Abstract
Objectives: Osteoarthritis, a chronic degenerative joint disorder, is characterized by joint pain. Emerging research demonstrates that a significant number of patients evidence central sensitization (CS), a hyper-excitability in nociceptive pathways, which is known to amplify and maintain clinical pain. The clinical correlates of CS in OA, however, are poorly understood. Insomnia is prevalent in older adults with OA and recent experiments suggest associations between poor sleep and measures of CS. Catastrophizing, a potent predictor of pain outcomes has also been associated with CS, but few studies have investigated possible interactions between catastrophizing, sleep and CS. Methods: We conducted a case controlled study of 4 well characterized groups of adults with insomnia and/or knee osteoarthritis. A total of 208 participants completed multimodal sleep assessments (questionnaire, diary, actigraphy, polysmnography) and extensive evaluation of pain using clinical measures and quantitative sensory testing to evaluate associations between CS, catastrophizing and insomnia. Descriptive characterization of each measure is presented, with specific focus on sleep efficiency and CS. Results: The KOA-Insomnia group demonstrated the greatest degree of CS compared to controls. In the overall sample, we found that catastrophizing moderated the relationship between sleep efficiency and CS. Specifically those with low sleep efficiency and high catastrophizing scores reported increased levels of CS. In addition, CS was significantly associated with increased clinical pain. Conclusions: These findings highlight the importance of assessing sleep efficiency, CS and catastrophizing in chronic pain patients and have important clinical implications for treatment planning.
Keywords: Sleep, Catastrophizing, Pain, Quantitative Sensory Testing, Central Sensitization
Pain is the most common symptom of Knee Osteoarthritis (KOA), a degenerative joint disease that is characterized by loss of cartilage in the knee, development of osteophytes and mobility limitation. KOA is one of the leading causes of pain and disability worldwide, affecting approximately one third of older adults(1;2). A growing literature has documented augmented central nervous system (CNS) processing in OA. In animal models, Central Sensitization (CS) is demonstrated as aberrant heighten spinal and supraspinal processing that increase afferent nociceptive input. Clinically it manifests as hypersensitivity to pain, that sometimes spreads beyond peripheral generators and is a marker for pain chronification(3;4). CS can be assessed through the application of noxious stimuli (e.g. temporal summation, pain after-sensations)(3). Both animal models and human laboratory and imaging studies have implicated CS of nociceptive pathways as a mechanism of clinical pain amplification in OA(5). Recent work from our group found substantial discordance between clinical pain reports and radiographic evidence of OA in KOA patients (e.g., increased reports of clinical pain were not corroborated by radiographic evidence of cartilage loss/OA). Patients with a high degree of clinical pain but minimal to mild radiographic evidence of joint disease, exhibited greater CS and conversely, patients reporting low clinical pain with moderate to severe radiographic evidence demonstrated reduced CS(6).
Sleep disturbance has become recognized as an important factor in determining pain perception. Systems that regulate both arousal and pain are neurobiologically intertwined, and may share common pathways(7). Sleep disruption in healthy individuals amplifies pain perception(8) and is associated with increased pain sensitivity and disability in chronic pain patients(9). Sleep disturbance also increases risk for developing pain complaints(10) and disrupted sleep following painful injury is associated with developing persistent post-injury pain(11).
Insomnia, defined as a subjective report of problems initiating or maintaining sleep, or non-restorative sleep, is the most common sleep disorder and similar to KOA, has increased prevalence in later life; almost 50% of older adults report insomnia complaints(7). Further, KOA and insomnia frequently co-occur and greater than 50% of patients with OA suffer from significant disturbances initiating or maintaining sleep(12). A relationship between sleep disturbance and pain severity in OA patients is consistently observed and sleep disturbances such as shortened sleep duration and fragmented sleep(12) have been specifically hypothesized to have direct effects on hyperalgesia in OA patients(13). Polysomnography (PSG), the gold standard sleep measure, has been applied in only a few small studies of KOA. Large multi-modal evaluations of sleep utilizing PSG, self-report and other measure of sleep are needed to better characterize and determine associations between sleep parameters, CS, and pain in KOA.
Catastrophizing, a persistently negative cognitive affective style characterized by helplessness, magnification and ruminative thoughts regarding one’s pain, is a potent predictor of negative pain-related outcomes in general(14) and OA pain specifically(15). Catastrophizing has been linked to both sleep disturbance(16) and indices of CS(17;18). Little empirical work has focused on the combined effects of catastrophizing and sleep disturbance on pain or CS, despite the fact that interventions designed to reduce pre-sleep pain-related catastrophizing cognitions are actively being tested in patients with comorbid insomnia and chronic pain (see(19) for review).
The current study sought to characterize sleep, pain, and their association in knee OA patients with and without insomnia versus matched controls with and without insomnia. This was accomplished by employing multi-modal evaluation using PSG, actigraphy, sleep questionnaires and diary measures, and multi-modal evaluation of pain using clinical measures and quantitative sensory testing (QST). With respect to sleep-related measures, we hypothesized that KOA-I patients would exhibit objectively worse sleep efficiency than insomnia patients and good-sleeping subjects. With respect to pain-related measures, we hypothesized that KOA-I patients would evidence greater clinical pain, as well as greater CS than good sleeping KOA patients and non-KOA controls. In order to reduce the number of comparisons presented and potential for family-wise error, we present descriptive information for all variables and have only conducted statistical analyses on our measures of specific interest: sleep efficiency, CS and questionnaires. Out of all of the sleep and pain variables we measured, sleep efficiency and CS were chosen as they are the strongest summary measures representing the overall quality of sleep continuity and hypersensitivity to pain.
In addition to the primary aims, we also explored the extent to which pain catastrophizing moderated the association of subjective sleep efficiency, which has well-documented associations with clinical and laboratory pain(20) as well as catastrophic thinking(14;21), and CS across subjects. The diary measure of sleep efficiency was chosen as it is widely used and well-validated in older adults(22) and represents individual’s perception of their own sleep.
Materials and Methods
The current case control study is the first phase of a project that involves a randomized controlled trial of CBT for Insomnia in Osteoarthritis. Participants were recruited via advertisements in community media outlets and physician offices. A total of 208 participants (72.1% female) were included in the current analyses. Participants were categorized into four groups based on diagnostic eligibility criteria: 1) OA patients meeting research diagnostic criteria (RDC) for insomnia disorder(23) (KOA-I, n=118); 2) OA patients meeting RDC criteria for normal sleep (KOA, n=31); 3) Healthy controls meeting RDC criteria for PI (PI, n=30); and 4) Healthy controls, without a pain syndrome, meeting RDC criteria for normal sleep (controls, n=29). KOA groups were matched on radiographic evidence of disease severity (Kellgren-Lawrence grading system(24)).
