Abstract
Aim
This proof of concept study examined if early trauma influences features of schizophrenia, consistent with HPA axis activation.
Methods
Early trauma and current perceived stress were assessed in 28 treated schizophrenia cases, along with salivary cortisol, brain volumes, cognition, and symptoms.
Results
Early trauma predicted more positive (r=.66, p=.005) and dysthymia symptoms (r-.65, p=.007), but less negative symptoms (r=−.56, p=.023), as well as reduced whole brain volumes (r=.50, p=.040) and increased amygdala to whole brain volume ratios (r=.56, p=.018). Larger volume reductions accompanied cortisol levels: evening values predicted smaller whole brain and hippocampal volumes, whereas afternoon levels only significantly predicted smaller brain volumes in females. Sex differences were demonstrated between early trauma and cognition, with better cognition in traumatized females than other females and no male effects. Current perceived stress was related to dysthymia (especially in females) and diminished sense of purpose and social drive (especially in males).
Conclusions
These results suggest that early trauma and current stress impact features of schizophrenia, consistent with stress sensitization and increased dopamine activity for treatment refractory positive symptoms, as well as the cascade of increased morning cortisol, reduced brain volumes and depressive and deficit symptoms. Conversely, cognitive deficits and negative symptoms may arise from a distinct diathesis. The sex differences accord with the literature on human HPA function and stress responses. Early trauma may be a stressor in the etiopathophysiology of schizophrenia, particularly for cases with treatment refractory positive symptoms, and may guide future treatment development.
Keywords: childhood trauma, neurobiology, brain volume, cortisol, schizophrenia
INTRODUCTION
In 1997, Walker and Diforio1 built on the psychologically oriented “diathesis-stressor” model for schizophrenia risk2 by proposing that stress-induced hypothalamic-pituitary-adrenal (HPA) changes could specifically contribute to the neurobiological substrates of psychosis vulnerability. Among stressful exposures, early trauma (ET) is particularly associated with later psychoses.3–7 Persistent alterations in HPA functioning from stress are referred to as sensitization and it is plausible that stress exposure plays a prominent role in the development or modulation of schizophrenia.8
As recently reviewed,9 the association of ET with HPA axis dysregulation, hippocampal atrophy, and cognitive changes are clearly demonstrated in animal models and human studies. An excess release of glucocorticoid stress hormones (cortisol in humans) produces atrophy of hippocampal neurons, which can further result in learning and memory deficits.10 A high proportion of individuals with schizophrenia exhibit dexamethasone nonsuppression or elevated basal cortisol levels.11 Hippocampal volume reductions12 and deficits in hippocampal-mediated memory13 are also prominent. Other data link ET to (positive) psychotic symptoms in first-episode schizophrenia,7 and demonstrate a dose-response relationship between childhood trauma and psychotic symptoms in cases with chronic psychosis.3 Only a few studies have investigated the association of ET and negative symptoms, although a meta-analysis5 reported this association in 3 of 9 studies reviewed. In our longitudinal study of prodromal youths at risk for psychosis,14 increased stress sensitivity was related to the emergence of the prodromal symptoms of schizophrenia over time, including negative symptoms, as well as conceptual disorganization, unusual thought content and suspiciousness, depression and anxiety, and declining function.
For decades researchers have considered aspects of the cortisol-neurotoxicity pathway in groups of persons with schizophrenia. Our proof-of-concept study included all of these elements in a group of exceptionally well-characterized schizophrenia patients. Additionally, there is evidence that males and females differ in emotional processing and stress related behavior15–18 and furthermore that sex differences in symptom presentation are commonly observed in schizophrenia.19 Therefore, we performed sex-stratified analyses to investigate whether ET might be related to distinct symptom profiles and other illness features for men and women. In line with the literature, we hypothesized that higher levels of ET and perceived stress would predict dysregulated cortisol activity, decreased hippocampal volumes, impaired cognitive performance, and more positive symptoms.
METHODS AND MATERIALS
Subjects with schizophrenia and schizoaffective disorder were recruited from the New York State Psychiatric Institute (NYSPI) Schizophrenia Research Unit (SRU) and the study was approved by the Institutional Review Board.20 All cases were on stable medication regimens. Subjects were on a smoke-free unit and none had smoked cigarettes for at least 2 weeks. Inclusion criteria required the capacity to provide informed consent, no history of substance dependence, and being medically healthy based on history, physical examination and routine laboratory tests, including a urine toxicology. Cases were right-handed on the Edinburgh inventory21 and had MRI scans that were read as normal by a neuroradiologist.
Master’s level clinicians assessed diagnoses and positive and negative symptoms using the Diagnostic Interview for Genetic Studies (DIGS)22 and Positive and Negative Syndrome Scale (PANSS) ratings,23 as well as the items from the Schedule for the Deficit Syndrome (SDS),24 which were rated dimensionally to provide better coverage of negative symptoms than the PANSS alone. Psychometric analyses demonstrate that the DIGS,22 PANSS, 23 and SDS24 are valid and reliable instruments.
Recent life stress and perceived stress were examined with the Perceived Stress Scale (PSS), which is a reliable and valid measure of appraised psychological stress.25 Childhood trauma was ascertained with the ET Inventory (ETI),26 a 56-item clinician-administered comprehensive assessment of physical, emotional, and sexual abuse, as well as general trauma experienced before and after the age of 18 years, including age of occurrence, frequency, identity of the perpetrators, and the impact of the event. Analyses of inter-rater reliability, test-retest reliability, internal consistency, and convergent validity all indicate that the ETI is a reliable and valid assessment for the measurement of reported childhood trauma. 26 An a priori analysis examined the associations to general trauma and total trauma. There was high inter-rater reliability (i.e. Kappa > .80 for individual symptom ratings, 95% agreement on diagnosis.
Cognition was assessed with the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III)27 to assess Verbal, Performance, and Full Scale IQ. The Wechsler Memory Scale – Revised (WMS-R)28 was used to assess Verbal, Visual, Attention, Delayed Recall, and General Memory indices. The Wisconsin Card Sort Test (WCST)29 was used to assess set-switching and executive functioning. The Trail making Test (TMT)30 examined processing speed, visual attention, and task switching. Verbal fluency was measured using the Controlled Word Association Test (COWAT)-FAS (the most common version of the COWAT, which uses the letters F, A, and S) and Animal Naming.31 The WAIS-lll, WCST, TMT, COWAT, Animal Naming, and WMS-R are all reported to have adequate reliability and validity.32 For cortisol assessments, saliva was collected in a Sarstedt (Germany) Salivette tube for each subject at awakening, 30 minutes after awakening, afternoon (postprandial for lunch) and evening (at 10 P.M.). Salivary cortisol was assayed by radioimmunoassay, as described in Gardner et al.33 MRIs were performed on a 1.5-T GE Signa system, and included a T2-weighted coronal MRI localizer sequence with TR=4500 ms, TE=105 ms, flip angle=90°. Two raters blinded to subject characteristics determined volumes in scans corrected for head rotation and evaluated perpendicular to the long axis of the hippocampus based on the ANALYZE program (Mayo Foundation, Rochester, Minn.).34 Two mid-hippocampal points that were separated by 15 mm were selected to construct along the axis of the hippocampus. A third mid-hippocampal point in the opposite hippocampus defined a plane parallel to the long axes of both hippocampi. A series of oblique images perpendicular to this plane created images orthogonal to the long axis of the hippocampus. The outlines of the hippocampus and amygdala were traced using a mouse-driven cursor. Cross sectional areas in each slice were, summed, and multiplied by the slice thickness to obtain the volumes of the hippocampus and amygdala. Automated techniques were used to measure whole brain size.
