Abstract
In this review of neuroanatomical studies of the genetically epilepsy-prone rat (GEPR), three main topics will be covered. First, the number of GABAergic neurons and total neurons in the inferior colliculus of GEPRs will be compared to those of the non-epileptic Sprague-Dawley rat. Next, the number of small neurons in the inferior colliculus will be described in both developmental and genetic analyses of GEPRs and their backcrosses. Last, results from two types of studies on the propagation pathways for audiogenic seizures in GEPRs will be shown. Together, these studies demonstrate a unique GABAergic, small neuron defect in the inferior colliculus of GEPRs that may play a vital role in the initiation and spread of seizure activity during audiogenic seizures.
Keywords: audiogenic seizures, epilepsy-prone rats, genetic model, inferior colliculus, GABAergic neurons
PART I. GABAergic Neurons in the Inferior Colliculus of GEPRs
Genetically epilepsy-prone rats (GEPRs) display seizures following auditory stimulation [1]. Bilateral lesions of the inferior colliculus of GEPRs block seizure activity while ablation of the auditory cortex or medial geniculate nucleus does not block seizures in these rats [2,3]. Neurons in the inferior colliculus of GEPRs may be less sensitive to GABA and benzodiazepine iontophoresis than neurons in the inferior colliculus of control rats [4]. Also, neurons in the inferior colliculus of GEPRs exhibit elevated thresholds to sound and a smaller degree of firing reduction during binaural inhibition than do units in the Sprague-Dawley rat [5]. In addition, neurons in the inferior colliculus of the GEPR show an increase in afterdischarge-like responses similar to those seen in other types of epilepsies and such afterdischarges are specific for the inferior colliculus but not from the cortex [6]. These data taken together indicate several abnormalities in the inferior colliculus of GEPRs, including a possible defect in GABA-mediated inhibition. It has been hypothesized that a decrease in the neurotransmitter, GABA, can cause a significant loss of inhibition and result in a hyperexcitable state [7]. Our previous neuroanatomical studies of a nonhuman primate model of focal epilepsy are consistent with this hypothesis because a preferential loss of GABAergic axon terminals and neurons occurs at epileptic foci created by alumina gel in monkeys [8,9]. Therefore, the studies on GEPRs, and specifically the inferior colliculus, that will be described in this section, were made to test the GABA hypothesis in this genetic model of audiogenic epilepsy.
An initial study was made in the inferior colliculus using immunocytochemistry to determine whether GABAergic neurons were altered in this region of GEPRs [10]. We employed antibodies to the GABA synthesizing enzyme, glutamate decarboxylase (GAD), and showed that the inferior colliculus of GEPRs displayed an increase in the number of GAD-positive neurons as compared to that of Sprague-Dawley rats [10]. These latter rats were chosen because the GEPR strain derives from Sprague-Dawley rats by selective breeding for susceptibility to audiogenic seizures. It should be noted that Sprague-Dawley rats did not have audiogenic seizures after loud auditory stimuli. The increase in the number of GAD-positive neurons was most evident in the midlevel of the central nucleus of the inferior colliculus [10]. Moreover, greater numbers of GAD-positive neurons are found throughout this central nucleus of GEPRs [10]. This increase in GAD-positive neurons was found for only the small (10-15 μm) and medium-sized (15-25 μm) GAD-positive neurons (Fig. 1), and the small GABAergic neurons were increased more than the medium-sized ones [10]. The increase was greater in the ventrolateral part of the central nucleus of the inferior colliculus than in the dorsomedial part. The increased number of GABAergic neurons in the GEPR also occurred in other subnuclei of the inferior colliculus. Paradoxically, the GEPRs were shown to have increased numbers of GABAergic neurons in the brain region where audiogenic seizures are generated. These findings are the opposite of the results found in the monkey model of focal epilepsy where the epileptic focus had decreased numbers of GAD-positive neurons [8,9].
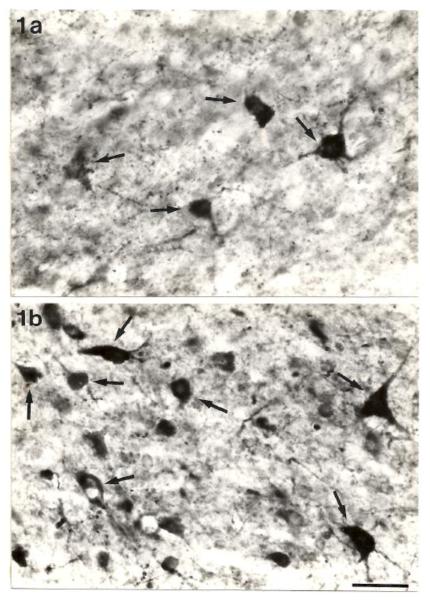
Fig. 1.
