Abstract
The retroviral oncoprotein Tax from human T cell leukemia virus type 1 (HTLV-1) induces persistent activation of IκB kinase (IKK)/NF-κB signaling, an essential step for initiating HTLV-1 oncogenesis. The regulation of the IKK/NF-κB signaling in HTLV-1-transformed T cells remains incompletely understood. In the present study, we showed that the autophagy molecule Beclin1 not only executed a cytoprotective function through induction of autophagy but also played a pivotal role in maintaining Tax-induced activation of two key survival factors, NF-κB and Stat3. Silencing Beclin1 in HTLV-1-transformed T cells resulted in diminished activities of NF-κB and Stat3 as well as impaired growth. In Beclin1-depleted cells, Tax failed to activate NF-κB and Stat3 at its full capacity. In addition, we showed that Beclin1 interacted with the catalytic subunits of IKK. Further, we observed that selective inhibition of IKK repressed the activities of both NF-κB and Stat3 in the context of HTLV-1-transformation of T cells. Our data, therefore, unveiled a key role of Beclin1 in maintaining persistent activities of both NF-κB and Stat3 in the pathogenesis of HTLV-1-mediated oncogenesis.
Keywords: HTLV-1 Tax, Beclin1, autophagy, IKK, NF-κB, Stat3
Introduction
Human T cell leukemia virus type 1 (HTLV-1) is the only human retrovirus that is etiologically linked to adult T cell leukemia and lymphoma (ATL) [1]. The viral genome of HTLV-1 encodes a transforming protein, named Tax, which plays a central role in transforming CD4+ T lymphocytes [2]. Expression of Tax is crucial not only for transactivating viral gene transcription but also for promoting survival and proliferation of virally infected human T lymphocytes [3,4]. Through dysregulation of cellular oncogenic signaling pathways, Tax promotes cell cycle progression, leading to aberrant proliferation of HTLV-1-infected T cells [4]. Notably, Tax stimulates IκB kinase complex (IKK), resulting in persistent activation of NF-κB [5]. Blockade of IKK/NF-κB signaling causes apoptosis of HTLV-1-transformed T cells, supporting the notion that the constitutive activation of NF-κB is a prerequisite for HTLV-1 transformation of T cells [6].
We have shown that Tax deregulates autophagy through activation of IKK, and this process is crucial for maintenance of HTLV-1-mediated T cell malignancy [16]. Tax directly targets the Beclin1-containing autophagy molecular complex to increase autophagic flux, while silencing Beclin1 affects survival and proliferation of HTLV-1-transformed T cells [7]. Autophagy is initiated to degrade aggregated cellular proteins or aged organelles through the autophagosome-lysosome degradation pathway, providing valuable energy source for cells under nutrient deprivation or other stress conditions [8]. Autophagy also plays key roles in anti-inflammation, viral infection, neurodegenerative disorder, autoimmune disease and tumor suppression by maintaining chromosomal integrity [8]. In certain contexts and stages of cancer, autophagy can function to promote tumor progression and metastasis [9].
Beclin1 is one of the key components of the Beclin1-PI3 kinase class III protein complex that mediates vesicular nucleation during formation of autophagosome [8]. In addition to the interaction of Tax with Beclin1, other viral proteins target at Beclin1 by either stimulating or inhibiting its activity [7,10]. Beclin1 is transcriptionally upregulated by NF-κB and is required for tumor necrosis factor alpha (TNFα)-mediated activation of NF-κB [11,12]. Given the observation that a persistent activation of NF-κB is essential for HTLV-1 oncogenesis, we reason that Beclin1 may play a role in NF-κB signaling in HTLV-1-transformed T cells. In the present study, we have shown that Beclin1 is crucial for maintaining constitutive activation of NF-κB and Stat3 induced by Tax.
Materials and Methods
Cell lines, antibodies and chemicals
MT-2 and Jurkat cell line were obtained from AIDS research and reference reagent program (NIAID, National Institutes of Health). SLB-1 and MT-1 cell lines were described previously [7]. These cells were cultured in RPMI1640 medium supplemented with 10% FBS. HEK293 cells were cultured in DMEM medium containing 10% FBS.
Antibodies for IKKα, IKKβ, IKKγ, Beclin1, SQSTM1/p62, HA and GST were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies for LC3, Atg5, PI3KC3, and cleaved caspase 3, 7, 9 and PARP were from Cell Signaling (Danvers, MA, USA). Anti-β-actin and anti-Flag were from Sigma (St Louis, MO, USA). Monoclonal anti-Tax antibody was obtained from AIDS Reagent Program. DMSO, U0126, LY294002 and BAY11-7805 were purchased from Sigma (St Louis, MO, USA). APC-Annexin V and 7-AAD staining solution were purchased from BD Biosciences (San Jose, CA, USA).
