Abstract
Objectives
β-Lactam antibiotics are commonly used in outpatient parenteral antimicrobial therapy (OPAT), but data regarding outcomes of long-term therapy are limited. The purpose of this study was to compare treatment success, readmission and antibiotic switch rates in patients treated with β-lactam antibiotics as OPAT.
Methods
We carried out a retrospective review of all patients, discharged from Tufts Medical Center with cefazolin, ceftriaxone, ertapenem or oxacillin, between January 2009 and June 2013. A competing risks analysis was used to compare the cumulative incidence of first occurrence of treatment success, antibiotic switch and 30 day readmission for each drug.
Results
Four hundred patients were identified (cefazolin n = 38, ceftriaxone n = 104, ertapenem n = 128 and oxacillin n = 130). Baseline demographics were similar. Treatment success rates were higher for ceftriaxone and ertapenem (cefazolin 61%, ceftriaxone 81%, ertapenem 73% and oxacillin 58%; P < 0.001). Thirty-day all-cause readmissions were similar (cefazolin 21%, ceftriaxone 14%, ertapenem 20% and oxacillin 15%; P = 0.46). In 400 OPAT courses, 37 out of 50 antibiotic switches were accomplished without readmission. Adverse drug events (ADEs) were the most common reason for outpatient antibiotic switches (31/37, 84%). The ADE rate was higher for the oxacillin group (cefazolin 2.0 versus ceftriaxone 1.5 versus ertapenem 2.9 versus oxacillin 8.4 per 1000 OPAT days; P < 0.001).
Conclusions
OPAT with β-lactam antibiotics is effective, but antibiotic switches for adverse events were more frequent with oxacillin use. Clinicians should be cognizant of the risk of readmissions and ADEs in OPAT patients, as the value of OPAT lies in reducing patient morbidity and readmissions by managing ADEs and preventing clinical failures.
Keywords: adverse drug events, ertapenem, OPAT
Introduction
Outpatient parenteral antibiotic therapy (OPAT) has become a standard medical therapy since its advent in the 1970s.1 By 1998, the estimated number of annual OPAT treatments in the USA was ∼250 000 patients. Since then, the practice has grown steadily in the USA as well as around the world.2,3 OPAT has been reported to be safe, effective, cost-saving and highly satisfactory to patients.4–6
Clinical cure is only one facet of OPAT's utility. OPAT also aims to prevent readmissions with early detection and outpatient management of adverse drug events (ADEs), clinical failures and catheter-related complications.7,8 Readmission occurs frequently in patients receiving OPAT. The reported rates of readmission vary significantly from 3.6% to 26%.9,10 Reducing unplanned 30 day readmissions and the associated economic burden is a national priority.11 In order to reduce OPAT-related readmissions and properly account for the clinical benefits of OPAT, further studies on readmissions and outpatient antibiotic switches are warranted.
β-Lactam antibiotics are commonly utilized in OPAT for treatment of skin and soft tissue infections (SSTIs), osteomyelitis, bacteraemia and endocarditis.1 β-Lactams performed similarly in terms of clinical efficacy (oxacillin versus ceftriaxone,12 cefazolin versus ceftriaxone13 and cefazolin versus nafcillin14). Higher rates of premature discontinuation are associated with semi-synthetic penicillins due to ADEs. However, the scope of these studies is limited to the treatment of MSSA infections. To our knowledge no previous studies have examined rates and causes of readmission in the broader context of OPAT using different β-lactams. Ertapenem, a newer agent, has been increasingly used in the OPAT setting.15 Previous studies reported successful clinical outcomes of ertapenem in OPAT.15–19 However, they had limited treatment indications15–19 and lacked data on long-term comparative tolerability and risk of readmission.
Because of these gaps in OPAT knowledge, the current study aims to compare the rates of treatment success, readmission and antibiotic switches in patients treated with commonly used β-lactam antibiotics (cefazolin, ceftriaxone, ertapenem or oxacillin) in OPAT.
Methods
Study design, setting and population
The retrospective cohort study utilized a previously published database of all patients who received intravenous antibiotics via the Tufts Medical Center (Tufts MC) OPAT programme (Boston, MA, USA).10 The Tufts MC OPAT programme was designed in 2008 in conjunction with published OPAT practice guidelines.1 In our institution, an infectious disease (ID) consultation and the OPAT monitoring programme have been strongly advised for all patients requiring outpatient antibiotic therapy to ensure patient safety and improve the quality of care transitions.
More than 90% of patients discharged from Tufts MC with parenteral antibiotic therapy were enrolled in the Tufts MC OPAT programme during an inpatient ID consultation. The OPAT patients were followed by an ID specialist, introduced during an inpatient consultation. Patients were seen in clinic by an ID specialist within 1–2 weeks of hospital discharge. For all of the OPAT patients discharged home, either the patients or their care providers were trained to self-administer antibiotics with the assistance of skilled home nursing care and infusion services prior to discharge, with follow-up teaching in the home setting. Surveillance laboratory studies were obtained in accordance with published guidelines1 under the supervision of ID physicians with the care coordinated by an OPAT administrator.
The antibiotic agent of choice and dosing regimen were determined by an inpatient ID consultant. The recommended dosing for the antibiotics is 2 g every 4–6 h for oxacillin with an option of continuous infusion via an electronic pump, 2 g every 8 h for cefazolin, 1–2 g daily for ceftriaxone and 1 g daily for ertapenem. In patients with renal insufficiency, the antibiotics were dose adjusted.