For inclusion in the KOA groups, all participants met American College of Rheumatology (ACR) criteria, based on history and physical exam, bilateral standing, and semi-flexion view radiographs, diagnosed by a board certified rheumatologist(25); had at least one knee rated at least 1 on the Kellgren-Lawrence scale for radiographic assessment of joint damage; had knee pain >2/10 on a near daily basis (>4 days/week) for at ≥6 months prior to entering the study; not be scheduled for arthoplasty during the study period; and (for those taking medications) maintain a stable dose for ≥one month prior to starting the study.
For inclusion in the insomnia groups, participants were further examined and categorized based on the Structured Interview for Sleep Disorders (SIS-D (26)) to confirm that subjects met both the American Academy of Sleep Medicine research diagnostic criteria(23) and DSM criteria for insomnia disorder. Additional sleep criteria included report of either latency to sleep onset >30 minutes, or ≥2 awakenings/night of >15 duration, or wake after sleep onset time >30 minutes as well as insomnia symptom frequency ≥3 night/week for ≥1 month. For inclusion into the “normal sleeper” group, participants were required to meet RDC (Insomnia) criteria for normal sleepers and a Pittsburgh Sleep Quality Index <5. Criteria for normal sleepers were required at intake (retrospective) and as an average profile (2–weeks of baseline diaries)(27).
Healthy control participants were required to be free from any acute pain, injury, or a history of chronic pain in the past 3 years (pain severity report >2 out of 10, >2 day/week for 6 months) and report good overall health with no unstable major medical/psychiatric illness. General exclusion criteria, applied to all groups, included having a serious medical illnesses, such as congestive heart disease, history of cerebral vascular accidents, cancer, and other chronic pain or rheumatic disorders; severe or unstable psychopathology, cognitive impairment/dementia, current/recent history (≤6 months) substance abuse disorder (or positive toxicology screening), or major medical illness. Participants were also excluded if they were found to have any untreated sleep disorders other than insomnia (e.g., OSA, PLMD, apnea/hypopnea Index (AHI) >15). Participants agreed to discontinue all pain-relieving and sedative medications 24 hours prior to the QST session.
Procedures
All participants completed informed consent procedures and were administered a clinical examination/interview, self-report questionnaires (The Western Ontario McMaster Universities Osteoarthritis Scale (WOMAC)(28), The Pain Catastrophizing Scale (PCS)(29), and The Center for Epidemiologic Studies Depression Scale (CES-D)(30)), sleep assessments (The Pittsburgh Sleep Quality Index (PSQI)(31), The Insomnia Severity Index (ISI)(32), The Epworth Sleepiness Scale (ESS)(33) electronic sleep diaries, actigraphy and in-home, ambulatory overnight PSG), electronic pain diary (similar to the Brief Pain Inventory(34)), and a series of quantitative sensory tests (described below). For those included in the KOA groups, bilateral knee x-rays were performed as well as a knee exam conducted by a rheumatologist, as previously described(6). The clinical interview included use of the SIS-D(26), a structured interview for sleep disorders according to the DSM-III-R(35), which demonstrates sound reliability and validity based on PSG and expert interviews. The interview generates current and lifetime DSM Axis-I and Axis-III sleep disorder diagnoses. Axis-III diagnoses such as obstructive sleep apnea were considered provisional until confirmed by the sleep laboratory findings.
Sleep Assessments
Diaries
A sleep diary was completed on a Palm Pilot (Tungsten E2, Palm, Sunnyvale, CA); specifically, participants entered the time they went to bed, latency to sleep onset, wake after sleep onset (WASO), final awakening, and time out of bed each morning for two weeks in order to create indexes of sleep continuity.
Wrist Actigraphy. Actigraphs are watch-like devices worn on the non-dominant wrist which continuously track movements. Participants were trained in wrist actigraphy monitoring and wore an Actigraph 2 (Philips Healthcare/Respironics, Bend, OR) for two weeks (while completing diaries) to provide an objective index of sleep efficiency and latency, WASO, and total sleep time (TST). Data were collected in 60-second epochs with medium sensitivity. We utilized a PSG-validated algorithm to estimate sleep continuity parameters(37).
Polysomnography
Following completion of actigraphy, participants underwent a one-night(38), in-home, ambulatory overnight PSG sleep study with demonstrated reliability(39), fully described by the Sleep Heart Healthy Study(40) (SHHS). Two highly trained PSG technicians used standard procedures to obtain the recording montage using standard placement of EEG, EOG, and EMG(41). PSG was scored in 30-second epochs according to standard R&K criteria(41). A subset (i.e., 10%) of all the records from the project was re-scored in order to asses inter-rater agreement.
Quantitative Sensory Testing (QST
Pain threshold
Heat pain threshold (HPTh) was assessed via a peltier-element-based stimulator (Medoc, Pathway, Advanced Thermal Stimulator (ATS) thermode), on the non-dominant ventral forearm and the patella of the index knee, with a 9 cm2 probe, using an ascending method of limits paradigm with a .5°C/sec rate of rise. Pressure pain threshold (PPTh) was assessed via algometer (SBmedic, 1 cm2 hard rubber probe) 2 times at the trapezius muscle (bilateral), and the insertion point of the quadriceps (index knee) according to standard procedures(42). Mean HpTh (in °C) and PpTh (in kilopascals) values were averaged across trials, respectively.
Temporal Summation
Thermal Temporal Summation (TTS) was assessed via response to three series (temperatures [randomized]: tailored (designed to be moderately painful), 49, 51°C), each of 10 heat pulses of equal temperature rated on a 0–100 scale, applied to the left dorsal forearm by the Medoc, Pathway Contact Heat-Evoked Potential Stimulator (CHEPS)(43). A 2.5 second inter stimulus interval (ISI) and a 70°C/sec rate of rise was employed. A TTS difference score (maximal rated pulse of the series minus first pulse of the series) was created for each temperature. One additional pain rating was obtained 15 seconds following the final pulse in each series to characterize after sensations. Mechanical Temporal Summation (MTS) was assessed at two weights via response to an initial single stimulus, and then to a sequence of 10 stimuli of identically weighted punctate probes applied on a flat contact area of 0.2 mm diameter with a force of 256 mN and 512mN, to the middle phalanx, dorsal surface of the non-dominant middle finger and the patella of the index knee (randomly assigned for non-OA patients). Each series was delivered with an ISI of 1 second, participants were instructed to rate the “peak” pain experienced over the train of 10 stimuli. A MTS difference score was calculated (peak rating minus initial stimulus rating) for each probe weight. Similar procedures assessing responses to repetitive suprathreshold noxious stimuli as an index of central sensitization have been previously employed in a variety of subjects(44).
