Abstract
Objective
To evaluate whether weight change since age 18, current body mass index (BMI), and BMI at age 18 are associated with fecundity.
Methods
Our study included 1,950 women currently attempting pregnancy in the Nurses’ Health Study 3 (2010–2014), a prospective cohort study. Height, current weight, and weight at age 18 were self-reported on the baseline questionnaire. Every 3 to 6 months thereafter, women reported the current duration of their pregnancy attempt. Multivariable accelerated failure time models were used to estimate the time ratios and 95% confidence intervals (CI).
Results
For every 5 kg increase in body weight from age 18, current duration of pregnancy attempt increased by 5% (95% CI 3, 7%). Compared to women who maintained weight, the adjusted median current duration was 0.5 months shorter in those who lost weight, 0.3 months longer for those who gained 4–9.9 kg and 10–19.9 kg, and 1.4 months longer for those who gained ≥20 kg (p-trend= <0.001). The adjusted time ratio (95% CI) for a 5 kg/m2 increase in current BMI was 1.08 (1.04, 1.12). After multivariable adjustment (including adjustment for current BMI), being underweight at age 18 (BMI < 18.5) was associated with a longer current duration of pregnancy attempt compared to normal weight women (time ratio: 1.25 95% CI 1.07, 1.47); however being overweight or obese at age 18 was not associated with fecundity.
Conclusions
Gaining weight in adulthood, being overweight or obese in adulthood, and being underweight at age 18 were associated with a modest reduction in fecundity.
Introduction
The effects of current body weight on fecundity are well recognized among women attempting pregnancy spontaneously and through infertility treatment (1). This has led to weight loss being strongly promoted as an effective means of increasing fertility in overweight or obese women (2). However, most of the evidence for weight loss as a treatment for subfecundity comes from small intervention studies in obese women prior to fertility treatment and observational studies among women undergoing bariatric surgery (3). Only two studies have evaluated the impact of weight change not as a result of surgery or clinical interventions on fecundity. Among overweight or obese women in the Danish National Birth Cohort (DNBC), every 1 kg decrement in weight between pregnancies was associated with 5.50 (95% CI: 1.35–9.65) days shorter TTP (4). Among all women in the DNBC, each 1-kg increment in weight was associated with 2.84 (95% CI: 1.33–4.35) days longer TTP. A study of Danish women planning pregnancy (n=1,651) showed that women who lost >5 kg since age 17 had slightly higher fecundability (fecundability ratio: 1.05, 95% CI 0.73, 1.52) and woman who gained ≥15 kg since age 17 had lower fecundability (fecundability ratio = 0.72, 95% CI = 0.59–0.88)(5).
Two studies found an inverse association between adolescent body weight and number of children (6, 7) and another found a positive association between adolescent body weight and risk of ovulatory infertility (8). Taken together, these studies suggest that body weight in adolescence might also be an important risk factor for adult fertility.
The objective of this study was to examine the relationship between weight change since age 18, current BMI, and BMI at age 18 and fecundity among a large cohort of US women.
Materials and Methods
The Nurses’ Health Study 3 (NHS3) is an ongoing Internet-based cohort study of female nurses in the United States and Canada. To be eligible for the study, women had to be either a registered nurse, licensed practical/vocational nurse or nursing student and born on or after January 1, 1965. As of September 2014, 38,016 women had joined the study and 26,798 women had completed at least one follow-up questionnaire, forming the base population for our analysis. Every 6 months, questionnaires are sent to participants to update lifestyle and medical characteristics. The response rate for the second questionnaire is currently 74%; for women who have completed at least two questionnaires, subsequent response rates exceed 80%. An overview of the study flow is illustrated in Figure 1. Briefly, women were eligible for this current analysis if they reported their height and weight on the baseline questionnaire and reported on any of the subsequent questionnaires that they were trying to get pregnant (n=1,970). We excluded women who reported that they were postmenopausal (n=16) or for whom we were missing information on duration of ongoing pregnancy attempt (n=4). After these exclusions, 1, 950 women were available for analysis. The study was approved by the Institutional Review Board of the Brigham and Women’s Hospital (Boston, Massachusetts). Completion of the web-based questionnaires implied informed consent.
Figure 1.
Flow diagram explaining the final cohort of women in the analysis.
Current height and weight as well as weight at age 18 were reported on the baseline questionnaire. Current BMI was calculated as weight (kg) divided by height squared (m2) and BMI at age 18 was calculated as weight at age 18 (kg) divided by height squared (m2). Weight change since age 18 years was calculated as the difference between current weight and weight at age 18 years. A previous validation study done in a similar cohort showed that self-reported height and weight were highly correlated with measured height (r=0.94) and weight (r=0.97) (9). Self-reported weight at age 18 was also highly correlated with weight recorded in medical records (r=0.84) (10).
