Abstract
Background:
Aromatase inhibitors (AIs) substantially reduce breast cancer mortality in clinical trials, but high rates of nonadherence to these long-term oral therapies have reduced their impact outside of trials. We examined the association of generic AI availability with AI adherence among a large national breast cancer cohort.
Methods:
Using a quasi-experimental prepost design, we examined the effect of generic AI introductions (7/2010 and 4/2011) on adherence among a national cohort of women with incident breast cancer in 2006 and 2007 who were enrolled in the Medicare D pharmaceutical coverage program. Medicare D claims were used to calculate AI adherence, defined as a medication possession ratio of 80% or more of eligible days, over 36 months. Multivariable logistic regression models estimated with generalized estimating equations were applied to longitudinal adherence data to control for possible confounders, including receipt of a Medicare D low-income subsidy, and to account for repeated measures. All statistical tests were two-sided.
Results:
Sixteen thousand four hundred sixty-two Medicare D enrollees were eligible. Adherence declined throughout the study. However, among women without a subsidy, the median quarterly out-of-pocket cost of anastrozole fell from $183 in the fourth quarter of 2009 to $15 in 2011, and declines in adherence were attenuated with generic AI introductions. Regression-adjusted adherence probabilities were estimated to be 5.4% higher after generic anastrozole was introduced in 2010 and 11% higher after generic letrozole/exemestane was introduced in 2011. Subsidy recipients had higher adherence rates throughout the study.
Conclusions:
The introduction of generic medications attenuated the decline in adherence to AIs over three years of treatment among breast cancer survivors not receiving low-income subsidies for Medicare D coverage.
There are over twenty oral antineoplastics currently approved in the United States and dozens more on the horizon (1). Adjuvant tamoxifen therapy for hormone receptor–positive breast cancer was one of the earliest developed long-term oncologic therapies. When taken for five to 10 years, it reduces long-term breast cancer mortality by one-third to one-half (2–4). The newer aromatase inhibitors anastrozole, letrozole, and exemestane reduce cancer recurrence by an additional 50% (5) but also need to be taken either alone or after tamoxifen for at least five total years of endocrine therapy (5).
Unfortunately, many breast cancer patients prescribed long-term oral therapies have trouble adhering to them (6). One-third to one-half of patients prescribed adjuvant endocrine therapy (ET) outside of clinical trials either discontinue or adhere poorly to their medication within the first three years of therapy (7–15). Endocrine therapy nonadherence has clinically important effects. In one large breast cancer cohort, poor adherence to tamoxifen or aromatase inhibitors as measured by prescription fills was associated with a reduction in survival of 4% to 8% (16).
There is increasing evidence that patient out-of-pocket costs play an important role in endocrine therapy nonadherence (17,18). The aromatase inhibitors (AIs) are substantially more costly than the generic tamoxifen, and many insurers are requiring that patients pay higher out-of-pocket costs for more expensive medications (17,18). In one recent study, 27% of patients in 2005 to 2008 with commercial insurance paid over $30 monthly out-of-pocket for their adjuvant aromatase inhibitor; adherence in this high-copay group was 18% to 28% lower than for patients paying $10 or less (17).
In July 2010, an opportunity for reversal of these trends arose with the patent expiration of anastrozole. In contrast to some generic medications that face little competition when first released, thirteen generic manufacturers had generic anastrozole approved by the month after patent expiration, offering the potential for competition by cost. Shortly thereafter (April 2011), generic letrozole and exemestane were also released.
Medicare D pharmaceutical program enrollees comprise an ideal national, population-based cohort in which to examine the adherence effects of this natural experiment. The Medicare D program required that all antineoplastic agents be on plan formularies. Furthermore, it provided a low-income subsidy (LIS) program to a substantial number of Medicare D enrollees, providing premium, deductible, and copayment support (including during the coverage gap or “donut hole” period) for enrollees with household incomes up to 150% of the federal poverty line. LIS recipients who experienced small or no effects upon out-of-pocket costs as generics became available thus could be compared with those without such supports, who experienced substantial changes in costs (18).
