Abstract
Vigna vexillata (L.) A. Rich. (tuber cowpea) is an underutilized crop for consuming its tuber and mature seeds. Wild form of V. vexillata is a pan-tropical perennial herbaceous plant which has been used by local people as a food. Wild V. vexillata has also been considered as useful gene(s) source for V. unguiculata (cowpea), since it was reported to have various resistance gene(s) for insects and diseases of cowpea. To exploit the potential of V. vexillata, an SSR-based linkage map of V. vexillata was developed. A total of 874 SSR markers successfully amplified single DNA fragment in V. vexillata among 1,336 SSR markers developed from Vigna angularis (azuki bean), V. unguiculata and Phaseolus vulgaris (common bean). An F2 population of 300 plants derived from a cross between salt resistant (V1) and susceptible (V5) accessions was used for mapping. A genetic linkage map was constructed using 82 polymorphic SSR markers loci, which could be assigned to 11 linkage groups spanning 511.5 cM in length with a mean distance of 7.2 cM between adjacent markers. To develop higher density molecular linkage map and to confirm SSR markers position in a linkage map, RAD markers were developed and a combined SSR and RAD markers linkage map of V. vexillata was constructed. A total of 559 (84 SSR and 475 RAD) markers loci could be assigned to 11 linkage groups spanning 973.9 cM in length with a mean distance of 1.8 cM between adjacent markers. Linkage and genetic position of all SSR markers in an SSR linkage map were confirmed. When an SSR genetic linkage map of V. vexillata was compared with those of V. radiata and V. unguiculata, it was suggested that the structure of V. vexillata chromosome was considerably differentiated. This map is the first SSR and RAD marker-based V. vexillata linkage map which can be used for the mapping of useful traits.
Introduction
Vigna vexillata (L.) A. Rich. is a perennial herb belonging to the genus Vigna and considered to be closely related to cowpea (Vigna unguiculata (L.) Walp.), the most important food legume in Africa [1]. A domesticated form of V. vexillata, which has large non-dormant seeds and non-shattering pods named “tuber cowpea”, was found cultivated for their flesh tubers and mature seeds in Bali and Timor, Indonesia [2]. Another cultivated form V. vexillata var. macrosperma was described from the specimen from Costa Rica [3]. The protein content of tuberous roots of V. vexillata is about three times higher (15%) than that of potato and about six times higher than that of cassava [4]. The tuberous roots of wild V. vexillata have been eaten like sweet potato by local people in Ethiopia and Sudan [5], in Himalayas and in the hills of eastern and northeastern India at altitude between 1,200 and 1,500m [6,7] and in tropical and subtropical Australia [8]. The wild plant has been used also as forage or cover crop in several African countries [9,10] and in Australia [11].
The wild V. vexillata distributed from Africa, Asia, Australia to Central and South America. Due to its worldwide distribution, V. vexillata shows a great morphological variation and several botanical varieties are recognized [3,12–14]. Wild V. vexillata is considered to be an important gene(s) source in cowpea breeding [15], because some of its accessions are highly resistant to the cowpea weevil [16], pod sucking bugs, flower thrips, Maruca vitrata (Fabricius) [17] and Striga gesnerioides (Scrophulariaceae) [18], powdery mildew [19] and cowpea mottle carmovirus [1,20,21]. In addition, it shows high levels of resistances to various environmental stresses, such as to prolonged water logging [11], to lateritic acid and aluminous soils [6], to infertile sandy loams to fertile heavy-textured clays and to alkaline cracking clay soil [8,22].
Genetic mapping is a powerful approach to understand the function of genes in a variety of biological processes [23]. Discovering genes that control morphological and physiological phenotypes is critical for understanding the mechanism of adaptive evolution and for plant breeding [24]. The SSR (simple sequence repeat) markers have particular advantages for characterization and mapping of gene(s) because of their high reproducibility, co-dominant inheritance, relative abundance, high polymorphism, and ease of genotyping [25]. The SNP (single nucleotide polymorphisms) markers have been developed based on genome sequence information and have been used for mapping gene(s). Among several methods of developing SNP markers, SNP discovery using sequenced RAD (Restriction site Associated DNA) markers have advantages for non-model organisms without prior reference genome sequence information [26,27]. By using the high-throughput sequencing of DNA fragments flanking the restriction sites (RAD tags), a huge number of SNPs could be identified as co-dominant markers efficiently.
To date, several SSR-based linkage maps of Vigna crops, including azuki bean (V. angularis (Willd.) Ohwi & H. Ohashi: [28], [29], [30]), black gram (V. mungo (L.) Hepper: [31]), mungbean (V. radiata (L.) R. Wilczek: [32]), rice bean (V. umbellata (Thunb.) Ohwi & H. Ohashi: [33]) and yardlong bean (V. unguiculata: [34]) were reported. Based on the comparative genome mapping study using the common SSR markers, it was revealed that all the four Vigna crops belonging to the subgenus Ceratotropis, i. e., azuki bean, black gram, mungbean and rice bean have highly conserved genome structures, and therefore the QTLs of domestication related traits among these 4 Ceratotropis crops could successfully be compared [35].
Recently, two sequencing-based linkage maps of azuki bean and mungbean [36,37]) were reported. However, there are no reports of the linkage map of V. vexillata consist of the 11 linkage groups which correspond to the number of chromosomes of this promising crops for the future [1,35].
Hence, the objectives of this study were to construct linkage map of V. vexillata (1) using SSR markers developed from related Vigna and Phaseolus crops, (2) using RAD markers developed by de novo RAD-sequencing and (3) to compare constructed linkage map with those of two most important Vigna crops, V. radiata and V. unguiculata.
Materials and Methods
Plant materials
An F2 mapping population was developed from a cross between wild V. vexillata accession V1 (from USDA, PI 406383, duplicate conserved as JP202334 in the NIAS genebank, National Institute of Agrobiological Sciences, Japan) and V5 (from the Botanic Garden, Meise, NI 936, conserved as JP235869). V1 is a salt tolerant accession collected from Paramaribo, Suriname while V5 is a salt sensitive accession collected at a site 35km E of Santa Marta, Columbia. The V1 was used as a male parent and V5 was a female parent in the cross to produced F1 seeds. The F1 plant was self-pollinated to produce 300 F2 plants which were grown in a greenhouse of NIAS from May 2013.