Women who report that they are actively trying to get pregnant are asked to report the current duration of their ongoing pregnancy attempt. Specifically, they are asked: “For how many months have you been actively trying to get pregnant?” Categories for response include: ≤ 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months, 12 months, 1–2 years, and ≥3 years. We took a woman’s first report of ongoing pregnancy attempt after the baseline questionnaire as her outcome. Validity of self-report of duration of pregnancy attempt has not been assessed in this population. However, there is an extensive literature documenting the reproducibility and validity of this outcome (11).
Information on potential confounding variables is assessed on the baseline questionnaire including age, race–ethnicity, lifetime pregnancy history, smoking history, marital status, and history of endometriosis and bariatric surgery. Menstrual cycle characteristics including current regularity and length of a usual menstrual cycle are reported on the first follow-up questionnaire. Information on physician diagnosed polycystic ovarian syndrome (PCOS), body hair, use of permanent and temporary hair removal solutions, and acne are also assessed on the second questionnaire. We assessed hirsutism with the simplified version of the Ferriman-Gallwey score, which uses a scale of 0–3 to assess the amount of hair on a woman’s chin, upper abdomen, and lower abdomen. Women with a summed score ≥3 were considered has having hirsutism as a previous study determined this cutoff be highly predictive (12).
Women were divided into groups according to the World Health Organization BMI classifications for current and age 18 BMI: <18.5 (underweight), 18.5–24.9 (normal weight), 25–29.9 (overweight), and ≥ 30 (obese). Weight change since age 18 was classified into categories based on its distribution in this cohort: lost 4 kg or more, stable weight (+/− 4 kg), gained 4–9.9 kg, gained 10–19.9 kg, and gained ≥20 kg. These variables were also analyzed as continuous variables. Differences in baseline characteristics by weight change since age 18 categories were compared using a chi-squared test for categorical variables and Kruskal-Wallis non-parametric tests for continuous variables
For our analysis, we applied a current duration approach that uses information collected in a cross-sectional fashion on current duration of ongoing pregnancy attempt to make inferences about actually realized waiting times to pregnancy (13). Numerous studies have utilized this approach to estimate the national prevalence of infertility (14) and the association of environmental factors on fecundity (15). Since couples who have long durations of attempting pregnancy are overrepresented in the current duration approach, appropriate statistical models are used to account for this length-biased sampling. The current duration approach and more generally backward recurrence time survival methods allow us to infer the relationship of characteristics to the (unobserved) total duration of pregnancy attempt by using the (observed) current duration of attempt via accelerated failure time models (13). Based on previous research (15), we chose an accelerated failure time model with log normal distribution to estimate the time ratios and 95% confidence intervals (CI). Other outcome distributions such as generalized gamma and Pareto were also explored. Since the log-normal distribution had the lowest −2 log likelihood and Akaike information criterion value, this was chosen as the outcome distribution. The log-normal accelerated failure time model is equivalent to linear regression on the log-transformed current durations. PROC GENMOD in SAS was used to model the effect of various exposures on current duration of pregnancy attempt. The time ratios correspond to exp([beta]) from the log-normal accelerated failure time models. These time ratios can be interpreted as the ratios of the median values of the duration of pregnancy attempts between the compared groups. A time ratio above 1 implies that a given exposure is associated with lower fecundity (e.g. prolongs the time to pregnancy) while a time ratio below 1 indicates a higher fecundity (e.g. shorter time to pregnancy). Tests for linear trend across categories were conducted by using the median values in each category as a continuous variable.
Confounding was evaluated using prior knowledge about suspected confounders and descriptive statistics from our cohort. Variables retained in the final multivariable models were age, race, smoking status, and marital status. Missing covariate data was rare- 2 women were missing data on smoking status and this was the only variable with missing data. To accommodate these missing responses, a categorical indicator was used. Weight change since age 18 models were further adjusted for BMI at age 18. Menstrual cycle characteristics, diagnosis of PCOS, and indicators of hyperandrogenism (such as acne and hirsutism), and current BMI (for BMI at age 18 models) were evaluated as potential mediators. Additional analyses were also performed to evaluate effect modification by age and gravidity. Separate analyses were carried out for weight change since age 18 in women with a low BMI (<25) or high BMI (≥25) at age 18. Women were also cross classified into categories based on WHO categories of BMI at age 18 and current BMI to address the impact of weight on fecundity depending on where women started in adolescence and ended up in adulthood. P values for heterogeneity were derived from the cross-product interaction term coefficient added to the main-effects multivariable model. SAS statistical software (version 9.3) was used for all analyses. A significance level of P < 0.05 was used for all analyses.