Data Analysis
All data were entered and verified using the SIR Database Management Software (SIR 2002, SIR Pty Ltd, Terrey Hills, Australia) and IBM/SPSS Statistics 20 was used for the analyses. The ETI scores were calculated for each domain by summing numbers of experiences endorsed, as more complex strategies (e.g., weighting scores by severity) are no more predictive of adult PTSD symptom severity.35 For each domain, all experiences rated as having a negative emotional impact were also totaled. The 30-items in the PANSS were assessed using the 5-factor model36 and items in the SDS were assessed continuously as to severity. Descriptive statistics and distributions of all measures were examined, whether continuous or categorical, to identify key features (e.g. non-normal distribution, outliers, skewness) that impacted inferential methods. The t-test statistic was used to examine the continuous measures across sex. Pearson correlation coefficients examined the associations between the ETI scales and the salivary cortisol, brain volumes, cognitive tests and the Deficit Syndrome items and PANSS Factors. All tests were two-tailed and alpha for significance was set at p < .050. Due to the hypothesis based proof-of-concept- nature of these analyses, correction for multiple testing was not applied. Finally, we examined the relationship of total trauma to positive symptoms, and then to negative symptoms, in multiple regression models.
RESULTS
Table 1 presents sex-specific and whole sample descriptive statistics for demographic measures, early trauma, perceived stress, brain volumes, diurnal salivary cortisol levels, cognitive tests, and symptoms. Males and females had similar ages, education, age of onset, and exposures and reports of childhood trauma on the ET inventory subscales. Further analysis of ET utilized the general events and total events scores only, the latter being twice as large as the former and permitting finer analyses. Notably, the numerical rating for the events for each of the subscales did not significantly differ from the score that also considered the negativity of the experiences. As a further consideration we examined sexual abuse, as it is reported to be particularly pathogenic. Current perceived stress did not differ by sex, nor did mean neural volumes, although whole brain volumes had significantly greater variability in the male than the female cases. Salivary cortisol was highest on awakening in males and higher after 30′ after awakening in females, with values that were half as high in the afternoon and only a tenth as high in the evening for both sexes, without significant sex differences in any cortisol measurement. Males and females also had similar cognitive test scores and symptoms, although males had consistently higher ratings on the trait related deficit syndrome items, as expected, which separated significantly from the mean female ratings for the “diminished sense of purpose” item.
Table 1.
Mean Demographics, Stress Measures, Brain Volumes, Cortisol, Cognition and Symptoms by Sex, with Sex Comparisons by T-tests.
| All Cases | Male Cases | Female Cases | M vs F T, p | |||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |||
| Age | 28 | 31.50 (9.70) | 20 | 31.45 (10.34) | 8 | 31.63 (8.54) | 0.04 | .967 |
| Education (Grade) | 27 | 13.15 (2.86) | 19 | 12.79 (2.70) | 8 | 14.00 (3.25) | 1.00 | .326 |
| Age of Onset | 26 | 22.00 (4.74) | 19 | 21.47 (4.56) | 7 | 23.43 (5.29) | 0.93 | .362 |
| ET Inventory Scores | ||||||||
| General events | 24 | 4.7 (2.8) | 16 | 4.75 (3.15) | 8 | 4.50 (1.93) | 0.21 | .840 |
| General events (−) ¥ | 24 | 3.7 (2.5) | 16 | 3.81 (2.88) | 8 | 3.50 (1.51) | 0.29 | .778 |
| Physical abuse events | 24 | 2.2 (1.8) | 16 | 1.94 (1.81) | 8 | 2.75 (1.91) | 1.02 | .319 |
| Physical abuse events (−) | 24 | 1.9 (1.9) | 16 | 1.81(1.91) | 8 | 2.13 (2.03) | 0.37 | .714 |
| Emotional abuse | 24 | 2.7 (2.7) | 16 | 2.06 (2.67) | 8 | 3.87 (2.59) | 1.58 | .128 |
| Emotional abuse (−) | 24 | 2.4 (2.7) | 16 | 1.94 (2.70) | 8 | 3.38 (2.77) | 1.22 | .235 |
| Sexual events | 24 | .88 (1.8) | 16 | .75 (1.73) | 8 | 1.13 (2.10) | 0.47 | .646 |
| Sexual events (−) | 24 | .79 (1.8) | 16 | .75 (1.73) | 8 | .87 (2.10) | 0.16 | .878 |
| Sum of All events | 24 | 10.4 (6.8) | 16 | 9.50 (7.60) | 8 | 12.25 (4.50) | 0.94 | .358 |
| Sum of All events (−) | 24 | 8.8 (6.5) | 16 | 8.31 (7.36) | 8 | 9.88 (4.36) | 0.55 | .588 |
| ¥ (−) = negative rated | ||||||||
| Perceived Stress | ||||||||
| Perceived Stress Total | 24 | 17.8 (6.0) | 18 | 17.67 (6.33) | 6 | 18.17 (5.56) | 0.17 | .865 |
| Brain Volumes (cm3) | ||||||||
| Whole Brain (WB) | 18 | 1423958 (86974) | 10 | 1448545 (101456) | 8 | 1393225 (56513) | 1.46 | .165† |
| Hippocampal:WB Ratio | 18 | .0060 (.00074) | 10 | .00602 (.00087) | 8 | .0060833 (.00058) | 0.17 | .865 |
| Amygdala:WB Ratio | 18 | .0029 (.00062) | 10 | .00295 (.00068) | 8 | .00288 (.00059) | 0.23 | .820 |
| Total Hippocampus | 18 | 8630 (1313) | 10 | 8749.09 (1593.83) | 8 | 8481.36 (936.25) | 0.42 | .681 |
| Total Amygdala | 18 | 4136 (846) | 10 | 4232.39 (844.25) | 8 | 4015.17 (890.64) | 0.53 | .604 |
