Abstract
Background
Extensive animal research has demonstrated the vulnerability of the brain to early life stress (ELS) with consequences for emotional development and mental health. However, the influence of moderate and common forms of stress on early human brain development is less well understood and precisely characterized. To date, most work has focused on severe forms of stress, and/or on brain functioning years after stress exposure.
Methods
In this report we focused on conflict between parents (interparental conflict), a common and relatively moderate form of ELS that is highly relevant for children's mental health outcomes. We used resting state functional connectivity MRI to examine the coordinated functioning of the infant brain (N=23; 6–12-months-of-age) in the context of interparental conflict. We focused on the default mode network (DMN) due to its well characterized developmental trajectory and implications for mental health. We further examined DMN strength as a mediator between conflict and infants’ negative emotionality.
Results
Higher interparental conflict since birth was associated with infants showing stronger connectivity between two core DMN regions, the posterior cingulate cortex (PCC) and the anterior medial prefrontal cortex (aMPFC). PCC to amygdala connectivity was also increased. Stronger PCC-aMPFC connectivity mediated between higher conflict and higher negative infant emotionality.
Conclusions
The developing DMN may be an important marker for effects of ELS with relevance for emotional development and subsequent mental health. Increasing understanding of the associations between common forms of family stress and emerging functional brain networks has potential to inform intervention efforts to improve mental health outcomes.
Keywords: Functional MRI (fMRI), infancy, stress, family functioning, brain development
Introduction
Early life stress (ELS) influences the development of neurobiological systems implicated in emotional functioning and mental health across the lifespan (Loman & Gunnar, 2010). Stress is posited to have a particularly pronounced impact during early periods of brain development, as rapid changes and specific properties of certain brain systems confer vulnerability to disorganizing influences (Tottenham & Sheridan, 2010). This phenomenon is referred to as developmental programming (Gluckman & Hanson, 2004).
Functional magnetic resonance imaging (fMRI) provides the capacity to examine brain systems that develop rapidly during infancy and are tightly linked to subsequent mental health. However, fMRI research examining effects of ELS on the brain has predominantly been conducted in older children and adults. Thus, it is difficult to differentiate effects of stress during early developmental periods from effects of subsequent ongoing adversity, the emergence of coping strategies, or symptoms of psychopathology. Moreover this work has frequently focused on more extreme sources of adversity, such as institutional rearing (Tottenham et al., 2011) or maltreatment (McCrory et al., 2013). There is currently limited understanding of how normative variation in familial stress influences early development of brain systems important for mental health outcomes.
Interparental Conflict and Mental Health Outcomes
As with other common forms of familial stress, conflict between parents (or interparental conflict) often increases after the birth of a child as the family system adjusts to the challenges of having a new infant (Cox & Paley, 2003). Although a common phenomenon in homes with children, interparental conflict is not benign, and has a well established impact on children's mental health (Cummings & Davies, 2002, 2010). For example, in a study of a large community sample, strong associations between interparental conflict and children's internalizing symptoms (anxiety and depression), externalizing symptoms (delinquency and impulsivity) and symptoms of posttraumatic stress disorder (PTSD) were evident, regardless of children's sex and ethnicity, and family socioeconomic status (El-Sheikh, Cummings, Kouros, Elmore-Staton, & Buckhalt, 2008).
Recent work has provided a link between interparental conflict during infancy and the functioning of brain regions implicated in mental health outcomes. A study employing fMRI with infants indicated that non-physical interparental conflict during the first year of life is associated with the brain’s ability to process and regulate stress and emotions. Specifically, higher levels of interparental conflict were associated with greater reactivity to a stress relevant stimulus (i.e., angry tone of voice), in the medial prefrontal cortex (MPFC)/rostral anterior cingulate cortex (rACC) and several subcortical regions, including the hypothalamus, thalamus and caudate (Graham, Fisher, & Pfeifer, 2013). These results suggest that early exposure to interparental conflict may influence mental health outcomes via effects on brain regions involved in stress and emotion regulation. More broadly, they indicate the potential for fMRI with infants to detect associations between moderate sources of familial stress and the functioning of brain systems tightly linked to mental health outcomes.
An important next step for this work is to move beyond examination of brain functioning in response to a specific, stressor relevant stimulus to understand how stressful events affect the integration of information between brain regions in vulnerable networks. There is now ample data to suggest such phenomenon can be examined in infants with resting-state functional connectivity MRI (rs-fcMRI;see Graham et al., 2015 for a review).
Capturing Early Functional Brain Development with Resting State Functional Connectivity MRI
Mental health, and social and emotional functioning more generally, are understood to rely on coordinated functioning among multiple brain regions organized into dissociable functional brain networks (Fox & Greicius, 2010; Whitfield-Gabrieli & Ford, 2012). These networks are activated to respond to task demands, but also demonstrate coordinated functioning in the absence of stimuli or tasks, and are therefore referred to as resting state networks (RSNs; Fox & Raichle, 2007). A wealth of highly influential work has led to an understanding that this intrinsic (resting), as opposed to reflexive (task-based), brain activity is a core feature of healthy brain functioning (Raichle, 2010).
Recent work demonstrates that specific RSNs relevant to mental health emerge during infancy, and develop particularly rapidly during the first year of life (Gao et al., 2013; Gao, Alcauter, & Smith, 2014). The default mode network (DMN) is one of these several systems, with multiple studies in infants documenting the existence of a network that bares remarkable resemblance to that observed in children and adults (Fransson et al., 2009; Gao, Alcauter, & Smith, 2014; Gao et al., 2009, 2011, 2013; Gao, Alcauter, Elton, et al., 2014; Gao, Elton, et al., 2014; Smyser et al., 2010; See Graham et al., 2014 for a review). The DMN is a group of brain regions originally described by the propensity to reduce activity in the presence of a stimulus or task (Raichle et al., 2001). Although the specific role of the DMN in behavioral functioning remains under investigation, the strength of the DMN has repeatedly been associated with mental health during childhood and adulthood, (Fox & Greicius, 2010), including depression (Berman et al., 2011; Sheline, Price, Yan, & Mintun, 2010) and attention deficit hyperactivity disorder (Fair et al., 2010, 2012).
The DMN also appears to be susceptible to ELS, including experiences of childhood abuse (Philip et al., 2013) and poverty (Sripada, Swain, Evans, Welsh, & Liberzon, 2014), and preterm birth (Smyser et al., 2010). However, the influence of familial stress on emerging DMN connectivity during the rapid developmental period of the first year of life has not been examined. Moreover, despite the well-documented associations between DMN strength and mental health in children and adults, links between the DMN and early indicators of emotional functioning during infancy have not been established.
