Abstract
Adult T cell leukemia and lymphoma (ATL) is a highly aggressive form of hematological malignancy and is caused by chronic infection of human T cell leukemia virus type 1 (HTLV-1). The viral genome encodes an oncogenic protein, Tax, which plays a key role in transactivating viral gene transcription and in deregulating cellular oncogenic signaling to promote survival, proliferation and transformation of virally infected T cells. Hence, Tax is a desirable therapeutic target, particularly at early stage of HTLV-1-mediated oncogenesis. We here show that niclosamide, an anti-helminthic molecule, induced apoptosis of HTLV-1-transformed T cells. Niclosamide facilitated degradation of the Tax protein in proteasome. Consistent with niclosamide-mediated Tax degradation, this compound inhibited activities of MAPK/ERK1/2 and IκB kinases. In addition, niclosamide downregulated Stat3 and pro-survival Bcl-2 family members such as Mcl-1 and repressed the viral gene transcription of HTLV-1 through induction of Tax degradation. Since Tax, Stat3 and Mcl-1 are crucial molecules for promoting survival and growth of HTLV-1-transformed T cells, our findings demonstrate a novel mechanism of niclosamide in inducing Tax degradation and downregulating various cellular pro-survival molecules, thereby promoting apoptosis of HTLV-1-associated leukemia cells.
Keywords: Niclosamide, HTLV-1, Tax, Down-regulation
Introduction
Chronic infection of human T cell leukemia virus type 1 (HTLV-1) causes adult T cell leukemia and lymphoma (ATL), a specific type of hematological malignancy with characteristic features of enlarged lymph nodes, skin lesion and elevated calcium[1; 2]. The leukemia develops following prolonged incubation period among 5% of infected patients, and the disease progresses aggressively. In addition to ATL, HTLV-1 infection is also associated with myelopathy (HAM)/tropical spastic paraparesis (TSP), uveitis and dermatitis[3]. Currently, there is no effective therapy for ATL[4].
HTLV-1 is the first human retrovirus ever discovered, and its viral genome encodes three major structural gene products including Gag, Pol and Env[3]. The pX region of the 3’-end of the viral genome encodes a viral transactivator, Tax. Expression of Tax is essential for promoting 5’-long terminal repeat (5’-LTR)-driven viral gene transcription through activation of the transcriptional factor CREB, and this process is crucial for productive viral replication, assembly of infectious viral particles and viral transmission by facilitating formation of virological synapse[5]. In addition, Tax deregulates a variety of cellular oncogenic signaling pathways including NF-κB, PI3K/Akt and Stat3[6], thereby promoting T cell survival, aberrant proliferation and ultimately, transformation once additional oncogenic events occur. Tax also upregulates an antisense gene product of the HTLV-1 genome, HBZ, a viral factor that exhibits an oncogenic activity in mice[7]. Targeting the oncogenic Tax protein, therefore, is a desirable therapeutic option for HTLV-1 infection to prevent development of HTLV-1-associated leukemia/lymphoma and neurological diseases.
We evaluated several small molecule inhibitors based on their reported activity in inhibiting oncogenic signaling pathways that are commonly seen in ATL. We identified niclosamide, an FDA-approved anti-helminthic compound, to be a promising therapeutic candidate for ATL. Niclosamide was previously reported to exhibit anti-tumorigenic activity in a number of human cancers by inhibiting NF-κB, Stat3, Wnt/β-catenin, Notch, ROS and S100A4 in addition to inhibiting mammalian target of rapamycin complex 1 (mTORC1)[8; 9; 10; 11; 12]. Since Stat3 and NF-κB are constitutively activated in ATL cells, these findings raise a possibility that niclosamide could act at these oncogenic targets, potentially causing apoptotic death of HTLV-1-transformed T cells. The present study has demonstrated that niclosamide is a potent therapeutic molecule for ATL by employing a novel mechanism through induction of proteasomal degradation of the viral oncoprotein Tax and downregulation of certain cellular pro-survival molecules.
Materials and Methods
Cell lines, antibodies and chemicals
MT-1, 2 and 4 cells were cultured in RPMI1640 supplemented with 10% of fetal bovine serum (FBS). HEK293 and HeLa cells (NIH AIDS Reagent Program) were cultured in DMEM containing 10% FBS. The Tax-immortalized human CD4+ T cell line, PTX4-1, was established by lentiviral transduction of the tax-gfp fusion gene[13], and was cultured in RPMI1640 containing 10% FBS and 100u/ml of recombinant IL-2 (AIDS Reagent Program). Antibodies for pERK1/2, ERK1/2, pMEK1, MEK1 and GST were purchased from Santa Cruz Biotechnology (Dallas, TX), and anti-Bcl-2, -Bcl-xL, -Mcl-1, -STAT3, -ubiquitin and -ubiquitin-K48 antibodies were from Cell Signaling (Boston, MA). Niclosamide, chloroquine and MG-132 were purchased from Sigma (St. Louis, MO).
