Abstract
Haematological malignancies, although a broad range of specific disease types, continue to show considerable overlap in classification, and patients are treated using similar chemotherapy regimes. In this review we look at the role of the CCN family of matricellular proteins and indicate their role in nine haematological malignancies including both myeloid and lymphoid neoplasms. The potential for further haematological neoplasms with CCN family associations is argued by summarising the demonstrated role of CCN family genes in the differentiation of haematopoietic stem cells (HSC) and mesenchymal stem cells. The expanding field of knowledge encompassing CCN family genes and cancers of the HSC-lineage highlights the importance of extracellular matrix-interactions in both normal physiology and tumorigenesis of the blood, bone marrow and lymph nodes.
Keywords: CCN, Cancer, Haematopoietic stem cell (HSC), Mesenchymal stem cell (MSC)
Introduction
Haematological malignancies represent cancers of the bone marrow, blood and lymph nodes. Trends for haematological malignancies show some variation geographically. Data from European cancer registries for the years 2000–2002 found an overall incidence rate of 39.37 per 100 000 people for all haematological malignancies (Sant et al. 2010). In the USA an incidence rate of 49.7 per 100 000 people was reported for 2011 (Surveillance, Epidemiology and End Results Program (SEER) Online Database). A comparison of the incidence of haematological malignancies between Japan and the USA from 1993 to 2008 found twice the incidence rate in the USA than Japan (Chihara et al. 2014).
Haematopoietic stem cells (HSCs) are multipotent, self-renewing progenitor cells that differentiate into all lymphoid and myeloid lineages of the blood and immune system (Fig. 1). Haematological malignancies arise from mutations that occur along the HSC differentiation pathway. Their classification has been one of the most complex as the criteria for disease definition has changed over time and across regions, along with the advances in immunophentoypic, molecular biological and cytogenetic techniques. Lymphoid neoplasms covers a large number of disease types, and prior to the early 1990s were primarily classified as leukaemia or lymphoma. However, the introduction of new techniques found significant overlap among the classification systems in place, and led to the proposal that the leukaemia/lymphoma and Hodgkin lymphoma/Non-Hodgkin lymphoma distinctions were not helpful. Further, cytogenetic studies revealed chromosomal translocations and other genetic markers could be used to further classify disease in a way that benefited clinicians and patients. Classification according to cell lineage (B-, T- or NK-cell) and precursor or mature cell status is currently implemented. Cancers of myeloid origin are largely chronic myelodysplastic or myeloproliferative diseases, and include chronic myeloid leukaemia (CML) and acute myeloid leukaemia (AML). The World Health Organisation regularly compiles and updates an international classification system for haematological malignancies. They stratify distinct diseases by cell lineage and their derivation from precursor or mature cells, and these standards have been applied to the classification of myeloid, lymphoid, mast cell and dendritic/histiocytic neoplasms (Ruhl et al. 2015; Swerdlow et al. 2008). It is a certainty, that as our knowledge of haematological malignancies increase, further important sub-divisions will be made (Campo et al. 2011).
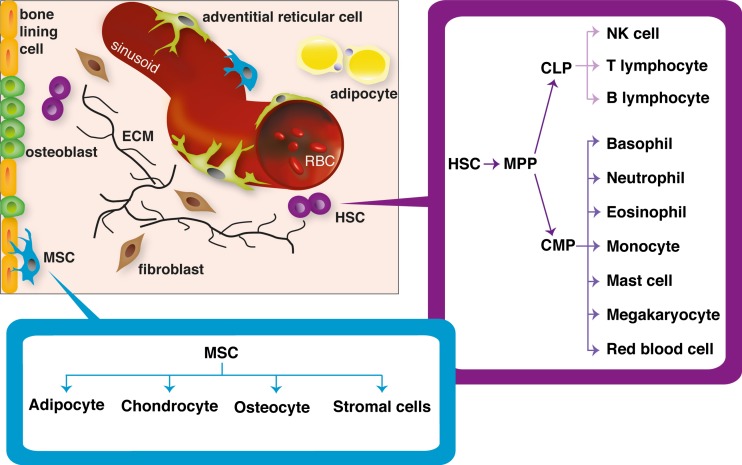
Fig. 1.
The bone marrow niche including HSC and MSC lineages. This schematic highlights that both HSCs and MSCs are found at two sites within the bone marrow, the perivascular (near the sinusoids) and endosteal niche (near the bone). HSCs ultimately differentiate into all constituents of the immune system comprising the lymphoid and myeloid lineages. MSCs are known to differentiate in vivo into fat cells (adipocytes), cartilage (chondrocytes), bone (osteoblasts/ osteocytes) and stromal cells such as fibroblasts and adventitial reticular cells. Bone lining cells are also though to arise from MSCs. HSC haematopoietic srem cell; MSC mesenchymal stem cell; ECM extracellular matrix; RBC red blood cell; MPP multipotent progenitor; CLP common lymphoid progenitor; CMP common myeloid progenitor
A unifying feature of cancers of the haematological lineages would be that they form in highly connected bodily tissues. Thus, at diagnosis these diseases are often disseminated through the blood, bone marrow, lymph nodes or spleen. It is for this reason that haematological malignancies were traditionally viewed as “liquid” tumours in contrast to their solid tumour counterparts. Yet the differences between solid and liquid tumours continue to diminish. Haematological neoplasms have been shown to harbour all the hallmarks of cancer (Hanahan and Weinberg 2011). The bone marrow microenvironment in particular is increasingly recognised as an important determinant of tumour development, where surrounding stroma and immune cells can influence the initiation and progression of haematological malignancies (Ayala et al. 2009; Burger et al. 2009; Riether et al. 2015). This is similar to normal haematopoiesis, where the microenvironment tightly regulates the maintenance and function of HSCs through their location at two distinct niches, surrounding the bone or vasculature, providing different cues for quiescence or differentiation (Fig. 1) (Morrison and Scadden 2014). The bone marrow microenvironment is comprised of cells largely derived from mesenchymal stem cells (MSCs), also referred to as mesenchymal stromal cells or multipotent stromal cells. Differences between in vitro culture and their natural tendencies in vivo have led to multiple definitions of MSCs (Bara et al. 2014; da Silva Meirelles et al. 2008). In vivo, MSCs have the ability to self renew and differentiate into bone, cartilage, fat deposits and stromal cells (Fig. 1). Another barrier to identification is that MSCs are phenotypically similar to adventitial reticular cells and fibroblasts, both found within the bone marrow microenvironment (Haniffa et al. 2009; Hematti 2012; Jones and McGonagle 2008). The unique identification of MSCs has been obscured due to the large overlap in expression markers with these other stromal cells and their own heterogeneous marker expression within the same tissue (Bühring et al. 2009; Siclari et al. 2013; Sudo et al. 2007). Recently, new methods of MSC identification by their adhesive properties has been suggested (Roncoroni et al. 2013). Fibroblasts are a long-studied cell type, traditionally viewed as ubiquitous adherent cells that were not endothelium, epithelium or haematopoietic in origin, they secrete extracellular matrix (ECM) precursors. The ECM provides the structural scaffold and spatial framework of the bone marrow and together with signalling from matricellular proteins play a critical role in haematological malignancy development and response to therapy (Chong et al. 2012).
In this review, we collate the evidence that one such matricellular signalling family, CCN, plays an important role across a diverse range of haematological malignancies. Then we provide support that further associations will be made with as-yet unstudied haematological malignancies, by highlighting the role CCN proteins play in regulating the fate and function of HSCs, MSCs and their lineages.
