Treatment of virologically suppressed human immunodeficiency virus-infected participants with low high-density lipoprotein cholesterol using extended-release niacin or fenofibrate for 24 weeks improved lipid measures. However, treatment did not improve arterial endothelial function measured by brachial artery flow-mediated dilation or inflammatory markers.
Keywords: HIV, niacin, fenofibrate, high-density lipoprotein, endothelial function
Abstract
Background. Low levels of high-density lipoprotein cholesterol (HDL-C) are common in individuals with human immunodeficiency virus (HIV) infection, persist during antiretroviral therapy (ART), and are associated with increased cardiovascular disease (CVD) risk.
Methods. Virologically controlled participants without CVD on stable ART with low HDL-C (men <40 mg/dL, women <50 mg/dL) and triglycerides >150 mg/dL were randomized to receive open-label extended-release niacin 1500 mg/day with aspirin 325 mg/day or fenofibrate 200 mg/day for 24 weeks. The primary endpoint was the week 24 within-arm change in brachial artery flow-mediated dilation (FMD) in participants with complete follow-up scans.
Results. Of 99 participants, 74 had complete data (35 niacin, 39 fenofibrate). Median age was 45 years, 77% were male, median CD4+ count was 561 cells/µL, and brachial FMD was 4.2%. Median HDL-C was 32 mg/dL for men and 38 mg/dL for women, low-density lipoprotein cholesterol was 103 mg/dL, and triglycerides were 232 mg/dL. In men, HDL-C increased a median of 3 mg/dL with niacin and 6.5 mg/dL with fenofibrate (P < .001 for both). In women, HDL-C increased a median of 16 mg/dL with niacin and 8 mg/dL with fenofibrate (P = .08 for both). After 24 weeks, there was no significant change in FMD in either arm; the median (interquartile range) change was +0.6% (−1.6 to 2.3) with niacin (P = .28) and +0.5% (−1.0 to 3.0) with fenofibrate (P = .19). Neither treatment significantly affected C-reactive protein, interleukin 6, or D-dimer levels.
Conclusions. Despite improvements in lipids, niacin or fenofibrate treatment for 24 weeks did not improve endothelial function or inflammatory markers in participants with well-controlled HIV infection and low HDL-C.
Clinical Trials Registration. NCT01426438.
In individuals infected with human immunodeficiency virus (HIV), low high-density lipoprotein cholesterol (HDL-C) is very common and is associated with disease progression, greater immunosuppression, and immune activation [1]. Antiretroviral therapy (ART) generally increases HDL-C levels; however, HDL-C levels do not return to normal even with prolonged ART. In the Multicenter AIDS Cohort Study, 55% of men had depressed HDL-C levels (<40 mg/dL) after 6–7 years of ART [2].
Depressed HDL-C levels predict future cardiovascular disease (CVD) events in HIV-infected patients [3, 4] and in the general population [5, 6]. However, current guidelines for managing dyslipidemia in the general [7] and in the HIV-infected population [8, 9] do not recommend drug intervention for low HDL-C; they focus on treating elevated levels of low-density lipoprotein cholesterol (LDL-C) and severe hypertriglyceridemia. Indeed, the clinical benefits of HDL-increasing therapies such as niacin and fibrates are not proven. Trials of fenofibrate [10, 11] and niacin [12, 13] among non-HIV-infected participants with well-controlled LDL-C on statins have not demonstrated a benefit on CVD events, and genetic studies have questioned the relationship between HDL-C and CVD risk [14–16].
Extended-release niacin [17–19] and fenofibrate [20, 21] are well tolerated and increase HDL-C and HDL particles in HIV-infected persons. Both drugs also reduce apolipoprotein B [17, 21], non–HDL-C, and triglyceride levels [17, 21, 22]. A pilot study of niacin in HIV-infected individuals [18] suggested that its use improved brachial artery endothelial function. To determine if treatment with extended-release niacin or fenofibrate could improve arterial endothelial function and cardiovascular inflammatory biomarkers, we performed a 24-week, open-label randomized trial in HIV-infected participants with low HDL-C and elevated triglycerides.
MATERIALS AND METHODS
Participants
HIV-infected individuals aged ≥18 years with a CD4+ count >100 cells/µL were eligible if they had fasting HDL-C <40 mg/dL (men) or <50 mg/dL (women) and also had fasting triglycerides 150–800 mg/dL and LDL-C <160 mg/dL. Participants were required to be on continuous ART for ≥48 weeks with no regimen changes within the prior 12 weeks, planned on continuing with their current regimen, and had HIV RNA below the limit of detection. Laboratory criteria included fasting glucose level <126 mg/dL, platelet count ≥50 000/µL, absolute neutrophil count >750 cells/µL, hemoglobin level >8 g/dL, liver aminotransferases <2.5 times the upper limit of normal, estimated creatinine clearance ≥60 mL/minute (Cockcroft-Gault), and uric acid <1.3 times the upper limit of normal. Participants were excluded if they had known coronary heart disease or a coronary heart disease risk equivalent condition [23], class III or IV heart failure, uncontrolled hypertension, acute gout, active peptic ulcer disease, untreated hypothyroidism, active or symptomatic gallbladder disease within the past year, active cancer within the past year, currently used any prescription or nonprescription lipid-lowering agents other than statins, or using niacin-containing products that contain >100 mg daily, systemic glucocorticoids above replacement levels, current or planned hepatitis C treatment during the study period, known intolerance of any of the study medications, severe substance abuse, active opportunistic infection, or other acute illness. Participants were randomized equally to niacin plus aspirin or fenofibrate with stratification by ongoing statin use and by screening HDL-C level (<30 vs 30–40 mg/dL for men, <40 vs 40–50 mg/dL for women). Those receiving a statin were required to be on a stable dose for at least 90 days prior to entry. All participants were provided with lipid-lowering diet and activity recommendations. Institutional review boards at each site approved the study. All participants provided written informed consent.