Eligible patients for the current study included individuals 18 years of age or older who had been discharged from hospital on the study antibiotics (cefazolin, ceftriaxone, ertapenem or oxacillin) and followed by the Tufts MC OPAT programme from January 2009 to June 2013. Only the first OPAT course for each patient was included in the study. The study excluded patients who: (i) initiated intravenous antibiotics as outpatients; (ii) were prescribed intravenous antibiotics intended for chronic suppression (as there is no planned end-date or cure in these patients); (iii) received a parenteral antibiotic(s) other than or in addition to study antibiotics; (iv) had a planned readmission within 30 days; (v) had infections with Pseudomonas, MRSA or VRE; or (vi) were not monitored in the Tufts OPAT programme or had no interaction with Tufts MC after discharge. This research was approved by the Institutional Review Board of Tufts Medical Center/Tufts University Health Sciences Campus.
Data collection and definitions
Patient data were extracted from medical charts into a secure electronic relational database using REDCap (Research Electronic Data Capture).20 All medical charts, including discharge summaries and outpatient electronic medical records, were reviewed by the investigators to assess the clinical outcomes and examine outpatient events such as readmissions, antibiotic switches and ADEs. Collected data included socio-demographic factors [age, disposition (home versus rehabilitation facility), insurance status], measures of healthcare utilization (length of stay, prior hospital admissions for any cause in the preceding 12 months), type of infection, severity of the infection (for pneumonia, urinary tract infections and osteoarticular infections), microbiology data, antibiotics prescribed (including oral agents), primary service, comorbidities at time of hospital admission, outpatient visits, antibiotic switches and reasons for the changes.
Microbiology data included type of specimen, Gram's stain results, speciation of the organism and susceptibility, including the presence of ESBLs. Positive Lyme serology was collected as a separate category. Categories of infectious diagnoses were extracted from previously published OPAT registry data and modified by the authors of the current study.4 Diagnoses were not exclusive—e.g. patients with endocarditis and vertebral osteomyelitis were included for both diagnoses. To assess comorbidities, a modified Charlson comorbidity score was calculated for each patient.21
Clinical cure was defined as the resolution of signs and symptoms of infection and discontinuation of antibiotic therapy. All 30 day readmissions and outpatient antibiotic changes were recorded. The reasons for readmission or antibiotic discontinuation were selected among multiple choices. Adverse drug reactions were entered as a binary outcome (present/not present) and further categorized as follows: diarrhoea, fever (a temperature >38.0°C or 100.4°F), transaminitis (alanine aminotransferase >42 U/L or aspartate aminotransferase >54 U/L and deemed clinically relevant by the treating physician), neutropenia (absolute neutrophil count <1500/μL), rash, acute renal injury (increase in serum creatinine of >0.5 mg/dL or 50% increase from baseline) and others, based on a previous review article.22
Outcomes
The primary outcome was the rate of treatment success—end of parenteral treatment as end of antibiotic therapy and transition to oral alternatives—at the time of initial antibiotic discontinuation. The secondary outcome measures included rates of readmissions and outpatient antibiotic switches. Incidence rates of specific ADEs were also calculated as events per 1000 patient-days on each medication. While the occurrence of ADEs cannot be definitively attributed to the first or subsequent antibiotics, for the purpose of statistical analysis, only the time from initiation of a new agent to antibiotic switch due to ADEs was included in the analysis.
Statistical analysis
Baseline characteristics are presented using means and standard deviations for continuous variables and frequencies and percentages for categorical characteristics. The clinical and demographic characteristics of each group were compared using ANOVA or Kruskal–Wallis tests for continuous variables and χ2 for categorical variables.
We examined the cumulative incidence of multiple treatment outcomes using the competing risk analysis approach proposed by Gray.23 This method was specifically designed for situations in which patients could experience one of a set of related treatment outcomes. Readmission and outpatient antibiotic switches due to treatment failure or ADEs before completion of OPAT were defined as competing risk events.
In order to estimate the sole effect of antibiotics on the outcome events, non-infection-related outpatient antibiotic changes, such as venous catheter problems or non-adherence, were censored. We calculated the cumulative incidence rates of the outcome events at fixed timepoints (60 days for treatment success and ADEs, and 30 days for readmissions) in the presence of competing risks and compared the incidence curves using a modified χ2 test.23 In addition, rates of ADEs per 1000 patient-days were calculated and compared among study antibiotic groups, with CIs constructed using exact Poisson methods.24
To adjust for the effects of a large number of potential confounding factors, including covariates potentially affecting treatment decision (e.g. microbiology or site of infection), we utilized a covariate balancing propensity score method.25,26 First, we performed bivariate analyses of each covariate and its association with treatment outcomes (Table S1, available as Supplementary data at JAC Online). Variables with P values ≤0.2 were included in the propensity score estimation. Due to their association with readmission, as demonstrated in the literature, both the Charlson comorbidity score and post-acute care (home versus rehabilitation) were forced into the propensity score modelling regardless of their statistical significance.27 Considering their strong association with specific antibiotics, which can reduce the precision of the propensity score model,28 ESBL and Lyme disease were removed from the propensity score model. The estimated propensity score for each subject reflects the probability of receiving one of the four study antibiotics. Then, we forced the estimated propensity score into the proportional hazards regression model of the treatment effect on the outcome. We also fitted a weighted regression model using the inverse probability weights obtained with the propensity score model. The cause-specific hazard for each study antibiotic was compared with ertapenem, including adjustment with the propensity score.