CCN structure and function
The CCN genes are a family of six members containing CYR61/CCN1 (cysteine-rich 61), CTGF/CCN2 (connective tissue growth factor), NOV/CCN3 (nephroblastoma overexpressed), and WISP-1/CCN4, WISP-2/CCN5, and WISP-3/CCN6 (Wnt-inducible-secreted proteins) (Bork 1993). The family members, except CCN5, each have four conserved cysteine rich-domains, with sequence homology to the insulin-like growth factor-binding proteins (IGFBP), the von Willebrand factor C (VWC) domain, Thrombospondin type 1 repeat (TSR), and a C-terminal domain with a cysteine-knot motif. CCN5 consists of the first 3 domains omitting the C-terminal cysteine knot. CCN genes as matricellular proteins are key orchestrators of cell behaviour and act in a context- and tissue-specific manner to influence a wide range of cellular activities. Under normal physiological conditions, CCN family members are involved in regulating endochondral ossification, survival of olfactory bulb interneurons, pancreatic β-cell development, suppression of hair follicle stem cells, possibly dental mesenchymal to epithelial cross-talk, retinal vasculature around the optic nerve, cardiac development and regulation of angiogenesis (Hall-Glenn and Lyons 2011; Kubota and Takigawa 2015; Rhodes and Simons 2007). In particular, CCN1 and CCN5 null mice show embryonic lethality and CCN2 null mice show perinatal lethality, indicating these CCN genes are essential for normal embryogenesis (Ivkovic et al. 2003; Mo et al. 2002; Russo and Castellot 2010). Studies on CCN family genes have commonly focused on human pathologies, and the family of genes has been shown to play a role in inflammation, wound healing, atherosclerosis, fibrosis, arthritis, retinopathy of the eye and tumourigenesis (Jun and Lau 2011; Kubota and Takigawa 2015; Kular et al. 2011). They have been shown to be powerful regulators of inflammatory modulators, such as chemokines, cytokines and other growth factors, in a context-specific fashion. Their range of functions is due to their modular nature that allows them to interact with a multitude of extracellular signals and cellular receptors. Ligands shown to interact with the CCN family include transforming growth factor β (TGFβ), bone morphogenic proteins (BMPs), vascular endothelial growth factor (VEGF), glycosominoglycans and ECM components (Desnoyers 2004; Kular et al. 2011; Leask and Abraham 2006). CCN family members bind to a large number of cell adhesion receptors, such as tropomyosin receptor kinase A (TrkA), Notch-1, low-density lipoprotein receptor-related proteins (LRPs), several specific dimers of integrins and heparan sulfate proteoglycans (Chen and Lau 2009; Rachfal and Brigstock 2005). Though for some cell types the receptor for CCN proteins remains unknown.
Role in myeloid neoplasms
Although no mast cell or dendritic/histiocytic neoplasms have been reported to have an association with CCN family members, two CCN proteins have been associated with myeloid neoplasms, CCN1 with AML and CCN3 with CML (Table 1).
Table 1.
Summary of CCN-associated haematological malignancies
| WHO classification | CCN family member | Haematological malignancy | CCN-expressing cell | Disease outcome | Reference (first cited) | |
|---|---|---|---|---|---|---|
| Myeloid neoplasms | CCN1 | Acute myelogenous leukaemia (AML) | Over-expressed in AML | Benefits neoplasm | Niu et al. 2014 | |
| CCN3 | Chronic myelogenous leukaemia (CML) | Under-expressed in BCR-ABL-positive CML | Benefits neoplasm | McCallum et al. 2006 | ||
| Lymphoid neoplasms | Precursor lymphoid neoplasms | CCN2 | B-cell precursor acute lymphoblastic leukaemia (ALL) | Over-expressed in B-cell precursor ALL | Benefits neoplasm | Vorwerk et al. 2000 |
| Mature B-cell neoplasms | CCN1 | Multiple myeloma | Over-expressed in tumour-associated MSCs | Inhibits neoplasm | Santra et al. 2011 | |
| CCN2 | Multiple myeloma | CCN2 whole protein reduced and N-terminal fragment increased in serum | Inhibits neoplasm | Munemasa et al. 2007 | ||
| Diffuse large B-cell lymphoma (DLBCL) | Over-expressed in DLBCL sub-types with stromal infiltration | Inhibits neoplasm | Blenk et al. 2007 | |||
| Hodgkin lymphoma | Over-expressed in nodular sclerosis Hodgkin lymphoma | Inhibits neoplasm | Birgersdotter et al. 2010 | |||
| Mantle cell lymphoma | Under-expressed in tumour cells | Unknown | Rizzatti et al. 2005 | |||
| CCN4 | Multiple myeloma | Under-expressed in tumour-associated MSCs | Unknown | Corre et al. 2007 | ||
| Mature T-cell and NK-cell neoplasms | CCN1 | Peripheral T-cell lymphoma | Over-expressed in tumour cells | Unknown | Mahadevan et al. 2005 | |
| Angioimmunoblastic lymphoma | Over-expressed in tumour cells | Unknown | Piccaluga et al. 2007b | |||
| CCN2 | Peripheral T-cell lymphoma | Over-expressed in tumour cells | Unknown | Mahadevan et al. 2005 |
CCN1
CCN1 was found to be over-expressed in bone marrow samples from AML patients, showing heterogenous levels of expression when compared by immunoblots with normal bone marrow samples (Niu et al. 2014). In the same study, CCN1 was reported to be over-expressed in AML cell lines U937 and Kasumi-1 compared with CML or T-cell ALL cell lines. A CCN1 monoclonal antibody or siRNA were shown to reduce proliferation and increase apoptosis in AML cell lines through up-regulation of Bcl-2-associated X protein (Bax) and down-regulation of B-cell lymphoma- extra large (Bcl-xl) and c-Myc, which were regulated through reduced phosphorylation of MEK and ERK. The CCN1 siRNA also showed increased AML cell apoptosis in combination with cytarabine treatment in vitro (Niu et al. 2014).
CCN3
The CCN3 gene has been linked to CML. Specifically, the BCR-ABL oncogene, a common feature of CML, was shown to reduce CCN3 gene and protein expression in the multipotent haematopoietic cell line FDCP-Mix (McCallum et al. 2006). After treatment with imatinib (a BCR-ABL tyrosine kinase inhibitor) or after complete remission, CML patient bone marrow samples were found with increased CCN3 expression, equivalent to normal healthy bone marrow. Further, when the K562 CML cell line was transfected with a full length CCN3 plasmid, cells were forced into growth arrest and had an enhanced susceptibility to imatinib (McCallum et al. 2009). McCallum et al. (2012) illustrated through in vitro studies that CCN3 upregulated integrin expression leading to increased cell adhesion and upregulated undetermined growth control mechanisms that prevented phosphorylation of Akt and ERK by BCR-ABL in CML cells. The ability of the BCR-ABL tyrosine kinase to downregulate CCN3 in CML cells was attributed to microRNA 130 a/b (Suresh et al. 2011). BCR-ABL was also shown to inhibit NOTCH1-CCN3 signalling contributing to the pathogenesis of CML (Suresh et al. 2013). CCN3 had previously been shown to be a non-canonical NOTCH1 ligand (Sakamoto et al. 2002), in CML the loss of CCN3 would result in a missing regulatory effect on the NOTCH1 signalling pathway.
Role in lymphoid neoplasms
In collating CCN family associations with lymphoid neoplasms we have included studies with gene expression profiling data identifying CCN family members without any subsequent validation, this is to expand on the small range of CCN-associated haematological malignancies and indicate possible avenues for future study. Only CCN2 has been associated with a precursor lymphoid neoplasm, CCN1, CCN2 and CCN4 have been associated with four mature B-cell neoplasms and CCN1 and CCN2 have been associated with two mature T-cell neoplasms (Table 1). CCN family associations with multiple myeloma, a disease of terminally differentiated plasma cells that accumulate in the bone marrow, are connected to bone osteolysis, or bone disease, which is due to the activation of osteoclasts and impairment of osteoblast differentiation by the myeloma cells.
