Abstract
OBJECTIVE
This study was conducted to determine if type 1 diabetes is associated with an increased risk of fracture across the life span.
RESEARCH DESIGN AND METHODS
This population-based cohort study used data from The Health Improvement Network (THIN) in the U.K. (data from 1994 to 2012), in which 30,394 participants aged 0–89 years with type 1 diabetes were compared with 303,872 randomly selected age-, sex-, and practice-matched participants without diabetes. Cox regression analysis was used to determine hazard ratios (HRs) for incident fracture in participants with type 1 diabetes.
RESULTS
A total of 334,266 participants, median age 34 years, were monitored for 1.9 million person-years. HR were lowest in males and females age <20 years, with HR 1.14 (95% CI 1.01–1.29) and 1.35 (95% CI 1.12–1.63), respectively. Risk was highest in men 60–69 years (HR 2.18 [95% CI 1.79–2.65]), and in women 40–49 years (HR 2.03 [95% CI 1.73–2.39]). Lower extremity fractures comprised a higher proportion of incident fractures in participants with versus those without type 1 diabetes (31.1% vs. 25.1% in males, 39.3% vs. 32% in females; P < 0.001). Secondary analyses for incident hip fractures identified the highest HR of 5.64 (95% CI 3.55–8.97) in men 60–69 years and the highest HR of 5.63 (95% CI 2.25–14.11) in women 30–39 years.
CONCLUSIONS
Type 1 diabetes was associated with increased risk of incident fracture that began in childhood and extended across the life span. Participants with type 1 diabetes sustained a disproportionately greater number of lower extremity fractures. These findings have important public health implications, given the increasing prevalence of type 1 diabetes and the morbidity and mortality associated with hip fractures.
Introduction
Type 1 diabetes is a lifelong condition of insulin deficiency resulting from autoimmune-mediated destruction of the pancreatic β-cells. The incidence of type 1 diabetes is highest during childhood (1). However, most of the comorbidities and end-organ effects do not manifest until adulthood. Large, multinational registry studies have consistently reported an increasing incidence of type 1 diabetes on the order of 2–5% per year (2,3). Improvements in medical care have also allowed patients with type 1 diabetes to live longer. These two factors have resulted in a growing number of patients living with type 1 diabetes who are at risk for the development of diabetes-related complications (4). There is an emerging awareness that diabetes adversely affects skeletal health and that type 1 diabetes affects the skeleton more severely than type 2 diabetes (5). Studies in humans and animal models have identified a number of skeletal abnormalities associated with type 1 diabetes, including deficits in bone mineral density (BMD) (6,7) and bone structure (8), decreased markers of bone formation (9,10), and variable alterations in markers of bone resorption (10,11).
Previous studies and two large meta-analyses reported that type 1 diabetes is associated with an increased risk of fracture (12–19). However, most of these studies were conducted in older adults and focused on hip fractures. Importantly, most affected individuals develop type 1 diabetes in childhood, before the attainment of peak bone mass, and therefore may be at increased risk of fracture throughout their life span. Moreover, because hip fractures are rare in children and young adults, studies limited to this outcome may underestimate the overall fracture burden in type 1 diabetes.
We used The Health Improvement Network (THIN) database to conduct a population-based cohort study to determine whether type 1 diabetes is associated with increased fracture incidence, to delineate age and sex effects on fracture risk, and to determine whether fracture site distribution is altered in participants with type 1 diabetes compared with participants without diabetes. THIN is ideally suited to this task, because it provides a valid source of diagnosis and fracture data and has been used to investigate other complications of type 1 diabetes (20) and to characterize fracture incidence in a variety of patient populations across the life span (21–23).
Research Design and Methods
Data Source
We obtained data for this retrospective cohort study from the THIN database, an anonymized longitudinal electronic medical records database from the U.K. THIN data collection represents a collaboration between InPractice Systems (London, U.K.), which provides the Vision software used in general practices, and CSD Medical Group (London, U.K.), which provides primary care data for medical research. THIN provides demographic, medical history, biochemical, and prescription data for more than 10 million patients, derived from the daily records of 587 participating practices (24). Medical diagnoses in THIN are recorded using Read codes, the standard classification system in the U.K. (25). Data collected from 1994 through 2012 were used for this analysis. The study protocol was reviewed and approved by the THIN Scientific Review Committee and was reviewed by the University of Pennsylvania Institutional Review Board and determined to meet eligibility criteria for institutional review board exemption authorized by 45 CFR 46.101, category 4.
Study Cohort
The study sample was taken from participants 0–89 years of age with acceptable records for research based on checks performed by the data vendor. Exposure to type 1 diabetes was defined by the presence of one or more Read codes specific for type 1 diabetes and the absence of a code specific for type 2 diabetes. Participants were also considered exposed if they had a nonspecific diabetes Read code (e.g., “diabetes mellitus”) and were <35 years of age at the first diabetes code, had a prescription for insulin within 12 months of the first diabetes code, and did not have a prescription for oral or other antidiabetic medication within 12 months of the first diabetes code. THIN participants without any diagnosis codes suggestive of diabetes were considered to be unexposed to diabetes. Each type 1 diabetes participant was matched with up to 10 randomly selected participants without any diabetes codes based on age of diabetes participants (3-year age-groups up to 30 years and 5-year age-groups thereafter) at the start of follow-up, sex, and practice. The final study sample therefore consisted of all THIN participants meeting the criteria for type 1 diabetes and a random sample of matched THIN participants without exposure to diabetes.
