Abstract
Multiple myeloma (MM) in patients aged greater than 80 years poses an increasingly common challenge for oncology providers. A multidisciplinary workshop was held in which MM-focused hematologists/oncologists, geriatricians, and associated health-care team members discussed the state of research for MM therapy, as well as themes from geriatric medicine that pertain directly to this patient population. A summary statement of our discussions is presented here, in which we highlight several topics. MM disproportionately affects senior adults, and demographic trends indicate that this trend will accelerate. Complex issues impact cancer in seniors, and although factors such as social environment, comorbidities, and frailty have been well characterized in nononcological geriatric medicine, these themes have been inadequately explored in cancers such as MM, despite their clear relevance to this field. Therapeutically, novel agents have improved survival for MM patients of all ages, but less so for seniors than younger patients for a variety of reasons. Lastly, both MM- and treatment-related symptoms and toxicities require special attention in senior adults. Existing research provides limited insight into how best to manage these often complex patients, who are often not reflected in typical clinical trial populations. We hence offer suggestions for clinical trials that address knowledge gaps in how to manage very old and/or frail patients with MM, given the complicated issues that often surround this patient population.
Multiple myeloma (MM) is an increasingly treatable yet incurable malignancy of plasma cells. It affects people of all ages, but the median age at diagnosis is 70 years, making it predominantly a disease of senior adults. As the population ages worldwide, issues related to advanced age will increasingly become a required component of MM research and clinical care.
From September 27 to 28, 2012, the Institute for Advanced Studies in Aging and Geriatric Medicine (IASIA) convened a working group of 14 participants in Reston, Virginia, to discuss MM issues relevant to patients aged 80 years and older (very old people with MM [VOP-MM]). IASIA is a nonprofit organization that focuses on education and research related to geriatric patients, particularly those with cancer. The panel was comprised of academic geriatricians and geriatric oncologists, MM-specialized medical oncologists (some with specific interest in MM in seniors), pharmaceutical representatives, and nurses. Our aim was to discuss the state of research and clinical care for VOP-MM, to highlight gaps in knowledge, to develop a research agenda designed to fill those gaps, to stimulate the generation of collaborative research, and eventually to develop evidence-based clinical guidelines for caring for VOP-MM. What follows is a summary of the group’s discussion and recommendations to the broader oncology and geriatric community.
MM Demographics
MM is becoming progressively more prevalent in seniors. Census data unmistakably demonstrate that the number of older people in the United States is rising (1). Worldwide, the number of people aged 80 years and older is growing at the highest rate among any age group relative to the general population (2). Furthermore, the prevalence of MM precursor conditions such as monoclonal gammopathy of undetermined significance and MM itself are associated directly with age (Figure 1). Recent data show that roughly 33% of MM patients are aged 75 years or older (3,4), and projections indicate that the number of VOP-MM will rise strikingly over the next two decades.
Figure 1.
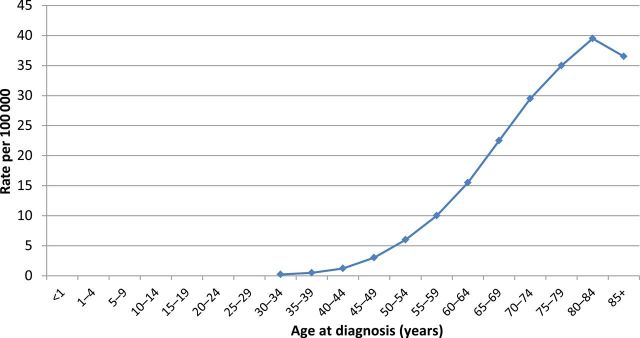
Age-specific (crude) incidence of multiple myeloma. Based on Surveillance Epidemiology and End Results database report of the incidence of multiple myeloma by age.
Perspectives From Geriatric Medicine
Geriatric medicine is highly relevant to oncology and particularly MM. Older adults with MM often have other chronic health problems and are likely to be receiving multiple medications that may influence their cancer treatment plan. Cultural beliefs, comorbidities, limited access to care, and ageism marginalize many and present barriers to appropriate health care. In patients with multiple health and psychosocial issues, the mismatch between the specialty-focused, acute-care orientation of today’s health-care system and the more holistic, chronic, multidisciplinary care needs of complex, senior patients is glaringly obvious and leads to insufficient and often ineffective care.
Despite the prevalence of cancer in seniors, we know surprisingly little about the effect of advanced age on the course of MM and its treatment. The majority of US cancer patients receive their care in community settings, where practice patterns are difficult to capture. In addition, although 3% of cancer patients are treated on clinical trials, an even lower percentage of older patients enroll in trials: 1.3% of patients aged between 65 and 74 years and 0.5% of patients aged 75 years or older (5). As a result, data on optimal management of VOP-MM remain incomplete.
Decision-Making
Deciding how aggressively to treat MM in a senior patient is a clinical dilemma that requires a thorough understanding of the indications and possible outcomes for the medical interventions being considered (6). Decision-making must be informed by knowledge of the natural history of MM, an estimate of life expectancy with and without MM, and an understanding of how MM and its therapy could affect a patient’s life in terms of symptoms, toxicities, and overall quality of life.