Present Study
The present study focuses on the DMN during the first year of life as a potential mediator between a common form of familial stress and infants’ negative emotionality. We focus on the DMN as a good starting point for investigation of this topic given the extensive literature focused on this network. However, the DMN is only one of multiple functional brain systems, and interacts with others under conditions of environmental stress (Veer et al., 2011). In fact, in addition to differences in resting state connectivity among midline DMN regions (PCC and MPFC), differences in connectivity between the DMN and amygdala have frequently been associated with exposure to environmental stress and emergence of stress related sympotomatology in adults (Bluhm et al., 2009; Lanius et al., 2010; Zhou et al., 2012). Our approach therefore focuses on the DMN, but does not restrict analyses to the DMN.
Higher levels of non-physical interparental conflict during the first year of life are hypothesized to predict differences in DMN connectivity strength. Previous literature indicates that early life stress may be associated with disruption of developing functional connectivity (e.g. effects of preterm birth [Smyser et al., 2010] and stress in the Neonatal Intensive Care Unit [G. C. Smith et al., 2011]), or with accelerated development of functional connectivity (e.g. effects of early adverse caregiving [Callaghan, Sullivan, Howell, & Tottenham, 2014; Gee et al., 2013; Tottenham, 2014]. We consider both possibilities.
Variability in the strength of functional connectivity is further hypothesized to mediate between higher levels of conflict and infants’ negative emotionality. Negative emotionality has frequently been associated with early life stress (Loman & Gunnar, 2010; Propper & Moore, 2006). DMN connectivity during represents a potential mediator of such effects due to its rapid development during infancy, vulnerability to ELS, and repeated links to internalizing disorders in research with children and adults (Berman et al., 2011; Gaffrey, Luby, Botteron, Repovš, & Barch, 2012; Sheline et al., 2010; Sylvester et al., 2012). The overarching hypothesis is that the DMN is vulnerable to variation in moderate forms of ELS during the first year of life, which in turn has implications for emotional development.
Methods
Participants
Families were recruited through advertisements on Craigslist and flyers posted in the community. The Problem Solving Communication subscale from the Marital Satisfaction Inventory-Revised (Snyder, 1997) was used during the screening process to assess level of interparental conflict, and obtain a range of conflict in the sample. The sample included healthy 6–12-month-old infants residing with both biological parents, and with no referrals or investigations involving any member of the family by a public child protective services agency (see Graham et al., 2013). The study was approved by the University of Oregon and Oregon Social Learning Center Institutional Review Boards, and parents of infants provided written informed consent.
Scans were attempted for 39 healthy infants (with no known significant illnesses, birth defects, neurological conditions or head injuries). Twenty-three infants (M=8.35 months, SD=1.94; 8 female) completed the scans for the present study. Twelve infants had difficulty falling asleep at the scan center, and 4 woke prior to or during the resting state scan. This is in line with previously reported success rates for natural sleep scanning (e.g. Blasi et al., 2011). There were no differences for the included versus excluded infants on any demographic variables (infant age, gender, race and ethnicity, maternal education, and household income), or on the dimensions of negative emotionality or conflict.
For the infants included in the study, race and ethnicity were representative of the community in which recruitment occurred (82.6% Caucasian and 17.4% other or more than one race; 21.7% Hispanic). Educational attainment for mothers was as follows: 8.7% did not complete high-school or a test equivalent, 21.7 % completed high-school or a test equivalent, 34.8% completed some community college, and 34.6% completed at least one year or more of a standard 4-year college. The median category for gross annual household income was $30,000–$39,999, based on a 12-point scale: 1 (less than $4,999 per year) to 12 ($100,000 or more per year).
Interparental Conflict Measures
Mothers reported on non-physical interparental conflict on the Psychological Aggression scale of the Revised Conflicts Tactics Scale (CTS2; Straus, Hamby, Boney-McCoy, & Sugarman, 1996). The CTS2 was adapted to focus on two time periods, pregnancy and since childbirth (postnatal). Participants indicated the frequency of postnatal aggressive behaviors on the following scale: 0 (this has never happened), 1 (once), 2 (twice), 3 (3–5 times), 4 (6–10 times), 5 (11–20 times), or 6 (more than 20 times). They indicated whether each behavior occurred during pregnancy with a ‘yes’ or ‘no.’ The CTS2 asks about psychological aggression toward and received from partner. These scores were highly correlated during pregnancy (r = .871, p < .001), and during the postnatal period (r = .941, p < .001). They were therefore averaged for each time period to form one pregnancy (α=.883), and one postnatal (α=.937) composite. The postnatal score was the predictor variable of interest.
Infant Negative Emotionality
Negative emotionality was assessed via maternal report on the Sadness and Distress to Limitations subscales from the Infant Behavior Questionnaire-Revised (Gartstein & Rothbart, 2003). Mothers rated infants’ engagement in specific behaviors on a scale from 1 (never) to 7 (always). These subscales have been found to load on the Negative Affectivity dimension of temperament (Gartstein & Rothbart, 2003), and were significantly correlated in this sample (r = .468, p < .05). They were therefore combined to form a negative emotionality composite (α=.749).
fMRI Data Acquisition
Neuroimaging data was collected during natural sleep on a Siemens Allegra 3.0T scanner with a phased array coil. An 8 minute high-resolution T1-weighted MP-RAGE scan was obtained (TR=2500ms, TE=4.38 ms, TI=1100ms, flip angle=8°, matrix size 256×192, FOV=256mm, 160 slices, 1mm in-plane resolution, 1mm thick), followed by two T2 weighted functional scans with auditory stimuli (Graham et al., 2013). An additional T2-weighted echoplanar functional scan with no stimuli was collected to obtain resting state data (TR=2000ms, TE=30ms, flip angle=80°, matrix size 64×64, FOV=200mm, 32 slices, 3.125mm in-plane resolution, 4mm thick, 180 whole brain volumes). Prospective acquisition correction (PACE) was applied to adjust slice position and orientation, as well as to re-grid residual volume-to-volume motion in real-time during data acquisition for the purpose of reducing motion-induced effects (Thesen, Heid, Mueller, & Schad, 2000).
fMRI Analysis
Data preprocessing
The MRIConvert program (http://lcni.uoregon.edu/~jolinda/MRIConvert/) was used to convert neuroimaging data to Neuroimaging Informatics Technology Initiative data format. Brain images were separated from the rest of the head tissue with the Brain Extraction Tool from the FMRIB Software Library (Beckmann et al., 2006; S. M. Smith, Bannister, Beckmann, & Brady, 2001; S. M. Smith, 2002) and the Brain Surface Extraction tool from BrainSuite09 (Sandor & Leahy, 1997; Shattuck, Sandor-Leahy, Schaper, Rottenberg, & Leahy, 2001). Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology, London, England) was used for realignment of functional images, registration to the anatomical scan, and normalization to a standard infant template (8- to 11-month age range) from the MRI Study of Normal Brain Development (Fonov et al., 2011; Fonov, Evans, Mckinstry, Almli, & Collins, 2009). This age appropriate infant template was registered to the Talairach coordinate system (Talairach & Tournoux, 1988) by aligning it to a custom atlas-transformed (Lancaster et al., 1995) target template (711-2B) using a series of affine transforms (Michelon, Snyder, Buckner, McAvoy, & Zacks, 2003).