Drug Treatment
Extended-release niacin (Niaspan, AbbVie) was supplied as 500-mg tablets. All participants initiated extended-release niacin at 500 mg before bedtime with 325 mg of plain aspirin daily. At weeks 4 and 8, they increased their doses to 1000 mg (2 tablets) and 1500 mg (3 tablets) daily, respectively, before bedtime. This dose was chosen because 95% of participants were able to tolerate it in our prior work, whereas only 70% tolerated 2000 mg/day [17]. Those who were unable to tolerate an increase continued at the previously tolerated dose for the duration of the study. Aspirin-intolerant participants had the option to use naproxen 225 mg instead of aspirin. Fenofibrate was supplied as 200-mg capsules. One capsule was taken orally once daily without regard to meals. No dosage adjustments were allowed.
Evaluations
Fasting serum glucose (defined as at least 8 hours with no food or beverage other than water), uric acid, creatinine and aminotransferase levels, inflammatory biomarkers, and standard lipid profiles were each obtained at entry and weeks 12 and 24. Adherence assessments were performed at weeks 4, 8, 12, and 24. A myopathy questionnaire [24] was administered at baseline and weeks 4, 8, 12, 16, and 24.
Assays
Centrally performed assays used specimens stored at −70°C and shipped on dry ice. Advanced lipoprotein testing used nuclear magnetic resonance spectroscopy [25] on ethylenediaminetetraacetic acid plasma (LipoScience NMR Lipoprofile, Raleigh, North Carolina). High-sensitivity C-reactive protein (hs-CRP) was measured by nephelometry (Siemens BNII Nephelometer, Siemens Health Care, Indianapolis, Indiana), at the University of Vermont Laboratory for Clinical Biochemistry, as were interleukin 6 (IL-6) and D-dimers. Plasma glucose, liver aminotransferase, and uric acid concentrations were measured locally at sites. All other assays were performed at the AIDS Clinical Trials Group central metabolic laboratory (Quest Diagnostics, Baltimore, Maryland). Serum insulin concentration was measured by an enzyme-labeled immunometric assay (DPC Immulite 2000). Total cholesterol, HDL-C, and triglycerides were measured enzymatically. LDL-C was calculated by the following equation: Total cholesterol - HDL cholesterol - Triglycerides/5 when triglycerides were <400 mg/dL. Non–HDL-C was calculated as total cholesterol minus HDL-C.
Brachial Artery Reactivity Testing
Brachial artery flow-mediated dilation (FMD) was measured by ultrasound prior to study treatment, then again after 12 and 24 weeks, as previously described [26, 27]. Participants were required to fast, not smoke, and not drink caffeinated products for at least 8 hours prior to testing. After resting supine for 10 minutes in a temperature-controlled room, a blood pressure cuff was placed on the widest part of the proximal right forearm approximately 1 cm distal to the antecubital fossa. Using a high-resolution linear array vascular ultrasound transducer, the brachial artery was located above the elbow and scanned in longitudinal sections with the focus zone set to the depth of the far wall. Extravascular landmarks were identified and labeled to assure that the imaged segment of the brachial artery was reproduced within and between studies. After recording baseline B-mode images of the brachial artery and spectral Doppler images of flow, the forearm cuff was inflated to 250 mm Hg for 5 minutes to induce reactive hyperemia. Immediately after deflation, spectral Doppler images are obtained to verify hyperemia. FMD of the brachial artery was measured 60 and 90 seconds after cuff deflation. FMD (%) was calculated as the ratio between the largest postcuff release and the baseline diameter. Images were sent electronically to the University of Wisconsin core laboratory for quality control and interpretation by a single, experienced technician using Access Point Web software (Freeland Systems, Westminster, Colorado). Blinded, paired readings of 25 FMD studies performed by sonographers in this study showed a median difference of 0.20% (1st–3rd quartile, −0.47% to 0.49%) [26].
Statistical Analysis
The primary hypothesis was that 24 weeks of treatment of low HDL-C with either extended-release niacin or fenofibrate will improve brachial artery FMD. The study had 80% power to detect a clinically relevant 1.5% mean difference in FMD within each arm after 24 weeks with 38 participants per arm. The primary analysis was as-treated and limited to participants with useable ultrasound scans at entry and week 24 and who were on study treatment at the time of the their week 24 scan. All values are reported as median (interquartile range [IQR]). Absolute changes over 24 weeks are reported from baseline. The Wilcoxon signed-rank test was used to assess within-arm changes. For the primary endpoint, a stratified, exact Wilcoxon signed-rank test was used. Between-group statistical comparisons used the Wilcoxon rank-sum test. Spearman correlations evaluated the relationship between pairs of continuous variables. The analysis did not adjust for multiple comparisons. A P value <.05 was considered statistically significant.
RESULTS
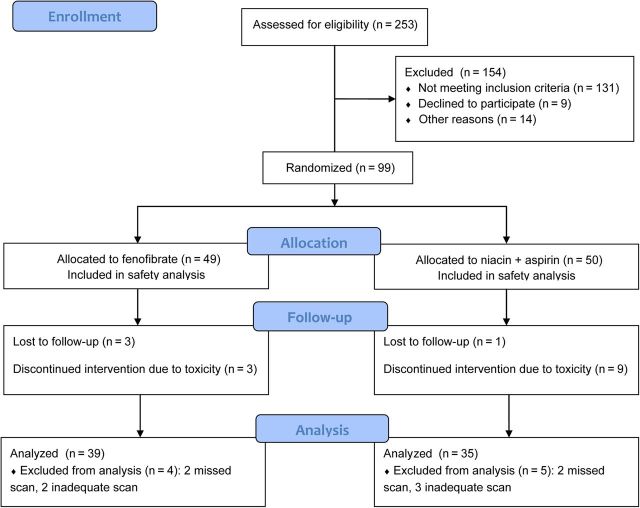
Participant flow through the study is shown in Figure 1. Fifty participants were assigned to niacin and 49 to fenofibrate. Nine participants discontinued niacin (mostly due to flushing) and 3 stopped fenofibrate (1 due to rash, 1 increased creatinine, 1 participant decision). Results are reported for the 35 niacin recipients and the 39 fenofibrate recipients who had complete data and completed study therapy. Baseline characteristics of participants not included in the primary analysis were similar (data not shown). Among the 40 participants who still were on niacin at week 24 (completed treatment), 1 was taking 500 mg, 4 were taking 1000 mg, and 35 were taking the full 1500-mg dose per day. Of the 9 who stopped niacin due to toxicity, 2 were taking the 500-mg dose, 3 the 1000-mg dose, and 4 the 1500-mg dose. Four participants switched from aspirin to naproxen because of flushing. All 49 participants receiving fenofibrate received 200 mg per day throughout the study; 3 stopped for toxicity and 3 for other reasons, and 43 completed treatment.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram.