The purpose of this study was to examine the effect of the introduction of generic versions of aromatase inhibitors on adherence to endocrine therapy among a nationally representative sample of elderly women with incident breast cancer using a quasi-experimental pre-post study design. We hypothesized that the well-documented rate of decline in adherence over time would be substantially attenuated after generic AIs became available. We further hypothesized that the effects would be larger for those not enrolled in the low-income subsidy program for pharmaceutical coverage.
Methods
Study Sample and Data Sources
From nationwide Medicare files, we identified women age 65 years and older with a 2006 or 2007 surgery for incident breast cancer (19). The study sample was identified using an algorithm that has an overall positive predictive value of 89% to 93%. Eligibility criteria included continuous enrollment in fee-for-service Medicare for at least one year prior to surgery and enrollment in a stand-alone Medicare D prescription drug plan at the time of surgery through the first month of 2009. To identify hormone therapy users, potential cohort members also were required to have at least one Medicare D claim for endocrine therapy with either an aromatase inhibitor (anastrozole, letrozole, or exemestane) or tamoxifen between July 1 and December 31, 2008. To be included in the final cohort, subjects were required to be taking an aromatase inhibitor during the study period (Supplementary Figures 1 and 2, available online). The few subjects who were reported by Medicare to have disenrolled from Medicare D for one or more calendar quarters (n = 216), as well as those who died or completed five years of therapy before the end of 2011, were included in analyses only for quarters where appropriate adherence data was available.
Medicare inpatient, outpatient, Carrier and Denominator files from 2005 through 2011 were used for cohort identification and for cohort members’ demographic characteristics, outpatient and inpatient diagnoses, and receipt of a Medicare D low-income subsidy. Medicare Prescription Drug Event (PDE) files were used for all measures of patients’ pharmaceutical use. The PDE files included variables for dispensed medications’ National Drug Code (NDC), the date prescriptions were dispensed, quantity dispensed, the number of days of supply, and the amount paid to the pharmacy for each medication by the prescription drug plan and the beneficiary. Patients who switched from traditional Medicare with a stand-alone Medicare D prescription drug plan to a Medicare Advantage plan with a prescription drug plan during the study period (4% of the total sample) continued to have prescription information recorded in beneficiary and PDE files (and thus remained in the cohort). All investigations were completed following approval from the Medical College of Wisconsin’s Internal Review Board (IRB).
Measures
All cohort members’ aromatase inhibitor prescriptions were measured from Medicare PDE files. Adherence was calculated by counting the number of days of medication received for time periods of interest (three-month periods as used in previous analyses) (20), between January 2009 and December 2011, and calculating a medication possession ratio (MPR) for each period. This measurement was used for: 1) aromatase inhibitors of any type and 2) individual AIs. When a medication was dispensed before the previous prescription should have run out, the new prescription was assumed to start the day after the previous prescription should have ended, so that patients who “stockpiled” pills at each prescription were considered adherent throughout the time they had any pills available (21). Adherence to therapy was defined as an MPR 80% or higher. Breast cancer mortality in one large cohort (16) was reduced by approximately 4% with adherence greater than or equal to this commonly used MPR threshold.
Out-of-pocket costs for medications were also identified from Medicare PDE files. CMS obtains this information from Medicare D insurers (plans) for each medication. Because patients pay different portions of medication costs during different “coverage periods” (deductible, initial coverage period, coverage gap, and catastrophic) and plans negotiate with pharmacies, these costs vary from month to month. Insurance premiums are not included in these costs.
Baseline (2008) covariates included demographics (age, race/ethnicity) (22) number of medications, and comorbidity score calculated using the breast-cancer specific National Cancer Institute algorithm for outpatient and inpatient diagnoses. To account for expected reductions in adherence with increasing treatment duration (7,8), time since medication initiation was also calculated. Receipt of a low-income subsidy was determined (yes/no) for each quarter as a time-varying covariate. In 2009, Medicare beneficiaries with yearly income below 150% of the Federal Poverty Line ($17 245/individual) and limited assets (<$12 510/individual) were eligible for the LIS. Eligibility thresholds are the same in all states and rise slightly each year based on cost of living. The subsidy, which is recorded on Medicare files monthly, provides help paying the Medicare D drug plan’s monthly premium, yearly deductible, and coinsurance/copayments and eliminates the coverage gap or “donut hole.”
