Abstract
BACKGROUND
Nocturnal blood pressure (BP) is associated with risk for cardiovascular events. However, the relationship between nocturnal BP in young adults and cognitive function in midlife remains unclear.
METHODS
We used data from the ambulatory BP monitoring substudy of the Coronary Artery Risk Development in Young Adults Study, including 224 participants (mean age 30 years, 45% men, 63% African Americans). At the 20-year follow-up, the Stroop test (executive function), Digit Symbol Substitution Test (psychomotor speed), and Rey Auditory Verbal Learning Test (verbal memory) were assessed.
RESULTS
Baseline mean office, daytime, and nocturnal BP were 109/73, 120/74, and 107/59mm Hg, respectively. Nocturnal BP dipping, calculated as (nocturnal systolic BP [SBP] − daytime SBP) × 100/daytime SBP, was divided into quartiles (Q1: −39.3% to −16.9%; Q2: −16.8% to −13.2%, Q3 [reference]: −13.1% to −7.8%, and Q4: −7.7% to +56.4%). In multiple regression analyses, the least nocturnal SBP dipping (Q4 vs. reference) and higher nocturnal diastolic BP level were associated with worse Stroop scores, with adjustments for demographic and clinical characteristics, and cumulative exposure to office BP during follow-up (β [standard error]: 0.37 [0.18] and 0.19 [0.07], respectively; all P < 0.05). Digit Symbol Substitution Test and Rey Auditory Verbal Learning Test were not significantly associated with nocturnal SBP dipping or nocturnal SBP/diastolic BP levels.
CONCLUSIONS
Among healthy young adults, less nocturnal SBP dipping and higher nocturnal diastolic BP levels were associated with lower executive function in midlife, independent of multiple measures of office BP during long-term follow-up.
Keywords: blood pressure, cognitive function, hypertension, midlife, nocturnal blood pressure, young adults.
As the population grows older worldwide, the challenge is not only to extend limits of lifespan but also to maintain normal activities of daily living in the later years of life. Cognitive dysfunction or dementia is a critical issue because it can cause a cascade of sequela such as falls, loss of independence, need for hospital care, and even cardiovascular- or noncardiovascular-related death.1 Efforts to prevent cognitive dysfunction have become a major public health goal. To do so, an understanding of the risk factors contributing to cognitive dysfunction, and identification those at younger age who may be at risk for developing cognitive dysfunction, are becoming top priorities.
The association between higher blood pressure (BP) measured in clinic/office and lower cognitive function has been well established.1–3 Additionally, ambulatory BP measures, in particular nocturnal BP abnormalities (i.e., higher absolute values and less nocturnal BP dipping), have been reported to be associated with cognitive dysfunction;4–10 most of these data were obtained in cross-sectional analyses for older populations, raising the possibility that comorbidities (e.g., cerebrovascular diseases with or without symptoms) could have affected both participants’ nocturnal BP and cognition.11,12 To our knowledge, no studies have examined whether nocturnal BP measured during young adulthood is associated with cognitive function in midlife, and the association is independent of long-term office BP values during follow-up. The Coronary Artery Risk Development in Young Adults (CARDIA) Study provides a unique opportunity to examine these issues, as CARDIA enrolled only young adults (18–30 years) with few comorbidities.
Using CARDIA data, we assessed whether nocturnal BP levels and BP dipping in young adults are associated with measures of cognitive function 20 year later in midlife, independent of long-term office BP values during follow-up.
METHODS
Overall design
The CARDIA Study is a multicenter longitudinal cohort of 5,115 young adults initially ages 18–30 years in 1985–1986 (see Supplementary Data). Black and white adults, who were healthy at enrollment, were recruited from 4 US cities and underwent baseline examination (Year 0: Y0) and follow-up examinations at Y2, Y5, Y7, Y10, Y15, Y20, and Y25. The CARDIA study and this CARDIA ambulatory blood pressure monitoring (ABPM) substudy were approved by the appropriate institutional review boards, and informed consent was obtained from each study participant.
As described previously,13 ABPM was performed in Y5 at 1 site (Birmingham, AL). A total of 316 people (147 men and 169 women; 112 whites and 204 blacks), selected randomly at the Birmingham center, completed this substudy.
BP and other measurements
The methods in office BP measures are shown in Supplementary Data. The baseline BP (Y5) and cumulative exposure of BP through Y5 to Y25 (Y5–25; mm Hg × year; the formula is shown in Supplementary Figure S1) were used as adjustment factors.
At Y5, ABPM was performed over a 24-hour period with a Suntech Accutracker II (Suntech Medical, Morrisville, NC),13 using an appropriately sized cuff inflated approximately every 20 minutes during the day (6:00 am–10:00 pm) and every 30 minutes during the night (10:00 pm–6:00 am). As done in a previous report of CARDIA ABPM,13 we defined daytime BP as average BP between 10:00 am and 10:00 pm and nocturnal BP between 12:00 am and 6:00 am. The magnitude of nocturnal BP dipping was calculated as (nocturnal SBP–daytime SBP) × 100/daytime SBP.14,15 Nocturnal BP dipping calculated by SBP is strongly correlated with that calculated by diastolic BP (DBP; r = 0.6, P < 0.0001). Most of the prior literature used SBP to calculate nocturnal BP dipping,14,15 so we did as well. We assessed “poor sleep quality” during the ABPM session as any report of being unable to fall asleep, being awakened ≥5 times, or not being able to sleep at all.13
Data on other factors including education, physical activity, and laboratory values were collected using standardized protocols and quality control across study centers and examinations (see Supplementary Data).