CCN1
CCN1 is a biomarker and possible therapeutic target in multiple myeloma. Immunohistochemistry on bone marrow spicules or from bone marrow core biopsies from multiple myeloma patients found CCN1 to be expressed strongly in the cytoplasm of lymphoid and myeloid cells but not plasma cells (Santra et al. 2011). A possible role of CCN1 in the MSC response to multiple myeloma indicated the protein could reduce bone lesions (Li et al. 2012). The study used a multiple myeloma cell line (Hg), injected directly onto subcutaneously engrafted rabbit bone in untreated SCID mice. When these multiple myeloma-engrafted SCID-rab mice were given intra-bone injections of MSCs, they had absence of bone disease and reduced multiple myeloma cell growth. Weekly intravenous injections of MSCs prevented bone disease with no effect on tumour growth. MSCs were shown to contain high levels of CCN1 by microarray, among other bone matrix proteins such as Decorin and Lumican, compared with HSCs and the HG multiple myeloma cell line. MSC-derived CCN1 was found to increase transcription and splicing of CCN1 pre-mRNA in the IL-6-dependent multiple myeloma cell line, INA-6 (Dotterweich et al. 2014). Splicing of all introns required the CT domain of CCN1 and an RGD-binding site on INA-6 cells, though the specific RGD-associated integrin receptor was not discovered. Increased splicing led to increased CCN1 protein expression from INA6 cells. These results indicate CCN1 is acting as a microenvironmental-derived anti-myeloma factor. The largest study found CCN1 expression was significantly higher in bone biopsies from 246 multiple myeloma patients compared with 24 healthy controls, measured by microarray analysis and validated by qRT-PCR (Johnson et al. 2014). CCN1 was highly expressed in cultured MSCs in healthy controls and multiple myeloma patients, but not detected in osteoclasts, B-lymphocytes and normal or multiple myeloma plasma cells. Further, CCN1 correlated with microenvironmental-specific gene expression and not genes associated with multiple myeloma cells. CCN1 protein levels, as detected by enzyme-linked immunosorbent assays (ELISA), where significantly higher in a subset of patients with multiple myeloma (including patients with disease, in complete remission and at relapse) as well as patients with asymptomatic multiple myeloma and monoclonal gammopathy of undetermined significance, both benign precursors to multiple myeloma. Univariate and multivariate stepwise Cox regression analysis found high expression of CCN1 was linked to improved overall survival. Johnson et al. (2014) found low CCN1 levels may also serve as a risk marker for progression from asymptomatic multiple myeloma and monoclonal gammopathy of undetermined significance to multiple myeloma or a longer time to complete remission after treatment. In a SCID-hu mouse model engrafted with the multiple myeloma cell line H929, CCN1 delivered as a recombinant protein or by multiple myeloma cell line over-expression acted to reduce osteolysis through increasing osteoblast numbers and inhibiting osteoclast proliferation, the underlying mechanism acted at least partially through integrin αVβ3 binding sites (Johnson et al. 2014).
CCN1 has been associated with T-cell lymphomas. CCN1 was found over-expressed 70-fold in unspecified peripheral T-cell lymphoma compared with normal T-cells or lymph node by gene expression array (Mahadevan et al. 2005). Two types of peripheral T-cell lymphomas, unspecified and angioimmunoblastic lymphoma, showed CCN1-positive staining in tumour cells in more than 95 % of patient tissue samples by immunohistochemistry (Piccaluga et al. 2007a, b). Some evidence of nuclear staining in addition to the cytoplasm was sporadically observed.
CCN2
CCN2 was found to be over-expressed in B-cell precursor acute lymphoblastic leukaemia (ALL) compared with normal HSCs and normal precursor B cells in both children and adult patient cohorts (Boag et al. 2007; Sala-Torra et al. 2007; Tesfai et al. 2012; Vorwerk et al. 2000). In children expression of CCN2 was part of a gene signature indicating worse outcome in high-risk stratified patients (Kang et al. 2010). CCN2 has been present in gene expression clusters for the TEL/AML1 subtype of B-cell precursor ALL (Gandemer et al. 2007) and MLL translocations in high-risk B-cell precursor ALL (Harvey et al. 2010). Silencing of CCN2 in two B-cell precursor ALL cell lines was shown to reduce proliferation in vitro through AKT/mTOR inactivation and increased cyclin independent kinase inhibitor protein 27 (CDKN1B) (Lu et al. 2013). In addition, a CCN2 monoclonal antibody showed evidence of improving chemotherapy response in a NOD/SCID xenograft mouse model of B-cell precursor ALL (Lu et al. 2013). In a study indicating CCN2 may play an inhibitory role to tumour growth, human CCN2-knockout MSCs were used to create an adipocyte-rich extramedullary bone marrow in the flanks of immune-compromised mice and led to a higher population of tail-vein injected leukaemia cell lines after 14 days compared to extramedullary bone marrow using control MSCs (Battula et al. 2013). This discordant result could be due to the mouse flank extramedullary sites lacking the full complement of microenvironment cell subsets for CCN2 to act through, CCN2 expression being lost from MSCs rather than leukaemia cells, or the function of CCN2 may be subtype-specific in B-cell precursor ALL.
CCN2 has been associated with four subtypes of mature B-cell neoplasms. These include mantle cell lymphoma, diffuse large B-cell lymphoma (DLBCL), Hodgkin lymphoma and multiple myeloma. In mantle cell lymphoma, CCN2 expression was found to be 7-fold lower compared with healthy naive B cells by gene expression analysis (Rizzatti et al. 2005). In DLBCL, CCN2 expression is associated with the less aggressive germinal centre B cell like subgroup (Blenk et al. 2007). CCN2 was also grouped in a gene set of stromal-like DLBCL, identifying tumours with high ECM deposition and infiltration of monocyte-derived cells (Lenz et al. 2008). CCN2, along with osteonectin (SPARC), was associated with hystiocytic infiltration in the lymphoma biopsy specimens. Expression of CCN2 significantly correlated with greater overall survival in DLBCL patients treated with chemotherapy, CCN2 was discovered in addition to 10 other genes by gene expression profiling on pre-treatment diagnostic biopsies (Rimsza et al. 2008). CCN2 has also been associated with classic Hodgkin lymphoma, specific to the less aggressive nodular sclerosis subtype and detected by immunohistochemistry with strong granular staining in Hodgkin-Reed-Sternberg tumour cells (Birgersdotter et al. 2010). CCN2 was also heterogeneously expressed in surrounding fibroblasts, macrophages and occasional lymphoid cells. Nodular sclerosis Hodgkins lymphoma is characterised by a higher infiltrate of activated and regulatory T cells and ECM deposits that form fibrotic bands surrounding the inflammatory and malignant cells. CCN2 is also an indicator of bone disease in multiple myeloma. The concentration of circulating full-length CCN2 protein was lower in the serum of multiple myeloma patients compared with healthy controls by sandwich ELISA (Munemasa et al. 2007). Further, the reduction of CCN2 full-length protein was accompanied by an increase in the detection of the N-terminal fragment of CCN2, indicating protease cleavage of the protein is occurring in multiple myeloma. The increase in N-terminal CCN2 fragments and decrease in full-length CCN2 in multiple myeloma patients were associated with increased bone disease, regardless of whether patients had undergone treatment. While it was suggested matrix metalloproteinases (MMPs) could be cutting the CCN2 protein at the hinge, a specific MMP was not identified (Munemasa et al. 2007). In another study, CCN2 was present in 37 % of non lineage-selected multiple myeloma bone marrow samples but not detectable in healthy donor bone marrow by gene expression array (Hose et al. 2009).
CCN2 was identified as aberrantly expressed in only one mature T-cell neoplasm, with 100-fold over-expression in unspecified peripheral T-cell lymphoma compared with normal T-cells or lymph node by gene expression array (Mahadevan et al. 2005).
Deregulated CCN2 is associated with sub-types of two mature B-cell lymphomas that have better patient outcomes, namely in DLBCL and Hodgkin lymphoma. These mature B-cell lymphomas, along with gallbladder cancer, are the only CCN2-deregulated cancers to be associated with a better survival outcome (Wells et al. 2014). There is currently no functional or patient outcome data for peripheral T-cell lymphoma or mantle cell lymphoma and CCN2. However, the role of CCN2 in multiple myeloma would depend on whether the absence of full-length protein or the increased circulation of the N-terminal fragment was contributing to decreased bone disease.