Statistical Analysis
Descriptive statistics were used for demographics and other baseline variables. Out-of-pocket cost variables were reported for all aromatase inhibitors. The percent of cohort members who had entered the Medicare coverage gap (donut hole) were also determined for each calendar quarter.
Unadjusted adherence measures (percent of patients with MPR ≥ 80% in each quarter) were calculated for all cohort patients in total and by medication type. Multivariable logistic regression models were used to examine the difference-in-differences effect of the introduction of generic AI alternatives among LIS and non-LIS beneficiaries, adjusting for subject-level covariates. The generalized estimating equations (GEE) method was used to estimate the models in order to account for longitudinal clustering of within-subject observations. Two prepost generic indicator variables were created and included in all models, the first for anastrozole (July 1, 2010) and the second for letrozole and exemestane (April 1, 2011). Data for patients who switched from one type of AI to another were excluded for the quarter where the switch occurred (less than 1% of the sample switched AI modalities). Receipt of a low-income subsidy was included in analyses as a binary indicator. The years since AI initiation were included to control for the previously described declining rate in endocrine therapy adherence over time (7–15). In addition, indicators for calendar quarters were included to account for the seasonal pattern in the unadjusted quarterly adherence consistent with deductible and donut hole effects (23–25). Given that LIS recipients had substantially smaller out-of-pocket costs and consequently minimal exposure to the coverage gap throughout the study period, potential interactions between LIS, quarterly effect, and generic availability were also examined and included where statistically significant. The GEE models also included controls for patients’ age, race, number of comorbid conditions, and overall number of prescription medications. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). All statistical tests were two-sided.
Three sensitivity analyses were performed to examine the effect of alternate specifications of key cohort and variable definitions. First, the model was refit after excluding patients with evidence of permanent discontinuation of medication (no pills available for ≥60 days). Next, in order to test the possibility that the effect of generics might be delayed, we examined the impact on our findings of redefining the post anastrozole generic indicator variable to at or after October 1, 2010. Next, LIS effects were dropped from the model entirely to examine whether the effect of generic introduction was detectable among the combined population of AI users with and without a low-income subsidy. Generic effects were not sensitive to these different specifications.
Results
There were 16 462 older Medicare D enrollees with a 2006 or 2007 incident breast cancer surgery who received aromatase inhibitors and were eligible for our cohort. Baseline information for the cohort is shown in Table 1. Over 33% of patients received a low-income subsidy.
Table 1.
Baseline characteristics of 2006–2007 US breast cancer patients age ≥65 years who received aromatase inhibitors*
| Characteristics | Total (%) (n = 16 462) |
|---|---|
| Age, y | |
| 65–74 | 8827 (53.6%) |
| 75–84 | 6424 (39.0%) |
| 85+ | 1211 (7.4%) |
| Race/ethnicity | |
| White | 13830 (84.0%) |
| Non-white | 2632 (16.0%) |
| Receipt of low-income subsidy (1/1–3/31/2009) | |
| Yes | 5568 (33.8%) |
| No | 10 894 (66.2%) |
| Comorbidity score (2008) | |
| Low (zero comorbidity) | 7760 (47.1%) |
| Moderate (0 – 0.7) | 4342 (26.4%) |
| High (≥ 0.7) | 3762 (22.9%) |
| Missing | 598 (3.6%) |
| Aromatase inhibitor | |
| Anastrozole | 10 647 (65.0%) |
| Letrozole or Exemestane | 5815 (35.0%) |
| Years since endocrine therapy initiation | |
| Mean (SD) | 1.77 (0.63) |
| (IQR) | (1.27–2.26) |
| Number of medications (1/1–3/31/2009) | |
| Mean (SD) | 4.31 (3.17) |
| (IQR) | (2.00–6.00) |
* All variables are at time of surgery, unless noted. IQR = interquartile range.