Cognitive function assessment
A battery of standardized tests to measure cognitive function was performed at the Y25 examination.16 Training and certification of CARDIA technicians for measurement of cognitive function were performed centrally by CARDIA investigators and coordinating center data quality assurance staff. The Stroop test evaluates the ability to view complex visual stimuli and to respond to one stimulus dimension while suppressing the response to another dimension, an executive skill largely attributed to frontal lobe function.17 The interference score provides a measure of how much additional executive processing is needed to respond to an incongruent trial; thus, a higher interference score indicates worse performance on the task. Each trial was scored by summing the number of errors and the time required to complete each trial. An interference score was calculated by subtracting the score on the incongruent trial from the second congruent trial. The Digit Symbol Substitution Test (DSST), a subtest of the Wechsler Adult Intelligence Scale (3rd edition), assesses an array of cognitive domains, most prominently visual motor speed, sustained attention, and working memory. The range of scores is 0–133, with increasing scores indicating better performance. The Rey Auditory Verbal Learning Test (RAVLT) assesses the ability to memorize and to retrieve words (verbal memory) after several presentations of the word list immediately one after another, and then after a delay of 10 minutes. Results from the long-delay (10 minutes) free recall were used in analyses. The range of scores is 0–15, with increasing scores indicating better performance.
Analysis
Among 316 participants, we excluded 14 participants with missing data on daytime BP or nocturnal BP, 60 participants who did not attend the follow-up examination at Y25, 2 participants with missing data on cognitive function, and 16 participants with missing data on covariates. As a result, we included 224 participants who attended the Y5, Y7, Y10, Y15, Y20, and Y25 examinations and completed ABPM at Y5 and cognitive tests at Y25. The included participants had a lower proportion of current smoking at Y5 (25.0 vs. 38.0 %; P = 0.02) compared with those not included (n = 92), but the mean age (30.2 vs. 29.6 years), gender distribution (55.4% vs. 48.9% women), racial distribution (63.4 vs. 67.4% African American), mean educational attainment (14.1 vs. 13.8 years), and mean office SBP/DBP levels at Y5 (108.7/73.1 vs. 110.3/73.1mm Hg; all P ≥ 0.24) were similar between the two groups. Compared with those in the entire CARDIA cohort (n = 4,891), the included participants were more likely to be African American (63.4% vs. 51.0%) but showed no differences in age, sex, or office BP levels (Supplementary Table S1).
Statistical analyses were performed using SAS software version 9.3 (SAS Institute Inc, Cary, NC). Demographic and clinical characteristics of the participants across quartiles were tested by analysis of variance or chi-squared test. Unadjusted and multivariable-adjusted linear regression models were used to assess the association of nocturnal BP with cognitive function measures. Because there is no standard definition on physiological nocturnal BP dipping in young adults, we defined Quartile 3, centered on −10% nocturnal BP dipping, as the reference group. This approach was used in a previous report of CARDIA ABPM substudy to test the association of nocturnal BP with coronary calcium.13 Our analyses were performed with sequential adjustment. In the first step, we carried out unadjusted analyses (Model 1). In the second step, we adjusted for demographic covariates including age (Y5), sex, race (black and white), and educational attainment in years (Model 2). In the last step, we further adjusted for the clinical characteristics at Y25 (i.e., body mass index, smoking, alcohol, physical activity, glucose and lipid parameters, use of antihypertensive drugs, and incidence of stroke) plus office BP at Y5 (Model 3), daytime BP at Y5 (Model 4), or cumulative exposure of office BP (Y5–25; Model 5). Statistical significance was defined by a P value of <0.05 on 2-sided tests.
RESULTS
Of the 224 participants, 45% were men, 63% were African American, their mean age was 30 years, and 0.5% were treated with antihypertensive drugs at baseline. Table 1 provides the demographic and clinical characteristics of the included participants stratified by the quartile of nocturnal SBP dipping. By definition, Quartile 1 showed the most nocturnal SBP dipping (−39.3% to −16.9%), and Quartile 4 (−7.7% to +56.4%) showed the least. The mean time of BP measures was 46±10 times in daytime and 14±2 times in nighttime. Across quartiles, age, sex, and educational attainment were similar. The proportion of African Americans was higher in the higher quartiles of nocturnal BP dipping. Supplementary Tables S2–S6 show the associations between nocturnal BP and clinical characteristics. Men, blacks, and higher body mass index were associated with higher nocturnal BP. No difference was observed in nocturnal BP between those with or without poor sleep quality. Office and ambulatory BP values across quartiles are shown in Table 2. Cumulative exposure of office SBP and DBP during follow-up was higher in Quartiles 2–4 vs. 1 of nocturnal dipping, but the trend was not statistically significant (P = 0.08).
Table 1.
