Abstract
Increased diacylglycerol (DAG) levels are observed in numerous pathologies, including conditions associated with bone loss. However, the effects of DAG accumulation on the skeleton have never been directly examined. Because DAG is strictly controlled by tissue specific diacylglycerol kinases (DGKs), we sought to examine the biological consequences of DAG accumulation on bone homeostasis by genetic deletion of DGKζ, a highly expressed DGK isoform in osteoclasts (OCs).
Strikingly, DGKζ−/− mice are osteoporotic due to a marked increase in OC numbers. In vitro, DGKζ−/− bone marrow macrophages (BMMs) form more numerous, larger and highly resorptive OCs. Surprisingly, while increased DAG levels do not alter RANK/RANKL osteoclastogenic pathway, DGKζ deficiency increases responsiveness to the proliferative and pro-survival cytokine M-CSF. We find that M-CSF is responsible for increased DGKζ−/− OC differentiation by promoting higher expression of the transcription factor c-Fos, and c-Fos knockdown in DGKζ−/− cultures dose-dependently reduces OC differentiation. Using a c-Fos luciferase reporter assay lacking the TRE responsive element, we also demonstrate that M-CSF induces optimal c-Fos expression through DAG production. Finally, to demonstrate the importance of the M-CSF/DGKζ/DAG axis on regulation of c-Fos during osteoclastogenesis, we turned to PLCγ2+/− BMMs, which have reduced DAG levels and form fewer OCs due to impaired expression of the master regulator of osteoclastogenesis NFATc1 and c-Fos. Strikingly, genetic deletion of DGKζ in PLCγ2+/− mice rescues OC formation and normalizes c-Fos levels without altering NFATc1 expression. To our knowledge, this is the first report implicating M-CSF/DGKζ/DAG axis as a critical regulator of bone homeostasis via its actions on OC differentiation and c-Fos expression.
Keywords: M-CSF, DGKζ, DAG, c-Fos, Osteoclasts
Introduction
Diacylglycerol (DAG) is an important second messenger involved in a variety of cellular responses including proliferation, differentiation, motility and secretion (1). DAG also acts as a precursor in the synthesis of triglycerides and certain membrane phospholipids (2). Thus, tight spatial and temporal regulation of DAG must be achieved for signal specificity. Accumulating evidence suggests the existence of three separate pools of DAG, differing in their fatty acid compositions and independently regulated. The most significant source of DAG originates from the phospholipase C (PLC) family of enzymes, which produce DAG from either phosphatidylinositol (PI) or phosphatidylcholine (PC) (3). Among them, PLCγ is known to generate DAG in response to receptor and non-receptor tyrosine kinases (3). A second source of DAG comes from phosphatidic acid (PA) dephosphorylation (3). Finally, the third source of DAG is generated by the activity of sphingomyelin synthases during the synthesis of sphingomyelin (3). The fatty acid compositions of the DAGs formed by these various routes reflect the composition of the parent phospholipids and dictate the specificity of DAG-mediated effects in vivo (4).
Bone loss is a key feature of numerous pathologies including osteoporosis, rheumatoid arthritis and bone metastases. Osteoclasts (OCs) are the specialized cells which resorb bone (5). Evidence suggests that DAG plays an important role in regulating OCs. Genetic deletion of PLCγ2, the enzyme that converts PIP2 into IP3 and DAG in the OC, leads to an osteopetrotic bone phenotype due to blockade of OC differentiation (6, 7). Ablation of DAG-dependent protein kinase C delta (PKCδ) leads to defective bone resorption and high bone mass (8). Similarly, inhibitors of classical and novel PKCs reduce bone resorption in vitro and in vivo (9, 10). Conversely, deletion of acyl-CoA-diacylglycerol acyltransferase (DGAT-1), the main catabolizing enzyme involved in DAG-mediated formation of triglycerides, leads to osteopenia owing to increased OC numbers (11). All these findings indirectly suggest that DAG is an important regulator of osteoclastogenesis and/or bone resorption. However, the direct effects of DAG on the OCs and bone metabolism have not been yet elucidated. DAG is a small molecule and it cannot be targeted by traditional genetic knockout approaches. So far pharmacological approaches using the synthetic DAG analog, phorbol ester myristate (PMA), or exogenous DAG species have revealed conflicting results regarding bone resorption and OC formation (12–14). Considering that increased diacylglycerol (DAG) levels are observed in conditions associated with bone loss, understanding the direct effects of DAG on the skeleton is mandatory but it requires an animal model of altered DAG metabolism.
Diacylglycerol kinases (DGK) are a class of enzymes that convert DAG into PA, thus providing strict modulation of DAG levels in the cell (15). Ten isozymes exist, each showing unique tissue expression and distinct subcellular localization (15). The diversity in expression, localization, and activity provides each DGK isoform the ability to modulate specific DAG species/pools and therefore to take part in different signaling axes (4, 15). We have identified that DGKζ is highly expressed in macrophages and OCs. Thus, analysis of DGKζ−/− mice would allow us to answer these critical questions: is DAG directly involved in OC differentiation and, if so, how? Can altered DAG levels in OC lineage cells affect bone homeostasis? And what signaling pathways modulate DAG levels in the OC? Herein, we demonstrate that accumulation of DAG through DGKζ deletion leads to a substantial osteoporotic bone phenotype owing to increased OC numbers. Surprisingly, increased DAG contributes to upregulation of the osteoclastogenic transcription factor c-Fos in response to M-CSF but not RANKL. We also demonstrate that restoring DAG levels in PLCγ2 haplo-insufficient OCs rescues OC formation and normalizes c-Fos expression. This is the first report to definitively establish the importance of this second messenger in OCs and thereby in bone homeostasis. Furthermore, our data also provide a paradigm shift demonstrating that M-CSF potently induces c-Fos expression through DAG production, thereby exacerbating OC differentiation in conditions where RANKL signaling alone would not have maximal effects.
Materials and methods
Mice
DGKζ−/− mice were kindly provided by Dr. Gary A Koretzky (Joan and Sanford I. Weill Department of Medicine, Weill Cornell Medical College, Ithaca, NY) and PLCγ2+/− by Dr. JN Ihle (St. Jude Children’s Research Hospital, Memphis, Tennessee, USA). All experiments were approved by the Washington University School of Medicine animal care and use committee. Male and female mice (C57BL/6 background), 4–8 weeks of age, were used in the study. Mice were housed in cages and were fed with food and water ad libitum, with a 12 h light and 12 h dark cycle.