Cold Pressor
Pain ratings were additionally assessed using cold pressor testing (CPT). Participants immersed the non-dominant hand in a circulating water bath maintained at 4°C. A total of 4 immersions lasting a maximum of 45 seconds were conducted. Participants were permitted to remove their hand prior to the completion of the trial if the pain became intolerable. Pain ratings on a 0–100 scale were obtained at 20 seconds, and participants removed their hand from the water bath, and (to characterize after sensations) additionally at 30 seconds, 1 minute and 2 minutes post hand removal. Pain ratings were averaged across the four trials.
Conditioned pain modulation
Two PpTh readings were obtained on the dominant side trapezius muscle immediately prior to commencing the CPT. At the 20 second point during each of the hand immersion trials for CPT, a PpTh reading was obtained on the contralateral trapezius muscle. A difference value was created for CPM, such that the 2 PpTh values obtained during each of the CPT trials were averaged and the average of the 2 baseline PpTh readings was subtracted from it (during-baseline to yield a positive number if threshold increased during hand immersion).
All QST methods were z-scored separately, reversed where appropriate, and combined to establish one “sensitivity index,” as previously described(45), where higher values represent greater sensitivity. Similarly, thermal and mechanical temporal summation, CPM and after sensation z-scores were combined to create one measure of central sensitization, which was the primary QST-derived variable of interest. Of note, difference scores were computed as opposed to index scores (for MTS, TTS and CPM), as creating index scores would have created missing data for subjects (in those with no baseline pain, by attempting to divide by 0), this would have primarily been problematic for MTS. We chose to keep this calculation consistent and use a difference score for all variables requiring such computation.
Statistical Analyses
All analyses were conducted using SPSS 20. Chi-Square tests were used to compare categorical demographic variables between the participant groups. In order to reduce multiple comparisons, a series of Analyses of Variance (ANOVA), to examine group differences, were only conducted on measures of sleep efficiency, CS (obtained from laboratory pain responses to specific tests), and questionnaires of interest. Pearson product-moment correlations were conducted to examine the relationship between these variables as well Differences between objectively (actigraphy) and subjectively (PDA) reported sleep were also evaluated within night for each person.. Pain testing measures thought to reflect CS were z-scored and combined to create an index. The CS measure included TTS, MT), CPM (CPM was reversed [multiplied by −1] in order to make the direction of sensitivity consistent and comparable across tests) and after sensations to thermal temporal summation and cold water hand immersion. A general QST Sensitivity Index was also created which combines every QST measure (reversed where appropriate). Planned contrasts were conducted to compare the CS variable between each group.
Hayes’(46) PROCESS macro was employed to examine the potential moderating effect of catastrophizing on the relationship between sleep and CS. An ordinary least squares or logistic regression-based path analytical framework is employed in this macro to analyze statistical models. Model 1, for simple moderation was used in the current analyses. Age was entered as a covariate to control for potential demographic confounds.
Results
Patient demographics and clinical characteristics for each group are presented in Table 1. All demographic characteristics of the sample were roughly equivalent between groups with the exception of age. The KOA group was significantly older than each of the other groups (p’s<0.001). Self-reported clinical measures varied by group in a generally predictable manner, with those in the KOA-I group generally endorsing the highest pain (WOMAC), depression, catastrophizing and BMI. Participants in the PI group also endorsed elevated depressive symptomatology and the poorest sleep quality (as measured by PSQI). In the entire sample, clinical pain report was significantly associated with each sleep questionnaire (r’s range from .3–.4;p’s<0.001), catastrophizing (r=.48;p< 0.001), depression (r=.32;p<0.001), diary sleep efficiency (r=−.32;p<0.001) and the CS measure (r=.32;p<0.001). Of note, diary sleep efficiency was correlated with each measure (r’s range from −.22 to −.70;p’s<0.04). Additionally, the CS measure was correlated with all variables (r’s range from −.22 to .32;p’s<0.02) other than sleepiness (ESS).
Table 1.
Demographic and Clinical Characteristics by Group [Mean (SD) / % (n)]
| Demographic and Clinical Variables |
Controls: No Pain Disorder | Knee Osteoarthritis (ACR) | Total Sample | ||
|---|---|---|---|---|---|
| RDC Good sleeper |
RDC Primary Insomnia |
RDC Good Sleeper |
RDC Insomnia | ||
| Age (mean ± SD) | 57.6 (7.3) | 58.9 (8.6) | 66.55 (10.6)*** | 59.2 (9.1) | 60.0 (9.4) |
| Female | 62.1 (18) | 80.0 (24) | 51.6 (16) | 78.0 (92) | 72.1 (150) |
| Race/Ethnicity | N = 29 | N = 30 | N = 31 | N = 118 | N = 208 |
| Non-Hispanic White American | 69.0 (20) | 50.0 (15) | 74.2 (23) | 55.1 (65) | 59.1 (123) |
| African American | 24.1 (7) | 46.7 (14) | 22.6 (7) | 41.5 (49) | 37.0 (77) |
| Asian American | 3.4 (1) | 3.3 (1) | 3.2 (1) | 2.5 (3) | 2.9 (6) |
| Multiracial | 3.4 (1) | 0 | 0 | .8 (1) | 1.0 (2) |