Plasmids, immunoblot, cell proliferation assay
The plasmids for Tax-HA, M22-HA, Tax-GFP and Tax shRNA lentivirus have been reported previously. The co-immunoprecipitation and GST pulldown assays were described previously [14]. Cell proliferation assay was performed using tetrazolium compound based CellTiter 96® AQueous One Solution Cell Proliferation (MTS) assay (Promega, Madison, WI) according to the manufacturer's instructions.
Real-time quantitative PCR
Total RNA was isolated using the RNeasy kit (Qiagen, Valencia, CA) and its concentration was determined using the NanoDrop1000 spectrophotometer (Thermo Scientific, Waltham, MA). Quality and integrity of total RNA was assessed on 1% formaldehyde-agarose gels. cDNA was synthesized using the Omniscript Reverse Transcriptase Kit (Qiagen) following the manufacturer's recommended protocol. Template samples were subjected in triplicate to real-time qPCR (Stratagene Mx3005P system, La Jolla, CA) using Power SYBR Green (Applied Biosystems, Carlsbad, CA).
Electrophoretic mobility gel shift assay (EMSA)
Nuclear extracts were prepared from various T cell lines using NE-PER nuclear and cytoplasmic extraction reagents (Pierce, Rockford, IL). The oligonucleotide was 5’-end labeled with biotin (Integrated DNA Technologies, Coralville, IA) and annealed to its complementary strand. The binding activities were examined by EMSA using Light Shift Chemiluminescent EMSA Kit (Pierce) following the protocol reported previously [14]. Consensus gel shift oligonucleotides are for Oct-1 (5’-TGTCGAATGCAAATCACTAGAA-3’) and Stat3 (5’-GATCCTTCTGGGAATTCCTAGATC-3’)
Fluorescence imaging
Tax-GFP and Ubiquitin-HA were transiently co-transfected into HeLa cells using FuGeneHD transfection reagent (Roche, Indianapolis, IN). 24 hours following transfection, the transfected cells were treated with DMSO, niclosamide or MG-132. For immunofluorescence staining, cells were fixed in 4% paraformaldehyde-PBS, blocked in 3% horse serum-PBS and stained with anti-HA primary antibodies overnight at 4°C, followed by incubation with fluorescence conjugated secondary antibodies and then mounted with DAPI (Invitrogen, Carlsbad, CS). Fluorescence images were taken using an OLYMPUS IX81 deconvolution microscope and analyzed with SlideBook 5.0 software (Intelligent Imaging Innovations, Denver, CO).
Results
Niclosamide induces apoptotic death of HTLV-1-transformed T cells
Recent screening of chemicals with autophagy-inducing capability revealed that niclosamide is a potent inducer of autophagy through inhibition of mTORC1[12], implicating its new potential in treating human cancer. Studies demonstrated that niclosamide inhibited multiple oncogenic pathways and suppressed colon cancer growth and metastasis in a mouse model[11].
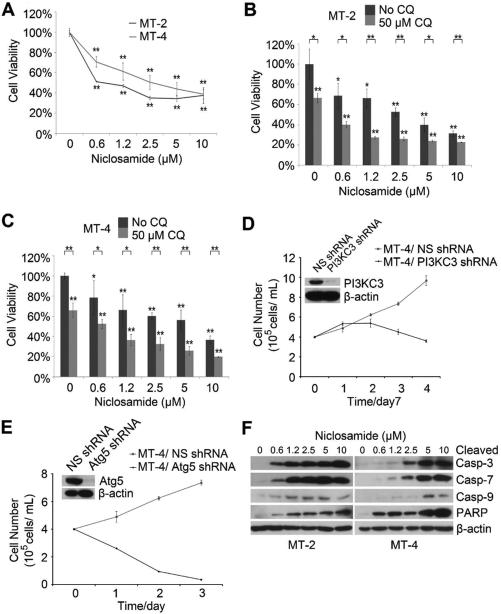
To determine whether niclosamide suppresses HTLV-1-transformed T cells, we treated MT-2 and MT-4 with various doses of niclosamide. We found that niclosamide effectively reduced cell viability of both T cell lines (Fig.1A). In cancer cells, autophagy is typically induced following chemotherapy, which was thought to play a cytoprotective role that contributes to chemotherapy resistance. However, excessive autophagy could also induce cell death. To understand the role of autophagy in niclosamide-mediated reduction of cell viability, we combined various amounts of niclosamide with chloroquine, an autophagy inhibitor that blocks fusion of autophagosome with lysosome. We found combination of niclosamide and chloroquine acted significantly better than niclosamide alone in suppressing the cell viability (Fig. 1B and 1C). Indeed, depletion of key autophagy molecules including PI3 kinase class III (PI3KC3) and Atg5 led to impaired growth in MT-4 cells (Fig. 1D and 1E), verifying a cytoprotective role of autophagy for HTLV-1-transformed T cells. We found that niclosamide induced dramatic caspase-mediated apoptosis as evidenced by detection of cleaved caspase-3, -7, -9 and PARP (Fig. 1F). These results suggested that niclosamide impaired survival of HTLV-1-transformed T cells through induction of caspase-dependent apoptosis.
