibbs P, Wong HL, Wong SF, Vilar E, Tie J, Broaddus R, Kopetz S, Desai J, Overman M. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H) Ann Oncol. 2014;25 (5:1032–1038. doi: 10.1093/annonc/mdu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Inoue Y, Komeda K, Shimizu T, Asakuma M, Hirokawa F, Miyamoto Y, Okuda J, Takeshita A, Shibayama Y, Tanigawa N. Clinicopathological analysis of recurrence patterns and prognostic factors for survival after hepatectomy for colorectal liver metastasis. BMC Surg. 2010;10:27. doi: 10.1186/1471-2482-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagkounis G, Torbenson MS, Daniel HD, Azad NS, Diaz LA, Jr, Donehower RC, Hirose K, Ahuja N, Pawlik TM, Choti MA. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. 2013;119 (23:4137–4144. doi: 10.1002/cncr.28347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359 (17:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- Kemeny N, Chou J, Capanu M, Gewirtz A, Cercek A, Kingham P, Jarnagin WR, Fong Y, DeMatteo RP, Allen PJ, Shia J, Ang C, Vakiani E, D'Angelica M. Association of KRAS mutation with worse recurrence-free survival and site of metastatic progression after resection of hepatic colorectal metastases. J Clin Oncol. 2013;31 (suppl; abstr 3609 [Google Scholar]
- Kim MJ, Lee HS, Kim JH, Kim YJ, Kwon JH, Lee JO, Bang SM, Park KU, Kim DW, Kang SB, Kim JS, Lee JS, Lee KW. Different metastatic pattern according to the KRAS mutational status and site-specific discordance of KRAS status in patients with colorectal cancer. BMC Cancer. 2012;12:347. doi: 10.1186/1471-2407-12-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM, McWilliams RR. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27 (22:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M, Rougier P, Penault-Llorca F, Laurent-Puig P. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66 (8:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- Maithel SK, Ginsberg MS, D'Amico F, DeMatteo RP, Allen PJ, Fong Y, Blumgart LH, Jarnagin WR, D'Angelica MI. Natural history of patients with subcentimeter pulmonary nodules undergoing hepatic resection for metastatic colorectal cancer. J Am Coll Surg. 2010;210 (1:31–38. doi: 10.1016/j.jamcollsurg.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris VK, Lucas FA, Overman MJ, Eng C, Morelli MP, Jiang ZQ, Luthra R, Meric-Bernstam F, Maru D, Scheet P, Kopetz S, Vilar E. Clinicopathologic characteristics and gene expression analyses of non-KRAS 12/13, RAS-mutated metastatic colorectal cancer. Ann Oncol. 2014;25 (10:2008–2014. doi: 10.1093/annonc/mdu252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77 (7:1254–1262. [PubMed] [Google Scholar]
- Peeters M, Oliner KS, Parker A, Siena S, Van Cutsem E, Huang J, Humblet Y, Van Laethem JL, Andre T, Wiezorek J, Reese D, Patterson SD. Massively parallel tumor multigene sequencing to evaluate response to panitumumab in a randomized phase III study of metastatic colorectal cancer. Clin Cancer Res. 2013;19 (7:1902–1912. doi: 10.1158/1078-0432.CCR-12-1913. [DOI] [PubMed] [Google Scholar]
- Primrose JN. Surgery for colorectal liver metastases. Br J Cancer. 2010;102 (9:1313–1318. doi: 10.1038/sj.bjc.6605659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247 (1:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- Richman SD, Seymour MT, Chambers P, Elliott F, Daly CL, Meade AM, Taylor G, Barrett JH, Quirke P. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27 (35:5931–5937. doi: 10.1200/JCO.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- Saridaki Z, Papadatos-Pastos D, Tzardi M, Mavroudis D, Bairaktari E, Arvanity H, Stathopoulos E, Georgoulias V, Souglakos J. BRAF mutations, microsatellite instability status and cyclin D1 expression predict metastatic colorectal patients' outcome. Br J Cancer. 2010;102 (12:1762–1768. doi: 10.1038/sj.bjc.6605694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirripa M, Cremolini C, Loupakis F, Morvillo M, Bergamo F, Zoratto F, Salvatore L, Antoniotti C, Marmorino F, Sensi E, Lupi C, Fontanini G, Gregorio VD, Giannini R, Basolo F, Masi G, Falcone A. Role of NRAS mutations as prognostic and predictive markers in metastatic colorectal cancer. Int J Cancer. 2014;136 (1:83–90. doi: 10.1002/ijc.28955. [DOI] [PubMed] [Google Scholar]