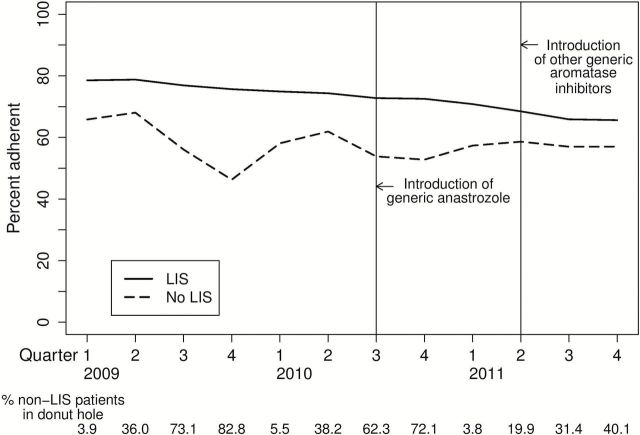
Unadjusted rates of adherence to aromatase inhibitors (percent of patients with MPR ≥ 80%), among women with and without a low-income subsidy are shown in Figure 1. Subjects receiving the subsidy had overall higher adherence throughout the study period, but adherence still declined substantially over time, consistent with previously described patterns. Among subjects not receiving a subsidy, there was an additional large seasonal drop in 2009 consistent with entrance into the coverage gap that was substantially attenuated after generic introductions in 2010 and 2011.
Figure 1.
Percent adherence to endocrine therapy. Unadjusted percent adherence (medication possession ratio ≥ 80%) to endocrine therapy for patients stratified by receipt of a low-income subsidy (restricted to patients with income ≤ 150% federal poverty level and low resources, see text for more detail). Anastrozole was available generically from July 1, 2010, and exemestane and letrozole from April 1, 2011. The percent of non-LIS patients in the coverage gap are shown below the figure. LIS = low-income subsidy.
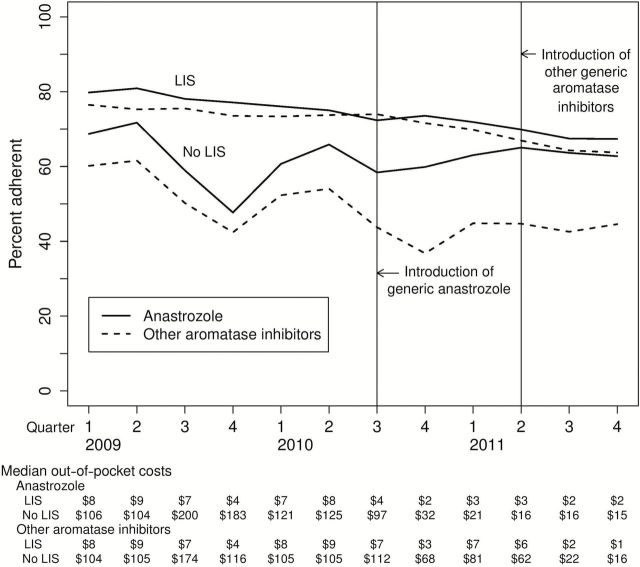
Figure 2 shows unadjusted adherence patterns by medication type. As expected, the decline in adherence to individual medications among LIS recipients showed no visible change after introduction of generic equivalents. In contrast, among non-LIS subjects, there was a sharp reduction in the rate of decline of anastrozole adherence with the 2010 generic anastrozole introduction. This was consistent with the large reductions in out-of-pocket costs shown below the figure. Among women without a subsidy, the median quarterly out-of-pocket cost of anastrozole fell from $183 in the fourth quarter of 2009 to $15 in 2011. A reduction in the rate of decline also occurred with exemestane and letrozole in 2011.
Figure 2.
Percent adherence to endocrine therapy by medication type. Figure 2 shows the unadjusted percent adherence (medication possession ratio ≥ 80%) to endocrine therapy by medication type among patients receiving Medicare D by receipt of a low-income subsidy (LIS). Exemestane and letrozole are grouped together in these figures. Median 90-day out-of-pocket costs are also shown. LIS = low-income subsidy.