To diminish the risk of misclassifying prevalent fractures as incident fractures, the start of the follow-up period for participants was the latest of 6 months after registration with the practice, the date that the practice started using the Vision software, and the date of the first diagnosis code meeting criteria for exposure. Follow-up for unexposed subjects started on the same date as that of their matched exposed participant. The follow-up period ended with the last collection date for the practice, and subjects were censored at the time of transfer out of their practice, death, or initial fracture event. Because the median observation period was 4.7 years, using age at the start of observation would generate misleading information regarding age-specific hazards. Therefore, the data set had multiple records for 92.8% of the participants, with each record representing the time followed up in a given year of life.
Primary Outcome
The primary outcome of incident fracture was defined as the first occurrence of a diagnosis code consistent with fracture during the study period. Incident fractures were further classified according to anatomic site as follows: vertebral, skull/face, pelvis, rib/thorax, clavicle/scapula, humerus/elbow, forearm/wrist, hand, femur/hip, lower leg/ankle, and foot. Fractures were coded as multisite if there were codes for two or more sites on the same date and categorized by the site-specific code if a site-specific and a nonspecific code were both entered on the same date. Surgically induced fractures and fractures attributed to birth trauma or metastatic bone disease were excluded. Secondary analyses defining incident hip fracture as the outcome of interest were also performed.
Covariates
Conditions identified by diagnosis codes as covariates of interest were hypothyroidism, hyperthyroidism, adrenal insufficiency, celiac disease, inflammatory bowel disease, vitamin D deficiency, fracture before the start of the follow-up period, diabetic retinopathy, and diabetic neuropathy. All variables, with the exception of prior fracture, were treated as time-varying covariates. Prescription codes were used to assess for an effect of exposure to corticosteroids.
Laboratory covariates analyzed included hemoglobin A1c (HbA1c), which was collected and averaged over the study period, and creatinine, which was used to define the presence or absence of chronic kidney disease (CKD) in participants ≥18 years. CKD was defined as two creatinine measures consistent with an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 separated by >90 days and treated as a time-varying covariate. eGFR was calculated using the Modification of Diet in Renal Disease study equation (26). The eGFR could not be reliably calculated in the pediatric population because of a large amount of missing height data, which is necessary for the calculation of eGFR in children. Given the extremely low incidence of CKD in the pediatric population (27,28), participants <18 years were considered unexposed to CKD. BMI within 1 year before the start of follow-up was included; the closest reading to the start of follow-up was analyzed for participants with more than one reading. Participants were considered to be exposed to smoking if they were identified as past or current smokers before the start of follow-up.
Analysis
Standard descriptive statistics were used to report participant and disease characteristics. Continuous variables are reported as median and interquartile range (IQR) and categorical variables as proportions. The χ2 test was used to assess for group differences between proportions.
Cox proportional hazards analysis was used to compare the incidence of fracture in participants with type 1 diabetes to that of matched unexposed participants. Multivariable Cox regression analysis was used to assess confounding by covariates of interest. Final models were stratified by age category (<20, 20–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years) after age was found to be a significant predictor of fracture and to violate the assumption of proportionality of hazards (using Schoenfeld residuals). Within each age stratum, models were again assessed for proportionality of hazards and further stratified where appropriate. Multivariable Cox regression analyses were also performed in only the participants with type 1 diabetes to determine if higher HbA1c was associated with an increased risk of fracture. Age was not found to violate the assumption of proportional hazards in these models, so it was included as a continuous variable.
All analyses were performed using Stata 12 software (StataCorp LP, College Station, TX), and a two-sided P value of <0.05 was used to define significance. To investigate the possibility of misclassification bias, we performed sensitivity analyses using only the participants identified as having type 1 diabetes based on the presence of a diagnosis code specific for type 1 diabetes and excluding participants with a diagnosis code for cystic fibrosis.
Results
Cohort Characteristics
We identified 30,394 participants with type 1 diabetes and 303,872 age-, sex-, and practice-matched participants without diabetes (Table 1). The median year for the start of follow-up was 2003 (IQR 2000–2007; range 1994–2012). The median follow-up time was 4.7 years (IQR 2–8.8; range 0.003–17.5), with a total follow-up time of 1.9 million person-years. The follow-up time was longer for participants without diabetes (4.7 years [IQR 2–9]) compared with those with type 1 diabetes (3.89 [IQR 1.5–7.7]). Males comprised 56.1% of the study cohort, consistent with the known higher prevalence of type 1 diabetes in males (29,30). The median average HbA1c was 8.5% (69 mmol/mol; IQR 7.6–9.5% [60–80 mmol/mol]) in the 24,533 type 1 diabetes participants with HbA1c data available for analysis. The median BMI was 25 kg/m2 in both groups. BMI data were available in 51.6% of participants with type 1 diabetes and in 14.4% of participants without diabetes. History of fracture before the start of the study follow-up was more common in participants with type 1 diabetes (19.6% vs. 17%). The prevalence of all other covariates of interest was higher in participants with type 1 diabetes, as anticipated. Diagnosis codes for diabetic retinopathy and diabetic neuropathy were present in 6,304 (20.7%) and 710 (2.3%) of participants with type 1 diabetes, respectively.
Table 1.