Histology and in vivo μCT
Histological analysis was performed on five-micron longitudinal sections of fixed, decalcified, paraffin-embedded tibiae stained with TRAP to detect OCs. Osteoblast activity was assessed in calcein labelled, non-decalcified, methacrylamide embedded-sections. Analysis was performed using a Nikon Eclipse 80i microscope and a 20X objective lens. Quantitative histological parameters were assessed using the BIOQUANT OSTEO software (BIOQUANT Image Analysis Corporation, Nashville, TN). For in vivo analysis, mice were anesthetized with isoflurane and bones were scanned at an x-ray potential of 70kV, a current of 114 μA, an integration time of 100 ms and at a voxel size of 21 μm using the viva μCT40 scanner (Scanco Medical AG, Bassersdorf, Switzerland). Three-dimensional models were constructed and a trabecular region 0.5 mm below the growth plate of the distal femur extending 100 sections was analyzed in Scanco image analysis software, in accordance to standard guidelines (16). All trabecular measurements were made by manually delineating cortical and trabecular bone, with an adaptive threshold of 155–255.
OC differentiation and bone resorption
Bone marrow was flushed from the long bones of 6–8 week old C57BL/6 WT or DGKζ−/− mice and cultured in α-minimum Eagle’s medium (α-MEM; Sigma-Aldrich, St. Louis, MO) containing 5% heat-inactivated fetal bovine serum, glutamine and 1/50 volume CMG 14–12 culture supernatant as source of M-CSF (17) for 3 days to generate BMMs. For osteoclastogenesis, BMMs were further cultured in the presence of 100 ng/ml GST-RANKL (purified from BL21 with pGEX-6-RANKL plasmid, using HOOK GST protein purification kit, G-BIOSCIENCES, St. Louis, MO) and 25 ng/ml M-CSF (Sigma-Aldrich, St. Louis, MO) for an additional 3–5 days. For TRAP staining, cells were fixed in 4% paraformaldehyde and stained using the leukocyte acid phosphatase kit (Sigma-Aldrich). For bone resorption, BMMs were cultured on tissue culture plates in the presence of osteoclastogenic medium for 2 days, lifted, and replated in equal number on bone slices for additional 2 days. Cells were scraped and bones stained with 20 μg/ml peroxidase-conjugated wheat-germ agglutinin (Sigma-Aldrich) for 1h at room temperature, followed by incubation with 3,3′-diaminobenzidine (Sigma-Aldrich) for 30 min. Bone resorptive pits were analyzed using a light microscope (Nikon) and quantified using Image J software.
Real-time PCR
Total RNA was isolated using RNeasy mini kit (Qiagen). 1 μg of total RNA was reverse-transcribed to cDNA using High Capacity cDNA Reverse Transcription Kit (life technologies, Grand Island, NY). For quantitative real-time PCR, SYBR Green PCR master mix (Life Technologies) and specific synthesized primers ((IDT Technologies, Coralville, IA) were used. The primer sequences are included in supplemental method table 1. mRNA expression levels were normalized to the respective cyclophilin A mRNA and the 2 −ΔΔCt method was used to determine the relative amounts of mRNA transcribed.
RANKL, M-CSF, PMA Stimulation and Immunoblotting
BMMs (cells in culture with 100ng/ml M-CSF) or preOCs (cells in culture with 100ng/ml RANKL and 25ng/ml M-CSF for 2–3 days) were serum and cytokine starved for 2 h and then stimulated with 100 ng/ml GST-RANKL, 100 ng/ml M-CSF or 1–500 nM PMA for the indicated times. In adhesion assays, cells were lifted and replated in equal numbers onto pRGD coated dishes for indicated times. Cells were harvested in lysis buffer containing protease-phosphatase inhibitor cocktail (Thermo SCIENTIFIC). To obtain nuclear extracts, cells were lysed with hypotonic buffer (10 mM HEPES, 1.5 mM MgCl2, 1 mM KCl, 1 mM DTT, and protease and phosphatase inhibitors) followed by the addition of 0.1% Nonidet P-40 (Sigma-Aldrich). After centrifugation, the supernatants were collected (cytosolic fraction), whereas the pellets (nuclear fraction) were suspended in high salt buffer (hypotonic buffer plus 400 mM NaCl). 25 μg of protein from each condition was subjected to immunoblotting. 1:500 dilutions of primary antibodies were used; DGKζ c-terminus (Santa Cruz Biotechnology, Dallas, Texas), actin, H3, GAPDH, IKBα, PKC isoforms, PKD2, ERK, JNK/SAPK, AKT, P38 and c-Fos (Cell Signaling Technology, Danvers, MA). Specific proteins were detected with Super Signal West Pico Chemiluminescent Substrate (Thermo SCIENTIFIC, Rockford, IL) and imaged using the SYNGENE chemiluminescent Imaging System (SYNOPTICS GROUP, Alexandria, VA).
AP1/c-Fos binding assay
Biotinylated complementary AP1 oligos (5′-CGCTTGATGACTCAGCCGGAA-3′) were synthesized (IDT Technologies, Coralville, IA) and annealed at 100 μM final concentration. 50 μl of biotinylated oligos were mixed with 100 μl streptavidin agarose beads (Sigma-Aldrich) for 1 h at 4°C, washed and re-suspended in 100 μl 1x binding buffer with DTT plus protease inhibitors. For c-Fos pull-down, 50 μg nuclear extract was mixed with 1 μl poly dIdC (1 μg/μl) (Sigma-Aldrich) and 5 μl AP1 oligo-beads. The mixture was rotated overnight at 4°C, washed, and resuspended in 50 μl 1x sample loading buffer (30 mM NaCl, 10 mM Tris pH7.4, 1 mM EDTA, 5% glycerol, 1 mg/ml BSA and β-Mercaptoethanol). The beads were boiled, and supernatants were subjected to immunoblotting.
Lipid extraction, liquid chromatography and mass spectrometry
BMMs were stimulated with 100 ng/ml M-CSF and homogenized in 600 μL of PBS using the Omni Bead Ruptor 24 (Omni International, Inc., Kennesaw, GA). A modified Bligh-Dyer extraction method (18) was used to extract lipids in the presence of internal standard DAG 15:0–15:0. Sample analysis was performed with a Shimadzu 10A HPLC system coupled to a TSQ Quantum Ultra triple quadrupole mass spectrometer operated in SRM mode under ESI (+). Data processing was conducted with Xcalibur software (Thermo SCIENTIFIC). The analyte concentration was calculated as the concentration of its corresponding internal standard multiplied by the peak area ratio of the analyte to the internal standard, based on the assumption that the MS responses of the analyte and internal standard are the same. The DAG species concentrations were normalized to respective DNA content.
Expression plasmids and transfection
Luciferase reporter genes, WT-c-Fos and ΔTRE-c-Fos plasmids were a gift from Dr. Ron Prywes (Department of Biological Sciences, Columbia University, New York, NY). Chimeric EpoR/c-Fms receptor construct, containing the external domain of erythropoietin fused to the cytoplasmic domain of M-CSF receptor, was a gift from Dr. Steven Teitelbaum (Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, MO). WT or mutant c-Fos luciferase reporters expressed in pGL3 vector and EpoR/c-Fms in pMX retrovirus vector were transiently transfected into HEK293T cells using PolyJet DNA in vitro Transfection Reagent (SignaGen Laboratories, Gaithersburg, MD). After 24 h the media was changed, cells were starved overnight, and stimulated with 100 nM PMA (Sigma-Aldrich), 5% serum or 100 ng/ml human recombinant erythropoietin (Sigma-Aldrich) for indicated time.
