Abstract
Introduction:
Smoking cessation pharmacotherapy is underutilized by people with mental illnesses, who smoke at high rates and die prematurely of smoking-related diseases. Educational outreach can improve prescribing, but distances impede widespread use of this practice. Little research has assessed whether videoconference can effectively deliver educational outreach. We conducted a randomized, controlled trial of in-person versus videoconference educational outreach for smoking cessation pharmacotherapy across a state mental health system.
Methods:
We randomly assigned clinics to receive in-person or videoconference educational outreach with audit and feedback for cessation pharmacotherapy. Prescribers completed brief questionnaires before and after the intervention. With segmented regression analysis of interrupted time series, we evaluated prescribing trends in Medicaid pharmacy claims for nicotine replacement therapy (NRT) and varenicline, with interaction terms for the effect of intervention type (in-person vs. videoconference).
Results:
With interaction terms in the model, filled NRT prescriptions increased after the intervention compared to before (p < .01). The pattern of fills after the intervention were different at centers receiving in-person compared to videoconference educational outreach (p < .02) without clearly favoring one over the other. Additionally, filled varenicline prescriptions increased after the intervention compared to before (p = .04), but type of intervention delivery did not influence varenicline fills. Prescriber satisfaction with the educational intervention was high and prescriber attitudes became more positive in both groups.
Conclusion:
This study suggests that single session educational outreach with audit and feedback can increase cessation pharmacotherapy utilization, and that videoconference delivery could be an effective, scalable approach to improve workforce capacity in systems serving mentally ill smokers.
Introduction
People with mental illness are much more likely to smoke than those without mental illness. The most recent national survey assessing mental illness diagnoses reported current smoking rates ranging from 34.3% to 59.1%, with the highest rates of smoking among those with schizophrenia and the lowest among those without mental illness (18.3%).1 A recent national survey confirmed that rates of smoking are double to triple in people with mental illnesses compared to those without (30.5% in those with severe mental illnesses, 20.0% in those with any mental illness, and 13.5% in those without mental illness).2 Further, this study showed the same pattern in every region of the country, as well as in large metro, small metro and nonmetro settings, and also across demographic groups (gender, race, educational level, and income level). People with severe mental illnesses such as schizophrenia experience disparate early morbidity and mortality due to smoking-related diseases.3–16 This group is therefore an important target for prevention and cessation treatment efforts.
Smoking cessation pharmacotherapy is effective but is underutilized, especially by smokers with mental illnesses,17–21 even though medication combined with behavioral treatment has been shown to dramatically improve their treatment outcomes.22,23 Psychiatric providers are ideally positioned to treat patients who smoke for nicotine dependence during routine office visits. However, psychiatrists and psychiatric nurse practitioners generally lack basic training regarding evidence-based treatment for nicotine dependence, and, in addition to knowledge and skills barriers, they may harbor attitudinal barriers to treating nicotine dependence.24 Effective and practical strategies are needed to ensure that the mental health workforce has the necessary knowledge and skills to address nicotine dependence among smokers with mental illness.
Educational outreach (also termed academic detailing or counter-detailing) uses brief visits by respected peers, such as physicians or clinical pharmacists, to provide physicians information regarding evidence-based prescribing or other clinical interventions, including the risks, benefits, and costs of alternate treatment options.25 The term is used to describe educational visits by noncommercial entities, in contrast to drug company detailing conducted by pharmaceutical companies. Compared to conventional strategies such as conferences, group lectures and mailed materials, educational outreach is one of the few interventions that consistently improves physician performance.25 A meta-analysis of 69 studies of educational outreach found an increase in compliance with desired practices (adjusted risk difference of 4.8%).26 In addition to educational outreach, providing clinicians with data regarding how their own prescribing compares to peers and/or guidelines results in modest improvements.27 Known as audit and feedback, this approach has the advantage of providing personalized information and is acceptable to clinicians.28 Educators can utilize audit and feedback to increase the impact of the educational outreach.
One challenge to the broad application of educational outreach is the distances separating the educator from physicians. Videoconference is commonly used to allow people separated by distance to meet for a variety of purposes. Randomized, controlled trials have shown that videoconference classes can be effective in medical education.29 Several30–32 but not all33 reports have suggested that videoconference-delivered education is satisfactory to clinicians. However, little research has evaluated whether educational outreach can be effectively provided via videoconference.34 As educational outreach relies on the interaction between the educator and the practicing physician, it is not known whether educational outreach via videoconference can result in changes in prescribing. To address this knowledge gap, we conducted a randomized trial comparing videoconference to in-person educational outreach with audit and feedback for smoking cessation pharmacotherapy within a state community mental health system. We hypothesized that (a) the intervention would increase receipt of smoking cessation pharmacotherapy, and (b) in-person delivery of the intervention would be more effective than videoconference delivery. We examined filled Medicaid pharmacotherapy claims as the primary outcome. In addition, we assessed self-reported prescriber satisfaction, knowledge, attitudes and beliefs as the secondary outcomes.