- Schmidt GP, Baur-Melnyk A, Haug A, Utzschneider S, Becker CR, Tiling R, Reiser MF, Hermann KA. Whole-body MRI at 1.5T and 3T compared with FDG-PET-CT for the detection of tumour recurrence in patients with colorectal cancer. Eur Radiol. 2009;19 (6:1366–1378. doi: 10.1007/s00330-008-1289-y. [DOI] [PubMed] [Google Scholar]
- Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, Ciardiello F, Hoff P, Kerr D, Kohne CH, Labianca R, Price T, Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R, Jordan K, Meshcheryakov A, Papamichail D, Pfeiffer P, Souglakos I, Turhal S, Cervantes A. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23 (10:2479–2516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- Stremitzer S, Stift J, Gruenberger B, Tamandl D, Aschacher T, Wolf B, Wrba F, Gruenberger T. KRAS status and outcome of liver resection after neoadjuvant chemotherapy including bevacizumab. Br J Surg. 2012;99 (11:1575–1582. doi: 10.1002/bjs.8909. [DOI] [PubMed] [Google Scholar]
- Teng HW, Huang YC, Lin JK, Chen WS, Lin TC, Jiang JK, Yen CC, Li AF, Wang HW, Chang SC, Lan YT, Lin CC, Wang HS, Yang SH. BRAF mutation is a prognostic biomarker for colorectal liver metastasectomy. J Surg Oncol. 2012;106 (2:123–129. doi: 10.1002/jso.23063. [DOI] [PubMed] [Google Scholar]
- Tie J, Lipton L, Desai J, Gibbs P, Jorissen RN, Christie M, Drummond KJ, Thomson BN, Usatoff V, Evans PM, Pick AW, Knight S, Carne PW, Berry R, Polglase A, McMurrick P, Zhao Q, Busam D, Strausberg RL, Domingo E, Tomlinson IP, Midgley R, Kerr D, Sieber OM. KRAS mutation is associated with lung metastasis in patients with curatively resected colorectal cancer. Clin Cancer Res. 2011;17 (5:1122–1130. doi: 10.1158/1078-0432.CCR-10-1720. [DOI] [PubMed] [Google Scholar]
- Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D'Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25 (29:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, Agarwal A, Maru DM, Sieber O, Desai J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117 (20:4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda Y, Nagasaka T, Mori Y, Sadamori H, Sun DS, Shinoura S, Yoshida R, Satoh D, Nobuoka D, Utsumi M, Yoshida K, Yagi T, Fujiwara T. Poor prognosis of KRAS or BRAF mutant colorectal liver metastasis without microsatellite instability. J Hepatobiliary Pancreat Sci. 2013;20 (2:223–233. doi: 10.1007/s00534-012-0531-9. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29 (15:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- Vauthey JN, Kopetz SE. From multidisciplinary to personalized treatment of colorectal liver metastases: 4 reasons to consider RAS. Cancer. 2013;119 (23:4083–4085. doi: 10.1002/cncr.28348. [DOI] [PubMed] [Google Scholar]
- Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou A, Curley SA, Aloia TA, Maru DM.2013RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases Ann Surg 258(4619–626.discussion 626-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaeger R, Cercek A, Chou JF, Sylvester BE, Kemeny NE, Hechtman JF, Ladanyi M, Rosen N, Weiser MR, Capanu M, Solit DB, D'Angelica MI, Vakiani E, Saltz LB. BRAF mutation predicts for poor outcomes after metastasectomy in patients with metastatic colorectal cancer. Cancer. 2014;120 (15:2316–2324. doi: 10.1002/cncr.28729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Ura T, Shibata N, Takahari D, Shitara K, Nomura M, Kondo C, Mizota A, Utsunomiya S, Muro K, Yatabe Y. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer. 2011;104 (5:856–862. doi: 10.1038/bjc.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.