Figure 1. Niclosamide induces apoptosis of HTLV-1-transformed T cells.
A) MT-2 and MT-4 cells were treated with niclosamide. % of cell viability was determined by comparison of niclosamide vs solvent treated cells 48 hours after treatment using MTT assay. Experiments were performed in triplicate. *, p<0.05. **, p<0.01 as determined by 2-tail student t-test. B) and C) MT-2 cells (B) or MT-4 cells (C) were treated with niclosamide alone or with chloroquine.% of cell viability was determined after 72 hours using the method described in (A). D) and E) MT-4 cells were transduced with lentiviruses expressing NS (non-specific) shRNA, PI3KC3 shRNA (D) or Atg5 shRNA (E), and the cell viability was determined by trypan-blue exclusion assay. F) MT-2 and MT-4 cells were treated with niclosamide, and total protein lysates were analyzed with immunoblot to detect cleaved forms of caspase-3 (casp-3), -7, -9 and PARP 24 hours after niclosamide treatment.
Niclosamide facilitates proteasomal degradation of Tax
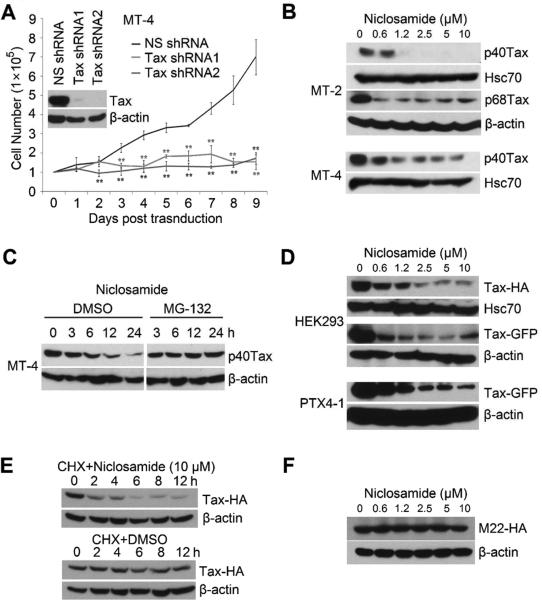
The retroviral oncoprotein Tax not only activates viral gene transcriptions that are necessary for viral replication, but also promotes survival and growth of HTLV-1-transformed T cells. Depletion of Tax by lentiviral transduction of the Tax specific shRNA led to growth retardation and death of Tax-expressing, HTLV-1-transformed MT-4 T cells (Fig. 2A), validating an essential role of Tax for survival of HTLV-1-transformed T cells. To determine whether niclosamide interferes with the stability and function of the Tax protein, MT-2 and MT-4 cells were treated with various doses of niclosamide. Niclosamide downregulated both the wild type p40Tax and p68Tax, an Env-Tax chimeric protein that was predominantly expressed in MT-2 cells. Similar pattern was observed in MT-4 cells that exclusively expressed p40Tax (Fig. 2B). To determine if niclosamide induces proteasomal degradation of Tax, we pre-treated MT-4 cells with MG-132, a proteasome inhibitor, followed by adding niclosamide at indicated time points. Niclosamide induced degradation of the Tax protein, while MG-132 blocked niclosamide-mediated down-regulation of Tax (Fig. 2C). Together, these data support the notion that niclosamide facilitates proteasomal degradation of the Tax protein in HTLV-1-transformed T cells.
Figure 2. Niclosamide induces proteasomal degradation of the retroviral oncoprotein Tax.