Baseline demographic, clinical, and laboratory features are shown in Table 1. Participants had a median age of 45 years (IQR, 38–51) years and 35% were smokers. Nine (12%) were receiving a statin at entry and none had a prior diagnosis of diabetes mellitus. Median time on current ART regimen was about 3 years. Approximately half of participants were taking a protease inhibitor. All participants had undetectable HIV RNA at entry. Median CD4 cell counts and hemoglobin levels were relatively normal, indicating a generally healthy population. Approximately 30% of participants had a very low HDL-C level. The median 10-year risk of coronary heart disease death or myocardial infarction was low at 3% (IQR, 1%–8%); only 15 (21%) had an estimated 10-year risk >10%.
Table 1.
Demographics and Baseline Characteristics: As-Treated, Primary Analysis Population
| Characteristic | Niacin + Aspirin (n = 35) | Fenofibrate (n = 39) |
|---|---|---|
| Age, median (IQR) | 46 (37–50) | 45 (38–51) |
| Male sex | 27 (77) | 30 (77) |
| Race/ethnicity | ||
| Hispanic | 15 (43) | 17 (44) |
| White non-Hispanic | 15 (43) | 15 (38) |
| Black non-Hispanic | 5 (14) | 6 (15) |
| Other | 0 | 1 (3) |
| CD4+ cells/µL, median (IQR) | 580 (421–798) | 539 (393–777) |
| Months on current ART (IQR) | 38 (28–62) | 34 (16–63) |
| Protease inhibitor–containing regimen | 19 (54) | 17 (44) |
| Abacavir-containing regimen | 4 (11) | 6 (15) |
| Current smoker | 10 (29) | 16 (41) |
| Statin use | 4 (11) | 5 (13) |
| Use of antihypertensive medications | 10 (29) | 9 (23) |
| Very low HDL-C (<30 mg/dL men, <40 mg/dL women) | 10 (29) | 12 (31) |
| LDL-C mg/dL, median (IQR) | 103 (88–117) | 101 (83–120) |
| Hemoglobin g/dL, median (IQR) | 15 (14–15) | 15 (14–16) |
| Body mass index kg/m2, median (IQR) | 26.9 (25.9–31.5) | 28.3 (24.6–34.4) |
| 10-year Framingham coronary heart disease risk, median (IQR) | 3% (1–6) | 3% (1–10) |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: ART, antiretroviral therapy; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol.
Lipids and Lipoproteins
Baseline values and their changes after 24 weeks are shown in Table 2. Triglyceride decreases were similar with both treatments: −65 mg/dL (IQR, −163 to 8 mg/dL) with niacin (P = .002) and −54 mg/dL (IQR, −113 to −10 mg/dL) with fenofibrate (P < .001). LDL-C did not change with either drug, nor did total LDL particles. Small LDL particles were high at entry, at levels comparable to the 90th percentile in the general population [28], and decreased significantly with both drugs, whereas LDL particle size increased with both treatments. Among men, HDL-C levels increased modestly in both groups, by a median of 3 mg/dL (IQR, 0–9 mg/dL) with niacin (P < .001) and by 6.5 mg/dL (IQR, 0–12 mg/dL) with fenofibrate (P < .001; P = .37 for between-groups difference). Women had numerically greater increases in HDL-C: 16 mg/dL (IQR, −1 to 22 mg/dL) with niacin and 8 mg/dL (IQR, 5–13 mg/dL) with fenofibrate (P = .08 for both), but these changes were based on only 16 female participants. Total HDL particles decreased significantly only with fenofibrate, whereas large HDL increased significantly only with niacin. Non–HDL-C decreased significantly only with niacin. Large very low-density lipoprotein (VLDL) particles decreased with both drugs.
Table 2.