DNA extraction
Total genomic DNA of the parents and F2 plants were extracted from fresh leaf tissue using the CTAB method [38] with a slight modification. The DNA was adjusted to 5 ng/μl for SSR marker analysis by comparing with known concentrations of standard λ–DNA on 1.5% agarose gel.
SSR marker analysis
A total of 1336 SSR markers consisting of 329 SSR markers of azuki bean [39], 480 EST-SSR markers of azuki bean [40], 487 SSR markers of cowpea [34,41], and 40 SSR markers of common bean [42–45] were screened (Table 1, S1 Table). For azuki bean SSR, common bean SSR, cowpea SSR (VM primers; Li et al. [41]), each PCR reaction mixture solution was prepared to a volume of 5 μL containing 5 ng DNA, 1x QIAGEN Multiplex PCR Master Mix and 5 pmol of forward and reverse primers. The 5’-end of the reverse primer was fluorescent labeled with one of the four following fluorescent dyes: 6-FAM (blue), VIC (green), NED (yellow) and PET (red) (Applied Biosystems). For azuki bean EST and cowpea SSR (cp primers; Kongjaimun et al. [34]), each PCR reaction mixture solution was prepared to a volume of 5 μL containing 5 ng DNA, 1x QIAGEN Multiplex PCR Master Mix, 1x Q-solution, 2 pmol of forward primer and 20 pmol of reverse primer. The 5’-end of the forward primer was fluorescent labeled with one of the three following fluorescent dyes: FAM (blue), HEX (green) and NED (yellow) (Applied Biosystems). PCR reactions were performed in a GeneAmp PCR System 9700 (Applied Biosystems) The PCR thermal cycling was programmed as follows: 95°C for 15 mins follow by 40 cycles of 94°C for 30 s, 55°C for 90 s, 72°C for 60 s, and a final cycle at 72°C for 10 mins. For azuki bean SSR, common bean SSR, cowpea SSR (VM primers; Li et al. [41]), 1 μL of ten times diluted PCR product was mixed with 8.5 μL of Hi-Di formamide and 0.125 μL of Gene Scan 500 LIZ size standard (Applied Biosystems). For azuki bean EST and cowpea SSR (cp primers; Kongjaimun et al. [34]), 1 μL of five times diluted PCR product was mixed with 8.5 μL of Hi-Di formamide and 0.125 μL of Gene Scan 500 ROX size standard (Applied Biosystems). The mixer was denatured at 95°C for 5 mins and run on an ABI Prism 3100 or 3130xl Genetic Analyzer (Applied Biosystems). Allele size for the highest stutter peak with the height ranging between 500 and 10,000 RFU was recorded and used to create bins for automatic assignment of genotypes. The genotyping was conducted by the GeneMapper 3.0 software (Applied Biosystems) with default settings. After marker screening, 6 or 7 primers with different labels and product sizes were put into a single PCR reaction mixture and amplified as a multiplex PCR using the same procedures described above.
Table 1. Summary of amplification of SSR and EST-SSR markers from three legumes in Vigna vexillata and polymorphic rate between parents V1 and V5.
| SSR sources | Marker type | Screened | Amplified (%) b | Polymorphic (%) c |
|---|---|---|---|---|
| Azuki bean | SSR a | 329 | 257 (78.1) | 28 (8.5) |
| Azuki bean | EST-SSR | 480 | 305 (63.5) | 19 (4.0) |
| Cowpea | SSR | 487 | 273 (56.1) | 35 (7.2) |
| Common bean | SSR | 40 | 39 (97.5) | 1 (2.5) |
| Total | 1,336 | 874 (65.4) | 83 (6.2) |
a One STS marker is included.
b (No. of SSR and EST-SSR primer pairs amplified/ No. of SSR and EST-SSR primer pairs screened)*100
c (No. of SSR and EST-SSR primer pairs polymorphic/ No. of SSR and EST-SSR primer pairs screened)*100.
SSR linkage map construction and comparison with related species
An SSR genetic linkage map was constructed with JoinMap ver. 4.0 [46]. The calculation was set with a minimum logarithm of the odds (LOD) of 4.0 and a maximum recombination frequency (r) of 0.25. Kosambi mapping function [47] was used to calculate the distance between SSR loci. For each marker, chi-square analysis was calculated for goodness of fit to a 1:1 segregation ratio of genotypic classes at P = 0.05, 0.01, and 0.001. Markers were assigned to an LG based on recombination frequencies and LOD values. The recombination frequencies were converted into map distances (cM) using the mapping function of Kosambi [47]. Double crossovers between adjacent loci were confirmed manually. The numbering of linkage groups was named following mungbean linkage map [32]. Based on common SSR markers, the structure of the linkage maps among mungbean (V. radiata), yardlong bean (V. unguiculata) and V. vexillata were compared. In case more than 2 SSR markers of a mungbean linkage group (e.g., LG1) were mapped with several SSR markers of another mungbean linkage group (e.g., LG5), it is estimated that a translocation is occurred.
RAD-seq analysis
RAD-seq analysis was performed based on the protocol of Matsumura et al. with minor modifications [48].
Preparation of adaptors
Adapter-1 for BamHI-digested DNA site was prepared by annealing the two synthesized oligonucleotides 5’-biotin-GTACAGGTTCAGAGTTCTACAGTCCGACGATCXXXXXX-3′ and 5′-GATCXXXXXXGATCGTCGGACTGTAGAACTCTGAACCTGT-3 (XXXXXX correspond to the variable index sequences to identify the individual DNA sample). Adapter 2 for NlaIII-digested DNA site was prepared by annealing of the two complementary oligonucleotides 5′-amino-CAAGCAGAAGACGGCATACGACATG-3′ and 5′-TCGTATGCCGTCTTCTGCTTG-3′.
Library construction and RAD sequencing
DNA library for RAD sequencing was constructed as follows. Each genomic DNA samples (100–300 ng) of parents and 286 F2 individuals were simultaneously digested with BamHI-HF and NlaIII and purified. Adaptor-1 and adaptor-2 were ligated to the digested DNA samples and purified. Adaptors ligated biotinylated DNA samples were collected using streptavidin coated magnetic beads (Dynabeads M270, Dynal). Adaptor ligated DNA on the beads was amplified by PCR using Phusion High-Fidelity DNA polymerase (Thermo Fisher Scientific) and the adapter primers. Size of the PCR amplified fragments were checked by electrophoresis in agarose gel. The 96 purified PCR products were pooled and sequenced using the Illumina HiSeq2000 system. The sequencing primer was 5′-CGACAGGTTCAGAGTTCTACAGTCCGACGATC.