Statistical analyses were performed using R statistical software (version 3.0.1, updated 26 May, 2013, copyright R Foundation, from http://www.r-project.org) for comparing cumulative incidence functions (cmprsk library) and developing the propensity score model (cbps library), and SAS (version 9.3, July 2011, copyright SAS Institute Inc., Cary, NC, USA) for cause-specific HR modelling. Statistical significance was determined using two-sided P < 0.05.
Results
Study population characteristics
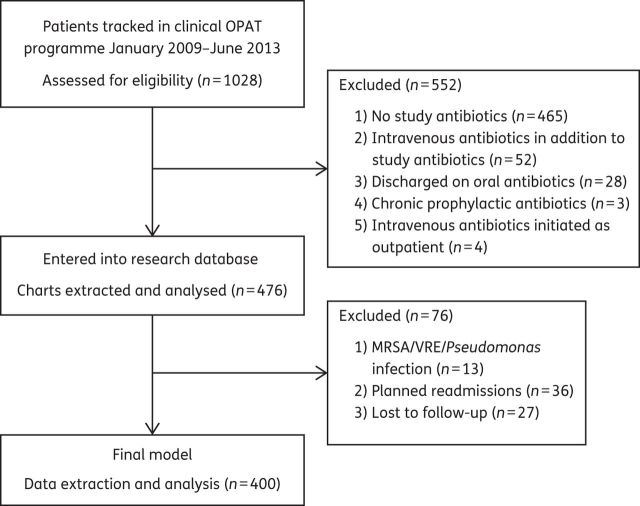
A total of 1028 patients receiving OPAT were screened for eligibility and 400 met the enrolment criteria (Figure 1). Twenty-seven patients (27/476, 5.7%) with no available outpatient records were excluded from the study. The cefazolin group had the lowest number of patients compared with the other groups.
Figure 1.
Patient selection for the retrospective cohort.
Demographics and clinical characteristics of the study cohort are shown in Table 1. The groups were similar in age and gender. The overall cure rate at the end of OPAT therapy was 331/400 (83%). The cefazolin and ertapenem groups had higher Charlson comorbidity scores. Sixteen patients (16/38, 42%) in the cefazolin group had preexisting chronic renal failure, and 15 of them were on renal replacement therapy. In terms of healthcare utilization, the cefazolin group had a longer length of hospital stay and a lower proportion of patients discharged to home.
Table 1.
Demographics and clinical characteristics of the study cohort (n = 400)
| Cefazolin (n = 38) | Ceftriaxone (n = 104) | Ertapenem (n = 128) | Oxacillin (n = 130) | P | |
|---|---|---|---|---|---|
| Demographics | |||||
| age (years), mean (SD) | 61 (16) | 57 (18) | 58 (17) | 56 (18) | 0.56 |
| male | 24 (63) | 59 (57) | 67 (52) | 73 (56) | 0.68 |
| Comorbidities | |||||
| Charlson, mean (SD)a | 2.8 (2.4) | 1.6 (2.1) | 2.4 (2.4) | 1.7 (2.0) | 0.002 |
| heart failure | 2 (5.3) | 9 (8.7) | 14 (10.9) | 20 (15.4) | 0.27 |
| diabetes mellitus | 12 (31.6) | 28 (26.9) | 36 (28.1) | 32 (24.6) | 0.83 |
| CKD | 16 (42.1) | 16 (15.4) | 30 (23.4) | 20 (15.4) | 0.002 |
| CKD with RRT | 15 (39.5) | 0 (0) | 2 (1.6) | 1 (0.8) | <0.0001 |
| liver dysfunction | 9 (23.7) | 10 (9.6) | 20 (15.6) | 14 (10.8) | 0.11 |
| immunosuppression | 9 (23.7) | 18 (17.3) | 40 (31.3) | 28 (21.5) | 0.08 |
| past drug-resistant organismsb | 5 (13.2) | 5 (4.8) | 10 (7.8) | 2 (1.5) | 0.02 |
| Healthcare utilization | |||||
| LOS (days), mean (SD) | 9.7 (7.9) | 6.0 (4.2) | 7.1 (6.9) | 8.6 (7.2) | 0.001 |
| prior admissions, mean (SD) | 1.4 (2.4) | 0.6 (1.2) | 1.0 (1.6) | 1.0 (1.8) | 0.18 |
| insurance | |||||
| Medicare | 15 (39.5) | 37 (35.6) | 50 (39.1) | 52 (40.0) | 0.91 |
| Medicaid | 8 (21.1) | 14 (13.5) | 19 (14.8) | 16 (12.3) | 0.59 |
| private | 15 (39.5) | 49 (47.1) | 58 (45.3) | 60 (46.2) | 0.88 |
| home versus rehab | 14 (36.8) | 61 (58.7) | 88 (68.8) | 76 (58.5) | 0.005 |
| Oral antibiotic usec | 9 (23.7) | 20 (19.2) | 24 (18.8) | 18 (13.8) | 0.48 |
CKD, chronic kidney disease (defined by practice guideline39); RRT, renal replacement therapy; LOS, length of stay.