Plasmids, immunoblot, glutathione S-transferase (GST) pulldown assay
The expression plasmids for GST-tagged IKKα, IKKβ, IKKγ, Beclin1 and HA-tagged Tax, M22 and M47 as well as Flag-tagged Beclin1 and PI3KC3 were described previously [7]. The mutants of Beclin1 were generated using a PCR-based mutagenesis method. Lentivirus vectors for IKKα- and IKKβ-specific shRNAs were described previously [7], and the lentivirus vectors for IKKγ- and Beclin1-specific shRNAs were purchased from Open Biosystems (Pittsburgh, PA, USA). Lentivirus vector for PI3KC3-specific shRNA was purchased from Thermo Scientific (Grand Island, NY, USA). Western Blot (WB), co-immunoprecipitation and GST pulldown assays were performed as described previously [7].
Electrophoretic gel mobility shift assay (EMSA)
Nuclear extracts were prepared from various cell lines using NE-PER nuclear and cytoplasmic extraction reagents (Pierce, IL, USA). The oligonucleotides were 5′-end labeled with biotin (Integrated DNA Technologies, Coralville, IA, USA) and annealed to their complementary strands. The binding activities were examined by EMSA using Light Shift Chemiluminescent EMSA Kit (Pierce, Rockford, IL, USA). The sequences of the probes for NF-κB, Stat3, AP-1 and OCT1 were previously reported [7].
Cell viability assay by trypan blue exclusion and FACS
Cells were transduced with recombinant lentiviruses that were produced and concentrated as previously described [41]. Three days post-transduction, cells were collected and re-suspended in fresh complete medium. An aliquot of cells was added to an equal volume of trypan blue (0.4%). The viable cells were measured by trypan blue exclusion assay. Each experiment was conducted in triplicate and the results were indicated as the mean ± SD.
The induction of apoptosis was determined using an APC-Annexin V/7-AAD double staining. NS (non-specific)-, Beclin1-depleted MT-2 and SLB-1 cells were harvested and washed twice with PBS buffer 6 days post-transduction. The cells were resuspended with 100μl binding buffer at 1 x 106 cells/ml, 5μl of APC-Annexin V and 5μl of 7-AAD were added and incubated for 15 min at room temperature at dark. The results were analyzed by flow cytometry.
Results
Silencing Beclin1 leads to growth arrest and apoptosis of HTLV-1-transformed T cells
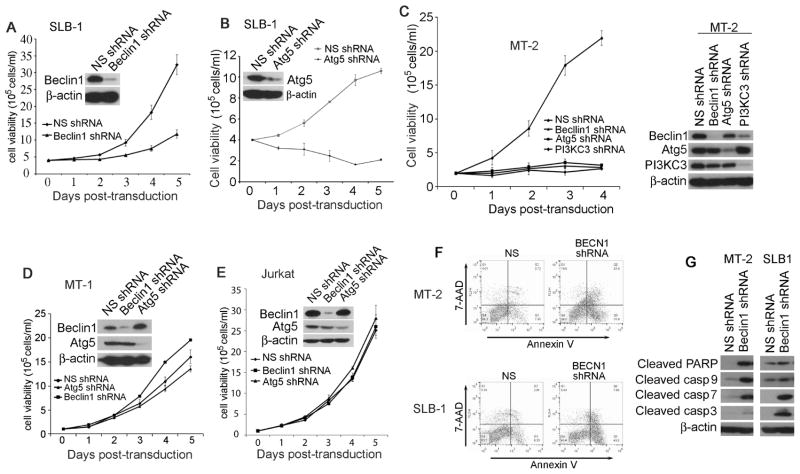
To determine the role of the autophagy molecules in promoting aberrant proliferation of HTLV-1-transformed T cells, we applied a lentiviral delivery of specific shRNA method to silence selected autophagic molecules including Beclin1, PI3KC3 and Atg5. We employed four model T cell lines including MT-2 and SLB-1 cell lines (HTLV-1-transformed human CD4+ T cells that express Tax), MT-1 cell line (adult T cell leukemia cells with lack of Tax expression) and Jurkat T cell line (non-HTLV-1-infected, CD4+ lymphoblastic leukemia cells). We found that knockdown of either Beclin1 or Atg5 in SLB-1 cells resulted in impaired survival and proliferation, and the growth of the Atg5-depleted cells were affected more severely (Fig. 1A and 1B). Similarly, silencing the key autophagy molecule Beclin1, Atg5 or PI3KC3 in MT-2 cells led to growth arrest (Fig. 1C). In contrast, depletion of either Beclin1 or Atg5 had no apparent growth impairment in MT-1 and Jurkat T cells (Fig. 1D and 1E). We found that silencing Beclin1 resulted in an increased annexin V staining in MT-2 and SLB-1 cells (Fig. 1F). Further, depletion of Beclin1 led to caspase-dependent apoptotic cell death as evidenced by increased production of the cleaved forms of caspase-3, -7, -9 and PARP (Fig. 1G). These findings support a key role of Beclin1 in promoting survival of HTLV-1- transformed T cells.