rs-fcMRI preprocessing
Additional rs-fcMRI preprocessing to account for signal stemming from non-neuronal processes (Fox & Raichle, 2007) followed established procedures described in previous work, including temporal band-pass filtering (0.009 Hz < f < 0.08 Hz), regression of rigid body head motion parameters in 6 directions, regression of the whole brain signal, regression of ventricular signal averaged from a ventricular region mask, regression of white matter signal averaged from a white matter mask and regression of first order derivative terms for the whole brain, ventricular, and white matter signals (Fair et al., 2009). As per Hallquist, Hwang, & Luna (2013), the frequencies of nuisance regressors and fMRI data matched prior to nuisance regression, which was conducted prior to bandpass filtering. (Note: analyses were also conducted without regression of the whole brain signal in order to ensure consistency of results.) Additional steps were taken to examine movement of a given frame relative to the previous frame, known as framewise displacement (FD; Fair et al., 2012; Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). We used a volume censoring approach, removing volumes associated with greater than .3 mm FD (and 1 preceding and 2 following to account for temporal blurring; Power et al., 2012). The maximum percentage of volumes removed from a scan was 22.5% (M=7.10, range=1.70–22.5%). See online Appendix S1 for more detailed information about fMRI data processing.
PCC region of interest
We focused on the posterior cingulate cortex (PCC), a key hub of the DMN (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Hagmann et al., 2008) that is highly interconnected with other regions in the DMN beginning in infancy (Gao et al., 2013), and the brain at large in later developmental stages (Hagmann et al., 2008). The PCC region of interest (ROI) was a 10mm sphere identified in previous research on the DMN (Talairach Coordinates −2, −36, 37; Fox et al., 2005), and used in work documenting the developmental trajectory of the DMN from childhood to adulthood (Fair et al., 2009).
Analysis Plan
Analysis involved two steps. First, we conducted a regression of postnatal conflict on PCC connectivity to examine variation in connectivity of the PCC with the rest of the brain depending on the level of interparental conflict. Postnatal conflict was entered as the independent variable with infant age (adjusted for gestational age at birth) and the pregnancy conflict score as covariates, and whole brain voxel-wise connectivity of the PCC as the dependent variable. Pregnancy conflict was included as a covariate due to previous work indicating that events prior to term age equivalent may also impact functional connectivity (G. C. Smith et al., 2011; Smyser et al., 2010). Please see Appendix S2 and supplementary Table S1 for information about the main effects of these covariates on PCC connectivity, and for analyses with additional covariates (supplementary Figure S1). Thresholding based on Monte Carlo simulation was implemented to account for multiple comparisons (Forman et al., 1995). Correction for p<0.05 voxel clusters required a threshold of 53 contiguous voxels with a Z-value> 2.25.
From the results of this whole brain regression, connections of interest were identified based on previous literature examining DMN integrity in the context of environmental stress. These connections were then examined in post-hoc regression models as predictors of infant negative emotionality (covarying for infant age). (See Appendix S3 regarding the methods for identifying and extracting connections from the whole brain regression). For connections found to significantly predict negative emotionality, a mediation model was run to examine connectivity as a mediator between postnatal interparental conflict and infant emotionality. Mediation analyses were conducted using the Indirect Macro for SPSS (IBM, 2012; Preacher & Hayes, 2008).
Results
Regression of Interparental Conflict on Whole Brain PCC Connectivity
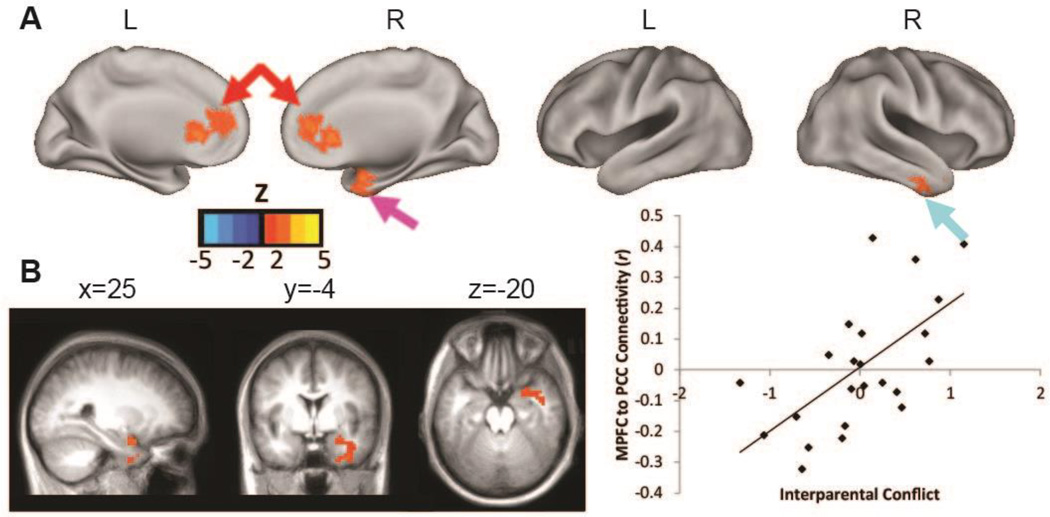
Higher interparental conflict was associated with greater positive resting state functional connectivity of PCC to two DMN regions, the rACC/anterior MPFC (rACC/aMPFC) and right inferior temporal gyrus (ITG), and two non-DMN regions, the right amygdala, and a region in the right cerebellum (Figure 1, FigureS S2 and S3, Table S2). The scatter-plot in Figure 1, Panel C illustrates that the association between interparental conflict and PCC to rACC/aMPFC connectivity is not driven by one or more outliers. Figure 2 illustrates the similarity between the rACC/aMPFC region identified in this analysis and the rACC region previously identified as showing greater reactivity to very angry versus neutral tone of voice for infants in homes with higher levels of interparental conflict (Graham et al., 2013). This indicates that interparental conflict is relevant for the reactivity of this region to stressor relevant stimuli, and to the incorporation of this region into an early developing functional brain network. Analyses without regression of the whole brain signal, and with inclusion of other potentially relevant covariates (remaining FD and completion of the functional activation portion of the study) indicated consistent results.
Figure 1. Interparental conflict is associated with infants’ DMN strength.
