Abstract
OBJECTIVE
To evaluate the effectiveness of monetary reinforcement to increase the frequency of self-monitoring blood glucose (SMBG).
RESEARCH DESIGN AND METHODS
Ten adolescents with poorly controlled diabetes enrolled in a 12-week program in which they earned monetary reinforcers based on SMBG frequency ($0.10 per test, with bonuses for ≥4 tests per day, and $251.40 maximum).
RESULTS
SMBG increased from 1.8 ± 1.0 to 4.9 ± 1.0 tests per day (P < 0.001) with 90% completing four or more tests per day. Mean A1C fell from 9.3 ± 0.9% to 8.4 ± 1.5% (P = 0.05). Adolescents and parents reported high satisfaction with procedures.
CONCLUSIONS
Reinforcing adolescents for SMBG may increase testing and improve A1C.
Introduction
Adolescents with type 1 diabetes (T1D) have difficulty carrying out the tasks needed to achieve target A1C levels (1,2), and decreased frequency of self-monitoring blood glucose (SMBG) is associated with increased A1C levels (3). Interventions to increase SMBG have had only limited success (4).
Behavioral economics involves provision of monetary-based reinforcers for behavior change. Studies in adults find this is an efficacious means of decreasing substance use (5), reducing weight (6), enhancing exercise (7), and improving medication adherence (8). A multicomponent procedure involving monetary reinforcers for parents and adolescents along with intensive counseling demonstrated promise in improving diabetes management (9), but the study design could not isolate effects of reinforcement. Raiff and Dallery (10) reported an increase in SMBG in four adolescents reinforced for submitting computer-generated videos of SMBG testing for 5 days, but this period is too short to assess effects on metabolic control. Anecdotally, some clinicians provide incentives to youth with T1D for SMBG, A1C levels, or other behaviors, suggesting acceptability of this approach, but the procedures are not standardized and have not been evaluated empirically. The goal of this proof-of-concept study was to assess the preliminary effectiveness of a novel and specific intervention in youth that reinforced SMBG directly via regular glucose meter uploads. The hypothesis was that youth reinforced for SMBG would increase SMBG frequency and A1C levels would decrease.
Research Design and Methods
Subjects were recruited from the Yale T1D clinic if they were 12–21 years old; diagnosed with T1D ≥12 months; had an average A1C during the past year >7.5% but ≤11% (to ensure reductions did not simply reflect regression to the mean); performed SMBG fewer than four times per day during the month before enrollment, using glucose meters with remote uploading possibilities; had a computer for uploading meters and cell phone for text messaging; and had Diabetes Knowledge Test (11) scores >12. Exclusion criteria were presence of a major psychiatric or neurocognitive disorder, a medical condition impacting diabetes management, or plans to switch insulin delivery mode. Subjects and parents of those under 18 years of age signed informed consent forms approved by the university Institutional Review Board.
Of the 13 subjects approached to participate, 1 declined prior to completing the baseline evaluation, 1 had an A1C level >11.0%, and 1 did not have computer access. Among the 10 subjects who initiated treatment, 1 withdrew after 3 weeks and 1 completed 12 weeks of study procedures but not the posttreatment evaluation.
Assessments
Number of SMBG tests per day for 84 days prior to and during the study were analyzed. At baseline and study end, A1C was measured by point-of-care DCA Vantage (Siemons, Inc.). Subjects completed Diabetes Quality Of Life for Youth (DQOLY) (12), Problem Areas In Diabetes (PAID) (13), and the Diabetes Empowerment Scale (DES) (14) pre- and posttreatment. Parents and subjects also completed a treatment satisfaction survey at study end. Clinic A1C levels were accessed 1 year posttreatment.
Intervention
At study initiation, subjects received instructions for uploading glucose meters to Diasend or Carelink and to do four or more blood tests/day spread out during the day, e.g., 8:00 a.m., 12:00 p.m., 5:00 p.m., and 10:00 p.m. Alarms on cell phones and/or meters reminded them of testing times.
The monetary reinforcement schedule provided $0.10 for each SMBG test, up to 6 per day, based on verification by meter uploads. For encouragement of sustained testing patterns, subjects earned bonuses when four consecutive tests fell within testing windows (±2 h) and were separated by ≥2 h. Bonuses started at $0.50 per day and increased by $0.25 per day up to a cap of $2.50 after 9 consecutive days of four or more daily SMBG checks at appropriate intervals. Maximal earnings over 12 weeks were $251.40. Staff texted subjects after each upload, noting earnings. Subjects were encouraged to text after each test, and if they did, staff sent return texts congratulating them for testing. Additionally, staff encouraged subjects to review SMBG results weekly and call regarding concerns about diabetes management or glucose levels, although few did.
Data Analysis
Primary outcomes were change from baseline in mean SMBG tests per day and A1C levels; paired t tests compared values pre- and posttreatment. For application of an intent-to-treat analysis, glucose meter readings and A1C levels from the most proximal clinical visit to the posttreatment evaluation were used for two subjects who did not complete the study follow-up evaluation.