As for treatment choices, it is important to clarify the goals of treatment at the outset. In addition to the usual primary aims of maximizing response and survival, seniors are likely to have expectations related to quality of life and especially their physical and intellectual independence. They may also have goals related to their family or friends.
Finally, while being mindful of medical recommendations, seniors and their family members must consider the additional treatment-associated burdens, such as financial costs and time spent in clinic, against a realistic assessment of the potential benefits that might be derived from that treatment. The discussion of treatment should include all alternatives, including palliative and complementary approaches and hospice care. Physicians are duty-bound to help the patient and, when appropriate, the patient’s family by appreciating the possible benefits and limitations of all available treatment options beyond standard chemotherapy.
Cause of Death: MM or Coexisting Morbidity
One major consideration is whether MM will end the patient’s life prematurely. To answer this important question requires a thorough understanding of the interplay between age and cancer. The natural history and unique biology of an individual patient’s cancer, comorbidities, and social environment are all relevant factors. Chronological age is one determinant that impacts cancer and its treatment, but it is not always the most reliable one. Although younger and older MM patients clearly differ in many ways, including in their overall life expectancy and their ability to respond to and tolerate therapy, heterogeneity is a hallmark of geriatric medicine, and some older patients tolerate therapy with minimal toxicity. In fact, predicting toxicity is the very focus of much of geriatric oncology; balancing expected benefits and risks of cancer treatment in light of the complex interactions between cancer and aging. The ultimate aim is to optimize an MM-management approach (which may or may not include chemotherapy) to achieve the best possible outcome for VOP-MM. That outcome includes survival, but only as one of several factors to consider.
Effect of Age on Patients
Despite advanced age, men and women who are relatively well will have a life expectancy measured in years. The average 80-year-old woman is likely to live another 9 years, and an 85 year-old is likely to live another 6 to 7 years (Figure 2). Even an unwell 75-year-old man will probably live 5 more years—long enough to experience cancer-related morbidity and mortality (7).
Figure 2.
Upper, middle, and lower quartiles of life expectancy for women (A) and men (B) at selected ages. Adapted from Walter and Covinsky (7).
Hence although comorbidity and functional limitations disproportionately affect older adults, advanced age alone should not be the sole determinant of how and when to use life-prolonging or palliative anticancer treatment (8,9). Population data are instructive, but for an individual, age alone is not reliable in estimating life expectancy, functional reserve, or risk of treatment complications (10). More relevant definitions include biological and functional limitations that increasingly (but variably) are associated with advanced age. Biological correlates of longevity include sarcopenia, body mass index, elevated serum inflammatory cytokine levels, and decreased glomerular filtration rate (11–15).
Frailty and Comprehensive Geriatric Assessment
Frailty is a recognized syndrome most prevalent in old age, for which one (of several) operational definitions includes the identification of deficiencies in three or more of the following domains: 1) unintentional weight loss (10 pounds or more in the last year), 2) self-reported exhaustion, 3) weakness (grip strength), 4) slow walking speed, and 5) low physical activity (16). The impact of cancer on frailty and the value of systematically defining the frail phenotype for patients with MM has yet to be determined, but in geriatric medicine, frail individuals are recognized to have decreased resistance to stressors and increased vulnerability to adverse outcomes, including falls, disability, hospitalization, and early mortality (16). In the future, it may be possible to assess frailty with biomarkers; inflammatory cytokines such as interleukin 6, for instance, are associated with the presence of the determinants of the frailty phenotype (12,17).
Frailty provides a useful conceptual framework for VOP-MM, particularly because once identified it may be possible to move frail patients into the “prefrail” or “nonfrail” categories through pharmacologic or other interventions. Nonpharmacologic interventions are currently under development within senior adult oncology programs that engage multidisciplinary teams of oncologists, geriatricians, psychiatrists, pharmacists, physiatrists, social workers, and dieticians to identify and reduce the stressors that lead to frailty and limit effective cancer treatment (18).
Multidisciplinary teams often use some form of the comprehensive geriatric assessment (CGA) to clinically assess frailty and related variables, some of which are listed in Table 1. The identification of comorbidities is paramount because a heavy burden of coexisting disease increases mortality in cancer patients (8). This effect is independent of functional status and appears to have very little impact on the behavior of the cancer itself. However, studies show that comorbidity has an adverse impact on treatment toxicity and outcomes (19).
Table 1.
Examples of common components of comprehensive geriatric assessment instruments
| Functional status |
| Comorbities |
| Cognition |
| Socioeconomic issues |
| Nutritional status |
| Polypharmacy |
| Risk of falls |
| Finances |
Growing evidence demonstrates that the variables examined in a CGA can predict morbidity and mortality in older patients with cancer and uncover problems relevant to cancer care that would otherwise go unrecognized (20). The International Society of Geriatric Oncology has recommended CGA as an essential component for improving cancer care and possibly survival in older cancer patients (21). Although the best form of the CGA in cancer patients remains to be defined, three promising and clinically feasible CGA tools have recently been published that predict severe chemotherapy toxicity (22–24). This new generation of CGA is now being tested systematically in MM (eg, clinicaltrials.gov trials NCT01729338 and NCT01782963). Information collected through CGAs conceivably could assist therapeutic decision-making in MM more effectively than rudimentary age cutoffs.