| Hippocampal: Amygdala Volume Ratio | 18 | 2.18 (.617) | 10 | 2.17 (.711) | 8 | 2.20 (.524) | 0.08 | .941 |
| Salivary Cortisol (ng/ml) | ||||||||
| Awakening Cortisol | 22 | 2.78 (1.68) | 15 | 2.51 (1.46) | 7 | 3.37 (2.07) | 1.13 | .270 |
| 30 min. post awakening | 23 | 3.58 (2.67) | 16 | 3.66 (2.92) | 7 | 3.40 (2.16) | 0.21 | .838 |
| Afternoon | 23 | 1.47 (.94) | 15 | 1.55 (1.08) | 8 | 1.31 (.620) | 0.58 | .569 |
| Evening | 20 | .42 (.36) | 15 | .44 (.40) | 5 | .34 (.217) | 0.53 | .600 |
| Cognitive Tests | ||||||||
| Trail Making Test | ||||||||
| Trail Making A (secs.) | 23 | 47.7 (15.00) | 16 | 47.25 (14.23) | 7 | 48.86 (17.92) | 0.23 | .820 |
| Trail Making B (secs.) | 23 | 116.4 (56.0) | 16 | 116.19 (57.99) | 7 | 117.00 (55.49) | 0.03 | .975 |
| Verbal Fluency Tests | ||||||||
| Animal names | 23 | 16.0 (4.4) | 16 | 15.88 (5.04) | 7 | 16.14 (2.54) | 0.13 | .896 |
| COWAT (FAS) | 23 | 32.9 (13.1) | 16 | 34.38 (13.00) | 7 | 29.43 (13.55) | 0.83 | .416 |
| Wechsler Memory Scale - Revised | ||||||||
| Verbal Index | 22 | 78.1 (16.9) | 15 | 77.27 (13.70) | 7 | 79.86 (23.65) | 0.33 | .747 |
| Visual Index | 22 | 91.5 (23.6) | 15 | 87.60 (24.91) | 7 | 100.00 (19.51) | 1.16 | .261 |
| Attention Index | 22 | 83.0 (17.6) | 15 | 81.73 (18.42) | 7 | 85.71 (16.91) | 0.48 | .634 |
| Delayed Recall Index | 22 | 77.9 (19.5) | 15 | 74.87 (18.41) | 7 | 84.43 (21.74) | 1.07 | .296 |
| General Memory Index | 22 | 77.4 (20.0) | 15 | 75.40 (17.37) | 7 | 81.71 (25.68) | 0.68 | .503 |
| Wechsler Adult Intelligence Scale III | ||||||||
| Full Scale IQ | 21 | 90.4 (13.3) | 14 | 90.93 (11.45) | 7 | 89.29 (17.51) | 0.26 | .798 |
| Performance IQ | 21 | 84.5 (12.9) | 14 | 84.00 (11.57) | 7 | 85.57 (16.26) | 0.26 | .800 |
| Verbal IQ | 21 | 96.7 (13.7) | 14 | 97.79 (12.03) | 7 | 94.43 (17.53) | 0.52 | .610 |
| Wisconsin Card Sort Test | ||||||||
| Error Percent | 22 | 37.1 (17.0) | 15 | 39.9 (17.3) | 7 | 31.0 (15.8) | 1.56 | 2.51 |
| Perseverative Responses (%) | 22 | 25.7 (20.8) | 15 | 27.7 (22.7) | 7 | 21.6 (16.9) | 0.63 | .502 |
| Non-perseverative Responses (%) | 22 | 15.3 (10.3) | ||||||
| Perseverative Errors (%) | 22 | 21.5 (15.2) | 15 | 16.5 (12.1) | 7 | 12.6 (4.2) | 0.82 | .422 |
| PANSS Symptom Factors | 15 | 23.1 (16.4) | 7 | 18.3 (12.6) | 0.68 | .502 | ||
| Positive | 21 | 8.9 (4.3) | 15 | 8.27 (4.76) | 6 | 10.33 (2.73) | 0.99 | .334 |
| Negative | 21 | 18.0 (5.9) | 15 | 18.13 (5.74 | 6 | 17.50 (6.77) | 0.22 | .830 |
| Activation | 21 | 7.4 (1.2) | 15 | 7.33 (1.23) | 6 | 7.67 (1.03) | 0.58 | .567 |
| Dysthymia | 21 | 9.3 (4.6 | 15 | 8.93 (5.13) | 6 | 10.33 (3.20) | 0.62 | .545 |
| Autistic Preoccupation | 21 | 10.3 (3.6) | 15 | 9.33 (2.50) | 6 | 12.83 (4.79) | 1.70 | .139_ |
| Deficit Syndrome Items | ||||||||
| Restricted Effect | 21 | 1.10 (1.04) | 15 | 1.20 (1.01) | 6 | .83 (1.17) | 0.72 | .481 |
| Diminished emotional range | 21 | 1.05 (.92) | 15 | 1.13 (.99) | 6 | .83 (.75) | 0.67 | .514 |
| Poverty of speech | 20 | .70 (.80) | 15 | .80 (.86) | 5 | .40 (.55) | 0.97 | .347 |
| Curbing of interests | 21 | .86 (.73) | 15 | .93 (.70) | 6 | .67 (.82) | 0.75 | .462 |
| Diminished sense of purpose | 21 | 1.05 (.97) | 15 | 1.33 (.90) | 6 | .33 (.82) | 2.36 | .029* |
| Diminished social drive | 21 | 1.43 (.87) | 15 | 1.53 (.83) | 6 | 1.17 (.98) | 0.87 | .397 |
-indicates significance,
separate variance estimate corrected
As predicted by activation of the stress cascade, and shown in Table 2, ET was associated with cortisol, although some of these associations varied by sex. Men with high general trauma showed lower afternoon cortisol whereas women showed significantly different associations by Fisher’s R to Z transformation tests, demonstrating non-significant positive associations. Also, increased emotional abuse predicted higher cortisol measured 30′ after awakening in the entire sample, and particularly in the males. Perceived stress was significantly and positively related to afternoon cortisol in all cases, and higher evening cortisol similarly predicted reduced whole brain volumes in the entire group. In contrast, it was afternoon cortisol that again showed sex differences, predicting higher brain volumes in men but significantly reduced volumes in women. A reduction in the hippocampal volume in comparison to the whole brain volume was present in men and women with higher evening cortisol levels, which is a crucial node in the stress cascade. More general traumatic events predicted reduced whole brain volumes and increased amygdala to whole brain volume ratios (Table 2). The effects were similar in both sexes but were stronger in men, as was the trend for decreased hippocampal to amygdala volume ratios for those with greater trauma. It is notable that general trauma was significantly associated with relatively larger amygdala volumes based on the increased amygdala to whole brain volume ratios, but also with reduced hippocampal volumes based on the decreased hippocampal to amygdala ratios. However, a significant relationship between trauma and hippocampal to whole brain volume ratio was not demonstrated.
Table 2.
