Abstract
Classical swine fever (CSF) is an economically important infectious disease of pigs caused by classical swine fever virus (CSFV). Pseudorabies (PR), which is caused by pseudorabies virus (PRV), is another important infectious disease of pigs and other animals. Coinfections of pigs with PRV and CSFV occur occasionally in the field. The modified live vaccine Bartha-K61 strain has played an important role in the control of PR in many countries, including China. Since late 2011, however, increasing PR outbreaks caused by an emerging PRV variant have been reported in Bartha-K61-vaccinated swine populations on many farms in China. Previously, we generated a gE/gI-deleted PRV (rPRVTJ-delgE) based on this PRV variant, which was shown to be safe and can provide rapid and complete protection against lethal challenge with the PRV variant in pigs. Here, we generated a new recombinant PRV variant expressing the E2 gene of CSFV (rPRVTJ-delgE/gI-E2) and evaluated its immunogenicity and efficacy in pigs. The results showed that rPRVTJ-delgE/gI-E2 was safe for pigs, induced detectable anti-PRV and anti-CSFV neutralizing antibodies, and provided complete protection against the lethal challenge with either the PRV TJ strain or the CSFV Shimen strain. The data indicate that rPRVTJ-delgE/gI-E2 is a promising candidate bivalent vaccine against PRV and CSFV coinfections.
INTRODUCTION
Classical swine fever (CSF), an economically important infectious disease of pigs, is caused by classical swine fever virus (CSFV), which belongs to the Pestivirus genus within the Flaviviridae family (1). At present, vaccination is still an important measure for the prevention and control of CSF in many countries (2). Efficacious and safe modified live vaccines (MLVs) have played a key role in the control of CSF, but MLVs have some disadvantages. Notably, MLVs do not allow differentiation of infected from vaccinated animals (DIVA) (3). On the other hand, coadministration of different MLVs confers less protection than does immunization with individual ones (4). Therefore, there is a need for the development of alternative vaccine strategies.
Pseudorabies (PR) or Aujeszky's disease (AD), caused by pseudorabies virus (PRV), also known as suid herpesvirus 1 (SHV-1), is another economically important viral disease of pigs and other animals in many regions, especially in many developing countries (5, 6). The disease is characterized by high mortality in newborn pigs, respiratory illness in growing pigs, and abortions and stillbirths in sows (5). PRV belongs to the Alphaherpesvirinae subfamily of the Herpesviridae family and has a number of features that make it an attractive candidate for a viral vector (7). The PRV genome is approximately 145 kb and composed of a unique long (UL) region, a unique short (US) region, large inverted repeat sequences, internal repeats (IRs), and terminal repeats (TRs). There exist many nonessential regions, such as genes coding for thymidine kinase (TK), gE, gG, gC, protein kinase (PK), ribonucleotide reductase (RR), and dUTPase. This means that these genes can be deleted or replaced by heterogeneous genes without affecting the in vitro and/or in vivo replication in most cases, instead resulting in reduced virulence in animals. Thus, PRV can be used to develop economical and promising vectored vaccines. A number of PRV recombinants vectored by several gene-deleted vaccines were generated to express foreign genes (7–12).
PR MLVs, such as the Bartha-K61 strain, have been used to control the disease successfully in many countries, including China (8). Since late 2011, however, PR has reemerged in a large number of Bartha-K61-vaccinated swine herds in many regions of China and caused great economic losses to the pig industry. Sequence analysis indicated that the recently emerging PRV isolates from various regions of China were clustered into an independent branch in the phylogenetic tree, which was relatively distant from earlier ones (13–16).
Recently, we showed that rPRVTJ-delgE, a gE/gI-deleted PRV mutant based on the emergent PRV variant, was safe for pigs and provided complete protection against lethal challenge with the PRV variant (17). In this study, we generated a PRV variant-based recombinant expressing the CSFV E2 protein and evaluated its safety, immunogenicity, and efficacy in pigs.
MATERIALS AND METHODS
Viruses and cells.
The PRV TJ strain (PRVTJ), a virulent PRV variant (15), and the highly virulent CSFV Shimen strain were used for PRV- and CSFV-specific neutralizing test and virus challenge. The gE- and gI-deleted PRV mutants rPRVTJ-delgE and rPRVTJ-delgE/gI-EGFP were described previously (Fig. 1) (17). The CSF C-strain vaccine (lot no. 2014001) was produced by Weike Biotech Co., Harbin, China. All PRV strains were propagated and titrated in PK-15 or Vero cells, which were grown at 37°C and 5% CO2 and maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco, USA), 100 μg/ml streptomycin, and 100 IU/ml penicillin.
FIG 1.
Schematic diagrams of the PRV recombinants rPRVTJ-delgE/gI-EGFP (A) and rPRVTJ-delgE/gI-E2 (B). The coding regions of glycoprotein I (gI) and glycoprotein E (gE) genes are deleted, and an EGFP or E2 expression cassette is inserted in the deleted region. hCMV, human cytomegalovirus; polyA, SV40 polyadenylation signal.
Construction of the recombinant transfer plasmid.
A universal transfer plasmid, pOK-LR (17), was used as a backbone to construct the recombinant transfer plasmid. The human cytomegalovirus (hCMV) promoter and the CSFV E2 gene were amplified with the primer pairs P1S/P1R and P2S/P2R (Table 1) from pEGFP-N1 (Clontech, USA) and pShuttle-E2 (18), respectively. To generate the transfer plasmid pOK-LR-CMV-E2, the E2 expression cassette under the control of the CMV promoter (CMV-E2) was amplified by PCR using the purified CMV and E2 fragments as the templates with primers P1S and P2R and cloned into the MluI site of pOK-LR. The recombinant transfer plasmid pOK-LR-CMV-E2 was verified by restriction enzyme analysis, sequencing, and immunofluorescence assay (IFA).
TABLE 1.