Effect of Age on MM
Neoplasia primarily occurs in older people, and both its incidence and prevalence increase remarkably with each decade of adult life. Yet there remains a notion that some forms of cancer—primarily solid tumors—exhibit less aggressive behavior in the very old (25,26). Unfortunately, that observation has proven irrelevant for most hematological malignancies. As an example, acute myeloid leukemia, clearly has more aggressive biology in the elderly, manifest as a higher prevalence of preexisting myelodysplasia and high-risk cytogenetics (27). Diffuse large B-cell lymphoma is similarly higher risk in the elderly. In fact, age greater than 60 years is a poor-prognosis marker in the International Prognostic Index (28). Hence more, not less, aggressive disease biology is often the pattern in geriatric malignant hematology. This is compounded in some diseases by seniors’ inability to tolerate the most effective therapies. In acute myeloid leukemia, for example, curative treatment includes highly intensive chemotherapy regimens such as cytarabine and an anthracycline—an approach for which most older patients are not candidates (29). With regard to MM, however, the impact of advanced age on survival, biology, and therapy selection is more mixed.
Recent studies have demonstrated improved survival in MM across all ages, but survival is shorter in older MM patients in general, and the recent gains seem to have benefitted younger more than older patients (30–32). For example, an analysis of the Surveillance Epidemiology and End Results database revealed that 5-year overall survival (OS) improved by 7.5% for all MM patients between 1998 and 2002 and 2003 and 2007, but the gain for patients aged 75 years and older was only 3.3% (Figure 3) (3). The largest epidemiological studies demonstrate that age correlates with poorer outcomes (33), even in the most recent studies incorporating novel agents (34). Arguing to the contrary, age was excluded from the standard prognostic model for MM, the International Staging System, in favor of other more powerful predictors of survival (35). Additionally, one recently presented abstract suggests that improvements in survival in MM may preferentially be benefiting older patients (36), although those data are not yet available in published form. Despite those reports, the balance of available data generally supports the fact that survival in older MM patients is inferior to survival in younger ones and that recent improvements in survival have primarily benefited the young.
Figure 3.
Five-year relative survival by patient age and chronological year at diagnosis. Based on Surveillance Epidemiology and End Results database-reported survival of multiple myeloma patients.
To what degree poorer survival in senior patients is impacted by disease biology vs inability to receive intensive therapies is unclear in MM. Regarding the former, one MM study showed that chromosomal hyperdiploidy, a standard risk (ie, not high risk) finding, is more common in elderly than younger MM patients (37). A separate study showed that t(4;14) [historically a high-risk prognostic marker, now likely less so if bortezomib is used (38)] is less common in older MM patients (39). On the other hand, other published data suggest that favorable International Staging System prognostic stages are more common in younger patients (32). Whether the latter point is simply a consequence of purely age-related impairment in renal function and/or hypoalbuminemia or a true reflection of more aggressive MM biology in seniors is unknown.
As for therapy intensity, unlike in acute myeloid leukemia, in which the dichotomy between curative and palliative therapies is clear and the few curative approaches are generally unavailable to seniors, the intensity of MM therapies falls along a more continuous spectrum, so VOP-MM may have numerous, albeit less intensive, therapeutic options. Although most physicians agree that older patients should be treated less intensively, there is no consensus gold standard for treatment for any MM patients, including VOP-MM. Therefore, the impact of reduced therapy intensity on survival in VOP-MM is difficult to discern. That said, a somewhat clearer distinction can be made for one particular intensive therapy—namely, high-dose chemotherapy with autologous hematopoietic stem cell transplantation. It was particularly evident before the advent of novel therapies that autologous transplant improved survival in patients who could receive it (40–42). However, that approach has generally been restricted to younger patients with MM. Attempts have been made to tailor transplant regimens for fit seniors by reducing the total chemotherapy dose or splitting high-dose therapy over more than 1 day with the aim of improving transplant tolerability while preserving efficacy (43–46). It appears that such approaches are feasible, but equivalent efficaciousness to standard approaches to high-dose therapy has not been rigorously demonstrated. Regardless, even with such modified transplants now commonly being employed for older, fit MM patients, the continued lack of feasibility of even modified transplants for most VOP-MM despite transplant’s historical survival benefit argues that inability to tolerate the most intensive MM therapies probably contributes to poorer survival in VOP-MM.
Taken together, these data demonstrate that although survival is increasing for MM patients of all ages, survival in seniors has improved less than in younger patients. The relative impact of differences between young and old in underlying cancer biology and therapeutic options remains unclear.
MM Therapy in the Very Old
The recent introduction of proteasome inhibitors and immunomodulatory agents has improved outcomes in MM dramatically, including for seniors.
Thalidomide, an immunomodulatory agent, was the first important new drug in MM since melphalan and prednisone (MP) became standard of care in the 1960s (47,48). When thalidomide was added to MP (MPT), older patients experienced increased overall response rates and progression-free survival; OS was improved in only some studies. Unfortunately, these advances were at the expense of markedly increased cardiac, thromboembolic, neuropathic, and gastrointestinal serious adverse events (49–56). Nonetheless, MPT remains a standard of care for older patients with a new diagnosis of MM.