Extraction of RAD-tag and bi-allelic RAD-marker detection
Extraction of RAD-tag sequence and bi-allelic RAD-marker detection were conducted using a software, Stacks ver. 1.12 [49]. Sequence reads of low quality and sequence reads with ambiguous six-base variable index sequences were discarded and 85 bp RAD-tag sequence reads were prepared. The RAD-tag sequence reads were classified into those of 2 parental accessions and F2 individuals based on the six-base variable index sequences. RAD-tags with the sequence reads of less than 2 sequence mismatch were grouped as a stack. Stacks of each parent with less than 3 sequence mismatch were estimated as the stacks derived from a homologous locus. A list of RAD-tag sequences and their count was constructed for each sample. For the genotyping of F2 individuals, stacks which have more than 20 RAD-tags (minimum stack depth of 20) were used as potential RAD-markers.
Linkage map construction using SSR and RAD-markers
Linkage group construction was conducted by a software R/qtl ver. 1.36.6 [50]. Potential RAD-markers which have genotype data of less than 100 F2 individuals, which show identical F2 individuals genotypes, and/or which show significant segregation distortion (chi-squared test, P < 10−5) were not used. Suitable values of recombination fractions and LOD scores were estimated using the est.rf command. After these steps, initial linkage group construction was carried out using the formLinkageGroups command with a maximum recombination fraction of 0.25 and LOD threshold of 15. Robustness of linkage groups was checked using plot.rf command. Marker order within a linkage group was estimated using a program TMAP [51]. Some RAD-markers mapped at less than 0.1cM locus were not used for linkage map construction.
Results
Linkage map based on SSR markers
Among 1,336 SSR markers screened, 874 SSR markers (65.4% on average) amplified single DNA fragment (Table 1). The percentage of SSR markers amplified ranged from 56.1% for cowpea SSR markers to 97.5% for common bean SSR markers. Among 874 amplified SSR markers, only 83 SSR markers (6.2%) showed polymorphism between parents. The percentage of polymorphic SSR markers ranged from 2.5% for common bean SSR markers to 8.5% for azuki bean SSR markers.
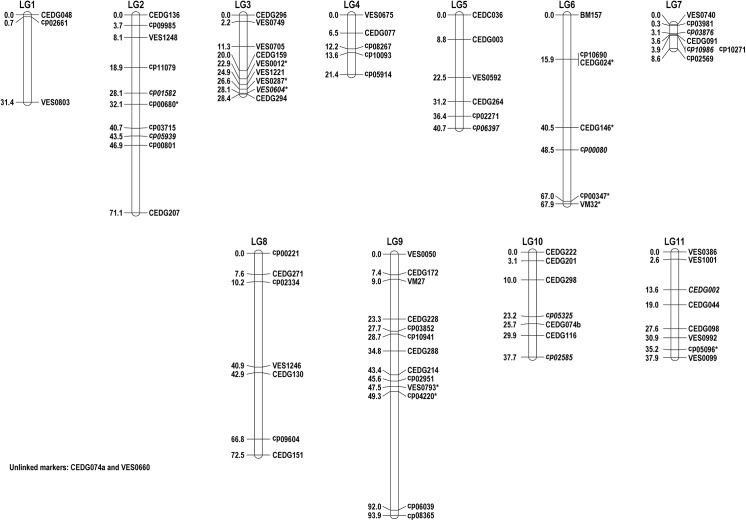
A total of 82 out of 84 polymorphic SSR loci could be assigned to 11 linkage groups (LGs) covering a total length of 510.5 cM of the V. vexillata genome at an average marker distance of 7.2 cM (Fig 1, Table 1). Two polymorphic markers (CEDG074a and VES0660) were unlinked and could not be mapped. The number of markers on each LG ranged from 3 (LG1) to 13 (LG9) (Table 2). The length of each LG ranged from 8.6 (LG7) to 93.9 cM (LG9). The average distance between two adjacent markers ranged from 1.4 (LG7) to 15.7 cM (LG1). The LGs 1, 2, 6, 8 and 9 had gaps greater than 15 cM between markers. Eight markers (9.8%) showed significant segregation distortion at 5% level (Fig 1).
Fig 1. A genetic linkage map of V. vexillata constructed from 300 F2 individuals of intraspecific cross between accessions V1 and V5.
Map distances and marker names are shown on the left and right side of the linkage groups, respectively. Marker names in italics indicate dominant loci. Markers showing significant deviation from the expected segregation ratio at 0.05 are indicated with*. SSR markers with prefixes CED are derived from azuki bean. Markers with prefixes cp and VM are derived from cowpea. BM157 (LG6) is derived from common bean. Markers with prefixes VES are EST-SSR markers derived from azuki bean.
Table 2. Comparison of Vigna vexillata linkage group with those of V. radiata and V. unguculata, and some characteristics of the constructed V. vexillata SSR linkage map.
| Linkage | Corresponding linkage groups of | Length | No. of loci | Average distance between two maker loci | ||||
|---|---|---|---|---|---|---|---|---|
| group | V. radiata and V. unguiculata maps | (cM) | Azuki bean SSR | Cowpea SSR | Common bean SSR | Azuki bean EST-SSR | Total | (cM) |
| 1 | 1 | 31.4 | 1 | 1 | 0 | 1 | 3 | 15.7 |
| 2 | 2 | 71.1 | 2 | 7 | 0 | 1 | 10 | 7.9 |
| 3 | 3 | 28.4 | 3 | 0 | 0 | 6 | 9 | 3.6 |
| 4 | 4 | 21.4 | 1 | 3 | 0 | 1 | 5 | 5.4 |
| 5 | 1 and 5 | 40.7 | 3 | 2 | 0 | 1 | 6 | 8.1 |
| 6 | 6 and 9 | 67.9 | 2 | 4 | 1 | 0 | 7 | 11.3 |
| 7 | 4 | 8.6 | 1 | 5 | 0 | 1 | 7 | 1.4 |
| 8 | 8 | 72.5 | 3 | 3 | 0 | 1 | 7 | 12.1 |
| 9 | 1 and 9 | 93.9 | 4 | 7 | 0 | 2 | 13 | 7.8 |
| 10 | 10 | 37.7 | 5 | 2 | 0 | 0 | 7 | 6.3 |
| 11 | 11 | 37.9 | 3 | 1 | 0 | 4 | 8 | 5.4 |
| Total | - | 511.5 | 28 | 35 | 1 | 18 | 82 | 7.2 |
Unlinked markers: CEDG074a and VES0660
Linkage map based on SSR and RAD markers
Illumina sequencing with HiSeq2000 yielded a total of 451,012,199 RAD-tag 85-base reads from 494,666,849 raw reads. The number of RAD-tags of parents (V1 and V5) and F2 individuals was 412,372, 1,138,645 and 450,040,150, respectively (Table 3). The average number of RAD-tags per F2 individual was 1,597,514.3. RAD-tags were aligned and clustered into 42,517 stacks. Among these stacks, 10,037 candidate RAD loci were inferred and genotyped for 286 individuals of F2 population. For the analysis of the F2 mapping population, 5,438 RAD markers which showed homozygote polymorphic genotype between parents were used. Among them, 735 RAD markers together with 84 SSR markers loci were used for the linkage map construction after discarding markers showing identical F2 genotypes, high level of segregation distortion, and less than 100 F2 genotypes.