Sequences of PCR primers used in the study
| Fragment | Primer | Sequence | Product size (bp) |
|---|---|---|---|
| CMV | P1S | 5′-CGACGCGTTAGTTATTAATAGTAATCAATT-3′ (introduced MluI site underlined) | 704 |
| P1R | 5′-CTGTCCTCTTAATACCATGGTGGCGACCGGTGGATCCCGGGCCCGCGGTA-3′ | ||
| E2 | P2S | 5′-TCCACCGGTCGCCACCATGGTATTAAGAGGACAGGTCGTGCAAGGTGTGAT-3′ | 1,210 |
| P2R | 5′-GCACGCGTTTAACCAGCGGCGAGCTGTTCTGTTAGAAC-3′ (introduced MluI site underlined) | ||
| Partial LRa | P3S | 5′-GATGATGGTGGCGCGCGACGTG-3′ | 532 |
| P3R | 5′-ACTTCCGGTTTCTCCGGATCGC-3′ | ||
| gE | P4S | 5′-GGGGTTGGACAGGAAGGACACCA-3′ | 1,897 |
| P4R | 5′-AACCAGCTGCACGCGCTCAA-3′ |
LR, left and right homology arms.
Rescue of the recombinant virus and plaque assay.
The recombinant virus was rescued as previously described (17). In brief, the genomic DNA of the PRV TJ mutant rPRVTJ-delgE/gI-EGFP was extracted, digested with PacI and PmeI, and cotransfected with pOK-LR-CMV-E2 into Vero cells. The rescued virus was subjected to at least six rounds of plaque assay. During each round of plaque purification, the presence of the E2 gene and the absence of the gE gene were verified by PCR using E2-specific (P2S/P2R) and gE-specific (P3S/P3R and P4S/P4R) primer pairs (Table 1).
In order to obtain a plaque isolate with high-level expression of the heterologous protein, better propagation traits, and genetic stability, another round of purification against the recombinant virus was performed by plaque assay and 10 plaque isolates were randomly picked out. Following freezing at −80°C and thawing at 37°C three times, all 10 plaque isolates were passaged 20 times on PK-15 cells and then were identified and compared by PCR, IFA, and flow cytometry as described below.
PCR.
After the 10 plaque isolates were passaged 20 times on PK-15 cells, the genomic DNA of different plaque isolates was extracted. The inserted CSFV E2 fragment was amplified by PCR with the primers P2S/P2R and sequenced to verify the stability of different plaque isolates during the passages.
Immunofluorescence assay.
To check the expression of the CSFV E2 protein, PK-15 cells were infected with different plaque isolates at a multiplication of infection (MOI) of 1 for 24 h. Cells were fixed with cold absolute ethyl alcohol for 20 min. The fixed cells were incubated with the anti-E2 monoclonal antibody (MAb) HQ06 (19) for 2 h at 37°C in a humidified chamber, followed by three washes with phosphate-buffered saline (PBS), and then incubated with fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG (Sigma-Aldrich, USA) for 1 h at 37°C. Following three washes with PBS, the cells were examined under a fluorescence microscope (Nikon TE200; Japan).
Flow cytometry.
Ten plaque isolates were picked out and passaged 20 times on PK-15 cells. PK-15 cells were infected with different plaque isolates at an MOI of 1 for 12 h and then digested with trypsin and filtered with a 300-mesh sieve to disperse the cells. The cells were washed three times with prechilled PBS, and the viable cells were detected by trypan blue staining; then, the cells were incubated with 500 μl of the anti-E2 MAb HQ06 (diluted 1:1,000) at 37°C for 2 h. After washing three times with prechilled PBS, the cells were incubated with 500 μl of FITC-labeled goat anti-mouse IgG (diluted 1:100) at 37°C for 1 h. Following washing three times with prechilled PBS, the cells were resuspended in 500 μl of PBS. Propidium iodide (PI) staining was used to exclude nonviable cells, and 104 viable cells were included to analyze the mean fluorescence intensities (MFI) by flow cytometry (FACSAria; BD, USA). The PK-15 cells infected with CSFV or rPRVTJ-delgE were used as positive or negative controls, respectively. The PK-15 cells infected with CSFV and incubated with FITC-conjugated anti-mouse IgG1 (Sigma-Aldrich, USA) were used as an isotype control.
The plaque isolate with the highest MFI was screened and named rPRVTJ-delgE/gI-E2 (Fig. 1).
Western blotting.
The expression of the CSFV E2 protein in rPRVTJ-delgE/gI-E2-infected PK-15 cells was further determined by Western blotting. Infected or uninfected PK-15 cells (106 cells/well) were treated with the lysis buffer (10 mM Tris-HCl, pH 7.4, 1 mM MgCl2, 0.5% NP-40, 20 μg/ml DNase I). The cell lysate was cleared of cell debris by centrifugation at 12,000 rpm for 5 min. Proteins in the lysate were separated by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and subsequently transferred onto a nitrocellulose membrane (Bio-Rad, USA). The membrane was incubated for 1 h at room temperature with the anti-E2 MAb HQ06 (1:2,000) in PBS, followed by incubation with IRDye 800CW-labeled goat anti-mouse secondary antibody (Li-Cor). The lysate of PK-15 cells infected with CSFV or rPRVTJ-delgE served as a positive or negative control, respectively. The E2 protein was visualized using the Odyssey infrared imaging system.
One-step growth curve.
The growth kinetics was determined for rPRVTJ-delgE/gI-E2, rPRVTJ-delgE, and PRVTJ as described previously (17). Briefly, PK-15 cells grown in 24-well culture plates were inoculated with each of the above viruses at an MOI of 10 and incubated on ice for 1 h. Thereafter, the inoculum was replaced with prewarmed fresh DMEM and the infected cells were further incubated for 1 h at 37°C. The extracellular virus was inactivated by low-pH treatment (20), and the cell culture was harvested at the indicated time points. After two freeze-thaw cycles, the cellular debris was removed by centrifugation and the supernatant was titrated on PK-15 cells as described previously (15). Average titers and standard deviations of two independent experiments were calculated.