Lenalidomide, thalidomide’s next-generation analog, improved both overall response rates and progression-free survival when tested against single agent high-dose dexamethasone in newly diagnosed MM. OS was not improved, but that study was closed early (57) because of publication of a separate study that unambiguously showed the efficacy of lenalidomide and dexamethasone in combination, with higher response rates and higher mortality in patients who received high-dose dexamethasone (4 days on, 4 days off) as opposed to low-dose (weekly) dexamethasone (58). A subgroup analysis of patients aged more than 70 years similarly showed 3-year OS was enhanced for patients who received low-dose dexamethasone (59). This critical finding exemplifies the potential for efficacy at the expense of toxicity or even mortality in older patients; high-dose dexamethasone augmented responses but also mortality. As a result of these studies, lenalidomide and weekly dexamethasone became a standard of care for older MM patients. More recently, one study demonstrated that adding lenalidomide to MP during induction and continuing lenalidomide as postinduction maintenance prolongs progression-free survival at the expense of more hematological toxicity in particular. An OS benefit has not been demonstrated to date (60). Currently, trials are ongoing that study, for example, lenalidomide and low-dose dexamethasone (Rd) versus MPT and suggest that MPT may be a less attractive option than Rd, but those data are only now forthcoming.
The first-in-class proteasome inhibitor bortezomib was approved in the United States and Europe based upon data showing an improved OS in patients with relapsed and refractory MM who received bortezomib when compared with high-dose dexamethasone (61). Subgroup analyses revealed that severe toxicity and 1-year OS were both similar in patients older and younger than 65 years (62). As for newly diagnosed MM, the VISTA trial demonstrated an OS benefit for all patients who received MP and bortezomib compared with MP alone, although patients aged more than 75 years still experienced a shorter median OS than younger patients. Peripheral neuropathy and gastrointestinal events resulted in many patients stopping bortezomib (63,64).
Fortunately, bortezomib’s toxicity in all patients, especially seniors, can be mitigated. In one trial, an approximately 30% reduction in both gastrointenstinal and neurologic adverse events was observed when patients received bortezomib subcutaneously instead of intravenously with no decrement in overall response rates or OS (65). A similar reduction in toxicity with equivalent efficacy was demonstrated for weekly, instead of standard twice-weekly, bortezomib in a separate study of a primarily older patient population receiving MP-based chemotherapy (66). Although MP and bortezomib using twice-weekly intravenous bortezomib dosing is still arguably the standard for the first-line treatment of many MM patients, numerous MM centers have moved toward regular use of subcutaneous and weekly bortezomib dosing for senior MM patients. Notably, however, this practice is based on an extrapolation from the available clinical data.
A recent review by the European Myeloma Network on personalized MM therapy included useful and specific dosing recommendations for older adults (67).
Newer drugs such as pomalidomide and carfilzomib have recently been approved by regulatory agencies and are also certain to impact MM care in the elderly, but data specific to seniors are not yet available.
Despite these advances, the improvements in survival in MM have primarily benefited younger patients. With the rising numbers of VOP-MM, focused research is critical to establish best practices for that group. Although trials have indeed been conducted, some of which have been discussed, existing research on MM in seniors is often irrelevant to many VOP-MM for three reasons:
-
1.
In the United States, most MM patients are treated in community practices, where access to clinical research programs may be limited. Among older patients, it is usually fit, younger seniors (eg, aged 65–70 years) with few comorbidities who are referred to academic centers for consideration of aggressive approaches such as transplant and/or clinical trials.
-
2.
In much of Europe, governmental policy permits access to certain health interventions such as transplant to only younger patients. Consequently, European studies of older MM patients often include many fit, “younger elderly” patients, who elsewhere would be candidates for aggressive treatment including transplant and not considered for senior protocols for MM.
-
3.
Despite the high prevalence of comorbid conditions in VOP-MM, clinical trials often exclude patients with conditions such as renal insufficiency and poor performance status.
Hence the combination of referral patterns, health policy, and clinical trial design often results in only the fittest, younger elderly accruing to trials and a dearth of data relevant to VOP-MM.
These factors deprive the MM community of precisely the data that are essential to help determine optimal therapy for VOP-MM. Fortunately, recent trials are beginning to fill the gap. For example, the community-based UPFRONT study (NCT00507416) is currently testing three bortezomib-based induction regimens in older MM patients. As of last report, it had enrolled 501 patients, with roughly 40% and 20% who are aged 75 years or older and 80 years or older, respectively. A notable proportion has at least one comorbidity, including diabetes and renal insufficiency (68). That trial provides proof of principle that studies targeting “real-world” MM patients, including VOP-MM, can accrue successfully. Such studies will likely provide useful information on how to best treat this growing patient population. To preserve their generalizability to VOP-MM, future clinical studies should have liberal inclusion criteria. Such trials should also incorporate some form of CGA to explore CGA as a possible means to more effectively stratify older patients for therapy intensity in a manner that is more reliable than simple cutoffs based on age, performance status, or clinical gestalt.
Special Considerations
When contemplating therapy for VOP-MM, it is critical to understand that seniors have an increased risk of toxicity by virtue of the aging process itself and impaired homeostasis, as well as factors related to frailty, comorbidity, or functional and socioeconomic limitations. The more impairments, the greater the risks. At some point, these burdens outweigh the potential benefits, and a comfort care/hospice approach may be more appropriate.