A) MT-4 cells were transduced with lentiviruses expressing NS shRNA or Tax-specific shRNA1 or shRNA2. The Tax knockdown efficiency was examined by anti-Tax immunoblot. The cell viability was determined by trypan blue exclusion assay in triplicate. *, p < 0.05. **, p < 0.01. B) MT-2 and MT-4 cells were treated with DMSO or niclosamide. Total protein lysates were collected 24 hours later for anti-Tax, anti-Hsc70 and anti-actin immunoblots. Hsc70 and actin were used for protein loading controls. C) MT-4 cells were pre-treated with DMSO or MG-132 (5μM), followed by adding niclosamide at indicated time points. Total protein lysates were examined with anti-Tax immunoblot. D) phEF/Tax-HA or phEF/Tax-GFP was transiently transfected into HEK293 cells. 24 hours following transfection, cells were treated with niclosamide. 24 hours after niclosamide treatment, total lysates were evaluated with immunoblot (upper panel). Meanwhile, Tax-GFP-immortalized human CD4 T cell line, PTX4-1, was treated with niclosamide and the Tax-GFP level was determined with anti-GFP blot. E) HEK293 cells were transiently transfected with phEF/Tax-HA. 24 hours following transfection, the cells were pre-treated with cyclohexamide (CHX, 10μg/ml) for 1 hour, followed by adding niclosamide or DMSO for indicated time points. The protein lysates were analyzed with anti-Tax immunoblot. F) The Tax mutant, M22-HA was transiently transfected into HEK293 cells. 72 hours following transfection, the cells were treated with niclosamide for 24 hours, and the total protein lysates were examined with anti-HA immunoblot.
To exclude the possibility that niclosamide interferes with Tax mRNA transcription through its native promoter, we constructed HA- or GFP-tagged Tax in a mammalian expression vector in which the human elongation factor promoter (phEF) drives Tax expression. phEF-Tax-HA or Tax-GFP was transiently transfected into HEK293 cells. 24 hours following transfectioncells were treated with niclosamide. Niclosamide induced degradation of Tax-HA (Fig. 2D, top panel) and Tax-GFP (middle panel) that were expressed from a heterologous promoter in non-lymphoid cells. Further, niclosamide facilitated degradation of Tax-GFP in PTX4-1 cell line, a Tax-GFP-immortalized human CD4+ T cell line in which the expression of Tax-GFP was driven from phEF promoter (bottom panel). Niclosamide destabilized the Tax protein as evidenced by its ability to shorten Tax's half-life in transiently transfected HEK293 cells (Fig. 2E). These results indicated that niclosamide promoted proteasomal degradation of the Tax protein and had no direct effect on the tax gene transcription. Further, we found that M22, a mutant form of Tax[14], resisted niclosamide-mediated degradation (Fig. 2F). Because M22 was known to be weakly ubiquitinated[16], it appeared that reduction of the Tax protein by niclosamide is executed through ubiquitination-mediated degradation.
Niclosamide increases formation of the protein aggregates of Tax and cellular signaling proteins
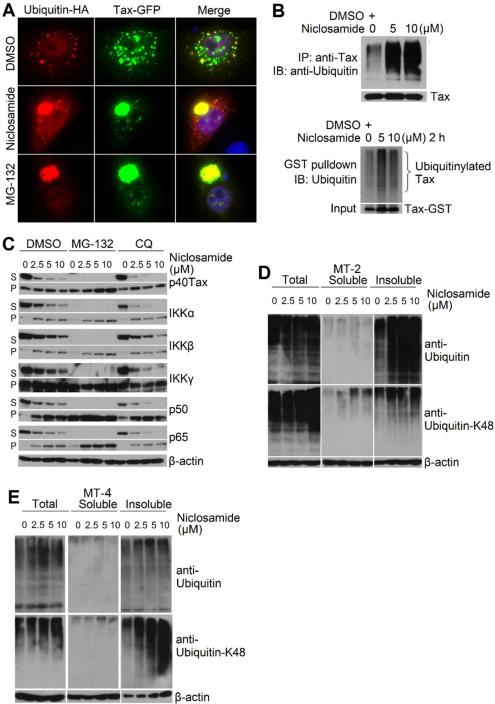
We applied dual-color fluorescence imaging technique to examine formation of the ubiquitinated Tax protein. Tax-GFP was co-transfected with ubiquitin-HA into HeLa cells. Tax-GFP was shown to distribute in both the cytoplasm and nucleus with speckled pattern, and a small portion of ubiquitin co-localized with Tax-GFP as seen with the yellow puncta in the cytoplasm from the merged picture (Fig. 3A, top panel). Niclosamide induced formation of the cytoplasmic aggregates of the Tax-ubiquitin complex (middle panel). As expected, inhibiting proteasome with MG-132 led to accumulation of the ubiquitinated Tax protein aggregates (bottom panel). Next, we employed immunoblot to detect poylubiquitinated Tax protein by co-transfecting Tax with ubiquitin-HA into HEK293 cells. 24 hours following transfection, the transfected cells were treated with niclosamide for 4 hours. We found that niclosamide increased the amount of the polyubiquitinated Tax protein aggregates (Fig. 3B, upper panel). To determine if Tax is conjugated with the endogenously expressed ubiquitin following niclosamide treatment, we expressed Tax-GST into HEK293 cells followed by the treatment with niclosamide. While GST itself was not ubiquitinated (data not shown), increased amounts of the ubiquitinated Tax-GST insoluble aggregates were detected following niclosamide treatment (lower panel). Further, correlating with increased ubiquitinated Tax proteins, we found that in addition to Tax, NF-κB signaling molecules, including IκB kinases and NF-κB subunits p65 and p50, were translocated from soluble compartments into insoluble aggregates (Fig. 3C). It became apparent that niclosamide not only affected Tax stability but also modulated subcellular redistribution of cellular signaling proteins, probably through interference of cellular ubiquitination system.