Participant characteristics
| Type 1 diabetes | No diabetes1 | ||
|---|---|---|---|
| n = 30,394 | n = 303,872 | P value | |
| Male, n (%) | 17,047 (56.1) | 170,421 (56.1) | |
| Age at start of follow-up, median (IQR), years | 35 (24–50) | 35 (24–50) | |
| Follow-up time, median (IQR), years | 3.8 (1.5–7.7) | 4.7 (2–9) | <0.001 |
| BMI,2 median (IQR), kg/m2 | 25.4 (22.7–28.7) | 25.5 (22.4–29.2) | 0.33 |
| Overweight,3 n (%) | 5,442 (34.7) | 14,249 (32.5) | <0.001 |
| Obese,4 n (%) | 3,025 (19.3) | 9,595 (21.9) | 0.01 |
| Smoking,5 n (%) | 9,512 (39.8) | 79,640 (37) | <0.001 |
| Prior fracture, n (%) | 5,952 (19.6) | 51,641 (17) | <0.001 |
| CKD,6 n (%) | 3,695 (12.2) | 14,064 (4.6) | <0.001 |
| Celiac disease, n (%) | 496 (1.6) | 698 (0.2) | <0.001 |
| Hypothyroidism, n (%) | 3,018 (9.9) | 8,913 (2.9) | <0.001 |
| Hyperthyroidism, n (%) | 620 (2) | 2,155 (0.7) | <0.001 |
| Adrenal insufficiency, n (%) | 101 (0.3) | 52 (0.02) | <0.001 |
| Cystic fibrosis, n (%) | 161 (0.5) | 122 (0.04) | <0.001 |
| Systemic corticosteroid exposure, n (%) | 5,489 (18.1) | 50,681 (16.7) | <0.001 |
| Diabetic retinopathy, n (%) | 6,304 (20.7) | – | – |
| Diabetic neuropathy, n (%) | 710 (2.3) | – | – |
| HbA1c,7 median (IQR), % | 8.5 (7.6–9.5) | 5.6 (5.3–6) | |
| HbA1c,7 median (IQR), mmol/mol | 69 (60–80) | 38 (34–42) | <0.001 |
1Matched on age, sex, and practice ID.
2Data for 15,686 participants with type 1 diabetes and 43,879 participants without diabetes.
3Overweight defined as BMI ≥25 and <30 kg/m2 for participants ≥18 years; BMI-Z ≥1.04 and BMI-Z <1.65 for participants <18 years.
4Obese defined as BMI ≥30 kg/m2 for participants ≥18 years; BMI-Z ≥1.65 for participants <18 years.
5Data for 23,931 participants with type 1 diabetes and 215,299 participants without diabetes.
6CKD defined in participants ≥18 using Modification of Diet in Renal Disease definition, creatinine, for 22,296 participants with type 1 diabetes and 118,859 participants without diabetes.
7Data for 24,533 participants with type 1 diabetes and 7,020 participants without diabetes.
Fracture Incidence
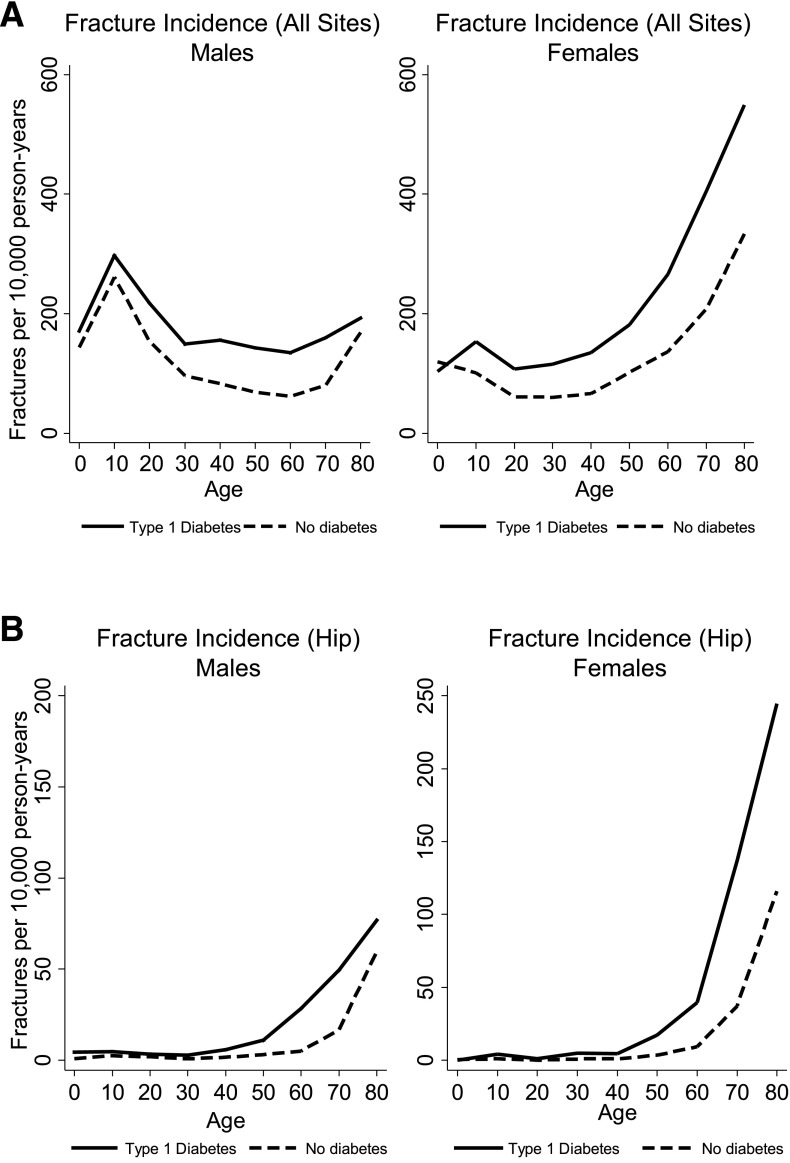
During the study period, incident fractures occurred in 2,615 participants (8.6%) with type 1 diabetes compared with 18,624 participants (6.1%) without diabetes. The fracture incidence per 10,000 person-years is shown for each decade of life for males and females in Fig. 1A. The incidence in males was greatest in the 10- to 20-year age bracket, at 297.2 and 261.3 fractures per 10,000 person-years in participants with and without type 1 diabetes, respectively. The fracture incidence in women was greatest in the 80- to 90-year age bracket, at 549.1 and 333.9 fractures per 10,000 person-years in participants with and without type 1 diabetes, respectively. Hip fracture incidence (Fig. 1B) was greatest in the 80- to 90-year age bracket for both sexes, at 76.7 and 59.6 fractures per 10,000 person-years in men and 244.5 and 116.1 fractures per 10,000 person-years in women, for participants with and without type 1 diabetes, respectively.