Immunization and challenge of pigs.
The animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals of Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, China. Thirty-five 6-week-old healthy piglets were obtained from a local farm without PR and CSF history. The piglets were tested and proven to be free of CSFV and PRV by serum neutralization assay, enzyme-linked immunosorbent assay (ELISA), and PCR. All the pigs were randomly divided into seven experimental groups of 5 pigs each (Table 2). Groups 1 to 3 were each inoculated intramuscularly (i.m.) with 1 ml of different doses (106, 105, and 104 50% tissue culture infective doses [TCID50]) of rPRVTJ-delgE/gI-E2; groups 4 and 5 were each inoculated i.m. with rPRVTJ-delgE (105 TCID50) or 1 ml of DMEM, respectively; groups 6 and 7, in a separate pen, were inoculated i.m. with one-dose C-strain vaccine or 1 ml of PBS, respectively. One week postimmunization, groups 1 to 5 were each challenged i.m. with 106 TCID50 of the PRV TJ strain in the neck. Following challenge, clinical signs and rectal temperatures were monitored daily throughout the experiment. At 14 days postchallenge (dpc), all surviving pigs in the rPRVTJ-delgE (group 4) and DMEM (group 5) groups were euthanized, and different tissues (brain, lung, liver, kidney, heart, spleen, bladder, tonsils, and lymph nodes) were collected and subjected to pathological and immunohistochemistry (IHC) examinations and PCR.
TABLE 2.
Animal experimental design
| Group | Vaccine | Dose (TCID50 unless otherwise specified) | No. of piglets | Boost interval (wk) | Challenge with strain: |
|||
|---|---|---|---|---|---|---|---|---|
| PRVTJ |
CSFV Shimen |
|||||||
| Interval (wk) | Dose (TCID50) | Interval (wk) | Dose (TCID50) | |||||
| 1 | rPRVTJ-delgE/gI-E2 | 106 | 5 | 1 | 106 | 5 | 106 | |
| 2 | rPRVTJ-delgE/gI-E2 | 105 | 5 | 3 | 1 | 106 | 5 | 106 |
| 3 | rPRVTJ-delgE/gI-E2 | 104 | 5 | 3 | 1 | 106 | 5 | 106 |
| 4 | rPRVTJ-delgE | 105 | 5 | 1 | 106 | |||
| 5 | DMEM | 1 ml | 5 | 1 | 106 | |||
| 6 | C-strain | One dose | 5 | 3 | 5 | 106 | ||
| 7 | PBS | 1 ml | 5 | 5 | 106 | |||
Three weeks after the first immunization, 105- and 104-TCID50-rPRVTJ-delgE/gI-E2 groups (groups 2 and 3), and the C-strain group (group 6), were boosted with the same vaccine and dose as those in the first immunization. Two weeks after the booster immunization, groups 1 to 3 and groups 6 and 7 were each challenged i.m. with 106 TCID50 of the CSFV Shimen strain. Following challenge, the animals were monitored daily for the presence of clinical signs, including anorexia, depression, cough, dyspnea, and fever. At 14 dpc, all surviving pigs were euthanized. Various tissues from all the pigs were collected and subjected to pathological and IHC examinations.
Blocking ELISA and serum-virus neutralizing test (SNT).
Serum samples at different time points were collected and tested for the production of the gB-, gE-, and E2-specific antibodies by blocking ELISA using the PRV and CSFV antibody detection kits (Idexx, USA) according to the manufacturer's instructions.
The serum samples were also tested by SNT for the PRV- and CSFV-specific serum neutralizing antibodies (NAbs) as described previously (17, 18). The titers of PRV- and CSFV-specific serum NAbs were determined and expressed as the reciprocal of the highest dilution at which infection of the PK-15 cells was inhibited in 50% of the culture wells.
Virus isolation.
Nasal and rectal swabs were collected daily postimmunization or postchallenge and subjected to virus isolation as described previously (17).
Real-time RT-PCR.
Different tissues, including brain, spleen, lung, kidney, urinary bladder, tonsils, and lymph nodes, were collected at 14 dpc and subjected to detection of the CSFV RNA by a real-time reverse transcription-PCR (RT-PCR) as described previously (21).
Statistical analysis.
Data were analyzed using the SPSS 14.0 software. One-way analysis of variance (ANOVA) followed by Duncan's multiple-range test was used to compare the parameters among the different groups.
RESULTS
Generation of the recombinant virus rPRVTJ-delgE/gI-E2.
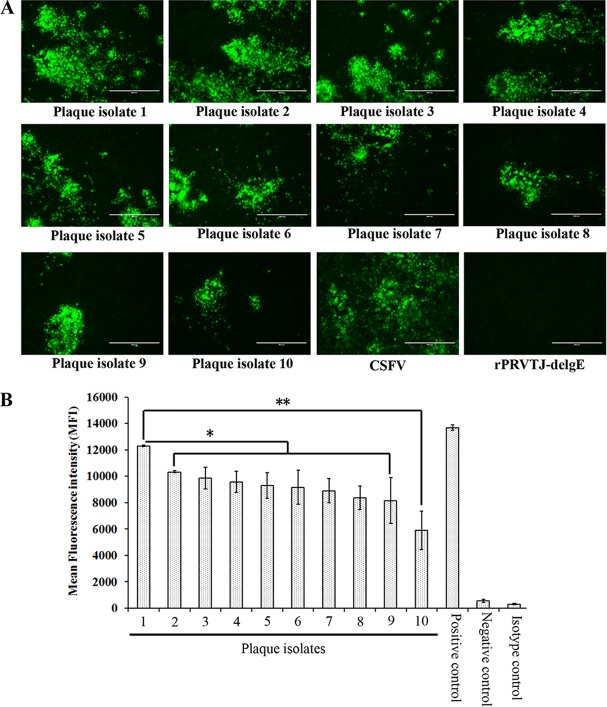
Following cotransfection with the pOK-LR-CMV-E2 plasmid and the digested genomic DNA of rPRVTJ-delgE/gI-EGFP, the recombinant virus was generated after six rounds of plaque purification. To obtain a recombinant virus strain with the highest expression levels of the CSFV E2 protein and the propagation trait and with genetic stability, the recombinant virus was subjected to another round of purification by plaque assay and 10 plaque isolates were randomly picked out; after 20 passages on PK-15 cells, the inserted CSFV E2 gene of each plaque isolate was verified by PCR and sequencing (data not shown). The CSFV E2 protein was also detected in PK-15 cells infected with all plaque isolates of the recombinant virus by IFA (Fig. 2A).