Data are presented as n (%) unless otherwise specified.
aThe Charlson comorbidity index includes 19 diseases listed in Table S1.
bThe list of organisms includes MRSA, VRE and Gram-negative bacteria with ESBLs.
cTherapeutic oral agents with a high level of systemic absorption were included in the analysis. The list of drugs used is as follows: azoles, doxycycline, fluoroquinolones, metronidazole, rifampicin and trimethoprim/sulfamethoxazole.
Table 2 presents the infectious diagnoses and microbiology data of the study cohort. Patients may have more than one diagnosis. The most commonly treated diagnoses were abscess, bacteraemia and osteoarticular infections. Ceftriaxone was also used for 13 patients who had Lyme disease, which is endemic in the catchment area of the study institution. Ertapenem was the most frequent antibiotic used to treat urinary tract and intra-abdominal infections. The study groups exhibited distinct patterns in microbiology: close to 90% of the cefazolin and oxacillin groups had MSSA infections. The ertapenem and ceftriaxone groups had a wider array of bacteriology. In the ertapenem group, 84/128 (66%) patients had infections with Gram-negative organisms; 35% (29/84) of the Gram-negative organisms were ESBL producers. Five patients (5/400, 1.3%) developed Clostridium difficile colitis during OPAT and four out of these five patients received ertapenem (4/128, 3.1%).
Table 2.
Infectious diagnoses and microbiological characteristics of the study cohort (n = 400)
| Cefazolin (n = 38) | Ceftriaxone (n = 104) | Ertapenem (n = 128) | Oxacillin (n = 130) | P | |
|---|---|---|---|---|---|
| Infection diagnosis | |||||
| abscess | 6 (15.8) | 16 (15.4) | 33 (25.8) | 32 (24.6) | 0.16 |
| bacteraemia without endocarditis | 18 (47.4) | 32 (30.8) | 23 (18.0) | 37 (28.5) | 0.03 |
| endocarditis | 5 (13.2) | 14 (13.5) | 1 (0.8) | 15 (11.5) | 0.0001 |
| intra-abdominal infection | 4 (10.5) | 9 (8.7) | 30 (23.4) | 6 (4.6) | <0.0001 |
| Lyme disease | 0 (0) | 13 (12.5) | 0 (0) | 0 (0) | <0.0001 |
| osteomyelitis | 14 (36.8) | 13 (12.5) | 25 (19.5) | 36 (27.7) | 0.004 |
| pneumonia | 6 (15.8) | 9 (8.7) | 13 (10.2) | 4 (3.1) | 0.03 |
| prosthetic joint infection | 4 (10.5) | 9 (8.7) | 3 (2.3) | 23 (17.7) | 0.0003 |
| SSTI and wound infection | 2 (5.3) | 4 (3.8) | 14 (10.9) | 22 (16.9) | 0.0075 |
| urinary tract infection | 2 (5.3) | 16 (15.4) | 39 (30.5) | 6 (4.6) | <0.0001 |
| Microbiology | |||||
| Gram-positive | 35 (92.1) | 53 (51.0) | 50 (39.1) | 128 (98.5) | <0.0001 |
| MSSA | 33 (86.8) | 5 (4.8) | 12 (9.4) | 121 (93.1) | <0.0001 |
| Streptococcus | 2 (5.3) | 46 (44.2) | 24 (18.8) | 4 (3.1) | <0.0001 |
| Gram-negative | 4 (10.5) | 30 (28.8) | 84 (65.6) | 5 (3.8) | <0.0001 |
| ESBL | 0 (0) | 0 (0) | 29 (22.7) | 0 (0) | <0.0001 |
| not ESBL | 3 (7.9) | 23 (22.1) | 23 (18.0) | 3 (2.3) | <0.0001 |
| some resistance | 1 (2.6) | 2 (1.9) | 16 (12.5) | 0 (0) | <0.0001 |
| no susceptibility | 0 (0) | 5 (4.8) | 17 (13.3) | 2 (1.5) | 0.004 |
| C. difficile infection in OPAT | 1 (2.6) | 0 (0) | 4 (3.1) | 0 (0) | NA |
NA, not applicable due to small sample size.
Treatment success, readmissions and antibiotic switches
Overall, 287/400 (72%) patients achieved treatment success when their initial antibiotics were stopped. Treatment success rates were higher for ceftriaxone and ertapenem (Table 3). A total of 67/400 patients (16.8%) were readmitted within 30 days. Reasons for readmissions are presented in Table 3. Thirty-day all-cause readmissions were similar among study groups. Sixty per cent (40/67) of readmissions were ID related; the most common indication was treatment failure. Antibiotic switches occurred in 12.5% of the subjects (50/400) and 74% (37/50) of antibiotic switches were achieved without readmission. ADEs account for the majority of outpatient switches (31/37, 84%). Figure 2 shows the results of competing risk analyses. The cumulative incidence rates of treatment success and outpatient switches were significantly different across all groups (P < 0.001); 30 day all-cause readmission rates were similar (P = 0.46). Nine patients with antibiotic changes due to other reasons (e.g. catheter problem, non-adherence or financial burden) were censored. Catheter-related complications accounted for two antibiotic switches (one in oxacillin and one in ertapenem).
Table 3.