Significant Associations of ET and Current Perceived Stress with Cortisol, Brain Volumes and PANSS Symptom Factor Scores
| All Cases r, N, p |
Males r, N, p |
Females r, N, p |
M/F R to Z‡ |
|
|---|---|---|---|---|
| Cortisol with general trauma and current perceived stress: | ||||
| General Events with: Afternoon Cortisol | −.157 (18) .534 | −.737 (8) .037 | .520 (8) .186 | .016 |
| Emotional Abuse with: Cortisol 30 “After Awakening | .511 (18) .030 | .662 (11) .027 | .368 (7) .416 | |
| Perceived Stress with: Afternoon Cortisol | 477 (18) .045 | .284 (11) .398 | .434 (6) .390 | |
| Brain volumes and general trauma: | ||||
| Whole Brain Volume | −.501 (17) .040 | −.686 (9) .041 | −.084 (8) .844 | |
| Amygdala: Whole Brain Volume Ratio | .564 (17) .018 | .724 (9) .027 | .139 (8) .742 | |
| Hippocampal:Amygdala Volume Ratio | −.468 (17) .058 | −.534 (9) .139 | −.264 (8) .524 | |
| Hippocampal:Whole Brain Volume Ratio (added for completion) | −.087 (17) .740 | −.062 (9) .941 | −.185 (8) .662 | |
| Brain volumes and cortisol levels: | ||||
| Whole Brain Volume with Evening Cortisol | −.844 (10) .002 | −.671 (4) .329 | −.884 (6) .020 | |
| Whole Brain Volume with Afternoon Cortisol | −.520 (14) .057 | .545 (5) .342 | −.728 (9) .026 | .060 |
| Hippocampal: Whole Brain Ratio with Evening Cortisol | −.728 (9) .026 | −.999 (4) .001 | −.624 (5) .261 | |
| Hippocampal Volume with Evening Cortisol | −.622 (10) .055 | −.955 (4) .045 | −.693 (6) .127 | |
| Hippocampal: Amygdala Volume with cortisol 30 “After Awakening | .555 (14) .040 | .715 (6) .110 | .499 (8) .209 | |
| Symptoms and total trauma: | ||||
| Positive Symptoms | .660 (16) .005 | .660 (10) .038 | .624 (6) .186 | |
| Negative Symptoms | −.563 (16) .023 | −.517 (10) .126 | −.614 (6) .195 | |
| Dysthymia | .649 (16) .007 | .703 (10) .023 | .456 (6) .364 | |
| Poverty of speech | −.550 (15) .034 | −.440 (10) .203 | −.722 (5) .169 | |
| Symptoms and current perceived stress: | ||||
| Diminished Sense of Purpose | .575 (17) .016 | .626 (13) .022 | .860 (4) .140 | |
| Diminished Social Drive | .559 (17) .020 | .648 (13) .017 | .399 (4) .601 | |
| Dysthymia | .533 (18) .023 | .449 (14) .107 | .973 (4) .027 | |
Indicates comparison of male and female correlations
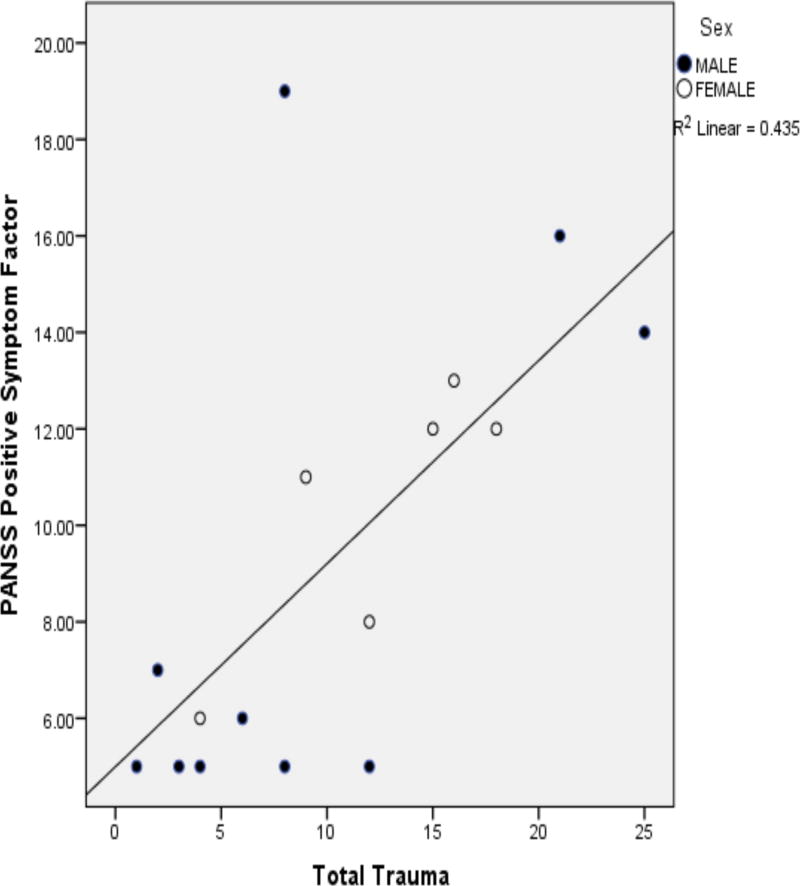
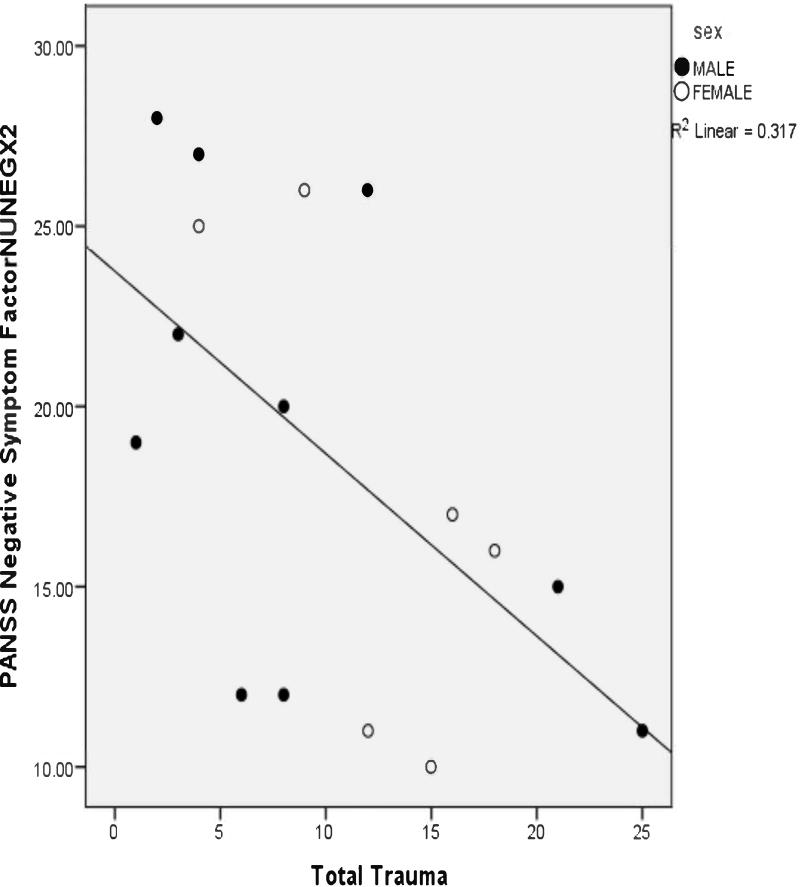
As shown in Table 2, positive symptoms were significantly and positively related to total trauma (depicted in Figure 1), as well as to general, physical, and emotional abusive events. Correspondingly, positive symptoms were significantly and positively associated with total traumatic events rated negatively, particularly in the males, in addition to general, physical, and emotional abuse events. The relationship between total trauma and positive symptoms remained significant in two separate multiple regression models, the first of which accounted for the effects of age and cortisol 30′ after awakening, and the second of which controlled for age and afternoon cortisol (Tables 3a and 3b). In contrast to positive symptoms, negative symptoms were significantly less in relation to total trauma and total trauma appraised negatively in all patients (Figure 2). The negative association between total trauma and negative symptoms remained significant in two multiple regression analyses that controlled for age and measures of cortisol (Tables 4a and 4b). Current perceived stress significantly predicted dysthymia, as well as diminished sense of purpose and social drive. The former was stronger in female cases and the latter two (trait deficits from the syndrome for deficit symptoms) were stronger for male cases.