Lentiviral c-Fos shRNA knockdown
Two c-Fos lentiviral ShRNA constructs were designed and purchased from Washington University RNAI CORE (Saint Louis, MO). shRNA1 targets the sequence GCGGAGACAGATCAACTTGAA (nucleotides 638 to 658) and shRNA2 CCTGTCAACACACAGGACTTT (nucleotides 266 to 286) of c-Fos mRNA (NCBI accession number NM_010234.2). Briefly, the c-Fos shRNAs (alone or in combination) or control shRNA (targeting LacZ sequence CGACCACGCAAATCAGCGATT) were co-transfected with the packaging plasmid (psPAX2) and the envelope plasmid (pMD2.G) into HEK293T cells. Medium containing the lentivirus was harvested 48 hrs after transfection, filtered, and was used to infect DGKζ−/− BMMs cultured in osteoclastogenesis medium (containing 100 ng/ml RANKL and 25 ng/ml M-CSF) to assess OC differentiation. A parallel set of cells was used to confirm c-Fos knocked-down by Western blot analysis.
Statistics
Data were analyzed using the GraphPad PRISM 5 software (GraphPad Software, Inc. La Jolla, CA). Student’s t-test was performed to determine the statistic P values of experimental data compared to a reference. P less than 0.05 was considered to be statistically significant.
Results
DGKζ is expressed in osteoclasts and controls bone homeostasis
To evaluate the importance of DAG in modulating bone homeostasis, we turned to the DGK family of kinases, which upon activation convert DAG into PA (19). Thus, removal of critical DGKs would allow us to examine the biological effects of increased DAG levels on bone mass. We first examined expression of all ten DGK isoforms by qRT-PCR in bone residing cells. DGKζ is highly expressed in BMMs and OCs (Figure 1A), although its levels gradually decrease in mature OCs compared to their precursors (Figure 1B). DGKζ is also expressed to a lesser extent in osteoblasts, T cells, neutrophils, and dendritic cells. Analysis of DGKζ protein levels reveals both cytoplasmic and nuclear localization. Short-term stimulation with M-CSF or RANKL, two cytokines that induce macrophage proliferation and OC differentiation respectively, does not alter the protein distribution (Figure 1C–D).
Figure 1. DGKζ is highly expressed in BMMs and OCs.
(A) qPCR of the relative mRNA levels of DGK isoforms in the cells and tissue indicated (BMM: Bone marrow derived macrophages, OC: Osteoclasts, Nφ: Neutrophils, OB: Osteoblasts, DC: Dendritic cells. (B) qPCR analysis of DGKζ in WT BMMs (black bar) and OCs (white bars) cultured in M-CSF alone (day0) or osteoclastogenic medium (M-CSF + RANKL) for 2, 4 or 6 days. Columns represent the average of three technical replicates normalized to the DGKζ mRNA at day zero (* and ** represent P < 0.05 and P <0.001 respectively). (C–D) Western blot for DGKζ expression in the nuclear and cytoplasmic fractions of WT BMMs treated with (C) RANKL (100 ng/ml) or (D) M-CSF (100 ng/ml) for 0, 1, 2 or 4 hours. GAPDH and H3 were used as loading controls.
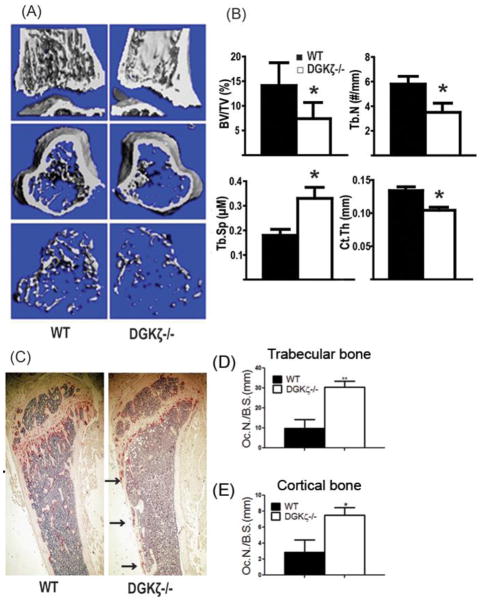
Next, we sought to determine the biological role of DGKζ on bone homeostasis in vivo. Strikingly, viva-CT scans of distal femurs from 4 and 8 week-old DGKζ−/− mice reveal an osteoporotic bone phenotype, with significant reduction in bone volume/total volume (BV/TV), cortical thickness, trabecular number (Tb. N.), and increased trabecular spacing (Tb. Sp) compared to WT littermates (Figure 2A–B and Supplemental Figure 1A–B). Thinning of the cortical bone is also observed in DGKζ−/− mice versus WT (Figure 2B; cortical thickness in μm WT: 0.134±0.01; KO: 0.104±0.01; p<0.001). Bone histomorphometric analysis of tartrate-resistant acid phosphatase (TRAP) stained tibia sections from 6 weeks old mice reveals increased OC number normalized to bone surface in the trabecular bone of KO mice compared to WT (Figure 2C, D p = 0.01) as well as increased number of OCs on the cortical bone (Figure 2E; p<0.01). Ex-vivo μCT analysis performed in the 6 weeks old set of mice confirms the osteoporotic bone phenotype detected by viva-CT (not shown). Because DGKζ is also expressed at low levels in osteoblasts, next we determined whether decreased osteoblast function might also contribute to the osteoporotic bone phenotype of DGKζ−/− mice. To this end, we measured bone formation rates (BFR) and mineralization apposition rates (MAR) in 4 week-old mice following two consecutive calcein injections at a 5 day interval. Our analysis reveals no differences between WT and DGKζ−/− animals in OB activities (Supplemental Figure 2A–B). Furthermore, mRNA levels of RANKL, OPG and M-CSF isolated from snap-frozen long bones are similar in both WT and KO mice, thus indicating that DGKζ deficiency impacts bone mass primarily by targeting intrinsic pathways in the OCs (Supplemental Figure 2C).
Figure 2. DGKζ−/− mice exhibit an osteoporotic bone phenotype due to increased OC numbers.
(A) Representative Viva-CT images of distal femurs from 4 week-old WT and DGKζ−/− mice. (B) Quantitative analysis of bone parameters including: bone volume per tissue volume (% BV/TV); trabecular number per bone perimeter (Tb.N), trabecular separation (Tb.Sp); and cortical thickness (Ct.Th). Data are means ± SEM; n=8 mice per group (* represents P < 0.05 vs. WT). (C) Representative images of TRAP stained histological sections of the proximal tibia of 6 week-old WT and DGKζ−/− mice. (D–E) Number of OCs per bone surface (OCn/BS) in trabecular bone (D) and cortical bone (E) of WT and KO mice in C (means ± SEM, n = 4 WT and 4 DGKζ−/− mice).