Figure 1.
Overall (A) and hip (B) fracture incidence rates by age and sex in participants with type 1 diabetes compared with participants without diabetes.
Fracture Site Distribution
The distribution of fracture site differed for males and females with type 1 diabetes compared with those without diabetes (Supplementary Fig. 1A). Fractures involving the lower extremity (hip/femur, lower leg/ankle, foot) comprised a greater percentage of all fractures in participants with type 1 diabetes compared with those without diabetes (31.1% vs. 25.1% in male subjects and 39.3% vs. 32% in females, P < 0.001 for both sexes; Supplementary Fig. 1B). Hip fractures alone comprised 5.5% and 11.6% of all fractures in males and females with type 1 diabetes, compared with 4.1% and 8.6% in males and females without diabetes (P = 0.04 for males and P = 0.001 for females). Participants with type 1 diabetes with a lower extremity fracture were more likely to have retinopathy (30% vs. 22.5%, P < 0.001) and neuropathy (5.4% vs. 2.9%, P = 0.001) compared with those with fractures at other sites. The median average HbA1c did not differ between the two groups.
Cox Regression Models
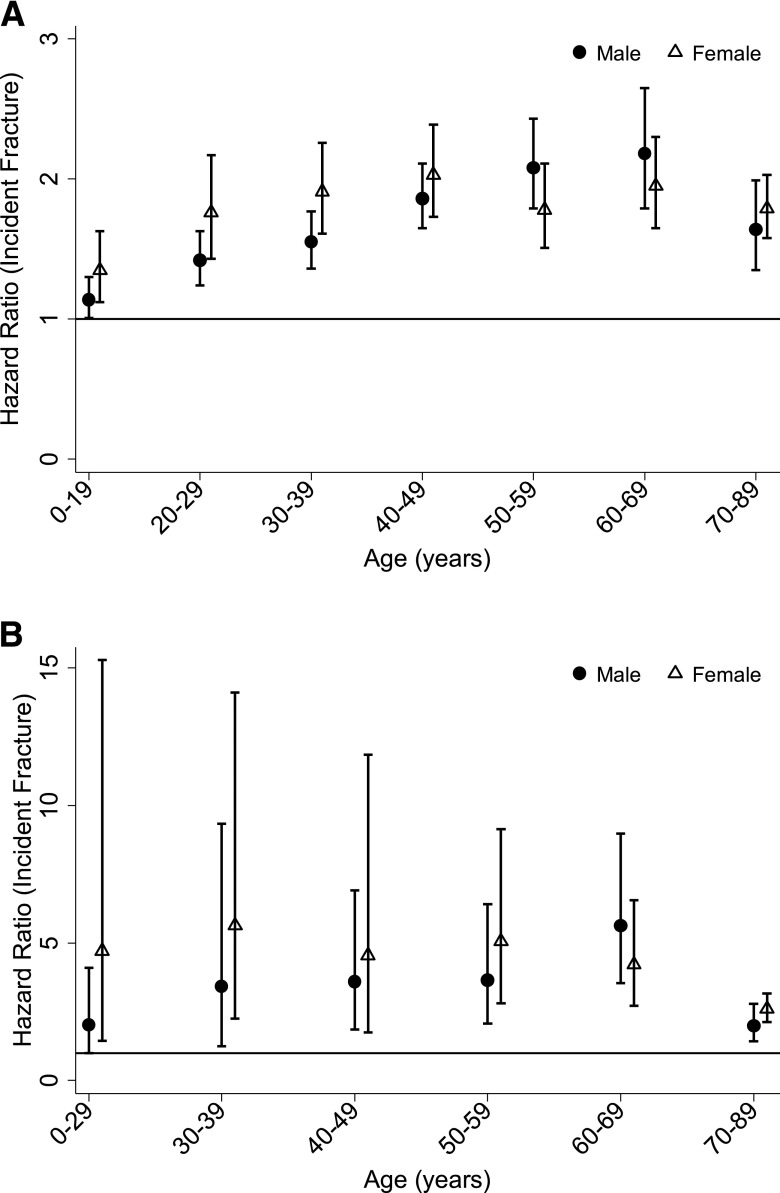
Crude hazard ratios (HRs) for incident fracture associated with type 1 diabetes are reported in Table 2A and Fig. 2A. Because of a significant interaction between sex, age, and diabetes status, results are presented stratified by sex and age category. Participants with type 1 diabetes had an increased risk of incident fracture for all age categories. The HR ranged from 1.14 (95% CI 1.01–1.29) in participants 0–19 years of age to a maximum of 2.18 (95% CI 1.79–2.65) in participants 60–69 years of age in males and from 1.35 (95% CI 1.12–1.63) in participants 0–19 years of age to a maximum of 2.03 (95% CI 1.73–2.39) in participants 40–49 years of age in females. Sensitivity analyses restricted to the participants with codes specific for type 1 diabetes and without codes for cystic fibrosis (n = 27,698) and their matched unexposed participants (n = 276,915) had a minimal effect on results, altering the HR by less than 5% across all age and sex categories.
