Abstract
Processing speed is considered a key cognitive resource and it has a crucial role in all types of cognitive performance. Some researchers have hypothesised the importance of white matter integrity in the brain for processing speed; however, the relationship at the whole-brain level between white matter volume (WMV) and processing speed relevant to the modality or problem used in the task has never been clearly evaluated in healthy people. In this study, we used various tests of processing speed and Voxel-Based Morphometry (VBM) analyses, it is involves a voxel-wise comparison of the local volume of gray and white, to assess the relationship between processing speed and regional WMV (rWMV). We examined the association between processing speed and WMV in 887 healthy young adults (504 men and 383 women; mean age, 20.7 years, SD, 1.85). We performed three different multiple regression analyses: we evaluated rWMV associated with individual differences in the simple processing speed task, word–colour and colour–word tasks (processing speed tasks with words) and the simple arithmetic task, after adjusting for age and sex. The results showed a positive relationship at the whole-brain level between rWMV and processing speed performance. In contrast, the processing speed performance did not correlate with rWMV in any of the regions examined. Our results support the idea that WMV is associated globally with processing speed performance regardless of the type of processing speed task.
Introduction
Processing speed is an individual cognitive ability measured by how fast an individual executes cognitive tasks, particularly elementary cognitive tasks [1], [2]. Moreover, processing speed is viewed as an overall measure of cognitive mechanisms that are widely used to support fluent execution of perceptual, cognitive and motor processes [3]. Indeed, processing speed is considered a key cognitive resource [4], [5], similar to attention, working memory and inhibition, underlying performance in various cognitive domains [2], [6], [7]. Accordingly, previous research showed that processing speed correlates with performance in various cognitive domains [4], [5], [8], [9], [10].
From the perspective of neuroscience, processing speed performance has traditionally been assumed to depend to a large extent on the properties of white matter [11], [12], [13]; the latter affects the speed of neural transmission. In fact, white matter includes all myelinated axons in the cerebrum, and the thickness of the myelin sheath is related to nerve conduction velocity; therefore, its relation to processing speed seems logical [11][14].
Previous neuroimaging studies have been focused on the two major structural properties of white matter: fractional anisotropy (FA), which reflects structural integrity of white matter[15], [16], in diffusion tensor imaging [5], [17], [18] and white matter volume (WMV) [11], [19], [20].
Previous studies of fractional anisotropy indicate that processing speed performance correlates with properties of regional white matter in brain areas relevant to the task. Processing speed is associated with visual choice reaction time in visual-related areas (in the number of posterior regions of the right hemisphere), the left middle frontal gyrus and the occipital and parietal areas during the digit–symbol task (fronto–parieto–occipital areas perform important functions in the execution of this task) [18], [21]. Moreover, the temporal lobe is associated with processing speed in the auditory reaction time task [22]. Conversely, there are studies that show a global association between processing speed tasks and white matter structure [17]; in fact, processing speed is related to white matter average FA of the whole brain [23].
Other studies indicate that processing speed performance correlates with total WMV (tWMV); in fact, processing speed is genetically related to tWMV[11]. There is also a relationship between reduced tWMV and impaired processing speed performance in patients with temporal lobe epilepsy [24].
Many studies have been focused on correlations between white matter integrity and processing speed in various tasks using diffusion tensor imaging [17], [18], [25], [26], [27]. Usually, studies on WMV involve specific populations, such as old adults [17–27], or specific diseases, such as temporal lobe epilepsy [24] or left-hemisphere stroke [5]. Additionally, previous anatomical studies did not verify the significance of regional WMV (rWMV), and this is one of the purposes of this study. To the best of our knowledge, no previous studies have observed a relationship between WMV and processing speed in a large sample of healthy young people. This study aimed to identify the rWMV correlates of processing speed in healthy young people. As we previously discussed [28], we consider that 1) FA and rWMV are moderately to weakly related, 2) the associations between them seem particularly weak in deep white matter areas [29] and 3) rWMV is known to significantly correlate with cognitive functions. Particularly, our previous studies that concurrently explored rWMV and FA correlates of cognitive functions showed more significant results of rWMV analyses in regions congruent with our hypothesis [30], [31]. In this study, we focus on rWMV correlates of processing speed using various tests of processing speed and Voxel-Based Morphometry (VBM) analyses that involves a voxel-wise comparison of the local volume of gray and white matter.
Based on previous studies, we are evaluating two sets of relatively opposite assumptions: 1) processing speed correlates with white matter structure globally; therefore it is rather problem independent and 2) processing speed correlates with white matter structure relevant to the modality or problem used in the task. Considering the contribution of processing speed to human cognitive activity, it is important to investigate the rWMV correlates with processing speed tasks in healthy adults compared with other neuroimaging methods. In fact, rWMV is widely accepted as the basis of individual intellectual abilities; the networks that underlie intellectual abilities can be identified by measuring WMV [32], [33], [34].
Materials and Methods
Subjects
Eight hundred eighty-seven healthy right-handed individuals (504 men and 383 women; mean age, 20.7 years, SD, 1.85 years) participated in this study as part of our ongoing project to explore the associations among brain imaging, cognitive functions, ageing, genetics and daily habits. All subjects were college students from Tohoku University in Japan. All subjects were undergraduate or postgraduate university students. All had normal vision and none had a history of neurological or psychiatric illness. None reported recent use of any psychoactive drugs or drugs that would be likely to negatively impact their cognitive abilities. The history of psychiatric illnesses and recent drug use were assessed using our laboratory’s routine questionnaire, in which each subject answered questions related to their current or previous experience with any of a list of illnesses and listed drugs they had taken recently. Handedness was evaluated using the Edinburgh Handedness Inventory [35]. The Ethics Committee of Tohoku University approved all procedures. Written informed consent was obtained from each subject for the projects in which they participated.
Psychological tests
For the measurement of processing speed we used three tasks. Moreover, we measured general intelligence and verbal and spatial working memory of the participant, to verified the relationship between processing speed and more complicate cognitive tasks. All psychological tests were performed in the same day, with 15 minutes break between each tests; after another hour of rest, we performed the MRI scan.
Simple processing speed tasks
The Tanaka B-type intelligence test (TBIT) [36] type 3B was used for the measurement of processing speed. TBIT is a simple test for measuring simple processing speed. In all subtests, the subjects had to solve as many problems as possible within a certain period (a few minutes). This factor involved three subtests: a displacement task [substitute a figure (nine figures) with a number (1–9) according to a model chart], identification versus same–different judgments (Japanese kana characters; decide whether a pair of meaningless Japanese strings are the same) and marking of figures [select shapes identical to three samples from a series (sequence) of eight different shapes]. These tasks do not require recognition of words, and instead require recognition of symbols, letters, numbers and the like. These tasks do not involve complex cognitive processes but constitute simple processing speed tasks.
Word–colour and colour–word tasks (processing speed tasks with words)
The Stroop [37] task is a widely used paradigm in psychology and clinical practice [38]. During Stroop paradigms, subjects experience cognitive interference when they resolve interferences, for example, identifying the ink colour of a printed word while ignoring the word’s identity [37]. As in a previous study [39], [40], we used Hakoda’s version of the Stroop task [41]. This version of the Stroop task is of the matching type, requiring subjects to choose and check correct answers as possible from five options in 1 min, unlike the traditional oral naming task. This type of task is rather similar to the button-pressing matching-type Stroop tasks used in neuroimaging studies [42]. The task consists of two control tasks, a word–colour task and a colour–word task and a reverse Stroop task and a Stroop task. In this study, we focused on processing speed and used the normalised sum of the word–colour task and the colour–word task. These tasks require recognition of words.