Clinical characteristics of the included participants according to quartiles of nocturnal SBP dipping (n = 224)
| Quartile: magnitude of nocturnal SBP dipping | ||||||
|---|---|---|---|---|---|---|
| Total (n = 224) | Q1: −39.3% to −16.9% (n = 56) | Q2: −16.8% to −13.2% (n = 56) | Q3: −13.1% to −7.8% (n = 56) | Q4: −7.7% to +56.4% (n = 56) | P values | |
| Demographic variables at Y5 (baseline) | ||||||
| Age at Y5, years | 30.2±3.8 | 30.8±3.9 | 29.6±3.8 | 30.3±3.8 | 30.3±3.5 | 0.40 |
| Men, % | 44.6 | 41.1 | 50.0 | 35.7 | 51.8 | 0.27 |
| Blacks, % | 63.4 | 44.6 | 53.6 | 73.2 | 82.1 | <0.0001 |
| Education, years | 14.1±2.1 | 13.7±2.0 | 14.4±2.1 | 14.5±2.4 | 13.7±2.0 | 0.07 |
| Poor sleep quality during ABPM, % | 16.7 | 13.0 | 14.8 | 13.0 | 25.9 | 0.21 |
| Clinical characteristics at Y25 (follow-up) | ||||||
| Body mass index, kg/m2 | 31.9±6.9 | 30.4±6.5 | 32.1±6.4 | 32.4±7.6 | 32.9±6.9 | 0.24 |
| Current smoker, % | 17.9 | 17.9 | 8.9 | 16.1 | 28.6 | 0.06 |
| Current drinkers, % | 41.5 | 35.7 | 42.9 | 42.9 | 44.6 | 0.78 |
| Physical activity, exercise units | 250.2±230.7 | 268.1±256.9 | 283.5±237.7 | 223.4±202.9 | 225.6±222.0 | 0.41 |
| Antihypertensive medication, % | 45.1 | 39.3 | 42.9 | 50.0 | 48.2 | 0.65 |
| Incident stroke, % | 0.9 | 0 | 0 | 3.6 | 0 | 0.11 |
| Fasting glucose, mg/dl | 99.6±31.5 | 96.7±30.6 | 96.9±20.1 | 98.2±24.9 | 106.7±44.5 | 0.28 |
| Total cholesterol, mg/dl | 182.5±34.6 | 184.2±36.5 | 178.0±39.8 | 183.3±30.4 | 184.6±31.5 | 0.72 |
| High-density lipoprotein, mg/dl | 54.7±16.1 | 57.5±19.2 | 52.0±16.7 | 56.1±13.1 | 53.1±14.5 | 0.23 |
Abbreviations: ABPM, ambulatory blood pressure monitoring; Q, quartile; SBP, systolic blood pressure.
Data are expressed as the means ± standard deviation or percentage. P values were obtained by analysis of variance or chi-squared test among quartile groups. The magnitude of nocturnal SBP dipping was calculated as (nocturnal SBP–daytime SBP) × 100/daytime SBP. Poor sleep quality was defined as any report of being unable to fall asleep, being awakened ≥5 times, or not being able to sleep at all during the ABPM session. Statistical significance was defined as P < 0.05.
Table 2.
BP parameters according to quartiles of nocturnal SBP dipping (n = 224)
| Quartile: magnitude of nocturnal SBP dipping | ||||||
|---|---|---|---|---|---|---|
| Total (n = 224) | Q1: −39.3% to −16.9% (n = 56) | Q2: −16.8% to −13.2% (n = 56) | Q3: −13.1% to −7.8% (n = 56) | Q4: −7.7% to +56.4% (n = 56) | P values | |
| BP at Y5 (baseline) | ||||||
| Office SBP, mm Hg | 108.7±9.8 | 105.1±9.0 | 110.3±10.0 | 111.2±9.9 | 108.1±9.3 | 0.004 |
| Office DBP, mm Hg | 73.1±9.1 | 69.9±8.8 | 74.8±9.1 | 74.9±8.2 | 73.0±9.7 | 0.01 |
| Daytime SBP, mm Hg | 120.4±11.0 | 121.2±9.1 | 122.3±12.0 | 119.5±11.8 | 118.6±10.9 | 0.28 |
| Daytime DBP, mm Hg | 73.5±7.4 | 74.6±6.1 | 74.4±8.3 | 73.0±8.0 | 71.9±6.9 | 0.18 |
| Nocturnal SBP, mm Hg | 106.5±14.8 | 95.9±7.3 | 104.0±10.1 | 106.7±11.2 | 119.5±17.8 | <0.0001 |
| Nocturnal DBP, mm Hg | 59.3±9.3 | 54.5±5.3 | 58.5±7.0 | 59.9±7.6 | 64.2±12.8 | <0.0001 |
| BP at Y25 (follow-up) | ||||||
| Office SBP, mm Hg | 122.7±18.0 | 120.2±15.7 | 122.9±19.6 | 124.0±18.0 | 123.7±18.6 | 0.67 |
| Office DBP, mm Hg | 75.3±10.9 | 73.3±9.3 | 75.0±11.5 | 76.2±11.3 | 76.7±11.4 | 0.36 |
| Cumulative exposure of office BP through Y5–Y25 | ||||||
| Cumulative exposure of office SBP, mm Hg × year | 2285.6±241.6 | 2227.2±214.0 | 2290.4±229.5 | 2336.3±244.4 | 2288.3±268.9 | 0.12 |
| Cumulative exposure of office DBP, mm Hg × year | 1488.2±156.3 | 1441.8±149.2 | 1499.8±151.6 | 1512.0±162.4 | 1499.2±156.3 | 0.08 |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; Q, quartile; SBP, systolic blood pressure.
Data are expressed as the means ± standard deviation. P values were obtained by analysis of variance.