Table 2 presents unadjusted (raw) quarterly averaged adherence measures among LIS and non-LIS beneficiaries by generic availability (column one) and the effects of generic availability in a multivariable regression (column 2). As indicated by the odds ratios presented in column 2, the pattern of improved adherence (reduced decline) among non-LIS recipients persisted with adjustment for age, comorbidities, years since AI initiation, and other possible confounders. Odds ratios for anastrozole reflect odds of adherence by quarter after generic introduction compared with the odds of adherence by quarter before generics, and ranged from 1.08 (95% confidence interval [CI] = 1.02 to 1.14) in Quarter 1 to 1.51 (95% CI = 1.44 to 1.58) in Quarter 4 (when traditionally adherence rates were at their lowest). For exemestane/letrozole, there was no difference in the odds ratio by quarter (ie, no interaction between quarter and generic), and the odds of adherence after generic introduction was 1.47 (95% CI = 1.40 to 1.55) times greater than the odds of adherence before these generics. In contrast, and consistent with expectations, there were minimal or no effects of generic medications in any quarters among LIS beneficiaries—for whom out-of-pocket expenditures were minimally or not at all affected by the availability of generic AIs.
Table 2.
Association of generic introductions and low-income subsidy status with adherence to endocrine therapy for breast cancer
| Low-income subsidy status | Average proportion of population adherent (unadjusted) | Model-based adjusted odds ratio (95% CI) comparing odds of adherence after generic available compared with odds of adherence before generics* | |||
|---|---|---|---|---|---|
| No generics available | Generic anastrozole available | Generic letrozole and exemestane available | Generic anastrozole available | Generic letrozole and exemestane available | |
| LIS | 0.94 (0.88 to 1.00) | ||||
| Quarter | |||||
| 1 | 76.73 | 70.82 | -† | 0.94 (0.88 to 1.01) | |
| 2 | 76.56 | - † | 68.47 | 0.95 (0.88 to 1.02) | |
| 3 | 76.90 | 72.77 | 65.88 | 0.93 (0.87 to 0.99) | |
| 4 | 75.67 | 72.55 | 65.61 | 0.99 (0.92 to 1.06) | |
| No LIS | 1.47 (1.40 to 1.55) | ||||
| Quarter | |||||
| 1 | 61.96 | 57.36 | -† | 1.08 (1.02 to 1.14) | |
| 2 | 64.98 | -† | 58.62 | 0.70 (0.66 to 0.74) | |
| 3 | 56.06 | 53.83 | 56.99 | 1.05 (1.00 to 1.10) | |
| 4 | 46.28 | 52.79 | 57.01 | 1.51 (1.44 to 1.58) | |
* Model was also adjusted for age, comorbidity, number of medications, time since introduction of endocrine therapy (all P < .001), race (P = .03), and appropriate two-way interactions. The model-based adjusted odds ratios shown represent: 1) the effect of the anastrozole generic introduction by low-income subsidy (LIS) status and by quarter as measured by the ratio of odds of adherence by quarter after anastrozole introduction compared with before generic introduction and 2) the effect of the other generic (exemestane, letrozole) introductions by LIS status as measured by the ratio of odds of adherence after introduction of other generics compared with before other generics’ introduction. No LIS by quarter interactions were observed in the letrozole/exemestane generic introduction period. CI = confidence interval; LIS = low-income subsidy.
† There were no second quarters where only generic anastrozole was available (by April 2011 letrozole and exemestane were also available), and there was no first quarter where letrozole and exemestane were also available (the study period ended before January 2012). All statistical tests were two-sided.
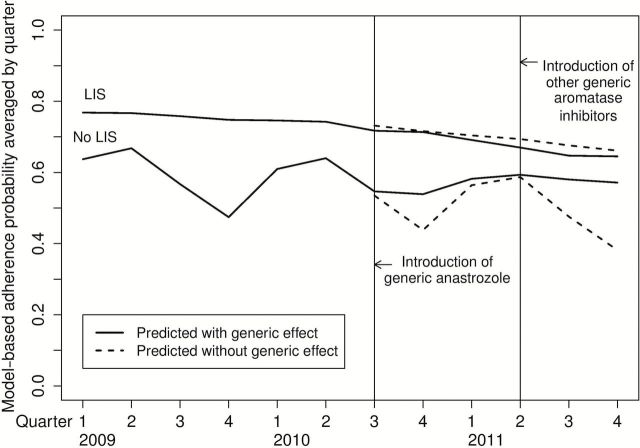
Regression-adjusted average predicted probabilities of adherence that demonstrate the effect of generics on the probability of adherence are shown in Figure 3. The average predicted probability of adherence among patients without LIS was 5.4% higher in the nine-month period after generic anastrozole was introduced than if no generic had been available and 11% higher in the subsequent nine months after generic letrozole and exemestane were introduced. There was no statistically significant change in the model-based probability of adherence among breast cancer survivors receiving a LIS.