| Education Level | N = 29 | N = 30 | N = 31 | N = 117 | N = 207 |
| High School/Some College | 31.0 (9) | 43.2 (13) | 32.3 (10) | 51.4 (60) | 44.4 (92) |
| College Graduate | 44.8 (13) | 30.0 (9) | 22.6 (7) | 21.4 (25) | 26.1 (54) |
| Graduate Studies | 24.1 (7) | 26.7(8) | 45.1 (14) | 27.4 (32) | 29.5 (61) |
| Occupational Status | N = 29 | N = 30 | N = 31 | N = 117 | N = 207 |
| Full-Time Employment | 34.5 (10) | 30 (9) | 25.8 (8) | 29.1 (34) | 29.5 (61) |
| Part-Time Employment | 20.7 (6) | 23.3 (7) | 16.1 (5) | 13.7 (16) | 16.4 (34) |
| Student | 3.4 (1) | 0 | 0 | 0 | 0.5 (1) |
| Homemaker | 3.4 (1) | 10.0 (3) | 3.2 (1) | 5.1 (6) | 5.3 (11) |
| Unemployed | 17.2 (5) | 13.3 (4) | 0 | 20.5 (24) | 15.9 (33) |
| Retired | 20.7 (6) | 23.3 (7) | 54.8 (17) | 31.6 (37) | 32.4 (67) |
| Marital Status | N = 29 | N = 30 | N = 31 | N = 117 | N = 207 |
| Single/Divorced/Separated/Widowed | 58.6 (17) | 53.4 (12) | 38.8 (12) | 53.8 (63) | 52.2 (108) |
| Living with partner | 3.4 (1) | 0 | 3.2 (1) | 2.6 (3) | 2.4 (5) |
| Married | 34.5 (10) | 46.7 (14) | 48.4 (15) | 41.9 (49) | 42.5 (88) |
| Yearly Household Income | N = 28 | N = 27 | N = 30 | N = 101 | N = 186 |
| $25,000 or less | 21.4 (6) | 18.5 (5) | 3.3 (1) | 28.7 (29) | 22.0 (41) |
| $25,001–$50,000 | 39.3 (11) | 18.5 (5) | 36.7 (11) | 31.7 (32) | 31.7 (59) |
| $50,001–$75,000 | 17.9 (5) | 25.9 (7) | 33.3 (10) | 13.9 (14) | 19.4 (36) |
| >$75,000 | 21.4 (6) | 37.0 (10) | 26.7 (8) | 25.7 (26) | 26.9 (50) |
| Clinical Variables | N = 28–29 | N = 26–31 | N = 29–30 | N = 114–118 | N = 198–208 |
| BMI | 26.0 (4.8) | 26.8 (5.1) | 29.1 (4.7)* | 31.2 (6.5)*** | 29.5 (6.2)*** |
| SBP | 124.8 (17.0) | 122.6 (17.5) | 132.4 (22.2) | 129.7 (19.3) | 128.3 (19.3) |
| DBP | 77.9 (12.4) | 77.9 (12.1) | 79.0 (12.4) | 81.3 (11.0) | 80.0 (11.6) |
| WOMAC (total score) | .11 (.1) | .22 (.2) | 3.0 (2.2)*** | 4.9 (2.2)*** | 3.3 (2.8)*** |
| Diary Pain (0–100) | 12.9 (9.2) | 18.1 (12.5) | 31.2 (18.8)*** | 46.0 (18.5)*** | 36.4 (21.3)*** |
| PSQI | 2.6 (1.8) | 12.5 (3.3)*** | 3.4 (2.2) | 11.9 (3.2)*** | 9.4 (5.1)*** |
| ISI | 1.3 (1.7) | 17.6 (5.1)*** | 2.2 (2.5) | 17.2 (4.9)*** | 12.8 (8.3)*** |
| ESS | 4.3 (3.0) | 7.1 (6.0)* | 5.6 (3.9) | 9.5 (5.1)*** | 7.8 (5.2)*** |
| PCS | 6.1 (8.4) | 6.9 (9.4) | 4.4 (4.4) | 15.2 (10.3)*** | 11.1 (10.4*** |
| CES-D | 3.1 (3.3) | 13.9 (8.2)*** | 3.5 (4.1) | 14.1 (8.5)*** | 10.9 (8.8)*** |
Differ from Non Pain Control Good Sleeper Reference group, two-tailed t-test
(p<.05),
(p<.01),
(p<.001).
Omnibus significance level is noted in the Total Sample column
Abbreviations: BMI: Body Mass Index; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; WOMAC: Western Ontario McMaster Universities Osteoarthritis Scale; PSQI: Pittsburgh Sleep Quality Index; ISI: Insomnia Severity Index; ESS: Epworth Sleepiness Scale; PCS: Pain Catastrophizing Scale; CES-D: Center for Epidemiologic Studies Depression Scale
Sleep
Measures of sleep continuity by measurement method, sleep architecture and clinical sleep disorder indices are presented in Table 2. The pattern of findings in sleep continuity measures appears to depend on assessment method. The KOA-I group demonstrated greater sleep discontinuity as measured by diminished sleep efficiency on Diary, Actigraphy and PSG measures. Both insomnia groups reported significantly lower sleep efficiency when compared to both non-insomnia groups (p<0.001) on their sleep diaries. KOA-I participants also experienced reduced sleep efficiency measured by actigraphy compared to healthy controls and, surprisingly, PI participants. KOA-I participants also had reduced sleep efficiency compared to controls on PSG. Controlling for age did not alter significance. Total sleep time (TST), sleep latency and wake after sleep onset (WASO) in the insomnia groups all appeared worse than good sleeping groups on diary measures. However, patterns appeared more inconsistent for actigraphy and PSG. Interestingly, on both Actigraphy and PSG measures of TST, all 4 groups demonstrated low TST that appeared about equivalent. The largest within-night differences between objective and subjective sleep measures were observed primarily in the KOA group. This group was observed to have the greatest difference between objective and subjective measures for SE (21% worse observed in actigraphy compared to PDA report), TST (108 more minutes slept reported in PDA) and WASO (102 minutes less reported in PDA). The PI group had the largest difference for SOL, believing it took on average 30 minutes longer to fall asleep (reported in PDA) than indicated by actigraphy. Sleep architecture and clinical sleep disorder indices were comparable between groups, except for periodic limb movement, which appeared substantially greater among the KOA-I group compared to controls.
Table 2.
Measures of Sleep by Group [Mean (SD)]
| Sleep Parameters | Controls: No Pain Disorder | Knee Osteoarthritis (ACR) | Total Sample (N=194) |
||
|---|---|---|---|---|---|
| RDC Good sleeper (n = 29) |
RDC Primary Insomnia (n = 28) |
RDC Good Sleeper (n = 30) |
RDC Insomnia (n = 116) |
||
|
Sleep Continuity | |||||
| Sleep Efficiency (%) | |||||
| Diary | .91 (.1) | .64 (.1)***a,b | .88 (.1) | .67 (.1)***a,b | .73 (.2)*** |
| Actigraphy | .73 (.1) | .71 (.2) | .66 (.1) | .65 (.2)*a,c | .67 (.1)* |
| PSG | .82 (.1) | .76 (.1) | .77 (.1) | .74 (.1)**a | .76 (.1)† |
| Total Sleep Time (min) | |||||
| Diary | 415.0 (49.6) | 311.0 (77.2) | 402.5 (68.3) | 324.3 (74.3) | 346.4 (80.9) |
| Actigraphy | 327.1 (50.1) | 335.7 (75.7) | 298.0 (71.7) | 306.7 (78.4) | 312.8 (74.3) |