Note. Panel A shows results of the regression with interparental conflict as the predictor, covariates for pregnancy conflict and infant age, and voxel-wise PCC connectivity as the dependent measure. Results indicate that higher conflict is associated with greater positive connectivity of PCC to rACC/aMPFC (red arrows), right inferior temporal gyrus (turquoise arrow), and right medial temporal lobe/amygdala (pink arrow). Panel B shows that higher levels of conflict are also associated with stronger PCC to right amygdala connectivity. See Figure S2 for presentation of the cerebellar finding, and the cortical findings in 2-dimensional slices. All results with monte carlo correction for multiple comparisons. For illustrative purposes, Panel C contains the scatter plot for the association between interparental conflict and PCC-rACC/aMPFC (6,39, 14) connectivity.
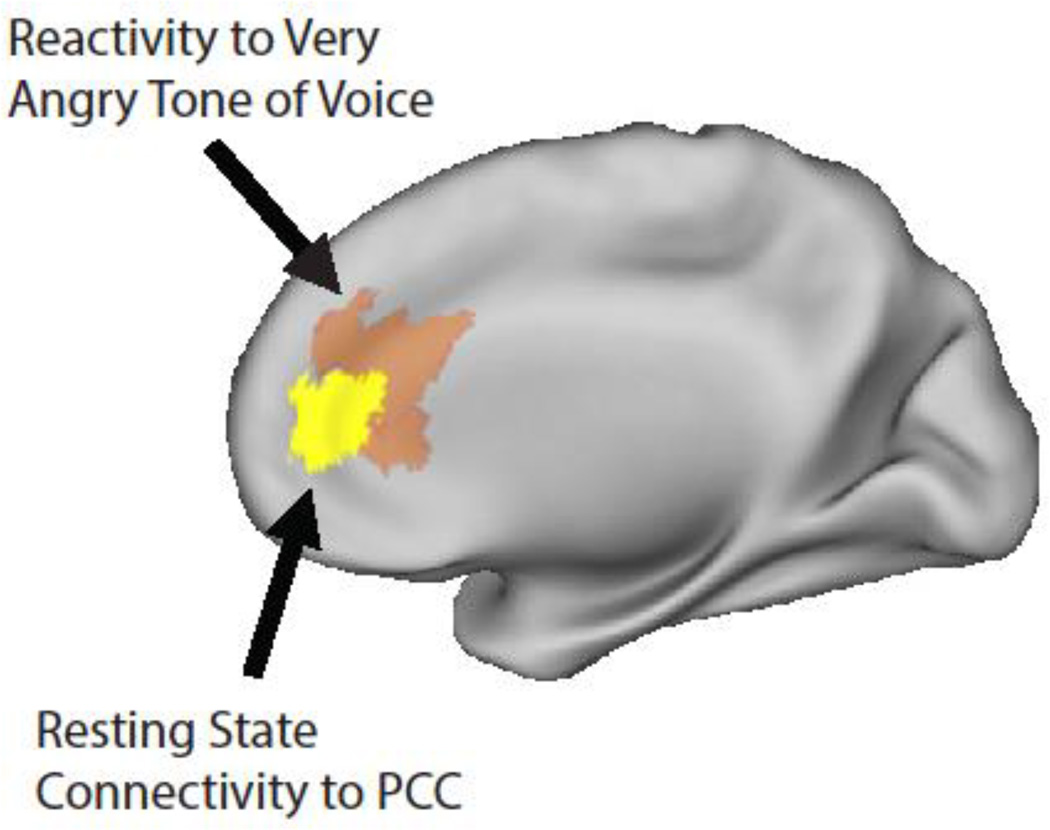
Figure 2. Interparental conflict is associated with both resting state functional connectivity and reactivity of the MPFC.
Note. Yellow= the rACC/aMPFC region (6, 39, 14) identified in the rs-fcMRI analysis as showing more positive connectivity to the PCC for infants in homes with greater conflict (based on the regression of interparental conflict on voxel-wise connectivity of the PCC). Orange=the rACC region (3,34,16) identified in the prior functional activation study as showing greater reactivity to very angry versus neutral tone of voice for infants in homes with higher levels of conflict (based on a whole-brain regression of interparental conflict on the contrast of very angry versus neutral tone of voice; Graham, Fisher, Pfeifer, 2013).
Test for Functional Connectivity as a Mediator Between Interparental Conflict and Infant Negative Emotionality
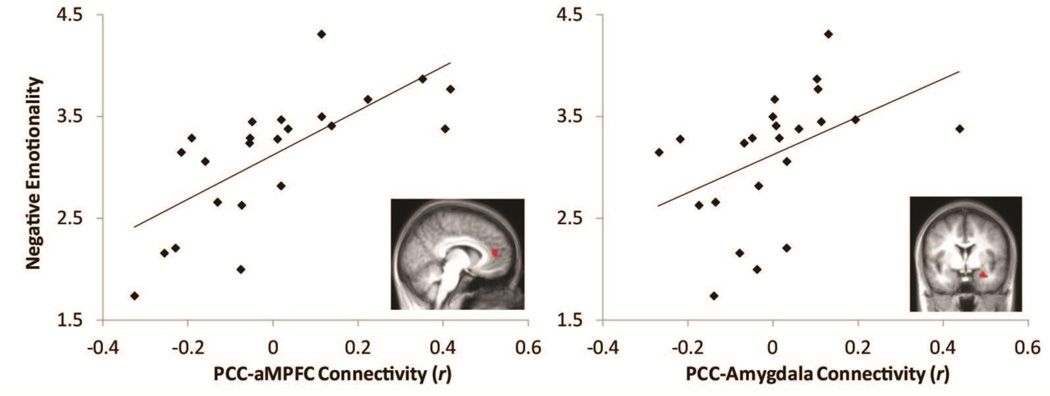
Of the findings from the whole brain regression, PCC to rACC/aMPFC and PCC to amygdala connectivity were identified as being most consistent with previous literature examining effects of stress on DMN connectivity (Bluhm et al., 2009; Lanius et al., 2010; Philip et al., 2013; Smyser et al., 2010; Zhou et al., 2012), and literature linking DMN connectivity to stress related symptomatology (Bluhm et al., 2009; Lanius et al., 2010; Zhou et al., 2012). Correlation coefficients (r) were therefore extracted for these two connections for post-hoc analyses (see online supplementary materials for details on defining the aMPFC and amygdala seed regions from the whole brain analysis results). It is also important to note that these were not the only connections associated with interparental conflict in the whole brain regression (Figure 1, Figure S2, Table S2). In the first regression model, greater PCC-rACC/aMPFC connectivity predicted greater negative emotionality (β= .703, SE = .477, p < .001). Age was not a significant predictor (β= .175, SE = .010, p >.10), and the model explained 52.5% of the variance in negative emotionality (Figure 3). In the second regression model, greater PCC-amygdala connectivity predicted greater negative emotionality (β= .455, SE = .837, p < .05). Age was not a significant predictor (β= .292, SE = .014, p =.167), and the model explained 22.4% of the variance in negative emotionality (Figure 3).