Lipid and Lipoprotein Values
| Variable | Niacin + Aspirin (n = 35) |
Fenofibrate (n = 39) |
Between-Groups P Value for Week 24 Change | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 24 Change | P Value | Baseline | Week 24 Change | P Value | ||
| Total cholesterol, mg/dL | 184.5 (171.0–212.0) | 9 (−26 to 3) | .037 | 184 (159–211) | −2 (−28 to 25) | .93 | .17 |
| Triglycerides | 253.5 (198.0–360.0) | −65 (−163 to 8) | .002 | 206 (171–293) | −54 (−113 to −10) | <.001 | .80 |
| LDL cholesterol | 103 (88–117) | −1 (−14 to 12) | .95 | 101.0 (83.5–120.0) | 7 (−13 to 26) | .20 | .38 |
| Total LDL particles, nmol/L | 1231 (1088–1470) | −113 (−273 to 62) | .07 | 1394 (1088–1560) | 35 (−123 to 225) | .77 | .15 |
| Small LDL particles | 1018 (803–1133) | −176 (−410 to −19) | <.001 | 1052 (823–1222) | −119 (−320 to −17) | <.001 | .52 |
| LDL size, nm | 19.7 (19.6–20.0) | 0.4 (0.0–1.0) | <.001 | 19.9 (19.7–20.3) | 0.6 (0.4–1.0) | <.001 | .26 |
| HDL cholesterol, men, mg/dL | 32 (30–35) (n = 27) | 3 (0–9) | <.001 | 36 (29–39) (n = 30) | 6.5 (0.0–12.0) | <.001 | .37 |
| HDL cholesterol, women, mg/dL | 37 (36–49) (n = 7) | 16 (−1 to 22) | .08 | 38 (35–42) (n = 9) | 8 (5–13) | .07 | 0.17 |
| Total HDL particles, µmol/L | 31.8 (28.4–35.4) | −1.7 (−4.3 to 2.1) | .14 | 30.2 (26.7–33.2) | 4.3 (1.8–7.2) | <.001 | <.001 |
| Large HDL particles | 2.1 (1.4–3.2) | 0.9 (0.1–3.3) | <.001 | 2.6 (1.5–3.7) | −0.3 (−0.9 to 0.5) | .27 | <.001 |
| HDL size, nm | 8.8 (8.5–9.0) | 0.2 (0.0–0.4) | <.001 | 8.7 (8.6–9.0) | −0.2 (−0.4 to 0.0) | .007 | <.001 |
| Non-HDL cholesterol, mg/dL | 150 (135–169) | −17 (−29 to 4) | .002 | 153 (123–175) | −4 (−28 to 17) | .27 | .26 |
| Large VLDL particles, nmol/L | 12.1 (7.8–24.9) | −5.4 (−12.2 to 0.6) | <.001 | 8.7 (3.4–14.6) | −1.7 (−6.5 to 0.7) | .009 | .13 |
Data are presented as median (interquartile range). Significant P values are shown in bold.
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein.
Inflammatory Biomarkers, Glucose Metabolism, and Renal Function
There were no statistically significant changes in the levels of D-dimer, IL-6, or hs-CRP with either drug treatment (Table 3). Baseline values for these biomarkers were not elevated. GlycA, a novel biomarker that reflects enzymatically glycated acute phase proteins [29, 30], decreased significantly with fenofibrate, whereas levels of GlycB increased with niacin and aspirin. Fasting glucose, insulin, and the homeostasis model assessment–insulin resistance (HOMA-IR) index increased significantly with niacin, consistent with other reports [17], and did not change with fenofibrate. Estimated creatinine clearance decreased by 13.1 mL/minute (IQR, −25.4 to −9.5) with fenofibrate (P < .001), consistent with the known reversible renal effects of fenofibrate [31, 32]. Only 1 participant stopped fenofibrate due to increased creatinine levels.
Table 3.
Inflammatory Biomarkers, Renal Function, and Insulin Resistance
| Variable | Niacin + Aspirin (n = 35) |
Fenofibrate (n = 39) |
Change at Week 24 Between Groups P Value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 24 Change | P Value | Baseline | Week 24 Change | P Value | ||
| Glucose, mg/dL | 91 (84–99) | 3 (−2 to 13) | .005 | 92 (89–97) | −4 (−10 to 6) | .45 | .006 |
| Insulin, µIU/mL | 12 (9–23) | 6 (1–11) | <.001 | 14 (12–24) | −1 (−5 to 5) | .79 | .016 |
| HOMA-IR | 2.5 (1.9–4.5) | 1.3 (0.0–3.0) | <.001 | 3.2 (2.6–5.3) | 0.3 (−1.2 to 1.2) | .74 | <.001 |
| Estimated creatinine clearance, mL/mina | 112.6 (91.9–142.8) | 0.0 (−11.9 to 6.8) | .99 | 119.8 (90.7–167.3) | −13.1 (−25.4 to −9.5) | <.001 | <.001 |
| hs-CRP, mg/L | 1.9 (1.0–5.4) | −0.6 (−3.0 to 2.6) | .16 | 1.5 (0.7–3.9) | 0.7 (−2.1 to 2.7) | .27 | .09 |
| D-dimer, pg/mL | 0.3 (0.2–0.4) | 0.1 (−0.1 to 0.2) | .18 | 0.3 (0.2–0.5) | 0.1 (−0.2 to 0.3) | .39 | .90 |
| Interleukin 6, pg/mL | 1.1 (0.8–2.2) | 0.1 (−1.1 to 0.8) | .99 | 1.5 (0.8–2.2) | 0.2 (−0.7 to 1.1) | .33 | .41 |
| GlycA, μmol/L | 405.7 (372.7–447.5) | 7.0 (−24.3 to 40.3) | .45 | 400.3 (354.7–459.6) | −31.5 (−51.2 to −4.9) | <.001 | .002 |
| GlycB, μmol/L | 112.6 (104.1–120.5) | 10.1 (−3.1 to 18.5) | .003 | 120.2 (100.7–135.1) | 7.1 (−8.5 to 14.9) | .29 | .25 |
Data are presented as median (interquartile range). Significant P values are shown in bold.
Abbreviations: HOMA-IR, homeostasis model assessment–insulin resistance; hs-CRP, high-sensitivity C-reactive protein.
a Estimated creatinine clearance by Cockroft-Gault.
Brachial Artery FMD
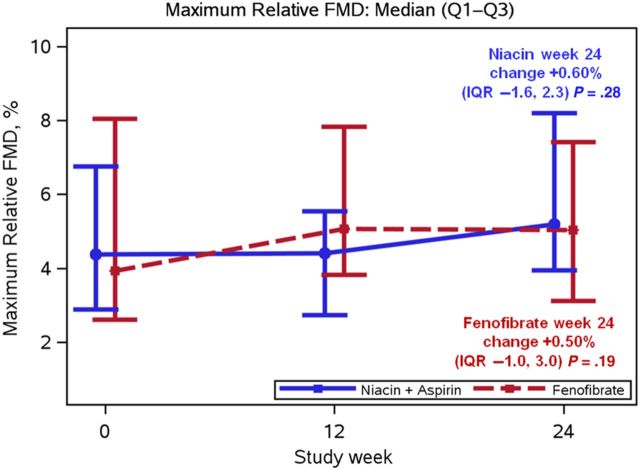
FMD over time is shown in Figure 2. Baseline FMD was normal and similar in both groups at baseline and did not change over time with niacin (+0.60% [IQR, −1.6 to 2.3]; P = .28]) or with fenofibrate (+0.50% [IQR, −1.0 to 3.0]; P = .19]) (Table 4). The standard deviation of the change in the week 24 relative FMD of 3.2% was consistent with the assumptions in our sample size estimates. There were no changes in brachial artery diameter, peak hyperemic flow velocity after cuff deflation, systolic or diastolic blood pressure, or heart rate with either drug treatment. In post hoc analyses, the change in FMD at week 24 did not differ by the HDL-C stratification factor at entry (<30 vs 30–40 mg/dL for men, <40 vs 40–50 mg/dL for women), the change in HDL-C at week 24 categorized into tertiles, protease inhibitor use, abacavir use, smoking status, antihypertensive medication use, or concurrent statin use (data not shown).