Figure 1. Beclin1 is required for promoting survival and growth of HTLV-1-transformed T cells.
Depletion of Beclin1 (A) or Atg5 (B) by lentiviral transduction of Beclin1- or Atg5-specific shRNA in SLB-1 cells was examined by anti-Beclin1 or anti-Atg5 immunoblot. The cell viability of SLB-1 cells transduced with non-specific (NS), Beclin1-specific shRNA (A) or Atg5-specific shRNA (B) was determined by trypan blue exclusion assay. (C) Beclin1, Atg5 or PI3KC3 was depleted by specific shRNAs in MT-2 cells, and the cell viability was measured accordingly (left panel). The knockdown efficiency of Beclin1-, Atg5- and PI3KC3 in MT-2 cells was determined by with immunoblot using relevant antibodies (right panel). Depletion of Beclin1 or Atg5 by lentiviral transduction of Beclin1- or Atg5-specific shRNA in MT-1 (D) or Jurkat (E) cells was examined by anti-Beclin1 or anti-Atg5 immunoblot. The cell viability was determined accordingly. The experiments were repeated at least three times. The mean ± s.e.m was shown in the figure. (F) Cell death analysis by flow cytometry with dual Annexin V/7-AAD cell labeling. NS-, Beclin1-, Atg5- or PI3KC3-depleted MT-2 or SLB-1 cells collected 6 days after transduction were dual-labeled with Annexin V and 7-AAD, followed by analysis with FACS. (G) Whole cell lysates of NS-, Beclin1-, Atg5- or PI3KC3-depleted MT-2 and SLB-1 cells were examined with immunoblot using anti-cleaved caspase-3, -7, -9 and PARP antibodies.
Beclin1 is required for Tax activation of NF-κB and Stat3
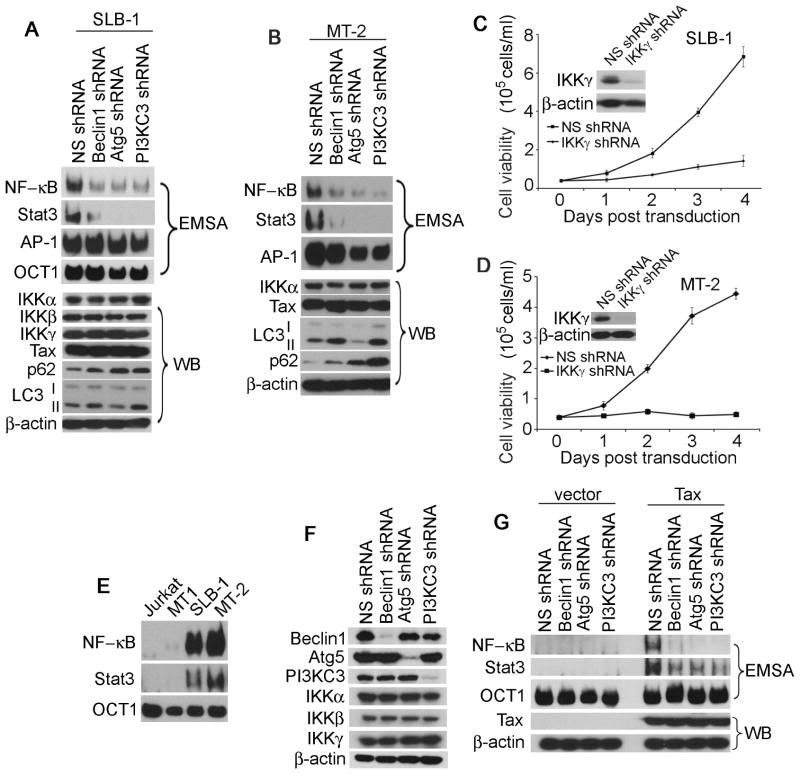
Maintenance of an optimal NF-κB activity is essential for survival and proliferation of HTLV-1-transformed T cells, particularly at early stage of oncogenesis. To elucidate a potential role of the autophagy molecules in regulating NF-κB activity, we silenced Beclin1, Atg5 or PI3KC3 with lentiviral transduction of the specific shRNAs in HTLV-1-transformed T cells. We found that depletion of Beclin1, Atg5 or PI3KC3 did not affect the protein levels of the IKK complex containing three subunits, IKKα, IKKβ and IKKγ as well as Tax (Fig. 2A). Next, we examined the expression of the autophagy marker proteins LC3 and p62/sequestosome1. LC3-II, the lipidated form of LC3-I, is generated during autophagy process, exhibiting a slightly faster mobility than LC3-I on SDS-PAGE. p62 is a master regulator of autophagy, which functions to conjugate ubiquitinated cellular proteins for targeting them into the autophagosome-lysosome degradation pathway. We observed that the LC3-II protein was accumulated in Beclin1- or PI3KC3-depleted T cells whereas it was reduced in Atg5-depleted cells (Fig. 2A and 2B). This result was consistent with the role of Atg5 at the early stage of vesicular nucleation during autophagy in which LC3-I was processed to generate the lipidated LC3-II. Silencing Beclin1 or PI3KC3 blocked fusion of autophagosome with lysosome, thereby preventing degradation of LC3-II. In contrast, p62 was accumulated in the autophagy molecule-depleted MT-2 and SLB-1 cells due to the blockade of autophagic flux (Fig. 2A and 2B).