Figure 3.
Model-based predicted average probability of adherence. The curves depict average multivariable logistic regression model–predicted probabilities with and without the generic effect in the postgeneric period. All statistical tests were two-sided.
Discussion
Downward trends in endocrine therapy adherence among a nationwide cohort of Medicare D enrollees with breast cancer improved substantially following July 1, 2010 when generic anastrozole became available and then were further improved as exemestane and letrozole generics were released in 2011. The differences in adherence following generic introduction were attributable primarily to better adherence trends among women who did not have the extra financial help provided by a low-income subsidy.
Our study findings for patients without subsidies add to prior research regarding the importance of copays in oncologic drug adherence (17,18,26,27). Our results are complementary to a recent study by Hershman et al. of younger and Medicare Advantage patients (27). Our study also takes advantage of a natural experiment to examine adherence longitudinally as generic, cheaper medicines became available. While our finding that the introduction of a generic slows the decline in adherence might seem intuitive, some studies outside of oncology that examined the effects of copay decreases have found disappointingly small effects, perhaps because patients who had discontinued medications because of high out-of-pocket costs did not learn about later cost decreases (28). Furthermore, costs to patients do not always decrease rapidly as generics are introduced; the initial introduction of a single generic simvastatin in the mid-2000s did not decrease costs substantially and had only small effects upon adherence (29).
The improvements in the probability of adherence among unsubsidized patients in our study with the introduction of generics (5.4% after anastrozole was introduced and 11% after letrozole/exemestane was introduced) were substantial. The larger observed effect after letrozole/exemestane may reflect a higher level of competition, but it is also possible that there was some delay in the full effect of generic anastrozole introduction. Nonetheless, the total effect is large, and its clinical significance is supported by prior studies. Several randomized trials support the importance of adherence to attain the full benefits of AIs (3), and a large cohort study found that patients who discontinued AIs early had 7.1% lower 10-year survival, and those with adherence under 80% had 3.9% lower 10-year survival (16).
The seasonal pattern of worsening AI adherence in the pregeneric period for non-LIS subjects is consistent with an effect of the “donut hole,” or coverage gap, upon adherence and the amelioration of that cyclic effect when generic introduction reduced patients’ out-of-pocket costs. In 2009, patients entered the coverage gap when their total medication costs reached $2700 (average out-of-pocket costs $896); in 2010 the threshold increased slightly to $2830. While gap coverage was available in some plans, only 6% of patients nationwide enrolled in such plans (30). The current plan to close the coverage gap (at an estimated $42.6 billion taxpayer price) is to transition incrementally to an endpoint in 2020 of 25% patient out-of-pocket cost for medications (31,32). Many new brand-name oncology drugs cost thousands of dollars monthly, and out-of-pocket costs under this strategy would thus still be substantial. Furthermore, cost concerns may even be expanding to older generic medications. Generic manufacturers increased prices of one-third of older generic medications in 2013, with several rising over 1000% (33,34). Our results suggest that the Food and Drug Administration should use incentives such as rapid regulatory reviews and reduced review fees to foster and maintain low-priced generics. Other governmental regulatory bodies could also require insurers to cover medications such as oral oncologics based on their mortality benefit (value-based insurance design).
Subjects with a low-income subsidy had higher adherence throughout the study. This novel finding supports the effectiveness of the Medicare D subsidy policy in improving disparities in endocrine therapy use by socioeconomic status. Socioeconomic disparities have been found throughout the continuum of breast cancer care, including in AI adherence (35). Their reversal in our study population provides evidence of a success of the LIS program. The findings are also consistent with, though somewhat larger than, a recent report that LIS recipients had higher adherence to hypertension and lipid-lowering medications (36). Because LIS is a heterogeneous group that includes “dual-eligible” patients (ie, enrolled in both Medicare and Medicaid) as well as women with incomes above their state’s Medicaid eligibility threshold but below 150% of poverty level, further research should examine the extent of the benefit in different low-income groups. Further research into the effects of reducing out-of-pocket costs for low-income patients with cancer could provide important information for the Medicare D program, for insurance exchanges created by the Affordable Care Act (17) and for the Food and Drug Administration.