Methods
Procedures
We randomly assigned the prescribers grouped within all of New Hampshire’s 10 community mental health centers (CMHCs) to receive group in-person or videoconference educational outreach with audit and feedback for smoking cessation pharmacotherapy. We used a computer-generated randomization table using groups of two, with site assignment blocked by CMHC location in urban area greater than 80,000 people. Consenting prescribers completed knowledge and attitudes questions before and after the intervention. Analyses assessed changes in knowledge, attitudes and prescribing trends before and after the intervention and evaluated whether outcomes differed at videoconference compared to in-person sites. The study was reviewed and approved by the Dartmouth Institutional Review Board. Research procedures were in compliance with the Declaration of Helsinki.
Setting and Context
Approximately 85 prescribers in 10 private, nonprofit mental health centers provided mental health care in the state. A total of 14,615 adult Medicaid recipients with mental illness received care at these mental health centers for a period of at least 6 months during the year prior to the intervention and were included in the pharmacotherapy analyses. In this state, the Medicaid insurance policy covered nicotine replacement therapy (NRT) by prescription without copayment or prior authorization, whereas the Medicaid insurance covered varenicline only if prior authorization was obtained and indicated other cessation treatments had failed. Buproprion was available without limits but was not evaluated in this study due to the ubiquitous use of this medication to treat mood disorders. Smoking cessation counseling was available through a state Quitline that did not offer NRT during the study period. Medicaid recipients could also access an incentive program for tobacco education and cessation treatment. In this program, small cash incentives were used to facilitate treatment involvement. Smokers who signed up for the program received $20 to interact with a web-based motivational decision support system. If they then decided they wanted cessation treatment, they could obtain $5–20 for talking with their prescriber about cessation medication, calling the state quit line, and/or using telephone cognitive behavioral therapy. The incentive program is ongoing. During the 2 years of this study, about 400 recipients had enrolled. We evaluated outcomes for the educational outreach study reported here both including and excluding the recipients in the incentive program (described in statistical analyses).
Intervention
Educators were experienced psychiatrists who provided each CMHC a single, 50-minute interactive lecture with slides and handouts. The session was completed during a regularly scheduled CMHC administrative meeting and continuing medical education credit was provided. Topics included facts about nicotine dependence in people with mental illness, nicotine withdrawal, and methods for prescribing evidence-based pharmacotherapy to smokers with mental illness disabilities (“educational outreach”). Educators also presented each group of CMHC prescribers with a chart of recent Medicaid claims data on amount of cessation pharmacotherapy prescribing by each CMHC (“audit and feedback”). Prescribers were encouraged to treat their patients’ nicotine dependence. After 6 months, prescribers received an email that showed them the amount of prescribing by center, and identified the top prescriber in the state mental health system.
At the five centers randomly assigned to in-person education, each group of providers met with the educator in-person, whereas prescribers at the five centers randomly assigned to videoconference intervention met with the educator via videoconference. For videoconference centers, the informed consent information sheet, printed slides, handouts and questionnaires were sent to the videoconference center via email in advance and printed by CMCH staff. Completed questionnaires were faxed back to the educator. All sessions included all components of the education with audit and feedback intervention. Educational materials were left for prescribers who did not attend the educational session.
Assessments
We obtained data from prescribers and from the state Medicaid claims database.
Prescriber Data
Consenting prescribers completed questionnaires with information about demographics and smoking history developed for this study. Before and after the intervention, they answered 10 additional questions: four items assessing knowledge and six items assessing attitudes/beliefs about treating nicotine dependence in people with mental illnesses. No identifying information was obtained.
Medicaid Claims Data
We obtained all state patient-level Medicaid medical and pharmaceutical claims for years 2011, 2012 and the first nine months of 2013. Further claims were not obtained due to a change in the Medicaid claims data acquisition and management processes as well as a transition to managed care insurance, both of which were expected to impair claim quality for a period of time. We defined our study cohort as Medicaid recipients with at least two community mental health center rehabilitation service claims separated by 6 months in a given year, indicating they were receiving longitudinal services for severe mental illness. The database provided age, gender and diagnosis code. Smoking status was not available. Claims for NRT and varenicline prescriptions filled by recipients served at the CMHCs each year were linked and analyses proceeded with de-identified claims.