Table 3. RAD-seq results in parents and F2 populations using HiSeq2000 platform.
| Samples | Number of reads | Number of RAD-tag | Mean coverage depth | Mean merged coverage depth |
|---|---|---|---|---|
| V. vexillata V1 | 442,165 | 412,372 | 12.0 | 12.7 |
| V5 | 1,176,089 | 1,138,645 | 24.0 | 25.2 |
| F2 populations (286 lines) | 470,584,049 | 450,040,150 | - | - |
| Average per F2 individual | 1,670,491 | 1,597,514.3 | 30.6 | 37.0 |
Linkage analysis identified 11 linkage groups (LG1–LG11) containing a total of 475 RAD markers loci and 84 SSR markers loci after the removal of unlinked markers (Table 4, Fig 2). Sequence and SNP position of each RAD-marker was summarized (S2 Table). The map spanned 973.9 cM, with a mean distance between markers of 1.8 cM. The lengths of linkage groups ranged from 71.7 (LG11) to 128.3 cM (LG9). The positions of all SSR markers in an SSR-RAD markers linkage map were consistent with those in SSR markers linkage map.
Table 4. Characteristics of a V. vexillata linkage map based on the SSR and RAD markers.
| Linkage group | Number of loci | Length (cM) | Average marker interval (cM) | ||
|---|---|---|---|---|---|
| Total | SSR | RAD | |||
| 1 | 54 | 3 | 51 | 84.5 | 1.6 |
| 2 | 56 | 10 | 46 | 109.4 | 2.0 |
| 3 | 58 | 10 | 48 | 91.4 | 1.6 |
| 4 | 41 | 5 | 36 | 78.0 | 1.9 |
| 5 | 50 | 6 | 44 | 75.4 | 1.5 |
| 6 | 54 | 7 | 47 | 89.7 | 1.7 |
| 7 | 53 | 8 | 45 | 78.8 | 1.5 |
| 8 | 43 | 7 | 36 | 90.8 | 2.2 |
| 9 | 55 | 13 | 42 | 128.3 | 2.4 |
| 10 | 56 | 7 | 49 | 75.9 | 1.4 |
| 11 | 39 | 8 | 31 | 71.7 | 1.9 |
| Total | 559 | 84 | 475 | 973.9 | 1.8 |
Fig 2. The SSR and RAD markers linkage map of V. vexillata constructed from 286 F2 individuals of intraspecific cross between accessions V1 and V5.
Map distances and marker names are shown on the left and right side of the linkage groups, respectively. Marker names in italics indicate dominant loci. Markers showing significant deviation from the expected segregation ratio at 0.05 and at 0.01 are indicated with* and **, respectively. SSR markers with prefixes CED are derived from azuki bean (red text). Markers with prefixes cp and VM are derived from cowpea (blue text). BM157 (LG6) is derived from common bean (green text). Markers with prefixes VES are EST-SSR markers derived from azuki bean (purple text). Markers without prefixes are RAD-markers derived from V. vexillata (black text).
Comparative linkage maps
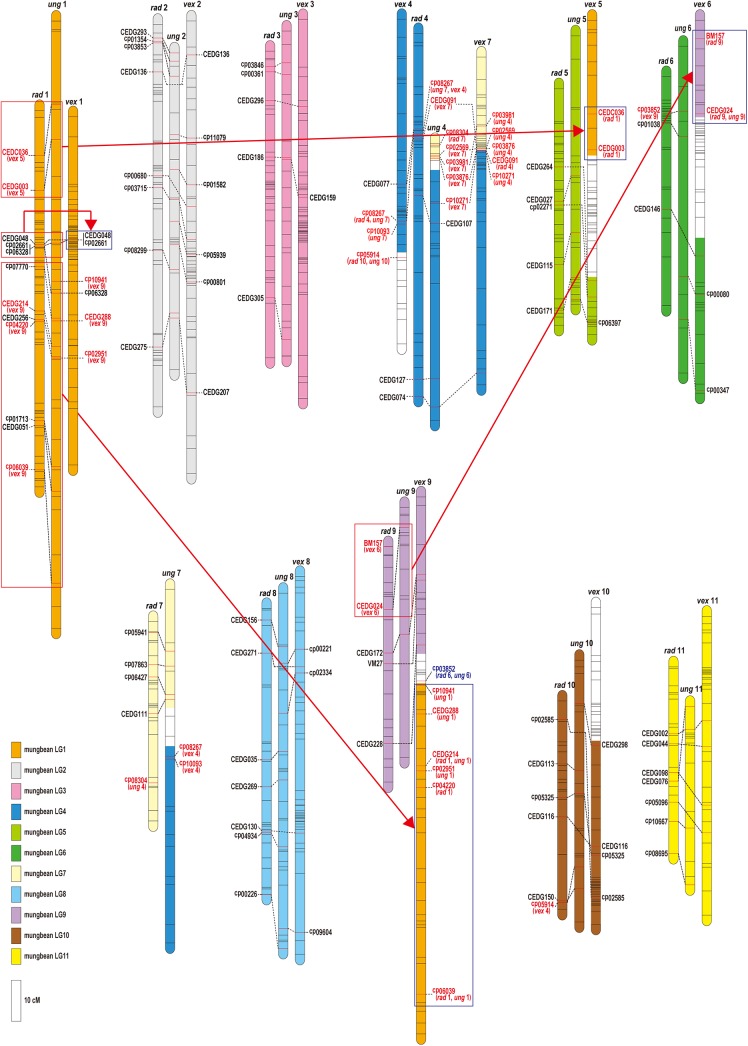
Comparative linkage maps based on the common SSR markers were developed (Fig 3). Linkage maps of V. radiata (left) and V. unguiculata (middle) were highly conserved. Most of the common markers were mapped on the same linkage group with the same order. One reciprocal translocation was found between LG4 and LG7. On the other hand, 3 translocations were detected in V. vexillata compared with V. radiata (Fig 3, Table 2). Among 11 LGs, the structure of 8 V. vexillata LGs (vex LGs 1, 2, 3, 4, 7, 8, 10, 11) was estimated to be conserved with those of V. radiata and V. unguiculata. Other 3 LGs of V. vexillata (vex LGs 5, 6, 9) were composed of the fragments of 2 LGs of V. radiata or V. unguiculata.