DGKζ deficiency increases osteoclast differentiation and bone resorption
To further characterize the DGKζ−/− OC phenotype, we turned to our in vitro culture system. DGKζ−/− BMMs form larger and more numerous OCs than WT in the presence of RANKL (100 ng/ml) and M-CSF (25 ng/ml) (Figure 3A–B). To assess OC functionality, we plated equal numbers of committed OCs (BMMs cultured in osteoclastogenic cytokines for 3 days) on bone slices for 48 h and resorptive area was measured following cell removal by peroxidase-conjugated wheat-germ agglutinin staining. Presence of equal numbers of OC was confirmed by counting the numbers of actin rings, the functional OC unit, prior to removal of the cells (Supplemental Figure 3). DGKζ−/− OCs show a 2.5-fold increase in the percentage of resorbed area, demonstrating a higher resorptive capacity than WT (Figure 3C). In agreement with the above observations, qRT-PCR analysis shows a significant increase in markers of OC differentiation (NFATc1, TRAP, Calcitonin receptor, c-Fos), fusion (DC-STAMP, ATP6V0D2), and function (Cathepsin K, MMP-9) in DGKζ−/− cultures compared to WT (Figure 3D).
Figure 3. DGKζ deficiency increases OC number, size and function in vitro.
(A) TRAP staining and quantification of multinucleated OCs derived from WT and DGKζ−/− marrow cultures following exposure to RANKL (100 ng/ml) and M-CSF (25 ng/ml) for 5 days. (B) Analysis of OC size in 10X magnification images from panel A. Data are presented as mean ± SEM from 3 sets of experiments performed in triplicate (* represents P < 0.05). (C) Staining of resorptive pits (areas contoured by black lines) and analysis of % of resorbed bone by WT and DGKζ−/− OCs (D) q-PCR analysis of OC differentiation markers (TRAP, NFATc1, CLCR, c-FOS), fusion markers (DC-STAMP and ATP6V0D2), and resorption markers (CTSK and MMP-9) in WT and DGKζ−/− cells during OC differentiation. Data represent the mean ± SEM of two experiments performed in triplicate (* represent P < 0.05 vs. WT). (E) TRAP staining and quantification of WT or DGKζ−/− OCs cultured with RANKL (25 ng/ml) and different doses of M-CSF (5 ng/ml and 25 ng/ml) (P < 0.05 vs. WT). Data represent the mean ± SEM from a representative experiment performed in triplicate.
To exclude that the higher rate of osteoclastogenesis in DGKζ−/− cultures is dependent on increased proliferation of precursor cells, we plated equal numbers of committed OCs (BMMs cultured in osteoclastogenic cytokines for 3 days) in the presence of different concentrations of M-CSF and a fixed amount of RANKL. Higher numbers of DGKζ−/− OCs form at every dose of M-CSF tested (Figure 3E). Taken together, these results indicate that DGKζ is a negative regulator of osteoclastogenesis.
DGKζ controls PKC activation in response to integrin-mediated adhesion
Next, we analyzed signaling pathways activated in DGKζ deficient cells that could account for enhanced OC differentiation and resorption. Among DAG downstream effectors, novel and classical PKC members control MAPK and NF-κB activation in various cell types, as well as integrin mediated-responses. Thus, we measured phosphorylation of various PKC isoforms, MAPKs, and the negative regulator of NF-κB, namely IκBα, in response to RANKL and M-CSF. We also analyzed nuclear translocation of classical and alternative NF-κB subunits, p65 and Rel-B, in WT and DGKζ−/− BMMs stimulated with the two osteoclastogenic cytokines. Surprisingly, no major differences in the above pathways are noted between the two genotypes in response to either cytokine (Supplemental Figure 4, and Supplemental Figure 5). Interestingly, however, activation of PKC pathway, detected by the pan-phospho PKC antibody, and phosphorylation of the novel PKC isoform PKCδ, are increased in DGKζ−/− BMMs plated on pRGD, an integrin substrate (Supplemental Figure 6). Considering the importance of PKCδ in the bone resorptive process (8), this result suggests that DGKζ deficiency controls OC activity, but not OC formation, via DAG-mediated PKC activation.
DGKζ−/− osteoclasts display increased c-Fos levels in response to M-CSF
Searching for the mechanism by which DGKζ deficiency enhances osteoclastogenesis, we turned to c-Fos, an early transcription factor upregulated during osteoclastogenesis (20) (21, 22), whose activation is dependent on PLCγ2 pathway (6). Thus, we hypothesized that DGKζ deficiency may increase osteoclastogenesis by modulating c-Fos levels. We found that c-Fos levels are elevated in OC cultures from DGKζ−/− cells compared to WT (not shown). Since c-Fos is an immediate early gene, that can be rapidly induced in response to stimuli (23), we also measured c-Fos induction in WT and DGKζ−/− BMMs stimulated with RANKL or M-CSF. RANKL exposure leads to similar levels of c-Fos expression in WT and DGKζ−/− cells (Figure 4A). To our surprise, M-CSF induces higher and prolonged accumulation of c-Fos in both the cytoplasm and nucleus of DGKζ−/− cells (Figure 4B–C).
Figure 4. Increased c-Fos levels in DGKζ−/− OCs in response to M-CSF.
Western blot analysis of c-Fos in cytoplasmic fraction of BMMs stimulated with (A) RANKL (100 ng/ml) or (B) M-CSF (100 ng/ml). (C) Western blot analysis of nuclear c-Fos levels in preOCs stimulated with M-CSF (100 ng/ml). (D) Western blot analysis of c-Fos in preOCs treated with RANKL, M-CSF, or both for 1 h. (E-F) AP1 oligonucleotide pull-down of nuclear c-Fos from WT and DGKζ−/− preOCs stimulated with RANKL (100 ng/ml) or M-CSF (100 ng/ml) as indicated. Representative experiments are shown.
To directly compare the effects of RANKL and M-CSF on c-Fos levels, WT and DGKζ−/− preOCs were incubated with both cytokines alone or in combination for one hour. c-Fos protein levels are significantly lower in cells stimulated by RANKL compared to M-CSF in both genotypes (Figure 4D). Interestingly, no further induction of c-Fos over the levels induced by M-CSF is observed in response to both cytokines together. Importantly, while RANKL-mediated c-Fos induction is similar in the two genotypes, M-CSF, alone or in combination with RANKL, induces a much stronger elevation of c-Fos in DGKζ−/− cells compared to WT.
The ability of c-Fos to recognize and bind to consensus sequences in chromosomal DNA is essential to drive osteoclastogenesis (24). To investigate whether M-CSF induces c-Fos binding to the DNA in a DAG-dependent manner, equal concentrations of nuclear lysates from RANKL- and M-CSF-stimulated WT and DGKζ−/− preOCs were incubated with biotinylated labeled AP1 double-stranded oligonucleotides. While c-Fos/AP1 association is no different upon RANKL stimulation, the binding of c-Fos to AP1 oligos after M-CSF exposure is higher in DGKζ−/− cells compared to WT (Figure 4E–F).