FIG 2.
Screening of the recombinant virus. (A) CSFV E2 protein expression in PK-15 cells infected with different plaque isolates of the recombinant virus by IFA. PK-15 cells were either mock infected or infected with CSFV or different plaque isolates of the recombinant virus expressing the E2 protein of CSFV at an MOI of 1. The cells were fixed 48 h postinfection and were analyzed by IFA using the anti-E2 MAb HQ06 as primary antibody and FITC-conjugated goat anti-mouse IgG as secondary antibody. Bars, 400 μm. (B) Comparison of E2 protein expression levels in PK-15 cells infected with different plaque isolates of the recombinant virus by flow cytometry analysis. PK-15 cells were either left uninfected or infected with different plaque isolates of the recombinant virus expressing the E2 protein of CSFV at an MOI of 1 for 12 h and then digested with trypsin and filtered with mesh sieve to disperse the cells. The cells were washed with prechilled PBS three times and then incubated with MAb HQ06 and FITC-conjugated goat anti-mouse IgG, respectively. The fluorescence intensities of the CSFV E2 protein were detected by flow cytometry to evaluate the E2 expression differences of different plaque isolates. Error bars represent the standard errors of the means from two replicates. *, significant difference between plaque isolates 1 and 2 to 9 (P < 0.05); **, very significant difference between plaque isolates 1 and 10 (P < 0.001).
PK-15 cells were infected with different plaque isolates of the recombinant virus at an MOI of 1. After a 12-h incubation, the infected cells were digested with trypsin and filtered with a 300-mesh sieve. After incubation with the anti-E2 MAb HQ06 and FITC-labeled goat anti-mouse IgG, respectively, the fluorescence intensity of protein expression was detected by flow cytometry. A plaque isolate, named rPRVTJ-delgE/gI-E2, with the highest expression of the E2 protein in infected PK-15 cells was screened (Fig. 2B). The E2 protein precipitated from lysates of rPRVTJ-delgE/gI-E2-infected PK-15 cells was similar in size to the native E2 protein precipitated from cells infected with CSFV (Fig. 3A). The one-step growth curve indicated that the in vitro growth of rPRVTJ-delgE/gI-E2 was comparable to that of rPRVTJ-delgE but different from that of the parent virus PRVTJ at the indicated time points. At 8 h postinfection (hpi), the titers of rPRVTJ-delgE and rPRVTJ-delgE/gI-E2 were only 104 TCID50/ml, in contrast with 106 TCID50/ml for the parent virus PRVTJ. There were significant differences between rPRVTJ-delgE, rPRVTJ-delgE/gI-E2, and PRVTJ (P < 0.05). Subsequently, the titers of each virus increased gradually and reached a peak at 14 to 18 hpi, with titers of 108 (PRVTJ) and 107 (rPRVTJ-delgE and rPRVTJ-delgE/gI-E2) TCID50/ml (Fig. 3B).
FIG 3.
Identification of rPRVTJ-delgE/gI-E2. (A) Western blotting of infected PK-15 cells with rPRVTJ-delgE/gI-E2. PK-15 cells were either infected with the CSFV Shimen strain or recombinant virus rPRVTJ-delgE/gI-E2 or left uninfected for 48 h and then lysed for Western blotting using the anti-E2 MAb HQ06. The arrow indicates the band of the CSFV E2 protein. (B) One-step growth curves of rPRVTJ-delgE/gI-E2. PK-15 cells grown in a 24-well plate were inoculated with PRVTJ, rPRVTJ-delgE/gI, or rPRVTJ-delgE/gI-E2 at an MOI of 10. The culture supernatant was collected at the indicated time points and used to determine the viral titers. Error bars represent the standard errors of the means from two replicates. *, significant difference between the PRV TJ strain and the two mutants (P < 0.05); **, very significant difference between the PRV TJ strain and the two mutants (P < 0.001).
Safety of rPRVTJ-delgE/gI-E2 for pigs.
Following vaccination with rPRVTJ-delgE/gI-E2 or rPRVTJ-delgE, all animals remained clinically healthy and showed no adverse reactions. No fever was observed in any immunized pigs prior to challenge. In addition, no virus was detected in the nasal or rectal swabs of any inoculated animals prior to challenge.
PRV-specific antibodies induced by rPRVTJ-delgE/gI-E2 in pigs.
At 6 days postimmunization (dpi), the gB-specific antibodies were detected in pigs immunized with rPRVTJ-delgE/gI-E2 and rPRVTJ-delgE. Following challenge with the PRV variant TJ strain, the gB-specific antibodies increased progressively and reached a peak at 9 dpc (Fig. 4A). The gE-specific antibodies were not detected in all pigs immunized with rPRVTJ-delgE/gI-E2 or rPRVTJ-delgE before 12 dpc but were detected in the DMEM group (3/5) or in the pigs immunized with 104 TCID50 of rPRVTJ-delgE/gI-E2 (3/5) at 12 dpc (Fig. 4B).
FIG 4.