Table 2.
Crude and adjusted HRs for incident fracture
| A. All fracture sites | ||||||
|---|---|---|---|---|---|---|
| Person-years (n1) | Crude HR (95% CI) | Adjusted HR2 (95% CI) | Person-years (n1) | Crude HR (95% CI) | Adjusted HR2 (95% CI) | |
| Age-group | Males | Females | ||||
| 0–19 years | 112,460 (30,623) | 1.14 (1.01–1.29)4 | 1.14 (1.01–1.29)4 | 98,851 (26,525) | 1.35 (1.12–1.63)5 | 1.35 (1.12–1.62)5 |
| 20–29 years | 132,639 (34,860) | 1.42 (1.24–1.63)4 | 1.40 (1.22–1.60)4 | 113,175 (32,587) | 1.76 (1.43–2.17)4 | 1.72 (1.39–2.12)4 |
| 30–39 years | 202,109 (42,878) | 1.55 (1.36–1.77)6 | 1.50 (1.31–1.71)6 | 158,358 (29,776) | 1.91 (1.61–2.26)6 | 1.77 (1.49–2.12)6 |
| 40–49 years | 224,241 (31,804) | 1.86 (1.65–2.11)6 | 1.78 (1.57–2.01)6 | 153,547 (19,337) | 2.03 (1.73–2.39)6 | 1.82 (1.53–2.16)6 |
| 50–59 years | 168,936 (20,957) | 2.07 (1.78–2.41)6 | 1.97 (1.69–2.31)6 | 106,941 (13,582) | 1.78 (1.51–2.11)6 | 1.69 (1.42–2.01)6 |
| 60–69 years | 117,089 (14,077) | 2.18 (1.79–2.65)6 | 2.00 (1.63–2.45)6 | 84,277 (11,235) | 1.95 (1.65–2.30)6 | 1.76 (1.49–2.10)6 |
| 70–89 years | 111,447 (12,268) | 1.64 (1.35–1.99)6 | 1.55 (1.27–1.89)6 | 106,952 (13,757) | 1.79 (1.58–2.03)6 | 1.69 (1.49–1.92)6 |
| B. Hip fracture | ||||||
|---|---|---|---|---|---|---|
| Person-years (n1) | Crude HR (95% CI) | Adjusted HR3 (95% CI) | Person-years (n1) | Crude HR (95% CI) | Adjusted HR2 (95% CI) | |
| Age-group | Males | Females | ||||
| 0–29 years | 263,584 (65,483) | 2.01 (0.99–4.10) | 1.90 (0.92–3.93) | 218,777 (59,112) | 4.71 (1.45–15.28)4 | 4.69 (1.44–15.23)4 |
| 30–39 years | 211,666 (42,878) | 3.42 (1.25–9.32)4 | 3.38 (1.24–9.25)4 | 162,501 (29,776) | 5.63 (2.25–14.11)6 | 4.16 (1.52–11.43)5 |
| 40–49 years | 234,658 (31,804) | 3.59 (1.86–6.91)6 | 2.56 (1.24–5.29)4 | 158,903 (19,337) | 4.55 (1.75–11.85)5 | 2.10 (0.65–6.78) |
| 50–59 years | 175,743 (20,957) | 3.64 (2.07–6.41)6 | 3.23 (1.79–5.84)6 | 111,739 (13,582) | 5.06 (2.80–9.14)6 | 4.38 (2.31–8.31)6 |
| 60–69 years | 120,950 (14,077) | 5.64 (3.55–8.97)6 | 5.21 (3.2–8.47)6 | 89,406 (11,235) | 4.22 (2.73–6.56)6 | 3.21 (2.00–5.16)6 |
| 70–89 years | 115,389 (12,268) | 1.99 (1.43–2.78)6 | 1.71 (1.21–2.4)5 | 116,740 (13,757) | 2.6 (2.13–3.18)6 | 2.34 (1.91–2.87)6 |
1Based on age at study entry.
2Adjusted for exposure to steroid medication, history of prior fracture, and presence of chronic kidney disease.
3Adjusted for exposure to steroid medication, history of prior fracture, presence of chronic kidney disease, and hypothyroidism.
4P < 0.05.
5P < 0.01.
6P < 0.001.
Figure 2.
Crude HRs for incident overall (A) and hip (B) fracture in participants with type 1 diabetes compared with no diabetes. Participants younger than the age of 30 years were collapsed into one age category for hip fractures due to the low incidence.
Age, history of prior fracture, exposure to corticosteroids, and CKD were all associated with an increased risk of incident fracture in univariate analyses and were included in the final multivariable models. Type 1 diabetes remained significantly associated with an increased risk of fracture after adjustment for these covariates, with minimal attenuation of HRs (ranging from 0 to 8% in males and 0 to 10% in females, across age strata; Table 2A).
Crude HRs for incident hip fracture are reported in Table 2B and Fig. 2B. Incident hip fracture risk was increased in all age categories for female participants with type 1 diabetes, and in age categories >30 years in men. Age, history of prior fracture, exposure to corticosteroids, CKD, and hypothyroidism (males only) were significant predictors of fracture and were included in the final models. Type 1 diabetes remained significantly associated with fracture after adjustment for covariates in all previously significant sex and age strata, with the exception of women aged 40–49.
Predictors of Fracture in Type 1 Diabetes
Results of multivariable analyses of predictors of fracture within participants with type 1 diabetes are reported in Supplementary Table 1. Each 1% (11 mmol/mol) greater average HbA1c level was associated with a 5% greater risk of incident fracture in males and an 11% greater risk of fracture in females. Diabetic neuropathy was a significant risk factor for incident fracture in males (HR 1.33; 95% CI 1.03–1.72) and females (HR 1.52; 95% CI 1.19–1.92); however, diabetic retinopathy was significant only in males (HR 1.13; 95% CI 1.01–1.28).