β-Lactam antibiotic types and initial treatment outcomesa
| Total (n = 400) | Cefazolin (n = 38) | Ceftriaxone (n = 104) | Ertapenem (n = 128) | Oxacillin (n = 130) | |
|---|---|---|---|---|---|
| Outcomeb | |||||
| treatment success | 287 (72) | 26 (68) | 84 (81) | 97 (76) | 80 (62) |
| readmission | 67 (17) | 8 (21) | 14 (13) | 25 (20) | 20 (15) |
| outpatient switch | 37 (9) | 4 (11) | 4 (4) | 4 (3) | 25 (19) |
| Reasons for readmission | |||||
| non-ID related | 27 (7) | 5 (13) | 5 (5) | 10 (8) | 7 (5) |
| catheter related | 3 (1) | 0 | 2 (2) | 0 | 1 (1) |
| ADE | 10 (3) | 0 | 0 | 4 (3) | 6 (5) |
| new infection | 9 (2) | 1 (3) | 2 (2) | 3 (2) | 3 (2) |
| treatment failure | 18 (5) | 2 (5) | 5 (5) | 8 (6) | 3 (2) |
| Reasons for outpatient switch | |||||
| ADE | 31 (8) | 2 (5) | 4 (4) | 4 (3) | 21 (16) |
| treatment failure | 6 (2) | 2 (5) | 0 | 0 | 4 (3) |
Data are presented as n (%).
aFirst-event outcomes were analysed.
bNine patients with non-ID-related changes are excluded from the table.
Figure 2.
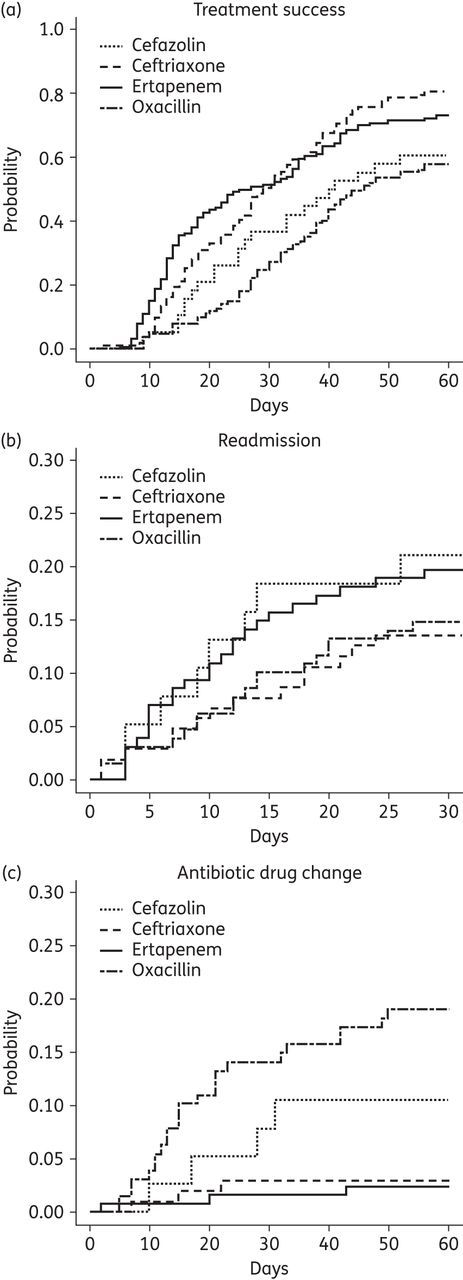
Cumulative incidence plot of treatment success (a), 30 day all-cause readmission (b) and outpatient antibiotic changes (c) due to treatment failure or adverse drug effects. (a) Cefazolin 60.5%, ceftriaxone 80.5%, ertapenem 73.1% and oxacillin 57.8% (P < 0.001). (b) Cefazolin 21.1%, ceftriaxone 13.5%, ertapenem 19.7% and oxacillin 14.8% (P = 0.46). (c) Cefazolin 10.5%, ceftriaxone 2.9%, ertapenem 2.4% and oxacillin 19.1% (P < 0.001).
The ADE rate was higher for the oxacillin group compared with counterparts (Table 4). Liver enzyme abnormality was the most common reason for discontinuation, followed by rash, acute renal injury and neutropenia. Six patients developed ADEs other than those listed: seizure (two in the ertapenem group), mental status change (two in the ertapenem group), nausea (one in the oxacillin group) and wheezing (one in the oxacillin group). Ten patients required readmissions for ADEs (Table 3).
Table 4.
β-Lactam antibiotic types and incidence of ADEs
| Cefazolin (n = 38) | Ceftriaxone (n = 104) | Ertapenem (n = 128) | Oxacillin (n = 130) | |
|---|---|---|---|---|
| Patient days | 1004 | 2624 | 3061 | 3831 |
| ADEsa,b | 2 (2.0) | 4 (1.5) | 9 (2.9) | 32 (8.4) |
| transaminitis | 0 | 2 (0.8) | 1 (0.3) | 15 (3.9) |
| neutropenia | 1 (1.0) | 1 (0.4) | 1 (0.3) | 3 (0.8) |
| rash | 0 | 1 (0.4) | 1 (0.3) | 6 (1.6) |
| acute renal injury | 0 | 0 | 1 (0.3) | 6 (1.6) |
| fever | 0 | 0 | 0 | 0 |
| diarrhoea | 1 (1.0) | 0 | 1 (0.3) | 0 |
| other | 0 | 0 | 4 (1.3) | 2 (0.5) |
aData are presented as n (incidence rate, per 1000 patient days).
bP < 0.001 for overall group difference estimated from Poisson model. All pairwise comparisons with oxacillin have P < 0.05.