Figure 3. Infants' resting state functional connectivity is associated with negative emotionality.
Note. Scatter plots showing positive associations between both PCC to rACC/aMPFC (left; R2=52.5%) and PCC to right amygdala (right; R2=22.4%) connectivity and maternal report of infant negative emotionality (controlling for infant age).
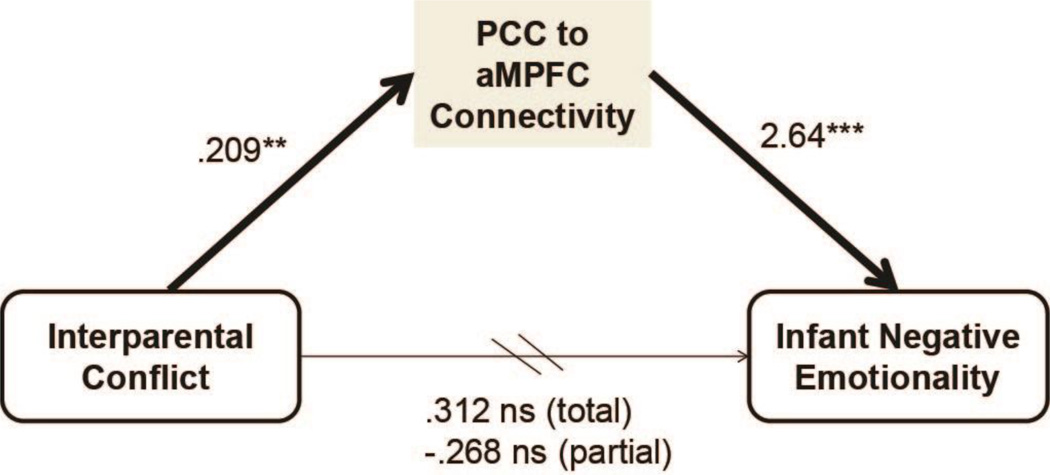
Based on these results, a mediation model was tested with interparental conflict as the independent variable, both PCC-rACC/aMPFC and PCC-amygdala connectivity as mediators, infant negative emotionality as the dependent variable and infant age and pregnancy conflict as covariates. Consistent with previous analyses, significant positive path coefficients from conflict to PCC-rACC/aMPFC connectivity (b=.209, SE=.059, p =.002) and to PCC-amygdala connectivity (b=.126, SE=.047, p = .015) were identified. However, in this full model only PCC-rACC/aMPFC connectivity significantly predicted negative emotionality (b=2.64, SE=.690, p = .001). PCC-amygdala connectivity no longer significantly predicted infant emotionality (b=.231, SE=.863, p > .05). The direct effect of conflict on infant negative emotionality was not significant (b=.312, SE=.219, p >.05 not adjusted; b=−.268, SE=.208, p >.05 adjusted). The indirect effects of conflict on infant negative emotionality through PCC-rACC/aMPFC and PCC-amygdala connectivity were estimated to be .552 (.579 mean from bootstrap samples) and .029 (.028) respectively. The confidence interval (CI) for the indirect effect of conflict on negative emotionality through PCC-rACC/aMPFC indicated mediation (99% CI: .118, 1.42) based on 5,000 bootstrap samples. The CI for the indirect effect of conflict on negative emotionality through PCC-amygdala connectivity did not support mediation (99% CI: −.229, .548). Thus greater interparental conflict was associated with higher levels of negative infant emotionality via greater PCC-rACC/aMPFC connectivity (Figure 4).
Figure 4. DMN connectivity mediates between interparental conflict and infant negative emotionality.
Note. Coefficients are unstandardized and derived from the full mediation model including PCC-amygdala connectivity as a second potential mediator, and covariates for infant age and pregnancy conflict. The indirect effect of interparental conflict on infant negative emotionality through PCC-rACC/MPFC connectivity was .552, and mediation was supported based on 5,000 bootstrap samples (99% CI: .118, 1.42). **=p<.005, ***=p<.001, ns=non-significant. Arrows indicate hypothesized direction of effects.
Discussion
The results of the present study indicate that variation in a common form of familial stress, non-physical interparental conflict, is relevant for infants’ functional brain organization during the first year of life. Moreover, variation in DMN connectivity appears to mediate between higher levels of interparental conflict and infants’ negative emotionality. This study provides further support for the conceptualization of the DMN as a potential marker of the effects of ELS (Daniels, Frewen, McKinnon, & Lanius, 2011). It extends previous work by focusing on variation in a common and moderate form of ELS during a time of early, rapid DMN development. Moreover, it provides initial evidence for the relevance of the early organization of the DMN for emotional functioning in infancy.
PCC to MPFC as a core DMN connection and mediator between ELS and infant negative emotionality
The DMN includes multiple MPFC regions, a ventral, anterior and dorsal portion (Andrews-Hanna et al., 2010). Consistent with previous work (Bluhm et al., 2009; Philip et al., 2013; Smyser et al., 2010), the findings in the present study indicate that PCC connectivity with the aMPFC node (which encompasses rACC) may be particularly vulnerable to ELS. This region, along with the PCC, appears to be one of two main hubs of the DMN that are most highly interconnected with other DMN regions (Andrews-Hanna et al., 2010). Individual differences in connectivity between these two regions may therefore have implications for development of the broader network.
Variation in DMN connectivity has repeatedly been associated with mental health status (Fair et al., 2010; Fox & Greicius, 2010; Whitfield-Gabrieli & Ford, 2012). Interestingly, major depressive disorder, a disorder characterized primarily by negative emotionality, has been associated with greater DMN connectivity specifically between the PCC and the ACC/MPFC in adults (Berman et al., 2011; Sheline et al., 2010) and children (Gaffrey et al., 2012). Among depressed and non-depressed individuals, higher ACC to PCC connectivity is associated with greater rumination (passive and repetitive focus on distress; Berman et al., 2011). In the present study, greater PCC to rACC/aMPFC connectivity mediated an association between higher levels of interparental conflict and higher negative emotionality in infants. The current findings do not speak to risk for depression, but indicate a potential role for DMN connectivity in linking ELS and negative emotionality beginning during the first year of life.
The rACC/aMPFC region identified in the present study overlaps substantially with the rACC region previously identified as more reactive to very angry tone of voice for infants in higher conflict homes (Graham et al., 2013). This suggests that even in the absence of this external, conflict-related trigger, the functioning of this region is associated with levels of conflict. As such, it may be more relevant for infants’ development and functioning across a variety of contexts. The strong association between the resting state connectivity of this region and infants’ negative emotionality is in line with this idea. The importance of this region for regulating stress and emotions, and mediating between early adversity and emotional difficulties is supported by extensive work in animal models (Liston et al., 2006; Sánchez, Ladd, & Plotsky, 2001; Uchida et al., 2010), and fMRI work in children and adults (Fonzo et al., 2010; Gee et al., 2013; Meyer-Lindenberg & Tost, 2012).