Statistical Analyses
We evaluated prescribing in Medicaid pharmacy claims for NRT and varenicline separately. To prepare data for analyses, study cohort monthly pharmacy claims for varenicline and NRT agents were aligned according to the time of intervention at each CMHC. Medication claims were aggregated as monthly proportions of individuals with at least one cessation medication fill per 1,000 individuals served in a given month. The study covered a 21-month study period, 1 year prior to and nine months after the intervention (month 13 corresponded to the beginning of the post-intervention period). Use of a proportion prevented fluctuations due to changes in the overall number of people served in the system.
To evaluate the longitudinal effect of the intervention, we applied segmented regression analysis of interrupted time series data.35–37 This method is ideal for evaluating the impact of policy changes or interventions across an entire system when outcomes are collected regularly over time. In this method the data are usually graphed first to visually display the impact of an intervention both immediately and over time. Analysts then specify the model that includes level and time trend before intervention, and change in level and time trend after intervention to assess immediate intervention effect (level change right after intervention), and gradual intervention effect (slope or time trend change in post intervention period).
Following this method, we first visually inspected the prescribing trends (slope of change in fills per month over time) in the 12 months before, directly after the intervention (level), and over the nine months after the intervention. The trend change is the difference in the slope of monthly prescription fills after the intervention compared with the slope before the intervention. We tested our models for autocorrelation using Durbin–Watson tests and did not find autocorrelations. We then used segmented regression models to compare prescribing level and trends for NRT and varenicline separately in the 12 months before and the nine months after the intervention. Next, we evaluated the effect of intervention type (in-person vs. videoconference) on trend change by including interaction terms in the model. In order to assess whether the statewide cessation treatment incentive program influenced prescribing outcomes, we repeated the segmented regressions excluding all recipients who participated in that program (N = 411).
We evaluated whether knowledge and attitudes changed after the intervention compared to before using McNemar’s and paired t tests. Prescriber satisfaction with the educational intervention, improvement in knowledge, and attitudes about prescribing were compared for prescribers receiving in-person versus videoconference sessions by chi-square tests and independent sample t tests. Data management and analyses were performed using SAS 9.3 (SAS Institute Inc.).
Results
Subjects
Overall, 14,502 adult Medicaid recipients using community mental health services were followed during the 21-month study period. Basic characteristics of Medicaid recipients are shown in Table 1. This cohort of Medicaid recipients was on average 42.5±14.9 years old and 36.7% male. All had mental illness diagnoses attached to their claims: 21.7% schizophrenia and other psychotic disorders, 16.7% bipolar disorders, 34.2% depressive disorders, 16.5% anxiety disorders, and 11.1% other disorders. Although the claims database did not provide any other demographic information, monthly income must be less than $590 to be eligible for the Medicaid benefit.
Table 1.
Characteristics of Prescribers and Medicaid Recipients Before Interventiona
| Prescribers in community mental health centers | ||
|---|---|---|
| Video (n = 25) | In-person (n = 18) | |
| Gender (% male) | 10 (38%) | 6 (35%) |
| Age under 55 | 11 (44%) | 9 (50%) |
| Adult Medicaid recipients with paid claims for rehabilitation services | ||
|---|---|---|
| Video (n = 8,250) | In-person (n = 6,365) | |
| Gender (% male) | 37.22% | 35.81% |
| Mean age (SD)* | 41.85 (14.34) | 43.37 (15.44) |
| Mental health diagnosis* | ||
| Schizophrenia | 22.16% | 20.97% |
| Bipolar | 14.70% | 19.40% |
| Depression | 33.03% | 35.62% |
| Anxiety | 18.99% | 13.18% |
| Substance abuse | 2.11% | 1.79% |
| Personality disorder | 2.28% | 0.58% |
| Other | 6.73% | 8.45% |
| Proportion filling smoking cessation pharmacotherapy (past year) | ||
| Varenicline | 1.02% | 0.88% |
| NRT | 5.84% | 5.95% |
| Varenicline and NRT | 0.35% | 0.41% |
| None | 92.79% | 92.76% |
Note. NRT = nicotine replacement therapy.
aThree prescribers did not provide demographic information.
*p < .0001.
Cessation Medication Utilization
The monthly proportions of individuals filling cessation pharmacotherapy prescriptions at in-person and videoconference CMHCs are shown in Figure 1A and 1B. Visual inspection of filled prescriptions graphs (Figure 1A and 1B) indicate shifts in the trends (the slopes of the monthly filled medications) indicating an increase in medication fills after the intervention compared to before for both NRT and varenicline. There was a rapid increase in NRT fills for the in-person group after the intervention from about 13th month to 14th month, then the trend slope tended to slightly negative, but there did not appear to be persisting differences between in-person and videoconference centers for NRT fills. Varenicline fills were declining before the intervention; after the intervention fills then declined more slowly, indicating a relative increase in prescribing after the intervention compared to before.
Figure 1.