Figure 3. Niclosamide induces formation of ubiquitinated Tax protein aggregate.
A) HeLa cells were co-transfected with Tax-GFP and Ubiquitin-HA, and the transfected cells were treated with DMSO, niclosamide (10 μM) or MG-132 (5 μM). 45 minutes after treatment, cells were stained with anti-HA and examined with fluorescence imaging. B) Upper panel: HEK293 cells were co-transfected with Tax and ubiquitin-HA. Transfected cells were treated with niclosamide for 4 hours, and the total lysates were immunoprecipitated with anti-Tax followed by anti-ubiquitin blot. Lower panel: HEK293 cells were transfected with Tax-GST. Transfected cells were treated with niclosamide for 2 hours, and the insoluble pellets were dissolved in 1% SDS/50mM Tris-Cl (pH7.6) and further diluted into RIPA buffer (the final concentration of SDS is 0.1%). The GST-pulldown was analyzed with anti-ubiquitin blot. C) MT-4 cells were pre-treated with DMSO, MG-132 (5 μM) or chloroquine (CQ, 50 μM), followed niclosamidetreatment. 24 hours later, the cells were lysed with 1% Triton X-100, and the remaining insoluble pellets (P) were dissolved in 1% SDS buffer. The cellular extracts from soluble fractions (S) and insoluble fractions (P) were examined with immunoblot. D) and E) MT-2 and MT-4 cells were treated with niclosamide. Total protein lysates, soluble proteins and insoluble protein extracts were examined with anti-ubiquitin and anit-ubiquitin-K48 immunoblots.
To examine this possibility, we evaluated polyubiquitination status of cellular proteins prior to and after niclosamide treatment. After MT-2 or MT-4 cells were treated with niclosamide, total protein lysates, soluble proteins and insoluble protein extracts were examined with anti-ubiquitin and anit-ubiquitin-K48 immunoblots. We found that niclosamide promoted K48-mediated ubiquitination in insoluble fractions in HTLV-1-transformed T cell lines and that this compound had no significant effect on ubiquitination of soluble proteins (Fig. 3D and 3E). The induction of polyubiquitination of cellular proteins by niclosamide appeared not to be lymphocyte specific as it was able to do so in non-lymphoid cell line HEK293 (data not shown). Together, we concluded that niclosamide was capable of inducing ubiquitination of Tax and cellular proteins, leading to the formation of the protein aggregates and their subsequent degradation in proteasome.