Figure 2.
Median brachial artery flow-mediated dilation (FMD) with interquartile range (IQR) over 24 weeks among evaluable participants receiving either extended-release niacin plus aspirin (n = 35) or fenofibrate (n = 39).
Table 4.
Brachial Ultrasound and Cardiovascular Measurements
| Variable | Niacin + Aspirin (n = 35) |
Fenofibrate (n = 39) |
Between-Groups P Value for Week 24 Change | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 24 Change | P Value | Baseline | Week 24 Change | P Value | ||
| Brachial flow mediated dilation, % | 4.38 (2.88–6.76) | 0.60 (−1.58 to 2.28) | .28 | 3.93 (2.61–8.05) | 0.50 (−0.97 to 2.28) | .19 | .99 |
| Brachial artery diameter, mm | 4.25 (3.88–4.66) | 0.08 (−0.11 to 0.21) | .22 | 4.46 (4.19–4.74) | −0.03 (−0.15 to 0.11) | .35 | .08 |
| Relative hyperemia brachial flow, mL/min | 801 (621–1040) | −7 (−130 to 142) | .84 | 857 (592–1100) | 22 (−106 to 126) | .97 | .77 |
| Systolic blood pressure, mm Hg | 120 (110–129) | 0 (−5 to 4) | .74 | 119 (114–124) | −2 (−14 to 10) | .74 | .66 |
| Diastolic blood pressure, mm Hg | 76 (72–81) | −1 (−7 to 3) | .22 | 76 (71–82) | −2 (−8 to 5) | .32 | .89 |
| Heart rate, beats/min | 69 (60–77) | −1 (−5 to 5) | .68 | 64 (58–71) | 2 (−5 to 6) | .49 | .45 |
Data are presented as median (interquartile range).
Correlations Between Changes in Variables and Changes in FMD
The strongest correlations with changes in FMD were for changes in non–HDL-C (r = −0.43, P < .001), total cholesterol (r = −0.40, P < .001), and total LDL particles (r = −0.39, P < .001) (Table 5). However, the correlation of change in FMD with change in HDL-C was weak (r = 0.23, P = .046). There was no statistically significant correlation between changes in FMD and changes in HDL particles, large HDL particles, HDL size, large VLDL particles, LDL size, HOMA-IR, estimated creatinine clearance, GlycA, GlycB, IL-6, hs-CRP, or D-dimer.
Table 5.
Spearman Correlations Between the Week 24 Change in Brachial Flow-Mediated Dilation and the Week 24 Changes in Lipid, Lipoprotein, Glucose Metabolism, and Inflammatory Markers
| Study Variable | No. | Spearman ρ | P Value |
|---|---|---|---|
| Total cholesterol | 73 | −0.40 | <.001 |
| Triglycerides | 73 | −0.26 | .03 |
| LDL cholesterol | 62 | −0.28 | .03 |
| Total LDL particles | 74 | −0.39 | <.001 |
| Small LDL particles | 74 | −0.24 | .04 |
| LDL size | 74 | 0.12 | .31 |
| HDL cholesterol | 73 | 0.23 | .05 |
| Total HDL particles | 74 | −0.08 | .50 |
| Large HDL particles | 74 | 0.21 | .07 |
| HDL size | 74 | 0.18 | .13 |
| Non-HDL cholesterol | 73 | −0.43 | <.001 |
| Large VLDL particles | 74 | −0.15 | .20 |
| HOMA-IR | 69 | −0.04 | .77 |
| Estimated creatinine clearance | 72 | −0.05 | .68 |
| High sensitivity C-reactive protein | 74 | −0.02 | .86 |
| D-dimer | 73 | −0.04 | .76 |
| Interleukin 6 | 65 | −0.09 | .46 |
| GlycA | 74 | 0.10 | .39 |
| GlycB | 74 | 0.19 | .10 |
Abbreviations: HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment–insulin resistance; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein cholesterol.
Tolerability
All participants who were randomized were included in the safety analysis. There were no grade 4 safety events. Thirty-six of 50 participants (72%) in the niacin arm reported at least 1 primary event of interest. In the fenofibrate arm, 21 of 49 participants (43%) reported at least 1 primary event (P = .007 compared with niacin). The most frequent, prespecified targeted event was flushing, which was experienced by 13 participants (26%) in the niacin arm. Five participants in the niacin arm had possible myopathy, as did 2 participants in the fenofibrate arm. There were no persistent symptoms of myopathy in either arm and there were no treatment discontinuations due to possible myopathy. There were no episodes of myositis.
DISCUSSION
In participants with well-controlled HIV infection and low HDL-C, 24 weeks of either niacin or fenofibrate led to significant improvements in HDL-C and atherogenic lipoproteins. However, despite these potentially beneficial effects, neither intervention improved endothelial function or most inflammatory biomarkers. The reasons for this lack of benefit are not clear, but these results are consistent with recent large-scale studies of the addition of niacin [12, 13] or fenofibrate [10, 11] to statin therapy as secondary CVD prevention among individuals with well-controlled LDL-C but without HIV infection. The current study had few participants who were receiving statins (12%) and had relatively normal levels of LDL-C but had high levels of small LDL particles and low levels of HDL-C. Despite improvements in these parameters with both study interventions, there still was no appreciable benefit in arterial endothelial function or cardiovascular inflammatory biomarkers. As such, it is unlikely that use of either intervention for patients with HIV infection with low levels of HDL–C will favorably impact CVD risk.