| PSG | 376.9 (77.5) | 370.6 (84.0) | 370.2 (60.3) | 351.5 (99.6) | 360.4 (89.9) |
| Sleep Latency (min) | |||||
| Diary | 14.8 (10.5) | 50.1 (25.6) | 16.1 (10.3) | 49.4 (35.2) | 39.9 (32.7) |
| Actigraphy | 14.9 (10.2) | 20.3 (21.1) | 14.1 (8.3) | 26.8 (24.3) | 22.4 (21.3) |
| PSG | 29.0 (43.3) | 25.1 (24.2) | 21.4 (29.8) | 31.9 (38.8) | 29.0 (36.5) |
| Wake After Sleep Onset (min) | |||||
| Diary | 14.4 (17.7) | 76.4 (38.3) | 25.4 (24.5) | 70.5 (43.3) | 56.9 (44.3) |
| Actigraphy | 94.0 (58.0) | 96.9 (62.4) | 123.1 (73.8) | 120.2 (75.9) | 113.4 (71.9) |
| PSG | 53.3 (37.8) | 94.8 (84.8) | 95.4 (51.3) | 86.2 (61.9) | 84.3 (62.6) |
|
Sleep Architecture | |||||
| NREM Stage 1% | 8.9 (4.3) | 8.4 (5.3) | 8.8 (5.0) | 8.9 (5.6) | 8.8 (5.3) |
| NREM Stage 2% | 56.1 (7.7) | 56.8 (10.5) | 53.4 (9.9) | 56.6 (10.8) | 56.1 (10.3) |
| NREM Stage 3 (slow wave sleep) | 13.1 (11.7) | 11.6 (9.6) | 16.1 (9.0) | 13.8 (13.3) | 13.7 (12.1) |
| REM % | 22.0 (7.3) | 23.2 (5.8) | 21.7 (6.3) | 21.3 (8.8) | 21.7 (7.9) |
| REM Latency (min, cycle 1) | 107.7 (52.6) | 117.2 (47.2) | 95.2 (44.5) | 132.9 (82.6) | 121.7 (71.2) |
|
Clinical Sleep D/O Indices | |||||
| Apnea Hypopnea Index | 4.6 (3.4) | 4.6 (3.9) | 5.0 (3.6) | 5.2 (4.6) | 5.0 (4.2) |
| Respiratory Disturbance Index | 10.2 (5.8) | 10.6 (6.4) | 9.7 (4.9) | 11.4 (6.3) | 10.9 (6.1) |
| Average SPO2 Low during sleep (min) | 85.7 (7.7) | 82.4 (16.9) | 85.6 (3.6) | 83.8 (10.7) | 84.1 (10.7) |
| Periodic Limb Movement Index | 4.3 (6.2) | 3.15 (5.2) | 19.6 (25.2) | 12.6 (18.9) | 11.2 (18.3) |
| Periodic Limb Movement with Arousal Index | .34 (.4) | .36 (.8) | 3.3 (6.5) | 2.2 (5.5) | 1.8 (5.0) |
| Spontaneous Arousal Index | 5.2 (4.1) | 5.6 (5.5) | 4.1 (2.8) | 6.7 (7.7) | 5.9 (6.5) |
| Overall Arousal Index | 12.6 (5.9) | 14.2 (10.2) | 13.6 (8.6) | 16.8 (12.7) | 15.4 (11.2) |
Two-tailed t-test
(.05<p<.10),
(p<.05),
(p<.01),
(p<.001).
different from control,
different from KOA good sleeper,
different from Primary Insomnia (no KOA). Omnibus significance level is noted in the Total Sample column.
RDC Good sleeper is defined by Edinger et al., 2004(23).
Quantitative Sensory Testing (QST
Laboratory pain measures are displayed for each group in Table 3. Additionally, z-scored values (makes laboratory measures with different response scales comparable) are presented in Figure 1. A significant difference was observed between groups on the CS measure, our primary QST measure of interest, with KOA-I patients being the most sensitive (p=0.01). Specifically, they were significantly more sensitive when compared to healthy controls (p=0.002) and marginally so when compared with PI patients (p=0.06). Identical significance levels were observed when controlling for age. The QST index appeared to differ between groups, again with KOA-I patients being the most sensitive. With regard to individual laboratory tests, only pressure pain threshold at the index knee and both after sensations appeared different between groups, with KOA-I being most sensitive. Surprisingly, for PPTh at the knee, the PI group appeared to be the least pain sensitive of all the groups.
Table 3.
QST Measures by Group [Mean (SD)]
| QST Measures | Controls: No Pain Disorder | Knee Osteoarthritis (ACR) | Total Sample (n = 203) |
||
|---|---|---|---|---|---|
| RDC Good sleeper (n = 15–29) |
RDC Primary Insomnia (n=21–30) |
RDC Good Sleeper (n = 18–27) |
RDC Insomnia (n = 93–117) |
||
|
Central Sensitization | |||||
| Central Sensitization Score | −0.2 (0.5) | −0.1 (0.5) | −0.02 (0.5) | 0.1 (0.6)**a | 0.03 (0.6)** |
| QST Index Score | −0.2 (.4) | −0.1 (0.5) | −0.1 (0.4) | 0.1 (0.5) | 0.02 (0.5) |
|
Thermal Pain Threshold | |||||
| Forearm | 42.3 (3.3) | 41.3 (3.8) | 42.1 (3.8) | 41.4 (3.7) | 41.6 (3.6) |
| Patella (Index knee) | 43.9 (3.6) | 44.0 (3.7) | 43.9 (3.6) | 43.0 (3.7) | 43.4 (3.6) |
|
Thermal Temporal Summation | |||||
| Forearm-Difference Score | 6.4 (6.8) | 6.0 (8.4) | 11.3 (12.5) | 7.5 (11.5) | 7.6 (10.7) |
| After Sensation Ratings | 10.0 (16.2) | 11.8 (19.7) | 6.8 (12.4) | 19.8 (26.9) | 15.5 (23.5) |
|
Pressure Pain Threshold | |||||
| Quadriceps (Index knee) | 564.7 (222.0) | 668.1 (257.0) | 519.2 (202.8) | 507.6 (234.2) | 541.0 (237.2) |
| Trapezius | 367.3 (160.3) | 423.1 (152.8) | 403.4 (183.4) | 355.9 (150.0) | 373.8 (157.6) |
|
Mechanical Temporal Summation | |||||
| Forearm-Difference Score | 12.3 (11.1) | 13.4 (11.6) | 14.9 (12.8) | 15.4 (12.5) | 14.6 (12.2) |
| Knee-Difference Score | 18.8 (20.4) | 26.2 (19.5) | 24.6 (16.0) | 26.0 (20.1) | 24.8 (19.6) |
|
Cold Pressor Tests | |||||
| Cold Water Pain | 60.2 (29.0) | 78.8 (17.6) | 66.3 (23.4) | 72.5 (24.8) | 71.4 (24.5) |
| CPM Difference Trapezius | 102.7 (99.1) | 92.4 (96.6) | 73.8 (85.2) | 74.4 (89.0) | 81.0 (91.1) |
| After Sensation Ratings | 19.8 (18.0) | 24.7 (17.2) | 19.6 (18.8) | 31.8 (21.7) | 27.4 (20.8) |
Two-tailed t-test
(p<.05),
(p<.01).
different from control
Omnibus significance level is noted in the Total Sample column. Difference Scores represent the maximal rated pulse (for Thermal Temporal Summation) or following the train of 10 stimuli (for Mechanical Temporal Summation) of the series minus first pulse of the series. CPM: Conditioned Pain Modulation, CPM Difference represents pressure pain thresholds at the trapezius obtained during water immersion of the hand minus baseline trapezius pressure pain thresholds.
Figure 1.
Z-scored means and standard error for laboratory pain methods displayed by group. Measures denoted with ‡ were reversed (multiplied by −1) in order to keep directionality consistent.