Among participants, mean ± standard deviation scores on the Stroop test, DSST, and RAVLT were 25.9±11.6seconds + errors (range = −21.0 to 92.0: higher score indicates worse function), 65.1±15.7 symbols (range = 8.0 to 114.0: lower score indicates worse function), 8.7±3.2 words (range = 0 to 15.0: lower score indicates worse function), respectively. Figure 1 shows mean scores of cognitive function test across quartiles of nocturnal dipping. Participants in Quartile 4 had the worst Stroop test scores, and worst DSST and RAVLT scores, whereas those in Quartile 3 showed the best Stroop and DSST scores.
Figure 1.
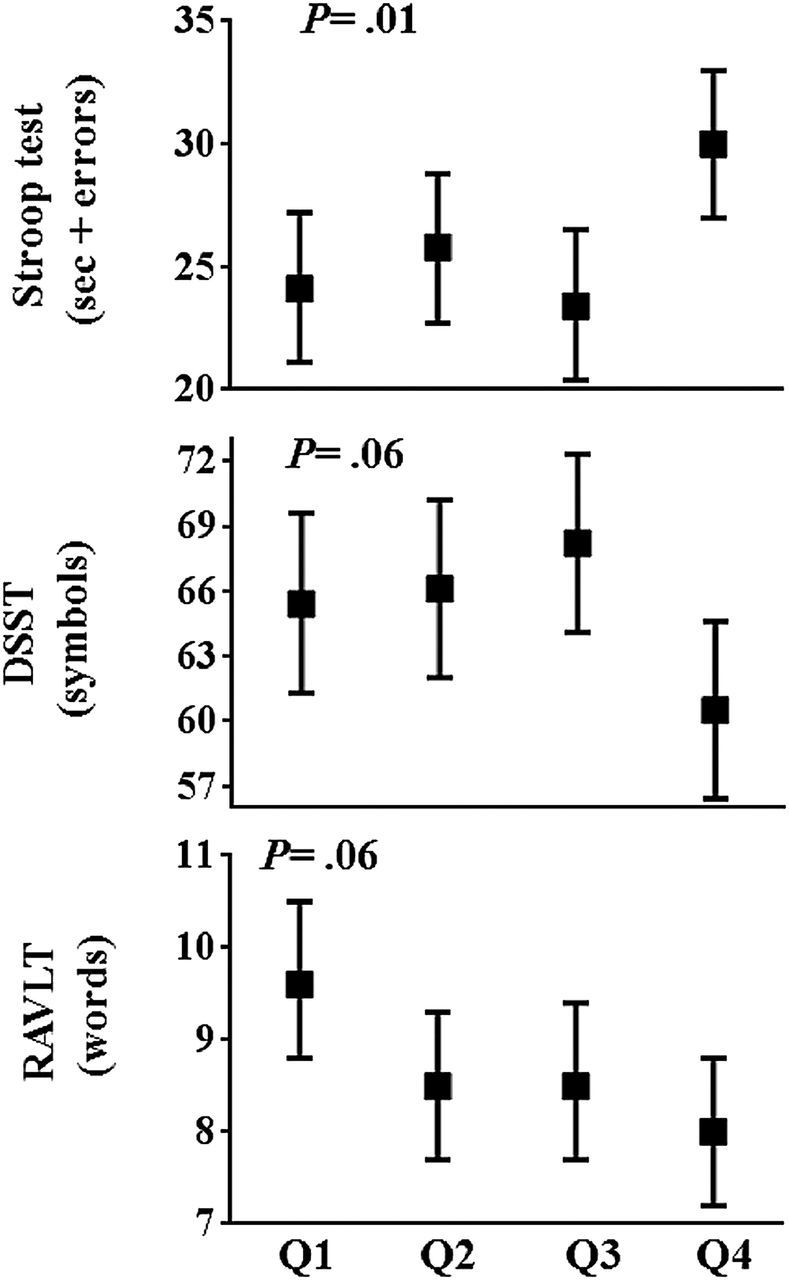
Cognitive function score according to the quartile of nocturnal SBP dipping. Mean (95% confidence intervals) of each cognitive function score according to the quartile of nocturnal SBP dipping are shown. Q1 indicates Quartile 1, and the magnitude of nocturnal BP dipping is as follows; Q1, −39.3% to −16.9% (n = 56); Q2, −16.8 to −13.2% (n = 56); Q3, −13.1% to −7.8% (n = 56); Q4, −7.7% to +56.4% (n = 56). P values were obtained by analysis of variance, and statistical significance was defined as P < 0.05. Abbreviations: BP, blood pressure; SBP, systolic blood pressure.
Table 3 shows unadjusted and multivariable-adjusted linear regression models examining the associations of nocturnal BP with cognitive function. Quartile 4 (vs. Quartile 3) was associated with worse Stroop and DSST scores (Model 1 in Table 3). Nocturnal SBP dipping, when assessed as a continuous variable, was not associated with cognitive function. Higher nocturnal DBP was associated with worse Stroop test scores, and worse DSST and RAVLT scores (Model 1 in Table 3). Adjustments for the demographic variables attenuated the associations (Model 2), but Quartile 4 (vs. Quartile 3) and higher nocturnal DBP remained significantly associated with worse Stroop test scores, with adjustments also for clinical characteristics, and office BP at Y5 (Model 3), daytime BP at Y5 (Model 4), or cumulative exposure of office BP (Y5–25; Model 5). In contrast, when the relationship of office or daytime BP to cognitive function was examined, office BP at Y5, daytime SBP or DBP at Y5, and cumulative exposure of office SBP or DBP (Y5–25) were not associated with the Stroop test, DSST, and RAVLT scores (Models 3–5 in Table 3; all P ≥ 0.09). When we adjusted for the presence of poor sleep quality during ABPM as covariates, the overall findings were similar (Supplementary Table S7). When we used clinical characteristics at Y5 instead of those at Y25 as adjusted factors, the results were mostly similar (Supplementary Table S8).