M-CSF induces c-Fos upregulation via DAG
To determine whether DAG controls c-Fos expression in OC precursors, BMMs were treated with various concentrations of the non-hydrolyzable DAG analog PMA. Exposure to PMA rapidly induces c-Fos in both WT and DGKζ−/− cells in a dose-dependent manner (Figure 5A), suggesting that c-Fos is directly downstream of DAG signaling. We further confirmed that PMA can potently induce c-Fos expression by using a WT c-Fos luciferase reporter construct in HEK293T cells and show a similar increase in luciferase expression following PMA and serum as control (Figure 5B).
Figure 5. DAG production by M-CSF is required for c-Fos upregulation.
(A) Western blot analysis of DGKζ and c-Fos levels in WT and DGKζ−/− preOCs treated with PMA for 30 minutes. (B) Dual luciferase assay in HEK293T cells expressing Fos-WT reporter construct and treated with PMA (100 nM) or serum (5%) for 3 hours. Cells expressing empty vector and stimulated with serum are used as negative control. Experiments were performed twice in triplicate and data are presented as means ± SEM; * P < 0.05. (C) DAG specie analysis in WT and DGKζ−/− BMMs stimulated with M-CSF (100 ng/ml) or RANKL (100 ng/ml) for 5 or 30 minutes. Experiment was repeated three times and data are pooled together and expressed as fold induction compared to unstimulated cells. * P < 0.05 (D) Dual luciferase assay in HEK293T cells transfected as indicated and treated with erythropoietin (Epo, 100 ng/ml) for 3 or 6 h. Experiments were performed twice in triplicate and data are presented as means ± SEM; * P < 0.05.
Having found that DAG can induce c-Fos expression, next we measured DAG levels in WT and DGKζ−/− OC precursors stimulated with M-CSF or RANKL using liquid chromatography–mass spectrometry (LC/MS). M-CSF induces the generation of diverse DAG species, containing both saturated and unsaturated fatty acyl chains. Experiments were performed in triplicate using three different sets of mice per genotype and data were pooled together and expressed as fold change induction compared to unstimulated cells. Higher levels of DAG with Oleic/Palmitic, Palmitic/Palmitoleic, Stearic/Oleic, Oleic/Oleic, and Stearic/Arachidonic fatty acyl chains are observed in M-CSF-treated DGKζ−/− cells compared to WT (Figure 5C). Interestingly, RANKL exposure only minimally affects the generation of DAG species compared to M-CSF in both genotypes (Figure 5C). DGKζ deficiency does not alter Palmitic-Eicosenoic, Palmitic-Stearic or Palmitic/Palmitic DAG species neither in response to RANKL or M-CSF (supplemental Figure 7). All together, these data further demonstrate that DGKζ regulates DAG levels in response to M-CSF and not RANKL in the OC.
To further investigate the mechanism by which M-CSF-mediated DAG production promotes c-Fos expression, we turned to the c-Fos promoter region. This region harbors four enhancer elements, which respond to a variety of growth factors, cytokines, and small molecules (25). One of these regions, the TRE site, has been shown to activate c-Fos transcription in response to DAG analogs (26). Thus, we hypothesized that the TRE region is responsible for the activation of c-Fos in response to M-CSF. Due to technical difficulties in generating primary BMMs expressing c-Fos luciferase reporter constructs, we transfected HEK293T cells with a luciferase reporter construct containing the full-length c-Fos promoter (WT-Fos) or lacking the TRE region (ΔTRE-Fos), a mutant known to abrogate c-Fos expression in response to DAG (25). Cells were also co-transfected with a chimeric M-CSF receptor (c-Fms), containing the intracellular tail of c-Fms and the extracellular domain of the erythropoietin receptor (EpoR), thus enabling us to study M-CSF-dependent induction of c-Fos in this model. Double transfected cells were then stimulated with erythropoietin (Epo) for 3–6 hours to activate c-Fms signaling. After 3 hour stimulation, cells expressing ΔTRE-Fos show approximately 50% less luciferase activity in response to Epo than cells expressing WT-Fos, and this difference is more striking following 6 hour Epo stimulation (Figure 5D). Taken together, these results indicate that DAG production and the TRE site in the c-Fos promoter region are required for optimal expression of c-Fos by M-CSF.
DGKζ deficiency rescues the PLCγ2+/− osteoclast differentiation defect by increasing c-Fos levels
Next, to demonstrate that DGKζ deficiency promotes osteoclastogenesis via DAG-mediated c-Fos induction, we used 2 different approaches. Our first approach consisted of knocking down c-Fos in DGKζ−/− BMMs. We used 2 c-Fos shRNA lentiviral constructs, alone or in combination, or control shRNA. Cells were then cultured for 7 days with RANKL and M-CSF to induce OC differentiation and western blot for c-Fos expression was performed at the end of the experiment. We find a dose-dependent inhibitory effect of c-Fos knockdown on osteoclastogenesis in DGKζ null cultures (Figure 6A–B), thus demonstrating that c-Fos is required for OC differentiation in the absence of DGKζ.
Figure 6. DGKζ deletion rescues PLCγ2+/− OC differentiation by restoring c-Fos expression.
(A) Representative TRAP staining of DGKζ−/− OCs transfected with lentiviral constructs encoding control ShRNA or 2 different c-Fos ShRNAs. (B) Western blot analysis of cells in A to detect c-Fos expression levels. β-actin is used as loading control. (C) Representative TRAP staining of OCs from WT, DGKζ−/−, PLCγ2+/−, and DGKζ−/−; PLCγ2+/− cells. (D) Western blot analysis of NFATc1, c-Fos and β-actin as control during osteoclastogenesis from indicated genotypes. Blots were run in parallel and exposed identically.
Our second approach consisted on targeting DGKζ in PLCγ2 deficient OC cultures. We have previously shown that PLCγ2, an enzyme that converts PIP2 into IP3 and DAG, is required for OC formation (6, 8, 27) via upregulation of NFATc1 and c-Fos transcription factors. Thus, we hypothesized that genetic deletion of DGKζ in the PLCγ2−/− background could rescue osteoclastogenesis through increasing DAG levels and restoring c-Fos expression. We were unsuccessful in generating DGKζ−/−; PLCγ2−/− mice due to embryonic lethality, however, DGKζ−/−; PLCγ2+/− mice are viable. As previously reported (6), PLCγ2+/− BMMs form fewer and poorly spread OCs compared to WT and display reduced NFATc1 and c-Fos expression (Figure 6C–D). Notably, genetic deletion of DGKζ−/− in PLCγ2+/− cultures rescues OC formation and c-Fos expression, without affecting NFATc1 levels (Figure 6C–D). These data demonstrate that DAG is an important regulator of osteoclastogenesis downstream of the PLCγ pathway via modulation of c-Fos levels.