Fig 3. Comparative linkage maps among Vigna radiata (left: rad), V. unguiculata (middle: ung), and V. vexillata (right: vex) based on common SSR markers.
Linkage group 4 (LG 4) of V. vexillata (vex 4) was placed to the left and LG 7 of V. vexillata (vex 7) was placed to the right of LG4 of V. radiata (rad 4) to show the relationships of common markers. The positions of common markers were connected by dotted lines. Markers which were mapped on different LGs among 3 Vigna species were written in red text. Major translocations were indicated by red arrows. The species abbreviation name and number in parentheses below red text marker names, e.g., (vex 5) indicate that the species and linkage group number on which corresponding marker was mapped.
Discussion
Transferability of SSR markers
The genetic linkage map developed in this study is the first SSR and RAD markers-based linkage map of V. vexillata. In addition, this is the first genetic linkage map consisting of 11 linkage groups that correspond to the haploid chromosome number of V. vexillata (n = 11). Former V. vexillata linkage map was constructed by 70 RAPD, 47 AFLP and one SSR marker, and was consisted of 14 linkage groups [1].
SSR markers developed in related species of Vigna and Phaseolus showed good amplification and a total of 874 markers were selected as potentially useful markers for V. vexillata (Table 1, S1 Table). However, there were only 83 polymorphic markers between the parents (V1 and V5). This might be explained by the fact that both of the parental accessions were from South America (V1 from Suriname and V5 from Columbia), where the genetic diversity of this species was reported to be low [3]. Baudoin and Maréchal [52] mentioned that there were two main centers of diversity, one in eastern and southern Africa (Zambezian district) and the other in Southeast Asia from Yunnan to Indonesia. Spinosa et al. [53] showed a lower degree of isozyme and RAPD variations in American accessions compared with African accessions. Vanderborght [10] reported that accessions from America showed epigeal germination which might be a recent evolutionary trend since most of the African accessions showed hypogeal germination which is considered ancestral type of germination in V. vexillata.
RAD-seq analysis for linkage map construction
SSR markers have been used as useful molecular markers and several SSR based linkage maps were constructed for Vigna crops [28–34]. However, we have encountered a problem of low polymorphism in SSR markers (6.2%) between V. vexillata parental accessions, hence resulting in a low density SSR linkage map. Recently, RAD-markers have been successfully used to construct a genetic linkage map and to perform QTL mapping in higher plant species [54–56].
By the application of RAD-seq analysis to the genetically close parental accessions of V. vexillata (V1 and V5), we could construct a high-density linkage map with 11 linkage groups with 475 RAD-markers (SNPs based of RAD-tag sequence) (S2 Table). Recently, a mungbean (V. radiata) and an azuki bean (V. angularis) linkage maps were constructed by SNPs using GBS method (genotyping-by-sequencing) [35,36]. However, since they used different enzyme (ApeKI) in library preparation, sequence of the DNA fragments flanking the restriction sites could not be compared with RAD-tag sequence of the present study.
Since the genetic linkage map based on the RAD and SSR markers could confirm the linkage group accuracy of SSR-based linkage map, comparative genomic analysis were performed using common SSR markers.
Translocations in Vigna vexillata
The present study suggested several translocations occurred in V. vexillata chromosomes, while genomic structure of V. radiata and V. unguiculata was basically conserved with only one translocation. The remarkable chromosomal re-arrangements in V. vexillata was surprising because molecular phylogenetic analysis based on RFLP and the sequences of nuclear ribosomal DNA (rDNA) regions suggested that V. vexillata was more closely aligned with V. unguiculata compared with V. radiata [18,57,58]. In addition, morphological studies suggested V. vexillata group (subgenus Plectrotropis) formed an evolutionary intermediate group between V. unguiculata group (subgenus Vigna) and V. radiata group (subgenus Ceratotrois) [52,59].
Similar phenomenon was reported in the comparative analyses among V. marina, V. radiata and V. unguiculata linkage maps [60]. Structure of linkage maps was completely conserved between V. marina and V. radiata, which belong to the different subgenera, i.e., subgenus Vigna and Ceratotropis, respectively. On the contrary, one reciprocal translocation between LG4 and 7 was found between V. marina and V. unguiculata, which belong to the same subgenus Vigna. However, since the number of common SSR markers used for developing the comparative linkage map was not sufficient in these studies, it should be confirmed by the comparison of higher density molecular maps or more directly by cytogenetic analysis. Gomathinayagam et al. [61] observed the meiotic chromosomes of a hybrid of V. vexillata x V. unguiculata and reported high frequency of univalent formation, hence suggested that the genomes of the two species are structurally differentiated.
In addition, it is unknown that the chromosomal re-arrangement of V. vexillata suggested in the present study is commonly seen in V. vexillata or only occurred in some local accessions (e.g. American accessions). In case of azuki bean (V. angularis), we also suggested reciprocal translocation between LG4 and LG6 based on the comparison of SSR-based linkage maps developed for 2 different mapping populations [29,30]. Recently, this translocation event was confirmed to be occurred on a Japanese wild azuki bean parent by BAC-fluorescence in situ hybridization (FISH) analyses [62]. In that study, 21 wild azuki bean accessions collected from various regions of Japan were analyzed and it was found that geographical distribution of accessions with translocated chromosomes were restricted to eastern and northern Japan.