Photomicrographs of the ventrolateral portion of the central nucleus of the inferior colliculus showing the typical distribution of GAD-positive somata (arrows) in the Sprague-Dawley rat (a) and the GEPR (b). Note the increase in the numbers of small GAD-positive neurons in the GEPR. Scale bar = 25μm. Published with permission from Roberts et al. [10].
Further studies were needed to confirm the results from the GAD immunocytochemical studies that showed an increase in the number of GABAergic neurons in the inferior colliculus of GEPRs. The first of these studies used Nissl preparations of the central nucleus of the inferior colliculus, and it revealed a heterogeneous population of small, medium-sized and large neurons in this brain region [10]. Consistent with the quantitative data on GAD-positive neurons, there were more neuronal somata in the Nissl preparations from GEPRs than from those obtained from Sprague-Dawley rats (Fig. 2). This increase in the numbers of small (100%) and medium-sized (30%) neurons in GEPRs was consistent with the magnitude of the increase in GAD-positive cell types. An analysis of the volume of the inferior colliculus in both GEPRs and Sprague-Dawley rats was made to determine whether there was shrinkage in the GEPRs of this brain structure that may explain the increase in the number of small and medium-sized neurons. However, no difference in the volume of the inferior colliculus was found [10]. Therefore, these data on the increase in small and medium-sized neuronal somata in the inferior colliculus of GEPRs confirm the observed increase in GABAergic neurons.
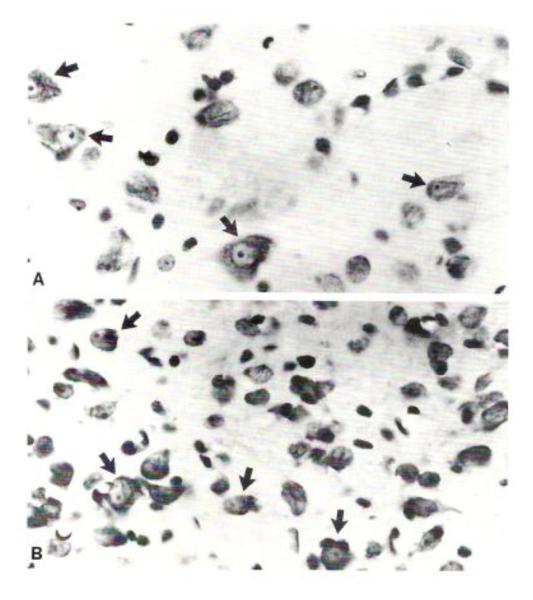
Fig. 2.
Nissl preparations of neurons (arrows) and glia from representative regions of the central nucleus of the inferior colliculus from a Sprague-Dawley rat (A) and a GEPR (B). The GEPR displays a greater number of small neuronal somata that the Sprague-Dawley rat. X900. Published with permission from Roberts et al. [10].
The Nissl preparations of the inferior colliculus of GEPRs often showed clusters of small neuronal somata. Electron microscopy was used to confirm this observation and electron micrographs of the inferior colliculus of GEPRs had clusters of 4-5 small neurons apposed to each other or separated by only a thin astrocytic process [11]. In contrast, such clusters were rarely observed in the inferior colliculus of Sprague-Dawley rats [12]. Therefore, the increase in cell number in GEPRs was confirmed by another neuroanatomical method, electron microscopy.
Another study that was made to confirm the results from the GAD immunocytochemical study utilized in situ hybridization and emulsion autoradiographic techniques to show the neurons with GAD mRNA, a molecule that is required to synthesize GAD [13]. Hybridization was performed using a 35S-labeled RNA probe complementary to a span of monkey GAD mRNA. Labeled cells in the central nucleus of the inferior colliculus were different sizes and corresponded to descriptions of the GAD-immunolabeled cells [14]. GEPRs had significantly greater numbers of GAD67 cRNA labeled neurons in the central nucleus of the inferior colliculus than Sprague-Dawley rats [13]. These data confirmed the finding of increased numbers of GAD-immunolabeled neurons in the inferior colliculus of GEPRs [10]. The specificity of this GAD mRNA defect for the inferior colliculus was evidenced by the finding that the frontal cortex, an area of the brain not involved in audiogenic seizures, showed no differences in hybridization between GEPRs and Sprague-Dawley rats [13].