Paired t tests compared responses on self-report inventories. Owing to the small sample size, effect sizes (Cohen d) are presented, with d > 0.50 indicating a medium effect size and d > 0.80 a large effect size. Data are presented as mean ± SD when appropriate.
Results
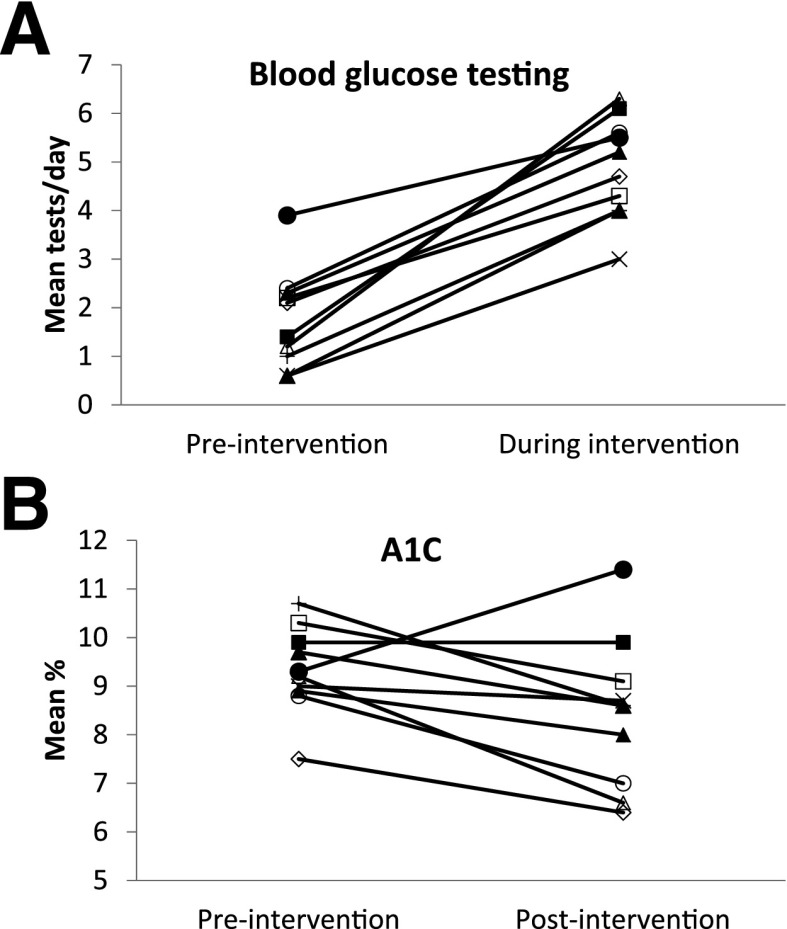
Ten subjects ranged in age from 12 to 19 years, seven were male, and three were Hispanic, one was mixed races, and six were non-Hispanic White. Duration of diabetes was 8.2 ± 4.5 years (range 3–15), and seven used insulin pumps and three injections. Figure 1 shows SMBG testing frequencies and A1C levels for each subject pre- and during treatment. SMBG increased from 1.8 ± 1.0 (range 0.6–3.9) to 4.9 ± 1.0 (range 3.0–6.4) tests per day (P < 0.001, Cohen d = 3.10). Nine of the 10 subjects reached the recommended threshold of four or more tests per day, and subjects tested at all four windows on 66 ± 17 of the 84 intervention days. Total earnings averaged $122 ± $76, with approximately $35 earned for $0.10/test and approximately $87 in bonuses for testing at four consecutive windows.
Figure 1.
A: Mean blood glucose tests per day in the 3 months prior to the intervention and during the 3-month intervention period. B: A1C (%) level immediately before starting the study and at the 3-month posttreatment evaluation at the end of the study. Each symbol represents one subject.
A1C levels fell from 9.3 ± 0.9% to 8.4 ± 1.5% (78.1 ± 7.7 mmol/mol vs. 68.3 ± 11.5 mmol/mol; P = 0.05, Cohen d = 0.73), with three subjects reaching A1C levels of <7.5% (Fig. 1). For eight subjects who remained at the clinic, A1C levels obtained at clinic visits approximately 1 year after study initiation averaged 8.4 ± 1.8%.
Supplementary Table 1 shows responses to self-report inventories. Almost all subjects (87.5%) and 100% of parents reported moderate to very high satisfaction with the study (Supplementary Table 2).
Conclusions
This pilot study was undertaken to determine the potential for monetary reinforcement to increase SMBG frequency in teenagers who matched the profile of many adolescents with T1D, namely, mean SMBG fewer than two times per day and A1C >9.0%. It is particularly noteworthy that the intervention had pronounced effects on increasing SMBG with respect to both testing frequency and timing. Almost all adolescents and parents reported high satisfaction with the program.