Future perspectives
Vigna vexillata is a promising food crop which could be grown under harsh environmental conditions [63]. There are domesticated accessions collected in Bali, which could be harvested in about 3 months and estimated tuber yield is 18–30 t/ha and seed yields of 0.7–1.2 t/ha [2]. There is another domesticated form (var. macrosperma) recognized having large seeds, non-dehiscent pods and bushy plant type [3,64]. V. vexillata var. macroperma showed earlier maturity with higher seed yield than Bali cultivated accessions [64]. Based on the hybridization study of these 2 domesticated forms (Bali and var. macrosperma) with African and Austronesian wild V. vexillata accesions, it was revealed that var. macrosperma and the wild accessions can be considered to belong to the same gene pool with strong genetic compatibility [65]. In contrast, there were various levels of genetic barriers between the cultivated Bali accessions and the wild accessions. Among several cross combinations tried, there was an Australian wild accession which could produce F1 plants with a cultivated Bali accession having vigorous growth, but it showed complete F1 sterility. However, backcrossing both to wild and Bali cultivated parents could produce BC1 plants, suggesting the possibility of backcross breeding of improving these domesticated forms [66]. As was mentioned in Introduction section, there are wild accessions showing high levels of resistance to various kinds of insects and diseases, and also to various kinds of abiotic stresses. Therefore, diverse wild V. vexillata could be used to incorporate adaptation gene(s) into these domesticated forms to grow under both biotic and abiotic stress environments.
In the breeding procedure, especially using wild germplasm, it is essential to map the position of useful traits and use marker assisted selection in the segregating population [67]. The SSR and RAD-marker based linkage map and SSR markers which produce single amplified DNA fragment in V. vexillata in the present study could be used as basic genome mapping resources.
Supporting Information
(XLSX)
(XLSX)
Acknowledgments
We appreciate Associate Professor, Dr. Somchai Chakhatrakan, Thammasat University, Thailand who encouraged the first author (RM) to conduct the present study in Japan.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Ph.D. scholarship program of Monbukagakusho (MEXT), Japan to RM, Genebank Project of the National Institute of Agrobiological Sciences (NIAS), Japna to NT, study design, data collection and analysis, publication, preparation of the manuscript.
References
- 1. Ogundiwin EA, Thottappilly G, Aken’Ova ME, Pillay M, Fatokun CA. A genetic linkage map of Vigna vexillata . Plant Breed. 2005; 124: 392–398. [Google Scholar]
- 2. Karuniawan A, Iswandi PRK, Heinzemann J, Grüneberg WJ. Vigna vexillata (L.) A. Rich. cultivated as a root crop in Bali and Timor. Genet Resour Crop Evol. 2006; 53: 213–217. [Google Scholar]
- 3. Maréchal R, Mascherpa JM, Stainier F. Etude taxonomique d’un groupe complexe d’especes des genres Phaseolus et Vigna (Palilionaceae) sur la base de donnes morphologiques et polliniques, traitees par l’analyse informatique. Boissiera. 1978; 28: 1–273. [Google Scholar]
- 4. Chandel KPS, Arora RK, Joshi BS. Vigna capensis Walp.–an edible root legume. Curr Sci. 1972; 41: 537. [Google Scholar]
- 5. Duke JA. Handbook of Legumes of World Economic Importance. 11th ed. Plenum Press, New York and London; 1981. 345 p. [Google Scholar]
- 6. United States of America. National Research Council (NRC). Vigna vexillata in Tropical Legumes: Resources for the Future. National Academy of Science; Washington, DC: 1979; 34–36. [Google Scholar]
- 7. Sasikumar B, Sardana S. Vigna vexillata (Fabaceae), a pulse cum tuber crop of Northeastern Hill Region of India. Econ Bot. 1988; 42(2): 292. [Google Scholar]
- 8. Lawn RJ, Cottrell A. Wild mungbean and its relatives in Australia. Biologist. 1988; 35(5): 267–273. [Google Scholar]
- 9. Maxted N, Mabuza Dlamini P, Moss H, Padulosi S, Jarvis A, Guarino L. African Vigna: an ecogeographic study International Plant Genetic Resources Institute (IPGRI), Rome, Italy: 2004. [Google Scholar]
- 10. Vanderborght T. Some observations on seedlings of Vigna vexillata (L.) A. Rich. (Fabaceae). Bull Jard Bot Nat Belg. 1989; 59: 179–187. [Google Scholar]
- 11. Miller IL, Williams WT. Tolerance of some tropical legumes to six months of simulated waterlogging. Trop Grasslands. 1981; 15(1): 39–43. [Google Scholar]
- 12. Verdcourt B. Studies in the Leguminosae Papilionoidae for the Flora of Tropical East Africa: 4. Kew Bull 1970; 24: 440–442. [Google Scholar]
- 13. Verdcourt B. Leguminosae In Milne-Redhead E and Polhill RM, editors. Flora of Tropical East Africa. Crown Agency for Overseas Governments, London: 1971; 4: 652–655. [Google Scholar]
- 14.Garba M, Pasquet RS. The Vigna vexillata (L.) A. Richi. gene pool. In Sorensen M, Estrella JE, Hermann EOJ, Luiz SAR, editors. Proceedings of the 2nd International Symposium on Tuber Legumes; 1996 Aug 5–8, Celaya Guanajuato, Mexico. MacKenzie: Copenhagen. 1998; 61–71.