*p < 0.05: Different from controls. HpTh = Heat Pain Threshold, PpTh = Pressure Pain Threshold, MTS = Mechanical Temporal Summation, TTS = Thermal Temporal Summation, CP = Cold Pressor, CPM = Conditioned Pain Modulation, AS = After Sensation, CS = Central Sensitization (combines MTS, TTS, CPM and AS).
Moderation Analysis
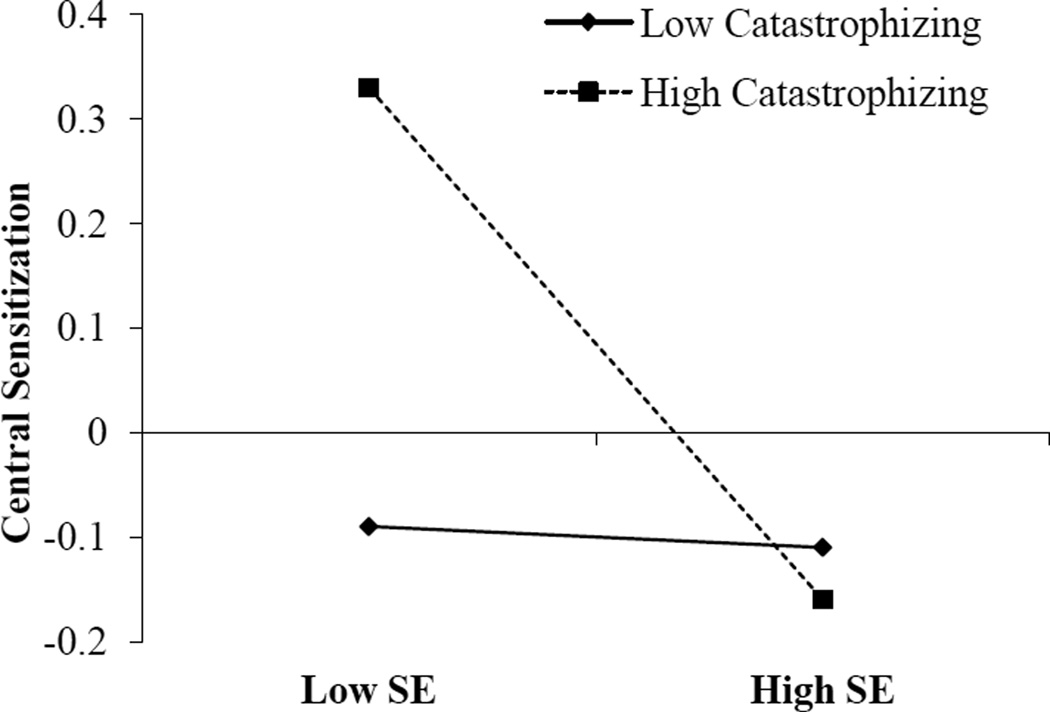
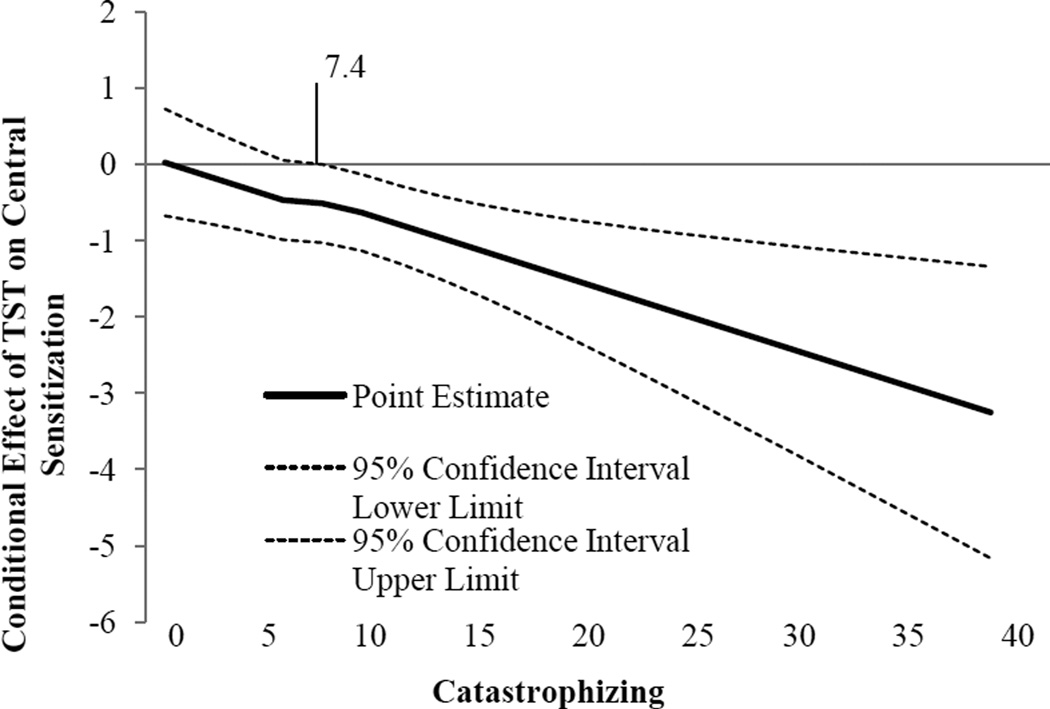
Catastrophizing was significantly associated with CS such that greater catastrophizing was associated with higher levels of CS (ß=0.009, p=0.02). Sleep efficiency (measured by diary and averaged over the assessment period) was significantly associated with CS (ß=−0.80, p=0.003). A significant interaction emerged between catastrophizing and sleep efficiency (ß=−0.07, p=0.007). This interaction is represented graphically in Figure 2, which depicts the simple slope of sleep efficiency for low (−1 standard deviation) and high (+1 standard deviation) catastrophizing. Simple slopes were tested across levels of sleep efficiency and only low sleep efficiency revealed a significant association between catastrophizing and CS (ß=.02, p=0.0001). Of note, when controlling for clinical pain, as well as sex, the pattern of significance does not change. We probed the interaction further by use of the Johnson-Neyman technique(46) to evaluate the regions of significance of the conditional effect. This allows for visualization of the range of values within the moderator where the interaction is significant. Figure 3 plots the conditional effect of sleep efficiency on CS across values of catastrophizing. The region of significance lies where the confidence interval does not include 0. Thus, sleep efficiency is associated with CS when catastrophizing is >7.4. Those with catastrophizing scores >7.4 accounted for 56.3% (n=116) of the sample (note, two participants were missing PCS scores). While only 22–33% of participants in each group (controls[n=9], KOA[n=7], PI[n=10]) endorsed catastrophizing above this level, it represented 77% of the KOA-I patients(n=90).