Production of PRV-specific antibodies in immunized/challenged pigs. Groups of pigs (n = 5) were inoculated with different doses of rPRVTJ-delgE/gI-E2 or rPRVTJ-delgE or DMEM and challenged with the PRV TJ strain at the indicated time points. At 0, 3, and 6 days post-first immunization (dpi) and 0, 3, 6, 9, and 12 days postchallenge (dpc), serum samples were collected and tested for the presence of the anti-gB (A) and anti-gE (B) antibodies by using PRV antibody detection kits (Idexx, USA) according to the manufacturer's instructions. ELISA values are given in S/N (sample OD650 value/negative OD650 value) ratios. Samples with S/N ratios less than 0.6 were scored positive. Standard deviations were shown as error bars.
The SNT results showed that anti-PRV NAbs were not detectable in all groups until 6 dpc. At 6, 9, and 12 dpc, the anti-PRV NAbs were detected in groups immunized with rPRVTJ-delgE/gI-E2 at different doses or with rPRVTJ-delgE. There were significant differences between different doses of rPRVTJ-delgE/gI-E2 (104, 105, and 106 TCID50) (P < 0.05), with no difference between groups immunized with 105 TCID50 of rPRVTJ-delgE/gI-E2 and rPRVTJ-delgE at any indicated time points (Table 3).
TABLE 3.
PRV-specific neutralizing antibodies in pigs following immunization with rPRVTJ-delgE/gI-E2 and challenge with PRV TJ strain
| Group | Vaccine (TCID50) | Titer of NAbsb at dpi (dpc): |
||||
|---|---|---|---|---|---|---|
| 0 | 10 (3) | 13 (6) | 16 (9) | 19 (12) | ||
| 1 | rPRVTJ-delgE/gI-E2 (106) | <2 | <2 | 9.5 ± 1.8 | 22 ± 3.7 | 30 ± 3.7 |
| 2 | rPRVTJ-delgE/gI-E2 (105) | <2 | <2 | 12.5 ± 3.2 | 14.5 ± 6.3 | 18 ± 4.8 |
| 3 | rPRVTJ-delgE/gI-E2 (104) | <2 | <2 | 6 ± 1.8a | 10.7 ± 2.1a | 14 ± 3.1 |
| 4 | rPRVTJ-delgE (105) | <2 | <2 | 14 ± 1.3 | 17.2 ± 4.1 | 20.8 ± 4.5 |
| 5 | DMEM | <2 | <2 | 2 ± 0 | 3.2 ± 1.0 | 5.6 ± 2.1 |
There were significant differences between different doses of rPRVTJ-delgE/gI-E2 (104, 105, and 106 TCID50) at 6 and 9 dpc (P < 0.05), and there was no difference between groups immunized with 105 TCID50 of rPRVTJ-delgE/gI-E2 and rPRVTJ-delgE at any indicated time points.
The titers of PRV-specific serum neutralizing antibodies (NAbs) are expressed as the reciprocal of the highest dilution at which infection of the PK-15 cells was inhibited in 50% of the culture wells.
Protection of pigs immunized with rPRVTJ-delgE/gI-E2 from virulent PRV challenge.
No clinical signs typical of PR were observed in pigs immunized with different doses of rPRVTJ-delgE/gI-E2 and rPRVTJ-delgE after the virulent PRV TJ strain challenge. In the DMEM group, all pigs displayed typical PR signs (depression, anorexia, cough, diarrhea, and neurological signs) with high fever (≥40.5°C) from 1 dpc to the end of the PRV challenge experiment. The challenge virus was isolated from the nasal and rectal swabs of all pigs in the DMEM group (5/5) and one pig in the group immunized with 104 TCID50 of rPRVTJ-delgE/gI-E2, whereas no challenge virus was isolated in other groups (Table 4).
TABLE 4.
Outcomes for immunized pigs following virulent PRV TJ strain challenge
| Group | Vaccine | Dose (TCID50 unless otherwise specified) | No. of days to fever onset | Fever incidencea | Survival rate (no. of pigs surviving/total no. of pigs) | Viral shedding (no. of pigs shedding/total no. of pigs) |
|---|---|---|---|---|---|---|
| 1 | rPRVTJ-delgE/gI-E2 | 106 | 0/75 | 5/5 | 0/5 | |
| 2 | rPRVTJ-delgE/gI-E2 | 105 | 0/75 | 5/5 | 0/5 | |
| 3 | rPRVTJ-delgE/gI-E2 | 104 | 0/75 | 5/5 | 1/5 | |
| 4 | rPRVTJ-delgE | 105 | 0/75 | 5/5 | 0/5 | |
| 5 | DMEM | 1 ml | 1 | 21/36 | 1/5 | 5/5 |
Total no. of days with any pig showing fever (≥40.5°C)/total days of monitoring for all pigs in a group following virulent challenge.
CSFV-specific antibodies induced by rPRVTJ-delgE/gI-E2 in pigs.
E2-specific antibodies were measured by blocking ELISA and SNT following vaccination. Four weeks after the first immunization, E2-specific antibodies were detectable in two pigs immunized once with high-dose (106-TCID5) rPRVTJ-delgE/gI-E2 and two pigs immunized twice with low-dose (105- or 104-TCID50) rPRVTJ-delgE/gI-E2, with the antibody titers increasing gradually. After virulent CSFV challenge, the antibody titers in pigs immunized with rPRVTJ-delgE/gI-E2 or the C-strain were increased markedly. There were significant differences in antibody titers between the C-strain and other groups and between the double-shot groups immunized with 105 TCID50 or 104 TCID50 of rPRVTJ-delgE/gI-E2, and the one-shot rPRVTJ-delgE/gI-E2 group (106 TCID50) at 28, 35, and 38 dpi (P < 0.05), but there were no significant differences in antibody titers between the C-strain group and groups immunized with different doses of rPRVTJ-delgE/gI-E2 after 9 dpc. None of the pigs in the PBS group produced detectable CSFV-specific antibodies throughout the experiment (Fig. 5).
FIG 5.