Table 3.
Associations of nocturnal BP in young adults with cognitive function in midlife: Unadjusted and multivariable-adjusted linear regression models (n = 224)
| Model 1 (unadjusted) | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (SE) | R 2, % | β (SE) | R 2, % | β (SE) | R 2, % | β (SE) | R 2, % | β (SE) | R 2, % | |
| Stroop test (seconds + errors) | ||||||||||
| Nocturnal SBP dipping | ||||||||||
| Q1 | 0.06 (0.19) | 3.6 | 0.11 (0.18) | 12.5 | 0.15 (0.19) | 17.9 | NA | NA | 0.12 (0.18) | 17.8 |
| Q2 | 0.20 (0.19) | 0.26 (0.18) | 0.24 (0.18) | NA | 0.24 (0.18) | |||||
| Q3 (reference) | Ref | Ref | Ref | NA | Ref | |||||
| Q4 | 0.57 (0.19)** | 0.41 (0.18)* | 0.38 (0.18)* | NA | 0.37 (0.18)* | |||||
| Nocturnal SBP dipping, % | 0.12 (0.07) | 1.1 | 0.07 (0.06) | 11.2 | 0.05 (0.06) | 17.1 | NA | NA | 0.05 (0.07) | 17.1 |
| Nocturnal SBP, mm Hg | 0.16 (0.07)* | 2.0 | 0.07 (0.07) | 11.5 | 0.04 (0.07) | 17.0 | 0.06 (.08) | 16.5 | 0.04 (0.07) | 17.0 |
| Nocturnal DBP, mm Hg | 0.30 (0.06)*** | 8.6 | 0.22 (0.06) *** | 15.7 | 0.20 (0.07)** | 20.2 | 0.21 (0.08)* | 20.1 | 0.19 (0.07)** | 20.6 |
| DSST (symbols) | ||||||||||
| Nocturnal SBP dipping | ||||||||||
| Q1 | −0.18 (0.19) | 2.0 | −0.18 (0.18) | 17.9 | −0.16 (0.19) | 18.6 | NA | NA | −0.21 (0.18) | 19.7 |
| Q2 | −0.13 (0.19) | −0.15 (0.18) | −0.16 (0.18) | NA | −0.16 (0.18) | |||||
| Q3 (reference) | Ref | Ref | Ref | NA | Ref | |||||
| Q4 | −0.49 (0.19)** | −0.26 (0.18) | −0.25 (0.18) | NA | −0.31 (0.18) | |||||
| Nocturnal SBP dipping, % | −0.06 (0.07) | 0.4 | −0.01 (0.06) | 17.8 | −0.002 (0.06) | 18.6 | NA | NA | −0.01 (0.06) | 19.3 |
| Nocturnal SBP, mm Hg | −0.12 (0.07) | 1.1 | −0.01 (0.07) | 17.8 | 0.001 (0.07) | 18.6 | 0.01 (0.08) | 18.5 | 0.03 (0.07) | 19.4 |
| Nocturnal DBP, mm Hg | −0.15 (0.07)* | 1.9 | −0.05 (0.06) | 18.0 | −0.03 (0.08) | 18.7 | 0.003 (0.08) | 18.6 | −0.005 (0.07) | 18.7 |
| RAVLT (words) | ||||||||||
| Nocturnal SBP dipping | ||||||||||
| Q1 | 0.34 (0.19) | 2.0 | 0.29 (0.18) | 14.3 | 0.30 (0.19) | 15.1 | NA | NA | 0.29 (0.19) | 15.1 |
| Q2 | −0.01 (0.19) | 0.002 (0.18) | 0.01 (0.18) | NA | 0.01 (0.18) | |||||
| Q3 (reference) | Ref | Ref | Ref | NA | Ref | |||||
| Q4 | −0.16 (0.19) | 0.05 (0.18) | 0.05 (0.19) | NA | 0.05 (0.19) | |||||
| Nocturnal SBP dipping, % | −0.17 (0.07)* | 2.4 | −0.11 (0.06) | 14.8 | −0.11 (0.07) | 15.7 | NA | NA | −0.11 (0.07) | 15.8 |
| Nocturnal SBP, mm Hg | −0.20 (0.07)** | 3.6 | −0.07 (0.07) | 14.2 | −0.07 (0.07) | 14.9 | −0.12 (0.08) | 15.8 | −0.07 (0.07) | 15.0 |
| Nocturnal DBP, mm Hg | −0.22 (0.07)** | 4.3 | −0.12 (0.06) | 15.2 | −0.10 (0.08) | 15.7 | −0.18 (0.08)* | 16.2 | −0.14 (0.07) | 15.7 |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; DSST, Digit Symbol Substitution Test; NA, not applicable; Q, quartile; RAVLT, Rey Auditory Verbal Learning Test; SBP, systolic blood pressure; SE, standard error.