Figure 2.
Interaction of Catastrophizing and Sleep Efficiency predicting Central Sensitization.
Figure 3.
Conditional effect of Sleep Efficiency on Central Sensitization across values of Catastrophizing. The region of significance lies where the confidence interval does not include 0. Thus, sleep efficiency is associated with central sensitization when catastrophizing is higher than 7.4
Discussion
To our knowledge, this is the largest multimodal sleep study in KOA patients to date and the only one to use in home measures. KOA-I showed strong evidence of sleep continuity disturbance measured across multiple modalities. These findings suggest various differences between knee OA patients with and without insomnia versus controls with and without insomnia in terms of sleep parameters, psychological factors, clinical pain and laboratory pain sensitivity. Participants in our study’s two insomnia groups varied in their subjective versus their objectively determined sleep parameters. Such discordance is not unusual in the larger literature(47). However, the non-insomnia groups (controls and particularly the KOA participants without insomnia), over-estimated their sleep efficiency and TST and underestimated there WASO. Participants in the PI group overestimated their sleep onset latency by approximately 30 minutes per night when compared with actigraphy. Additionally, we observed evidence of high rates of periodic limb movement in KOA. One other report has suggested a relationship between osteoarthritis and excessive night-time movement(48); however, these data should be examined in light of their potential contribution to sleep difficulty and contribution to pain in OA patients. While we were underpowered to fully examine all possible data described, we hope that these data serve as a platform for future studies to adequately power themselves, and explore potential differences.
Catastrophizing is an increasingly recognized factor influencing clinical pain in OA patients(15). Several reports have found a relationship between catastrophizing and temporal summation of pain, a marker of CS(17;18). A number of published reports have documented the relationship between poor/disturbed sleep and hyperalgesia(7;20). In addition, one study by members of our group found that a significant proportion of variance in clinical pain severity and pain-related interference attributable to pain catastrophizing was actually mediated by sleep disturbance in a facial pain population(16). Catastrophizing, sleep efficiency and an interaction between sleep efficiency and catastrophizing significantly moderated the severity of CS in participants. These results provide preliminary evidence in support of a combined effect of catastrophizing and sleep resulting in increased pain sensitivity in OA. CS has been hypothesized to account for the hypersensitivity observed in a number of chronic pain conditions(3). Support for this theory has been evidenced by enhanced temporal summation, after sensations, secondary hyperalgesia, and/or tactile allodynia among patients with various conditions, including OA and rheumatoid arthritis, temporomandibular joint disorder, fibromyalgia, headache, neuropathic pain, chronic musculoskeletal pain, and chronic visceral pain(3).
Not surprisingly, clinical pain differed between groups. In terms of laboratory pain measures, enhanced CS was primarily observed in the KOA-I group only. This suggests that insomnia in KOA might be an easily identified clinical marker for the possibility of CS. This could be used clinically to conduct formal testing or may hint that central pain agents may be useful in treating OA hyperalgesia. Of note, neither clinical pain nor sex, both factors known to be associated with CS, impacted the interaction between sleep and catastrophizing on CS. Clinical implications of these findings suggest that treatment options for OA patients could include sleep and/or intervention for catastrophizing, both modifiable risk factors, may aid in reducing CS and decrease clinical pain. A recent study found that a CBT intervention to reduce catastrophizing was effective in OA patients scheduled for total knee replacement(49).
Relative to controls, arthritis patients exhibit greater pain sensitivity across a variety of anatomical sites, including both affected and non-affected tissues(5). Contrary to our findings, recent work has indicated that knee OA patients rate suprathreshold mechanical stimuli on the upper body as more intensely painful than controls(50). However, not surprisingly, KOA patients did report enhanced sensitivity to pressure pain thresholds on the knee. Most of the published KOA reports are based on findings of reduced pain threshold or tolerance, measures that do not provide information about abnormalities in CS or CNS pain modulatory processes. The measures used in the current study to examine CS included thermal and mechanical temporal summation, CPM and after sensations. These are well-established markers for CS, assess central pain modulation(3) and may be more robustly linked with clinical pain(51). Clinical pain report was significantly associated with the CS measure, as was BMI, depression, insomnia and catastrophizing. Of note, the after-sensation component of the CS measure created here may have driven the group differences effect, given that the greatest difference between groups was observed in after-sensations.
Several limitations should be considered when interpreting the current findings. First, while we attempted to match groups based on demographic characteristics, a significant age difference emerged. This may have impacted the current findings. Indeed, the KOA good sleeping group (the oldest group) exhibited the greatest thermal temporal summation, but the lowest after sensation ratings to this task. Despite these limitations, our findings contribute additional evidence that insomnia is a significant pain-related issue. Compared to KOA patients without insomnia, those with insomnia endorse greater pain, depression and pain-related catastrophizing. Participants with primary insomnia (non-KOA) also endorsed elevated depressive symptomatology and the poorest sleep quality. This is the first examination, of which we are aware, that demonstrates the interactive effect of catastrophizing and sleep duration on CS. While it is important to note that no causal pathways may be determined from this cross-sectional study, these data suggest that those with low sleep efficiency and higher catastrophizing have the greatest CS. Manipulations involving experimental sleep deprivation or restriction may be a useful next step in elucidating the underlying mechanisms by which catastrophizing and sleep shape the experience of pain. This preliminary work supports a promising line of inquiry in understanding the relationship between sleep and catastrophizing, two constructs known to have substantial impact in pain outcomes. Collectively, additional study is warranted to characterize the relationship between sleep, catastrophizing and pain.
Significance and Innovations.
This is the largest and most comprehensive description of sleep and laboratory pain in osteoarthritis patients; descriptive characterization of each measure is presented.
The KOA-Insomnia group demonstrated the greatest degree of central sensitization compared to controls.
Catastrophizing moderated the relationship between sleep efficiency and central sensitization. Specifically those with low sleep efficiency and high catastrophizing scores reported increased levels of CS.
Central sensitization was also significantly associated with increased clinical pain.
Acknowledgements
This study was supported by the NIH, National Institutes of Arthritis and Musculoskeletal and Skin Disease grants: R01 AR05487 & AR059410 (Smith) and the National Institutes of Health (K23 NS070933, CMC).
Footnotes
Conflicts: The authors have no conflicts of interest to report.