The presence of celiac disease was associated with an increased risk of fractures in females, with an HR of 1.8 (95% CI 1.18–2.76), but not in males. A higher BMI was protective against fracture. Smoking was a risk factor for fracture in males in the 13,763 participants with type 1 diabetes with smoking and BMI data available for analysis. Calendar year at the start of follow-up was not significantly associated with fracture risk among participants with type 1 diabetes overall (males: HR 1.01 [95% CI 0.99–1.03]; females: HR 0.99 [95% CI 0.98–1.01]) or when stratified by age category.
Conclusions
This population-based cohort study found that participants with type 1 diabetes of all ages had an increased risk of fracture. In addition, fractures in participants with type 1 diabetes were more likely to involve the lower extremity.
To our knowledge, this is the first study to show that the increased fracture risk in type 1 diabetes begins in childhood. This finding has important implications for researchers planning future studies and for clinicians caring for patients in this population. Although peak bone mass is attained by the end of the third decade of life, peak bone accrual occurs in adolescence in conjunction with the pubertal growth spurt (31). This critical time for bone accrual may represent a period of increased skeletal vulnerability and also a window of opportunity for the implementation of therapies to improve bone formation (32). This is an especially important consideration in the population with type 1 diabetes, because the incidence of this disease peaks in early adolescence. Three-quarters of individuals will develop the condition before 18 years of age, and therefore before attainment of peak bone mass (33). The development and evaluation of therapies aimed at increasing bone formation and strength in adolescence may lead to a lifelong reduction in fracture risk. The development of screening guidelines to identify patients of all ages who may be at increased risk of fracture should be considered.
Few of the previous studies assessing fracture risk in type 1 diabetes have looked at all fracture sites. A recent cohort study reported an HR of 1.22 (95% CI 1.1–3.26) for incident fracture at any site in participants with type 1 diabetes in an adult Taiwanese population (18), and a previous case-control study reported an odds ratio of 1.93 (95% CI 1.82–2.05) for type 1 diabetes in a Danish population (14). In contrast to our study, the previous reports did not stratify fracture risk by age or sex and did not provide results for the pediatric population.
Most of the previous studies investigating fracture risk in type 1 diabetes have focused on hip fracture in older adults. We found that participants with type 1 diabetes had a markedly increased risk of incident hip fracture compared with those without diabetes. Although direct comparisons are limited by differences in participant population and methodology, the magnitude of hip fracture risk associated with type 1 diabetes in our study differed compared with previous large cohort studies. Miao et al. (13) reported standardized hospital ratios (observed-to-expected hospitalizations) for incident hip fracture hospitalization of 7.6 (95% CI 5.9–9.6) in males and 9.8 (95% CI 7.3–12.9) in females. This contrasts with a more recent study by Hothersall et al. (17) that reported incident rate ratios for hip fracture of 3.28 (95% CI 2.52–4.26) in males and 3.54 (95% CI 2.75–4.57) in females. The higher risk observed in the Miao et al. (13) study may be attributable to a bias toward the inclusion of more severely affected participants with type 1 diabetes, because only participants admitted to the hospital were classified as exposed. Compared with these previous studies, our findings more completely describe the effects of type 1 diabetes on fracture risk by evaluating all fracture sites in both sexes across the entire age range.
The underlying mechanism for the increased fracture risk in patients with type 1 diabetes is not fully understood. Current evidence suggests that bone quantity and quality may both be abnormal in this condition. Clinical studies using dual-energy X-ray absorptiometry and peripheral quantitative computed tomography have identified mild to modest deficits in BMD and bone structure in both pediatric and adult participants with type 1 diabetes (6,8,34). Deficits in BMD are unlikely to be the only factor contributing to skeletal fragility in type 1 diabetes, however, as evidenced by a recent meta-analysis that found that the increased fracture risk seen in type 1 diabetes could not be explained by deficits in BMD alone (16). Recent cellular and animal models have shown that insulin signaling in osteoblasts and osteoblast progenitor cells promotes postnatal bone acquisition, suggesting that the insulin deficiency inherent in type 1 diabetes is a significant contributor to the pathogenesis of skeletal disease (35). Other proposed mechanisms contributing to skeletal fragility in type 1 diabetes include chronic hyperglycemia (36), impaired production of IGF-1 (37), and the accumulation of advanced glycation end products in bone (38). Our results showed that a higher average HbA1c was associated with an increased risk of fracture in participants with type 1 diabetes, supporting the hypothesis that chronic hyperglycemia and its sequelae contribute to skeletal fragility.
Given the morbidity, mortality, and cost to society associated with hip fractures, our additional finding that participants with type 1 diabetes had a disproportionately greater burden of lower extremity fractures compared with those without diabetes also warrants further study. The mechanism for this discrepancy is unclear; however, we identified a higher prevalence of both retinopathy and neuropathy among participants with lower extremity fractures. One possibility is that these diabetes-related comorbidities may lead to a greater number of falls, which could affect the lower extremities disproportionately.
A major strength of our study is the large sample size that encompassed the entire age range. This allowed stratification by sex and age-group to more comprehensively evaluate the effect of type 1 diabetes on fracture risk compared with previous reports. In addition, the use of an outpatient electronic medical records database to define exposure and outcome provides a more representative sample than might be obtained using inpatient hospital databases. The latter strategy could overrepresent fractures in type 1 diabetes by virtue of the tendency toward inpatient management of these more complex patients.