Simple speed tasks
Our simple arithmetic task is similar to that constructed by Grabner et al. [43]. This task measures multiplication performance consisting of two forms of one-digit times one-digit multiplication problems (a simple arithmetic task with numbers between 2 and 9). The two forms of each task are the same, but the numbers used in the problems are different. Each form of the simple arithmetic task is presented with a 30-s time limit. The average of the performance on two forms was used. This task requires simple arithmetic calculations.
Raven's Advanced Progressive Matrix
Raven's Advanced Progressive Matrix (RAPM) [44], which is a psychometric measure of general intelligence [44], was used to assess general intelligence. The test contains 36 nonverbal items requiring fluid reasoning ability. Each item consists of a 3×3 matrix with a missing piece to be completed by selecting the best of 8 alternatives. The score of this test (number of correct answers in 30 min) was used as a psychometric index of individual intelligence. The RAPM was administered in a group setting in this study. The RAPM tests can be administered individually by a psychologist or trained test administrator, or administered on a group basis (Raven, 1998).
Verbal working memory task
Computerized forward and backward digit span tests were used to assess verbal WMC, as in our previous study [45]. Subjects were asked to view a progressively increasing number of random digits visually presented one-digit per second on a computer screen. They were then asked to repeat the sequence by pressing numbered buttons on the screen in the presented order (digit-span forward) or in the reverse order (digit-span backward), starting from two digits. Three sequences were given at each level, until the participants responded incorrectly to all three sequences, at which point the task was ended. The score of each test is equal to the sum of the number of digits correctly repeated in the digit span forward and digit span backward tasks.
Visuospatial working memory task
A (computerized) visuospatial WM task [46]. In the visuospatial WM task, circles were presented one by one at a rate of 1/s in a four-by-four grid-like interface. Participants had to remember the location and order of the stimuli. After the presentation of stimuli, participants indicated the location and order of the presented stimuli by clicking the grid-like interface on a computer screen with a mouse in the stimuli’s presented order (forward visuospatial WM task) or in the reverse order (backward visuospatialWMtask).
The number of items to be remembered started with two items and progressively increased. Three sequences were given at each level, until the participants responded incorrectly to all three sequences, at which point the task was ended. The score of each test was equal to the sum of the number of items correctly repeated in both the forward visuospatial WM task and the backward visuospatial WM task.
Image acquisition
All MRI data were acquired using a 3-T Philips Intera Achieva scanner (Philips Medical Systems, Best, The Netherlands). High-resolution T1-weighted structural images (240 × 240 matrix; repetition time, 6.5 ms; echo time, 3 ms; field of view, 24 cm; 162 slices; slice thickness, 1.0 mm) were collected using a magnetisation-prepared rapid gradient echo (MPRAGE) sequence [47], [48].
Pre-processing of structural data
Pre-processing of structural data was performed using the Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (Mathworks Inc., Natick, MA, USA). Using the new segmentation algorithm implemented in SPM8, T1-weighted structural images of each individual were segmented into six tissues. In this process, the grey matter tissue probability map (TPM) was manipulated using maps implemented in the software so that the signal intensity of voxels with (grey matter tissue probability of the default tissue grey matter TPM + white matter tissue probability of the default TPM) > 0.25 became 0. When this adjusted grey matter TPM is used, the dura matter is less likely to be classified as grey matter (compared with when the default grey matter TPM is used) without other substantial segmentation problems. In this new segmentation process, default parameters were used, except that affine regularisation was performed with the International Consortium for Brain Mapping template for East Asian brains. We then proceeded to the diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) registration process implemented in SPM8. In this process, we used DARTEL import of images of the five grey matter TPMs from the abovementioned new segmentation process. First, the template for the DARTEL procedures was created using imaging data from 63 subjects who participated in an experiment in our laboratory [46]. Using this existing template, the DARTEL procedures were performed for all of the subjects in the present study. In these procedures, default parameter settings were used. The resulting images were spatially normalised to the Montreal Neurological Institute (MNI) space to produce images with 1.5 × 1.5 × 1.5 mm3 voxels. Additionally, we performed volume change correction (modulation) by modulating each voxel with the Jacobian determinants derived from spatial normalisation, which allowed us to determine regional differences in the absolute amount of brain tissue [49]. Then, all images were smoothed by convolving them with an isotropic Gaussian kernel of 8 mm full width at half maximum for the reasons described below.
Statistical analysis
The behavioural data were analysed using the statistical software SPSS 20.0 (SPSS Inc., Chicago, IL, USA). Associations between psychological variables were analysed using Pearson’s correlation analysis. Moreover, we performed three different multiple regression analyses (non-voxelwise analyses) in which the dependent variable was tWMV and age, sex and the performance on each cognitive test were independent variables.
First, we assessed rWMV associated with individual differences in simple processing speed task, word–colour and colour–word tasks (processing speed tasks with words) and simple arithmetic task. Statistical analyses of morphological data were performed using the VBM8 software, an extension of SPM8.
In the analyses, we included only voxels that showed rWMV > 0.1 in all subjects. The primary purpose for using white matter thresholds was to cut the periphery of the white matter areas and effectively limit the areas for analyses. We performed this procedure by limiting the areas for analyses to those likely to be white matter. The voxels outside the brain areas are more likely to be affected by signals outside the brain through smoothing. Masking the analysis to brain areas was performed in fMRI analyses of SPM8 by default.
With the whole brain data, we performed three separate multiple regressions for regression analyses to test the relationship between the following: a) simple processing speed and rWMV, b) processing speed tasks with words and rWMV and c) simple arithmetic speed and rWMV. The analyses were performed with sex and age as additional covariates. According to our hypothesis, we performed two analyses: First, we did not use tWMV as a covariate because we were wanted to test whether processing speed globally (non-specifically) correlates with WMV. Second, we analysed the data using tWMV as a covariate to evaluate regional differences in the relationship of WMV with processing speed.
The voxel level threshold was set to P = 0.05 [corrected for false discovery rate (FDR)]. Multiple comparison correction was performed using the FDR approach [50]. FDR-based methods have been shown to be more powerful and sensitive than other available approaches to multiple statistical testing [50–51]).
Finally, anatomical labelling of significant areas was performed using the ICBM-DTI-81 Atlas [52].
We analysed data only for the participants who completed the tasks. This amounted to the data from 831, 887 and 883 participants for the simple processing speed task, simple arithmetic task and Stroop task, respectively.