PCC to amygdala connectivity as a potential early emerging consequence of stress
The association between higher interparental conflict and increased PCC to amygdala connectivity is noteworthy given the extensive literature indicating an important role for the amygdala in the effects of ELS on emotional development and mental health (Sánchez et al., 2001; Tottenham, 2012, 2014). Although not examined in human infants, research in animal models indicates that early life stress is associated with accelerated functional development of the amygdala, and heightened negative emotionality (Callaghan & Richardson, 2011, 2013; Moriceau, Roth, & Sullivan, 2010). More mature patterns of functional connectivity between the amygdala and cortical regions have been observed both in children and in animal models, and hypothesized to serve an adaptive role in regulating the preciously developing amygdala and associated emotional reactivity (Callaghan et al., 2014; Gee et al., 2013; Tottenham, 2014). Due to our cross-sectional research design, and the lack of literature documenting the development of amygdala functional connectivity during infancy, we cannot speak to whether these findings fit in with a pattern of accelerated development. However, the association between PCC to amygdala connectivity and infants’ negative emotionality provides some support for the hypothesized links between early life stress, emotional reactivity and developing functional connectivity.
These findings also fit within a growing literature in adults indicating that variability in PCC to amygdala connectivity is associated with environmental stress and with behavioral functioning. Increased resting state functional connectivity between the amygdala, PCC and MPFC has been observed one hour after a social stressor (Veer et al., 2011). Further, variability in PCC to amygdala functional connectivity has been associated with PTSD related to childhood abuse (Bluhm et al., 2009), and predicts emergence of PTSD symptoms following an accident (Lanius et al., 2010; Zhou et al., 2012). Results of the present study extend this work down to infancy, and indicate the potential vulnerability of this connection to environmental influences during the first year of life.
However, the association between PCC to amygdala connectivity and infant negative emotionality did not remain significant after accounting for the association between PCC to rACC/aMPFC connectivity and emotionality. The link between PCC to amygdala connectivity and emotion related functioning may become more pronounced over the course of development as more complex emotion regulatory behaviors emerge that depend on connectivity between the amygdala and PCC among other cortical regions (Stein et al., 2007).
Implications of timing of ELS for functional brain development
While the results are consistent with previous research regarding specific functional connections that appear to be sensitive to environmental stress, the direction of the PCC to rACC/aMPFC finding was, at first, surprising. Prior work shows a developmental pattern of increasing strength of connectivity between DMN regions over the first two years of life (Gao et al., 2009, 2013), and less connectivity between the MPFC and PCC associated with preterm birth (Smyser et al., 2010). Yet higher postnatal interparental conflict is associated with greater PCC connectivity with two cortical DMN regions, the aMPFC and ITG, which appears to be a more ‘mature’ pattern. This may be due to the focus of the current study on interparental conflict after birth. In contrast, previous work focused on preterm birth (Smyser et al., 2010), a stressor that occurs prior to term age equivalent. The timing of stress exposure appears to be important for understanding its consequences, with animal models indicating that stress during the pre- versus the postnatal period can have opposing effects on biological systems (Love & Williams, 2008). DMN development may be similarly disrupted by exposure to stress in an earlier developmental period and accelerated by exposure in a later period. The data provided in this report in conjunction with the current literature supports this view. The findings of the covariate measures (pregnancy conflict and infant age at scan) also lend support to this model (see Table S1).
The direction of the findings in the present study should also be considered within the context of a growing literature indicating that accelerated development is one potential consequence of exposure to ELS (Gee et al., 2013; Tottenham, 2014). Accelerated development may be adaptive under conditions of stress, but has also been hypothesized to occur at the expense of extended periods of plasticity, and therefore result in reduced learning potential (Tottenham, 2014). This hypothesis is supported by longitudinal research indicating that an extended period of plasticity in the development of cortical thickness is associated with greater intellectual ability (Shaw et al., 2006).
Limitations and Conclusions
Several limitations of the present study warrant attention. First, while the putative DMN in infants bares remarkable resemblance to the network observed in children and adults with regard to the pattern of functionally connected regions (Fransson et al., 2009; Gao, Alcauter, & Smith, 2014; Gao et al., 2009, 2011, 2013; Gao, Alcauter, Elton, et al., 2014; Gao, Elton, et al., 2014), and interaction with other functional brain networks (Gao, Alcauter, & Smith, 2014; Gao et al., 2013), it has yet to be determined whether the system serves the same function across age groups. Future research will be required to begin to address this important topic. The small, and disproportionately male, sample is an additional limitation to be addressed in future studies.
Additionally, interparental conflict and infant temperament were assessed via maternal report. Multi-method assessment of these constructs including observational measures will be important for future research. However, the measures used in the present study have been widely used, and demonstrated moderate inter-rater reliability (Gartstein & Rothbart, 2003; Parade & Leerkes, 2008; Smith Slep & O’Leary, 2005), and good predictive validity (Gartstein, Putnam, & Rothbart, 2012; Graham, Kim, & Fisher, 2012; Shortt, Capaldi, Kim, & Owen, 2006). Moreover, strong associations between questionnaire measures and more methodologically complex and expensive indices of brain functioning (such as rs-fcMRI) indicate the intriguing possibility of easily capturing aspects of the environment and behavior that are closely tied to brain development. The cross-sectional nature of this study also does not allow for causal inferences, and raises the possibility that infant emotionality influences interparental conflict through effects on infants' brain connectivity as opposed to the alternative pathway. Future prospective longitudinal research identifying changes in functional brain organization and behavior that follow changes in ELS will be needed to build on these findings.
Despite the limitations, this study represents a first step in linking the family environment, an early developing functional brain network and infant emotionality. The effects of the early environment on brain development and emotional functioning are often extrapolated from research in older children and adults, or inferred from peripheral measures of nervous system functioning during infancy. Rs-fcMRI allows for studying functional brain networks during infancy that appear to be highly relevant for emotional functioning and mental health throughout the lifespan. The specific connections identified contribute to the conceptualization of posterior to anterior DMN connectivity as sensitive to ELS. Moreover, despite extensive empirical and theoretical work focused on the role of the amygdala in linking ELS and mental health (Sánchez et al., 2001; Tottenham & Sheridan, 2010; Tottenham, 2012), the results of this study provide important initial empirical evidence for an association between ELS and the functional connectivity of the amygdala in human infants. More broadly, this study indicates the importance of considering early exposure to common and moderate forms of familial stress as a factor that may influence subsequent vulnerability to mental health difficulties via effects on rapidly developing brain systems.
Supplementary Material
Key points.
Functional brain networks that support mental health and emotional functioning develop rapidly during the first year of life.