Our study has several limitations. Like many prior studies using pharmacy data, we were unable to examine patients who never started their medications (37), and it is possible that there is substantial “primary nonadherence” that we could not identify. Nonetheless, our study’s strong design as a natural experiment on individual patients and our ability to make comparisons between LIS and non-LIS patients all enhance its validity. Information about extent of disease was unavailable in our study, and therefore patients of all stages were included. Because few patients with stage 4 disease have surgery, we estimate that only about 50% of stage 4 patients or a total of 325 women were enrolled in the study (19); however, they are more likely than those with lower-stage disease to have changes made to their medications because of progression of disease. Nevertheless, it is unlikely that cancer stage could explain the relative differences in adherence found in our study. Although prescription fills are strongly associated with adherence (21), they are an imperfect proxy as they do not measure actual patient medication use. Our study was limited to three years, including 18 months of information before and after anastrozole generics were released and nine months after letrozole and exemestane. Finally, although patients who switched medication were examined in the study, we did not directly examine how switching medications might play a role in adherence.
In conclusion, there were important improvements in adherence trends to breast cancer endocrine therapy by Medicare D enrollees as generic medications became available. Our findings highlight the inadequacy of the unsubsidized Medicare D program in providing the most effective medications for a group of patients with life-threatening illness. The other finding of our study, that receipt of a low-income subsidy is associated with increased adherence, supports the potential for governmental and regulatory bodies to improve patient supports in the future. Attention to such strategies is important; alternative behavioral interventions to improve adherence have been costly, complicated and labor intensive, and have had small effects at best (38,39). Regulatory efforts to enhance rapid and continuing competition by generics, promote legislative coverage of drugs that reduce mortality, or directly reduce out-of-pocket costs would likely improve patient medication adherence.
Funding
This work was supported by the American Cancer Society (RSG-11-098-01-CPHPS to JMN) and the National Institutes of Health (NIH-NCI R01-CA127648 to LEP).
Supplementary Material
The funders have not participated in the conduct of this study. None of the authors have any conflicts to declare with this manuscript.
References
- 1. Weingart S, Brown E, Bach P, et al. NCCN Task Force Report: Oral chemotherapy. J Natl Compr Canc Netw. 2008;6:S1-S14. [PubMed] [Google Scholar]
- 2. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. [DOI] [PubMed] [Google Scholar]
- 4. Early Breast Cancer Trialists’ Collaborative Group. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gibson L, Lawrence D, Dawson C, et al. Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Syst Rev. 2009;(4):1–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. [DOI] [PubMed] [Google Scholar]
- 7. Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–606. [DOI] [PubMed] [Google Scholar]
- 8. Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26(4):556–562. [DOI] [PubMed] [Google Scholar]
- 9. Kimmick G, Anderson R, Camacho F, et al. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27(21):3445–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ziller V, Kalder M, Albert U-S, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. 2009;20(3):431–436. [DOI] [PubMed] [Google Scholar]
- 11. Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 12. Lash TL, Fox MP, Westrup JL, et al. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99(2):215–220. [DOI] [PubMed] [Google Scholar]
- 13. Partridge AH, Avorn J, Wang PS, et al. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94(9):652–661. [DOI] [PubMed] [Google Scholar]
- 14. McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99(11):1763–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barron TI, Connolly R, Bennett K, et al. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer. 2007;109(5):832–839. [DOI] [PubMed] [Google Scholar]