Figure 2. Beclin1 maintains persistent activity of NF-κB and Stat3 in HTLV-1-transformed T cells.
Whole cell lysates of NS-, Beclin1-, Atg5- or PI3KC3-depleted SLB-1 (A) and MT-2 (B) cells were examined with Western Blot (WB) using antibodies indicated in the figure. The activities of NF-κB, Stat3 and AP-1 in NS-, Beclin1-, Atg5- or PI3KC3-depleted SLB-1 (A) and MT2 (B) cells were measured by EMSA. IKKγ was silenced by the specific shRNA in SLB-1 (C) and MT-2 (D) cells, and the cell viability was determined accordingly. (E) The activities of NF-κB and Stat3 in Jurkat, MT1, MT2 and SLB-1 cells were analyzed with EMSA. (F) Whole cell lysates of NS-, Beclin1-, Atg5- or PI3KC3-depleted HEK293 cells were analyzed with immunoblot using the antibodies indicated in the figure. (G) NS-, Beclin1-, Atg5- or PI3KC3-depleted HEK293 cells were transfected with vector or Tax-HA. EMSA was applied to determine the activities of NF-κB and Stat3 (upper panel). The expression level of the Tax protein was shown in the lower panel as indicated in the figure.
We determined if inhibition of autophagy affects the activity of NF-κB in HTLV-1- transformed T cells expressing Tax. We found that in Beclin1-, Atg5- or PI3KC3-depleted SLB-1 and MT-2 cells, the activity of both NF-κB and Stat3 was diminished (Fig. 2A and 2B). AP-1 was highly activated in HTLV-1-transformed T cells and its activity was not significantly altered by depleting the autophagy molecules (Fig. 2A and 2B). Indeed, inhibition of the NF-κB signaling by silencing IKKγ, the regulatory subunit of the IKK complex, led to drastic growth impairment of SLB-1 and MT-2 cells (Fig. 2C and 2D). In contrast, Jurkat and MT-1 cells displayed barely detectable levels of the NF-κB and Stat3 activities (Fig. 2E), and the growth of these cells was less affected by loss of Beclin1 (Fig. 1D and 1E).
Further, we evaluated if the autophagy molecules are required for Tax-induced activation of NF-κB and Stat3. Similar to the approaches employed in T cells, we depleted the autophagy molecules in HEK293, followed by transfection of Tax or a control vector (Fig. 2F and 2G). We found that in Beclin1-, Atg5- or PI3KC3-depleted HEK293 cells, Tax failed to activate either NF-κB or Stat3 at its full capacity (Fig. 2G). Thus, our results indicated that the autophagy molecules contributed significantly to Tax activation of NF-κB and Stat3.