Discussion
Although DAG production occurs downstream of numerous signals known to control bone mass (6, 23, 28, 29), its role in bone homeostasis or whether increased DAG levels contribute to bone fragility or osteopenia has never been investigated. Our study demonstrates that the accumulation of DAG in the OC has deleterious consequences for the maintenance of healthy bone mass.
Investigating the in vivo role of DAG has been extremely difficult because of its short half-life (30, 31). In addition, genetic deletion of this second messenger cannot be achieved, and pharmacological approaches using synthetic DAG or non-hydrolyzable compounds (i.e. PMA) may not provide an accurate representation of the complex nature of DAG signaling. To overcome this caveat, we turned to DGKζ, the enzyme that catabolizes DAG to PA (19), which is highly expressed by OC precursors. Accumulation of DAG in DGKζ−/− OC precursors leads to severe osteopenia due to accelerated OC differentiation. Surprisingly, DAG does not appear to regulate signaling pathways downstream of the RANK/RANKL axis but modulates M-CSF-dependent activation of the osteoclastogenic transcription factor c-Fos (Model in Figure 7). These findings establish the role of DAG as a significant regulator of bone mass via its actions in the OCs and further expand the current role of M-CSF beyond cell proliferation and survival, demonstrating its importance in OC differentiation. M-CSF and RANKL are key osteoclastogenic cytokines.
Figure 7. Working model for M-CSF/DAG/c-Fos pathway during osteoclastogenesis.
M-CSF induces the generation of DAG, which is required for c-Fos expression during osteoclastogenesis. DGKζ deficiency induces DAG accumulation leading to higher c-Fos levels in response to M-CSF stimulation. The end result is a net increase in osteoclastogenesis.
M-CSF provides proliferative and survival cues, while RANKL governs OC maturation (32–34). Both cytokines induce activation of PLCγ, a class of hydrolytic enzymes that generate DAG (6, 35). We have previously shown that loss of PLCγ2 leads to severe osteopetrosis (6, 7). While the main mechanism of OC dysfunction in the context of PLCγ2 deficiency has been primarily attributed to impaired calcium-mediated NFATc1 expression, our new data suggest an additional mechanism of PLCγ2-mediated regulation of osteoclastogenesis through DAG signaling. Indeed, we demonstrate that impaired OC differentiation in PLCγ2 haploinsufficient mice can be rescued by deletion of DGKζ, which increases DAG levels and restores defective c-Fos expression, while NFATc1 levels remain unaltered. These findings stress the importance of DAG-mediated signaling during the OC differentiation process. While our study indicates that the pool of DAG metabolized by DGKζ controls osteoclastogenic pathways downstream of M-CSF, we cannot exclude that additional DAG pools, generated through other pathways, could modulate osteoclastogenesis and bone homeostasis. For example, mice lacking DGAT-1, an enzyme involved in DAG-mediated synthesis of triglycerides, develop osteopenia due to increased OC differentiation (11). Although the authors did not fully investigate the mechanism by which DGAT-1 deficiency induces accelerated osteoclastogenesis, our findings would suggest that increased DAG levels in DGAT-1−/− OC precursors might be responsible for this phenotype.
Previous studies on the role of DAG in OCs, including ours, have focused on targeting downstream DAG effectors, such as classical and novel PKC family members. These reports indicated that inhibiting PKC activation impairs OC resorptive activity (8, 10). Similarly, we observe that phosphorylation of PKCδ is increased in DGKζ−/− cells compared to WT in response to adhesion to an integrin substrate. This result is in agreement with the importance of PKCδ in the bone resorptive process and provides important insights into the mechanism by which DGKζ deficiency may regulate bone resorption. However, activation of PKCδ is highly unlikely to be responsible for increased osteoclastogenesis, as PKCδ−/− OCs develop normally (8). Indeed, we did not find differences in PKC phosphorylation between DGKζ−/− and WT cells in response to RANKL and M-CSF, suggesting an alternative mechanism for DAG effects during osteoclastogenesis. This result was unexpected since studies in other cell types lacking DGKζ, such as T cells and cardiomyocytes, showed that increased PKC activation was responsible for T cell development and cardiac dysfunction, respectively (36, 37). Interestingly, it has been reported that activation of PKCα in response to increased calcium levels decreases its sensitivity to further activation by DAG (38). Thus, it is possible that increased calcium oscillations during osteoclastogenesis drive PKC activation in the OC and prevent DAG from inducing further activation of the kinase. Recent reports have also suggested that the functional significance of DAG is not restricted to PKC pathway, and DAG also activates several proteins including Ras GRP, protein kinase D, and transient receptor potential proteins that could account for DAG specific effects in the cell (1). Nevertheless, this finding left us with the remaining question: how does accumulation of DAG control OC differentiation independently of PKC?
c-Fos is a critical transcription factor involved in OC differentiation, whose expression increases during the early stages of osteoclastogenesis, and can also be rapidly regulated by stimuli. c-Fos knockout mice develop a severe osteopetrotic phenotype due to absence of OCs (20). The current paradigm places expression of c-Fos primarily downstream of RANKL (22). Surprisingly, in this study we demonstrate that M-CSF, but not RANKL, induces maximal c-Fos upregulation through DAG production. Our findings also reveal that RANKL is a weaker inducer of c-Fos expression than M-CSF, and that RANKL does not provide further additive effects over the optimal levels of c-Fos induced by M-CSF alone. Perhaps the most surprising aspect of this finding is that although both cytokines could generate DAG via activation of PLCγ, only M-CSF utilizes this second messenger to induce c-Fos transcription. Accumulating evidence suggests the existence of separate pools of DAG, different in their fatty acyl compositions and independently regulated. For example, only DAG molecules that contain unsaturated fatty acyls (oleic, linoleic, linolenic) are able to activate PKC, whereas DAGs containing saturated fatty acyls (such as palmitic and stearic) cannot (39). We find that M-CSF produces high levels of DAG containing stearic/arachidonic, oleic-oleic, stearic-oleic and oleic-palmitic acyls in WT cells, and the levels are even higher in DGKζ−/−cells. Surprisingly, our data also show that RANKL produces very low levels of these DAG species, at least at the time points tested in our experiments, and that DAG levels are similar in both WT and KO cells. This important observation supports the notion that DGKζ specifically controls the DAG pool generated downstream of M-CSF in the OC.
Another possible explanation for the distinct requirement of DAG during c-Fos transcription is that RANKL and M-CSF activate different PLCγ isoforms, which could further explain why M-CSF and RANKL have different effects on the generation of DAG species. We previously demonstrated that RANKL activates PLCγ2 to induce osteoclastogenesis and PLCγ1 does not compensate for lack of PLCγ2 in this process (6). We also observed that M-CSF, but not RANKL, activates PLCγ1 (6) and its deletion by shRNA arrests osteoclastogenesis, despite normal expression levels of PLCγ2 (data not shown). While PLCγ2 controls NFATc1 in response to RANKL, it is plausible that PLCγ1 is activated downstream of M-CSF to generate DAG species involved in c-Fos expression. This assumption is further supported by the observation that DGKζ deletion in PLCγ2+/− preOCs rescues osteoclastogenesis by normalizing c-Fos but not NFATc1 levels.