CCN4
CCN4 has been associated with one mature B-cell neoplasm, multiple myeloma. CCN4 mRNA was reported 3-fold under-expressed in bone marrow MSCs of multiple myeloma patients compared with healthy aged-matched controls (Corre et al. 2007). No further validation or functional studies were performed.
Role in bone marrow HSCs and their lineages
There is abundant evidence that CCN family proteins interact with HSC cells and their myeloid and lymphoid lineages. CCN2 and CCN3 have been associated with HSC differentiation and long-term repopulation. Further, CCN1, CCN2 and CCN3 have been shown to alter the chemotaxis of mature lymphoid and myeloid immune cells in a tissue-specific manner. There is very little overlap between studies on the interaction of CCN genes with immune cell lineages, which suggests that in the haematopoietic system, CCN members are more likely to be acting with other matricellular proteins, cytokines and chemokines than their fellow CCN family members. An exception to this would be the migration and activation of macrophages, where three family members have shown to have influence in tissue-specific contexts.
CCN1
In mice with experimental autoimmune myocarditis CCN1 expression was increased in liver-specific cells, consequently circulating CD11b+ macrophages and CD3+ T cells showed reduced migration to the heart (Rother et al. 2010). In contrast, liver cell expression of CCN1, during nonalcoholic fatty liver inflammation disease in mice, enhanced the chemotaxis of F4/80-positive macrophages through integrin αM binding and MEK/ERK signalling pathways (Bian et al. 2013). CCN1 was also shown to increase neutrophil chemotaxis through fibroblast-like synoviocytes in a mouse model of collagen-induced arthritis (Zhu et al. 2013). Circulating human T-cells, B-cells, NK-cells and monocytes bind to CCN1 ex vivo and intracellular CCN1 expression can be increased in response to inflammatory signals (Löbel et al. 2012). Immune cell subsets present in human peripheral blood mononuclear cells where found to express intracellular CCN1, specifically CCN1 protein was evident in vesicles associated with the golgi apparatus (Löbel et al. 2012). In mice, CCN1 was found to bind to macrophages through integrin αMβ2 and Syndecan 4, inducing them to a pro-inflammatory M1 phenotype (Bai et al. 2010; Thorne et al. 2014). CCN1 and CCN2 both adhere to monocytes presenting an integrin αMβ2 binding site in atherosclerotic plaques (Schober et al. 2002) and also bound activated platelets through integrin αVβ3 (Jedsadayanmata et al. 1999).
CCN2
Only one study to date has discovered a role for CCN2 in HSC differentiation to the mature B-cell lineage. Using a mouse chimeric foetal liver transplantation model, loss of CCN2 in the bone marrow stromal compartment resulted in reduced B-cell maturation past the pro-B cell stage and CCN2 was capable of enhancing pro-B to pre-B cell maturation in the presence of interleukin-7 in vitro (Cheung et al. 2014). There is considerably evidence for the role CCN2 plays in the regular functioning of mature immune cells. CCN2 is expressed in γδ T-cells, important in wound healing and fibrotic responses, after TGFβ/interleukin-15 stimulation, but not in CD4-positive αβ T-cells (Workalemahu et al. 2003). These γδ T-cells have been shown to have an anti-lymphoma effect (Kunzmann and Wilhelm 2005). Further, CCN2 was heterogeneously expressed by macrophages in several mature B-cell lymphomas, including Hodgkin, Burkitt and mantle cell lymphoma (Birgersdotter et al. 2010), suggesting CCN2 may be inhibiting mature B-cell lymphomas through tumour cell and immune cell secretion. CCN2 can also prolong survival of dendritic cells in vivo (Cheng et al. 2008). Megakaryocytes have been shown to secrete signals that induce CCN2 secretion by chondrocytes, secreted CCN2 is then taken up by platelets for their use in tissue regeneration and wound repair (Sumiyoshi et al. 2010).
CCN3
An integral role for CCN3 in HSC maintenance and differentiation has been outlined in several studies. CCN3 regulates cell cycling and Notch activation in CD34-positive primitive progenitor cells from cord blood (Gupta et al. 2007). CCN3 has a positive effect on mouse lineage- Sca1+c-kit+ CD150+CD34− HSC long-term repopulation, which is dependent on integrin αVβ3 activation by Thrombopoietin (Ishihara et al. 2014). CCN3 expression increases along the myeloid cell lineage in an opposing gradient to Notch expression in humans (McCallum and Irvine 2009). A role for CCN3 in mature immune cell function was also seen in prostate cancer, where CCN3 was shown to induce M2 macrophage chemotaxis and promote the M1 to M2 phenotype, with increased AKT/NFκB signalling resulting in increased VEGF expression that contributed to angiogenesis at the tumour site (Chen et al. 2014a).
Role in bone marrow MSCs and their lineages
The first comprehensive look at the CCN family of genes and their involvement in MSC differentiation was performed in primary cultures of human bone marrow MSCs (Schutze et al. 2005). Where CCN1, CCN2, CCN5 and CCN6 were expressed at detectable mRNA levels by RT-PCR, with differences in expression upon commitment to specific differentiation pathways (Table 2). CCN1 expression was reduced upon differentiation into all three examined lineages: adipogenic, chondrogenic and osteogenic. CCN2 was slightly reduced during adipogenic and chondrogenic differentiation. CCN5 expression was reduced during adipogenic differentiation and CCN6 expression during chondrogenic differentiation. The study by Schutze et al. (2005) highlighted that these four CCN genes are likely to play a role in maintenance of bone marrow MSCs, but it remained to seen whether they acted co-operatively or independently. Further studies have shown the role CCN genes play is more complex and many may play a transient role in MSC differentiation, acting between the initial and final stages of differentiation (Table 2).
Table 2.
CCN family involvement in human MSC differentiation
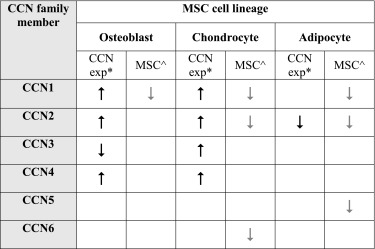
aCCN expression promotes ( ) or inhibits (
) or inhibits ( ) MSC or committed progenitor differentiation into osteoblast, chondrocyte or adipocyte
) MSC or committed progenitor differentiation into osteoblast, chondrocyte or adipocyte
bLoss of CCN expression ( ) in undifferentiated MSCs upon differentiation to osteoblast, chondrocyte or adipocyte, as described by Schutze et al. (2005)
) in undifferentiated MSCs upon differentiation to osteoblast, chondrocyte or adipocyte, as described by Schutze et al. (2005)
In the developing mouse embryo, CCN1 is transiently expressed in cells of mesenchymal origin undergoing chondrogenesis. Further, CCN1 actively promotes chrondrogenesis in mouse limb bud-derived mesenchymal cells (Wong et al. 1997). CCN1 also promotes osteogenesis in human mononuclear cells in vitro by inhibiting osteoclast proliferation and increasing osteoblast proliferation through Wnt-3A signalling (Crockett et al. 2007; Si et al. 2006). CCN2 has been involved with all three MSC differentiation lineages, supporting both osteogenesis and chondrogenesis during normal skeletal development (Ivkovic et al. 2003; Kubota and Takigawa 2007; Safadi et al. 2003; Wang et al. 2015). CCN2 expression has been associated with mesenchymal progenitor cells within the bone marrow, that were also positive for the MSC markers Twist2 and Osterix (Wang et al. 2015). However, expression of CCN2 was diminished in these mesenchymal progenitor cells during osteoblast maturation suggesting its importance in osteoblast differentiation is transient. CCN2 was shown to inhibit adipogenesis in humanised extramedullary bone marrow within an immune-compromised mouse. In this model CCN2 expressed from human MSCs reduced adipocyte differentiation (Battula et al. 2013). MSCs can also differentiate into fibroblasts and recombinant CCN2 has been shown to promote human MSC differentiation to fibroblasts in vitro, when administered in combination with ascorbic acid (Lee et al. 2010). Fibroblasts derived from MSCs grown with recombinant CCN2 and ascorbic acid were identified by decreased expression of mesenchymal surface epitopes CD44 and alkaline phosphatase and increased production of collagen I, collagen III protein and increased gene expression of fibroblast-specific protein 1 (FSP1), decorin, elastin and HA synthase 3 (Tong et al. 2011). CCN3 has been shown to inhibit osteoblast differentiation though stimulation of Notch signalling and down-regulation of BMP signalling (Katsube et al. 2009; Minamizato et al. 2007). CCN3 has also been linked to chondrocyte differentiation, present in embryonic murine pre-hypertrophic and early hypertrophic chondrocytes and shown to act in opposition to CCN2 in terminal differentiation of chondrocytes (Kawaki et al. 2008; Yu et al. 2003). CCN4 was shown to prevent apoptosis in human bone marrow MSCs by inhibiting the TNF-related apoptosis-inducing ligand 1 (TRAIL) pathway (Schlegelmilch et al. 2014). Studies have also indicated that CCN4 is involved in later differentiation into mature chondrocytes and osteoblasts, in part through regulation of TGF-β1 signalling (French et al. 2004; Inkson et al. 2008; Yanagita et al. 2007). Other transmembrane signalling pathways contributing to MSC maintenance that CCN family genes could be interacting with include the Leptin, Wnt, TGFb/BMP and Delta like kinase (Dlk)/Pref-1 pathways (Gimble et al. 2006). Additional evidence of the important role CCN family genes perform in MSC maintenance and differentiation is their association with bone pathologies. All CCN family members, except CCN5, have been associated with primary bone cancers, such as osteosarcoma, Ewing’s sarcoma and chondrosarcoma, or metastatic bone cancers derived from primary breast or prostate tumours (Chen et al. 2014b).