- 16. Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29(18):2534–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riley GF, Warren JL, Harlan LC, et al. Endocrine therapy use among elderly hormone receptor-positive breast cancer patients enrolled in Medicare Part D. Medicare Medicaid Res Rev. 2011;1(4):E1-E26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nattinger AB, Laud PW, Bajorunaite R, et al. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res. 2004;39(6 Pt 1):1733–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chernew ME, Shah MR, Wegh A, et al. Impact of decreasing copayments on medication adherence within a disease management environment. Health Affair. 2008;27(1):103–112. [DOI] [PubMed] [Google Scholar]
- 21. Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: Methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–116. [DOI] [PubMed] [Google Scholar]
- 22. Donohue JM, Cevasco M, Rosenthal MB. A decade of direct-to-consumer advertising of prescription drugs. N Engl J Med. 2007;357(7):673–681. [DOI] [PubMed] [Google Scholar]
- 23. Schneeweiss S, Patrick AR, Pedan A, et al. The effect of Medicare Part D coverage on drug use and cost sharing among seniors without prior drug benefits. Health Affairs. 2009;28(2):w305-w316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gu Q, Zeng F, Patel BV, et al. Part D coverage gap and adherence to diabetes medications. Am J Manag Care. 2009;16(12):911–918. [PubMed] [Google Scholar]
- 25. Fung V, Mangione CM, Huang J, et al. Falling into the coverage gap: Part D drug costs and adherence for Medicare Advantage prescription drug plan beneficiaries with diabetes. Health Serv Res. 2010;45(2):355–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dusetzina SB, Winn AN, Abel GA, et al. Cost Sharing and Adherence to Tyrosine Kinase Inhibitors for Patients With Chronic Myeloid Leukemia. J Clin Oncol. 2014;32(4):306–311. [DOI] [PubMed] [Google Scholar]
- 27. Hershman DL, Tsui J, Meyer J, et al. The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. J Natl Cancer Inst. 2014;106(11):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loewenstein G, Asch DA, Volpp KG. Behavioral economics holds potential to deliver better results for patients, insurers, and employers. Health Affairs. 2013;32(7):1244–1250. [DOI] [PubMed] [Google Scholar]
- 29. Sedjo RL, Cox ER. Lowering copayments: impact of simvastatin patent expiration on patient adherence. Am J Manag Care. 2008;14(12):813–818. [PubMed] [Google Scholar]
- 30. Hoadley J, Summer L, Hargrave E, Cubanski J, Neuman T. Analysis of Medicare Prescription Drug Plans in 2012 and Key Trends Since 2006. The Henry J. Kaiser Foundation, 2012. http://kaiserfamilyfoundation.files.wordpress.com/2013/01/8357.pdf. Accessed November 2012.
- 31. U.S. Congress. 111th Congress. 2nd Session. H. R. 4872, The Health Care and Education Reconciliation Act Washington: Government Printing Office, 2010. [Google Scholar]
- 32. Weaver C. Closing Medicare drug gap helps Democrats sell reform. Kaiser Health News. 2010. http://www.kaiserhealthnews.org/stories/2010/march/29/health-reform-doughnut-hole.aspx. Accessed January 2015.
- 33. Alpern JD, Stauffer WM, Kesselheim AS. High-cost generic drugs--implications for patients and policymakers. N Engl J Med. 2014;371(20):1859–1862. [DOI] [PubMed] [Google Scholar]
- 34. Perrone M. Soaring generic drug prices draw Senate scrutiny. Associated Press. Nov 20, 2014. http://news.yahoo.com/soaring-generic-drug-prices-draw-senate-scrutiny-141933064--finance.html. Accessed January 2015.
- 35. Yen TWF, Czypinski LK, Sparapani RA, et al. Socioeconomic factors associated with adjuvant hormone therapy use in older breast cancer survivors. Cancer. 2011;117(2):398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li P, McElligott S, Bergquist H, et al. Effect of the Medicare Part D coverage cap on medication use among patients with hypertension and hyperlipidemia. Ann Intern Med. 2012;156(11):776–784. [DOI] [PubMed] [Google Scholar]
- 37. Tamblyn R, Eguale T, Huang A, et al. The incidence and determinants of primary nonadherence with prescribed medication in primary care: a cohort study. Ann Intern Med. 2014;160(7):441–450. [DOI] [PubMed] [Google Scholar]
- 38. Kripalani S, Yao X, Haynes R. Interventions to enhance medication adherence in chronic medical conditions: A systematic review. Arch Intern Med. 2007;167(6):540–549. [DOI] [PubMed] [Google Scholar]
- 39. McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions. J Am Med Assoc. 2002;288(22): 2868–2879, correction. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.