Our study is limited by an inability to explore for an effect of racial/ethnic group, falls, or diabetes duration on fracture risk because these data are not available in THIN. We could not directly assess for an association between hypoglycemia and fall-related fractures. If hypoglycemia was a major contributing factor, we might have expected a negative effect of HbA1c on fracture risk; our data indicated the opposite. The effects of BMI on fracture risk may not be fully captured in our analyses because a considerable amount of BMI data were missing, especially in unexposed participants. It is possible that we underestimated the prevalence of CKD by assuming all participants <18 years of age were unaffected; however, the incidence of CKD in childhood is quite low, as evidenced by the fact that there are no cases of type 1 diabetes leading to CKD in the nearly 900 individuals enrolled in the Chronic Kidney Disease in Childhood cohort study (personal communication, S. Furth). Likewise the prevalence of retinopathy and neuropathy in our cohort may be underestimated because our study only included codes specific to diabetes.
Our study may also be subject to misclassification bias because some participants identified as having type 1 diabetes may actually have type 2 diabetes. Given that previous studies have shown that type 2 diabetes has less of an effect on fracture risk than type 1 diabetes (15,16), misclassification in our study would likely bias results toward the null. Sensitivity analyses using stricter definitions of type 1 diabetes did not appreciably alter our results, suggesting that our initial strategy was reasonable.
We did not investigate the effect of bisphosphonates therapy on fracture risk due to concerns about confounding by indication. A previous meta-analysis found no difference in the relationship between bisphosphonate use and fracture risk in participants with and without diabetes (39). The potential therapeutic role for bisphosphonates in the management of skeletal fragility related to type 1 diabetes is not known and warrants future study.
In summary, our study found that participants of all ages with type 1 diabetes were at increased risk of fracture. The adverse effect of type 1 diabetes on the skeleton is an underrecognized complication that is likely to grow into a significant public health burden given the increasing incidence and prevalence of this disease. Further research is needed to elucidate the natural history and pathophysiology of skeletal fragility in type 1 diabetes. Our novel finding that children with type 1 diabetes were already at increased risk of fracture suggests that therapeutic interventions aimed at children and adolescents may have an important effect on reducing lifelong fracture risk.
Supplementary Material
Article Information
Acknowledgments. The authors acknowledge Thomas Jemielita (University of Pennsylvania) for his help with statistical analyses.
Funding and Duality of Interest. This study was funded by National Institute of Child Health and Human Development grant K12-HD-068373 (D.R.W.) and National Institute of Diabetes and Digestive and Kidney Diseases grants K12-DK-094723 (D.R.W., S.M.W.), K24-DK-076808 (M.B.L.), and K23-DK-093556 (M.R.D.). The authors receive(d) funding from the following sponsors for research outside the submitted work: The NephCure Foundation-American Society of Nephrology Research Grant, Genentech, Inc., and the National Kidney Foundation/Amgen Kidney Disease Outcomes Quality Initiative Research Fellowship (M.R.D.), HealthCore Inc. (K.H.), AstraZeneca PLC (K.H.), Bristol-Myers Squibb (S.M.W.), Takeda (S.M.W.), and Novo Nordisk (S.M.W.).
M.B.L. reports consultancy agreements with Amgen, Inc., Johnson & Johnson, and Novartis and serves as an Associate Editor for the Journal of Bone and Mineral Research and on a National Institutes of Health Data and Safety Monitoring Board. S.M.W. reports a consultancy agreement with MannKind Corp. M.R.D. reports a consultancy agreement with Infiniti Medical. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. D.R.W., K.H., M.B.L., S.M.W., and M.R.D. were responsible for study conception and design. D.R.W., K.H., and M.R.D. were responsible for data collection and management and statistical analysis. D.R.W. and M.R.D. were responsible for the manuscript draft. All authors critically reviewed the manuscript, had authority over approval of the final manuscript version and the decision to submit for publication, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. D.R.W., K.H., and M.R.D. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as an abstract at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-0783/-/DC1.