To assess the robustness of our results, we conducted a more typical time to pregnancy analysis in NHS3 women who enrolled in the Maternal Health Study (MHS). Details of this analysis can be found in the supplemental Methods (Appendix 1, available online at http://links.lww.com/xxx).
Results
On average, participants who gained the most weight since age 18 were older and heavier, more likely to be current smokers, and less likely to be Caucasian and nulligravid (Table 1). Women in the highest category of weight gain were also more likely to have been diagnosed with polycystic ovarian syndrome (PCOS), have irregular menstrual cycles, higher amounts of body hair, and greater use of temporary hair removals. No women in our cohort reported history of bariatric surgery. The correlation between BMI at age 18 and current BMI was 0.68. The estimated proportions of women not pregnant after 12 and 24 months were 16% and 5%, respectively.
Table 1.
Baseline demographic characteristics by categories of weight change since age 18 among 1,950 Nurses' Health Study 3 women planning pregnancy.
| Lost ≥ 4 kg | Maintained Weight |
Gained 4–9.9 kg |
Gained 10–19.9 kg |
Gained ≥20 kg | p-value | |
|---|---|---|---|---|---|---|
| Number of Women | 129 | 513 | 551 | 401 | 347 | |
| Age, yrs | 32 [29, 36] | 32 [30, 35] | 33 [30, 37] | 34 [31, 38] | 36 [32, 40] | <0.001 |
| Current BMI, kg/m2 | 22.6 [20.8, 25.7] | 21.3 [20.0, 22.8] | 23.2 [21.5, 25.5] | 26.6 [24.6, 29.5] | 34.7 [31.2, 40.6] | <0.001 |
| BMI at 18, kg/m2 | 25.7 [23.0, 29.1] | 21.0 [19.7, 22.3] | 21.0 [19.4, 22.9] | 21.5 [19.4, 24.2] | 23.6 [20.8, 26.6] | <0.001 |
| Caucasian Race, n (%) | 117 (90.7) | 485 (94.5) | 511 (92.7) | 366 (91.3) | 311 (89.6) | 0.08 |
| Married, n (%) | 96 (74.4) | 390 (76.0) | 410 (74.4) | 298 (74.3) | 276 (79.5) | 0.43 |
| Smoking Status, n (%) | <0.001 | |||||
| Never | 101 (78.3) | 423 (82.5) | 439 (79.7) | 304 (75.8) | 237 (68.3) | |
| Former | 9 (7.0) | 16 (3.1) | 15 (2.7) | 24 (6.0) | 24 (6.9) | |
| Current | 19 (14.7) | 72 (14.0) | 95 (17.2) | 73 (18.2) | 85 (24.5) | |
| Nulligravity, n (%) | 81 (62.8) | 338 (65.9) | 346 (62.8) | 215 (53.6) | 151 (43.5) | <0.001 |
| Regular Menstrual Cycles, n (%) | 62 (73.8) | 336 (91.3) | 303 (81.2) | 272 (92.2) | 248 (88.6) | 0.08 |
| Normal Length Menstrual Cycles*, n (%) | 80 (91.0) | 296 (80.7) | 366 (93.4) | 236 (80.0) | 220 (78.9) | 0.28 |
| Current Acne, n (%) | 0.89 | |||||
| None | 34 (28.1) | 136 (27.5) | 125 (23.7) | 97 (25.7) | 85 (25.7) | |
| 1–4 pimples in last 3 months | 69 (57.0) | 293 (59.2) | 322 (61.1) | 222 (58.7) | 192 (58.0) | |
| ≥5 pimples in last 3 months | 18 (14.9) | 66 (13.3) | 80 (15.2) | 59 (15.6) | 54 (16.3) | |
| Acne at Worst, n (%) | 0.76 | |||||
| None | 33 (27.3) | 161 (32.5) | 154 (29.2) | 108 (28.6) | 89 (26.8) | |
| Mild | 37 (30.6) | 142 (28.7) | 161 (30.5) | 121 (32.0) | 101 (30.4) | |
| Moderate | 45 (37.2) | 176 (35.6) | 193 (36.6) | 130 (34.4) | 122 (36.8) | |
| Severe | 6 (7.4) | 16 (3.2) | 20 (3.8) | 19 (5.0) | 20 (6.0) | |
| Simplified Ferriman-Gallwey Score ≥ 3† | 8 (6.7) | 16 (3.3) | 21 (4.1) | 23 (6.1) | 47 (14.6) | <0.001 |
| Permanent Hair Removal‡, n (%) | 14 (11.6) | 58 (11.8) | 54 (10.3) | 36 (9.5) | 31 (9.3) | 0.74 |
| Temporary Hair Removal‡, n (%) | 45 (37.2) | 170 (34.4) | 203 (38.4) | 159 (42.1) | 169 (51.1) | <0.001 |
| Ever Diagnosed with PCOS, n (%) | 11 (9.1) | 25 (5.0) | 41 (7.8) | 42 (11.0) | 80 (24.0) | <0.001 |
| Ever Diagnosed with Laproscopically Confirmed Endometriosis | 3 (2.3) | 10 (2.0) | 17 (3.1) | 23 (5.7) | 15 (4.3) | 0.03 |
Data are median [25th, 75th percentile] unless otherwise specified.