Results
Mean (M) score and SD of behavioural data are shown in Table 1. The results of correlation analysis are also shown in Table 1. The processing speed task significantly and positively correlated with the simple arithmetic task (P = 0.0001, r = 0.360), the Stroop word–colour task (P < 0.0001, r = 0.515), the Stroop colour–word task (P < 0.0001, r = 0.578), the RAPM (P < 0.0001, r = 0.340), the Verbal working memory task (P < 0.001, r = 0.242) and Visuospatial working memory task (P < 0.0001, r = 0.332). The simple arithmetic speed task significantly and positively correlated with the Stroop word–colour task (P < 0.0001, r = 0.457),Stroop colour–word task (P < 0.0001, r = 0.384), the Verbal working memory task (P < 0.001, r = 0.203) and the Visuospatial working memory task (P < 0.001, r = 0.137). The simple arithmetic speed task did not correlate with the RAPM (P = 0.893, r = 0.005).
Table 1. Pearson's correlation among Processing speed task, Simple arithmetic task, Stroop Word–Colour task and Stroop Colour–Word task, and means (M) and standard deviations (SD).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Processing speed task | — | ||||||
| 2. Simple arithmetic task | .360*** | — | |||||
| 3. Stroop Word–Colour task | .515*** | .457*** | — | ||||
| 4. Stroop Colour–Word task | .578*** | .384*** | .620*** | — | |||
| 5. Raven's Advanced Progressive Matrix | .340*** | .005 | .162** | .204** | — | ||
| 6. Verbal working memory task | .242** | .203** | .208** | .227** | .293** | — | |
| 7. Visuospatial working memory task | .332*** | .137** | .172** | .169** | .363*** | .372*** | — |
| M | 49.3 | 31.4 | 70.7 | 52.3 | 28.1 | 35.7 | 28.3 |
| SD | 7.1 | 5.3 | 7.4 | 6.7 | 4.9 | 7.1 | 4.4 |
**p < .001
***p < .0001
The Stroop word–colour task significantly and positively correlated with the Stroop colour–word task (P < 0.0001, r = 0.620; Table 1), the RAPM (P < 0.001, r = 0.162), the Verbal working memory task (P < 0.001, r = 0.208) and the Visuospatial working memory task (P < 0.001, r = 0.172). The Stroop Colour–Word task significantly and positively correlated with the RAPM (P < 0.001, r = 0.204), the Verbal working memory task (P < 0.001, r = 0.227) and the Visuospatial working memory task (P < 0.001, r = 0.169). Raven's Advanced Progressive Matrix (RAPM) significantly and positively correlated with the Verbal working memory task (P < 0.001, r = 0.293) and the Visuospatial working memory task (P < 0.0001, r = 0.363). Verbal working memory task significantly and positively correlated with Visuospatial working memory task (P < 0.0001, r = 0.372)Regarding the three multiple regression analyses (non-voxelwise analyses), we verified the relationship between the predictors (cognitive task performance) and the outcome (tWMV), and the relationship was positive and significant (simple processing speed: β = 0.093, P < 0.001, R2 = 0.358; Stroop task: β = 0.098, P < 0.0001, R2 = 0.356; simple arithmetic speed: β = 0.089, P < 0.001, R2 = 0.355).
Correlation of rWMV and the processing speed task
After adjusting for age and sex, multiple regression analysis (FDR correction, P = 0.05) revealed a positive and significant correlation between performance on the simple processing speed task and rWMV across the whole white matter areas (Fig 1 and Table 2). Only in the left uncinate fasciculus we did not find a correlation between rWMV and performance on the simple processing speed task.
Fig 1. White matter regions showing a correlation between rWMV and Simple Processing Speed task performance.
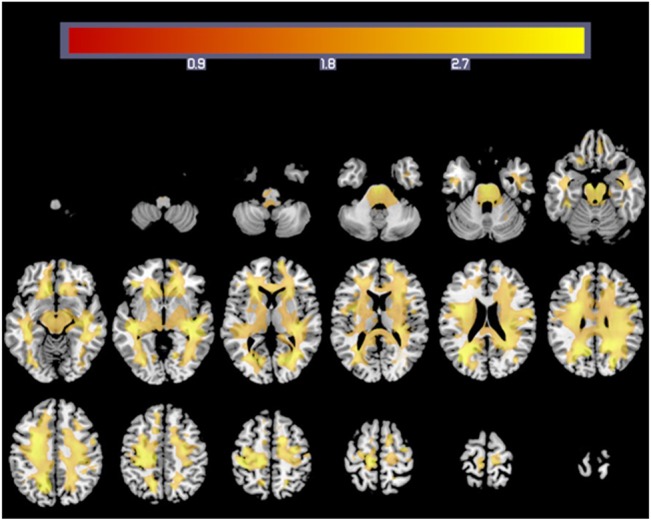
Colourbar indicates the t-values for the regression slopes.
Table 2. Brain regions of significant correlation between rWMV and Simple Processing Speed task.
| Region | N of significant voxel (Total N of voxel) | Peak t-value | Corrected p value (FDR) | MNI peak coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Middle cerebellar peduncle | 1691 (5391) | 3.60 | 0.007 | -2 | -16 | -32 |
| Pontine crossing tract | 424 (522) | 3.36 | 0.010 | 0 | -21 | -24 |
| Genu of corpus callosum | 2036 (3077) | 3,25 | 0.011 | -14 | 24 | -8 |
| Body of corpus callosum | 2785 (4759) | 3.31 | 0.010 | -20 | 3 | 31 |
| Splenium of corpus callosum | 2923 (4182) | 4.3 | 0.004 | -23 | -52 | -22 |
| Fornix | 5 (271) | 2.74 | 0.019 | -3 | -21 | 18 |
| Corticospinal tract right | 350 (495) | 3.31 | 0.010 | -5 | -21 | -33 |
| Corticospinal tract left | 317 (497) | 3.37 | 0.010 | 5 | -18 | -26 |