- 15. Fatokun CA, Perrino P, Ng NQ. Wide crossing in African Vigna species In Singh BB, Mohan Raj DR, Dashiell KE, Jackai LEN, editors. Advances in cowpea research. JIRCAS & IITA, Ibadan, Nigeria; 1997. p. 50–57. [Google Scholar]
- 16. Vincenzo L, Terzano R, Cicco N, Cardinali A, Di Venere D, Linsalata V. Seed coat tannins and bruchid resistance in stored cowpea seeds. J Sci Food Agr. 2005; 85: 839–846. [Google Scholar]
- 17. Jackai LEN, Padulosi S, Ng Q. Resistance to the legume pod borer, Maruca vitrata Fabricius, and the probable modalities involved in wild Vigna . Crop Prot. 1996; 753–761. [Google Scholar]
- 18. Fatokun CA, Danesh D, Young ND, Stewart EL. Molecular taxonomic relationships in the genus Vigna based on RFLP analysis. Theor Appl Genet. 1993; 86: 97–104. 10.1007/BF00223813 [DOI] [PubMed] [Google Scholar]
- 19. James AT, Lawn RJ. Inheritance of selected traits in accessions of Vigna vexillata (L.) A. Rich of Australian and African origin. Austral J Bot. 1991; 39: 415–429. [Google Scholar]
- 20. Thottappilly G, Ng NQ, Rossel HW. Screening germplasm of Vigna vexillata for resistance to cowpea mottle virus. Int J Trop Plant Dis. 1994; 12: 75–80. [Google Scholar]
- 21. Ogundiwin EA, Thottappilly G, Aken’Ova ME, Ekpo EJA, Fatokun CA. Resistance to cowpea mottle carmovirus in Vigna vexillata . Plant Breed. 2002; 121: 517–520. [Google Scholar]
- 22. Lawn RJ, Watkinson AR. Habitat, morphological diversity and distribution of the genus Vigna Savi in Australia. Aust J Agric Res. 2002; 53: 1305–1316. [Google Scholar]
- 23. Baired NA, Etter PD, Atwood TS, Currey MC, Shiver AL, Lewis ZA, et al. Rapid SNP Discovery and Genetic Mapping Using Sequenced RAD Markers. PLoS ONE. 2008; 3 (10): e3376 10.1371/journal.pone.0003376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baxter SW, Davey JW, Johnson JS, Shelton AM, Heckal DG, Jiggins CD, et al. Linkage Mapping and Comparative Genomics Using Next-Generation RAD Sequencing of a Non-Model Organism. PLoS ONE. 2011; 6 (4): e19315 10.1371/journal.pone.0019315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Varshney RK, Graner A, Sorrells ME. Genic microsatellite markers in plants: features and applications. Trends Biotechnol. 2005; 23: 48–55. [DOI] [PubMed] [Google Scholar]
- 26. Miller M, Dunham J, Amores A, Cresko W, Johnson E. Rapid and cost effective polymorphism identification and genotyping using restriction site associated DNA (RAD) markers. Genome Res. 2007; 17: 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baird NA, Etter PD, Atwood TS, Currey MC, Shiver AL, et al. Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS ONE. 2008; 3:e3376 10.1371/journal.pone.0003376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han OK, Kaga A, Isemura T, Wang XW, Tomooka N, Vaughan DA. A genetic linkage map of azuki bean [Vigna angularis (Willd.) Ohwi & Ohashi]. Theor Appl Genet. 2005; 111(7):1278–1287. 10.1007/s00122-005-0046-8 . [DOI] [PubMed] [Google Scholar]
- 29. Isemura T, Kaga A, Konishi S, Ando T, Tomooka N, Han O, et al. Genome dissection of traits related to domestication in azuki bean (Vigna angularis) and comparison with other warm season legumes. Ann Bot. 2007; 100 (5): 1053–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaga A, Isemura T, Tomooka N, Vaughan DA. The genetics of domestication of the azuki bean (Vigna angularis). Genetics. 2008; 178(2):1013–1036. 10.1534/genetics.107.078451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chaitieng B, Kaga A, Tomooka N, Isemura T, Kuroda Y, Vaughan DA. Development of a black gram [Vigna mungo (L.) Hepper] linkage map and its comparison with an azuki bean [Vigna angularis (Willd.) Ohwi and Ohashi] linkage map. Theor Appl Genet. 2006; 113: 1261–1269. [DOI] [PubMed] [Google Scholar]
- 32. Isemura T, Kaga A, Tabata S, Somta P, Srinives P, Shimizu T, et al. Construction of genetic linkage map and genetic analysis of domestication related traits in mungbean (Vigna radiata). PLos ONE. 2012; 7(8): e41304 10.1371/journal.pone.0041304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Isemura T, Kaga A, Tomooka N, Shimizu T, Vaughan DA. The genetics of domestication of rice bean, Vigna umbellata . Annals of Botany. 2010; 106: 927–944. 10.1093/aob/mcq188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kongjaimun A, Kaga A, Tomooka N, Somta P, Shimizu T, Shu Y, et al. An SSR-based linkage map of yardlong bean (Vigna unguiculata (L.) Walp. subsp. unguiculata Sesquipedalis Group) and QTL analysis of pod length. Genome. 2012; 55: 81–92. 10.1139/G11-078 [DOI] [PubMed] [Google Scholar]
- 35. Tomooka N, Isemura T, Naito K, Kaga A, Vaughan D. Vigna species In Singh M, Bisht IS, Dutta M, editors. Broadening the Genetic Base of Grain Legumes. Springer; New Delhi Heidelberg New York Dordrecht London; 2014b. Chapter 9, 175–208. [Google Scholar]
- 36. Kang YJ, Satyawan D, Shim S, Lee T, Lee J, et al. Draft genome sequence of adzuki bean, Vigna angularis . Sci Rep. 2015; 5: 8069 10.1038/srep08069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kang YJ, Kim SK, Kim MY, Lestari P, Kim KH et al. Genome sequence of mungbean and insights into evolution within Vigna species. Nat Commun. 2014; 5: 5443 10.1038/ncomms6443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lodhi MA, Ye GN, Weeden NF, Reisch BI. A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol Bio Rep. 1994; 12: 6–13. [Google Scholar]
- 39. Wang XW, Kaga A, Tomooka N, Vaughan DA. The development of SSR markers by a new method in plants and their applicationto gene flow studies in azuki bean [Vigna anguicularis (Willd.) Ohwi & Ohashi]. Theor Appl Genet. 2004; 109: 352–360. [DOI] [PubMed] [Google Scholar]
- 40. Chankaew S, Isemura T, Isobe S, Kaga A, Tomooka N, Somta P, et al. Detection of genome domor species of neglected tetraploid crop Vigna reflexo-pilosa (créole bean), and genetic structure of diploid species based on newly developed EST-SSR markers from azuki bean (Vigna angularis). PLos ONE. 2014. a; 9 (8): e104990 10.1371/journal.pone.0104990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li CD, Fatokun CA, Ubi B, Singh BB, Scoles GJ. Determining genetic similarities and relationships aming cowpea breeding lines and cultivars by microsatellite markers. Crop Sci. 2001; 41: 189–197. [Google Scholar]