Observational studies have reported an inverse relationship between SMBG frequency and A1C levels (2,15) but not whether an intervention aimed at increasing SMBG per se would lower A1C. Therefore, an important and novel finding of this study was the sharp reduction in A1C observed in the majority of subjects by an intervention focused on improving SMBG frequency directly. Study limitations include the small sample and nonrandomized design. It also remains to be determined whether additional reinforcement for using SMBG data to make self-adjustments of treatment might result in even greater improvements in A1C and whether all intervention aspects (alarms, texts, encouraging clinic calls) are critical to its effectiveness.
While A1C levels remained reduced in many subjects a year after study completion, a criticism of reinforcement interventions is that they are costly. In the case of T1D, savings in preventing acute and long-term vascular complications might recoup the relatively low $10 per week costs of the intervention, even if provided long-term. These results show the effectiveness of monetary rewards to sharply increase SMBG and lower A1C and provide a compelling rationale for randomized studies in much larger samples over longer periods to evaluate the efficacy and cost-effectiveness of this intervention.
Supplementary Material
Article Information
Acknowledgments. The authors thank Marcia DeSousa (University of Connecticut School of Medicine) for expert assistance in conducting this study and Lori Carria (Yale University School of Medicine) for support with study initiation and procedures.
Funding. This research was funded by National Institutes of Health grant DP3-DK097705, with additional support by UL1-TR000142. J.A.W. also receives support from R01-MD005879 and the American Diabetes Association (7-13-TS-31).
Duality of Interest. W.V.T. is a consultant for Novo Nordisk, Sanofi, and Medtronic. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. N.M.P. designed the study, conducted analyses, and wrote the manuscript. E.C., J.A.W., and W.V.T. assisted in the design of study and reviewed and edited the manuscript. K.W. and E.T. contributed to the conduct of the study and reviewed and edited the manuscript. N.M.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-0765/-/DC1.
References
- 1.Borus JS, Laffel L. Adherence challenges in the management of type 1 diabetes in adolescents: prevention and intervention. Curr Opin Pediatr 2010;22:405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller KM, Beck RW, Bergenstal RM, et al.; T1D Exchange Clinic Network . Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care 2013;36:2009–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell MS, Schatz DA, Chen V, et al.; T1D Exchange Clinic Network . A contrast between children and adolescents with excellent and poor control: the T1D Exchange clinic registry experience. Pediatr Diabetes 2014;15:110–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreland EC, Volkening LK, Lawlor MT, Chalmers KA, Anderson BJ, Laffel LM. Use of a blood glucose monitoring manual to enhance monitoring adherence in adults with diabetes: a randomized controlled trial. Arch Intern Med 2006;166:689–695 [DOI] [PubMed] [Google Scholar]
- 5.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 2006;101:192–203 [DOI] [PubMed] [Google Scholar]
- 6.Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA 2008;300:2631–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petry NM, Andrade LF, Barry D, Byrne S. A randomized study of reinforcing ambulatory exercise in older adults. Psychol Aging 2013;28:1164–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petry NM, Alessi SM, Byrne S, White WB. Reinforcing adherence to antihypertensive medications. J Clin Hypertens (Greenwich) 2015;17:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanger C, Ryan SR, Delhey LM, et al. A multicomponent motivational intervention to improve adherence among adolescents with poorly controlled type 1 diabetes: a pilot study. J Pediatr Psychol 2013;38:629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raiff BR, Dallery J. Internet-based contingency management to improve adherence with blood glucose testing recommendations for teens with type 1 diabetes. J Appl Behav Anal 2010;43:487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzgerald JT, Funnell MM, Hess GE, et al. The reliability and validity of a brief diabetes knowledge test. Diabetes Care 1998;21:706–710 [DOI] [PubMed] [Google Scholar]
- 12.Skinner TC, Hoey H, McGee HM, Skovlund SE; Hvidøre Study Group on Childhood Diabetes . A short form of the Diabetes Quality of Life for Youth questionnaire: exploratory and confirmatory analysis in a sample of 2,077 young people with type 1 diabetes mellitus. Diabetologia 2006;49:621–628 [DOI] [PubMed] [Google Scholar]
- 13.Weissberg-Benchell J, Antisdel-Lomaglio J. Diabetes-specific emotional distress among adolescents: feasibility, reliability, and validity of the problem areas in diabetes-teen version. Pediatr Diabetes 2011;12:341–344 [DOI] [PubMed] [Google Scholar]
- 14.Anderson RM, Funnell MM, Fitzgerald JT, Marrero DG. The Diabetes Empowerment Scale: a measure of psychosocial self-efficacy. Diabetes Care 2000;23:739–743 [DOI] [PubMed] [Google Scholar]
- 15.Shalitin S, Gil M, Nimri R, de Vries L, Gavan MY, Phillip M. Predictors of glycaemic control in patients with Type 1 diabetes commencing continuous subcutaneous insulin infusion therapy. Diabet Med 2010;27:339–347 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.