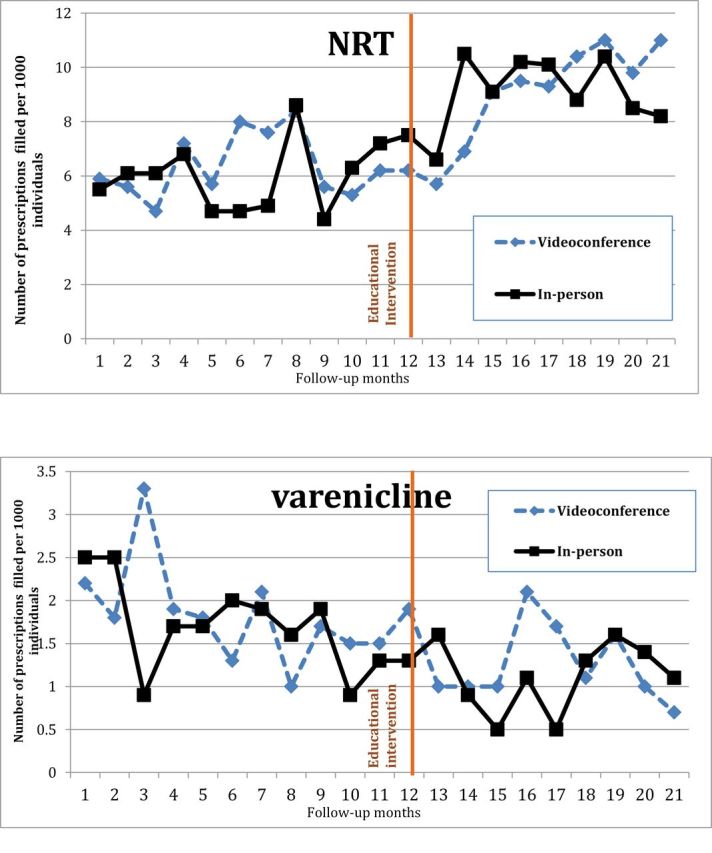
Individuals per 1,000 who filled a prescription 12 months before and 9 months after a 50-minute educational intervention. (A) Filled nicotine replacement therapy (NRT) prescriptions. (B) Filled varenicline prescriptions.
Based on model fit, we report the results for the multivariate segmented regression model with interaction terms for NRT prescriptions, and the main effect model for varenicline prescriptions in Table 2 (as recommended, interaction terms for varenicline were not presented in the Table because they were not statistically significant). In the initial main effect segmented regression model, filled NRT prescriptions increased prior to the intervention and the trend did not change after the intervention (corresponding graph not shown). When interaction terms were added, the model showed that the time trend for filled NRT prescriptions in the videoconference group increased after the intervention compared to before (p < .01), indicating an effect of the intervention. In terms of statistical testing, the post-intervention trend (or slope) for in-person group is negative relative to video-conference group, (p = .02), representing the flattening of the slope after the initial surge in NRT prescription fills.
Table 2.
Monthly Trends in Nicotine Replacement Therapy (NRT) and Varencline-Filled Prescriptions Before and After In-Person or Videoconference Educational Outreach With Audit and Feedback for Prescribersa
| NRT | Coefficients | Standard error | t | p value |
|---|---|---|---|---|
| Pre-intervention level (intercept) | 6.0803 | 0.74966 | 8.11 | <.0001 |
| Pre-intervention monthly trend | 0.0441 | 0.10186 | 0.43 | .6681 |
| Level of change immediately after intervention | −0.3034 | 1.10478 | −0.27 | .7853 |
| Trend change after the intervention | 0.5326 | 0.18736 | 2.84 | .0075 |
| Intervention type (in-person vs. videoconference) | −0.7682 | 1.06018 | −0.72 | .4737 |
| Difference in pre-intervention monthly trend between intervention groups | 0.0720 | 0.14405 | 0.5 | .6203 |
| Difference in level of change immediately after intervention between intervention groups | 2.6205 | 1.56239 | 1.68 | .1027 |
| Difference in trend change after the intervention between videoconference and in-person groups | −0.6220 | 0.26496 | −2.35 | .0249 |
| R 2 = 0.69* | ||||
| Varenicline | Coefficients | Standard error | t | p value |
|---|---|---|---|---|
| Pre-intervention level (intercept) | 2.28333 | 0.16616 | 13.74 | <.0001 |
| Pre-intervention monthly trend | −0.08077 | 0.02258 | −3.58 | .0023 |
| Level of change after intervention | −0.17521 | 0.24488 | −0.72 | .484 |
| Trend change after the intervention | 0.09077 | 0.04153 | 2.19 | .0431 |
| R 2 = 0.68* | ||||
Note. aReference group includes recipients at centers receiving videoconference intervention. *p < .05
In the initial main effect segmented regression model for varenicline (Table 2), the overall trend for filled varenicline prescriptions declined prior to intervention. After the intervention this trend changed significantly (p = .04) and filled varenicline prescriptions still declined, but at a slower rate, indicating a positive effect for the educational intervention. Type of intervention delivery did not influence varenicline prescription fills, again indicating no advantage for in-person over videoconference delivery.