Regulatory sequences in the c-Fos promoter have been extensively studied (26). Among them, the TRE site has been shown to activate c-Fos transcription in response to DAG analogs (26). In agreement with this observation, we show that PMA increases c-Fos protein levels in preOCs. More importantly, we demonstrate that M-CSF induces luciferase activity in HEK293T cells transfected with a full-length c-Fos promoter. M-CSF-triggered effects on c-Fos luciferase activity are significantly reduced in cells expressing the ΔTRE-c-Fos promoter. Unfortunately, we could not perform this experiment in primary BMMs because of technical difficulties in expressing luciferase constructs in primary cells. Nevertheless, the result from the luciferase assay in HEK293T cells, in conjunction with the data showing higher DAG and c-Fos levels in DGKζ−/−cells following M-CSF, but not RANKL, treatment support the importance of the M-CSF/DGKζ/DAG axis on regulation of c-Fos levels during osteoclastogenesis.
We have previously found that high doses of M-CSF, rather than increased RANKL concentrations, can overcome the OC differentiation defects in the context of αvβ3, Vav and Syk deficiencies; all pathways upstream of PLCγ1 and PLCγ2 activation (40–42). We have also observed that exposure to high concentrations of M-CSF increases ERK phosphorylation and rescues c-Fos expression in αvβ3−/− cells (40). Although not measured at that time, it is plausible that high doses of M-CSF generate higher levels of DAG required for optimal c-Fos expression. Our previous observations together with our current findings demonstrate that M-CSF not only supports precursor survival and proliferation, but also controls OC differentiation through a different mechanism than RANKL. Elevated M-CSF levels are observed in various pathologies associated with bone loss, such as rheumatoid arthritis (43–46). Considering that increased DAG species can also induce T cell hyperactivity (36, 37), as observed in mice lacking DGKζ and DGKα, targeting DAG production in rheumatic conditions could have beneficial effects for both the inflammatory and osteolytic components of the disease.
In conclusion, we demonstrate that augmented DAG levels in the OC have a profound impact on bone mass. To our knowledge, this is the first report positioning DAG, and its regulatory kinase DGKζ, as critical regulators of osteoclastogenesis in vitro and in vivo and also suggests that altered DAG levels in disease conditions such as diabetes should be considered as a potential mechanism for abnormal bone homeostasis.
Acknowledgments
We gratefully thank Dr. Gary Koretzky (Weill Cornell Medical College, Ithaca, NY) for the DGKζ−/− mice. This work was supported by National Institute of Health Grant 5R01 AR053628 to RF, Shriners Hospital Grant 85120 to RF, Arthritis Foundation Grant to RF and T32 Institutional Metabolic Skeletal Disorders Training Grant to CD. The histological and μCT analysis were supported by The Center for Musculoskeletal Biology and Medicine at Washington University, Award Number P30AR057235 from the National Institute of Arthritis, Musculoskeletal and Skin Diseases.
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jbmr.2533]
Additional supplemental data have been included with the submission.
Disclosures
All authors state that they have no conflicts of interest.
Authors’ roles: AZ designed and performed the experiments and wrote the paper; CD and VC performed some of the experiments; LH helped with the in vitro experiments; DVN participated in data discussion and manuscript preparation; RF designed the experiments and wrote the paper. AZ and RF take responsibility for the integrity of the data analysis.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Carrasco S, Merida I. Diacylglycerol, when simplicity becomes complex. Trends in biochemical sciences. 2007;32(1):27–36. doi: 10.1016/j.tibs.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 2004;43(2):134–76. doi: 10.1016/s0163-7827(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 3.Baldanzi G. Inhibition of diacylglycerol kinases as a physiological way to promote diacylglycerol signaling. Advances in biological regulation. 2014;55:39–49. doi: 10.1016/j.jbior.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Wakelam MJ. Diacylglycerol--when is it an intracellular messenger? Biochimica et biophysica acta. 1998;1436(1–2):117–26. doi: 10.1016/s0005-2760(98)00123-4. [DOI] [PubMed] [Google Scholar]
- 5.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 6.Mao D, Epple H, Uthgenannt B, Novack DV, Faccio R. PLCgamma2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. The Journal of clinical investigation. 2006;116(11):2869–79. doi: 10.1172/JCI28775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epple H, Cremasco V, Zhang K, Mao D, Longmore GD, Faccio R. Phospholipase Cgamma2 modulates integrin signaling in the osteoclast by affecting the localization and activation of Src kinase. Molecular and cellular biology. 2008;28(11):3610–22. doi: 10.1128/MCB.00259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cremasco V, Decker CE, Stumpo D, Blackshear PJ, Nakayama KI, Nakayama K, et al. Protein kinase C-delta deficiency perturbs bone homeostasis by selective uncoupling of cathepsin K secretion and ruffled border formation in osteoclasts. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27(12):2452–63. doi: 10.1002/jbmr.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SW, Kwak HB, Chung WJ, Cheong H, Kim HH, Lee ZH. Participation of protein kinase C beta in osteoclast differentiation and function. Bone. 2003;32(3):217–27. doi: 10.1016/s8756-3282(02)00976-6. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen MG, Karsdal MA, Dziegiel MH, Boutin JA, Nosjean O, Henriksen K. Screening of protein kinase inhibitors identifies PKC inhibitors as inhibitors of osteoclastic acid secretion and bone resorption. BMC Musculoskelet Disord. 2010;11:250. doi: 10.1186/1471-2474-11-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drosatos-Tampakaki Z, Drosatos K, Siegelin Y, Gong S, Khan S, Van Dyke T, et al. Palmitic acid and DGAT1 deficiency enhance osteoclastogenesis, while oleic acid-induced triglyceride formation prevents it. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(5):1183–95. doi: 10.1002/jbmr.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murrills RJ, Stein LS, Horbert WR, Dempster DW. Effects of phorbol myristate acetate on rat and chick osteoclasts. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1992;7(4):415–23. doi: 10.1002/jbmr.5650070409. [DOI] [PubMed] [Google Scholar]
- 13.Moonga BS, Stein LS, Kilb JM, Dempster DW. Effect of diacylglycerols on osteoclastic bone resorption. Calcif Tissue Int. 1996;59(2):105–8. doi: 10.1007/s002239900095. [DOI] [PubMed] [Google Scholar]
- 14.Abraham DC, Wadkins CL, Conaway HH. Enhancement of fetal rat limb bone resorption by phorbol ester (PMA) and ionophore A-23187. Calcif Tissue Int. 1988;42(3):191–5. doi: 10.1007/BF02556333. [DOI] [PubMed] [Google Scholar]