Conclusion
The field of study for CCN family genes and haematological malignancies is currently relatively small. While the breadth of neoplasms associated with CCN is quite diverse (Wells et al. 2014), most haematological diseases still need robust patient cohort validation and insight into CCN function. Even so, some generalisations can already be made and there is room for hypothesis on future directions in the field.
The first finding relates to CCN-specificity. Inclusion of gene expression profiling studies, even in the absence of functional mechanisms or disease outcomes, highlights the independent nature of CCN proteins in the haematological system. Largely, there is one solitary CCN gene in each newly uncovered gene signature. Only one of the studies presented here, using large scale gene or protein arrays, has identified multiple CCN genes, and that was in peripheral T-cell lymphoma, with CCN1- and CCN2-positive protein expression. CCN gene association with lymphoid or myeloid neoplasms appears to have little redundancy or co-operation among CCN family members.
The second finding is that the cell type producing CCN may determine the disease outcome, with MSC-derived CCN expression inhibiting neoplastic growth and HSC-lineage expression of CCN supporting neoplastic growth. As is seen with MSC-derived CCN1 expression in multiple myeloma, which acts to reduce osteolysis. It is unknown if CCN2 proteins found in multiple myeloma patient serum was from a haematopoietic or mesenchymal cell compartment, though if CCN2 was to work in a similar way to CCN1 in this disease it is plausible mesenchymal cells are at least partly responsible as bone osteolysis is also reduced in these patients. More indirectly, MSC-lineage cells may be involved in DLBCL and Hodgkin lymphoma, where CCN2 expression was associated with stromal infiltration of the tumour and in both these cases was associated with disease subtypes with better patient outcomes. CCN4 is under-expressed in MSCs of multiple myeloma patients, and whether they follow this pattern and then promote bone osteolysis or not will be of interest in determining if all MSC-expressed CCN proteins behave similarly in haematopoietic malignancies to inhibit neoplastic growth. The CCN2 gene was shown to be involved in the normal as well as the pathological signalling of the HSC B-cell lineage. CCN2 was shown to be involved in normal early B-cell differentiation and in B-cell precursor ALL. This suggests that the functions of CCN2 in B-cell precursor ALL is a corruption of its role in normal HSC differentiation.
While the full scope of neoplasms influenced by CCN family members is yet to be fully discovered, current research indicates CCN genes have the potential to be used as biomarkers or therapeutic targets in several haematological malignancies. Future studies to uncover these potential translational benefits are much anticipated.
Abbreviations
- ALL
Acute lymphoblastic leukaemia
- AML
Acute myeloid leukaemia
- Bax
Bcl-2-associated X protein
- Bcl-xl
B-cell lymphoma- extra large
- BMP
Bone morphogenic protein
- CML
Chronic myeloid leukaemia
- CDKN1B
Cyclin independent kinase inhibitor protein 27
- CLP
Common lymphoid progenitors
- CMP
Common myeloid progenitor
- CTGF/CCN2
Connective tissue growth factor
- CYR61/CCN1
Cysteine-rich 61
- DLCBL
Diffuse large B-cell lymphoma
- Dlk
Delta like kinase
- ECM
Extracellular matrix
- ELISA
Enzyme-linked immunosorbent assays
- FSP1
Fibroblast-specific protein 1
- HSC
Haematopoietic stem cell
- IGFBP
Insulin-like growth factor-binding proteins
- LRP
Low-density lipoprotein receptor-related protein
- MCIJ
Monitoring of Cancer Incidence in Japan
- MMP
Matrix metalloproteinase
- MPP
Multipotent progenitor
- MSC
Mesenchymal stem cell
- NOV/CCN3 NOV
Nephroblastoma overexpressed
- RBC
Red blood cell
- SEER
Surveillance, epidemiology and end results program (SEER)
- TGFβ
Transforming growth factor β
- TRAIL
TNF-related apoptosis-inducing ligand 1
- TrkA
Tropomyosin receptor kinase A
- TSR
Thrombospondin type 1 repeat
- VEGF
Vascular endothelial growth factor
- VWC
Von Willebrand factor C
- WISP-1/CCN4
Wnt-inducible-secreted protein 1
- WISP-2/CCN5
Wnt-inducible-secreted protein 2
- WISP-3/CCN6
Wnt-inducible-secreted protein 3
References
- Ayala F, Dewar R, Kieran M, Kalluri R. Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia. 2009;23(12):2233–2241. doi: 10.1038/leu.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai T, Chen C-C, Lau LF. Matricellular protein Ccn1 activates a proinflammatory genetic program in murine macrophages. J Immunol. 2010;184(6):3223–3232. doi: 10.4049/jimmunol.0902792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bara JJ, Richards RG, Alini M, Stoddart MJ. Concise review: bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem Cells. 2014;32(7):1713–1723. doi: 10.1002/stem.1649. [DOI] [PubMed] [Google Scholar]
- Battula VL, Chen Y, Cabreira MG, Ruvolo V, Wang Z, Ma W, et al. Connective tissue growth factor regulates adipocyte differentiation of mesenchymal stromal cells and facilitates leukemia bone marrow engraftment. Blood. 2013;122(3):357–366. doi: 10.1182/blood-2012-06-437988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Z, Peng Y, You Z, Wang Q, Miao Q, Liu Y, et al. Ccn1 expression in hepatocytes contributes to macrophage infiltration in nonalcoholic fatty liver disease in mice. J Lipid Res. 2013;54(1):44–54. doi: 10.1194/jlr.M026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgersdotter A, Baumforth KRN, Wei W, Murray PG, Sjöberg J, Björkholm M, et al. Connective tissue growth factor is expressed in malignant cells of Hodgkin lymphoma but not in other mature B-cell lymphomas. Am J Clin Pathol. 2010;133(2):271–280. doi: 10.1309/AJCPG7H0SSRYKNKH. [DOI] [PubMed] [Google Scholar]
- Blenk S, Engelmann J, Weniger M, Schultz J, Dittrich M, Rosenwald A, et al. Germinal center B cell-like (Gcb) and activated B cell-like (Abc) type of diffuse large B cell lymphoma (Dlbcl): analysis of molecular predictors, signatures, cell cycle state and patient survival. Cancer Informat. 2007;3:399. [PMC free article] [PubMed] [Google Scholar]
- Boag JM, Beesley AH, Firth MJ, Freitas JR, Ford J, Brigstock DR, et al. High expression of connective tissue growth factor in pre-B acute lymphoblastic leukaemia. Br J Haematol. 2007;138(6):740–748. doi: 10.1111/j.1365-2141.2007.06739.x. [DOI] [PubMed] [Google Scholar]
- Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993;327(2):125. doi: 10.1016/0014-5793(93)80155-N. [DOI] [PubMed] [Google Scholar]
- Bühring H-J, Treml S, Cerabona F, De Zwart P, Kanz L, Sobiesiak M. Phenotypic characterization of distinct human bone marrow-derived Msc subsets. Ann N Y Acad Sci. 2009;1176(1):124–134. doi: 10.1111/j.1749-6632.2009.04564.x. [DOI] [PubMed] [Google Scholar]
- Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114(16):3367–3375. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo E, Swerdlow S, Harris N, Pileri S, Stein H, Jaffe E. The 2008 who classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-C, Lau LF. Functions and mechanisms of action of Ccn matricellular proteins. Int J Biochem Cell Biol. 2009;41(4):771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P-C, Cheng H-C, Wang J, Wang S-W, Tai H-C, Lin C-W, et al. Prostate cancer-derived Ccn3 induces M2 macrophage infiltration and contributes to angiogenesis in prostate cancer microenvironment. Oncotarget. 2014;5(6):1595. doi: 10.18632/oncotarget.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P-C, Cheng H-C, Yang S-F, Lin C-W, Tang C-H (2014b) The Ccn family proteins: modulators of bone development and novel targets in bone-associated tumors. Biomed Res Int 2014(437096):11 p. doi:10.1155/2014/437096 [DOI] [PMC free article] [PubMed]
- Cheng W, Chang M, Sun W, Lee C, Lin H, Su Y, et al. Connective tissue growth factor linked to the E7 tumor antigen generates potent antitumor immune responses mediated by an antiapoptotic mechanism. Gene Ther. 2008;15(13):1007–1016. doi: 10.1038/gt.2008.25. [DOI] [PubMed] [Google Scholar]
- Cheung LC, Strickland DH, Howlett M, Ford J, Charles AK, Lyons KM, et al. Connective tissue growth factor is expressed in bone marrow stromal cells and promotes Il-7-dependent B lymphopoiesis. Haematologica. 2014;99:1149–1156. doi: 10.3324/haematol.2013.102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara D, Ito H, Matsuda T, Shibata A, Katsumi A, Nakamura S, et al. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol. 2014;164(4):536–545. doi: 10.1111/bjh.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong H, Tan C, Huang R, Tan N. Matricellular proteins: a sticky affair with cancers. J Oncol. 2012;2012:1. doi: 10.1155/2012/351089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corre J, Mahtouk KN, Attal M, Gadelorge ML, Huynh A, Fleury-Cappellesso S, et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia. 2007;21(5):1079–1088. doi: 10.1038/sj.leu.2404621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett JC, SchuÃàtze N, Tosh D, Jatzke S, Duthie A, Jakob F, et al. The matricellular protein Cyr61 inhibits osteoclastogenesis by a mechanism independent of Αvβ3 and Αvβ5. Endocrinology. 2007;148(12):5761–5768. doi: 10.1210/en.2007-0473. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26(9):2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- Desnoyers L. Structural basis and therapeutic implication of the interaction of Ccn proteins with glycoconjugates. Curr Pharm Des. 2004;10(31):3913–3928. doi: 10.2174/1381612043382567. [DOI] [PubMed] [Google Scholar]
- Dotterweich J, Ebert R, Kraus S, Tower RJ, Jakob F, Schütze N. Mesenchymal stem cell contact promotes Ccn1 splicing and transcription in myeloma cells. Cell Commun Signal. 2014;12(1):36. doi: 10.1186/1478-811X-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French DM, Kaul RJ, D’souza AL, Crowley CW, Bao M, Frantz GD, et al. Wisp-1 is an Osteoblastic regulator expressed during skeletal development and fracture repair. Am J Pathol. 2004;165(3):855–867. doi: 10.1016/S0002-9440(10)63348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandemer V, Rio A-G, de Tayrac M, Sibut V, Mottier S, Ly Sunnaram B, et al. Five distinct biological processes and 14 differentially expressed genes characterize Tel/Aml1-positive leukemia. BMC Genomics. 2007;8(1):385. doi: 10.1186/1471-2164-8-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98(2):251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- Gupta R, Hong D, Iborra F, Sarno S, Enver T. Nov (Ccn3) functions as a regulator of human hematopoietic stem or progenitor cells. Science. 2007;316(5824):590–593. doi: 10.1126/science.1136031. [DOI] [PubMed] [Google Scholar]
- Hall-Glenn F, Lyons K. Roles for Ccn2 in normal physiological processes. Cell Mol Life Sci. 2011;68(19):3209–3217. doi: 10.1007/s00018-011-0782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Haniffa MA, Collin MP, Buckley CD, Dazzi F. Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica. 2009;94(2):258–263. doi: 10.3324/haematol.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RC, Mullighan CG, Wang X, Dobbin KK, Davidson GS, Bedrick EJ, et al. Identification of novel cluster groups in pediatric high-risk B-precursor acute lymphoblastic leukemia with gene expression profiling: correlation with genome-wide DNA copy number alterations, clinical characteristics, and outcome. Blood. 2010;116(23):4874–4884. doi: 10.1182/blood-2009-08-239681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy. 2012;14(5):516–521. doi: 10.3109/14653249.2012.677822. [DOI] [PubMed] [Google Scholar]
- Hose D, Moreaux JRM, Meissner T, Seckinger A, Goldschmidt H, Benner A, et al. Induction of angiogenesis by normal and malignant plasma cells. Blood. 2009;114(1):128–143. doi: 10.1182/blood-2008-10-184226. [DOI] [PubMed] [Google Scholar]
- Inkson CA, Ono M, Kuznetsov SA, Fisher LW, Robey PG, Young MF. Tgf-Β1 and wisp-1/Ccn-4 can regulate each other’s activity to cooperatively control osteoblast function. J Cell Biochem. 2008;104(5):1865–1878. doi: 10.1002/jcb.21754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara J, Umemoto T, Yamato M, Shiratsuchi Y, Takaki S, Petrich BG, et al. Nov/Ccn3 regulates long-term repopulating activity of murine hematopoietic stem cells via integrin Αvβ3. Int J Hematol. 2014;99(4):393–406. doi: 10.1007/s12185-014-1534-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, et al. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130(12):2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedsadayanmata A, Chen C-C, Kireeva ML, Lau LF, Lam SC-T. Activation-dependent adhesion of human platelets to Cyr61 and Fisp12/mouse connective tissue growth factor is mediated through integrin Αiibβ3. J Biol Chem. 1999;274(34):24321–24327. doi: 10.1074/jbc.274.34.24321. [DOI] [PubMed] [Google Scholar]
- Johnson SK, Stewart JP, Bam R, Qu P, Barlogie B, van Rhee F, et al. Cyr61/Ccn1 overexpression in the myeloma microenvironment is associated with superior survival and reduced bone disease. Blood. 2014;124(13):2051–2060. doi: 10.1182/blood-2014-02-555813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford) 2008;47(2):126–131. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- Jun J-I, Lau LF. Taking aim at the extracellular matrix: Ccn proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10(12):945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Chen I-M, Wilson CS, Bedrick EJ, Harvey RC, Atlas SR, et al. Gene expression classifiers for relapse-free survival and minimal residual disease improve risk classification and outcome prediction in pediatric B-precursor acute lymphoblastic leukemia. Blood. 2010;115(7):1394–1405. doi: 10.1182/blood-2009-05-218560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsube K-I, Ichikawa S, Katsuki Y, Kihara T, Terai M, Lau LF, et al. Ccn3 and bone marrow cells. J Cell Commun Signal. 2009;3(2):135–145. doi: 10.1007/s12079-009-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaki H, Kubota S, Suzuki A, Lazar N, Yamada T, Matsumura T, et al. Cooperative regulation of chondrocyte differentiation by Ccn2 and Ccn3 shown by a comprehensive analysis of the Ccn family proteins in cartilage. J Bone Miner Res. 2008;23(11):1751–1764. doi: 10.1359/jbmr.080615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. Role of Ccn2/Ctgf/Hcs24 in bone growth. Int Rev Cytol. 2007;257:1–41. doi: 10.1016/S0074-7696(07)57001-4. [DOI] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. Cellular and molecular actions of Ccn2/Ctgf and its role under physiological and pathological conditions. Clin Sci. 2015;128(3):181–196. doi: 10.1042/CS20140264. [DOI] [PubMed] [Google Scholar]