In order to assess whether the presence of the statewide incentive program impacted the results, we repeated the analyses, excluding the 411 Medicaid recipients who had enrolled in the statewide tobacco incentive program. The analyses results were similar. We therefore report the graphs and analyses for the entire group only.
Prescribers
All 46 prescribers (psychiatrists and advanced nurse practitioners) who attended the educational session consented and completed pre- and post-intervention questionnaires; 63% were female and 47.8% were under 55 years of age (Table 1). Over a third (38.6%) had smoked cigarettes in the past, one (2%) smoked currently.
Prescriber Satisfaction, Knowledge, Attitudes, and Beliefs
Prescribers’ overall satisfaction with the educational session was high (90% were satisfied or very satisfied), and over 90% found the session useful. Satisfaction ratings were not statistically significantly different between prescribers who received in-person and videoconference educational outreach.
Prescriber’s attitudes, beliefs and knowledge assessed before and after the educational intervention are reported in Table 3. Answers to most items indicated that prescribers’ attitudes/beliefs became, on average, significantly more positive about smoking cessation treatment for people with mental illness. However, prescribers’ ability to apply the new knowledge after the intervention (assessed by four vignettes) did not increase. The post-intervention knowledge and attitudes among prescribers receiving the intervention in-person were not statistically different from those of prescribers who received the intervention via videoconference.
Table 3.
Effect of Education Intervention on Prescribers’ Smoking Cessation Attitudes, Beliefs and Knowledge
| N = 46 | Pre-education | Post-education | p value |
|---|---|---|---|
| Mean (SD) agreement score for attitudes/beliefs (1–5)a | |||
| Most people with mental illness do not want to quit smoking | 2.5 (1.0) | 2.0 (0.9) | <.0001 |
| Cessation treatment is best managed by primary care doctors | 2.3 (0.8) | 1.8 (0.6) | .0007 |
| Providing smoking cessation treatment is outside of my scope of practice | 2.1 (1.1) | 2.0 (1.1) | .44 |
| State Medicaid insurance covers all smoking cessation medications | 2.7 (0.8) | 3.8 (1.1) | <.0001 |
| Smoking cessation medication improves cessation outcomes | 3.9 (0.7) | 4.5 (0.7) | .0002 |
| Use of behavioral treatment improves cessation outcomes | 4.3 (0.7) | 4.5 (0.7) | .19 |
| Number (%) with correct answer to knowledge vignettes | |||
| Vignette 1 | 31 (67.4) | 23 (50.0) | .12 |
| Vignette 2 | 34 (73.9) | 40 (87.0) | .07 |
| Vignette 3 | 11 (23.9) | 19 (41.3) | .14 |
| Vignette 4 | 28 (60.9) | 36 (78.3) | .12 |
Note. a1 = strongly disagree, 5 = strongly agree.
Discussion
In the study we report here, a single session of group educational outreach with audit and feedback to mental health prescribers in a statewide community mental health system was associated with changes in cessation pharmacotherapy utilization trends among Medicaid recipients with severe mental illness, suggesting a relative increase in prescribing. These data suggest that tailored brief educational interventions may be able to expand delivery of pharmacotherapy in such settings. This is key, because pharmacotherapy is an important component of evidence-based cessation treatment for this population.38
To our knowledge, this is the first randomized trial comparing videoconference to in-person educational outreach with audit and feedback aimed at improving prescribing practices in a state mental health system serving people with severe mental illnesses. Similar to at least one previous feasibility report,34 clinicians were satisfied with the technology-delivered educational intervention. In this study, prescribing outcomes were not consistently different among patients whose clinicians who received the intervention in-person compared to those whose clinicians received it via videoconference. These data suggest that videoconference technology can be used for educational outreach and has the potential to increase the reach while also reducing the cost of evidence-based interventions aiming to change physician behavior.