- 42. Yu K, Park J, Poysa V, Gepts P. Integation of simple sequence repeat (SSR) markers into a molecular linkage map of common bean (Phaseolus vulgaris L.). J Hered. 2000; 91: 429–434. [DOI] [PubMed] [Google Scholar]
- 43. Gaitán-Solís E, Duque MC, Edwards KJ, Thome J. Microsatellite repeats in common bean (Phaseolus vulgaris L.): isolation, characterization, and cross-species amplification in Phaseolus ssp. Crop Sci. 2002; 42: 2128–2136. [Google Scholar]
- 44. Blair MW, Pedraza F, Buendia HF, Gaitán-Solís E, Beebe SE, Gepts P, et al. Development of a genome-wide anchored microsatellite map of common bean (Phaseolus vulgaris L.). Theor Appl Genet. 2003; 107: 1362–1374. [DOI] [PubMed] [Google Scholar]
- 45. Guerra-Sanz JM. New SSR markers of Phaseolus vulgaris from sequence databases. Plant Breed. 2004; 123, 87–89. [Google Scholar]
- 46. Van Ooijen JW. JoinMap Version 4.0. Software for the calculation of genetic linkage maps Kyazma BV, Wageningen: 2006. [Google Scholar]
- 47. Kosambi DD. The estimation of map distances from recombination values. Ann Eugen. 1944; 12: 172–175. [Google Scholar]
- 48. Matsumura H, Miyagi N, Taniai N, Fukushima M, Tarora K, et al. Mapping of the gynoecy in bitter gourd (Momordica charantia) using RAD-Seq analysis. PLoS ONE. 2014; 9(1): e87138 10.1371/journal.pone.0087138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Catchen JM, Amores A, Hohenlohe P, Cresko W, Postlethwait JH. Stacks: building and genotyping Loci de novo from short-read sequences. G3 (Bethesda). 2011; 1(3): 171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arends D, Prins P, Jansen RC, Broman KW. R/qtl: High-throughput multiple QTL mapping. Bioinformatics. 2010; 26: 2990–2992 10.1093/bioinformatics/btq565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cartwright DA, Troggio M, Velasco R, Gutin A. Genetic mapping in the presence of genotyping errors. Genetics. 2007; 176(4): 2521–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baudoin JP, Maréchal R. Taxonomy and evolution of the genus Vigna. In “Mungbean” Proceedings of the Second International Symposium; 1987 Nov 16–20; Bangkok, Thailand. Shanhua, Taiwan: AVRDC; 1988.
- 53. Spinosa A, Pignone D, Sonnante G. Assessment of genetic variation in a working collection of Vigna vexillata A. Rich. By isozyme and RAPD analyses. Genet Resour Crop Evol. 1998; 45: 347–354. [Google Scholar]
- 54. Hegarty M, Yadav R, Lee M, Armstead I, Sanderson R, et al. Genotyping by RAD sequencing enables mapping of fatty acid composition traits in perennial ryegrass (Lolium perenne (L.)). Plant Biotechnol. 2013; 11: 572–581. 10.1111/pbi.12045 [DOI] [PubMed] [Google Scholar]
- 55. Barchi L, Lanteri S, Portis E, Valè G, Volante A, et al. A RAD tag derived marker based eggplant linkage map and the location of QTLs determining anthocyanin pigmentation. PLoS One. 2012; 7: e43740 10.1371/journal.pone.0043740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pfender WF, Saha MC, Johnson EA, Slabaugh MB. Mapping with RAD (restriction-site associated DNA) markers to rapidly identify QTL for stem rust resistance in Lolium perenne . Theor Appl Genet. 2011; 122: 1467–1480. 10.1007/s00122-011-1546-3 [DOI] [PubMed] [Google Scholar]
- 57. Goel S, Raina SN, Ogihara Y. Molecular evolution and phylogenetic implications of internal transcribed spacer sequences of nuclear ribosomal DNA in the Phaseolus-Vigna complex. Mol Phylogenet Evol. 2002; 22(1): 1–19. [DOI] [PubMed] [Google Scholar]
- 58. Choi HW, Kim MY, Lee SH, Sultana S, Bang JW. Molecular cytogenetic analysis of the Vigna species distributed in Korea. Genes Genom. 2013; 35: 257–264. [Google Scholar]
- 59. Tateishi Y, Ohashi H. Systematics of the azuki bean group in the genus Vigna In Fujii K, Gatehouse AMR, Jhonson CD, Mitchel R, Yoshida T, editors. Bruchids and Legumes: Economics, Ecology and Coevolution. Kluwer Academic Publishers; Netherlands: 1990; 189–199. [Google Scholar]
- 60. Chankaew S, Isemura T, Naito K, Ogiso-Tanaka E, Tomooka N, Somta P, et al. QTL mapping for salt tolerance and domestication–related traits in Vigna marina subsp. oblonga, a halophytic species. Theor Appl Genet. 2014b. 10.1007/s00122-013-2251-1 [DOI] [PubMed] [Google Scholar]
- 61. Gomathinayagam P, Ganesh ram S, Rathnaswamy R, Ramaswamy NM. Interspecific hybridization between Vigna unguiculata (L.) Walp. and V. vexillata (L.) A. Rich. through in vitro embryo culture. Euphytica. 1998; 102: 203–209. [Google Scholar]
- 62. Wang L, Kikuchi S, Muto C, Naito K, Isemura T, Ishimoto M, et al. Reciprocal translocation identified in Vigna angularis dominates the wild population in East Japan. J Plant Res. 2015. 10.1007/s10265-015-0720-0 [DOI] [PubMed] [Google Scholar]
- 63. Tomooka N, Naito K, Kaga A, Sakai H, Isemura T, Ogiso-Tanaka E, et al. Evolution, domestication and neo-domestication of the genus Vigna . Plant Genet Resour. 2012a; 12(S1): S168–S171. [Google Scholar]
- 64. Damayanti F, Lawn RJ, Bielig M. Genotypic variation in domesticated and wild accessions of the tropical tuberous legume Vigna vexillata (L.) A. Rich. Crop Pasture Sci. 2010a; 61 (10): 771–784. [Google Scholar]
- 65. Damayanti F, Lawn RJ, Bielig M. Genetic compatibility among domesticated and wild accessions of the tropical tuberous legume Vigna vexillata (L.) A. Rich. Crop Pasture Sci. 2010b; 61 (10): 786–797. [Google Scholar]
- 66. Damayanti F, Lawn R, Bielig M. Expression of qualitative and quantitative traits in hybrids between domesticated and wild accessions of the tropical tuberous legume Vigna vexillata (L.) A. Rich. Crop Pasture Sci. 2010c; 61 (10): 798–811. [Google Scholar]
- 67. Ashraf M, Foolad MR. Crop breeding for salt tolerance in the era of molecular markers and marker-assisted selection. Plant Breed. 2013; 132: 10–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.