References
- 1.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010;39:481–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imkampe AK, Gulliford MC. Trends in Type 1 diabetes incidence in the UK in 0- to 14-year-olds and in 15- to 34-year-olds, 1991-2008. Diabet Med 2011;28:811–814 [DOI] [PubMed] [Google Scholar]
- 3.DIAMOND Project Group . Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med 2006;23:857–866 [DOI] [PubMed] [Google Scholar]
- 4.González EL, Johansson S, Wallander MA, Rodríguez LA. Trends in the prevalence and incidence of diabetes in the UK: 1996-2005. J Epidemiol Community Health 2009;63:332–336 [DOI] [PubMed] [Google Scholar]
- 5.Hamann C, Kirschner S, Günther KP, Hofbauer LC. Bone, sweet bone--osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol 2012;8:297–305 [DOI] [PubMed] [Google Scholar]
- 6.Strotmeyer ES, Cauley JA, Orchard TJ, Steenkiste AR, Dorman JS. Middle-aged premenopausal women with type 1 diabetes have lower bone mineral density and calcaneal quantitative ultrasound than nondiabetic women. Diabetes Care 2006;29:306–311 [DOI] [PubMed] [Google Scholar]
- 7.Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology 2007;148:198–205 [DOI] [PubMed] [Google Scholar]
- 8.Roggen I, Gies I, Vanbesien J, Louis O, De Schepper J. Trabecular bone mineral density and bone geometry of the distal radius at completion of pubertal growth in childhood type 1 diabetes. Horm Res Paediatr 2013;79:68–74 [DOI] [PubMed] [Google Scholar]
- 9.Pater A, Sypniewska G, Pilecki O. Biochemical markers of bone cell activity in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2010;23:81–86 [DOI] [PubMed] [Google Scholar]
- 10.Motyl K, McCabe LR. Streptozotocin, type I diabetes severity and bone. Biol Proced Online 2009;11:296–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abd El Dayem SM, El-Shehaby AM, Abd El Gafar A, Fawzy A, Salama H. Bone density, body composition, and markers of bone remodeling in type 1 diabetic patients. Scand J Clin Lab Invest 2011;71:387–393 [DOI] [PubMed] [Google Scholar]
- 12.Nicodemus KK, Folsom AR; Iowa Women’s Health Study . Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care 2001;24:1192–1197 [DOI] [PubMed] [Google Scholar]
- 13.Miao J, Brismar K, Nyrén O, Ugarph-Morawski A, Ye W. Elevated hip fracture risk in type 1 diabetic patients: a population-based cohort study in Sweden. Diabetes Care 2005;28:2850–2855 [DOI] [PubMed] [Google Scholar]
- 14.Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia 2005;48:1292–1299 [DOI] [PubMed] [Google Scholar]
- 15.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 2007;166:495–505 [DOI] [PubMed] [Google Scholar]
- 16.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int 2007;18:427–444 [DOI] [PubMed] [Google Scholar]
- 17.Hothersall EJ, Livingstone SJ, Looker HC, et al. Contemporary risk of hip fracture in type 1 and type 2 diabetes: a national registry study from Scotland. J Bone Miner Res 2014;29:1054–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao CC, Lin CS, Shih CC, et al. Increased risk of fracture and postfracture adverse events in patients with diabetes: two nationwide population-based retrospective cohort studies. Diabetes Care 2014;37:2246–2252 [DOI] [PubMed] [Google Scholar]
- 19.Forsén L, Meyer HE, Midthjell K, Edna TH. Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trøndelag Health Survey. Diabetologia 1999;42:920–925 [DOI] [PubMed] [Google Scholar]
- 20.Martín-Merino E, Fortuny J, Rivero E, García-Rodríguez LA. Validation of diabetic retinopathy and maculopathy diagnoses recorded in a U.K. primary care database. Diabetes Care 2012;35:762–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry SD, Zhu Y, Choi H, Kiel DP, Zhang Y. Diuretic initiation and the acute risk of hip fracture. Osteoporos Int 2013;24:689–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schelleman H, Pollard JR, Newcomb C, et al. Exposure to CYP3A4-inducing and CYP3A4-non-inducing antiepileptic agents and the risk of fractures. Pharmacoepidemiol Drug Saf 2011;20:619–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denburg MR, Leonard MB, Haynes K, et al. Risk of fracture in urolithiasis: a population-based cohort study using the health improvement network. Clin J Am Soc Nephrol 2014;9:2133–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.THIN Data Statistics [article online], 2014. Available from http://csdmruk.cegedim.com/. Accessed 8 August 2014
- 25.Chisholm J. The Read clinical classification. BMJ 1990;300:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 27.Bell S, Fletcher EH, Brady I, et al. End-stage renal disease and survival in people with diabetes: a national database linkage study. QJM 2015;108:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toppe C, Möllsten A, Schön S, Jönsson A, Dahlquist G. Renal replacement therapy due to type 1 diabetes; time trends during 1995-2010--a Swedish population based register study. J Diabetes Complications 2014;28:152–155 [DOI] [PubMed] [Google Scholar]
- 29.Diabetes UK. Diabetes in the UK 2011/2012 [article online], 2012. Available from http://www.diabetes.org.uk/documents/reports/diabetes-in-the-uk-2011-12.pdf. Accessed 24 March 2014
- 30.Currie CJ, Peyrot M, Morgan CL, et al. The impact of treatment non-compliance on mortality in people with type 1 diabetes. J Diabetes Complications 2013;27:219–223 [DOI] [PubMed] [Google Scholar]
- 31.Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res 2011;26:1729–1739 [DOI] [PubMed] [Google Scholar]
- 32.Tsampalieros A, Gupta P, Denburg MR, et al. Glucocorticoid effects on changes in bone mineral density and cortical structure in childhood nephrotic syndrome. J Bone Miner Res 2013;28:480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Diabetes Association . (11) Children and adolescents. Diabetes Care 2015;38(Suppl. 1):S70–S76 [DOI] [PubMed] [Google Scholar]
- 34.Mastrandrea LD, Wactawski-Wende J, Donahue RP, Hovey KM, Clark A, Quattrin T. Young women with type 1 diabetes have lower bone mineral density that persists over time. Diabetes Care 2008;31:1729–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thrailkill K, Bunn RC, Lumpkin C Jr, et al. Loss of insulin receptor in osteoprogenitor cells impairs structural strength of bone. J Diabetes Res 2014;2014:703589 [DOI] [PMC free article] [PubMed]
- 36.Botolin S, McCabe LR. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem 2006;99:411–424 [DOI] [PubMed] [Google Scholar]
- 37.Fowlkes JL, Nyman JS, Bunn RC, et al. Osteo-promoting effects of insulin-like growth factor I (IGF-I) in a mouse model of type 1 diabetes. Bone 2013;57:36–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann T, Lodes S, Kästner B, et al. High serum pentosidine but not esRAGE is associated with prevalent fractures in type 1 diabetes independent of bone mineral density and glycaemic control. Osteoporos Int 2014;25:1527–1533 [DOI] [PubMed] [Google Scholar]
- 39.Vestergaard P, Rejnmark L, Mosekilde L. Are antiresorptive drugs effective against fractures in patients with diabetes? Calcif Tissue Int 2011;88:209–214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.