Interparental conflict is a known risk factor for mental health difficulties in children.
Results of this study indicate that interparental conflict may impact risk for mental health difficulties via effects on early developing functional brain networks.
Efforts to reduce the burden of mental health difficulties in childhood should consider the sensitivity of early developing brain networks to common forms of familial stress.
Acknowledgments
Support for this work was provided by the Center for Drug Abuse Prevention in the Child Welfare System (1-P30-DA023920); the Early Experience, Stress, and Neurobehavioral Development Center (1-P50-MH078105); F31 MH 094000 (AG); F32 MH 105283 (AG); R00 MH091238 (DF); and R01 MH096773 (DF). The authors are grateful for the significant contributions of Kyndal Howell and Kristen Greenley (both of the Stress Neurobiology and Prevention Laboratory), and Scott Watrous (Lewis Center for Neuroimaging) to successfully running this study.
Footnotes
Conflict of interest statement: No conflicts declared.
Supporting Information
Additional Supporting Information is provided along with the online version of this article.
Appendix S1. Additional Information Regarding fMRI Data Processing
Appendix S2. Additional Information Regarding Effects of Covariates
Appendix S3. Additional Information Regarding Extraction of Connections and Post-Hoc Analyses
Table S1. Interparental conflict is associated with infants’ PCC connectivity: Results of the voxel-wise regression
Table S2. Effects of the two covariates, infant age and pregnancy conflict, on infants’ PCC connectivity: results of voxel-wise regression
Figure S1. Regression of interparental conflict on PCC connectivity with additional covariate for infant race/ethnicity
Figure S2. Regression of interparental conflict on PCC connectivity displayed on two-dimensional slices
Figure S3. Closer examination of right amygdala finding
References
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Woolrich MW, Behrens TEJ, David E, Devlin JT, Smith SM. Applying FSL to the FIAC Data: Model-Based and Model-Free Analysis of Voice and Sentence Repetition Priming. Human Brain Mapping. 2006;27(5):380–391. doi: 10.1002/hbm.20246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Social Cognitive and Affective Neuroscience. 2011;6(5):548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi A, Mercure E, Lloyd-Fox S, Thomson A, Brammer M, Sauter D, Murphy DGM. Early Specialization for Voice and Emotion Processing in the Infant Brain. Current Biology. 2011;21(14):1–5. doi: 10.1016/j.cub.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch Ea, Frewen Pa, Stevens TK, Boksman K, Lanius RA. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. Journal of Psychiatry & Neuroscience. 2009;34(3):187–194. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2674971&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behavioral Neuroscience. 2011;125(1):20–28. doi: 10.1037/a0022008. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. Early experiences and the development of emotional learning systems in rats. Biology of Mood & Anxiety Disorders. 2013;3:8. doi: 10.1186/2045-5380-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Sullivan RM, Howell B, Tottenham N. The international society for developmental psychobiology sackler symposium: Early adversity and the maturation of emotion circuits-A cross-species analysis. Developmental Psychobiology. 2014 doi: 10.1002/dev.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MJ, Paley B. Understanding families as systems. Current Directions in Psychological Science. 2003;12(5):193–196. [Google Scholar]
- Cummings EM, Davies PT. Effects of marital conflict on children: recent advances and emerging themes in process-oriented research. Journal Of Child Psychology And Psychiatry. 2002;43(1):31–63. doi: 10.1111/1469-7610.00003. [DOI] [PubMed] [Google Scholar]
- Cummings EM, Davies PT. Marital Conflict and Children: An Emotional Security Perspective. New York: The Guilford Press; 2010. p. 320. [Google Scholar]
- Daniels JK, Frewen P, McKinnon MC, Lanius RA. Default mode alterations in posttraumatic stress disorder related to early-life trauma: a developmental perspective. Journal of Psychiatry & Neuroscience. 2011;36(1):56–59. doi: 10.1503/jpn.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Cummings EM, Kouros CD, Elmore-Staton L, Buckhalt JA. Marital psychological and physical aggression and children’s mental and physical health: Direct, mediated, and moderated effects. Journal of Consulting and Clinical Psychology. 2008;76(1):138–148. doi: 10.1037/0022-006X.76.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NUF, Church JA, Miezin FM, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NUF, Milham MP. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Frontiers in Systems Neuroscience. 2012 Feb;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Posner J, Nagel BJ, Bathula D, Dias TGC, Mills KL, Nigg JT. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2010;68(12):1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov VS, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54(1):313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov VS, Evans AC, Mckinstry RC, Almli CR, Collins DL. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. 2009;47(Supplement 1):S102. [Google Scholar]
- Fonzo Ga, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biological Psychiatry. 2010;68(5):433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved Assessment of Significant Activation in Functional Magnetic Resonance Imaging (fMRI): Use of a Cluster-Size Threshold. Magnetic Resonance in Medicine. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fox MD, Greicius MD. Clinical applications of resting state functional connectivity. Frontiers in Systems Neuroscience. 2010;4:1–13. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Skiöld B, Engström M, Hallberg B, Mosskin M, Aden U, Blennow M. Spontaneous brain activity in the newborn brain during natural sleep--an fMRI study in infants born at full term. Pediatric Research. 2009;66(3):301–305. doi: 10.1203/PDR.0b013e3181b1bd84. [DOI] [PubMed] [Google Scholar]
- Gaffrey MS, Luby JL, Botteron K, Repovš G, Barch DM. Default mode network connectivity in children with a history of preschool onset depression. Journal of Child Psychology and Psychiatry. 2012;53(9):964–972. doi: 10.1111/j.1469-7610.2012.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Elton A, Hernandez-Castillo CR, Smith JK, Ramirez J, Lin W. Functional Network Development During the First Year: Relative Sequence and Socioeconomic Correlations. Cerebral Cortex. 2014:1–10. doi: 10.1093/cercor/bhu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Smith JK. Development of human brain cortical network architecture during infancy. Brain Structure and Function. 2014 doi: 10.1007/s00429-014-0710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Elton A, Zhu H, Alcauter S, Smith JK, Gilmore JH, Lin W. Intersubject Variability of and Genetic Effects on the Brain’s Functional Connectivity during Infancy. The Journal of Neuroscience. 2014;34(34):11288–11296. doi: 10.1523/JNEUROSCI.5072-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Giovanello KS, Smith JK, Shen D, Zhu H, Lin W. Temporal and spatial evolution of brain network topology during the first two years of life. PloS One. 2011;6(9):e25278. doi: 10.1371/journal.pone.0025278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Shen D, Smith JK, Zhu H, Lin W. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cerebral Cortex. 2013;23(3):594–603. doi: 10.1093/cercor/bhs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, Lin W. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proceedings of the National Academy of Sciences. 2009;106(16):6790–6795. doi: 10.1073/pnas.0811221106. Retrieved from http://www.pnas.org/content/106/16/6790.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Putnam S, Rothbart MK. Etiology of preschool behavior problems: Contributions of temperament attributes in early childhood. Infant Mental Health Journal. 