Detection of CSFV-specific antibodies in immunized/challenged pigs using blocking ELISA. Groups of pigs (n = 5) were inoculated with different doses of rPRVTJ-delgE/gI-E2, C-strain, or PBS and challenged with the CSFV Shimen strain at the indicated time points. Serum samples were collected at weekly intervals after immunization and at 2-day intervals after challenge (days 0 to 12) and tested for the presence of the CSFV-specific NAbs using an Idexx HerdChek CSFV antibody test kit (a blocking ELISA based on competition between peroxidase-labeled anti-E2 neutralizing MAb and serum NAbs for binding CFSV antigens), according to the manufacturer's instructions. Standard deviations are shown as error bars.
Anti-CSFV NAbs were first detected in double-shot rPRVTJ-delgE/gI-E2 (105- or 104-TCID50) groups at 28 dpi and the one-shot rPRVTJ-delgE/gI-E2 (106-TCID50) group at 35 dpi. There were no significant differences between double-shot rPRVTJ-delgE/gI-E2 groups and the one-shot rPRVTJ-delgE/gI-E2 group postchallenge, but there were significant differences between the C-strain group and other groups at any time points from 28 dpi to 41 dpi (Table 5).
TABLE 5.
CSFV-specific neutralizing antibodies in pigs following immunization and challenge with lethal CSFV
| Group | Vaccine (TCID50) | Antibody titerc at dpi (dpc): |
||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 28 | 35 (0) | 38 (3) | 41 (6) | 44 (9) | 47 (12) | ||
| 1 | rPRVTJ-delgE/gI-E2 (106) | <8 | <8 | 32 ± 12 | 56 ± 25 | 108 ± 56 | 384 ± 136 | 512 ± 0 |
| 2 | rPRVTJ-delgE/gI-E2 (105) | <8 | 20 ± 12 | 40 ± 15 | 60 ± 22 | 80 ± 30 | 384 ± 167 | 480 ± 59 |
| 3 | rPRVTJ-delgE/gI-E2 (104) | <8 | 26 ± 28 | 42 ± 16 | 53 ± 8 | 74 ± 16 | 426 ± 132 | 512 ± 0 |
| 6 | C-strain | <8 | 47 ± 11a | 67 ± 9a | 112 ± 23b | 336 ± 29b | 512 ± 0 | 512 ± 0 |
| 7 | PBS | <8 | <8 | <8 | <8 | <8 | 10 ± 4 | 29 ± 27 |
There were significant differences between the C-strain group and other groups at 28 and 35 dpi (P < 0.05).
There were very significant differences between the C-strain group and other groups at 38 and 41 dpi (P < 0.001).
The titers of CSFV-specific serum neutralizing antibodies (NAbs) are expressed as the reciprocal of the highest dilution at which infection of the PK-15 cells was inhibited in 50% of the culture wells.
Protection of pigs immunized with rPRVTJ-delgE/gI-E2 from lethal CSFV challenge.
No clinical signs were observed following challenge in all groups, except the PBS group. In the PBS group, all pigs displayed typical CSF signs (anorexia, depression, chill, prostration, incoordination, and constipation followed by diarrhea, locomotor ataxia, and posterior paresis) with high fever (≥40.5°C) from 2 dpc. All the pigs in the PBS group died from 6 to 9 dpc (Table 6).
TABLE 6.
Outcomes for immunized pigs following virulent CSFV challenge
| Group | Vaccine | Dose (TCID50 unless otherwise specified) | No. of days to fever onset | Fever incidencea | Survival rate (no. of pigs surviving/total no. of pigs) |
|---|---|---|---|---|---|
| 1 | rPRVTJ-delgE/gI-E2 | 106 | 0/75 | 5/5 | |
| 2 | rPRVTJ-delgE/gI-E2 | 105 | 0/75 | 5/5 | |
| 3 | rPRVTJ-delgE/gI-E2 | 104 | 0/75 | 5/5 | |
| 6 | C-strain | One dose | 0/75 | 5/5 | |
| 7 | PBS | 1 ml | 2 | 17/35 | 0/5 |
Total number of days with any pig showing fever (≥40.5°C)/total number of days of monitoring for all pigs in a group following virulent challenge.
At 14 dpc, all surviving pigs were euthanized and subjected to pathological examination. None of the pigs immunized with 105 or 106 TCID50 of rPRVTJ-delgE/gI-E2 or the C-strain showed pathological changes. Some (2/5) pigs immunized with 104 TCID50 of rPRVTJ-delgE/gI-E2 showed mild lesions (including slight hemorrhages in the lymph nodes, bladder, heart, and spleen). The pigs in the PBS group all showed severe pathological changes, including hemorrhages with necrotic foci in the tonsils, enlargement and hemorrhages of the lymph nodes, infarcts in the spleen, extensive petechiae in the kidney and bladder, and button-like ulcers in the ileocecal valve (data not shown). Examination of brain, spleen, lungs, kidney, bladder, tonsils, or lymph nodes by real-time RT-PCR showed that no CSFV RNA was detectable in these tissues of any animal of the vaccination groups 1, 2, 3, and 6 at 14 dpc. As expected, the tissues of all nonvaccinated control animals (group 7) contained between 7.2 × 104 and 6.3 × 107 CSFV RNA copies (data not shown).
DISCUSSION
Vaccination represents one of the most effective prophylactic measures to protect pigs against viral infections. Among the various types of vaccines available, MLVs are often preferred over inactivated or subunit ones, because MLVs are able to induce long-lasting humoral and cell-mediated immunity (22).
Despite tremendous efforts invested in controlling PR and CSF, the diseases continue to plague the swine industry in many countries. It has been demonstrated that pigs are occasionally coinfected with PRV and CSFV or other viruses, such as porcine reproductive and respiratory syndrome virus (PRRSV), porcine circovirus type 2 (PCV2), or porcine parvovirus (PPV), in the field in China (23), and coinfections usually cause more severe wasting diseases. So, using PRV as a vector to develop bivalent or multivalent vaccines will be of great significance.