Beclin1 interacts with the IKK complex
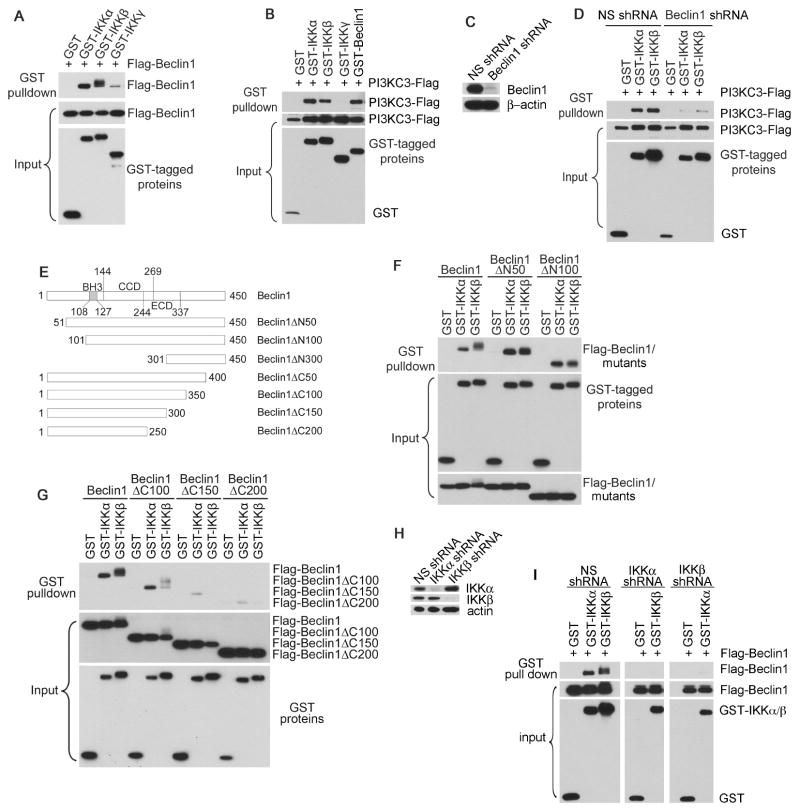
Tax was previously reported to interact with the IKK complex and Beclin1, and these viral-cellular protein interactions are critical for T cell survival and growth [5,7]. To investigate the molecular mechanism of the autophagy molecular complex Beclin1-PI3KC3 in mediating Tax-induced activation of NF-κB, we first performed co-immunoprecipitation (IP) assay by transient co-transfection with various combination of cDNA constructs as indicated in Fig. 3A. We observed that Beclin1 was co-precipitated with the catalytic subunits of IKK, IKKα and IKKβ, and interacted less efficiently with the regulatory subunit IKKγ (Fig. 3A). Using similar approach, we found that PI3KC3 was also co-precipitated with IKKα and IKKβ, but not with IKKγ (Fig. 3B). Depletion of Beclin1 resulted in the impairment of co-precipitation of PI3KC3 with IKKα and IKKβ (Fig. 3C and 3D), suggesting that the interaction of PI3KC3 with the IKK complex was dependent on Beclin1 and that Beclin1 directly interacted with the IKK complex. We next generated various mutants of Beclin1 by sequential deletion of N- or C-terminal fragments to determine the binding motifs for IKKα and IKKβ. Beclin1 constitutes three major domains, the BH3 domain that mediates binding to Bcl-2, the coiled coil domain (CCD) and the evolutionarily conserved domain (ECD) as depicted in Fig. 5E. It was shown that the C-terminal 150 amino acids of Beclin1 appeared to be critical for the interaction of Beclin1 with IKKα and IKKβ (Fig. 3F and 3G). To differentiate the role of IKKα from IKKβ for the interaction with Beclin1, we silenced IKKα or IKKβ followed by co-IP analysis. It was shown that the interaction of Beclin1 with the IKK complex required co-presence of IKKα and IKKβ (Fig. 3H and 3I), correlating with the crucial role of these catalytic kinases in Tax induction of NF-κB activation. Because it has been previously shown that Tax is able to interact with IκB kinases and Beclin1, this leads to the notion that Tax engages a molecular crosstalk between the IKK complex and Beclin1.
Figure 3. Beclin1 interacts with the components of the IKK complex.