The last of our studies of GABAergic structures in the inferior colliculus of GEPRs was an analysis of the GABAergic axon terminals in electron microscopic preparations [11]. Using immunocytochemical preparations, GABAergic synapses were labeled using both GAD and GABA antibodies [11]. GABA- or GAD-positive reaction product was localized to only axon terminals that made symmetric synapses and these axon terminals contained several mitochondria [11]. These GABAergic axon terminals from GEPRs were similar to those shown in a previous study of the inferior colliculus of Sprague-Dawley rats [14]. Unfortunately, the GABA receptors were not analyzed at these GABAergic synapses because the probes for such receptors and the methods needed to quantify the number of receptors were not available at the time these studies were being completed. Such data would be helpful in understanding the reason why more GABA inhibition is needed in the inferior colliculus of GEPRs.
In summary, the neuroanatomical studies of the GABAergic system in GEPRs showed greater numbers of small, GABAergic neurons in the central nucleus of the inferior colliculus of GEPRs as compared to Sprague-Dawley rats using both immunocytochemical and in situ hybridization methods. The data obtained from Nissl preparations showing increased numbers of small neurons in the inferior colliculus of GEPRs are consistent with the increase in GABAergic neurons in this structure. The GABAergic axon terminals in the inferior colliculus of GEPRs were shown to form the typical inhibitory type of synapse, symmetrical, that was also found in Sprague-Dawley rats. These data taken together with the finding that it takes more GABA in the inferior colliculus of GEPRs to produce the same amount of inhibition in Sprague-Dawley rats [4], suggests that a deficiency is present in GABAergic transmission in the inferior colliculus of GEPRs. This deficiency at the GABA synapse may involve a GABA receptor mechanism in GEPRs. Further studies need to be performed to test this hypothesis that a specific subunit of the GABA receptor may be lacking at synapses in the GEPR inferior colliculus.
PART II – Developmental and Genetic Studies in GEPRs
It remains to be determined whether the increase in the number of small, GABAergic neurons in the inferior colliculus of GEPRs is the cause of the seizures or is a compensatory mechanism in response to seizure activity. The first approach that was used to resolve this issue was a study of young GEPRs prior to the age at which they were responsive to audiogenic seizures [15]. For this developmental analysis, the numbers of small neurons in the inferior colliculus of young (4-6 and 8-10 postnatal days) GEPR offspring were analyzed in light microscopic preparations. The results showed an increase in the number of small neurons in the inferior colliculus of both ages analyzed of the young offspring of GEPRs as compared to age-matched Sprague-Dawley rats [15]. Nissl preparations were used for this analysis. The substantial increase in the number of neuronal cell bodies in young GEPRs as compared to the Sprague-Dawley rats indicated that the increase in cell number observed in the adult GEPR inferior colliculus is not compensatory for the seizure activity because this increase is present prior to the time the GEPRs respond to audiogenic seizure stimuli. Instead, the data indicate that the increase is genetically programmed because it is present prior to the time when audiogenic seizures begin. This conclusion is consistent with a GABA receptor defect in the inferior colliculus that may be present prior to the age at which audiogenic seizures begin.
The second approach that was used to determine whether an increase in small cell number in the inferior colliculus of GEPRs is genetically associated with audiogenic seizure behavior involved an analysis of the inheritance of audiogenic seizures in the progeny of GEPRs and Sprague-Dawley rats [16]. Offspring of GEPRs crossed with Sprague-Dawley rats as well as backcrosses made with F1 rats and either GEPRs or Sprague-Dawley rats were examined in a classic genetic analysis [16]. Small neurons were counted in the inferior colliculus from cresyl violet-stained sections from adult GEPRs, Sprague-Dawley rats, F1 rats and F2 (4 types of backcrosses) rats to determine if an increase in neuron number in the inferior colliculus is correlated with seizure behavior. All of the rats were tested for audiogenic seizures and a response score was given on a scale of 0 to 9 with 9 being the typical full seizure with wild running and clonic-tonic movements and 0 being no seizure [1].