Published studies in the general population with niacin [33–38] and fenofibrate [39–50] generally have supported a benefit with regard to their effects on endothelial function, albeit at higher doses of niacin and in individuals with more severe dyslipidemia. A recent meta-analysis of randomized niacin trials [51] suggests an overall benefit of niacin on FMD, particularly when used at doses ≥2000 mg/day and when used in primary CVD prevention. In meta-regression, observed improvements in FMD with niacin were not related to changes in atherogenic lipoproteins [51]. We identified significant correlations between changes in atherogenic lipoproteins and FMD, but no significant effect of either treatment on FMD and no major differences between the arms. The reason for the lack of FMD benefit in our study may be related to the moderate dose of niacin used, or that the milieu of inflammation and immune activation in patients with treated HIV infection is not amenable to intervention with either niacin or fenofibrate. Alternatively, it is possible that the relatively low CVD risk of our participants contributed to the null result.
The magnitude of lipid and lipoprotein changes in this trial were similar to other studies involving HIV-infected participants using niacin [17], fenofibrate [20], or both [52]. Differing participant populations, treatment durations, methods of data presentation, and drug dosages, however, make direct comparisons between these studies difficult. In the Heart Positive study, a trial that compared a usual-care group to an intensive diet and exercise intervention combined with fenofibrate, niacin, both drugs, or placebo, the combination of fenofibrate and niacin had the greatest increases in HDL-C and decreases in non–HDL-C [52]. However, no cardiovascular imaging was included in that study. We did not include a combination niacin plus fenofibrate treatment group in our study.
Endothelial dysfunction is associated with increased CVD risk and adverse risk factors in individuals with HIV infection [18, 26, 27]. We were unable to confirm the results of Chow et al with the same dose of extended-release niacin plus aspirin, in a very similar HIV-infected study population, and using the same FMD methods and core reading center [18]. The increase in FMD with 12 weeks of niacin in that study was 0.91%, which was not statistically different from the change in controls and was not dramatically different than that seen at 24 weeks in the present study with niacin (0.60%). However, that study was small (N = 19) and of only 12 weeks’ duration, the control arm experienced a decrease in FMD, and the between-arms difference was of borderline statistical significance and only emerged after adjustment, suggesting that the observations of Chow et al [18] may have been due to chance.
We observed relatively strong correlations (r = 0.39–0.43) between changes in total cholesterol, non–HDL-C, and LDL particles and changes in FMD. However, the association of changes in HDL-C with change in FMD was weak and only of borderline statistical significance. This suggests that changes in atherogenic lipoproteins such as non–HDL-C may influence endothelial function more so than changes in HDL-C. Thus, interventions with greater non–HDL-C–lowering potency should be considered for study in this population.
Limitations of our trial include lack of a blinded placebo control arm and the variability inherent in a multicenter study that employs precise ultrasound measurements. However, we were not able to demonstrate a benefit with either therapy even with an as-treated analysis using an objective endpoint. Test variability is less of a concern given that the standard deviation of the change in FMD that we observed was within the expected range, the longitudinal within-subject nature of the primary endpoint, and central reading of brachial ultrasound scans with excellent reproducibility. We also identified expected relationships between changes in lipids and changes in FMD. It is possible, although unlikely, that use of aspirin and/or naproxen in the niacin arm may have masked a salutary effect of niacin.
The approach to patients with well-controlled HIV infection who are not candidates for a statin, yet have low levels of HDL-C, is unclear. A consensus statement from the National Lipid Association [53] and current treatment guidelines in the general population [7] do not consider low HDL-C to be a therapeutic target with currently available interventions. In view of these and recent trials in secondary CVD prevention [10–13], as well as the current study, there appears to be no major role for either niacin or fenofibrate for treatment of low HDL-C in patients with HIV.
Notes
Acknowledgments. We are indebted to the study participants, without whom this project would not have been possible. Protocol support was provided by Jennifer Rothenberg, MS. Karin L. Klingman, MD, provided oversight from the Division of AIDS. Data management was overseen by Linda Wieclaw, BS. Baiba Berzins, MPH, served as AIDS Clinical Trials Group (ACTG) Field Representative. William Pfaff served as the Community Scientific Subcommittee Representative for this protocol.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the NIH (award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701). The protocol received support from the ACTG, the Site Data Management Center (grant number UM1AI68634), and the 14 participating clinical research sites. From the sites we acknowledge the following personnel and AIDS Clinical Trials Unit grants: University of Southern California CRS (Site 1201): Frances Canchola, RN, Mussolini Africano, PA-C. Cincinnati CRS (Site 2401): Connie E. McCoy, RVT and Jenifer Baer RN, BSN, ACTG Grant UM1 AI069501. New York University HIV/AIDS CRS (Site 401). Harbor-University of California, Los Angeles (UCLA) CRS (Site 603): Eric Daar and Ruben Lopez, ACTG Grant A1069424, CTSI UL1TR000124. Chapel Hill CRS (Site 3201): Elizabeth Lindsey, RN, and Tamara James. Alabama CRS (Site 31788): ACTG Grant 2UM1AI069452-08. Duke University Medical Center Adult (Site 1601). University of Washington AIDS CRS (Site 1401). Greensboro CRS (Site 3203). New Jersey Medical School CRS (Site 31786): Sally Hodder, MD, and Susana Rivera, RN, ACTG Grant 2UM1AI069419. UCLA CARE Center CRS (Site 601): Ardis Moe, MD, Khai Nguyen, ACTG Grant AI069424. Case CRS (Site 2501): Trisha Walton, RN, and Jane Baum, RN, ACTG Grant AID 069501. Northwestern University CRS (Site 2701): Baiba Berzins, MPH, and Babafemi Taiwo, MBBS, ACTG Grant UM1AI069471. University of Colorado Hospital CRS (Site 6101).