Bold values suggest significant associations. β means standardized regression coefficient, and R 2 means a measure for the model prediction. Quartile of nocturnal SBP dipping consists of Q1 (−39.3% to −16.9%), Q2 (−16.8% to −13.2%), Q3 (−13.1% to −7.8%), and Q4 (−7.7% to +56.4%). A higher score of the Stroop test indicates worse performance on the task, and a lower score of DSST and RAVLT indicates worse performance on the task. As adjusted factors, each model includes some covariates as follows: Model 2 includes demographic variables (age at baseline, sex, race, and education); Model 3 includes demographic variables + clinical characteristics at Y25 (body mass index, smoking, drinking, physical activity, fasting glucose, total cholesterol/high-density lipoprotein, use of antihypertensive drugs, incidence of stroke) + office BP at Y5; Model 4 includes demographic variables + clinical characteristics at Y25 + daytime BP at Y5; Model 5 included demographic variables + clinical characteristics at Y25 + cumulative exposure of office BP (Y5–25); statistical significance was defined as P < 0.05. *P < 0.05; **P < 0.01; ***P < 0.001.
There were no significant interactions between race and any of the nocturnal BP variables (nocturnal SBP dipping, nocturnal SBP levels, or nocturnal DBP levels) in associations with the Stroop test, DSST, and RAVLT scores (all P ≥ 0.15).
DISCUSSION
Among healthy young adults (mean age 30 years and mean office BP 109/73mm Hg), we show that nocturnal SBP dipping less than 7.7% (compared with that between 13.1% and 7.8%) and higher nocturnal DBP levels were associated with lower executive function (i.e., Stroop test) 20 years later in midlife. The associations were independent of multiple measures of office BP during long-term follow-up.
The finding in this study of a stronger association of nocturnal BP than office or daytime BP with cognitive function raises questions about mechanisms. Some plausible, albeit speculative, explanations are suggested. First, cerebrovascular responsiveness is reduced during sleep, and cerebral blood flow decreases by approximately 30% at least during nonrapid eye movement stages.18,19 The neurovascular unit may be particularly vulnerable to BP burden during sleep.20 Second, in terms of physical and mental activity as well as body position, the nocturnal BP compared with office or daytime BP is better standardized without being influenced by environmental stimuli.14,15 Third, nocturnal BP compared with office or daytime BP could reflect concurrent pathophysiology, such as sympathovagal imbalance, volume retention, impaired salt excretion, and disturbed breathing during sleep.21–25 Obstructive sleep apnea in particular could affect both nocturnal BP increase and cognitive dysfunction via nocturnal hypoxia and resultant sympathetic nerve activation.24,25 Nocturnal BP dipping is further affected by sporadic factors, such as physical activity during daytime and orthostatic BP changes.14,15 From our data, whether less nocturnal SBP dipping and higher nocturnal DBP in young adults are simply markers of concurrent pathophysiology or related in a causal pathway to pathogenesis in cognitive dysfunction remains uncertain and promotes us to further explore whether improving nocturnal BP abnormalities in young adults can prevent lower cognitive function in those middle-aged or over.
We found that nocturnal SBP was not associated with cognitive function after multivariable adjustment. The relative importance of DBP and SBP to cardiovascular disease risk differs with aging; DBP is more predictive for cardiovascular disease events than SBP in younger adults.26 Among adolescents ages 7–20 years, higher 24-hour DBP rather than 24-hour SBP, even within the normotensive range, was associated with impaired cerebrovascular reactivity.27 Our findings may complement the data, by showing a prospective association of higher nocturnal DBP in young adulthood with lower cognitive function in midlife.
Nocturnal SBP dipping and nocturnal DBP level were associated with Stroop score, a test reflecting white matter integrity in the frontal lobe,17,28 but not with RAVLT and DSST scores. Our sample size was small, so the null associations of the RAVLT and DSST tests might be a consequence of lack of power. However, the associations of higher BP with white matter disease have been shown to be greater for the frontal lobe than other areas.2,20,29 The exact mechanism of the regional specificity remains unclear, but the type and size of vessels, myogenic reactivity, vasodilator capacity, neurovascular units, and innervation of the cerebral blood vessels may vary by brain location.3,20 Different components of ambulatory BP are known to affect different parts of cerebral structure and function.4,8,10 Among older individuals, higher nocturnal SBP and less nocturnal SBP dipping were specifically associated with frontal lobe atrophy and subsequently lower executive function.8,10 Further investigations using brain magnetic resonance imaging may allow us to understand the underlying pathways (e.g., vascular dysfunction, brain atrophy, or impaired brain integrity) between higher nocturnal BP and cognitive dysfunction, and whether brain regional specificity exists in relation to nocturnal BP.
In our study, 82% of those with the least nocturnal BP dipping (i.e., Quartile 4) were African Americans. African Americans are known to have higher nocturnal BP and less dipping compared with whites.30–32 The mechanisms remain uncertain, but salt sensitivity, abnormal autonomic function, socioeconomic factors, and psychological factors have each been associated with altered diurnal BP variation14,15 and are more common in African Americans compared with whites.21,30,32–36 Our sample size was not large enough to allow robust race-specific analyses, but we observed no significant interactions between race and any of the nocturnal BP variables in association with cognitive function.