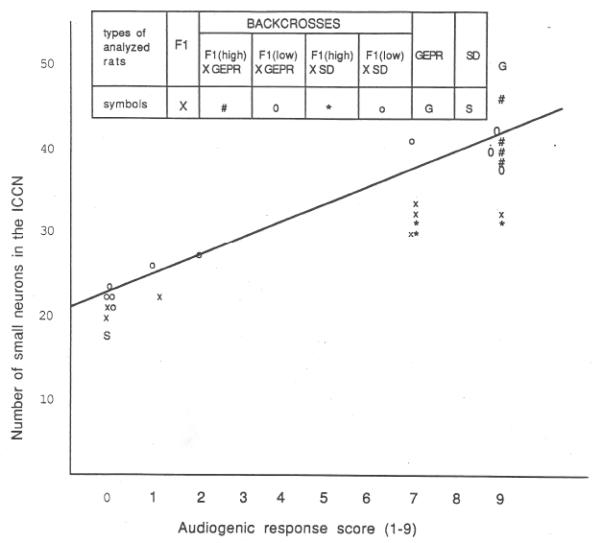
The results of the genetic studies indicated a polygenetic inheritance of this autosomal dominant trait of audiogenic seizure susceptibility because the F1 generation had a ratio of greater than 3:1 of audiogenic seizure susceptibility [15]. For the quantitative study of neuronal density in the inferior colliculus, statistically significant increases in the number of both small and medium-sized neurons were found in rats with high audiogenic seizure scores (7-9) as compared to the F2 and Sprague-Dawley rats that had no audiogenic seizures [15]. In addition, offspring from the F1 cross to GEPRs that had high audiogenic seizure scores had increased numbers of small neurons as compared to the offspring from F1 rats crossed with Sprague-Dawley rats that had no audiogenic seizures [15]. Finally, the data from the 25 rats in this study generated a 0.9 coefficient of linear correlation between audiogenic seizure scores and the number of small neurons in the central nucleus of the inferior colliculus (Fig. 3). These data showed that the inheritance of audiogenic seizures is closely linked to the number of small neurons in the central nucleus of the inferior colliculus.
Fig. 3.
A graph that shows the relationship between the number of small neurons in the central nucleus of the inferior colliculus and the audiogenic response scores of 28 rats from a genetic study. The coefficient of linear correlation was calculated to be 0.91. Published with permission from Ribak et al. [16].
PART III. Seizure Propagation Pathways with Origin in the Central Nucleus of the Inferior Colliculus of GEPRs
Previous studies showed a loss of audiogenic seizures following bilateral lesions of the inferior colliculus or bilateral lesions of ascending auditory pathways to the inferior colliculus [2,3]. Later studies showed that bilateral lesions of the reticularis pontis oralis nucleus of the brainstem reticular formation blocked the tonic motor component of audiogenic seizures [17,18]. Therefore, this nucleus in the reticular formation appears to be an important site for the common motor outflow to the spinal cord for eliciting the distinctive motor activity and clonic-tonic movements that characterize audiogenic seizures in GEPRs. It is important to note that the major ascending projections of the inferior colliculus are to the medial geniculate nucleus in the thalamus and to the deep layers of the superior colliculus [19]. Also, no direct projection from the inferior colliculus to the reticularis pontis oralis nucleus was shown to exist [20]. The following studies [21,22] used neuroanatomical methods to determine the projections from the inferior colliculus that play a role in seizure propagation in GEPRs following audiogenic stimuli.
The first study analyzed the rostral projections from the central nucleus of the inferior colliculus using bilateral knife cuts of both the superior and inferior colliculi to determine their effect on audiogenic seizures in GEPRs [21]. Two GEPRs with knife cuts between the central and external nuclei within the inferior colliculus had their seizures blocked. Five other GEPRs with knife cuts between the inferior and superior colliculi reduced the severity of audiogenic seizures. Several of these intercollicular knife cut cases had a retrograde tracer, HRP, injected into the knife cuts [21].