The toxic effects of cancer treatment are frequently more burdensome in older adults. In addition to the standard side effects of cancer treatment, there are important age-related toxicities to consider. As discussed, most of these are more a function of frailty than chronological age, but even the fittest senior cannot avoid the physiological effects of aging. These include sometimes subtle reductions in bone marrow, renal, hepatic and gastrointestinal function, and changes in body fluid and muscle-fat composition. Those factors in turn affect not only the homeostatic reserve that enables a patient to endure chemotherapy toxicity but also the pharmacokinetic and pharmacodynamic properties of many drugs used in treating cancer. In short, the drugs themselves behave differently in older patients (69).
Polypharmacy is quite prevalent in the geriatric oncology population. Drug–drug interactions increase with the number of medications taken (70). Polypharmacy affects many organs. Like MM itself, numerous drugs affect renal function, for example. Agents such as nonsteroidal anti-inflammatory drugs and bisphosphonates are well-reported nephrotoxins, especially in MM.
In seniors, minimal stress will disrupt hematopoiesis. Myelosuppression can be more pronounced and prolonged than in younger MM patients. Anemia is especially common in the seniors, especially if they are also frail (71,72). Often aggravated by both MM and many MM therapies, anemia can decrease the efficacy of anticancer treatment, alter the pharmacokinetics of chemotherapeutic agents, increase morbidity (fatigue, functional and cognitive decline), and potentially alter tumor biology (73). Maintaining adequate hemoglobin levels has proven effective in minimizing many of these side effects in senior cancer patients, although how best to do that (ie, transfusions vs growth factors) is controversial (74). Neutropenia with resultant infections can also be problematic in seniors, and consideration should be given to both chemotherapy dose reductions and/or usage of growth factors such as filgrastim when highly myelosuppressive chemotherapy is planned or when patients have disease-related cytopenias already.
Chemotherapeutic agents exert central nervous system effects by direct injury, endothelial injury, and inflammation (75,76). This form of central nervous system toxicity (“chemo-brain”) can be particularly important in cognitively impaired patients, but this issue has not been extensively studied in older patients. Many chemotherapy adjuncts are neurotoxins, such as diphenhydramine and certain antiemetics. Corticosteroid-induced delirium, psychosis, and depression are well described. Corticosteroids can also cause borderline diabetics to become symptomatically hyperglycemic and exacerbate chronic gastritis and gastroesophageal reflux disease.
Peripheral neuropathy is a common side effect of many MM therapies, such as thalidomide and bortezomib. Impaired proprioception, sensation, and autonomic neuropathy may predispose senior patients to thermal injuries, falls, and foot ulcers. Many MM patients present with peripheral neuropathy as a consequence of preexisting diabetes.
Mucositis and diarrhea are often more severe in older patients receiving MM therapy that affects gastrointestinal motility, such as bortezomib or radiation. Early diagnosis and appropriate treatment may prevent malnutrition, sepsis, renal insufficiency, electrolyte abnormalities, and dehydration. Older patients are particularly predisposed to dehydration and constipation. In addition to age-related diminished intracellular water, older individuals may have inadequate fluid intake and or be prescribed diuretics. Furthermore, many MM therapies and ancillary medications, such as narcotics, can result in debilitating constipation. Attention to adequate hydration, early intervention with bowel regimens, and consideration of dose modifications for therapies inducing severe cases of constipation are vital.
Falls that may result in bone fractures or bleeding are not uncommon in older cancer patients, especially those who are dehydrated or receiving neurotoxic agents. The high prevalence of vitamin D deficiency may further predispose older adults to a higher injury rate with falls (77).
Judicious selection of chemotherapy and adjuncts is critical in those at high risk for falls. Risk factors include a history of falls; preexisting neurocognitive, gait, or balance problems; deconditioning or diminished muscle mass; and impaired vision or mobility (78,79). Physical and occupational therapy evaluation (including home safety visits) are often effective at addressing these and other relevant risk factors for falls.
Falls and bleeding risk are also key considerations in decisions regarding aspirin or anticoagulant prophylaxis for venous thromboembolic events in VOP-MM. Immunomodulatory agents such as thalidomide or lenalidomide are popular choices for older patients but are also associated with an elevated risk of thromboembolism, especially if administered with high-dose dexamethasone (58,80,81). Routine prophylaxis for such events is generally as appropriate in VOP-MM as much as in younger patients, but the elevated potential for hemorrhage and falls in these patients should be contemplated as part of risk/benefit calculations in individual patients.
Fatigue is a near universal complaint of senior cancer patients, even in those without marked anemia. It can occur as a symptom of cancer itself, as a side effect of MM therapy, or as a consequence of other conditions associated with a cancer diagnosis, such as depression. Fatigue is particularly a problem for those who are socially isolated or dependent upon others in activities of daily living or instrumental activities of daily living. Depression is quite common in senior adults in general (82,83). CGA serves an essential role in the early diagnosis and management of many of these symptoms and syndromes.
Specific Recommendations
We make the following specific recommendations:
-
1.