Niclosamide inhibits oncogenic pathways in HTLV-1-transformed T cells
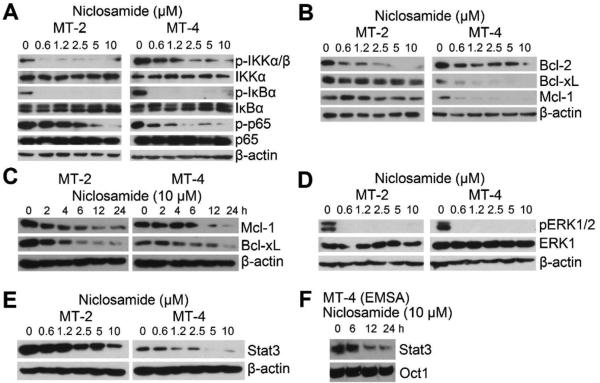
Since Tax deregulates multiple oncogenic pathways, we reasoned that degradation of Tax by niclosamide would affect these signaling pathways. NF-κB signaling in MT-2 and MT-4 was examined. Tax is known to activate IκB kinase complex (IKK), which is composed of two highly homologous catalytic subunits, IKKα and IKKβ, and one regulatory subunit, IKKγ [17; 18; 19]. The activated IKKs then phosphorylate their substrate IκBα, the inhibitor of NF-κB, resulting in its ubiquitination-dependent degradation. The activated IKKs also phosphorylate p65 subunit of the NF-κB heterodimer[20]. Expectedly, IKKα and IKKβ were constitutively phosphorylated in HTLV-1-transformed cells, correlating with their persistent activity in phosphorylating IκBα and p65 (Fig. 4A). The phosphorylation of IKKα/β, IκBα and p65 was reduced following the treatment of niclosamide in a dose-dependent manner (Fig. 4A), indicating that the activity of IKKα and/or IKKβ was inhibited. Next, we found that pro-survival Bcl-2 family proteins including Mcl-1 and BclxL were downregulated in HTLV-1-transformed T cells following niclosamide treatment in dose-and time-dependent manner (Fig. 4B and 4C). In addition, MAPK/ERK was persistently activated as seen with presence of phosphorylated ERK1/2 in HTLV-1-transformed T cell lines. Niclosamide potently suppressed ERK1/2 phosphorylation (Fig. 4D). Moreover, Tax is also known to activate Stat3, and we observed that Stat3 was downregulated in both MT-2 and MT-4 cells that were treated with increasing doses of niclosamide (Fig. 4E), resulting in reduced binding activity of Stat3 to its cis-element (Fig. 4F). The ability of niclosamide to affect multiple oncogenic signaling molecules correlated with its induction of ubiquitination of Tax and cellular proteins. We further confirm that niclosamide also induces apoptotic death of HTLV-1-associated ATL cells (MT-1 cells) and represses HTLV-1 viral gene transcription (supplement Fig.S1 and S2).
Figure 4. Niclosamide inhibits multiple oncogenic signaling molecules.
A) Immunoblot of MT-2 and MT-4 cells treated with niclosamide. B) Total protein lysates from (A) were examined with anti-Bcl-2, -Bcl-xL and -Mcl-1 immunoblot. C) MT-2 and MT-4 cells were treated with niclosamideand the cellular protein extracts harvested at indicated time points were examined with immunoblot. D) Total protein lysates from (A) were examined with anti-p-ERK1/2 and anti-ERK1 immunoblot. E) Total protein lysates from (A) were examined with anit-Stat3 immunoblot. F) MT-4 cells were treated with 10 μM of niclosamide, and the nuclear extracts from indicated time points were examined with Stat3 probe EMSA. Oct-1 was used for control.
Discussion
The present study demonstrated that niclosamide, an FDA-approved, anti-parasitic compound, potently induced apoptotic death of HTLV-1-transformed T cells and adult T cell leukemia cells. Niclosamide induces the degradation of the retroviral oncoprotein Tax and cellular growth-promoting factors. Consequently, the activities of a broad range of oncogenic signaling molecules such as Stat3, MAPK/ERK and IKK/NF-κB were diminished in HTLV-1-transformed T cells.
Tax is essential for establishing productive replication of HTLV-1 and for initiating oncogenesis of virally infected CD4+ T cells, though roughly 50% of clinical cases of ATL lost Tax expression. We showed that depletion of Tax in Tax-expressing, HTLV-1-transformed T cell lines resulted in cell death, supporting a crucial role of Tax in the early stage of leukemogenesis, which provided an ideal target for therapeutic intervention. We found that niclosamide targeted the Tax for degradation. Although niclosamide is a potent inducer of autophagy, Tax was apparently degraded in proteasome rather than in lysosome, since the inhibition of proteasome abrogated niclosamide-mediated degradation of Tax whereas blockade of autolysosome had no such effects. M22, the ubiquitination-resistant mutant of Tax, was not degraded by niclosamide, which suggests that Tax degradation is likely ubiquitination-dependent. Indeed, niclosamide induced formation of the polyubiquitinated Tax protein aggregate prior to its degradation. Furthermore, our data showed that downregulation of Tax was not related to the repression of viral gene transcription since Tax, when expressed from a heterologous promoter, was still degraded in proteasome following the treatment of niclosamide. Since Tax is the viral transactivator, reduction of the Tax protein level would indirectly cause lower transcriptional activity of the viral 5’-LTR, consequently resulting in decreased assembly of infectious viral particles. Together, our data support that niclosamide-mediated degradation of Tax in proteasome is through ubiquitination-dependent mechanism. Considering the controversial reports about maintenance of the Tax stability through ubiquitination [16; 21; 22; 23; 24; 25], further study is necessary to elucidate the mechanism that niclosamide induces ubiquitination of certain cellular proteins and Tax for degradation.