In these cases, HRP-labeled neurons were found in the external nucleus of the inferior colliculus, and labeled axons were located in the deep layers of the superior colliculus. Together, these data suggested a propagation pathway for audiogenic seizures in GEPRs that starts in the central nucleus of the inferior colliculus, then projects to the external nucleus of the inferior colliculus, and then to the deep layers of the superior colliculus [21]. In the second study, a c-fos mRNA method was used to examine seizure propagation pathways in GEPRs [22]. The use of c-fos mRNA, a proto-oncogene and a marker of elevated levels of neuronal activity, revealed brain structures involved in other models of epilepsy [23]. An in situ hybridization method for c-fos was employed for this study because it provides a more sensitive method to detect the expression of this immediate early gene than immunocytochemical localization [24]. Briefly, GEPRs were given four audiogenic seizures in one hour because a previous study used only one audiogenic seizure and did not show c-fos immunolabeling in the superior colliculus [25]. The in situ hybridization data for c-fos showed that both the intermediate and deep layers of the superior colliculus expressed high levels for c-fos mRNA following audiogenic seizures in GEPRs (Fig. 4) These data provided further support that the superior colliculus is involved in the propagation of seizure activity in GEPRs. Later work by Faingold and Randall [26] provided physiological proof for the involvement of the deep layers of the superior colliculus in the seizure propagation pathway for audiogenic seizures in GEPRs. Thus, based on these studies, the superior colliculus is involved in the propagation of seizure activity in GEPRs.
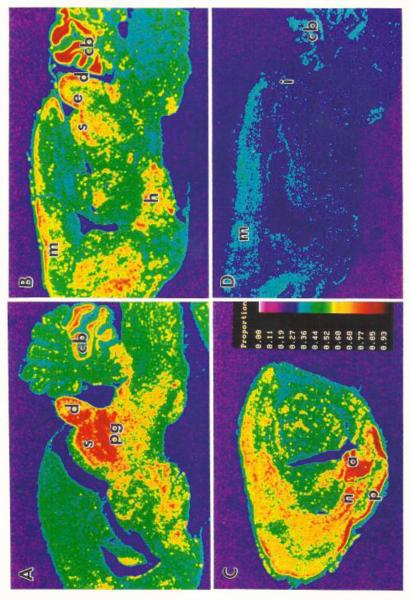
Fig. 4.
A - C are pseudo-color images from sagittal sections of c-fos mRNA 30 min after 4 seizures in one hour for a GEPR. A shows high labeling in the dorsal cortex of the inferior colliculus (d), deep layers of the superior colliculus (s) and periaqueductal grey (pg). B shows dense labeling in d and s as well as the external nucleus of the inferior colliculus (e). C has limbic structures with dense label; amygdala (a), piriform cortex (p) and endopiriform nucleus (n). D is a control section from an ‘unstimulated’ GEPR. X10. Published with permission from Ribak et al. [22].
The c-fos labeling showed interesting findings in the inferior colliculus [22]. Labeling in the dorsal cortex and external nucleus of the inferior colliculus was greater than that found in the central nucleus of this structure (Fig. 4). This result suggests that the presumed site of seizure initiation, the central nucleus, is not activated as greatly as the brain regions involved in seizure propagation, such as the external nucleus and dorsal cortex of the inferior colliculus [22].
Conclusions
In our initial papers on GEPRs, we showed that the number of GABAergic neurons, mainly the small neurons in the 10-15 μm size, were increased in the inferior colliculus. Subsequent studies showed that the GABAergic synapses in electron microscopic sections of GEPR inferior colliculus appeared normal but small neurons clustered together. Developmental and genetic studies indicated that the increase in small neurons in the GEPR inferior colliculus was present prior to seizure activity and that the increase in small neurons in the inferior colliculus showed a high correlation with audiogenic seizure score. The knife cut and c-fos mRNA labeling data provided evidence for a seizure pathway emanating from the central nucleus of the inferior colliculus, to its external nucleus and dorsal cortex, and then to the deep layers of the superior colliculus. The propagation of seizure activity is presumed to continue by an ipsilateral projection from the deep layers of the superior colliculus to the reticularis pontis oralis nucleus, and from here to the motor neurons of the spinal cord via the reticulospinal tract. These results have provided important data for our understanding of the neurotransmitters and structures that form the basis for audiogenic seizures in GEPRs.
Highlights.