Multivariable analysis model
The results of cause-specific hazards for each study antibiotic are shown in Table 5. The HRs of treatment success, readmission and outpatient antibiotic switch showed no meaningful change after covariate adjustment with the propensity score. Compared with the ertapenem group, the oxacillin group had a lower treatment success rate (HR 0.53, 95% CI 0.39, 0.74) and higher outpatient antibiotic switches due to ADEs or treatment failure (HR 5.06, 95% CI 1.74, 14.75). There was no statistically significant difference in treatment success, switches for ADEs or treatment failure, or in readmission rates between the ertapenem group and the cefazolin or ceftriaxone groups.
Table 5.
Propensity-adjusted associations between antibiotic type and treatment success, readmission and outpatient switch
| Ertapenem HR (95% CI) | Cefazolin HR (95% CI) | Ceftriaxone HR (95% CI) | Oxacillin HR (95% CI) | |
|---|---|---|---|---|
| Treatment success | 1.0 (reference) | |||
| unadjusted | 0.73 (0.47, 1.12) | 0.95 (0.71, 1.28) | 0.56 (0.42, 0.76) | |
| PS adjusted | 0.76 (0.49, 1.19) | 0.90 (0.71, 1.28) | 0.53 (0.39, 0.74) | |
| IPT weighted | 0.68 (0.49, 0.93) | 1.05 (0.76, 1.44) | 0.61 (0.44, 0.86) | |
| Readmission | 1.0 (reference) | |||
| unadjusted | 0.92 (0.42, 2.05) | 0.59 (0.31, 1.13) | 0.64 (0.35, 1.15) | |
| PS adjusted | 0.98 (0.38, 2.25) | 0.59 (0.31, 1.14) | 0.66 (0.34, 1.13) | |
| IPT weighted | 1.16 (0.61, 2.20) | 0.69 (0.33, 1.43) | 0.79 (0.40, 1.54) | |
| Outpatient switch | 1.0 (reference) | |||
| unadjusted | 2.73 (0.68, 10.94) | 1.07 (0.27, 4.29) | 4.52 (1.57, 13.02) | |
| PS adjusted | 2.25 (0.55, 9.18) | 1.10 (0.27, 4.40) | 5.06 (1.74, 14.75) | |
| IPT weighted | 5.62 (1.30, 24.28) | 8.63 (2.11, 35.33) | 5.41 (1.27, 23.05) |
IPT, inverse probability; PS, propensity score.
Discussion
This study evaluated patients treated with β-lactam antibiotics in the OPAT setting and analysed reasons for the first parenteral antibiotic change: treatment success, readmission and outpatient switches. Overall, outcomes were favourable for all study antibiotics. Our study indicates that oxacillin is less likely to accomplish treatment success without antibiotic switches. In our comparison of ertapenem with other commonly used β-lactams, accounting for potential confounding by indication, the only statistically significant finding was that patients treated with oxacillin remained at higher risk for antibiotic switches due to ADEs and treatment failure (Table 5). This finding is consistent with previous literature. Compared with cefazolin or ceftriaxone, semi-synthetic penicillins have been associated with an absolute risk increase of 15%–20% for premature discontinuation due to ADEs in adult patients.10,12,29,30 Although OPAT with β-lactams carries a considerable risk of ADEs and treatment failure, our findings suggest that we can mitigate its burden with close follow-up and timely antibiotic switches guided by ID specialists. In our cohort, 37 outpatient antibiotic switches prevented readmission, thus lowering the risks of nosocomial infections and functional declines while avoiding additional costs to both patients and the healthcare system.31 This should be considered as one of OPAT's valuable contributions. Successful switches require comprehensive understanding of the tolerability of alternatives and cross-reactivity data. A recent study suggested that patients with non-IgE-mediated ADEs from nafcillin can be safely switched to cefazolin.32 Taking these together, further studies to develop evidence-based guidance on the management of ADEs in OPAT and antibiotic switches would be helpful.
This study sheds additional light on the use of ertapenem in the OPAT setting. Our study suggests that ertapenem is safe and tolerable in the OPAT setting compared with other commonly used β-lactam antibiotics. ESBL infections accounted for a small portion of the ertapenem group. Other factors likely to favour clinician choice of ertapenem included: (i) polymicrobial infection; (ii) anaerobic infection; (iii) intra-abdominal infection, including anastomotic leak; and (iv) once-daily administration for home discharge. The rate of ADEs associated with ertapenem was comparable to those for ceftriaxone and cefazolin and lower than the rate of ADEs with oxacillin. Four patients showed CNS manifestations during ertapenem therapy. Ertapenem was discontinued in two patients after a seizure; however, both patients were being treated for brain abscesses, which predisposed them to seizures. Two patients were readmitted due to delirium of unclear aetiology. The discharge summaries attributed delirium to ertapenem as no other causes were identified after extensive workup. Carbapenems are associated with increased risk of seizure, even though the absolute risk is very low.33 The risk of seizure increases with advanced age, history of seizure disorder, renal dysfunction and low body weight.34 The risk of CNS toxicity other than seizure is unclear and only one case report described two patients who had mental status changes on ertapenem.35 Our incidence rate of C. difficile-associated diarrhoea (CDAD) in the OPAT setting was 5/400 (1.3%), while in the ertapenem group it was 4/128 (3.1%), higher than in previous studies.6,36,37 We speculate that the higher incidence of CDAD in the ertapenem group can be accounted for by: (i) its broader spectrum of coverage, compared with other OPAT agents, leading to more disruption of the intestinal microbiota; (ii) probable longer duration of therapy; and (iii) a more complex patient population with various treatment indications.38 The association between CDAD and ertapenem is not definite. Our finding warrants future studies involving larger patient pools on different broad-spectrum antibiotics.