Survey current community management of myeloma in VOP-MM. Catalog existing knowledge gaps that identify and highlight the need for coordinated research in this clinical domain.
-
2.
Develop, find sponsorship, and coordinate programs to increase awareness among health-care providers of the expected rapid increase in MM.
-
a.
Educational programs directed at primary care physicians. Perhaps a satellite program at national meetings of societies relevant to this issue (eg, American Geriatrics Society, American Academy of Family Physicians, American College of Physicians, American Society of Internal Medicine).
-
b.
A large-scale international meeting on MM in senior adults, attracting interested physicians from hematology/oncology, geriatric medicine, and palliative care, as well as oncology nursing, affiliated social sciences, and community advocates.
-
a.
-
3.
Establish a consortium of hematologist/oncologists and geriatricians to design and implement clinical trials for VOP-MM.
-
a.
Establish a registry of currently active clinical trials that do not exclude VOP-MM and that are a priori relevant to this age group.
-
b.
Identify and recommend common instruments for CGA for application in future consortium trials.
-
c.
Identify relevant outcomes for VOP-MM and provide recommendations regarding assessments of those outcomes. OS is likely not the optimal endpoint for this patient population, and other endpoints such as quality of life and preservation of functional status and independence warrant consideration for prioritization in clinical trials for VOP-MM.
Conclusions
VOP-MM are a unique patient population. They are growing in number and challenging to treat not only because of the multiple complex factors that impact cancer in senior adults but also because of the lack of relevant and robust clinical trial data. Therefore, it is essential that clinical trials be designed to specifically test treatment regimens for VOP-MM who choose treatment. These trials must measure outcomes that are most important to VOP-MM who, unlike younger MM patients, may value quality of life more than longevity. A holistic, proactive approach to the management of VOP-MM is essential to maximize the benefits of management of their MM, while limiting treatment-related toxicity and symptoms from MM itself.
Funding
SAT’s contribution to this manuscript was supported by NIH, KL2 RR024127.
References
- 1. United States Census Bureau. US Census Age and Sex Composition 2010: 2010 Census Briefs Issued May 2011 http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf Accessed October 1, 2012.
- 2. United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2010 Revision, Highlights and Advance Tables http://esa.un.org/unpd/ppp/index.htm. Accessed February 1, 2013.
- 3. Pulte D, Gondos A, Brenner H. Improvement in survival of older adults with multiple myeloma: results of an updated period analysis of SEER data. Oncologist. 2011;16(11):1600–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program and the National Center for Health Statistics http://www.cancer.gov/aboutnci/servingpeople/snapshots/myeloma.pdf. Accessed September 15, 2012.
- 5. MVH, Krumholz HM, Gross CP.. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. [DOI] [PubMed] [Google Scholar]
- 6. Jonsen AR, Siegler M, Winslade WJ. Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine. 5th ed. New York: McGraw Hill, Medical Pub. Division; 2002. [Google Scholar]
- 7. Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756. [DOI] [PubMed] [Google Scholar]
- 8. Yancik R, Ganz PA, Varricchio CG, et al. Perspectives on comorbidity and cancer in older patients: approaches to expand the knowledge base. J Clin Oncol. 2001;19(4):1147–1151. [DOI] [PubMed] [Google Scholar]
- 9. Saltzstein SL, Behling CA. 5- and 10-year survival in cancer patients aged 90 and older: a study of 37,318 patients from SEER. J Surg Oncol. 2002;81(3):113–116; dicussion 117. [DOI] [PubMed] [Google Scholar]
- 10. Wedding U, Honecker F, Bokemeyer C, et al. Tolerance to chemotherapy in elderly patients with cancer. Cancer Control. 2007;14(1):44–56. [DOI] [PubMed] [Google Scholar]
- 11. Bijlsma AY, Meskers CG, Westendorp RG, et al. Chronology of age-related disease definitions: osteoporosis and sarcopenia. Ageing Res Rev. 2012;11(2):320–324. [DOI] [PubMed] [Google Scholar]
- 12. Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. [DOI] [PubMed] [Google Scholar]
- 13. Grabowski DC, Ellis JE. High body mass index does not predict mortality in older people: analysis of the Longitudinal Study of Aging. J Am Geriatr Soc. 2001;49(7):968–979. [DOI] [PubMed] [Google Scholar]
- 14. Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev. 2008;7(1):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newby PK, Muller D, Hallfrisch J, et al. Dietary patterns and changes in body mass index and waist circumference in adults. AM J Clin Nutr. 2003;77(6):1417–1425. [DOI] [PubMed] [Google Scholar]
- 16. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56(3):M146–M156. [DOI] [PubMed] [Google Scholar]
- 17. Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114(3):180–187. [DOI] [PubMed] [Google Scholar]
- 18. Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25(14):1824–1831. [DOI] [PubMed] [Google Scholar]