2012;33(2):197–211. doi: 10.1002/imhj.21312. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior and Development. 2003;26(1):64–86. [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Tottenham N. Early developmental emergence of human amygdala - prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. doi:10.1073/pnas.1307893110/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Graham AM, Fisher PA, Pfeifer JH. What Sleeping Babies Hear: A Functional MRI Study of Interparental Conflict and Infants’ Emotion Processing. Psychological Science. 2013;24(5):782–789. doi: 10.1177/0956797612458803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Kim HK, Fisher Pa. Partner aggression in high-risk families from birth to age 3 years: associations with harsh parenting and child maladjustment. Journal of Family Psychology. 2012;26(1):105–114. doi: 10.1037/a0026722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Pfeifer JH, Fisher PA, Lin W, Gao W, Fair DA. The potential of infant fMRI research and the study of early life stress as a promising exemplar. Developmental Cognitive Neuroscience. 2015;12:12–39. doi: 10.1016/j.dcn.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biology. 2008;6(7):e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox PT. A Modality-Independent Approach to Spatial Normalization of Tomographic Images of the Human Brain. Human Brain Mapping. 1995;3(1995):209–223. [Google Scholar]
- Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Théberge J, Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatrica Scandinavica. 2010;121(1):33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(30):7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loman MM, Gunnar MR. Early experience and the development of stress reactivity and regulation in children. Neuroscience and Biobehavioral Reviews. 2010;34(6):867–876. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love OP, Williams TD. Plasticity in the adrenocortical response of a free-living vertebrate: the role of pre- and post-natal developmental stress. Hormones and Behavior. 2008;54(4):496–505. doi: 10.1016/j.yhbeh.2008.01.006. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito Sa, Kelly Pa, Bird G, Sebastian CL, Mechelli A, Viding E. Amygdala activation in maltreated children during pre-attentive emotional processing. The British Journal of Psychiatry. 2013;202(4):269–276. doi: 10.1192/bjp.bp.112.116624. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nature Neuroscience. 2012;15(5):663–668. doi: 10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- Michelon P, Snyder AZ, Buckner RL, McAvoy M, Zacks JM. Neural correlates of incongruous visual information An event-related fMRI study. NeuroImage. 2003;19(4):1612–1626. doi: 10.1016/s1053-8119(03)00111-3. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Sullivan RM. Rodent model of infant attachment learning and stress. Developmental Psychobiology. 2010;52(7):651–660. doi: 10.1002/dev.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parade SH, Leerkes EM. The reliability and validity of the Infant Behavior Questionnaire-Revised. Infant Behavior & Development. 2008;31(4):637–646. doi: 10.1016/j.infbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, Price LH, Bloom RF, Carpenter LL. Decreased default network connectivity is associated with early life stress in medication-free healthy adults. European Neuropsychopharmacology. 2013;23(1):24–32. doi: 10.1016/j.euroneuro.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Propper C, Moore GA. The influence of parenting on infant emotionality: A multi-level psychobiological perspective. Developmental Review. 2006;26:427–460. [Google Scholar]
- Raichle ME. Two views of brain function. Trends in Cognitive Sciences. 2010;14(4):180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Macleod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez MM, Ladd COCO, Plotsky PMPM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sandor S, Leahy R. Surface-based labeling of cortical anatomy using a deformable atlas. IEEE Transactions on Medical Imaging. 1997;16(1):41–54. doi: 10.1109/42.552054. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. NeuroImage. 2001;13(5):856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun Ma. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortt JW, Capaldi DM, Kim HK, Owen LD. Relationship separation for young, at-risk couples: prediction from dyadic aggression. Journal of Family Psychology. 2006;20(4):624–631. doi: 10.1037/0893-3200.20.4.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GC, Gutovich J, Smyser CD, Pineda R, Newnham C, Tjoeng TH, Inder T. Neonatal intensive care unit stress is associated with brain development in preterm infants. Annals of Neurology. 2011;70(4):541–549. doi: 10.1002/ana.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Bannister P, Beckmann C, Brady M. FSL: New tools for functional and structural brain image analysis. NeuroImage. 2001;(13) 2001. Retrieved from http://c3s2i.free.fr/cv/fsl.pdf. [Google Scholar]
- Smith Slep AM, O’Leary SG. Parent and partner violence in families with young children: rates, patterns, and connections. Journal of consulting and clinical psychology. 2005;73(3):435–444. doi: 10.1037/0022-006X.73.3.435. [DOI] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal Analysis of Neural Network Development in Preterm Infants. Cerebral Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder DK. Marital Satisfaction Inventory—Revised. CA: Western Psychological Services; 1997. [Google Scholar]
- Sripada RK, Swain JE, Evans GW, Welsh RC, Liberzon I. Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology. 2014;39(9):2244–2251. doi: 10.1038/npp.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. NeuroImage. 2007;36(3):736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The Revised Conflict Tactics Scales (CTS2) Journal of Family Issues. 1996;17(3):283–316. [Google Scholar]
- Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, Lenze EJ. Functional network dysfunction in anxiety and anxiety disorders. Trends in Neurosciences. 2012;35(9):527–535. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach P, Tournoux J. Co-planar Stereotaxic Altas of the Human Brain. Stuttgart, Germany: Georg Thieme Verlag; 1988. [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magnetic Resonance in Medicine. 2000;44(3):457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10975899. [DOI] [PubMed] [Google Scholar]
- Tottenham N. Human amygdala development in the absence of species expected caregiving. Developmental Psychobiology. 2012;54(6):598–611. doi: 10.1002/dev.20531. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/dev.20531/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. The Importance of Early Experiences for Neuro-Affective Development. In: Pine DS, Andersen SL, editors. Current Topics in Behavioral Neuroscience, Volume 16: The Neurobiology of Childhood. Berlin: Springer-Verlag; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience. 2010;3(68):1–18. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, Watanabe Y. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. The Journal of Neuroscience. 2010;30(45):15007–15018. doi: 10.1523/JNEUROSCI.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer IM, Oei NYL, Spinhoven P, van Buchem Ma, Elzinga BM, Rombouts SaRB. Beyond acute social stress: increased functional connectivity between amygdala and cortical midline structures. NeuroImage. 2011;57(4):1534–1541. doi: 10.1016/j.neuroimage.2011.05.074. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang Z, Qin L, Wan J, Sun Y, Su S, Xu J. Early altered resting-state functional connectivity predicts the severity of post-traumatic stress disorder symptoms in acutely traumatized subjects. PloS one. 2012;7(10):e46833. doi: 10.1371/journal.pone.0046833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.