- Kular L, Pakradouni J, Kitabgi P, Laurent M, Martinerie C. The Ccn family: a new class of inflammation modulators? Biochimie. 2011;93(3):377–388. doi: 10.1016/j.biochi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Kunzmann V, Wilhelm M. Anti-lymphoma effect of Γδ T cells. Leuk Lymphoma. 2005;46(5):671–680. doi: 10.1080/10428190500051893. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. All in the Ccn family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119(23):4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Lee CH, Shah B, Moioli EK, Mao JJ. Ctgf directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 2010;120(9):3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359(22):2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ling W, Khan S, Yaccoby S. Therapeutic effects of intrabone and systemic mesenchymal stem cell cytotherapy on myeloma bone disease and tumor growth. J Bone Miner Res. 2012;27(8):1635–1648. doi: 10.1002/jbmr.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löbel M, Bauer S, Meisel C, Eisenreich A, Kudernatsch R, Tank J, et al. Ccn1: a novel inflammation-regulated biphasic immune cell migration modulator. Cell Mol Life Sci. 2012;69(18):3101–3113. doi: 10.1007/s00018-012-0981-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Kojima K, Battula V, Korchin B, Shi Y, Chen Y et al. (2013) Targeting connective tissue growth factor (Ctgf) in acute lymphoblastic leukemia preclinical models: anti-Ctgf monoclonal antibody attenuates leukemia growth. Ann Hematol 93(3):485–492 [DOI] [PMC free article] [PubMed]
- Mahadevan D, Spier C, Della Croce K, Miller S, George B, Riley C, et al. Transcript profiling in peripheral T-cell lymphoma, not otherwise specified, and diffuse large B-cell lymphoma identifies distinct tumor profile signatures. Mol Cancer Ther. 2005;4(12):1867–1879. doi: 10.1158/1535-7163.MCT-05-0146. [DOI] [PubMed] [Google Scholar]
- McCallum L, Irvine A. Ccn3 - a key regulator of the hematopoietic compartment. Blood Rev. 2009;23(2):79–85. doi: 10.1016/j.blre.2008.07.002. [DOI] [PubMed] [Google Scholar]
- McCallum L, Price S, Planque N, Perbal B, Pierce A, Whetton AD, et al. A novel mechanism for Bcr-Abl action: stimulated secretion of Ccn3 is involved in growth and differentiation regulation. Blood. 2006;108(5):1716–1723. doi: 10.1182/blood-2006-04-016113. [DOI] [PubMed] [Google Scholar]
- McCallum L, Lu W, Price S, Lazar N, Perbal B, Irvine AE. Ccn3: a key growth regulator in chronic myeloid leukaemia. J Cell Commun Signal. 2009;3(2):115–124. doi: 10.1007/s12079-009-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum L, Lu W, Price S, Lazar N, Perbal B, Irvine AE. Ccn3 suppresses mitogenic signalling and reinstates growth control mechanisms in chronic myeloid leukaemia. J Cell Commun Signal. 2012;6(1):27–35. doi: 10.1007/s12079-011-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamizato T, Sakamoto K, Liu T, Kokubo H, Katsube K-I, Perbal B, et al. Ccn3/Nov inhibits Bmp-2-induced osteoblast differentiation by interacting with Bmp and notch signaling pathways. Biochem Biophys Res Commun. 2007;354(2):567–573. doi: 10.1016/j.bbrc.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Mo F-E, Muntean AG, Chen C-C, Stolz DB, Watkins SC, Lau LF. Cyr61 (Ccn1) is essential for placental development and vascular integrity. Mol Cell Biol. 2002;22(24):8709–8720. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Sakai A, Kuroda Y, Okikawa Y, Katayama Y, Asaoku H, et al. Connective tissue growth factor is an indicator of bone involvement in multiple myeloma, but matrix metalloproteinase‐9 is not. Br J Haematol. 2007;139(1):41–50. doi: 10.1111/j.1365-2141.2007.06721.x. [DOI] [PubMed] [Google Scholar]
- Niu C-C, Zhao C, Yang Z, Zhang X-L, Pan J, Si W-K. Inhibiting Ccn1 blocks Aml cell growth by disrupting the Mek/Erk pathway. Apoptosis. 2014;19:21. doi: 10.1186/s12935-014-0074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccaluga PP, Agostinelli C, Califano A, Carbone A, Fantoni L, Ferrari S, et al. Gene expression analysis of angioimmunoblastic lymphoma indicates derivation from T follicular helper cells and vascular endothelial growth factor deregulation. Cancer Res. 2007;67(22):10703–10710. doi: 10.1158/0008-5472.CAN-07-1708. [DOI] [PubMed] [Google Scholar]
- Piccaluga PP, Agostinelli C, Califano A, Rossi M, Basso K, Zupo S, et al. Gene expression analysis of peripheral T cell lymphoma, unspecified, reveals distinct profiles and new potential therapeutic targets. J Clin Invest. 2007;117(117 (3)):823–834. doi: 10.1172/JCI26833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachfal AW, Brigstock DR. Structural and functional properties of Ccn proteins. Vitam Horm. 2005;70:69–103. doi: 10.1016/S0083-6729(05)70003-0. [DOI] [PubMed] [Google Scholar]
- Rhodes JM, Simons M. The extracellular matrix and blood vessel formation: not just a scaffold. J Cell Mol Med. 2007;11(2):176–205. doi: 10.1111/j.1582-4934.2007.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riether C, Schürch C, Ochsenbein A. Regulation of hematopoietic and leukemic stem cells by the immune system. Cell Death Differ. 2015;22(2):187–198. doi: 10.1038/cdd.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimsza LM, LeBlanc ML, Unger JM, Miller TP, Grogan TM, Persky DO, et al. Gene expression predicts overall survival in paraffin-embedded tissues of diffuse large B-cell lymphoma treated with R-chop. Blood. 2008;112(8):3425–3433. doi: 10.1182/blood-2008-02-137372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzatti EG, Falcão RP, Panepucci RA, Proto-Siqueira R, Anselmo-Lima WT, Okamoto OK, et al. Gene expression profiling of mantle cell lymphoma cells reveals aberrant expression of genes from the Pi3k-akt, Wnt and Tgfβ signalling pathways. Br J Haematol. 2005;130(4):516–526. doi: 10.1111/j.1365-2141.2005.05630.x. [DOI] [PubMed] [Google Scholar]
- Roncoroni L, Maerz J, Angres B, Steuer H, Benz K. Adhesion to extracellular matrix proteins can differentiate between human bone marrow derived mesenchymal stem cells and fibroblasts. J Tissue Sci Eng. 2013;11:2. [Google Scholar]
- Rother M, Krohn S, Kania G, Vanhoutte D, Eisenreich A, Wang X, et al. Matricellular signaling molecule Ccn1 attenuates experimental autoimmune myocarditis by acting as a novel immune cell migration modulatorclinical perspective. Circulation. 2010;122(25):2688–2698. doi: 10.1161/CIRCULATIONAHA.110.945261. [DOI] [PubMed] [Google Scholar]
- Ruhl J, Adamo M, Dickie L. Hematopoietic and lymphoid neoplasm coding manual. Bethesda, MD: National Cancer Institute; 2015. [Google Scholar]