Reference List
- 1.Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Caspian J Intern Med. 2011;2(2):205–212. [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Osteoarthritis. 5-6-2014. Ref Type: Online Source. [Google Scholar]
- 3.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev. 2004;27(8):729–737. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Malfait AM, Schnitzer TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nat Rev Rheumatol. 2013;9(11):654–664. doi: 10.1038/nrrheum.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, et al. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013;65(2):363–372. doi: 10.1002/art.34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30(9):1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8(2):119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Silman AJ, Ray D, Morriss R, Dickens C, Macfarlane GJ, et al. The role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based study. Rheumatology (Oxford) 2007;46(4):666–671. doi: 10.1093/rheumatology/kel363. [DOI] [PubMed] [Google Scholar]
- 11.Smith MT, Klick B, Kozachik S, Edwards RE, Holavanahalli R, Wiechman S, et al. Sleep onset insomnia symptoms during hospitalization for major burn injury predict chronic pain. Pain. 2008;138(3):497–506. doi: 10.1016/j.pain.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilcox S, Brenes GA, Levine D, Sevick MA, Shumaker SA, Craven T. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. J Am Geriatr Soc. 2000;48(10):1241–1251. doi: 10.1111/j.1532-5415.2000.tb02597.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith MT, Quartana PJ, Okonkwo RM, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: a conceptual model. Curr Pain Headache Rep. 2009;13(6):447–454. doi: 10.1007/s11916-009-0073-2. [DOI] [PubMed] [Google Scholar]
- 14.Campbell CM, Edwards RR. Mind-body interactions in pain: the neurophysiology of anxious and catastrophic pain-related thoughts. Transl Res. 2009;153(3):97–101. doi: 10.1016/j.trsl.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. 2011;7(4):216–224. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- 16.Buenaver LF, Quartana PJ, Grace EG, Sarlani E, Simango M, Edwards RR, et al. Evidence for indirect effects of pain catastrophizing on clinical pain among myofascial temporomandibular disorder participants: the mediating role of sleep disturbance. Pain. 2012;153(6):1159–1166. doi: 10.1016/j.pain.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Edwards RR, Mensing G, Cahalan C, Greenbaum S, Narang S, Belfer I, et al. Alteration in pain modulation in women with persistent pain after lumpectomy: influence of catastrophizing. J Pain Symptom Manage. 2013;46(1):30–42. doi: 10.1016/j.jpainsymman.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain. 2006;22(8):730–737. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- 19.Finan PH, Buenaver LF, Coryell VT, Smith MT. Cognitive-Behavioral Therapy for Comorbid Insomnia and Chronic Pain. Sleep Med Clin. 2014;9(2):261–274. doi: 10.1016/j.jsmc.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell CM, Bounds SC, Kuwabara H, Edwards RR, Campbell JN, Haythornthwaite JA, et al. Individual Variation in Sleep Quality and Duration Is Related to Cerebral Mu Opioid Receptor Binding Potential during Tonic Laboratory Pain in Healthy Subjects. Pain Med. 2013 doi: 10.1111/pme.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodin BR, Fillingim RB, Machala S, McGuire L, Buenaver LF, Campbell CM, et al. Subjective sleep quality and ethnicity are interactively related to standard and situation-specific measures of pain catastrophizing. Pain Med. 2011;12(6):913–922. doi: 10.1111/j.1526-4637.2011.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levenson JC, Troxel WM, Begley A, Hall M, Germain A, Monk TH, et al. A quantitative approach to distinguishing older adults with insomnia from good sleeper controls. J Clin Sleep Med. 2013;9(2):125–131. doi: 10.5664/jcsm.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 24.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 26.Schramm E, Hohagen F, Grasshoff U, Riemann D, Hajak G, Weess HG, et al. Test-retest reliability and validity of the Structured Interview for Sleep Disorders According to DSM-III-R. Am J Psychiatry. 1993;150(6):867–872. doi: 10.1176/ajp.150.6.867. [DOI] [PubMed] [Google Scholar]
- 27.Morin CM, Kowatch RA, Barry T, Walton E. Cognitive-behavior therapy for late-life insomnia. J Consult Clin Psychol. 1993;61(1):137–146. doi: 10.1037//0022-006x.61.1.137. [DOI] [PubMed] [Google Scholar]
- 28.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 29.Sullivan MJ, Bishop S, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- 30.Haringsma R, Engels GI, Beekman AT, Spinhoven P. The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry. 2004;19(6):558–563. doi: 10.1002/gps.1130. [DOI] [PubMed] [Google Scholar]
- 31.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 33.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 34.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 35.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3-Revised ed. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 36.Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18(4):288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- 37.Lichstein KL, Stone KC, Donaldson J, Nau SD, Soeffing JP, Murray D, et al. Actigraphy validation with insomnia. Sleep. 2006;29(2):232–239. [PubMed] [Google Scholar]
- 38.Edinger JD, Fins AI, Sullivan RJ, Jr, Marsh GR, Dailey DS, Hope TV, et al. Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers. Sleep. 1997;20(12):1119–1126. doi: 10.1093/sleep/20.12.1119. [DOI] [PubMed] [Google Scholar]
- 39.Whitney CW, Gottlieb DJ, Redline S, Norman RG, Dodge RR, Shahar E, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21(7):749–757. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 40.Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21(7):759–767. [PubMed] [Google Scholar]
- 41.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, D.C.: US Government Printing Office; 1968. [Google Scholar]
- 42.Brennum J, Kjeldsen M, Jensen K, Jensen TS. Measurement of human pressure-pain thresholds on fingers and toes. Pain. 1989;38:211–217. doi: 10.1016/0304-3959(89)90240-6. [DOI] [PubMed] [Google Scholar]
- 43.Vierck CJJ, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78(2):992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- 44.Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 45.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders--pathways of vulnerability. Pain. 2006;123(3):226–230. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 46.Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling [White paper] 2012 http://www.afhayes.com. [Google Scholar]
- 47.Watson NF, Kapur V, Arguelles LM, Goldberg J, Schmidt DF, Armitage R, et al. Comparison of subjective and objective measures of insomnia in monozygotic twins discordant for chronic fatigue syndrome. Sleep. 2003;26(3):324–328. doi: 10.1093/sleep/26.3.324. [DOI] [PubMed] [Google Scholar]
- 48.Leigh TJ, Bird HA, Hindmarch I, Wright V. Measurement of nocturnal body motility: behaviour of osteoarthritic patients and healthy controls. Rheumatol Int. 1988;8(2):67–70. doi: 10.1007/BF00271837. [DOI] [PubMed] [Google Scholar]
- 49.Somers TJ, Blumenthal JA, Guilak F, Kraus VB, Schmitt DO, Babyak MA, et al. Pain coping skills training and lifestyle behavioral weight management in patients with knee osteoarthritis: a randomized controlled study. Pain. 2012;153(6):1199–1209. doi: 10.1016/j.pain.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee YC, Lu B, Bathon JM, Haythornthwaite JA, Smith MT, Page GG, et al. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res (Hoboken ) 2011;63(3):320–327. doi: 10.1002/acr.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edwards RR, Sarlani E, Wesselmann U, Fillingim RB. Quantitative assessment of experimental pain perception: multiple domains of clinical relevance. Pain. 2005;114(3):315–319. doi: 10.1016/j.pain.2005.01.007. [DOI] [PubMed] [Google Scholar]