Potential conflicts of interest. M. P. D. has served as a consultant to Gilead and AstraZeneca and receives research support through his university from Gilead, Serono, Merck, and ViiV. C. J. F. receives research funding from Gilead, Pfizer, Enterahealth, and Cubist. E. T. O. has served as an advisor for Gilead Sciences and receives research support through his university from Gilead, BMS, Vertex, Merck, AbbVie, and ViiV. J. S. C. has served as a consultant for Gilead and has received research funding from Merck. J. H. S. is Principal Investigator on a research grant from Gilead to the University of Wisconsin for a core ultrasound lab, and has served on data and safety monitoring committees for Abbott, Lilly, and Takeda. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab 1992; 74:1045–52. [DOI] [PubMed] [Google Scholar]
- 2.Riddler SA, Li X, Chu H, et al. Longitudinal changes in serum lipids among HIV-infected men on highly active antiretroviral therapy. HIV Med 2007; 8:280–7. [DOI] [PubMed] [Google Scholar]
- 3.DAD Study Group; Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–35. [DOI] [PubMed] [Google Scholar]
- 4.Duprez DA, Kuller LH, Tracy R, et al. Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis 2009; 207:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA 1986; 256:2835–8. [PubMed] [Google Scholar]
- 6.Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989; 79:8–15. [DOI] [PubMed] [Google Scholar]
- 7.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2889–934. [DOI] [PubMed] [Google Scholar]
- 8.Dubé MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidemia in HIV-infected adults receiving antiretroviral therapy. Recommendations of the HIV Medical Association of the Infectious Diseases Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis 2003; 37:613–27. [DOI] [PubMed] [Google Scholar]
- 9.Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:1–10. [DOI] [PubMed] [Google Scholar]
- 10.Accord Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005; 366:1849–61. [DOI] [PubMed] [Google Scholar]
- 12.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–67. [DOI] [PubMed] [Google Scholar]
- 13.HPS2-Thrive Collaborative Group; Landray MJ, Haynes R, Hopewell JC, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014; 371:203–12. [DOI] [PubMed] [Google Scholar]
- 14.Burgess S, Freitag DF, Khan H, Gorman DN, Thompson SG. Using multivariable Mendelian randomization to disentangle the causal effects of lipid fractions. PLoS One 2014; 9:e108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Do R, Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet 2013; 45:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet 2012; 380:572–80. [Erratum appears in Lancet 2012; 380:564]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubé MP, Wu JW, Aberg JA, et al. Safety and efficacy of extended-release niacin for the treatment of dyslipidaemia in patients with HIV infection: AIDS Clinical Trials Group Study A5148. Antivir Ther 2006; 11:1081–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Chow DC, Stein JH, Setoa TB, et al. Short-term effects of extended-release niacin on endothelial function in HIV-infected patients on stable antiretroviral therapy. AIDS 2010; 24:1019–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber MT, Mondy KE, Yarasheski KE, et al. Niacin in HIV-infected individuals with hyperlipidemia receiving potent antiretroviral therapy. Clin Infect Dis 2004; 39:419–25. [DOI] [PubMed] [Google Scholar]
- 20.Aberg JA, Zackin RA, Brobst SW, et al. A randomized trial of the efficacy and safety of fenofibrate versus pravastatin in HIV-infected subjects with lipid abnormalities: AIDS Clinical Trials Group Study 5087. AIDS Res Hum Retroviruses 2005; 21:757–67. [DOI] [PubMed] [Google Scholar]
- 21.Fichtenbaum CJ, Yeh T-M, Evans SR, Aberg JA. Treatment with pravastatin and fenofibrate improves atherogenic lipid profiles but not inflammatory markers in ACTG 5087. J Clin Lipidol 2010; 4:279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerber JG, Kitch DW, Fichtenbaum CJ, et al. Fish oil and fenofibrate for the treatment of hypertriglyceridemia in HIV-infected subjects on antiretroviral therapy: results of ACTG A5186. J Acquir Immune Defic Syndr 2008; 47:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–97. [DOI] [PubMed] [Google Scholar]
- 24.Stein E. Cerivastatin in primary hyperlipidemia—a multicenter analysis of efficacy and safety. Atherosclerosis 1998; 139:S15–22. [DOI] [PubMed] [Google Scholar]
- 25.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. In: Rifai N, Warnick GR, Dominiczak M, eds. Handbook of lipoprotein testing. 3rd ed Washington, DC: AACC Press, 2000:497–508. [Google Scholar]
- 26.Stein JH, Brown TT, Ribaudo HJ, et al. Ultrasonographic measures of cardiovascular disease risk in antiretroviral treatment-naive individuals with HIV infection. AIDS 2013; 27:929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: the ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol 2008; 52:569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mora S, Szklo M, Otvos JD, et al. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2007; 192:211–7. [DOI] [PubMed] [Google Scholar]
- 29.Muhlestein JB, May H, Winegar D, et al. GlycA and GlycB, novel NMR biomarkers of inflammation, strongly predict future cardiovascular events, but not the presence of coronary artery disease (CAD), among patients undergoing coronary angiography: the Intermountain Heart Collaborative Study. J Am Coll Cardiol 2014; 63:A1389. [Google Scholar]