| Medial lemniscus right | 198 (265) | 2.95 | 0.015 | -5 | -34 | -26 |
| Medial lemniscus left | 204 (264) | 3.02 | 0.014 | 3 | -33 | -26 |
| Inferior cerebellar peduncle right | 92 (377) | 2.31 | 0.032 | -9 | -40 | -35 |
| Inferior cerebellar peduncle left | 81 (367) | 2.21 | 0.36 | 9 | -40 | -33 |
| Superior cerebellar peduncle right | 140 (372) | 2.90 | 0.016 | -6 | -34 | -24 |
| Superior cerebellar peduncle left | 144 (379) | 3.04 | 0.014 | 5 | -33 | -23 |
| Cerebral peduncle right | 654 (833) | 3.21 | 0.012 | -6 | -18 | -21 |
| Cerebral peduncle left | 717 (827) | 3.19 | 0.012 | 5 | -16 | -21 |
| Anterior limb of internal capsule right | 783 (1111) | 3.52 | 0.008 | -23 | 21 | -0631 |
| Anterior limb of internal capsule left | 631 (1086) | 3.16 | 0.012 | 21 | 21 | 3 |
| Posterior limb of internal capsule right | 943 (1320) | 2.60 | 0.022 | -26 | -6 | 18 |
| Posterior limb of internal capsule left | 1026 (1312) | 3.16 | 0.012 | 27 | -27 | -18 |
| Retrolenticular part of internal capsule right | 672 (881) | 3.67 | 0.007 | -41 | -27 | -3 |
| Retrolenticular part of internal capsule left | 739 (859) | 3.92 | 0.006 | 36 | -24 | -3 |
| Anterior corona radiata right | 1571 (2368) | 3.70 | 0.007 | -12 | 29 | -12 |
| Anterior corona radiata left | 1987 (2338) | 3.35 | 0.010 | 15 | 39 | -3 |
| Superior corona radiata right | 2177 (2448) | 4.33 | 0.004 | -26 | 3 | 34 |
| Superior corona radiata left | 2098 (2479) | 3.07 | 0.014 | 24 | -4 | 37 |
| Posterior corona radiata right | 1018 (1301) | 4.58 | 0.003 | -27 | -57 | 22 |
| Posterior corona radiata left | 1102 (1305) | 4.07 | 0.005 | 21 | -52 | 28 |
| Posterior thalamic radiation (include optic radiation) right | 926 (1376) | 4.13 | 0.005 | -33 | -57 | 18 |
| Posterior thalamic radiation (include optic radiation) left | 1044 (1386) | 3.69 | 0.007 | 35 | -63 | 3 |
| Sagittal stratum right | 625 (773) | 3.76 | 0.006 | -42 | -27 | -6 |
| Sagittal stratum left | 602 (783) | 4.03 | 0.005 | 39 | -21 | -6 |
| External capsule right | 518 (1329) | 3.49 | 0.008 | -24 | 21 | -0 |
| External capsule left | 713 (1299) | 3.55 | 0.008 | 33 | -19 | -3 |
| Cingulum (cingulate gyrus) right | 111 (923) | 2.98 | 0.015 | -11 | -37 | -33 |
| Cingulum (cingulate gyrus) left | 440 (1086) | 2.97 | 0.015 | 9 | -27 | 30 |
| Cingulum (hippocampus) right | 10 (467) | 2.18 | 0.037 | -12 | -46 | 9 |
| Cingulum (hippocampus) left | 51 (452) | 2.39 | 0.029 | 20 | -25 | -21 |
| Fornix (cres) / Stria terminalis right | 232 (435) | 2.79 | 0.018 | -32 | -30 | -5 |
| Fornix (cres) / Stria terminalis left | 311 (426) | 3.82 | 0.006 | 32 | -24 | -6 |
| Superior longitudinal fasciculus right | 45 (2340) | 4.22 | 0.004 | -36 | -58 | 19 |
| 1094 | 3.88 | 0.007 | -32 | 2 | 28 | |
| Superior longitudinal | 1756 (2332) | 3.52 | 0.008 | 29 | -48 | 30 |
| fasciculus left | ||||||
| Superior fronto-occipital fasciculus right | 119 (175) | 3.17 | 0.012 | -23 | 0 | 24 |
| Superior fronto-occipital fasciculus left | 125 (165) | 2.63 | 0.022 | 21 | 12 | 19 |
| Inferior fronto-occipital fasciculus right | 168 (693) | 3.25 | 0.011 | -21 | 21 | -5 |
| Inferior fronto-occipital fasciculus left | 74 (638) | 3.62 | 0.007 | 35 | -19 | -16 |
| Uncinate fasciculus right | - | - | - | - | - | - |
| Uncinate fasciculus left | 39 (150) | 2.50 | 0.025 | 36 | -4 | -18 |
| Tapatum right | 77 (219) | 3.42 | 0.009 | -30 | -52 | 15 |
| Tapatum left | 161 (239) | 3.23 | 0.011 | 24 | -46 | 22 |
In contrast, after adjusting for age, sex and tWMV, multiple regression analysis revealed that performance on the processing speed task did not correlate with rWMV in any of the regions.
Correlation of rWMV and the simple arithmetic task
After adjusting for age and sex, multiple regression analysis (FDR correction, P < 0.05) revealed a positive and significant correlation between performance on the simple arithmetic task and rWMV across the whole white matter areas (Fig 2 and Table 3). Only in the left inferior cerebellar peduncle, the fornix, the left cingulum (hippocampus) and the right uncinate fasciculus we did not find a correlation between WMV and performance on the simple arithmetic task.
Fig 2. White matter regions showing a correlation between rWMV and Simple arithmetic task performance.
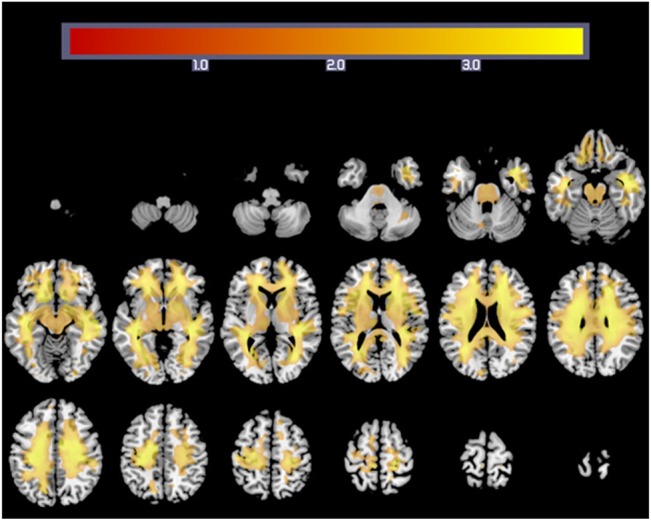
Colourbar indicates the t-values for the regression slopes.
Table 3. Brain regions of significant correlation between rWMV and Simple arithmetic task.