Defined as having a menstrual cycle that lasts on average 21 to 39 days.
Women ranked amount of body hair on a scale of 0 to 3 in 3 different areas- chin, lower abdomen and upper abdomen. The score represents the sum of the scores in each area (minimum of 0, maximum of 9). A score ≥3 is highly predictive of hirsutism.
Only applies to hair on the face, chest, or abdomen (excludes hair removal on arms, legs, armpits, or pubic area.
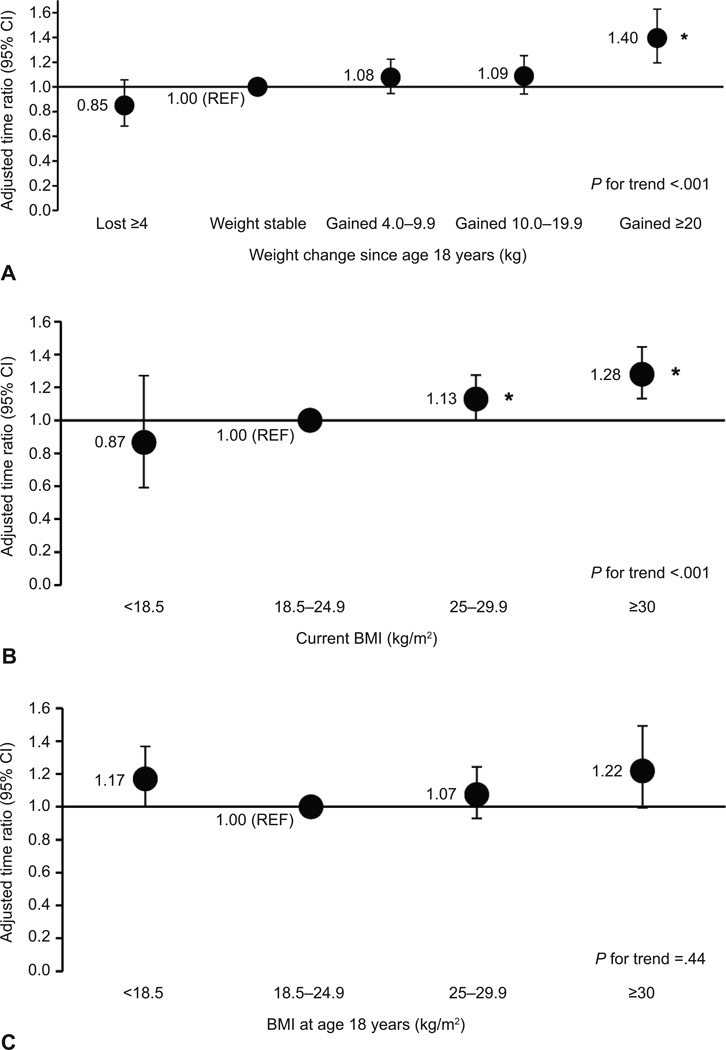
Every 5 kg increase in body weight from age 18 was associated with a 5% (95% CI 3, 7%) increase in median current duration of pregnancy attempt. Compared to women who maintained their weight since age 18, the multivariable adjusted time ratios (95% CIs) were 0.85 (0.68, 1.06) for women who lost ≥4 kg, 1.08 (0.95, 1.22) for women who gained 4–9.9 kg, 1.09 (0.94, 1.25) for women who gained 10–19.9 kg, and 1.40 (1.19, 1.63) for women who gained ≥20 kg (p-trend= <0.001) (Figure 2). Translated onto an absolute level, compared to women who maintained weight, the adjusted median current duration was 0.5 months shorter in those who lost weight (p-difference=0.14), 0.3 months longer for those who gained 4–9.9 kg (p-difference=0.26) and 10–19.9 kg (p-difference=0.25), and 1.4 months longer for those who gained ≥20 kg (p-difference=<0.001). Current BMI was also significantly associated with fecundity (p-trend=<0.001). The adjusted effects of being overweight and obese corresponded to 13% (95% CI 0, 27%) and 28% (95% CI 13, 45%) increases in the median duration of pregnancy attempt compared to women of normal BMI. The effect of overweight and obesity translated into an average of 0.5 and 1.0 month longer current duration of pregnancy attempt compared to women of normal BMI. When analyzed on a continuous level, the adjusted time ratio for a 5 kg/m2 increase in current BMI was 1.08 (95% CI 1.04, 1.12). BMI at age 18 appeared to have a U-shaped relationship with fecundity in multivariable adjustment as both underweight (time ratio: 1.17 95% CI 1.00, 1.37) and obese women (time ratio: 1.22 95% CI 1.00, 1.49) at age 18 had longer current duration of pregnancy attempts compared to normal weight women. We further adjusted these models for current BMI to evaluate whether the relationship between BMI at age 18 and later fecundity was solely due to the fact that a woman’s BMI tracked into adulthood After further adjustment for current BMI, only underweight women at age 18 had a longer current duration of pregnancy attempt compared to normal weight women at age 18 (time ratio: 1.25 95% CI 1.07, 1.47).