- 30.Akinkuolie AO, Buring JE, Ridker PM, Mora S. A novel protein glycan biomarker and future cardiovascular disease events. J Am Heart Assoc 2014; 3:e001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forsblom C, Hiukka A, Leinonen ES, Sundvall J, Groop P-H, Taskinen M-R. Effects of long-term fenofibrate treatment on markers of renal function in type 2 diabetes: the FIELD Helsinki substudy. Diabetes Care 2010; 33:215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hottelart C, El Esper N, Rose F, et al. Fenofibrate increases creatininemia by increasing metabolic production of creatinine. Nephron 2002; 92:536–41. [DOI] [PubMed] [Google Scholar]
- 33.Warnholtz A, Wild P, Ostad MA, et al. Effects of oral niacin on endothelial dysfunction in patients with coronary artery disease: results of the randomized, double-blind, placebo-controlled INEF study. Atherosclerosis 2009; 204:216–21. [DOI] [PubMed] [Google Scholar]
- 34.Benjo AM, Maranhao RC, Coimbra SR, et al. Accumulation of chylomicron remnants and impaired vascular reactivity occur in subjects with isolated low HDL cholesterol: effects of niacin treatment. Atherosclerosis 2006; 187:116–22. [DOI] [PubMed] [Google Scholar]
- 35.Kuvin JT, Ramet ME, Patel AR, Pandian NG, Mendelsohn ME, Karas RH. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am Heart J 2002; 144:165–72. [DOI] [PubMed] [Google Scholar]
- 36.Bregar U, Jug B, Keber I, Cevc M, Sebestjen M. Extended-release niacin/laropiprant improves endothelial function in patients after myocardial infarction. Heart Vessels 2014; 29:313–9. [DOI] [PubMed] [Google Scholar]
- 37.Philpott AC, Hubacek J, Sun YC, Hillard D, Anderson TJ. Niacin improves lipid profile but not endothelial function in patients with coronary artery disease on high dose statin therapy. Atherosclerosis 2013; 226:453–8. [DOI] [PubMed] [Google Scholar]
- 38.Lee JMS, Robson MD, Yu L-M, et al. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function a randomized, placebo-controlled, magnetic resonance imaging study. J Am Coll Cardiol 2009; 54:1787–94. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton SJ, Chew GT, Davis TME, Watts GF. Fenofibrate improves endothelial function in the brachial artery and forearm resistance arterioles of statin-treated type 2 diabetic patients. Clin Sci 2010; 118:607–15. [DOI] [PubMed] [Google Scholar]
- 40.Capell WH, DeSouza CA, Poirier P, et al. Short-term triglyceride lowering with fenofibrate improves vasodilator function in subjects with hypertriglyceridemia. Arterioscler Thromb Vasc Biol 2003; 23:307–13. [DOI] [PubMed] [Google Scholar]
- 41.Koh KK, Han SH, Quon MJ, Yeal Ahn J, Shin EK. Beneficial effects of fenofibrate to improve endothelial dysfunction and raise adiponectin levels in patients with primary hypertriglyceridemia. Diabetes Care 2005; 28:1419–24. [DOI] [PubMed] [Google Scholar]
- 42.Koh KK, Quon MJ, Han SH, et al. Additive beneficial effects of fenofibrate combined with atorvastatin in the treatment of combined hyperlipidemia. J Am Coll Cardiol 2005; 45:1649–53. [DOI] [PubMed] [Google Scholar]
- 43.Malik J, Melenovsky V, Wichterle D, et al. Both fenofibrate and atorvastatin improve vascular reactivity in combined hyperlipidaemia (fenofibrate versus atorvastatin trial—FAT). Cardiovasc Res 2001; 52:290–8. [DOI] [PubMed] [Google Scholar]
- 44.Wang T-D, Chen W-J, Lin J-W, Cheng C-C, Chen M-F, Lee Y-T. Efficacy of fenofibrate and simvastatin on endothelial function and inflammatory markers in patients with combined hyperlipidemia: relations with baseline lipid profiles. Atherosclerosis 2003; 170:315–23. [DOI] [PubMed] [Google Scholar]
- 45.Kilicarslan A, Yavuz B, Guven GS, et al. Fenofibrate improves endothelial function and decreases thrombin-activatable fibrinolysis inhibitor concentration in metabolic syndrome. Blood Coagul Fibrinolysis 2008; 19:310–4. [DOI] [PubMed] [Google Scholar]
- 46.Koh Koh K, Yeal Ahn J, Hwan Han S, et al. Effects of fenofibrate on lipoproteins, vasomotor function, and serological markers of inflammation, plaque stabilization, and hemostasis. Atherosclerosis 2004; 174:379–83. [DOI] [PubMed] [Google Scholar]
- 47.Ghani RA, Bin Yaakob I, Wahab NA, et al. The influence of fenofibrate on lipid profile, endothelial dysfunction, and inflammatory markers in type 2 diabetes mellitus patients with typical and mixed dyslipidemia. J Clin Lipidol 2013; 7:446–53. [DOI] [PubMed] [Google Scholar]
- 48.Koh KK, Quon MJ, Shin K-C, et al. Significant differential effects of omega-3 fatty acids and fenofibrate in patients with hypertriglyceridemia. Atherosclerosis 2012; 220:537–44. [DOI] [PubMed] [Google Scholar]
- 49.Shirinsky I, Polovnikova O, Kalinovskaya N, Shirinsky V. The effects of fenofibrate on inflammation and cardiovascular markers in patients with active rheumatoid arthritis: a pilot study. Rheumatol Int 2013; 33:3045–8. [DOI] [PubMed] [Google Scholar]
- 50.Walker AE, Kaplon RE, Lucking SMS, Russell-Nowlan MJ, Eckel RH, Seals DR. Fenofibrate improves vascular endothelial function by reducing oxidative stress while increasing endothelial nitric oxide synthase in healthy normolipidemic older adults. Hypertension 2012; 60:1517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sahebkar A. Effect of niacin on endothelial function: a systematic review and meta-analysis of randomized controlled trials. Vasc Med 2014; 19:54–66. [DOI] [PubMed] [Google Scholar]
- 52.Balasubramanyam A, Coraza I, Smith EOB, et al. Combination of niacin and fenofibrate with lifestyle changes improves dyslipidemia and hypoadiponectinemia in HIV patients on antiretroviral therapy: results of “heart positive,” a randomized, controlled trial. J Clin Endocrinol Metab 2011; 96:2236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toth PP, Barter PJ, Rosenson RS, et al. High-density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol 2013; 7:484–525. [DOI] [PubMed] [Google Scholar]