The major strengths of this study include long-term, repeated examinations of well-characterized participants from young adulthood to middle age and application of a comprehensive standardized cognitive test battery. However, there are limitations. First, we could not assess changes in cognitive function from baseline to follow-up, and we cannot conclude whether the low cognitive function scores reflect cognitive decline. Further CARDIA examinations including cognitive function would enable us to explore the time course of cognitive function in participants with abnormal nocturnal BP. Second, although our findings were statistically significant, whether nocturnal BP measurement in young adults, particularly in those who had normal office BP with minimum cardiovascular risks, is clinically meaningful remains uncertain. Further investigations are warranted, including analyses of the cost-effectiveness, availability, and invasiveness (e.g., with respect to sleep disturbances, discomfort, and restrictions in daily activities) of ABPM, as well as analyses identifying clinical characteristics (e.g., risk factors) in which ABPM is recommended. Third, we have only a single measurement of ABPM, and the reproducibility was limited. Some participants might have had sleep deprivation during the overnight BP monitoring. We adjusted for sleep quality and the results were similar. Also, it can be difficult to discern the transition from daytime to nocturnal BP measurements (i.e., bedtime), though several studies similarly used fixed clock-time intervals.14,15 Fourth, a number of participants from the original cohort were excluded, so there is a potential selection bias. Fifth, we did not assess what classes of antihypertensive drug were used in this study, and some of these drugs can affect cognitive function.37 Finally, our sample consists of black and white adults, so our findings cannot be generalized to other race/ethnic groups.
Among healthy young adults, less nocturnal SBP dipping and higher nocturnal DBP were associated with lower executive function 20 years later in midlife. Our results may help to identify young adults at risk for developing lower cognitive function in midlife. Replications in different studies/cohorts with larger samples size are warranted. Continued follow-up in CARDIA may allow us to discern the association of nocturnal BP in young adulthood with aging-related cognitive decline and dementia through older age. Such evidence may help to direct potential strategies for preventing lower cognitive function in those middle-aged or over.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
ACKNOWLEDGMENTS
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN26 8201300027C, HHSN268201300028C, HHSN2682013 00029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005). Y.Y. received the Manpei Suzuki International Prize for Diabetes Research Grants and AHA Strategically Focused Research Network (SFRN) Fellow Grant. A.J.V. has a research grant from the National Heart, Lung, and Blood Institute (R01 HL098604) to study ambulatory blood pressure monitoring. He has also served on the medical advisory board of Suntech Medical, manufacturer of a brand of ambulatory blood pressure monitor. D.A.C. received research support from the NIH (P30DK092926 and K23AG040278). None of the other authors has any potential conflict of interest to disclose.
REFERENCES
- 1. Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011; 42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, Carmichael O, Wolf PA, DeCarli C. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol 2012; 11:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalaria RN. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke 2012; 43:2526–2534. [DOI] [PubMed] [Google Scholar]
- 4. Goldstein IB, Bartzokis G, Guthrie D, Shapiro D. Ambulatory blood pressure and the brain: a 5-year follow-up. Neurology 2005; 64:1846–1852. [DOI] [PubMed] [Google Scholar]
- 5. White WB, Wolfson L, Wakefield DB, Hall CB, Campbell P, Moscufo N, Schmidt J, Kaplan RF, Pearlson G, Guttmann CR. Average daily blood pressure, not office blood pressure, is associated with progression of cerebrovascular disease and cognitive decline in older people. Circulation 2011; 124:2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J Hypertens 2008; 26:1636–1641. [DOI] [PubMed] [Google Scholar]
- 7. Yano Y, Inokuchi T, Hoshide S, Kanemaru Y, Shimada K, Kario K. Association of poor physical function and cognitive dysfunction with high nocturnal blood pressure level in treated elderly hypertensive patients. Am J Hypertens 2011; 24:285–291. [DOI] [PubMed] [Google Scholar]
- 8. Celle S, Annweiler C, Pichot V, Bartha R, Barthélémy JC, Roche F, Beauchet O. Association between ambulatory 24-hour blood pressure levels and brain volume reduction: a cross-sectional elderly population-based study. Hypertension 2012; 60:1324–1331. [DOI] [PubMed] [Google Scholar]
- 9. Birns J, Morris R, Jarosz J, Markus H, Kalra L. The structural and functional consequences of diurnal variations in blood pressure in treated patients with hypertensive cerebrovascular disease. J Hypertens 2009; 27:1042–1048. [DOI] [PubMed] [Google Scholar]
- 10. Hajjar I, Zhao P, Alsop D, Abduljalil A, Selim M, Novak P, Novak V. Association of blood pressure elevation and nocturnal dipping with brain atrophy, perfusion and functional measures in stroke and nonstroke individuals. Am J Hypertens 2010; 23:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamamoto Y, Akiguchi I, Oiwa K, Satoi H, Kimura J. Diminished nocturnal blood pressure decline and lesion site in cerebrovascular disease. Stroke 1995; 26:829–833. [DOI] [PubMed] [Google Scholar]