This finding is especially important in the context of the rapid growth of videoconferencing and telehealth to deliver care and provide education in rural regions,39 but the adoption of its use for administrative and training purposes is unknown. Private companies now market real time Internet with video communications (e.g., Webex and Adobe Connect). One randomized study of a single session intervention delivered in-person or via webinar using Adobe Connect (Adobe Systems Incorporated) demonstrated that there was no difference in effectiveness between delivery via Adobe Connect and the in-person delivery, and they both appeared to be satisfactory to clinicians.40
Two factors related to prescribing to disadvantaged smokers with mental illness (e.g., those with low education, income or employment) could have influenced our findings. First, this study addressed smokers with mental illness disabilities whose income is typically quite low, such that these smokers typically do not feel they can afford to buy NRT over the counter in pharmacies. Encouraging prescribers to provide medication prescriptions potentially increases accessibility of the medication to low-income groups. Provision of NRT through Quitlines could have the same effect, but the local Quitline did not provide NRT during the intervention period. Second, varenicline is available by prescription only. Multiple studies have demonstrated its safety and efficacy in smokers with mental illness, but this medication is more likely to cause side effects and may be reserved for smokers who have failed other approaches. Notably, the smokers in the mental health system studied here did increase utilization of this medication as well as NRT after the single session intervention with prescribers. About half of the prescribers in the system attended the session. Better attendance could have resulted in a more robust change in prescribing practices.
Our findings contrast with two other recent studies of single session educational interventions. In a controlled trial of a 50-minute educational session for primary care physicians, cessation pharmacotherapy prescribing did not increase in the education group compared with the control group and also did not increase in either group after the intervention compared to before.41 In a pre-post study of a 1-hour educational session with audit and feedback for inpatient hospitalists, patient acceptance of NRT did not increase although there was an increase in documentation of offered treatment.42 Several features of educational outreach with audit and feedback as implemented in this study may be important. First, our intervention included audit and feedback provided by a mental health authority, a potentially powerful endorsement of provider behavior change. Second, in the intervention studied here, educators spent 20 minutes reviewing practical instructions for how to prescribe cessation pharmacotherapy to people with mental illness, which was our target behavioral outcome. Third, the educators trained prescribers together with their CMHC peers, who may have been able to provide support to and/or competition with each other for behavior change, whereas the primary care physician training in the previous study included only one physician in the entire practice.41 While the study reported here was not designed to provide information about effective ingredients of the intervention, previous research suggests that audit and feedback, tailored information, and group education all have strengths.
Several limitations require discussion. This study did not evaluate any patient-level smoking and quitting behaviors. However, since use of evidence-based pharmacotherapy is a key strategy for cessation in this group, the ability of the intervention to increase filling of prescriptions is an important outcome for this disparity group with poor health outcomes. Further, the intervention was delivered across an entire state mental health system and our primary outcome, a filled prescription, is a highly valid outcome measure for the behavior of all prescribers in the system. Second, a Medicaid-funded incentive program was implemented during the study period. While our study results remained significant when we excluded recipients in that program, the implementation of the program could have influenced prescribers. Future studies could evaluate the educational intervention implemented here with and without broader cessation programs. Nevertheless, since both the patients at the in-person and the videoconference CMHCs could access the incentive program, we do not believe the presence of the incentive program deterred our ability to test the efficacy of videoconference delivery of the intervention. Further, we evaluated a brief intervention consisting of a single 50-minute educational session coupled with audit and feedback on prescribing. While brief interventions such as the one studied here are practical, acceptable and less costly, lengthier training might be associated with greater skills acquisition and more robust outcomes. A previous study of a 6-hour training for health professionals did result in a greater quit rate than no training.43 Further, higher participation or a more robust intervention may have enabled us to see potential small differences between videoconference and in-person delivery of the intervention that we were not able to detect in this study. Finally, we tracked outcomes in Medicaid claims, evaluating changes in trends for filled smoking cessation medications. Impact on people with other insurances or no insurance is not known.
Educational outreach with audit and feedback is a well-studied strategy to change physician behavior. It is not known how commonly this strategy is used to improve or maintain tobacco treatment quality in public mental health and addiction treatment systems. New York State has broadly implemented a quality improvement system for integrated treatment of co-occurring mental illness and addiction that utilizes a web-based educational strategy in addition to teleconferences.44 Further examination of optimal implementation strategies, implementation costs, and the facilitators and barriers of implementing videoconference educational interventions is needed.
This study of group educational outreach with audit and feedback demonstrated increased smoking cessation pharmacotherapy utilization in Medicaid recipients with severe mental illnesses compared to before the intervention. Our finding that the in-person intervention was not better than videoconference educational outreach with audit and feedback suggests that videoconference could be a practical and scalable approach to broad implementation of educational programs with the potential to improve workforce capacity to help disadvantaged smokers. Further research is needed to replicate these findings, test other technology-enhanced educational strategies to change physician behavior, and to evaluate optimal implementation strategies.
Funding
This work was supported by the New Hampshire Department of Health and Human Services and by R01 MH 089811 to SB from the National Institute of Mental Health.
Declaration of Interests
None declared.