- Russo JW, Castellot JJ., Jr Ccn5: biology and pathophysiology. J Cell Commun Signal. 2010;4(3):119–130. doi: 10.1007/s12079-010-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safadi FF, Xu J, Smock SL, Kanaan RA, Selim AH, Odgren PR, et al. Expression of connective tissue growth factor in bone: its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J Cell Physiol. 2003;196(1):51–62. doi: 10.1002/jcp.10319. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Yamaguchi S, Ando R, Miyawaki A, Kabasawa Y, Takagi M, et al. The nephroblastoma overexpressed gene (Nov/Ccn3) protein associates with Notch1 extracellular domain and inhibits myoblast differentiation via notch signaling pathway. J Biol Chem. 2002;277(33):29399–29405. doi: 10.1074/jbc.M203727200. [DOI] [PubMed] [Google Scholar]
- Sala-Torra O, Gundacker HM, Stirewalt DL, Ladne PA, Pogosova-Agadjanyan EL, Slovak ML, et al. Connective tissue growth factor (Ctgf) expression and outcome in adult patients with acute lymphoblastic leukemia. Blood. 2007;109(7):3080–3083. doi: 10.1182/blood-2006-06-031096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, et al. Incidence of hematological malignancies in Europe by morphological subtype: results of the Haemacare project. Blood. 2010;116(19):3724–3734. doi: 10.1182/blood-2010-05-282632. [DOI] [PubMed] [Google Scholar]
- Santra M, Shaughnessy J, Jr, Bellamy W. Expression of multiple myeloma associated markers in bone marrow spicules using a novel immunohistochemical technique. Biotech Histochem. 2011;86(2):119–123. doi: 10.3109/10520290903565978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K, Keller A, Zehe V, Hondke S, Schilling T, Jakob F, et al. Wisp 1 is an important survival factor in human mesenchymal stromal cells. Gene. 2014;551(2):243–254. doi: 10.1016/j.gene.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Schober JM, Chen N, Grzeszkiewicz TM, Jovanovic I, Emeson EE, Ugarova TP, et al. Identification of integrin Αmβ2 as an adhesion receptor on peripheral blood monocytes for Cyr61 (Ccn1) and connective tissue growth factor (Ccn2): immediate-early gene products expressed in atherosclerotic lesions. Blood. 2002;99(12):4457–4465. doi: 10.1182/blood.V99.12.4457. [DOI] [PubMed] [Google Scholar]
- Schutze N, Noth U, Schneidereit J, Hendrich C, Jakob F. Differential expression of Ccn-family members in primary human bone marrow-derived mesenchymal stem cells during osteogenic, chondrogenic and adipogenic differentiation. Cell Commun Signal. 2005;3(1):5. doi: 10.1186/1478-811X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si W, Kang Q, Luu HH, Park JK, Luo Q, Song W-X, et al. Ccn1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3a-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol. 2006;26(8):2955–2964. doi: 10.1128/MCB.26.8.2955-2964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siclari VA, Zhu J, Akiyama K, Liu F, Zhang X, Chandra A, et al. Mesenchymal progenitors residing close to the bone surface are functionally distinct from those in the central bone marrow. Bone. 2013;53(2):575–586. doi: 10.1016/j.bone.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo K, Kanno M, Miharada K, Ogawa S, Hiroyama T, Saijo K, et al. Mesenchymal progenitors able to differentiate into osteogenic, chondrogenic, and/or adipogenic cells in vitro are present in most primary Fibroblas-like cell populations. Stem Cells. 2007;25(7):1610–1617. doi: 10.1634/stemcells.2006-0504. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi K, Kubota S, Furuta RA, Yasui K, Aoyama E, Kawaki H, et al. Thrombopoietic-mesenchymal interaction that may facilitate both endochondral ossification and platelet maturation via Ccn2. J Cell Commun Signal. 2010;4(1):5–14. doi: 10.1007/s12079-009-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh S, McCallum L, Lu W, Lazar N, Perbal B, Irvine AE. Micrornas 130a/B are regulated by Bcr-Abl and downregulate expression of Ccn3 in Cml. J Cell Commun Signal. 2011;5(3):183–191. doi: 10.1007/s12079-011-0139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh S, McCallum L, Crawford LJ, Lu WH, Sharpe DJ, Irvine AE. The matricellular protein Ccn3 regulates Notch1 signalling in chronic myeloid leukaemia. J Pathol. 2013;231(3):378–387. doi: 10.1002/path.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. World health organization classification of tumours of haematopoietic and lymphoid tissues. 4. Lyon: World Health Organization; 2008. [Google Scholar]
- Tesfai Y, Ford J, Carter KW, Firth MJ, O’Leary RA, Gottardo NG, et al. Interactions between acute lymphoblastic leukemia and bone marrow stromal cells influence response to therapy. Leuk Res. 2012;36(3):299–306. doi: 10.1016/j.leukres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Thorne A, Meisen W, Russell L, Yoo J, Bolyard C, Lathia J, et al. Role of cysteine-rich 61 protein (Ccn1) in macrophage-mediated Oncolytic herpes simplex virus clearance. Mol Ther. 2014;22:1678–1687. doi: 10.1038/mt.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z, Sant S, Khademhosseini A, Jia X. Controlling the fibroblastic differentiation of mesenchymal stem cells via the combination of fibrous scaffolds and connective tissue growth factor. Tissue Eng A. 2011;17(21–22):2773–2785. doi: 10.1089/ten.tea.2011.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorwerk P, Wex H, Hohmann B, Oh Y, Rosenfeld RG, Mittler U. Ctgf (Igfbp-Rp2) is specifically expressed in malignant lymphoblasts of patients with acute lymphoblastic leukaemia (All) Br J Cancer. 2000;83(6):756. doi: 10.1054/bjoc.2000.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Strecker S, Liu Y, Wang L, Assanah F, Smith S, et al. Connective tissue growth factor reporter mice label a subpopulation of mesenchymal progenitor cells that reside in the trabecular bone region. Bone. 2015;71:76–88. doi: 10.1016/j.bone.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JE, Howlett M, Cole CH, Kees UR. Deregulated expression of connective tissue growth factor (Ctgf/Ccn2) is linked to poor outcome in human cancer. Int J Cancer. 2014 doi: 10.1002/ijc.28972. [DOI] [PubMed] [Google Scholar]
- Wong M, Kireeva ML, Kolesnikova TV, Lau LF. Cyr61, product of a growth factor-inducible immediate-early gene, regulates chondrogenesis in mouse limb Bud mesenchymal cells. Dev Biol. 1997;192(2):492–508. doi: 10.1006/dbio.1997.8766. [DOI] [PubMed] [Google Scholar]
- Workalemahu G, Foerster M, Kroegel C, Braun RK. Human Γδ-T lymphocytes express and synthesize connective tissue growth factor: effect of Il-15 and Tgf-Β1 and comparison with Αβ-T lymphocytes. J Immunol. 2003;170(1):153–157. doi: 10.4049/jimmunol.170.1.153. [DOI] [PubMed] [Google Scholar]
- Yanagita T, Kubota S, Kawaki H, Kawata K, Kondo S, Takano-Yamamoto T, et al. Expression and physiological role of Ccn4/Wnt-induced secreted protein 1 Mrna splicing variants in chondrocytes. FEBS J. 2007;274(7):1655–1665. doi: 10.1111/j.1742-4658.2007.05709.x. [DOI] [PubMed] [Google Scholar]
- Yu C, Le AÄ, Yeger H, Perbal B, Alman BA. Nov (Ccn3) regulation in the growth plate and Ccn family member expression in cartilage neoplasia. J Pathol. 2003;201(4):609–615. doi: 10.1002/path.1468. [DOI] [PubMed] [Google Scholar]
- Zhu X, Xiao L, Huo R, Zhang J, Lin J, Xie J, et al. Cyr61 is involved in neutrophil infiltration in joints by inducing Il-8 production by fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res Ther. 2013;15(6):R187. doi: 10.1186/ar4377. [DOI] [PMC free article] [PubMed] [Google Scholar]