- 12. Goldstein IB, Bartzokis G, Hance DB, Shapiro D. Relationship between blood pressure and subcortical lesions in healthy elderly people. Stroke 1998; 29:765–772. [DOI] [PubMed] [Google Scholar]
- 13. Viera AJ, Lin FC, Hinderliter AL, Shimbo D, Person SD, Pletcher MJ, Jacobs DR., Jr Nighttime blood pressure dipping in young adults and coronary artery calcium 10-15 years later: the coronary artery risk development in young adults study. Hypertension 2012; 59:1157–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yano Y, Kario K. Nocturnal blood pressure and cardiovascular disease: a review of recent advances. Hypertens Res 2012; 35:695–701. [DOI] [PubMed] [Google Scholar]
- 15. Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension 2011; 57:3–10. [DOI] [PubMed] [Google Scholar]
- 16. Yano Y, Ning H, Allen N, Reis JP, Launer LJ, Liu K, Yaffe K, Greenland P, Lloyd-Jones DM. Long-term blood pressure variability throughout young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Hypertension. 2014;64:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull 1991; 109:163–203. [DOI] [PubMed] [Google Scholar]
- 18. Sakai F, Meyer JS, Karacan I, Derman S, Yamamoto M. Normal human sleep: regional cerebral hemodynamics. Ann Neurol 1980; 7:471–478. [DOI] [PubMed] [Google Scholar]
- 19. Mohsenin V. Sleep-related breathing disorders and risk of stroke. Stroke 2001; 32:1271–1278. [DOI] [PubMed] [Google Scholar]
- 20. Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension 2013; 62:810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higashi Y, Oshima T, Ozono R, Nakano Y, Matsuura H, Kambe M, Kajiyama G. Nocturnal decline in blood pressure is attenuated by NaCl loading in salt-sensitive patients with essential hypertension: noninvasive 24-hour ambulatory blood pressure monitoring. Hypertension 1997; 30:163–167. [DOI] [PubMed] [Google Scholar]
- 22. Grassi G, Bombelli M, Seravalle G, Dell’Oro R, Quarti-Trevano F. Diurnal blood pressure variation and sympathetic activity. Hypertens Res 2010; 33:381–385. [DOI] [PubMed] [Google Scholar]
- 23. de la Sierra A, Gorostidi M, Banegas JR, Segura J, de la Cruz JJ, Ruilope LM. Nocturnal hypertension or nondipping: which is better associated with the cardiovascular risk profile? Am J Hypertens 2014; 27:680–687. [DOI] [PubMed] [Google Scholar]
- 24. Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011; 306:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kapa S, Sert Kuniyoshi FH, Somers VK. Sleep apnea and hypertension: interactions and implications for management. Hypertension 2008; 51:605–608. [DOI] [PubMed] [Google Scholar]
- 26. Franklin SS. The importance of diastolic blood pressure in predicting cardiovascular risk. J Am Soc Hypertens 2007; 1:82–93. [DOI] [PubMed] [Google Scholar]
- 27. Wong LJ, Kupferman JC, Prohovnik I, Kirkham FJ, Goodman S, Paterno K, Sharma M, Brosgol Y, Pavlakis SG. Hypertension impairs vascular reactivity in the pediatric brain. Stroke 2011; 42:1834–1838. [DOI] [PubMed] [Google Scholar]
- 28. Wolf D, Zschutschke L, Scheurich A, Schmitz F, Lieb K, Tüscher O, Fellgiebel A. Age-related increases in Stroop interference: delineation of general slowing based on behavioral and white matter analyses. Hum Brain Mapp 2014; 35:2448–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci 2003; 117:1169–1180. [DOI] [PubMed] [Google Scholar]
- 30. Profant J, Dimsdale JE. Race and diurnal blood pressure patterns. A review and meta-analysis. Hypertension 1999; 33:1099–1104. [DOI] [PubMed] [Google Scholar]
- 31. Spruill TM, Gerin W, Ogedegbe G, Burg M, Schwartz JE, Pickering TG. Socioeconomic and psychosocial factors mediate race differences in nocturnal blood pressure dipping. Am J Hypertens 2009; 22:637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muntner P, Lewis CE, Diaz KM, Carson AP, Kim Y, Calhoun D, Yano Y, Viera AJ, Shimbo D. Racial differences in abnormal ambulatory blood pressure monitoring measures: results from the coronary artery risk development in young adults (CARDIA) study. Am J Hypertens 2014; e-pub ahead of print 4 November 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harshfield GA, Alpert BS, Pulliam DA, Willey ES, Somes GW, Stapelton FB. Sodium excretion and racial differences in ambulatory blood pressure patterns. Hypertension 1991; 18:813–818. [DOI] [PubMed] [Google Scholar]
- 34. Rodriguez CJ, Jin Z, Schwartz JE, Turner-Lloveras D, Sacco RL, Di Tullio MR, Homma S. Socioeconomic status, psychosocial factors, race and nocturnal blood pressure dipping in a Hispanic cohort. Am J Hypertens 2013; 26:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adefurin A, Ghimire LV, Kohli U, Muszkat M, Sofowora GG, Paranjape SY, Stein CM, Kurnik D. Blacks have a greater sensitivity to α1-adrenoceptor-mediated venoconstriction compared with whites. Hypertension 2013; 61:915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Flack JM, Sica DA, Bakris G, Brown AL, Ferdinand KC, Grimm RH, Jr, Hall WD, Jones WE, Kountz DS, Lea JP, Nasser S, Nesbitt SD, Saunders E, Scisney-Matlock M, Jamerson KA; International Society on Hypertension in Blacks. Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension 2010; 56:780–800. [DOI] [PubMed] [Google Scholar]
- 37. Levi Marpillat N, Macquin-Mavier I, Tropeano AI, Bachoud-Levi AC, Maison P. Antihypertensive classes, cognitive decline and incidence of dementia: a network meta-analysis. J Hypertens 2013; 31:1073–1082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