References
- 1. McClave AK, McKnight-Eily LR, Davis SP, Dube SR. Smoking characteristics of adults with selected lifetime mental illnesses: results from the 2007 National Health Interview Survey. Am J Public Health. 2010;100:2464–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glasheen C, Hedden SL, Forman-Hoffman VL, Colpe LJ. Cigarette smoking behaviors among adults with serious mental illness in a nationally representative sample. Ann Epidemiol. 2014;24:776–780. [DOI] [PubMed] [Google Scholar]
- 3. Birkenaes AB, Opjordsmoen S, Brunborg C, et al. The level of cardiovascular risk factors in bipolar disorder equals that of schizophrenia: a comparative study. J Clin Psychiatry. 2007;68:917–923. [DOI] [PubMed] [Google Scholar]
- 4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212–217. [DOI] [PubMed] [Google Scholar]
- 5. Carney CP, Jones LE. Medical comorbidity in women and men with bipolar disorders: a population-based controlled study. Psychosom Med. 2006;68:684–691. [DOI] [PubMed] [Google Scholar]
- 6. Chwastiak LA, Rosenheck RA, McEvoy JP, et al. The impact of obesity on health care costs among persons with schizophrenia. Gen Hosp Psychiatry. 2009;31:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3:A42. [PMC free article] [PubMed] [Google Scholar]
- 8. Dickey B. Medical morbidity, mental illness, and substance use disorders. Psychiatr Serv. 2002;53:861–867. [DOI] [PubMed] [Google Scholar]
- 9. Filik R, Sipos A, Kehoe PG, et al. The cardiovascular and respiratory health of people with schizophrenia. Acta Psychiatr Scand. 2006;113:298–305. [DOI] [PubMed] [Google Scholar]
- 10. Himelhoch S, Lehman A, Kreyenbuhl J, Daumit G, Brown C, Dixon L. Prevalence of chronic obstructive pulmonary disease among those with serious mental illness. Am J Psychiatry. 2004;161:2317–2319. [DOI] [PubMed] [Google Scholar]
- 11. Hoang U, Stewart R, Goldacre MJ. Mortality after hospital discharge for people with schizophrenia or bipolar disorder: retrospective study of linked English hospital episode statistics, 1999–2006. BMJ. 2011;343:d5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones DR, Macias C, Barreira PJ, Fisher WH, Hargreaves WA, Harding CM. Prevalence, severity, and co-occurrence of chronic physical health problems of persons with serious mental illness. Psychiatr Serv. 2004;55:1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mauer B. Morbidity and mortality in people with serious mental illness. In: Parks J, Svendsen D, Singer P, Foti ME. eds. Technical Reports. Alexandria, VA: National Association of State Mental Health Program Directors, Medical Directors Council; 2006. [Google Scholar]
- 14. Miller BJ, Paschall CB, III, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv. 2006;57:1482–1487. [DOI] [PubMed] [Google Scholar]
- 15. Sokal J, Messias E, Dickerson FB, et al. Comorbidity of medical illnesses among adults with serious mental illness who are receiving community psychiatric services. J Nerv Ment Dis. 2004;192:421–427. [DOI] [PubMed] [Google Scholar]
- 16. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374:620–627. [DOI] [PubMed] [Google Scholar]
- 17. Davis K, Brunette MF, Vorhies V, Ferron JC, Whitley R. A qualitative study of how individuals with severe mental illness assess smoking risks. Mental Health and Substance Abuse: Dual Diagnosis. 2010;3:110–123. [Google Scholar]
- 18. Dixon L, Medoff DR, Wohlheiter K, et al. Correlates of severity of smoking among persons with severe mental illness. Am J Addict. 2007;16:101–110. [DOI] [PubMed] [Google Scholar]
- 19. Lucksted A, Dixon L, Sembly JB. A focus group pilot study of tobacco smoking among psychosocial rehabilitation clients. Psychiatr Serv. 2000;51:1544–1548. [DOI] [PubMed] [Google Scholar]
- 20. Mann-Wrobel MC, Bennett ME, Weiner EE, Buchanan RW, Ball MP. Smoking history and motivation to quit in smokers with schizophrenia in a smoking cessation program. Schizophr Res. 2011;126:277–283. [DOI] [PubMed] [Google Scholar]
- 21. Morris CD, Waxmonsky JA, May MG. What do persons with mental illnesses need to quit smoking? Mental health consumer and provider perspectives. Psychiatr Rehabil J. 2009;32:276–284. [DOI] [PubMed] [Google Scholar]