Figure 1.
Scatterplot of the Correlation Between Total Early Trauma and Positive Symptoms (Pearson r=.660, p=.005).
Table 3a.
Regression Table: Dependent variable = PANSS Positive Symptom Factor; covariates are Age and 30′ After Awakening Cortisol. Independent measure of interest = Total Trauma.
| Model | Standardized Coefficients Beta | t | Sig. | 95.0% Confidence Interval for B | ||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 1 | (Constant) | 1.130 | .291 | −4.565 | 13.333 | |
| Age 30′ After | .323 | 1.418 | .194 | −.119 | .498 | |
| Awakening Cortisol | −.561 | −2.402 | .043 | −2.498 | −.051 | |
| Total Trauma | .637 | 2.720 | .026 | .064 | .777 | |
Table 3b.
Regression Table: Dependent variable = PANSS Positive Symptom Factor; covariates are Age and Afternoon Cortisol. Independent measure of interest = Total Trauma.
| Model | Standardized Coefficients Beta | t | Sig. | 95.0% Confidence Interval for B | ||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 1 | (Constant) | −.454 | .661 | −6.165 | 4.105 | |
| Age | .338 | 2.318 | .046 | .003 | .288 | |
| Afternoon Cortisol | .026 | .160 | .877 | −2.500 | 2.880 | |
| Total Trauma | .843 | 5.767 | .000 | .284 | .650 | |
Figure 2.
Scatterplot of the Correlation Between Total Early Trauma and Negative Symptoms (Pearson r=−.563, p=.023).
Table 4a.
Regression Table: Dependent variable = PANSS Negative Symptom Factor; covariates are Age and 30′ After Awakening Cortisol. Independent measure of interest = Total Trauma.
| Model | Standardized Coefficients Beta | t | Sig. | 95.0% Confidence Interval for B | ||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 1 | (Constant) | 2.743 | .025 | 3.063 | 35.406 | |
| Age 30′ After | .134 | .470 | .651 | −.444 | .672 | |
| Awakening Cortisol | −.264 | .907 | .391 | −1.341 | 3.080 | |
| Total Trauma | .675 | −2.311 | .050 | −1.290 | −.002 | |
Table 4b.
Regression Table: Dependent variable = PANSS Negative Symptom Factor; covariates are Age and Afternoon Cortisol. Independent measure of interest = Total Trauma.
| Model | Standardized Coefficients Beta | t | Sig. | 95.0% Confidence Interval for B | ||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 1 | (Constant) | 3.987 | .003 | 11.533 | 41.791 | |
| Age | .405 | 1.367 | .205 | −.166 | .673 | |
| Afternoon Cortisol | −.542 | −1.644 | .134 | −13.687 | 2.164 | |
| Total Trauma | −.787 | −2.647 | .027 | −1.171 | −.092 | |
Early trauma also did not predict cognitive impairments and, to the contrary (Table 5), female cases with greater trauma had significantly better performance on FAS verbal fluency, verbal memory index, attention index, delayed memory index, general memory index, full scale and verbal IQ scores than other females. Also in the females, total trauma was significantly associated with better performance on the attention index and with better general memory at the trend level. Perceived stress was significantly and positively associated with performance on animal naming and the WMS-R attention index in the sample. For the females, perceived stress was significantly and positively associated with delayed memory index, and marginally associated with visual and general memory indices. The associations of stress and better cognition for female subjects were significantly different from the respective associations in the males, whose correlations were in the low negative direction, but did not reach statistical significance.
Table 5.
Significant Associations of ET and Perceived Stress with Cognitive Measures
| All Cases | Male Cases | Female Cases | M/F R to Z | |
|---|---|---|---|---|
| General Events and Cognitive Measures: | ||||
| FAS | .193 (18) .442 | −.009 (11) .979 | .852 (7) .015 | .038 |
| Verbal Memory Index | .299 (17) .243 | −.052 (10) .886 | .766 (7) .045 | |
| Attention Index | .226 (17) .384 | −.058 (10) .873 | .880 (7) .009 | |
| Delayed Memory Index | .162 (17) .534 | −.167 (10) .644 | .779 (7) .039 | |
| General Memory Index | .197 (17) .448 | −.215 (10) .552 | .806 (7) .029 | .034 |
| Full Scale IQ | .212 (16) .431 | −.174 (9) .654 | .822 (7) .023 | .039 |
| Verbal IQ | .198 (16) .462 | −.219 (9) .572 | .837 (7) .019 | .027 |
| Current Perceived Stress and Cognitive Measures: | ||||
| Animal Names | .480 (19) .038 | .407 (14) .148 | .854 (5) .066 | |
| Visual Memory Index | .022 (19) .928 | −.255 (14) .380 | .871 (5) .054 | .038 |
| Attention Index | .461 (19) .047 | .385 (14) .175 | .661 (5) .225 | |
| Delayed Memory Index | .184 (19) .450 | −.149 (14) .611 | .928 (5) .023 | .020 |
| General Memory Index | .292 (19) .226 | −.009 (14) .975 | .875 (5) .052 | .076 |
Additional analyses of ET subscales demonstrated that the magnitude of exposure to sexual abuse predicted lesser negative symptoms. Sexual abuse was reported by 31.3% (5/16) subjects, including 3/10 males and 2/6 females.