- 15.Shulga YV, Topham MK, Epand RM. Regulation and functions of diacylglycerol kinases. Chem Rev. 2011;111(10):6186–208. doi: 10.1021/cr1004106. [DOI] [PubMed] [Google Scholar]
- 16.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25(7):1468–86. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 17.Takeshita S, Kaji K, Kudo A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2000;15(8):1477–88. doi: 10.1359/jbmr.2000.15.8.1477. [DOI] [PubMed] [Google Scholar]
- 18.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 19.Topham MK, Prescott SM. Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. The Journal of biological chemistry. 1999;274(17):11447–50. doi: 10.1074/jbc.274.17.11447. [DOI] [PubMed] [Google Scholar]
- 20.Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266(5184):443–8. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo K, Owens JM, Tonko M, Elliott C, Chambers TJ, Wagner EF. Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat Genet. 2000;24(2):184–7. doi: 10.1038/72855. [DOI] [PubMed] [Google Scholar]
- 22.Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416(6882):744–9. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- 23.Schutze S, Machleidt T, Kronke M. The role of diacylglycerol and ceramide in tumor necrosis factor and interleukin-1 signal transduction. Journal of leukocyte biology. 1994;56(5):533–41. doi: 10.1002/jlb.56.5.533. [DOI] [PubMed] [Google Scholar]
- 24.Wagner EF, Matsuo K. Signalling in osteoclasts and the role of Fos/AP1 proteins. Annals of the rheumatic diseases. 2003;62(Suppl 2):ii83–5. doi: 10.1136/ard.62.suppl_2.ii83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson LM, Kerppola TK, Vendrell M, Luk D, Smeyne RJ, Bocchiaro C, et al. Regulation of c-fos expression in transgenic mice requires multiple interdependent transcription control elements. Neuron. 1995;14(2):241–52. doi: 10.1016/0896-6273(95)90282-1. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Prywes R. Activation of the c-fos enhancer by the erk MAP kinase pathway through two sequence elements: the c-fos AP-1 and p62TCF sites. Oncogene. 2000;19(11):1379–85. doi: 10.1038/sj.onc.1203443. [DOI] [PubMed] [Google Scholar]
- 27.Cremasco V, Benasciutti E, Cella M, Kisseleva M, Croke M, Faccio R. Phospholipase C gamma 2 is critical for development of a murine model of inflammatory arthritis by affecting actin dynamics in dendritic cells. PloS one. 2010;5(1):e8909. doi: 10.1371/journal.pone.0008909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veis N, Hamilton JA. Colony stimulating factor-1 stimulates diacylglycerol generation in murine bone marrow-derived macrophages, but not in resident peritoneal macrophages. J Cell Physiol. 1991;147(2):298–305. doi: 10.1002/jcp.1041470215. [DOI] [PubMed] [Google Scholar]
- 29.Song JG, Pfeffer LM, Foster DA. v-Src increases diacylglycerol levels via a type D phospholipase-mediated hydrolysis of phosphatidylcholine. Molecular and cellular biology. 1991;11(10):4903–8. doi: 10.1128/mcb.11.10.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oancea E, Teruel MN, Quest AF, Meyer T. Green fluorescent protein (GFP)-tagged cysteine-rich domains from protein kinase C as fluorescent indicators for diacylglycerol signaling in living cells. The Journal of cell biology. 1998;140(3):485–98. doi: 10.1083/jcb.140.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. The Journal of cell biology. 2003;161(5):899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuller K, Owens JM, Jagger CJ, Wilson A, Moss R, Chambers TJ. Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J Exp Med. 1993;178(5):1733–44. doi: 10.1084/jem.178.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190(12):1741–54. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura I, Lipfert L, Rodan GA, Le TD. Convergence of alpha(v)beta(3) integrin- and macrophage colony stimulating factor-mediated signals on phospholipase Cgamma in prefusion osteoclasts. The Journal of cell biology. 2001;152(2):361–73. doi: 10.1083/jcb.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, et al. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat Immunol. 2003;4(9):882–90. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Zhang H, Shao Z, O’Hara KA, Kopilas MA, Yu L, et al. Suppression of endothelin-1-induced cardiac myocyte hypertrophy by PPAR agonists: role of diacylglycerol kinase zeta. Cardiovasc Res. 2011;90(2):267–75. doi: 10.1093/cvr/cvq401. [DOI] [PubMed] [Google Scholar]
- 38.Stensman H, Raghunath A, Larsson C. Autophosphorylation suppresses whereas kinase inhibition augments the translocation of protein kinase Calpha in response to diacylglycerol. The Journal of biological chemistry. 2004;279(39):40576–83. doi: 10.1074/jbc.M405560200. [DOI] [PubMed] [Google Scholar]
- 39.Madani S, Hichami A, Legrand A, Belleville J, Khan NA. Implication of acyl chain of diacylglycerols in activation of different isoforms of protein kinase C. FASEB J. 2001;15(14):2595–601. doi: 10.1096/fj.01-0753int. [DOI] [PubMed] [Google Scholar]
- 40.Faccio R, Takeshita S, Zallone A, Ross FP, Teitelbaum SL. c-Fms and the alphavbeta3 integrin collaborate during osteoclast differentiation. The Journal of clinical investigation. 2003;111(5):749–58. doi: 10.1172/JCI16924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, et al. Vav3 regulates osteoclast function and bone mass. Nature medicine. 2005;11(3):284–90. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- 42.Faccio R, Zou W, Colaianni G, Teitelbaum SL, Ross FP. High dose M-CSF partially rescues the Dap12−/− osteoclast phenotype. J Cell Biochem. 2003;90(5):871–83. doi: 10.1002/jcb.10694. [DOI] [PubMed] [Google Scholar]
- 43.Kitaura H, Zhou P, Kim HJ, Novack DV, Ross FP, Teitelbaum SL. M-CSF mediates TNF-induced inflammatory osteolysis. The Journal of clinical investigation. 2005;115(12):3418–27. doi: 10.1172/JCI26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang PT, Kasai H, Xiao WG, Zhao LJ, He LM, Yamashita A, et al. Increased expression of macrophage colony-stimulating factor in ankylosing spondylitis and rheumatoid arthritis. Annals of the rheumatic diseases. 2006;65(12):1671–2. doi: 10.1136/ard.2006.054874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korkosz M, Bukowska-Strakova K, Sadis S, Grodzicki T, Siedlar M. Monoclonal antibodies against macrophage colony-stimulating factor diminish the number of circulating intermediate and nonclassical (CD14(++)CD16(+)/CD14(+)CD16(++)) monocytes in rheumatoid arthritis patient. Blood. 2012;119(22):5329–30. doi: 10.1182/blood-2012-02-412551. [DOI] [PubMed] [Google Scholar]
- 46.Paniagua RT, Chang A, Mariano MM, Stein EA, Wang Q, Lindstrom TM, et al. c-Fms-mediated differentiation and priming of monocyte lineage cells play a central role in autoimmune arthritis. Arthritis research & therapy. 2010;12(1):R32. doi: 10.1186/ar2940. [DOI] [PMC free article] [PubMed] [Google Scholar]