- 19. Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–2447. [DOI] [PubMed] [Google Scholar]
- 20. Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25(14):1824–1831. [DOI] [PubMed] [Google Scholar]
- 21. Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol. 2005;55(3):241–252. [DOI] [PubMed] [Google Scholar]
- 22. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muffly LS, Boulukos M, Swanson K, et al. Pilot study of comprehensive geriatric assessment (CGA) in allogeneic transplant: CGA captures a high prevalence of vulnerabilities in older transplant recipients. Biol Blood Marrow Transplant. 2013;19(3):429–434. [DOI] [PubMed] [Google Scholar]
- 24. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118(13):3377–3786. [DOI] [PubMed] [Google Scholar]
- 25. Ershler WB, Longo DL. Aging and cancer: issues of basic and clinical science. J Natl Cancer Inst. 1997;89(20):1489–1497. [DOI] [PubMed] [Google Scholar]
- 26. Kanapuru B, Posani K, Muller D, et al. Decreased cancer prevalence in the nursing home. J Am Geriatr Soc. 2008;56(11):2165–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wahlin A, Markevärn B, Golovleva I, et al. Prognostic significance of risk group stratification in elderly patients with acute myeloid leukaemia. Br J Haematol. 2001;115(1):25–33. [DOI] [PubMed] [Google Scholar]
- 28. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. New Engl J Med. 1993;329(14):987–994. [DOI] [PubMed] [Google Scholar]
- 29. Balducci L, Ershler WB. Cancer and ageing: a nexus at several levels. Nat Rev Cancer. 2005;5(8):655–662. [DOI] [PubMed] [Google Scholar]
- 30. Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111(5):2521–2526. [DOI] [PubMed] [Google Scholar]
- 32. Ludwig H, Durie BGM, Bolejack V, et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the International Myeloma Working Group. Blood. 2008;111(8):4039–-–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. [DOI] [PubMed] [Google Scholar]
- 34. Bringhen S, Mateos MV, Zweegman S, et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica. 2013;98(6);980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420. [DOI] [PubMed] [Google Scholar]
- 36. Kumar SK, Dispenzieri A, Gertz MA, et al. Continued improvement in survival in multiple myeloma and the impact of novel agents. ASH Ann Meeting Abstr. 2012;120(21):3972. [Google Scholar]
- 37. Ross FM, Ibrahim AH, Vilain-Holmes A, et al. Age has a profound effect on the incidence and significance of chromosome abnormalities in myeloma. Leukemia. 2005;19(9):1634–1642. [DOI] [PubMed] [Google Scholar]
- 38. Avet-Loiseau H, Leleu X, Roussel M, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p). J Clin Oncol. 2010;28(30):4630–4634. [DOI] [PubMed] [Google Scholar]
- 39. Avet-Loiseau H, Hulin C, Campion L, et al. Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: the intergroupe francophone du myélome experience. J Clin Oncol. 2013;31(22):2806–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Attal M, Harousseau J-L, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. New Engl J Med. 2003;349(26):2495–2502. [DOI] [PubMed] [Google Scholar]
- 41. Attal M, Harousseau J-L, Stoppa A-M, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. New Engl J Med. 1996;335(2):91–97. [DOI] [PubMed] [Google Scholar]
- 42. Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–1883. [DOI] [PubMed] [Google Scholar]
- 43. Palumbo A, Bringhen S, Petrucci MT, et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood. 2004;104(10):3052–3057. [DOI] [PubMed] [Google Scholar]
- 44. Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370(9594):1209–1218. [DOI] [PubMed] [Google Scholar]
- 45. Bashir Q, Shah N, Parmar S, et al. Feasibility of autologous hematopoietic stem cell transplant in patients aged ≥70 years with multiple myeloma. Leukemia Lymphoma. 2012;53(1):118–122. [DOI] [PubMed] [Google Scholar]
- 46. Kumar SK, Dingli D, Lacy MQ, et al. Autologous stem cell transplantation in patients of 70 years and older with multiple myeloma: results from a matched pair analysis. Am J Hematol. 2008;83(8):614–617. [DOI] [PubMed] [Google Scholar]
- 47. Alexanian R, Haut A, Khan AU, et al. Treatment for multiple myeloma. Combination chemotherapy with different melphalan dose regimens. JAMA. 1969;208(9):1680–1685. [DOI] [PubMed] [Google Scholar]
- 48. Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. New Engl J Med. 1999;341(21):1565–1571. [DOI] [PubMed] [Google Scholar]
- 49. Kapoor P, Rajkumar SV, Dispenzieri A, et al. Melphalan and prednisone versus melphalan, prednisone and thalidomide for elderly and/or transplant ineligible patients with multiple myeloma: a meta-analysis. Leukemia. 2011;25(4):689–696. [DOI] [PubMed] [Google Scholar]
- 50. Palumbo A, Waage A, Hulin C, et al. Safety of thalidomide in newly diagnosed elderly myeloma patients: an individual patient data meta-analysis of six randomized trials. Haematologica. 2012;98(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fayers PM, Palumbo A, Hulin C, et al. Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood. 2011;118(5):1239–1247. [DOI] [PubMed] [Google Scholar]
- 52. Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370(9594):1209–1218. [DOI] [PubMed] [Google Scholar]
- 53. Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664–3670. [DOI] [PubMed] [Google Scholar]