DISCUSSION
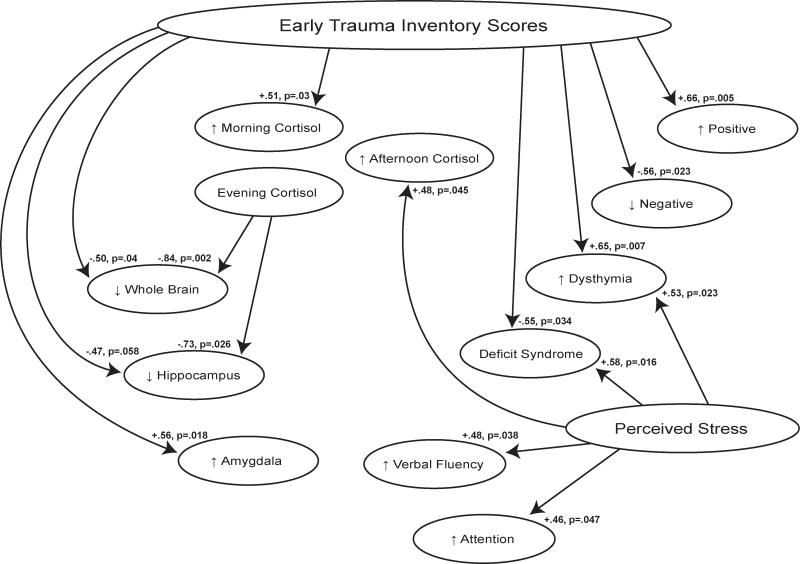
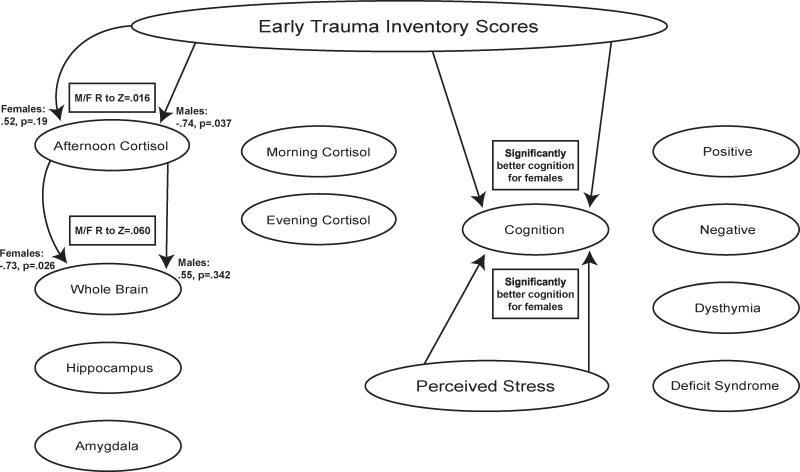
These findings demonstrate associations of early trauma with symptoms, cortisol levels, and neural volumes in chronic schizophrenia cases (Figure 3), supporting the value of further studies of HPA axis dysfunction in the ontology of the disease. Trauma exposure was significantly associated with positive symptoms and, given that the patients were all treated, these were treatment refractory psychotic symptoms. Furthermore, ET and perceived stress predicted aberrant daily cortisol patterns, and ET was significantly related to reduced whole brain and relative hippocampal volumes, as well as increased amygdala volumes. Trauma exposure and perceived stress also predicted higher depression scores, measured by the PANSS dysthymia factor. Conversely, ET was associated with significantly less negative symptoms and lower scores on some deficit items. Current perceived stress, on the other hand, predicted more deficit symptoms for diminished sense of purpose and social drive, with stronger effects for male cases. Although ET was unrelated to cognitive performance for the all cases group, it predicted significantly better cognition for the females. A number of measures showed sex differences (Figure 4), which is consistent with the large literature on sex differences in HPA function and responses to stress, as recently reviewed.37 Notably, the significant positive correlations between ET and cognitive performance in the females were significantly different from the non-significant negative correlations found between ET and cognition in the males.
Figure 3.
The effects of stress on features of schizophrenia in all patients: shows significant positive and negative relationships between stress (early trauma and perceived stress) and features of schizophrenia for all cases in the sample.
Figure 4.
Significant differences between the sexes: shows significant differences between the males and the females for the associations of stress with features of schizophrenia.
This study suggests a role for ET in the development of positive psychotic symptoms in schizophrenia cases with treatment refractory positive symptoms, which is consistent with other findings supporting the relevance of ET for psychosis.3;5;6 The relationship of stress and cortisol values with reduced whole brain and relative hippocampal volumes (but not amygdala volumes) is congruous with negative glucocorticoid influences on neurogenesis, a major component of the stress cascade. Walker et al.38 recently demonstrated hypercortisolemia in at-risk adolescents who developed psychosis; others propose hypercortisolemia contributes to schizophrenia risk by elevating dopaminergic activity, 39–40 which is consistent with the excessive positive symptoms in our chronic cases and in other samples. The relationship between stress and depression was also not unexpected;41 increased or dysregulated HPA axis function figures prominently in depression,41 particularly for psychotic depression.42–43
Our finding that emotional abuse is related to cortisol measured 30′ after awakening adds to a conflicted literature on the relationship of cortisol with ET in psychosis, although our results include more detailed assessments of ET.44–46 These discrepant findings echo other conflicting results on HPA activity in schizophrenia. A 2010 review found increased basal cortisol in 44.2% of studies and reports of decreased levels by 5.2% of 77 studies.11 These varying results may reflect patient heterogeneity, quantity, timing and frequency of ET or other methodological factors. It is well demonstrated that ET can lead to an eventual hypofunction of cortisol secretion. This decrease may be a “counterregulative adaption”47 or “over-adjustment”48 after prolonged and frequent exposure to trauma. Sex differences may also play a role in our varying results. There are reports of males exhibiting greater cortisol and stress responses to traumatic recollections15 and to psychosocial stress,16 which may be explained by their psychological and emotional processing16 and coping strategies17–18. For example, there is evidence that females rely more heavily upon more adaptive “tend-and-befriend” styles to cope with stressful exposures, as opposed to the fight-flight response, which males tend to use17–18. This response may be regulated by oxytocin release17–18, which can diminish HPA stress responding.49 If men are in fact more vulnerable to the detrimental effects of stress, this may help to explain why the positive relationship between emotional abuse and cortisol measured 30′ after awakening only remained significant in the males when our sample was broken up by sex.
The hippocampal reductions from stress and cortisol accord with human50 and animal studies,51–52 and support the contention that some portion of the well-recognized reductions in hippocampal volumes in schizophrenia are related to ET. Other genetic and environmental insults also compromise hippocampal function in the disease, which may explain why hippocampal-dependent memory was not negatively associated with trauma. Nonetheless, vigilance was increased, as seems appropriate for stress activation.
The relatively increased amygdala volumes are interesting. The amygdala is the center for sensory input that initiates the stress cascade53 and is involved in emotional learning, including fear processing. Over-activation of the amygdala is commonly reported in PTSD54–55, although the volume sizes may be reduced.56 One study found larger amygdala volumes in PTSD, but adult exposures including greater severity of combat exposure and an interaction of combat severity exposure with childhood trauma predicted smaller amygdala volumes.57 Whole brain volume reductions are also consistently reported in schizophrenia,58–59 and our findings suggest that some of the reduction may be attributable to early adversity, which should be considered in brain imaging studies.