| Region | N of significant voxel (Total N of voxel) | Peak t-value | Corrected p value (FDR) | MNI peak coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Middle cerebellar peduncle | 606 (5391) | 2.58 | 0.015 | -2 | -15 | -30 |
| Pontine crossing tract (a part of MCP) | 348 (522) | 2.47 | 0.018 | -2 | -24 | -23 |
| Genu of corpus callosum | 2601 (3077) | 4.00 | 0.003 | 18 | 24 | 22 |
| Body of corpus callosum | 4076 (4759) | 4,56 | 0.003 | 12 | -6 | 36 |
| Splenium of corpus | 2978 (4182) | 4.08 | 0.003 | -29 | -57 | 9 |
| callosum | 75 | 3.45 | 0.003 | 26 | -58 | 12 |
| Fornix | - | - | - | - | - | - |
| Corticospinal tract right | 273 (495) | 2.46 | 0.019 | -9 | -22 | -23 |
| Corticospinal tract left | 270 (497) | 2.37 | 0.022 | 5 | -24 | -23 |
| Medial lemniscus right | 149 (265) | 2.22 | 0.028 | -5 | -33 | -26 |
| Medial lemniscus left | 113 (264) | 2.21 | 0.028 | 3 | -33 | -26 |
| Inferior cerebellar peduncle right | 7 (377) | 1.91 | 0.048 | -8 | -42 | -41 |
| Inferior cerebellar peduncle left | - | - | - | - | - | - |
| Superior cerebellar peduncle right | 87 (372) | 2.29 | 0.025 | -5 | -30 | -20 |
| Superior cerebellar peduncle left | 94 (379) | 2.55 | 0.016 | 6 | -33 | -15 |
| Cerebral peduncle right | 654 (833) | 2.56 | 0.016 | -12 | -15 | -18 |
| Cerebral peduncle left | 678 (827) | 2.70 | 0.012 | 12 | -6 | -5 |
| Anterior limb of internal capsule right | 889 (1111) | 3.43 | 0.003 | -21 | 12 | 18 |
| Anterior limb of internal capsule left | 882 (1086) | 3.75 | 0.003 | 20 | 18 | 16 |
| Posterior limb of internal capsule right | 348 (1320) | 2.47 | 0.018 | -2 | -24 | -23 |
| Posterior limb of internal capsule left | 1029 (1312) | 3.06 | .006 | 24 | -6 | 18 |
| Retrolenticular part of internal capsule right | 722 (881) | 4.28 | 0.003 | -36 | -33 | -3 |
| Retrolenticular part of internal capsule left | 720 (859) | 4.30 | 0.003 | 38 | -30 | -3 |
| Anterior corona radiata right | 2035 (2368) | 3.68 | 0.003 | -21 | 14 | 27 |
| Anterior corona radiata left | 2035 (2338) | 4.27 | 0.003 | 17 | 27 | 25 |
| Superior corona radiata right | 2206 (2448) | 4.43 | 0.003 | -26 | 2 | 30 |
| Superior corona radiata left | 2218 (2479) | 4.20 | 0.003 | 17 | -6 | 36 |
| Posterior corona radiata right | 1151 (1301) | 4.10 | 0.003 | -32 | -55 | 19 |
| Posterior corona radiata left | 1071 (1305) | 3.99 | 0.003 | 21 | -30 | 30 |
| Posterior thalamic radiation (include optic radiation) right | 1212 (1376) | 4.12 | 0.003 | -32 | -55 | 18 |
| Posterior thalamic radiation (include optic radiation) left | 1228 (1386) | 3.85 | 0.003 | 30 | -66 | 3 |
| Sagittal stratum right | 625 (773) | 4.32 | 0.003 | -38 | -33 | -5 |
| Sagittal stratum ùleft | 625 (783) | 4.28 | 0.003 | 38 | -30 | -5 |
| External capsule right | 82 (1329) | 3.50 | 0.003 | -36 | -16 | -3 |
| 535 | 3.18 | 0.005 | -27 | 8 | 18 | |
| External capsule left | 915 (1299) | 3.59 | 0.003 | 26 | 9 | 18 |
| Cingulum (cingulate gyrus) right | 565 (923) | 3.15 | 0.005 | -12 | -36 | 33 |
| Cingulum (cingulate gyrus) left | 674 (1086) | 4.52 | 0.003 | 9 | -4 | 37 |
| Cingulum (hippocampus) right | 8 (467) | 2.19 | 0,030 | -9 | -45 | 7 |
| Cingulum (hippocampus) left | - | - | - | - | - | - |
| Fornix (cres) / Stria terminalis right | 298 (435) | 3.39 | 0.004 | -35 | -15 | -11 |
| Fornix (cres) / Stria terminalis left | 227 (426) | 3.32 | 0.004 | 35 | -12 | -17 |
| Superior longitudinal fasciculus right | 1883 (2340) | 4.35 | 0.003 | -30 | -24 | 40 |
| Superior longitudinal fasciculus left | 1930 (2332) | 3.90 | 0.003 | 2 | -12 | 36 |
| Superior fronto-occipital fasciculus right | 125 (175) | 4.01 | 0.003 | -21 | 5 | 24 |
| Superior fronto-occipital fasciculus left | 125 (165) | 3.76 | 0.003 | 20 | 17 | 19 |
| Inferior | 181 (693) | 3.90 | 0.003 | -18 | 14 | -12 |
| fronto-occipital fasciculus right | 166 | 3.70 | 0.003 | -38 | -15 | -8 |
| Inferior | 251 (638) | 3.25 | 0.004 | 36 | -12 | -12 |
| fronto-occipital fasciculus left | 137 | 3.19 | 0.005 | 17 | 14 | -12 |
| Uncinate fasciculus right | - | - | - | - | - | - |
| Uncinate fasciculus left | 111 (150) | 3.57 | 0.003 | 36 | -1 | -21 |
| Tapatum right | 183 (219) | 3.90 | 0.003 | -32 | -52 | 15 |
| Tapatum left | 154 (239) | 3.30 | 0.004 | 29 | -54 | 7 |
Additionally, in this case, after adjusting for age, sex and tWMV, multiple regression analysis revealed that performance on the simple arithmetic task did not correlate with rWMV in any of the regions.
Correlation of rWMV and Stroop task
First, we created a normalised sum of scores on the word–colour and colour–word tasks. After adjusting for age and sex, multiple regression analysis (FDR correction, P < 0.05) showed a positive and significant correlation between performance on the Stroop task and rWMV across the whole white matter areas (Fig 3 and Table 4). Furthermore, in this case, we did not find a correlation between performance on the Stroop task and rWMV in the left and right inferior cerebellar peduncle and in the left cingulum (hippocampus). Moreover, after adjusting for age, sex and tWMV, multiple regression analysis showed that performance on the Stroop task did not correlate with rWMV in any of the regions.
Fig 3. White matter regions showing a correlation between rWMV and Stroop task performance.
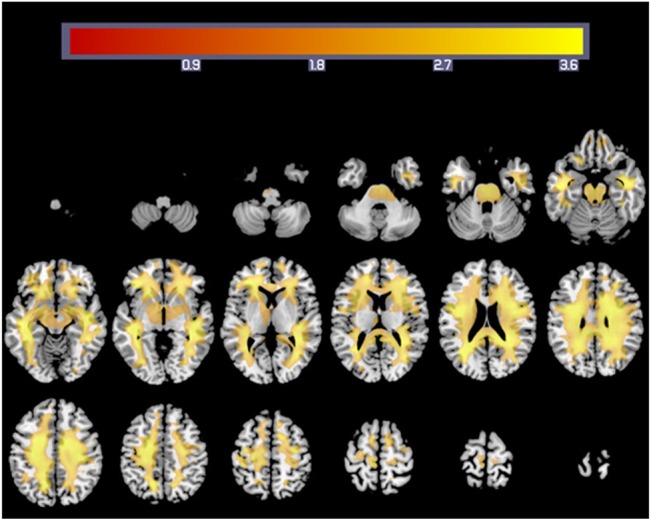
Colourbar indicates the t-values for the regression slopes.
Table 4. Brain regions of significant correlation between rWMV and Stroop task.