Figure 2.
Association between weight change since age 18 years (A), current body mass index (BMI) (B), and BMI at age 18 years (C) and current duration of pregnancy attempt. Accelerated failure time models were used to estimate the time ratios and 95% confidence intervals (CI). All models are adjusted for age (years), race (white compared with other), smoking status (never, former, current, or missing), and marital status (married or not married). Weight change models were further adjusted for BMI at age 18 years. *P<.05 for the comparison of a time ratio for a specific exposure group compared to the reference group.
The association between weight change and fecundity was similar in women who were <25 kg/m2 and >25 kg/m2 at age 18 (p-interaction=0.33) although this analysis was limited by the low number women who were overweight or obese at age 18 (n=370). When we cross-classified women into categories based on WHO categories of BMI at age 18 and current BMI it became apparent that weight gain was particularly detrimental in underweight women at age 18 (Figure 3). Moreover, weight gain was detrimental even if women did not end up being classified as overweight or obese in adulthood (time ratio for being underweight at age18 and normal weight in adulthood = 1.20 95% CI 1.00, 1.43). There was no evidence of effect modification of the weight change, current BMI, or BMI at age 18 and fecundity association by age (<37, ≥37 yrs), smoking status (ever vs. never smoker), or gravidity (nulligravid vs. gravid) (Appendix 2, available online at http://links.lww.com/xxx). Associations between weight change and current BMI and fecundity were slightly attenuated albeit still significant after adjustment for regular menstrual cycles, diagnosis of PCOS, and hirsutism.
Figure 3.
Association between changes in body mass index (BMI) category between age 18 years and present and current duration of pregnancy attempt. Accelerated failure time models were used to estimate the time ratios and 95% confidence intervals (CI). All models are adjusted for age (years), race (white compared with other), smoking status (never, former, current, or missing), and marital status (married or not married). The following groups were not presented in the figure due to low numbers: women with a BMI of 18.5–24.9 kg/m2 at age 18 years and a BMI of <18.5 kg/m2 in adulthood (n=19) and women with a BMI of ≥25 kg/m2 at age 18 years and a BMI of <18.5 kg/m2 in adulthood (n=0). *P<.05 for the comparison of a time ratio for a specific exposure group compared to the reference group.
The results of the sensitivity analysis in pregnant women who retrospectively reported their time to pregnancy were consistent with the main analysis with the exception of BMI at age 18, which was not associated with fecundability in this cohort (Table 2). Every 5 kg increase in body weight since age 18 was associated with a 3% (95% CI 1, 6%) decrease in fecundability. A significant linear trend was also observed across categories of weight change since age 18 and current BMI. Overall, compared to the current duration results, effect estimates were slightly weaker for the weight change and current BMI analyses in this cohort, although both were still statistically significant. Of note, women in the retrospective pregnancy cohort were significantly younger, thinner, more likely to be never smokers, married, Caucasian, and less likely to be nulligravid, diagnosed with PCOS, and have regular menstrual cycles (p-value < 0.05) compared to women in the current duration cohort (Appendix 3, available online at http://links.lww.com/xxx).
Table 2.