Recently, we constructed a gE/gI-gene-deletion PRV mutant, rPRVTJ-delgE, which is defective for the gE and gI genes. We showed that rPRVTJ-delgE was safe and immunogenic for pigs and was able to protect immunized pigs from lethal PRV challenge (17). This suggests that rPRVTJ-delgE may be used as a biologically safe vaccine vector for the expression of other viral antigens. To verify this, we inserted the gene encoding the envelope glycoprotein E2 of CSFV into the genome of rPRVTJ-delgE under the transcriptional control of the human cytomegalovirus (hCMV) immediate-early promoter. It has been shown that E2 is highly immunogenic and can provide protection for pigs against CSF (18, 24).
To develop a virus vector-based bivalent vaccine, it is important that the recombinant virus still retains the growth ability and immunogenicity of the vector virus. In this study, we demonstrated that there was no difference in virus titers between rPRVTJ-delgE/gI-E2 and the parent virus rPRVTJ-delgE, indicating that the insertion of the E2 gene in the gE/gI locus did not influence the growth of the vector virus.
Following vaccination with rPRVTJ-delgE/gI-E2, all animals remained clinically healthy and showed no adverse reactions, and no virus shedding was detected in the nasal or rectal swabs of all inoculated animals. In a recent study, we demonstrated that a maximum amount up to 104 TCID50/μl of PRV shedding could be detected in the pigs infected with PRVTJ at 4 to 6 dpc (unpublished data).
Pigs immunized with different doses of rPRVTJ-delgE/gI-E2 induced strong PRV-specific humoral immune responses, which are dose dependent; rPRVTJ-delgE/gI-E2 and rPRVTJ-delgE immunizations at the same dose induced comparable levels of PRV-specific antibodies (Table 3) and full protection from lethal PRV challenge, which indicates that the insertion of a foreign gene did not influence the immunogenicity of the vector virus.
Several CSF marker vaccines, such as pSFV1CS-E2 (25), pcdSW (26), or rAdV-E2 (18), have been shown to provide incomplete protection from lethal CSFV challenge. In contrast, rPRVTJ-delgE/gI-E2 showed a much greater efficacy, since it was able to provide complete protection even when administered as a single dose, which was comparable to that of rAdV-SFV-E2 (24) or CP7_E2alf (27).
In order to determine the dose that yields full protection against PRV and CSFV challenge, pigs were immunized with three different titers of rPRVTJ-delgE/gI-E2, and all pigs vaccinated with different doses of rPRVTJ-delgE/gI-E2 showed full protection in PRV and CSFV challenge experiments. The minimum dose of rPRVTJ-delgE/gI-E2 protective against PR is expected to be lower than 104 TCID50. In subsequent experiments, we will evaluate the immune efficacy of lower doses.
In this study, a rapid increase in CSFV- or PRV-specific neutralizing antibody titers was observed in pigs immunized with different doses of rPRVTJ-delgE/gI-E2 following virulent challenge, indicating that immunization with rPRVTJ-delgE/gI-E2 could establish an immunological memory. In pigs, rPRVTJ-delgE/gI-E2 was safe even when used for inoculation at 106 TCID50. Thus, the recombinant virus rPRVTJ-delgE/gI-E2 has a potential to be developed as a bivalent vaccine.
Nevertheless, the present data demonstrated the efficacy of the recombinant virus only in piglets. Prior to practical application, it is necessary to further study whether rPRVTJ-delgE/gI-E2 can protect pregnant sows against reproductive failure and confer sterilizing immunity in sows and their offspring. It is also necessary to further study this vaccine candidate using different immunization methods, such as intradermal and parenteral routes. In addition, the latency of this new recombinant should be evaluated.
In summary, we describe here a recombinant PRV expressing the CSFV E2 protein vectored by a gE/gI-deleted PRV variant with high safety and strong immunogenicity in pigs. This recombinant virus might be a promising bivalent vaccine candidate against PRV and CSFV coinfections of pigs.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (grant 31200690) and the National 863 Projects of China (grant 2011AA10A208).
REFERENCES
- 1.Moennig V, Floegel-Niesmann G, Greiser-Wilke I. 2003. Clinical signs and epidemiology of classical swine fever: a review of new knowledge. Vet J 165:11–20. doi: 10.1016/S1090-0233(02)00112-0. [DOI] [PubMed] [Google Scholar]
- 2.de Smit AJ, van Gennip HG, Miedema GK, van Rijn PA, Terpstra C, Moormann RJ. 2000. Recombinant classical swine fever (CSF) viruses derived from the Chinese vaccine strain (C-strain) of CSF virus retain their avirulent and immunogenic characteristics. Vaccine 18:2351–2358. doi: 10.1016/S0264-410X(00)00027-X. [DOI] [PubMed] [Google Scholar]
- 3.Dong XN, Chen YH. 2007. Marker vaccine strategies and candidate CSFV marker vaccines. Vaccine 25:205–230. doi: 10.1016/j.vaccine.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Suradhat S, Intrakamhaeng M, Damrongwatanapokin S. 2001. The correlation of virus-specific interferon-gamma production and protection against classical swine fever virus infection. Vet Immunol Immunopathol 83:177–189. doi: 10.1016/S0165-2427(01)00389-0. [DOI] [PubMed] [Google Scholar]
- 5.Mettenleiter TC. 2000. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis—state of the art, June 1999. Vet Res 31:99–115. [DOI] [PubMed] [Google Scholar]