| Region | N of significant voxel (Total N of voxel) | Peak t-value | Corrected p value (FDR) | MNI peak coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Middle cerebellar peduncle | 1231 (5391) | 3.30 | 0.006 | 2 | -19 | -27 |
| Pontine crossing tract (a part of MCP) | 376 (522) | 3.27 | 0.006 | 0 | -21 | -24 |
| Genu of corpus callosum | 1994 (3077) | 3.48 | 0.005 | 17 | 20 | 25 |
| Body of corpus callosum | 2881 (4759) | 4.55 | 0.004 | 14 | -3 | 37 |
| Splenium of corpus callosum | 3542 (4182) | 4.43 | 0.004 | -27 | -54 | 19 |
| Fornix | 17 (271) | 2.45 | 0.022 | -2 | 3 | 1 |
| Corticospinal tract right | 313 (495) | 3.20 | 0.006 | -6 | -18 | -26 |
| Corticospinal tract left | 312 (497) | 3.26 | 0.006 | 5 | -19 | -29 |
| Medial lemniscus right | 72 (265) | 2.54 | 0.019 | -5 | -33 | -26 |
| Medial lemniscus left | 85 (264) | 2.54 | 0.019 | 3 | -33 | -26 |
| Inferior cerebellar peduncle right | - | - | - | - | - | - |
| Inferior cerebellar peduncle left | - | - | - | - | - | - |
| Superior cerebellar peduncle right | 81 (372) | 2.55 | 0.018 | -6 | -30 | -21 |
| Superior cerebellar peduncle left | 95 (379) | 2.68 | 0.015 | 5 | -30 | -21 |
| Cerebral peduncle right | 670 (833) | 2.96 | 0.009 | -6 | -19 | -21 |
| Cerebral peduncle left | 550 (827) | 2.97 | 0.009 | 5 | -21 | -21 |
| Anterior limb of internal capsule right | 744 (1111) | 3.38 | 0.005 | -23 | 21 | -0 |
| Anterior limb of internal capsule left | 372 (1086) | 3.14 | 0.007 | 21 | 15 | 18 |
| Posterior limb of | 186 (1320) | 2.43 | 0.023 | -14 | -3 | -2 |
| internal capsule right | 97 | 2.41 | 0.024 | -26 | -6 | 18 |
| Posterior limb of | 108 (1312) | 2.63 | 0.016 | 27 | -27 | 18 |
| internal capsule left | 33 | 2.07 | 0.041 | 12 | -4 | -3 |
| Retrolenticular part of internal capsule right | 568 (881) | 4.46 | 0.004 | -41 | -30 | -3 |
| Retrolenticular part of internal capsule left | 595 (859) | 3.76 | 0.004 | 41 | -36 | -3 |
| Anterior corona radiata right | 2013 (2368) | 3.80 | 0.004 | -27 | 35 | 3 |
| Anterior corona radiata left | 2004 (2338) | 3.68 | 0.004 | 15 | 17 | 30 |
| Superior corona radiata right | 2206 (2448) | 4.61 | 0.004 | -24 | -1 | 37 |
| Superior corona radiata left | 2209 (2479) | 4.49 | 0.004 | 14 | -3 | 39 |
| Posterior corona radiata right | 1151 (1301) | 4.53 | 0.004 | -30 | -55 | 21 |
| Posterior corona radiata left | 1132 (1305) | 4.19 | 0.004 | 27 | -43 | 21 |
| Posterior thalamic radiation (include optic radiation) right | 1197 (1376) | 4.46 | 0.004 | -30 | -55 | 18 |
| Posterior thalamic radiation (include optic radiation) left | 1221 (1386) | 4.07 | 0.004 | 29 | -45 | 18 |
| Sagittal stratum ùright | 625 (773) | 4.80 | 0.004 | -42 | -30 | -6 |
| Sagittal stratum ùleft | 617 (783) | 3.80 | 0.004 | 39 | -12 | -17 |
| External capsule right | 406 (1329) | 3.48 | 0.005 | -24 | 21 | -3 |
| 36 | 2.62 | 0.016 | -35 | -22 | 1 | |
| External capsule left | 477 (1299) | 3.19 | 0.007 | 27 | 9 | 18 |
| 36 | 2.55 | 0.018 | 33 | -22 | 1 | |
| Cingulum (cingulate gyrus) right | 256 (923) | 3.64 | 0.004 | -9 | -34 | 33 |
| 61 | 2.34 | 0.027 | -11 | 9 | 31 | |
| 23 | 2.17 | 0.035 | -9 | 32 | 12 | |
| Cingulum (cingulate gyrus) left | 800 (1086) | 4.02 | 0.00 | 9 | -6 | 36 |
| Cingulum (hippocampus) right | 19 (467) | 2.50 | 0.020 | -12 | -46 | 9 |
| Cingulum (hippocampus) left | - | - | - | - | - | - |
| Fornix (cres) / Stria terminalis right | 182 (435) | 3.07 | 0.008 | -36 | -12 | -17 |
| Fornix (cres) / Stria terminalis left | 141 (426) | 3.26 | 0.006 | 33 | -7 | -18 |
| Superior longitudinal fasciculus right | 1659 (2340) | 4.33 | 0.004 | -36 | -1 | 27 |
| Superior longitudinal fasciculus left | 1946 (2332) | 3.97 | 0.004 | 33 | 2 | 27 |
| Superior fronto-occipital fasciculus right | 125 (175) | 3.40 | 0.005 | -23 | 0 | 24 |
| Superior fronto-occipital fasciculus left | 117 (165) | 3.50 | 0.005 | 20 | 9 | 24 |
| Inferior fronto-occipital fasciculus right | 450 (693) | 3.4 | 0.005 | -24 | 21 | -5 |
| Inferior fronto-occipital fasciculus left | 424 (638) | 3.16 | 0.007 | 23 | 21 | -6 |
| Uncinate fasciculus right | 43 (145) | 2.50 | 0.020 | -35 | -4 | -14 |
| Uncinate fasciculus left | 109 (150) | 3.40 | 0.005 | 36 | -4 | -18 |
| Tapatum right | 183 (219) | 4.02 | 0.004 | -30 | -52 | 15 |
| Tapatum left | 167 (239) | 4.18 | 0.004 | 24 | -45 | 22 |
Discussion
To the best of our knowledge, this is the first study to explore the associations between rWMV and processing speed tasks in healthy adults at the whole brain level. First, as in previous studies [2], [6], [7], correlation analyses showed a positive correlation between processing speed tasks and working memory and general intelligent tasks. This result are on line with the literature, considering that more are complex the processing speed tasks, stronger is the relationship between processing speed and intelligence and vice versa [53]. Second, VBM analyses showed a positive relationship across the whole white matter between WMV and processing speed performance. Our results support the assumption that WMV is globally related to processing speed performance regardless of the type of processing speed tasks. However, in our results some WM area are not correlated with the processing speed performances. This could be possible considering the high specialization and the lateralization of this areas that consequently they are not involved in processing speed activity. These results do not support the other assumption, i.e., processing speed performance is related to volume of a specific white matter area.
Considering the physiological functions of white matter, we found evidence that WMV might be essential for processing speed, which is often viewed as a key variable of cognitive architecture[2], [6]. In fact, processing speed in each task is likely to largely depend on the properties of white matter that are essential for performance on that task [5–46]. White matter consists mostly of glial cells and myelinated axons; it controls the signals that neurons share, coordinating the cooperative work of brain regions [52]. In fact, the speed of neural signals is associated with the thickness and degree of myelination of axons [16], [54], [55]. As we previously discussed [29], [42], enhancement of these physiological components, which is presumably secondary to increased myelination [56] or increased axonal calibre [57], may be associated with greater effectiveness of neural circuit communication and consequently may facilitate cognitive functions [47].
Our results suggest that globally distributed white matter structures support performance on different processing speed tasks, and this finding is in agreement with the following studies. Previous psychological studies showed that processing speed correlates with performance in various cognitive domains during development and healthy ageing [58], [59]. Furthermore, general white matter integrity is considered a lifelong stable biological foundation of processing speed throughout the lifespan [2], [15], [17]. Simultaneously, performance on so-called processing speed tasks is actually considered to depend on various cognitive activities. For example, the digit symbol test [8], which is a typical test of processing speed, is considered to be affected by psychomotor speed, attention, perceptual organisation, motor persistence and visual short-term memory [60], [61]. Thus, various white matter structures of brain areas may be responsible for processing speed. We may assume that processing speed is a fundamental component of cognitive efficiency or cognitive proficiency, and our results show that data from global WMV partly support this idea and help to explain that variable.