Our results concerning negative and cognitive symptoms are consistent with the notion that these symptoms arise from a distinct diathesis with separate underpinnings. Neurodevelopmental deficits are commonly hypothesized for this diathesis. Notably, the females with high ET had distinctly better cognition, suggesting that trauma may be more sufficient in triggering their mental illness. The positive association between general trauma and cognition in females was unexpected and conflicts with two other studies.60–61 They reported worse cognition in schizophrenia cases exposed to trauma; e.g., deficits in working memory60–61, episodic narrative memory61, and information processing speed.60 One61 found no significant differences for sex for those with moderate to high trauma exposure compared to those with low or no trauma, but this sample consisted of mostly male subjects, and the other study60 only included male subjects. Sex differences in stress reactivity may also help to explain these results. If males exhibit increased stress and cortisol responses to stressful exposures in comparison with females (as above), this suggests less damage to the hippocampus in females, and may partially account for why higher levels of trauma predicted better neuropsychological performance in the females in our sample (in contrast to the males, who showed non-significant negative correlations between these variables). Additionally, our finding that perceived stress predicted better attention and vigilance is consistent with increased arousal and stress, giving some support to our findings.
The relationship of certain deficit syndrome items with perceived stress suggests that either these deficits make it more stressful to be in an intense milieu or that the symptoms may themselves be defensive against current stressors. Findings that these symptoms are persistent and trait-like support the former hypothesis.
Notably, ET was frequent in the current sample. Compared to subjects in another study that used the Early Trauma Inventory Self Report (ETI-SR),35 which has 6 more questions about ET than the clinician-administered ETI that we used, our sample had 2 to 3 times the trauma as healthy subjects or those with depression, bipolar disorder or post-traumatic stress disorder. Trauma, therefore, is clearly coloring features of disease and may be a crucial exposure for the development of schizophrenia, particularly for females who evidence less global cognitive deficits in the setting of exposure to ET.
A major strength of this study was the ETI instrument, which considers the age of occurrence, frequency, identity of the perpetrators, and the impact of the event.26,35 In contrast to self-report formats, which limit the ability of the clinician to determine the patient’s comprehension of the assessment questions, the version of the ETI that we used was clinician-administered. The tradeoff is that our information was retrospective and based only on subject reporting, which can bias the reporting of events based on arousal and stress. A major weakness of this study was its small sample size, as it was designed as a proof of concept pilot study. We also recognize the lack of a matched healthy control group in our study, although our findings are consistent with many studies of healthy and other samples. Finally, because this was a cross-sectional study, we could not track the progression of cortisol levels, brain volumes, cognition, and symptoms from childhood onward, limiting the extent to which we could corroborate the relationships we found between these variables and ET.
In conclusion, our findings provide support for the hypothesis that ET and stress can play a role in the development of the treatment refractory positive symptoms of schizophrenia via stress sensitization, whereas negative and cognitive symptoms may reflect other components of vulnerability. This pilot study indicates the importance of future studies that utilize larger samples and longitudinal designs to consider early trauma and stress sensitization in the development of features of schizophrenia. Finally, it may be important to consider treating stress in individuals with schizophrenia who are incomplete responders with positive symptoms, and utilizing early intervention in children and adolescents who experienced trauma.
Acknowledgments
This work was supported by NIMH K23MH066279 (CC) and K24MH001699 (DM) and was presented at the Society of Biological Psychiatry’s 69th Annual Scientific Meeting in 2014. The authors thank Jonathan Slavuter for designing the figures and Stephanie Polito, Kevin McMahon, and Marisa Gorovitz for editorial assistance.
Footnotes
FINANCIAL DISCLOSURES
None of the authors have any financial disclosures or conflicts of interest.
References
- 1.Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- 2.Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- 3.Heins M, Simons C, Lataster T, et al. Childhood trauma and psychosis: a case-control and case-sibling comparison across different levels of genetic liability, psychopathology, and type of trauma. Am J Psychiatry. 2011;168:1286–1294. doi: 10.1176/appi.ajp.2011.10101531. [DOI] [PubMed] [Google Scholar]
- 4.Matheson SL, Sheperd AM, Pinchbeck RM, Laurens KR, Carr VJ. Childhood adversity in schizophrenia: a systematic meta-analysis. Psychol Med. 2013;43:225–238. doi: 10.1017/S0033291712000785. [DOI] [PubMed] [Google Scholar]
- 5.Read J, van Os J, Morrison AP, Ross CA. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112:330–350. doi: 10.1111/j.1600-0447.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 6.Varese F, Smeets F, Drukker M, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient- control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38:661–71. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ucok A, Bikmaz S. The effects of childhood trauma in patients with first-episode schizophrenia. Acta Psychiatr Scand. 2007;116:371–377. doi: 10.1111/j.1600-0447.2007.01079.x. [DOI] [PubMed] [Google Scholar]
- 8.Yuii K, Suzuki M, Kurachi M. Stress sensitization in schizophrenia. Ann N Y Acad Sci. 2007;1113:276–290. doi: 10.1196/annals.1391.013. [DOI] [PubMed] [Google Scholar]
- 9.Ruby E, Polito S, McMahon K, Gorovitz M, Corcoran C, Malaspina D. Pathways associating childhood trauma to the neurobiology of schizophrenia. Front Psychol Behav Sci. 2014;3:1–17. [PMC free article] [PubMed] [Google Scholar]
- 10.Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 11.Bradley AJ, Dinan TG. A systematic review of hypothalamic-pituitary-adrenal axis function in Schizophrenia: implications for mortality. J Psychopharmacol. 2010;24:91–118. doi: 10.1177/1359786810385491. [DOI] [PubMed] [Google Scholar]
- 12.Adriano F, Caltagirone C, Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. The Neuroscientist. 2012;18:180–200. doi: 10.1177/1073858410395147. [DOI] [PubMed] [Google Scholar]
- 13.Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 14.Devylder JE, Ben-David S, Schobel SA, Kimhy D, Malaspina D, Corcoran CM. Temporal association of stress sensitivity and symptoms in individuals at clinical high risk for psychosis. Psychol Med. 2013;43:259–268. doi: 10.1017/S0033291712001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dekel S, Ein-Dor T, Gordon KM, Rosen JB, Bonanno GA. Cortisol and PTSD symptoms among male and female high-exposure 9/11 survivors. J Trauma Stress. 2013;26:621–625. doi: 10.1002/jts.21839. [DOI] [PubMed] [Google Scholar]
- 16.Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Klein LC, Corwin EJ. Seeing the unexpected: how sex differences in stress responses may provide a new perspective on the manifestation of psychiatric disorders. Curr Psychiatry Rep. 2002;4:441–448. doi: 10.1007/s11920-002-0072-z. [DOI] [PubMed] [Google Scholar]