(A) Flag-Beclin1 was co-transfected with GST or with GST-tagged IKKα, IKKβ or IKKγ in HEK293 cells. The interaction of these proteins was examined with GST pulldown, followed by anti-Flag immunoblot to detect Flag-Beclin1 (top panel). Protein inputs were shown in the middle and bottom panels. (B) PI3KC3-Flag was co-transfected with GST or with GST-tagged IKKα, IKKβ, IKKγ or Beclin1 in HEK293 cells, followed by GST pulldown assay and anti-Flag immunoblot. (C) The knockdown efficiency of the endogenous Beclin1 with lentiviral transduction of Beclin1-specific shRNAs in HEK293 cells was determined by anti-Beclin1 immunoblot. (D) The interaction of PI3KC3 with IKKα or IKKβ was examined in transfected NS- or Beclin1- depleted HEK293 cells as shown in the figure. (E) Schematic structures of the Beclin1 mutants, which were generated using PCR-based mutagenesis method. (F) and (G) The IKK-binding motifs on Beclin1 were evaluated using Flag-Beclin1 and its mutants, which were co-transfected with GST-tagged IKKα or IKKβ in HEK293 cells. (H) The knockdown efficiency of endogenous IKKα or IKKβ in HEK293 cells was determined by immunoblot with relevant antibodies. (I) The interaction of Beclin1 with IKKα or IKKβ was examined in transfected NS-, IKKα- or IKKβ-depleted HEK293 cells using the method as above.
Stat3 cooperates with NF-κB in HTLV-1-transformed T cells
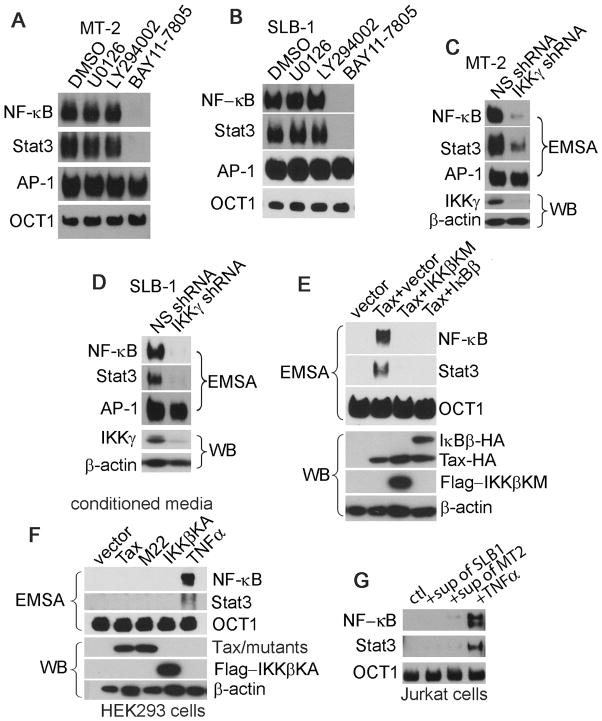
In HTLV-1-transformed T cells that express Tax, the constitutive activities of both NF-κB and Stat3 can be detected. Tax is found to induce production and secretion of pro-inflammatory cytokines, leading to the activation of Stat3 [13]. In other context, NF-κB is found to directly activate Stat3 [14]. In MT-2 and SLB-1 cells treated with a selective inhibitor of IKK, BAY11-7805, the activities of both NF-κB and Stat3, but not AP-1, were inhibited (Fig. 4A and 4B). Consistently, silencing IKKγ resulted in the reduction of both NF-κB and Stat3 activities in MT-2 and SLB-1 cells (Fig. 4C and 4D). We next observed that co-transfection of Tax with IKKβKM, a dominant-negative mutant of IKKβ, or with IκBβ, a native cellular inhibitory protein for NF-κB, led to suppression of both NF-κB and Stat3 activities (Fig. 4E).
Figure 4. Stat3 cooperates with NF-κB in the context of HTLV-1 -transformed T cells.
(A) MT-2 or (B) SLB-1 cells were treated with DMSO, U0126, LY294002 or BAY11-7805 (5μM) for 4 hours. The activities of NF-κB, Stat3 and AP-1 were evaluated with EMSA. EMSA was employed to determine the activities of NF-κB, Stat3 and AP-1 in NS- or IKKγ-depleted MT-2 (C) and SLB-1 (D) cells. (E) Tax was co-transfected with vector, IKKβKM (the dominant-negative mutant of IKKβ) or IκBβ in HEK293 cells. The nuclear extracts were examined for the activities of NF-κB and Stat3 with EMSA. The NF-κB and Stat3 activities were shown in the upper panel. The total lysates were analyzed with Western Blot (WB) (lower panel). (F) Parental HEK293 cells were stimulated with TNFα (20ng/ml) or the conditioned culture media from the HEK293 cells transfected with Tax, M22 or IKKβKA (the constitutively active form of IKKβ) for 4 hours. The activities of NF-κB and Stat3 were analyzed by EMSA (upper panel). Proteins expression was shown in the lower panel. (G) Jurkat T cells were stimulated with the conditioned culture media from SLB-1 or MT-2 or with TNFα (20ng/ml) for 4 hours. The activities of NF-κB and Stat3 were analyzed by EMSA. Sup: supernatant; Ctl: the fresh complete medium for control.
