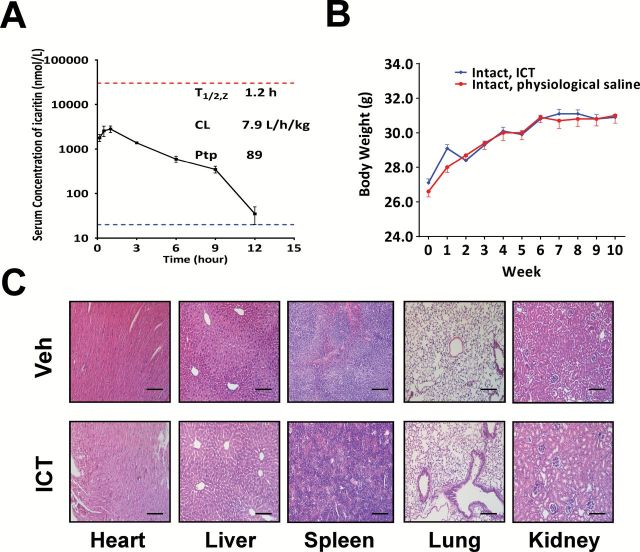
Figure. 6.
ICT has favorable pharmacokinetics and safety profiles. (A) The serum concentrations of ICT in SCID mice-bearing LNCaP tumors were quantified after intraperitoneal administration of ICT at doses of 33mg/kg. The mean serum concentrations of ICT (nmol/l) are expressed as mean ± SD (n = 2). The lower dotted line indicates the lower limit of quantitation (20 nmol/l) of ICT in the mouse serum. The upper dotted line indicates 30 µmol/l ICT, the effective concentrations associated with anti-proliferative effects. The terminal serum half-life (T 1/2, z), the serum clearance (CL) and the tissue-to-plasma partition coefficient of ICT were assessed using non-compartmental analysis with WinNonLin 6.2.1. (B) Mean body weight-time profiles were computed following treatment of intact SCID mice with intraperitoneally injected physiological saline (n = 5) or 33mg/kg ICT (n = 10) 5 times per week for 10 weeks. Bars represents mean ± SEM. (C) The figures show hematoxylin and eosin staining of representative sections (×200) of heart, liver, spleen, lung and kidney harvested at the end of the experiment from mice receiving intraperitoneal delivery of vehicle control (top) or 33mg/kg ICT (bottom). Scale bars represent 100 µm in all micrographs.