Fecundability odds ratios and 95% CIs of the association between weight change since age 18, current BMI, and BMI at age 18 and retrospective time to pregnancy among women with planned pregnancies in the Maternal Health Study (n=2,148).
| Exposure Categories | n (%) | Adjusted Fecundability OR (95% CI)* |
|---|---|---|
| Current BMI (kg/m2) | ||
| Underweight | 44 (2.0) | 1.02 (0.71, 1.48) |
| Normal | 1308 (60.9) | 1.0 (REF) |
| Overweight | 461 (21.5) | 0.86 (0.76, 0.98) |
| Obese | 335 (15.6) | 0.86 (0.74, 0.99) |
| p-trend | 0.01 | |
| BMI at Age 18 (kg/m2) | ||
| Underweight | 219 (10.3) | 0.96 (0.81, 1.15) |
| Normal | 1567 (73.4) | 1.0 (REF) |
| Overweight | 269 (12.6) | 0.93 (0.79, 1.08) |
| Obese | 81 (3.8) | 0.95 (0.73, 1.23) |
| p-trend | 0.49 | |
| Weight Change Since Age 18 | ||
| Lost ≥ 4 kg | 153 (7.2) | 1.07 (0.85, 1.34) |
| Weight Stable | 666 (31.2) | 1.0 (REF) |
| Gained 4–9.9 kg | 658 (30.8) | 1.01 (0.89, 1.15) |
| Gained 10–19.9 kg | 416 (19.5) | 0.97 (0.83, 1.12) |
| Gained ≥20 kg | 244 (11.4) | 0.82 (0.68, 0.98) |
| p-trend | 0.04 |
Cox proportional hazard models for discrete time were used to estimate the fecundability odd ratios (FOR) and 95% confidence intervals (CI). FORs estimate the odds of becoming pregnant each month for a given exposure, conditional on not being pregnant in the previous month. FORs <1 denote a reduction in fecundity or a longer TTP, and FORs >1 denote a shorter TTP.
Models are adjusted for age (years), race (white vs. other), smoking status (never, former, current, missing), and marital status (married, not married). Weight change models are further adjusted for BMI at age 18.
Discussion
In this ongoing cohort of women planning pregnancy, weight gain of ≥20 kg since age 18, a current BMI ≥25 kg/m2, and a BMI <18.5 kg/m2 at age 18 were associated with lower fecundity although all associations were modest in effect size. For instance, compared to women who maintained weight since age 18, women who gained ≥20 kg experienced, on average, a 1.4 months longer current duration of pregnancy attempt. The association between weight gain and fecundity was similar in women who were <25 kg/m2 and ≥25 kg/m2 at age 18 and appeared to be independent of current BMI.
Our results for adult weight change and fecundity agree with previous studies that have also shown slightly shorter waiting time to pregnancy in women who lost weight since age 17 (5) or between consecutive pregnancies (4) and longer waiting time to pregnancy in women who gained weight in adulthood (5) or between pregnancies (4). They are also in agreement with the smaller, clinical studies of weight loss and improved ovulatory function among women with PCOS and women undergoing surgery for weight loss (3). We did not find any difference in effect among younger or older women, suggesting that weight loss is beneficial and weight gain is detrimental to fecundity, regardless of timing. One unexpected finding, however, was that gaining weight was particularly detrimental in women who were underweight in adolescence. This suggested to us a critical window of exposure where being underweight at age 18 significantly impacts fecundity to an extent that exacerbated by later weight gain.
Our results were similar after adjusting for current menstrual cycle characteristics, ever diagnosis of PCOS, and other markers of hyperandrogenism such as body hair patterns, suggesting that mechanisms other than clinical or subclinical PCOS or anovulation are driving this association. Weight loss prior to in vitro fertilization has been linked to better quality oocytes (16) and many studies have shown that weight loss improves insulin sensitivity (17). As both of these pathways have been linked to higher fecundity, one or both of these mechanisms could be driving this association (18, 19). In regards to our findings on weight gain, since our results remained significant after adjusting for current BMI, this suggests that effect of weight gain on fecundity is not completely mediated through current body fatness. An animal study showed that adult female mice reared on chronic caloric restriction who were allowed to eat ad libitum for 1 month (resulting in weight gain) produce fewer good quality oocytes and had a lower number of embryos reaching blastocyst stage than female mice that are kept on caloric restriction (20). Thus, impaired oocyte quality could be mediating this relationship as well.
The finding of reduced fecundity in overweight and obese women has been observed in many other studies (4, 5, 21–23). Our study expanded on previous research by directly quantifying the magnitude of this effect on an interpretable scale and suggests that effects of overweight and obesity on fertility are small (e.g. on the order of 0.5–1 month delays). Similar to the results on weight change, the effects of current BMI on fecundity did not appear to be completely mediated through menstrual cycle disturbances, PCOS, or markers of hyperandrogenism which has been shown previously (5). Instead, a growing body of literature suggests that obesity may also exert its negative consequences on oocyte quality (24) and thus lengthen time to pregnancy. For example, a recent meta-analysis found that among egg donor recipients, the chance of pregnancy after IVF is no different in obese recipients versus normal weight recipients (25).