- 22. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams JM, Steinberg ML, Zimmermann MH, et al. Comparison of two intensities of tobacco dependence counseling in schizophrenia and schizoaffective disorder. J Subst Abuse Treat. 2010;38:384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tong EK, Strouse R, Hall J, Kovac M, Schroeder SA. National survey of U.S. health professionals’ smoking prevalence, cessation practices, and beliefs. Nicotine Tob Res. 2010;12:724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soumerai SB. Principles and uses of academic detailing to improve the management of psychiatric disorders. Int J Psychiatry Med. 1998;28:81–96. [DOI] [PubMed] [Google Scholar]
- 26. O’Brien MA, Rogers S, Jamtvedt G, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2007;4:CD000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivers N, Jamtvedt G, Flottorp, et al. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2006. 2012;6:CD000259. doi:10.1002/14651858.CD000259.pub3 [DOI] [PubMed] [Google Scholar]
- 28. Swartz SH, Cowan TM, DePue J, Goldstein MG. Academic profiling of tobacco-related performance measures in primary care. Nicotine Tob Res. 2002;4(suppl 1):S38–44. [DOI] [PubMed] [Google Scholar]
- 29. Chipps J, Brysiewicz P, Mars M. A systematic review of the effectiveness of videoconference-based tele-education for medical and nursing education. Worldviews on Evidence-Based Nursing, 2012;9:78–87. [DOI] [PubMed] [Google Scholar]
- 30. de Godoy S, Mendes IAC, Hayashida M, Nogueira MS, Alves LMM. In-service nursing education delivered by videoconference. J Telemed Telecare. 2004;10:303–305. [DOI] [PubMed] [Google Scholar]
- 31. Fitzgerald A, Bailey M, Smith AC, et al. Child development services: a multidisciplinary approach to professional education via videoconference. J Telemed Telecare. 2002;8(suppl 3):S3:19–S3:21. [DOI] [PubMed] [Google Scholar]
- 32. Greenwood J, Williams R. Continuing professional development for Australian rural psychiatrists by videoconference. Australas Psychiatry. 2008;16:273–276. [DOI] [PubMed] [Google Scholar]
- 33. Kroeker K. Residency training via videoconference—satisfaction survey. Telemed J E Health. 2000;6:425–428. [DOI] [PubMed] [Google Scholar]
- 34. Hartung DM, Hamer A, Middleton L, Haxby D, Fagnan LJ. A pilot study evaluating alternative approaches of academic detailing in rural family practice clinics. BMC Fam Pract. 2012;13129. 10.1186/1471-2296-13-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taljaard M, McKenzie JE, Ramsay CR, Grimshaw JM. The use of segmented regression in analysing interrupted time series studies: an example in pre-hospital ambulance care. Implement Sci. 2014;9. 10.1186/1748-5908-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. [DOI] [PubMed] [Google Scholar]
- 37. Zuckerman IH, Lee E, Wutoh AK, Xue Z, Stuart B. Application of regression-discontinuity analysis in pharmaceutical health services research. Health Serv Res. 2006;41:550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferron JC, Alterman AI, McHugo GJ, Brunette MF, Drake RE. A review of research on smoking cessation interventions for adults with schizophrenia spectrum disorder. Mental Health and Substance Abuse: Dual Diagnosis. 2009;2:65–80. [Google Scholar]
- 39. Kvedar J, Coye MJ, Everett W. Connected health: a review of technologies and strategies to improve patient care with telemedicine and telehealth. Health Aff (Millwood). 2014;33:194–199. [DOI] [PubMed] [Google Scholar]
- 40. Gilkey MB, Moss JL, Roberts AJ, Dayton AM, Grimshaw AH, Brewer NT. Comparing in-person and webinar delivery of an immunization quality improvement program: a process evaluation of the adolescent AFIX trial. Implement Sci. 2014;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Verbiest ME, Crone MR, Scharloo M, et al. One-hour training for general practitioners in reducing the implementation gap of smoking cessation care: a cluster-randomized controlled trial. Nicotine Tob Res. 2014;16:1–10. [DOI] [PubMed] [Google Scholar]
- 42. Kisuule F, Necochea A, Howe EE, Wright S. Utilizing audit and feedback to improve hospitalists’ performance in tobacco dependence counseling. Nicotine Tob Res. 2010;12:797–800. [DOI] [PubMed] [Google Scholar]
- 43. Olano-Espinosa E, Matilla-Pardo B, Minué C, Antón E, Gómez-Gascón T, Ayesta FJ. Effectiveness of a health professional training program for treatment of tobacco addiction. Nicotine Tob Res. 2013;15:1682–1689. [DOI] [PubMed] [Google Scholar]
- 44. Covell NH, Margolies PJ, Smith MF, Merrens MR, Essock SM. Distance training and implementation supports to scale up integrated treatment for people with co-occurring mental heatlh and substance use disorders. J Dual Diagn. 2011;7:162–172. [Google Scholar]