Neuroanatomical studies of the genetically epilepsy-prone rat (GEPR)
Increased number of GABAergic neurons in the inferior colliculus of GEPRs
Small neuronal number correlates with audiogenic seizure scores of GEPR backcrosses
Studies on the propagation pathways for audiogenic seizures in GEPRs
Unique GABAergic, small neuron defect in the inferior colliculus of GEPRs
This defect may play a role in the initiation of audiogenic seizures in GEPRs
Acknowledgements
The author gratefully acknowledges the critical reading of the manuscript by Dr. Lee A. Shapiro. This review is based on collaborative work performed by many who worked with me in my laboratory, including Drs. Rosalinda C. Roberts, Michael Y. Byun, Howard L. Kim, Marco Navetta, Vandhana Khurana, Allan Manio, Catherine L. Morin and Nha T. Lien. In addition, collaborations with other laboratories included the following: Drs. Wolfgang H. Oertel, Christine M. Gall and Julie C. Lauterborn. This research was supported by NIH grant NS-15669.
Abbreviations
- GEPRs
Genetically epilepsy-prone rats
- GAD
glutamate decarboxylase
Footnotes
Conflict of Interest Statement
I hereby disclose that there are no actual or potential conflicts of interest that may involve any financial, personal or other relationships with other people or organizations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jobe PC, Picchioni AL, Chin L. Role of brain 5-hydroxytryptamine in audiogenic seizures in the rat. Life Sci. 1973;13:1–13. [PubMed] [Google Scholar]
- [2].Kesner RP. Subcortical mechanisms of audiogenic seizures in the rat. Exp Neurol. 1966;15:192–205. doi: 10.1016/0014-4886(66)90045-8. [DOI] [PubMed] [Google Scholar]
- [3].Wada JA, Terao A, White B, Jung E. Inferior colliculus lesion and audiogenic seizure susceptibility. Exp Neurol. 1970;28:326–32. doi: 10.1016/0014-4886(70)90240-2. [DOI] [PubMed] [Google Scholar]
- [4].Faingold CL, Gehrlach GT, Caspary DM. Decreased effectiveness of GABA-mediated inhibition in the inferior colliculus of the genetically epilepsy-prone rat. Exp Neurol. 1986;93:145–59. doi: 10.1016/0014-4886(86)90154-8. [DOI] [PubMed] [Google Scholar]
- [5].Faingold CL, Travis MA, Gehrlach G, Hoffmann WE, Jobe PC, Laird HE, II, Caspary DM. Neuronal response abnormalities in the inferior colliculus of the genetically epilepsy-prone rat. Electroencephalogr Clin Neurophysiol. 1986;63:296–305. doi: 10.1016/0013-4694(86)90097-0. [DOI] [PubMed] [Google Scholar]
- [6].McCown TJ, Greenwood RS, Frye GD, Breese GR. Electrically elicited seizures from the inferior colliculus: a potential site for epilepsy. Exp Neurol. 1984;86:527–42. doi: 10.1016/0014-4886(84)90087-6. [DOI] [PubMed] [Google Scholar]
- [7].Meldrum B. Epilepsy and GABA-mediated inihibition. Int Rev Neurobiol. 1975;17:1–36. doi: 10.1016/s0074-7742(08)60205-6. [DOI] [PubMed] [Google Scholar]
- [8].Ribak CE, Harris AB, Vaughn JE, Roberts E. Inhibitory, GABAergic nerve terminals decrease at sites of focal epilepsy. Science. 1979;205:211–4. doi: 10.1126/science.109922. [DOI] [PubMed] [Google Scholar]
- [9].Ribak CE, Hunt CA, Bakay RA, Oertel WH. A decrease in the number of GABAergic somata is associated with the preferential loss of GABAergic terminals at epileptic foci. Brain Res. 1986;363:78–90. doi: 10.1016/0006-8993(86)90660-8. [DOI] [PubMed] [Google Scholar]
- [10].Roberts RC, Ribak CE, Oertel WH. Increased numbers of GABAergic neurons occur in the inferior colliculus of an audiogenic model of genetic epilepsy. Brain Res. 1985;361:324–38. doi: 10.1016/0006-8993(85)91303-4. [DOI] [PubMed] [Google Scholar]
- [11].Roberts RC, Ribak CE. The ultrastructure of the central nucleus of the inferior colliculus of the genetically epilepsy-prone rat. Epilepsy Res. 1988;2:196–214. doi: 10.1016/0920-1211(88)90057-5. [DOI] [PubMed] [Google Scholar]