- 8.Taylor SE, Klein LC, Lewis BP, Grunwald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 19.Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22:417–428. doi: 10.3109/09540261.2010.515205. [DOI] [PubMed] [Google Scholar]
- 20.Malaspina D, Corcoran C, Fahim C, et al. Paternal age and sporadic schizophrenia: evidence for de novo mutations. Am J Med Genet B Neuropsychiatr. 2002;114:299–303. doi: 10.1002/ajmg.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologica. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 22.Nurnberger JI, Jr, Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 23.Kay SR, Opler LA, Lindenmayer JP. The positive and negative syndrome scale (PANSS): rationale and standardisation. Br J Psychiatry. 1989;155:59–65. [PubMed] [Google Scholar]
- 24.Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT., Jr The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30:119–123. doi: 10.1016/0165-1781(89)90153-4. [DOI] [PubMed] [Google Scholar]
- 25.Cohen S, Karmack T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 26.Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the ET Inventory. Depress Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 27.Wechsler D. Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) administration and scoring manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 28.Wechsler D. Wechsler Memory Scale, Revised (WMS-R) San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 29.Heaton RK. Wisconsin Card Sorting Test: Computer version-2 research edition. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- 30.Reitan R. Validity of the Trail Making Test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 31.Miller E. Verbal fluency as a function of a measure of verbal intelligence in relation to different types of cerebral pathology. Br J Clin Psychol. 1984;23:53–57. doi: 10.1111/j.2044-8260.1984.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 32.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd edn. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 33.Gardner MP, Lightman S, Sayer AA, et al. Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and physical performance at older ages: an individual participant meta-analysis. Psychoneuroendocrinology. 2013;38:40–9. doi: 10.1016/j.psyneuen.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robb RA, Hanson DP, Karwoski RA, Larsson AG, Workman EL, Stacy MC. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph. 1989;13:433–454. doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- 35.Bremner JD, Bolus R, Mayer EA. Psychometric properties of the ET Inventory-Self Report. J Nerv Ment Dis. 2007;195:211–218. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White L, Harvey P, Opler L, Lindenmayer JP. Empirical assessment of the factorial structure of clinical symptoms in schizophrenia. A multisite, multimodel evaluation of the factorial structure of the Positive and Negative Syndrome Scale. The PANSS Study Group. Psychopathology. 1997;30:263–274. doi: 10.1159/000285058. [DOI] [PubMed] [Google Scholar]
- 37.Goel N, Workman JL, Lee TT, Innala L, Viau V. Sex differences in the HPA axis. Compr Physiol. 2014;4:1121–1125. doi: 10.1002/cphy.c130054. [DOI] [PubMed] [Google Scholar]
- 38.Walker EF, Brennan PA, Esterberg M, Brasfield J, Pearce B, Compton MT. Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. J Abnorm Psychol. 2010;119:401–408. doi: 10.1037/a0018399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schatzberg AF, Rothschild AJ, Langlais PJ, Bird ED, Cole JO. A corticosteroid/dopamine hypothesis for psychotic depression and related states. J Psychiatr Res. 1985;19:57–64. doi: 10.1016/0022-3956(85)90068-8. [DOI] [PubMed] [Google Scholar]
- 40.Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- 41.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinolog. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Nelson JC, Davis JM. DST studies in psychotic depression: a meta-analysis. Am J Psychiatry. 1997;154:1497–1503. doi: 10.1176/ajp.154.11.1497. [DOI] [PubMed] [Google Scholar]
- 43.Anton RF. Urinary free cortisol in psychotic depression. Biol Psychiatry. 1987;22:24–34. doi: 10.1016/0006-3223(87)90126-0. [DOI] [PubMed] [Google Scholar]
- 44.Braehler C, Holowka D, Brunet A, et al. Diurnal cortisol in schizophrenia patients with childhood trauma. Schizophr Res. 2005;79:353–354. doi: 10.1016/j.schres.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Valiquette LF. Association between self-reported childhood maltreatment and cortisol profiles in psychotic patients. Ann Arbor, MI: Proquest, UMI Dissertations Publishing; 2008. [Google Scholar]
- 46.Mondelli V, Dazzan P, Hepgul N, et al. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: The role of stress and of antipsychotic treatment. Schizophr Res. 2010;116:234–242. doi: 10.1016/j.schres.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carpenter LL, Carvalho JP, Tyrka AR, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Legros JJ. Inhibitory effect of oxytocin on corticotrope function in humans: are vasopressin and oxytocin ying-yang neurohormones? Psychoneuroendocrinology. 2001;26:649–655. doi: 10.1016/s0306-4530(01)00018-x. [DOI] [PubMed] [Google Scholar]
- 50.Frodl T, O'Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis. 2013;52:24–37. doi: 10.1016/j.nbd.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Sutanto W, Rosenfeld P, de Kloet ER, Levine S. Long-term effects of neonatal maternal deprivation and ACTH on hippocampal mineralocorticoid and glucocorticoid receptors. Brain Res Dev Brain Res. 1996;92:156–163. doi: 10.1016/0165-3806(95)00213-8. [DOI] [PubMed] [Google Scholar]
- 52.Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen JA, Perel JM, Debellis MD, Friedman MJ, Putnam FW. Treating traumatized children: clinical implications of the psychobiology of posttraumatic stress disorder. Trauma Violence Abuse. 2002;3:91–108. [Google Scholar]
- 54.Liberzon I, Taylor SF, Amdur R, et al. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 55.Rauch SL, van der Kolk BA, Fisler RE. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 56.Morey RA, Gold AL, LaBar KS, et al. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch Gen Psychiatry. 2012;69:1169–1178. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuo JR, Kaloupek DG, Woodward SH. Amygdala volume in combat-exposed veterans with and without posttraumatic stress disorder: a cross-sectional study. Arch Gen Psychiatry. 2012;69:1080–1086. doi: 10.1001/archgenpsychiatry.2012.73. [DOI] [PubMed] [Google Scholar]
- 58.Giedd JN, Jeffries NO, Blumenthal J, et al. Childhood-onset schizophrenia: progressive brain changes during adolescence. Biol Psychiatry. 1999;46:892–898. doi: 10.1016/s0006-3223(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 59.Gur RE, Cowell P, Turetsky BI, et al. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55:145–152. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- 60.Lysaker PH, Meyer P, Evans JD, Marks KA. Neurocognitive and symptom correlates of self-reported childhood sexual abuse in schizophrenia spectrum disorders. Ann Clin Psychiatry. 2001;13:89–92. doi: 10.1023/a:1016667624487. [DOI] [PubMed] [Google Scholar]
- 61.Shannon C, Douse K, McCusker C, Feeney L, Barrett S, Mulholland C. The association between childhood trauma and memory functioning in schizophrenia. Schizophr Bull. 2011;37:531–537. doi: 10.1093/schbul/sbp096. [DOI] [PMC free article] [PubMed] [Google Scholar]