To verify a potential role of soluble factors in the involvement of NF-κB-dependent activation of Stat3, we collected culture media from the HEK293 cells transfected with Tax, M22 (a Tax mutant defective in inducing NF-κB activity) or IKKβKA (a constitutively active form of IKKβ) to stimulate the parental HEK293 cells. We found that none of these conditioned media was able to activate either NF-κB or Stat3. In contrast, the treatment of HEK293 cells with TNFα activated both NF-κB and Stat3 (Fig. 4F). Next, we utilized the conditioned media from SLB-1 or MT-2 cells to stimulate Jurkat T cells, and we observed that none of the conditioned media had the capacity to induce the activity of NF-κB and Stat3 (Fig. 4G). Taken these results together, we concluded that Stat3 cooperates with NF-κB via an intracellular mechanism and that Beclin1 plays a crucial role in maintaining sustainable activities of NF-κB and Stat3 in the context of HTLV-1-transformed T cells.
Discussion
In the present study, we show that the autophagy molecule Beclin1 maintains the constitutive activities of both NF-κB and Stat3. Because both NF-κB and Stat3 are crucial in supporting T cell survival and malignancy, we conclude that Beclin1 promotes aberrant proliferation of HTLV-1-transformed T cells through regulation of Tax-mediated activation of NF-κB and Stat3. Our study supports the notion that the autophagosome may serve as a signal transduction platform where Tax recruits IκB kinases to this subcellular structure for activation. Indeed, a portion of the Tax protein co-localizes with the cytoplasmic LC3+ foci, recruiting IκB kinases together with autophagy molecules Beclin1 and Bif-1 to the lipid raft microdomains within these compartments [7]. Alternatively, Beclin1, Atg5 or PI3KC3 may participate in the process of Tax-mediated activation of NF-κB via distinct mechanisms independent of autophagosome. At the advanced disease stage, Tax is present in roughly 40% of ATL cases and this viral protein remains to be a determining factor for the maintenance of T cell malignancy. However, loss of Tax occurs in 50–60% of ATL cases, suggesting that Tax is no longer required for the maintenance of T cell malignancy probably due to accumulative oncogenic events. Indeed, we notice that in MT-1 cells that do not express Tax, silencing Beclin1 even slightly facilitates their growth. The NF-κB activity is very low in MT-1 cells, suggesting that a persistent NF-κB activity is also not needed for maintaining malignant phenotype at the Tax-independent stage of leukemia. Since autophagy can function to limit genome instability, a lower autophagic activity in MT-1 cells may be beneficial for facilitating leukemia progression by enhancing genome damage, allowing accumulation of multiple oncogenic proteins.
The ability of Beclin1 in assisting Tax-mediated activation of NF-κB may be correlated with its binding to the catalytic subunits of the IKK complex via its C-terminus, and Beclin1 itself may be phosphorylated by IKKβ. A higher molecular weight protein of Beclin1 is observed in cells ectopically expressing IKKβ (Fig. 5A and 5G), and this larger form of Beclin1 is disappeared when the IKKβ immunoprecipitates are treated with calf intestinal phosphatase (data not shown). Thus, the function of Beclin1 in Tax-mediated activation of NF-κB may serve two lines of biological significances contributing to HTLV-1 oncogenesis. First, Tax is known to induce cell senescence through hyper-activation of NF-κB. By fine-tuning NF-κB activity, Beclin1 could alleviate Tax-mediated cytotoxicity, allowing Tax to override T cell senescence, to promote cell cycle progression and ultimately to transform T cells. This assumption is supported by the observation that HBZ, an antisense gene product of HTLV-1, tunes NF-κB activity and assists Tax in overcoming cell senescence [15]. By extension, Beclin1 could act as a cellular modulator that fine-tunes the NF-κB activity in HTLV-1-infected T cells. Second, the Beclin1-containing autophagy complex is able to execute a cytoprotective role in promoting T cell survival particularly at the Tax-dependent stage of T cell oncogenesis by maintaining the optimal activities of NF-κB and Stat3.