- [12].Ribak CE, Roberts RC. The ultrastructure of the central nucleus of the inferior colliculus of the Sprague-Dawley rat. J Neurocytol. 1986;15:421–38. doi: 10.1007/BF01611726. [DOI] [PubMed] [Google Scholar]
- [13].Ribak CE, Lauterborn JC, Navetta MS, Gall CM. The inferior colliculus of GEPRs contains greater numbers of cells that express glutamate decarboxylase (GAD67) mRNA. Epilepsy Res. 1993;14:105–13. doi: 10.1016/0920-1211(93)90015-y. [DOI] [PubMed] [Google Scholar]
- [14].Roberts RC, Ribak CE. An electron microscopic study of GABAergic neurons and terminals in the central nucleus of the inferior colliculus of the rat. J Neurocytol. 1987;16:333–45. doi: 10.1007/BF01611345. [DOI] [PubMed] [Google Scholar]
- [15].Roberts RC, Kim HL, Ribak CE. Increased numbers of neurons occur in the inferior colliculus of the young genetically epilepsy-prone rat. Brain Res. 1985;355:277–81. doi: 10.1016/0165-3806(85)90050-1. [DOI] [PubMed] [Google Scholar]
- [16].Ribak CE, Roberts RC, Byun MY, Kim HL. Anatomical and behavioral analyses of the inheritance of audiogenic seizures in the progeny of genetically epilepsy-prone and Sprague-Dawley rats. Epilepsy Res. 1988;2:345–55. doi: 10.1016/0920-1211(88)90046-0. [DOI] [PubMed] [Google Scholar]
- [17].Browning RA, Nelson DK, Mogharreban N, Jobe PC, Laird HE., II Effect of midbrain and pontine tegmental lesions on audiogenic seizures in genetically epilepsy-prone rats. Epilepsia. 1985;26:175–83. doi: 10.1111/j.1528-1157.1985.tb05402.x. [DOI] [PubMed] [Google Scholar]
- [18].Browning RA. Neuroanatomical localization of structures responsible for seizures in the GEPR: lesion studies. Life Sci. 1986;39:857–67. doi: 10.1016/0024-3205(86)90367-x. [DOI] [PubMed] [Google Scholar]
- [19].Cadusseau J, Roger M. Afferent projections to the superior colliculus in the rat, with special attention to the deep layers. J Hirnforsch. 1985;26:667–81. [PubMed] [Google Scholar]
- [20].Shammah-Lagnado SJ, Negrão N, Silva BA, Ricardo JA. Afferent connections of the nuclei reticularis pontis oralis and caudalis: a horseradish peroxidase study in the rat. Neuroscience. 1987;20:961–89. doi: 10.1016/0306-4522(87)90256-9. [DOI] [PubMed] [Google Scholar]
- [21].Ribak CE, Khurana V, Lien NT. The effect of midbrain collicular knife cuts on audiogenic seizure severity in the genetically epilepsy-prone rat. J Hirnforsch. 1994;35:303–11. [PubMed] [Google Scholar]
- [22].Ribak CE, Manio AL, Navetta MS, Gall CM. in situ hybridization for c-fos mRNA reveals the involvement of the superior colliculus in the propagation of seizure activity in genetically epilepsy-prone rats. Epilepsy Res. 1997;26:397–406. doi: 10.1016/s0920-1211(96)01011-x. [DOI] [PubMed] [Google Scholar]
- [23].Simler S, Hirsch E, Danober L, Motte J, Vergnes M, Maresceaux C. c-fos expression after single and kindled seizures in Wistar rats. Neurosci Lett. 1994;175:58–62. doi: 10.1016/0304-3940(94)91077-4. [DOI] [PubMed] [Google Scholar]
- [24].Dragunov M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–5. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- [25].Chakravarty DN, Faingold CL, Terry WJ, Naritoku DK. Audiogenic seizures in the genetically epilepsy-prone rat induce c-fos proto-oncogene expression in the inferior colliculus and periaqueductal grey. Soc Neurosci Abstr. 1993;19:605. [Google Scholar]
- [26].Faingold CL, Randall ME. Neurons in the deep layers of superior colliculus play a critical role in the neuronal network for audiogenic seizures: mechanisms for production of wild running behavior. Brain Res. 1999;815:250–8. doi: 10.1016/s0006-8993(98)01136-6. [DOI] [PubMed] [Google Scholar]