The present findings are expected to stimulate further research in this area, in particular how the relationship between processing speed and WMV can change during cognitive development and ageing process. Moreover, it seems important to test how the relationship between rWMV and improvements in processing speed via training [46] changes in healthy people and patients with cognitive impairment.
This study has some limitations. One limitation is shared with our previous studies and studies by others that involve college cohorts [39], [44], [47], [62], [63]. As mentioned above, we tested young healthy subjects at a relatively high educational level. Limited sampling of the full range of intellectual abilities is a common problem when sampling from college cohorts [57]. Limited sampling may be an important step to rule out the effects of age or the education level that could strongly influence brain structures and increase sensitivity of the analyses. In fact, processing speed correlates with age-related changes in cognition in the course of childhood development [15], [64], [65] (and healthy ageing [2], [15]
This study seems to be the first study to explore the associations between rWMV and processing speed in a large sample of young adults. Supporting our hypothesis, the results confirm that WMV is related globally to performance on different tests of processing speed. Our results support the notion that global WMV could help to explain in detail the influence of white matter on processing speed performance.
Supporting Information
(SAV)
Acknowledgments
We thank Yuki Yamada for operating the MRI scanner, Haruka Nouchi for conducting the psychological tests, all other assistants for helping with the experiments and the study, and the study participants and all our other colleagues at IDAC, Tohoku University for their support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by JST/RISTEX, JST/CREST, a Grant-in-Aid for Young Scientists (B) (KAKENHI 23700306) and a Grant-in-Aid for Young Scientists (A) (KAKENHI 25700012) from the Ministry of Education, Culture, Sports, Science, and Technology. JSPS Postdoctoral Fellowship Program for Foreigner Researchers FY2012. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological review. 1996;103(3):403 [DOI] [PubMed] [Google Scholar]
- 2. Salthouse TA, Madden DJ. 10 Information processing speed and aging. Information Processing Speed in Clinical Populations. 2013:221. [Google Scholar]
- 3. Lezak MD. Neuropsychological assessment: Oxford university press; 2004. [Google Scholar]
- 4. DeLuca J, Kalmar JH . Information processing speed in clinical populations: Psychology Press; 2013. [Google Scholar]
- 5. Turken AU, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JDE. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42(2):1032–44. 10.1016/j.neuroimage.2008.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kail R, Salthouse TA. Processing speed as a mental capacity. Acta psychologica. 1994;86(2):199–225. [DOI] [PubMed] [Google Scholar]
- 7. Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biological psychology. 2000;54(1):1–34. [DOI] [PubMed] [Google Scholar]
- 8. Li S-C, Lindenberger U, Hommel B, Aschersleben G, Prinz W, Baltes PB. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science. 2004;15(3):155–63. [DOI] [PubMed] [Google Scholar]
- 9. Salthouse TA. Relations between cognitive abilities and measures of executive functioning. Neuropsychology. 2005;19(4):532 [DOI] [PubMed] [Google Scholar]
- 10. Wechsler D. WAIS-III: Wechsler adult intelligence scale: Psychological Corporation San Antonio, TX; 1997. [Google Scholar]
- 11. Posthuma D, Baaré WFC, Hulshoff Pol HE, Kahn RS, Boomsma DI, De Geus EJC. Genetic correlations between brain volumes and the WAIS-III dimensions of verbal comprehension, working memory, perceptual organization, and processing speed. Twin Research. 2003;6(02):131–9. [DOI] [PubMed] [Google Scholar]
- 12. Borghesani PR, Madhyastha TM, Aylward EH, Reiter MA, Swarny BR, Schaie KW, et al. The association between higher order abilities, processing speed, and age are variably mediated by white matter integrity during typical aging. Neuropsychologia. 2013;51(8):1435–44. 10.1016/j.neuropsychologia.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobs HIL, Leritz EC, Williams VJ, Van Boxtel MPJ, Elst Wvd, Jolles J, et al. Association between white matter microstructure, executive functions, and processing speed in older adults: the impact of vascular health. Human brain mapping. 2013;34(1):77–95. 10.1002/hbm.21412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gutiérrez R, Boison D, Heinemann U, Stoffel W. Decompaction of CNS myelin leads to a reduction of the conduction velocity of action potentials in optic nerve. Neuroscience letters. 1995;195(2):93–6. [DOI] [PubMed] [Google Scholar]
- 15. Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Shenton ME, Kennedy JL, et al. Age-related decline in white matter tract integrity and cognitive performance: a DTI tractography and structural equation modeling study. Neurobiology of aging. 2012;33(1):21–34. 10.1016/j.neurobiolaging.2010.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. Journal of Molecular Neuroscience. 2008;34(1):51–61. [DOI] [PubMed] [Google Scholar]
- 17. Penke L, Maniega SM, Murray C, Gow AJ, Hernández MCV, Clayden JD, et al. A general factor of brain white matter integrity predicts information processing speed in healthy older people. The Journal of Neuroscience. 2010;30(22):7569–74. 10.1523/JNEUROSCI.1553-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(34):12212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poldrack RA, Temple E, Protopapas A, Nagarajan S, Tallal P, Merzenich M, et al. Relations between the neural bases of dynamic auditory processing and phonological processing: evidence from fMRI. Journal of Cognitive Neuroscience. 2001;13(5):687–97. [DOI] [PubMed] [Google Scholar]
- 20. Wessels AM, Rombouts S, Remijnse PL, Boom Y, Scheltens P, Barkhof F, et al. Cognitive performance in type 1 diabetes patients is associated with cerebral white matter volume. Diabetologia. 2007;50(8):1763–9 [DOI] [PubMed] [Google Scholar]
- 21. Usui N, Haji T, Maruyama M, Katsuyama N, Uchida S, Hozawa A, et al. Cortical areas related to performance of WAIS Digit Symbol Test: a functional imaging study. Neuroscience letters. 2009;463(1):1–5. 10.1016/j.neulet.2009.07.048 [DOI] [PubMed] [Google Scholar]
- 22. Catani M, Jones DK, Donato R. Occipito‐temporal connections in the human brain. Brain. 2003;126(9):2093–107. [DOI] [PubMed] [Google Scholar]
- 23. Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, et al. White matter microstructural integrity and cognitive function in a general elderly population. Archives of General Psychiatry. 2009;66(5):545–53. 10.1001/archgenpsychiatry.2009.5 [DOI] [PubMed] [Google Scholar]
- 24. Dow C, Seidenberg M, Hermann B. Relationship between information processing speed in temporal lobe epilepsy and white matter volume. Epilepsy & Behavior. 2004;5(6):919–25. [DOI] [PubMed] [Google Scholar]
- 25. Deary IJ, Bastin ME, Pattie A, Clayden JD, Whalley LJ, Starr JM, et al. White matter integrity and cognition in childhood and old age. Neurology. 2006;66(4):505–12. [DOI] [PubMed] [Google Scholar]
- 26. Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. American Journal of Neuroradiology. 2007;28(2):226–35. [PMC free article] [PubMed] [Google Scholar]