Our study has several limitations. First, this study is a single-centre retrospective review; antibiotic switches were determined solely by the clinicians caring for the patients without the knowledge of the current study. This is particularly germane in the response to abnormal laboratory values such as transaminases, as the switches based on laboratory abnormalities were made in their clinical context. Therefore, the risk of information bias is present, as some information could have been lost during information transfer. To minimize the bias, the investigators reviewed all medical records and excluded patients whose outpatient treatment could not be evaluated. Second, due to the inherent limitations of this retrospective observational study, there is a risk of residual and unmeasured confounding. We utilized a propensity score analysis to balance the effect of measured confounders. The difference between the unadjusted and adjusted HRs was small, but residual confounding due to covariate misclassification and the impact of unmeasured confounders remains a risk. Matching is often the favoured approach to applying the propensity score to mitigate the effect of confounding by indication; however, matching could not be efficiently performed due to our relatively small sample size of antibiotic subgroups. Third, inpatient antibiotic switches occurring before discharge were not collected; IgE-mediated reactions or early non-IgE-mediated ADEs might have been underestimated in all study groups.
The greatest strength of our study is that it reflects the complexity and variety of a real-world clinical setting with a large OPAT cohort. The details captured in our study can be applied to other OPAT settings in the context of tertiary care that commonly utilizes β-lactam antibiotics. Our study is also valuable in that it is the first to evaluate antibiotic switches and their potential impact on readmission prevention during OPAT. Our findings suggest that readmission avoidance via timely outpatient antibiotic switches may be an important indicator in demonstrating the value of OPAT as directed by ID specialists.
In conclusion, our study suggests that OPAT with β-lactam antibiotics is effective and safe with close monitoring. In addition to efficacy, the distinctive side effect profiles of β-lactam antibiotics need to be considered when selecting antibiotics for OPAT. This study reaffirms the necessity of close monitoring and treatment adjustment in the outpatient setting.1 Readmission avoidance by timely outpatient antibiotic switches can serve as an important outcome metric. Clinicians should be vigilant about the risk of readmissions and ADEs in OPAT patients, as the value of OPAT lies in reducing patient morbidity, readmissions and costs by overseeing ADEs and clinical failures.
Funding
This work was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Numbers UL1TR000073 and UL1TR001064 (J. K. P., J. B.) and was also supported by the National Center for Research Resources Award Number UL1RR025752 and the National Center for Advancing Translational Sciences, National Institutes of Health, Award Numbers UL1TR000073 and UL1TR001064 (REDCap database). This work was also upported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. G. M. A.'s role in the project described was also supported by the National Center for Research Resources Award Number UL1RR025752, now the National Center for Advancing Translational Sciences, National Institutes of Health Award Number UL1TR000073; and the National Cancer Institute, Award Number KM1CA156726.
Transparency declarations
None to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp.
Supplementary data
Table S1 is available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
Acknowledgements
We gratefully acknowledge Dr Helen Boucher for her thoughtful review of the manuscript prior to submission.
References
- 1.Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis 2004; 38: 1651–72. [DOI] [PubMed] [Google Scholar]
- 2.Portez D. Evolution of outpatient parenteral antibiotic therapy. Infect Dis Clin N Am 1998; 12: 827–34. [DOI] [PubMed] [Google Scholar]
- 3.Paladino JA, Portez D. Outpatient parenteral antimicrobial therapy today. Clin Infect Dis 2010; 51 Suppl 2: S198–208. [DOI] [PubMed] [Google Scholar]
- 4.Nathwani D, Tice A. Ambulatory antimicrobial use: the value of an outcomes registry . J Antimicrob Chemother 2002; 49: 149–54. [DOI] [PubMed] [Google Scholar]
- 5.Kunkel MJ. Quality assurance and outcomes in outpatient parenteral antibiotic therapy . Infect Dis Clin N Am 1998; 12: 1023–34. [DOI] [PubMed] [Google Scholar]
- 6.Chapman AL, Dixon S, Andrews D, et al. Clinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): a UK perspective. J Antimicrob Chemother 2009; 64: 1316–24. [DOI] [PubMed] [Google Scholar]
- 7.Muldoon EG, Snydman DR, Penland EC, et al. Are we ready for an outpatient parenteral antimicrobial therapy bundle? A critical appraisal of the evidence. Clin Infect Dis 2013; 57: 419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman AL, Seaton RA, Cooper MA, et al. Good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults in the UK: a consensus statement. J Antimicrob Chemother 2012; 67: 1053–62. [DOI] [PubMed] [Google Scholar]
- 9.MacKenzie M, Rae N, Nathwani D. Outcomes from global adult outpatient parenteral antimicrobial therapy programmes: a review of the last decade. Int J Antimicrob Agents 2014; 43: 7–16. [DOI] [PubMed] [Google Scholar]