The weaker association with BMI that we observed in the retrospective time-to-pregnancy cohort was expected, given that women who are involuntary infertile and women experiencing pregnancy losses prior to 20 weeks were excluded by design. As both of these conditions have been linked to obesity (26), this would support the stronger effects we observed in the current duration cohort. Moreover, due to the design of his cohort, women had to retrospectively report their time-to-pregnancy. While retrospective report of time-to-pregnancy is considered reasonably valid, it has been documented that women who experienced longer observed time-to-pregnancy are more likely to under-report their time-to-pregnancy later (27). The predicted effect of this underestimation is also bias towards the null (28).
Limitations of this study include the possibility of planning bias, residual confounding, and the use of only one exposure assessment while the strengths include the ability to include women with both high fertility and those who are involuntarily infertile, to address menstrual cycle characteristics and sublinical markers of PCOS as possible mechanisms of effect, and to compare our results to a typical retrospective time to pregnancy analysis. These strengths and limitations have been described in more detail in the supplemental Discussion section (Appendix 4, available online at http://links.lww.com/xxx)
The main clinical implications of this study concern the absolute magnitudes of effect for the associations between current BMI, weight change since age 18, and underweight at age 18. While previous studies have consistently documented lower fecundity among women who are overweight and obese most were unable to quantify the exact magnitude of effect on an interpretable scale (e.g. due to non-parametric modelling) and in a representative population (e.g. not just women who become pregnant). As we have shown here, the effect of current overweight and obesity on duration of pregnancy attempt are on the order of 0.5 and 1.0 months respectively. The effects of weight gain, even at extreme levels of ≥20 kg since adolescence on current duration of pregnancy attempt are also modest, 1.4 months. This information can provide valuable guidance to women planning pregnancy who wish to understand the absolute magnitude of body weight’s impact on fecundity.
Supplementary Material
Acknowledgments
Supported by NIH grant T32HD060454 and grants from the Breast Cancer Research Foundation and Nutricia Early Life Nutrition US.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.Jungheim ES, Travieso JL, Hopeman MM. Weighing the impact of obesity on female reproductive function and fertility. Nutr Rev. 2013;71(Suppl 1):S3–S8. doi: 10.1111/nure.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson SM, Fleming R. Obesity and reproduction: impact and interventions. Curr Opin Obstet Gynecol. 2007;19:384–389. doi: 10.1097/GCO.0b013e32825e1d70. [DOI] [PubMed] [Google Scholar]
- 3.Sim KA, Partridge SR, Sainsbury A. Does weight loss in overweight or obese women improve fertility treatment outcomes? A systematic review. Obes Rev. 2014;15:839–850. doi: 10.1111/obr.12217. [DOI] [PubMed] [Google Scholar]
- 4.Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sorensen TI, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22:1634–1637. doi: 10.1093/humrep/dem035. [DOI] [PubMed] [Google Scholar]
- 5.Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod. 2010;25:253–264. doi: 10.1093/humrep/dep360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jokela M, Elovainio M, Kivimaki M. Lower fertility associated with obesity and underweight: the US National Longitudinal Survey of Youth. Am J Clin Nutr. 2008;88:886–893. doi: 10.1093/ajcn/88.4.886. [DOI] [PubMed] [Google Scholar]
- 7.Jokela M, Kivimaki M, Elovainio M, Viikari J, Raitakari OT, Keltikangas-Jarvinen L. Body mass index in adolescence and number of children in adulthood. Epidemiol. 2007;18:599–606. doi: 10.1097/EDE.0b013e3181257158. [DOI] [PubMed] [Google Scholar]
- 8.Rich-Edwards JW, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, Manson JE. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171:171–177. doi: 10.1016/0002-9378(94)90465-0. [DOI] [PubMed] [Google Scholar]
- 9.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiol. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19:570–572. [PubMed] [Google Scholar]
- 11.Joffe M, Villard L, Li Z, Plowman R, Vessey M. Long-term recall of time-to-pregnancy. Fertil Steril. 1993;60:99–104. doi: 10.1016/s0015-0282(16)56044-0. [DOI] [PubMed] [Google Scholar]