- 27. Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, et al. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiology of aging. 2008;29(7):1070–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takeuchi H, Taki Y, Thyreau B, Sassa Y, Hashizume H, Sekiguchi A, et al. White matter structures associated with empathizing and systemizing in young adults. Neuroimage. 2013;77:222–36. 10.1016/j.neuroimage.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 29. Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, et al. Relating imaging indices of white matter integrity and volume in healthy older adults. Cerebral Cortex. 2008;18(2):433–42. [DOI] [PubMed] [Google Scholar]
- 30. Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, et al. Working memory training using mental calculation impacts regional gray matter of the frontal and parietal regions. PLoS One. 2011;6(8):e23175 10.1371/journal.pone.0023175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, et al. Brain structures associated with executive functions during everyday events in a non-clinical sample. Brain Structure and Function. 2013;218(4):1017–32. 10.1007/s00429-012-0444-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Colom R, Jung RE, Haier RJ. General intelligence and memory span: evidence for a common neuroanatomic framework. Cognitive Neuropsychology. 2007;24(8):867–78. [DOI] [PubMed] [Google Scholar]
- 33. Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. Structural brain variation and general intelligence. NeuroImage. 2004;23(1):425–33. [DOI] [PubMed] [Google Scholar]
- 34. Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. The neuroanatomy of general intelligence: sex matters. NeuroImage. 2005;25(1):320–7. [DOI] [PubMed] [Google Scholar]
- 35. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 36. Tanaka K, Okamoto K. Manual of New Tanaka B Type Intelligence Test. Tokyo: Kaneko Syobo; 2003. [Google Scholar]
- 37. Stroop JR. Studies of interference in serial verbal reactions. Journal of experimental psychology. 1935;18(6):643. [Google Scholar]
- 38. MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological bulletin. 1991;109(2):163 [DOI] [PubMed] [Google Scholar]
- 39. Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, et al. Verbal working memory performance correlates with regional white matter structures in the frontoparietal regions. Neuropsychologia. 2011;49(12):3466–73. 10.1016/j.neuropsychologia.2011.08.022 [DOI] [PubMed] [Google Scholar]
- 40. Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Nagase T, et al. Regional gray and white matter volume associated with Stroop interference: evidence from voxel-based morphometry. Neuroimage. 2012;59(3):2899–907. 10.1016/j.neuroimage.2011.09.064 [DOI] [PubMed] [Google Scholar]
- 41. Hakoda Y, Sasaki M. Group version of the Stroop and reverse-Stroop test: the effects of reaction mode, order and practice. Kyoiku Shinrigaku Kenkyu (JPN J Educ Psychol). 1990;38:389–94. [Google Scholar]
- 42. Langenecker SA, Nielson KA, Rao SM. fMRI of healthy older adults during Stroop interference. Neuroimage. 2004;21(1):192–200. [DOI] [PubMed] [Google Scholar]
- 43. Grabner RH, Ansari D, Reishofer G, Stern E, Ebner F, Neuper C. Individual differences in mathematical competence predict parietal brain activation during mental calculation. Neuroimage. 2007;38(2):346–56. [DOI] [PubMed] [Google Scholar]
- 44. Raven JC, John Hugh C. Raven's progressive matrices and vocabulary scales: Oxford Psychologists Press; 1998. [Google Scholar]
- 45. Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, et al. Failing to deactivate: the association between brain activity during a working memory task and creativity. Neuroimage. 2011;55(2):681–7. 10.1016/j.neuroimage.2010.11.052 [DOI] [PubMed] [Google Scholar]
- 46. Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, et al. Effects of training of processing speed on neural systems. The Journal of Neuroscience. 2011;31(34):12139–48. 10.1523/JNEUROSCI.2948-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, et al. Regional gray matter volume of dopaminergic system associate with creativity: evidence from voxel-based morphometry. Neuroimage. 2010;51(2):578–85. 10.1016/j.neuroimage.2010.02.078 [DOI] [PubMed] [Google Scholar]
- 48. Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, et al. White matter structures associated with creativity: evidence from diffusion tensor imaging. Neuroimage. 2010;51(1):11–8. 10.1016/j.neuroimage.2010.02.035 [DOI] [PubMed] [Google Scholar]
- 49. Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11(6):805–21. [DOI] [PubMed] [Google Scholar]
- 50. Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–8. [DOI] [PubMed] [Google Scholar]
- 51. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological). 1995:289–300. [Google Scholar]
- 52. Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X, et al. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage. 2008;43(3):447–57. 10.1016/j.neuroimage.2008.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Conway ARA, Cowan N, Bunting MF, Therriault DJ, Minkoff SRB. A latent variable analysis of working memory capacity, short-term memory capacity, processing speed, and general fluid intelligence. Intelligence. 2002;30(2):163–83. [Google Scholar]
- 54. Fields RD. White matter matters. Scientific American. 2008;298(3):54–61. [PubMed] [Google Scholar]
- 55. Tolhurst DJ, Lewis PR. Effect of myelination on the conduction velocity of optic nerve fibres. Ophthalmic and Physiological Optics. 1992;12(2):241–3. [DOI] [PubMed] [Google Scholar]
- 56. Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle & nerve. 1980;3(2):141–50. [DOI] [PubMed] [Google Scholar]
- 57. Beaulieu C. The basis of anisotropic water diffusion in the nervous system–a technical review. NMR in Biomedicine. 2002;15(7‐8):435–55. [DOI] [PubMed] [Google Scholar]
- 58. Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–39. [DOI] [PubMed] [Google Scholar]
- 59. Peters BD, Ikuta T, DeRosse P, John M, Burdick KE, Gruner P, et al. Age-related differences in white matter tract microstructure are associated with cognitive performance from childhood to adulthood. Biological psychiatry. 2014;75(3):248–56. 10.1016/j.biopsych.2013.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Salthouse TA. Relations between cognitive abilities and measures of executive functioning. Neuropsychology. 2005;19(4):532 [DOI] [PubMed] [Google Scholar]
- 61. Schear JM, Sato SD. Effects of visual acuity and visual motor speed and dexterity on cognitive test performance. Archives of Clinical Neuropsychology. 1989;4(1):25–32. [PubMed] [Google Scholar]
- 62. Ellingsen DG, Bast-Pettersen R, Efskind J, Thomassen Y. Neuropsychological effects of low mercury vapor exposure in chloralkali workers. Neurotoxicology. 2001;22(2):249–58. [DOI] [PubMed] [Google Scholar]
- 63. Jung RE, Segall JM, Jeremy Bockholt H, Flores RA, Smith SM, Chavez RS, et al. Neuroanatomy of creativity. Human brain mapping. 2010;31(3):398–409. 10.1002/hbm.20874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Song M, Zhou Y, Li J, Liu Y, Tian L, Yu C, et al. Brain spontaneous functional connectivity and intelligence. Neuroimage. 2008;41(3):1168–76. 10.1016/j.neuroimage.2008.02.036 [DOI] [PubMed] [Google Scholar]
- 65. Kail R. Developmental change in speed of processing during childhood and adolescence. Psychological bulletin. 1991;109(3):490 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(SAV)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