- 10.Allison GM, Muldoon EG, Kent DM, et al. Prediction model for 30-day hospital readmissions among patients discharged receiving outpatient parenteral antibiotic therapy. Clin Infect Dis 2014; 58: 812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials . JAMA Intern Med 2014; 174: 1095–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wieland BW, Marcantoni JR, Bommarito KM, et al. A retrospective comparison of ceftriaxone versus oxacillin for osteoarticular infections due to methicillin-susceptible Staphylococcus aureus. Clin Infect Dis 2012; 54: 585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winans SA, Luce AM, Hasbun R. Outpatient parenteral antimicrobial therapy for the treatment of methicillin-susceptible Staphylococcus aureus: a comparison of cefazolin and ceftriaxone. Infection 2013; 41: 769–74. [DOI] [PubMed] [Google Scholar]
- 14.Youngster I, Shenoy ES, Hooper DC, et al. Comparative evaluation of the tolerability of cefazolin and nafcillin for treatment of methicillin-susceptible Staphylococcus aureus infections in the outpatient setting. Clin Infect Dis 2014; 59: 369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qureshi ZA, Syed A, Doi Y. Safety and efficacy of long-term outpatient ertapenem therapy. Antimicrob Agents Chemother 2014; 58: 3437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bazaz R, Chapman AL, Winstanley TG. Ertapenem administered as outpatient parenteral antibiotic therapy for urinary tract infections caused by extended-spectrum-β-lactamase-producing gram-negative organisms. J Antimicrob Chemother 2010; 65: 1510–3. [DOI] [PubMed] [Google Scholar]
- 17.Forestier E, Gros S, Peynaud D, et al. Ertapenem administered intravenously or subcutaneously for urinary tract infections caused by ESBL producing enterobacteriacea. Med Mal Infect 2012; 42: 440–3. [DOI] [PubMed] [Google Scholar]
- 18.Goswami ND, Johnson MD, Chu VH. Ertapenem for treatment of osteomyelitis: a case series. BMC Res Notes 2011; 4: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berg ML, Crank CW, Philbrick AH, et al. Efficacy of ertapenem for consolidation therapy of extended-spectrum β-lactamase-producing gram-negative infections: a case series report . Ann Pharmacother 2008; 42: 207–12. [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–9. [DOI] [PubMed] [Google Scholar]
- 22.Robinson JL, Hameed T, Carr S. Practical aspects of choosing an antibiotic for patients with a reported allergy to an antibiotic. Clin Infect Dis 2002; 35: 26–31. [DOI] [PubMed] [Google Scholar]
- 23.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–54. [Google Scholar]
- 24.Cameron AC, Trivedi PK. Regression Analysis of Count Data. New York: Cambridge University Press, 1998. [Google Scholar]
- 25.Imai K, Ratkovic R. Covariate balancing propensity score. J R Stat Soc 2014; 76: 243–63. [Google Scholar]
- 26.Rosenbaum PR. Model-based direct adjustment. JASA 1987; 82: 387–94. [Google Scholar]
- 27.Silverstein MD, Qin H, Mercer SQ, et al. Risk factors for 30-day hospital readmission in patients ≥65 years of age. Proc (Bayl Univ Med Cent) 2008; 21: 363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol 2006; 15: 1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Choe PG, Song KH, et al. Is cefazolin inferior to nafcillin for treatment of methicillin-susceptible Staphylococcus aureus bacteremia? Antimicrob Agents Chemother 2011; 11: 5122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Echevarria KL, Hughes DW, et al. Comparison of cefazolin versus oxacillin for treatment of complicated bacteremia caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 2014; 58: 5117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donzé J, Lipsitz S, Bates DW, et al. Causes and patterns of readmissions in patients with common comorbidities: retrospective cohort study. BMJ 2013; 367: f7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blumenthal KG, Youngster I, Shenoy ES, et al. Tolerability of cefazolin after immune-mediated hypersensitivity reactions to nafcillin in the outpatient setting. Antimicrob Agents Chemother 2014; 58: 3137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cannon JP, Lee TA, Clark NM, et al. The risk of seizures among the carbapenems: a meta-analysis. J Antimicrob Chemother 2014; 69: 2043–55. [DOI] [PubMed] [Google Scholar]
- 34.Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 2011; 72: 381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duquaine S, Kitchell E, Tate T, et al. Central nervous system toxicity associated with ertapenem use. Ann Pharmacother 2011; 45: e6. [DOI] [PubMed] [Google Scholar]
- 36.Wong KK, Fraser TG, Shrestha NK, et al. Low incidence of Clostridium difficile infection (CDI) in patients treated with outpatient parenteral antimicrobial therapy (OPAT). Infect Control Hosp Epidemiol 2015; 36: 110–2. [DOI] [PubMed] [Google Scholar]
- 37.Barr DA, Semple L, Seaton RA. Outpatient parenteral antimicrobial therapy (OPAT) in a teaching hospital-based practice: a retrospective cohort study describing experience and evolution over 10 years. Int J Antimicrob Agents 2012; 39: 407–13. [DOI] [PubMed] [Google Scholar]
- 38.Owens RC, Jr, Donskey CJ, Gaynes RP, et al. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis 2008; 46 Suppl 1: S19–31. [DOI] [PubMed] [Google Scholar]
- 39.Levey AS, De Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011; 80: 17–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.