- 12.Cook H, Brennan K, Azziz R. Reanalyzing the modified Ferriman-Gallwey score: is there a simpler method for assessing the extent of hirsutism? Fertil Steril. 2011;96:1266–1270. e1. doi: 10.1016/j.fertnstert.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keiding N, Kvist K, Hartvig H, Tvede M, Juul S. Estimating time to pregnancy from current durations in a cross-sectional sample. Biostatistics. 2002;3:565–578. doi: 10.1093/biostatistics/3.4.565. [DOI] [PubMed] [Google Scholar]
- 14.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99:1324–1331. e1. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slama R, Ducot B, Carstensen L, Lorente C, de La Rochebrochard E, Leridon H, Keiding N, Bouyer J. Feasibility of the current-duration approach to studying human fecundity. Epidemiol. 2006;17:440–449. doi: 10.1097/01.ede.0000221781.15114.88. [DOI] [PubMed] [Google Scholar]
- 16.Chavarro JE, Ehrlich S, Colaci DS, Wright DL, Toth TL, Petrozza JC, Hauser R. Body mass index and short-term weight change in relation to treatment outcomes in women undergoing assisted reproduction. Fertil Steril. 2012;98:109–116. doi: 10.1016/j.fertnstert.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holte J, Bergh T, Berne C, Wide L, Lithell H. Restored insulin sensitivity but persistently increased early insulin secretion after weight loss in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1995;80:2586–2593. doi: 10.1210/jcem.80.9.7673399. [DOI] [PubMed] [Google Scholar]
- 18.Terriou P, Sapin C, Giorgetti C, Hans E, Spach JL, Roulier R. Embryo score is a better predictor of pregnancy than the number of transferred embryos or female age. Fertil Steril. 2001;75:525–531. doi: 10.1016/s0015-0282(00)01741-6. [DOI] [PubMed] [Google Scholar]
- 19.Wang TH, Chang CL, Wu HM, Chiu YM, Chen CK, Wang HS. Insulin-like growth factor-II (IGF-II), IGF-binding protein-3 (IGFBP-3), and IGFBP-4 in follicular fluid are associated with oocyte maturation and embryo development. Fertil Steril. 2006;86:1392–1401. doi: 10.1016/j.fertnstert.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 20.Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci U S A. 2011;108:12319–12324. doi: 10.1073/pnas.1018793108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nohr EA, Vaeth M, Rasmussen S, Ramlau-Hansen CH, Olsen J. Waiting time to pregnancy according to maternal birthweight and prepregnancy BMI. Hum Reprod. 2009;24:226–232. doi: 10.1093/humrep/den357. [DOI] [PubMed] [Google Scholar]
- 22.Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22:414–420. doi: 10.1093/humrep/del400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolumar F, Olsen J, Rebagliato M, Saez-Lloret I, Bisanti L. Body mass index and delayed conception: a European Multicenter Study on Infertility and Subfecundity. Am J Epidemiol. 2000;151:1072–1079. doi: 10.1093/oxfordjournals.aje.a010150. [DOI] [PubMed] [Google Scholar]
- 24.Purcell SH, Moley KH. The impact of obesity on egg quality. J Assist Reprod Genet. 2011;28:517–524. doi: 10.1007/s10815-011-9592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jungheim ES, Schon SB, Schulte MB, DeUgarte DA, Fowler SA, Tuuli MG. IVF outcomes in obese donor oocyte recipients: a systematic review and meta-analysis. Hum Reprod. 2013;28:2720–2727. doi: 10.1093/humrep/det292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaskins AJ, Rich-Edwards JW, Colaci DS, Afeiche MC, Toth TL, Gillman MW, Missmer SA, Chavarro JE. Prepregnancy and early adulthood body mass index and adult weight change in relation to fetal loss. Obstet Gynecol. 2014;124:662–669. doi: 10.1097/AOG.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooney MA, Buck Louis GM, Sundaram R, McGuiness BM, Lynch CD. Validity of self-reported time to pregnancy. Epidemiol. 2009;20:56–59. doi: 10.1097/EDE.0b013e31818ef47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baird DD, Weinberg CR, Rowland AS. Reporting errors in time-to-pregnancy data collected with a short questionnaire. Impact on power and estimation of fecundability ratios. Am J Epidemiol. 1991;133:1282–1290. doi: 10.1093/oxfordjournals.aje.a115840. [DOI] [PubMed] [Google Scholar]
- 29.Brunner Huber LR, Stanley WA, Broadhurst L, Dmochowski J, Vick TM, Scholes D. No association between body size and frequency of sexual intercourse among oral contraceptive users. Ann Epidemiol. 2014;24:655–659. doi: 10.1016/j.annepidem.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abrevaya J, Tang H. Body mass index in families: spousal correlation, endogeneity, and intergenerational transmission. Empir Econ. 2010;41:841–864. [Google Scholar]
- 31.Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, Van Wely M, Cao J, Martini AC, Eskandar M, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19:221–231. doi: 10.1093/humupd/dms050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.