Tax appears not to be a direct target of niclosamide as this compound is capable of inducing drastic K48-linked polyubiquitination of cellular proteins in non-Tax expressing ATL cells, causing their apoptotic cell death. The selective cellular target of niclosamide remains elusive. Several studies demonstrated that niclosamide suppressed growth of a variety of human cancer cells in vitro and in mouse model by interfering with various oncogenic signaling pathways including NF-κB, Stat3, Wnt/β-catenin, Notch, ROS and S100A4 in addition to inhibiting mTORC1[8; 9; 10; 11; 12]. Moreover, niclosamide exhibited anti-tumor activity in colon cancers by downregulating expression of Dishevelled-2 (Dvl2), a component of Wnt signaling. Furthermore, this compound was found to suppress growth of acute myelogenous leukemia (AML) cells in culture and in mice. It has been proposed that niclosamide inhibits activities of two key regulators of NF-κB pathway, TAK1 and IKK, and both kinases play essential roles in inducing constitutive activation of NF-κB, which is crucial for supporting survival and proliferation of human AML blast cells.
To reconcile the discrepancies for lack of selective cellular target for niclosamide in cancer cells, our data suggest that niclosamide affects stability and function of multiple cellular signaling molecules by deregulating ubiquitination and deubiquitination process, probably by targeting cellular deubiquitinase. The specific deubiquitinase that is potentially targeted by niclosadmie is currently not clear, however, it is known that the stability of the Tax protein is regulated by STAMBPL1, a cellular deubiquitinase in which its depletion results in polyubiquitination and subsequent proteasomal degradation of Tax[26]. Furthermore, the stabilities of cellular signaling molecules, such as IκB kinases, Stat3 and Bcl-2 family members, are regulated by ubiquitination and deubiquitination system[27; 28; 29]. Identification of a specific cellular target of niclosamide will provide insights paving the way for personalized therapy.
Supplementary Material
Highlights.
Niclosamide is a promising therapeutic candidate for adult T cell leukemia.
Niclosamide employs a novel mechanism through proteasomal degradation of Tax.
Niclosamide downregulates certain cellular pro-survival molecules.
Acknowledgements
We thank Douglas Richman for MT-2 and MT-4 cell lines, and Atsushi Koito and Takeo Ohsugi for MT-1 cell line
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
XD, YY and LC performed experiments. XD, XL, CB and HC analyzed data. HC wrote the manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415–9. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallo RC, Blattner WA, Reitz MS, Jr., Ito Y. HTLV: the virus of adult T-cell leukaemia in Japan and elsewhere. Lancet. 1982;1:683. [PubMed] [Google Scholar]
- 3.Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7:270–80. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- 4.Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453–9. doi: 10.1200/JCO.2008.18.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnard AL, Igakura T, Tanaka Y, et al. Engagement of specific T-cell surface molecules regulates cytoskeletal polarization in HTLV-1-infected lymphocytes. Blood. 2005;106:988–95. doi: 10.1182/blood-2004-07-2850. [DOI] [PubMed] [Google Scholar]
- 6.Currer R, Van Duyne R, Jaworski E, et al. HTLV tax: a fascinating multifunctional co-regulator of viral and cellular pathways. Front Microbiol. 2012;3:406. doi: 10.3389/fmicb.2012.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuoka M, Jeang KT. Human T-cell leukemia virus type 1 (HTLV-1) and leukemic transformation: viral infectivity, Tax, HBZ and therapy. Oncogene. 2011;30:1379–89. doi: 10.1038/onc.2010.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin Y, Lu Z, Ding K, et al. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer Res. 2010;70:2516–27. doi: 10.1158/0008-5472.CAN-09-3950. [DOI] [PubMed] [Google Scholar]
- 9.Ren X, Duan L, He Q, et al. Identification of Niclosamide as a New Small-Molecule Inhibitor of the STAT3 Signaling Pathway. ACS Med Chem Lett. 2010;1:454–9. doi: 10.1021/ml100146z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen M, Wang J, Lu J, et al. The anti-helminthic niclosamide inhibits Wnt/Frizzled1 signaling. Biochemistry. 2009;48:10267–74. doi: 10.1021/bi9009677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sack U, Walther W, Scudiero D, et al. Novel effect of antihelminthic Niclosamide on S100A4-mediated metastatic progression in colon cancer. J Natl Cancer Inst. 2011;103:1018–36. doi: 10.1093/jnci/djr190. [DOI] [PubMed] [Google Scholar]