- 54. Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112(8):3107–3114. [DOI] [PubMed] [Google Scholar]
- 55. Waage A, Gimsing P, Fayers P, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116(9):1405–1412. [DOI] [PubMed] [Google Scholar]
- 56. Wijermans P, Schaafsma M, Termorshuizen F, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study. J Clin Oncol. 2010;28(19):3160–3166. [DOI] [PubMed] [Google Scholar]
- 57. Zonder JA, Crowley J, Hussein MA, et al. Lenalidomide and high-dose dexamethasone compared with dexamethasone as initial therapy for multiple myeloma: a randomized Southwest Oncology Group trial (S0232). Blood. 2010;116(26):5838–5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jacobus S, Callander N, Siegel D, et al. Outcome of elderly patients 70 years and older with newly diagnosed myeloma in the ECOG randomized trial of lenalidomide/high-dose dexamethasone (RD) versus lenalidomide/low-dose dexamethasone (Rd). Haematologica. 2010;95(Suppl 2):S149. [Google Scholar]
- 60. Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. New Engl J Med. 2012;366(19):1759–1769. [DOI] [PubMed] [Google Scholar]
- 61. Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. New Engl J Med. 2005;352(24):2487–2498. [DOI] [PubMed] [Google Scholar]
- 62. Richardson PG, Sonneveld P, Schuster MW, et al. Safety and efficacy of bortezomib in high-risk and elderly patients with relapsed multiple myeloma. Br J Haematol. 2007;137(5):429–435. [DOI] [PubMed] [Google Scholar]
- 63. San-Miguel JF, Richardson PG, Sonneveld P, et al. Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leukemia. 2008;22(4):842–849. [DOI] [PubMed] [Google Scholar]
- 64. Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28(13):2259–2266. [DOI] [PubMed] [Google Scholar]
- 65. Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–440. [DOI] [PubMed] [Google Scholar]
- 66. Bringhen S, Larocca A, Rossi D, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116(23):4745–4753. [DOI] [PubMed] [Google Scholar]
- 67. Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood. 2011;118(17):4519–4529. [DOI] [PubMed] [Google Scholar]
- 68. Niesvizky R, Flinn IW, Rifkin R, et al. Efficacy and safety of three bortezomib-based combinations in elderly, newly diagnosed multiple myeloma patients: results from all randomized patients in the community-based, phase 3b UPFRONT study. ASH Ann Meeting Abstr. 2011;118(21):478. [Google Scholar]
- 69. Lichtman SM, Wildiers H, Chatelut E, et al. International Society of Geriatric Oncology Chemotherapy Taskforce: evaluation of chemotherapy in older patients--an analysis of the medical literature. J Clin Oncol. 2007;25(14):1832–1843. [DOI] [PubMed] [Google Scholar]
- 70. Launay-Vacher V, Chatelut E, Lichtman SM, et al. Renal insufficiency in elderly cancer patients: International Society of Geriatric Oncology clinical practice recommendations. Ann Oncol. 2007;18(8):1314–1321. [DOI] [PubMed] [Google Scholar]
- 71. Artz AS, Fergusson D, Drinka PJ, et al. Prevalence of anemia in skilled-nursing home residents. Arch Gerontol Geriatr. 2004;39(3):201–206. [DOI] [PubMed] [Google Scholar]
- 72. Beghe C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):3S–10S. [DOI] [PubMed] [Google Scholar]
- 73. Van Belle SJ, Cocquyt V. Impact of haemoglobin levels on the outcome of cancers treated with chemotherapy. Crit Rev Oncol Hematol. 2003;47(1):1–11. [DOI] [PubMed] [Google Scholar]
- 74. Rizzo JD, Brouwers M, Hurley P, et al. American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol. 2010;28(33):4996–5010. [DOI] [PubMed] [Google Scholar]
- 75. Briones TL, Woods J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci. 2011;12(124):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mitchell T, Turton P. “Chemobrain”: concentration and memory effects in people receiving chemotherapy—a descriptive phenomenological study. Eur J Cancer Care. 2011;20(4):539–548. [DOI] [PubMed] [Google Scholar]
- 77. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31(1):48–54. [DOI] [PubMed] [Google Scholar]
- 78. Richardson JK. Factors associated with falls in older patients with diffuse polyneuropathy. J Am Geriatr Soc. 2002;50(11):1767–1773. [DOI] [PubMed] [Google Scholar]
- 79. Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. J Am Geriatr Soc. 1999;47(1):30–39. [DOI] [PubMed] [Google Scholar]
- 80. Zangari M, Anaissie E, Barlogie B, et al. Increased risk of deep-vein thrombosis in patients with multiple myeloma receiving thalidomide and chemotherapy. Blood. 2001;98(5):1614–1615. [DOI] [PubMed] [Google Scholar]
- 81. Gay F, Hayman SR, Lacy MQ, et al. Lenalidomide plus dexamethasone versus thalidomide plus dexamethasone in newly diagnosed multiple myeloma: a comparative analysis of 411 patients. Blood. 2010;115(7):1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kurtz ME, Kurtz JC, Stommel M, et al. Physical functioning and depression among older persons with cancer. Cancer Pract. 2001;9(1):11–18. [DOI] [PubMed] [Google Scholar]
- 83. Stommel M, Kurtz ME, Kurtz JC, et al. A longitudinal analysis of the course of depressive symptomatology in geriatric patients with cancer of the breast, colon, lung, or prostate. Health Psychol. 2004;23(6):564–573. [DOI] [PubMed] [Google Scholar]