Our study has also shown that Stat3 cooperates with NF-κB in HTLV-1-transformed T cells. The Stat3 activity in ATL cells has not been well studied. A previous report suggests that the Stat3 activity is stimulated by a soluble IL-6 receptor in HTLV-1-transformed T cells [13]. We have observed that Stat3 is co-activated with NF-κB, as evidenced by EMSA and the specific phosphorylation of Stat3 in Tax-expressing, HTLV-1-transformed T cells, and that Tax can co-activate Stat3 and NF-κB in transfected HEK293 cells and in Tax-immortalized T cells. It is well known that co-activation of NF-κB and Stat3 correlates with poor prognosis in a number of human cancers and contributes to resistance to chemotherapy [14,16]. Furthermore, it has been shown that NF-κB and Stat3 can crosstalk [14]. In this study, we show that the cooperation of NF-κB and Stat3 is mediated through an intracellular mechanism, which is not related to any soluble factor. Collectively, our study demonstrates a dynamic molecular crosstalk between NF-κB/Stat3 and the Beclin1-containing autophagy protein complex in the context of HTLV-1-mediated transformation.
Acknowledgments
We thank Atsushi Koito and Takeo Ohsugi for MT-1 cell line. Research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under award number R01AI090113 to Hua Cheng.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Authors’ contributions
LC and DL designed, performed experiments and analyzed the data. LC wrote the manuscript. YZ and HZ conducted parts of the experiments. HC analyzed the data and modified the manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77(12):7415–9. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grassmann R, Berchtold S, Radant I, Alt M, Fleckenstein B, Sodroski JG, et al. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J Virol. 1992;66(7):4570–5. doi: 10.1128/jvi.66.7.4570-4575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felber BK, Paskalis H, Kleinman-Ewing C, Wong-Staal F, Pavlakis GN. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985;229(4714):675–9. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- 4.Marriott SJ, Semmes OJ. Impact of HTLV-I Tax on cell cycle progression and the cellular DNA damage repair response. Oncogene. 2005;24(39):5986–95. doi: 10.1038/sj.onc.1208976. [DOI] [PubMed] [Google Scholar]
- 5.Sun SC, Ballard DW. Persistent activation of NF-kappaB by the tax transforming protein of HTLV-1: hijacking cellular IkappaB kinases. Oncogene. 1999;18(49):6948–58. doi: 10.1038/sj.onc.1203220. [DOI] [PubMed] [Google Scholar]
- 6.Sun SC, Yamaoka S. Activation of NF-kappaB by HTLV-I and implications for cell transformation. Oncogene. 2005;24(39):5952–64. doi: 10.1038/sj.onc.1208969. [DOI] [PubMed] [Google Scholar]
- 7.Ren T, Takahashi Y, Liu X, Loughran TP, Sun SC, Wang HG, Cheng H. HTLV-1 Tax deregulates autophagy by recruiting autophagic molecules into lipid raft microdomains. Oncogene. 2015;34(3):334–45. doi: 10.1038/onc.2013.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–21. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306(5698):990–5. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang H, Da L, Mao Y, Li Y, Li D, Xu Z, et al. Hepatitis B virus X protein sensitizes cells to starvation-induced autophagy via up-regulation of beclin 1 expression. Hepatology. 2009;49(1):60–71. doi: 10.1002/hep.22581. [DOI] [PubMed] [Google Scholar]
- 11.Copetti T, Bertoli C, Dalla E, Demarchi F, Schneider C. p65/RelA modulates BECN1 transcription and autophagy. Mol Cell Biol. 2009;29(10):2594–608. doi: 10.1128/MCB.01396-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Criollo A, Chereau F, Malik SA, Niso-Santano M, Marino G, Galluzzi L, et al. Autophagy is required for the activation of NFkappaB. Cell cycle. 2012;11(1):194–9. doi: 10.4161/cc.11.1.18669. [DOI] [PubMed] [Google Scholar]
- 13.Horiuchi S, Yamamoto N, Dewan MZ, Takahashi Y, Yamashita A, Yoshida T, et al. Human T-cell leukemia virus type-I Tax induces expression of interleukin-6 receptor (IL-6R): Shedding of soluble IL-6R and activation of STAT3 signaling. Int J Cancer. 2006;119(4):823–30. doi: 10.1002/ijc.21918. [DOI] [PubMed] [Google Scholar]
- 14.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21(1):11–9. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhi H, Yang L, Kuo YL, Ho YK, Shih HM, Giam CZ. NF-kappaB hyper-activation by HTLV-1 tax induces cellular senescence, but can be alleviated by the viral anti-sense protein HBZ. PLoS Pathog. 2011;7(4):e1002025. doi: 10.1371/journal.ppat.1002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greten FR, Weber CK, Greten TF, Schneider G, Wagner M, Adler G, et al. Stat3 and NF-kappaB activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterology. 2002;123(6):2052–63. doi: 10.1053/gast.2002.37075. [DOI] [PubMed] [Google Scholar]