- 12.Balgi AD, Fonseca BD, Donohue E, et al. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PLoS One. 2009;4:e7124. doi: 10.1371/journal.pone.0007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren T, Takahashi Y, Liu X, et al. HTLV-1 Tax deregulates autophagy by recruiting autophagic molecules into lipid raft microdomains. Oncogene. 2013;34:334–45. doi: 10.1038/onc.2013.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Ren T, Guan H, et al. HTLV-1 Tax is a critical lipid raft modulator that hijacks IkappaB kinases to the microdomains for persistent activation of NF-kappaB. J Biol Chem. 2009;284:6208–17. doi: 10.1074/jbc.M806390200. [DOI] [PubMed] [Google Scholar]
- 15.Craig P, Ito A. Intestinal cestodes. Curr Opin Infect Dis. 2007;20:524–32. doi: 10.1097/QCO.0b013e3282ef579e. [DOI] [PubMed] [Google Scholar]
- 16.Chiari E, Lamsoul I, Lodewick J, et al. Stable ubiquitination of human T-cell leukemia virus type 1 tax is required for proteasome binding. J Virol. 2004;78:11823–32. doi: 10.1128/JVI.78.21.11823-11832.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun SC, Ballard DW. Persistent activation of NF-kappaB by the tax transforming protein of HTLV-1: hijacking cellular IkappaB kinases. Oncogene. 1999;18:6948–58. doi: 10.1038/sj.onc.1203220. [DOI] [PubMed] [Google Scholar]
- 18.Li XH, Murphy KM, Palka KT, et al. The human T-cell leukemia virus type-1 Tax protein regulates the activity of the IkappaB kinase complex. J Biol Chem. 1999;274:34417–24. doi: 10.1074/jbc.274.48.34417. [DOI] [PubMed] [Google Scholar]
- 19.Yamaoka S, Courtois G, Bessia C, et al. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell. 1998;93:1231–40. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai H, Chiba H, Miyoshi H, et al. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–6. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 21.Yan P, Qing G, Qu Z, et al. Targeting autophagic regulation of NFkappaB in HTLV-I transformed cells by geldanamycin: implications for therapeutic interventions. Autophagy. 2007;3:600–3. doi: 10.4161/auto.4761. [DOI] [PubMed] [Google Scholar]
- 22.Ikebe E, Kawaguchi A, Tezuka K, et al. Oral administration of an HSP90 inhibitor, 17-DMAG, intervenes tumor-cell infiltration into multiple organs and improves survival period for ATL model mice. Blood Cancer J. 2013;3:e132. doi: 10.1038/bcj.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peloponese JM, Jr., Iha H, Yedavalli VR, et al. Ubiquitination of human T-cell leukemia virus type 1 tax modulates its activity. J Virol. 2004;78:11686–95. doi: 10.1128/JVI.78.21.11686-11695.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan P, Fu J, Qu Z, et al. PDLIM2 suppresses human T-cell leukemia virus type I Tax-mediated tumorigenesis by targeting Tax into the nuclear matrix for proteasomal degradation. Blood. 2009;113:4370–80. doi: 10.1182/blood-2008-10-185660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao L, Harhaj EW. HSP90 protects the human T-cell leukemia virus type 1 (HTLV-1) tax oncoprotein from proteasomal degradation to support NF-kappaB activation and HTLV-1 replication. J Virol. 2013;87:13640–54. doi: 10.1128/JVI.02006-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavorgna A, Harhaj EW. An RNA interference screen identifies the Deubiquitinase STAMBPL1 as a critical regulator of human T-cell leukemia virus type 1 tax nuclear export and NF-kappaB activation. J Virol. 2012;86:3357–69. doi: 10.1128/JVI.06456-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skaug B, Jiang X, Chen ZJ. The role of ubiquitin in NF-kappaB regulatory pathways. Annu Rev Biochem. 2009;78:769–96. doi: 10.1146/annurev.biochem.78.070907.102750. [DOI] [PubMed] [Google Scholar]
- 28.Beverly LJ, Lockwood WW, Shah PP, et al. Ubiquitination, localization, and stability of an anti-apoptotic BCL2-like protein, BCL2L10/BCLb, are regulated by Ubiquilin1. Proc Natl Acad Sci U S A. 2012;109:E119–26. doi: 10.1073/pnas.1119167109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulane CM, Rodriguez JJ, Parisien JP, et al. STAT3 ubiquitylation and degradation by mumps virus suppress cytokine and oncogene signaling. J Virol. 2003;77:6385–93. doi: 10.1128/JVI.77.11.6385-6393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.