- 6.Pomeranz LE, Reynolds AE, Hengartner CJ. 2005. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol Mol Biol Rev 69:462–500. doi: 10.1128/MMBR.69.3.462-500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Zijl M, Wensvoort G, de Kluyver E, Hulst M, van der Gulden H, Gielkens A, Berns A, Moormann R. 1991. Live attenuated pseudorabies virus expressing envelope glycoprotein E1 of hog cholera virus protects swine against both pseudorabies and hog cholera. J Virol 65:2761–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu HJ, Tian ZJ, Tong GZ, Zhou YJ, Ni JQ, Luo YZ, Cai XH. 2005. Protective immunity induced by a recombinant pseudorabies virus expressing the GP5 of porcine reproductive and respiratory syndrome virus in piglets. Vet Immunol Immunopathol 106:309–319. doi: 10.1016/j.vetimm.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, Fang L, Xiao S, Zhang H, Pan Y, Luo R, Li B, Chen H. 2007. Immunogenicity and protective efficacy of recombinant pseudorabies virus expressing the two major membrane-associated proteins of porcine reproductive and respiratory syndrome virus. Vaccine 25:547–560. doi: 10.1016/j.vaccine.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 10.Hong Q, Qian P, Li XM, Yu XL, Chen HC. 2007. A recombinant pseudorabies virus co-expressing capsid proteins precursor P1-2A of FMDV and VP2 protein of porcine parvovirus: a trivalent vaccine candidate. Biotechnol Lett 29:1677–1683. doi: 10.1007/s10529-007-9459-6. [DOI] [PubMed] [Google Scholar]
- 11.He Y, Qian P, Zhang K, Yao Q, Wang D, Xu Z, Wu B, Jin M, Xiao S, Chen H. 2008. Construction and immune response characterization of a recombinant pseudorabies virus co-expressing capsid precursor protein (P1) and a multiepitope peptide of foot-and-mouth disease virus in swine. Virus Genes 36:393–400. doi: 10.1007/s11262-008-0204-6. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Liu R, Tang H, Jin M, Chen H, Qian P. 2008. Induction of protective immunity in swine by immunization with live attenuated recombinant pseudorabies virus expressing the capsid precursor encoding regions of foot-and-mouth disease virus. Vaccine 26:2714–2722. doi: 10.1016/j.vaccine.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 13.An TQ, Peng JM, Tian ZJ, Zhao HY, Li N, Liu YM, Chen JZ, Leng CL, Sun Y, Chang D, Tong GZ. 2013. Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg Infect Dis 19:1749–2755. doi: 10.3201/eid1911.130177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu R, Bai C, Sun J, Chang S, Zhang X. 2013. Emergence of virulent pseudorabies virus infection in northern China. J Vet Sci 14:363–365. doi: 10.4142/jvs.2013.14.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Y, Li N, Cong X, Wang CH, Du M, Li L, Zhao B, Yuan J, Liu DD, Li S, Li Y, Sun Y, Qiu HJ. 2014. Pathogenicity and genomic characterization of a pseudorabies virus variant isolated from Bartha-K61-vaccinated swine population in China. Vet Microbiol 174:107–115. doi: 10.1016/j.vetmic.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Yu X, Zhou Z, Hu D, Zhang Q, Han T, Li X, Gu X, Yuan L, Zhang S, Wang B, Qu P, Liu J, Zhai X, Tian K. 2014. Pathogenic pseudorabies virus, China, 2012. Emerg Infect Dis 20:102–104. doi: 10.3201/eid2001.130531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang CH, Yuan J, Qin HY, Luo Y, Cong X, Li Y, Chen J, Li S, Sun Y, Qiu HJ. 2014. A novel gE-deleted pseudorabies virus (PRV) provides rapid and complete protection from lethal challenge with the PRV variant emerging in Bartha-K61-vaccinated swine population in China. Vaccine 32:3379–3385. doi: 10.1016/j.vaccine.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Liu DF, Wang YF, Liang BB, Cheng D, Li N, Qi QF, Zhu QH, Qiu HJ. 2010. Generation and efficacy evaluation of a recombinant adenovirus expressing the E2 protein of classical swine fever virus. Res Vet Sci 88:77–82. doi: 10.1016/j.rvsc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Peng WP, Hou Q, Xia ZH, Chen D, Li N, Sun Y, Qiu HJ. 2008. Identification of a conserved linear B-cell epitope at the N-terminus of the E2 glycoprotein of classical swine fever virus by phage-displayed random peptide library. Virus Res 135:267–272. doi: 10.1016/j.virusres.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Mettenleiter TC. 1989. Glycoprotein gIII deletion mutants of pseudorabies virus are impaired in virus entry. Virology 171:623–625. doi: 10.1016/0042-6822(89)90635-1. [DOI] [PubMed] [Google Scholar]
- 21.Zhao JJ, Cheng D, Li N, Sun Y, Shi Z, Zhu QH, Tu C, Tong GZ, Qiu HJ. 2008. Evaluation of a multiplex real-time RT-PCR for quantitative and differential detection of wild-type viruses and C-strain vaccine of classical swine fever virus. Vet Microbiol 126:1–10. doi: 10.1016/j.vetmic.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 22.Peeters B, Bienkowska-Szewczyk K, Hulst M, Gielkens A, Kimman T. 1997. Biologically safe, non-transmissible pseudorabies virus vector vaccine protects pigs against both Aujeszky's disease and classical swine fever. J Gen Virol 78:3311–3315. [DOI] [PubMed] [Google Scholar]
- 23.Cao SJ, Wen XT. 2004. Studies on the detection of PRV antibody using Dot-PPA-ELISA in pig serum. Chin J Prev Vet Med 26:213–215. (In Chinese.) [Google Scholar]
- 24.Sun Y, Li HY, Tian DY, Han QY, Zhang X, Li N, Qiu HJ. 2011. A novel alphavirus replicon-vectored vaccine delivered by adenovirus induces sterile immunity against classical swine fever. Vaccine 29:8364–8372. doi: 10.1016/j.vaccine.2011.08.085. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Qiu HJ, Zhao JJ, Li Y, Wang MJ, Lu BW, Han CG, Hou Q, Wang ZH, Gao H, Peng WP, Li GX, Zhu QH, Tong GZ. 2007. A Semliki Forest virus replicon vectored DNA vaccine expressing the E2 glycoprotein of classical swine fever virus protects pigs from lethal challenge. Vaccine 25:2907–2912. doi: 10.1016/j.vaccine.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Tu C, Li H, Hu R, Chen C, Li Z, Zhang M, Yin Z. 2001. DNA-mediated protection against classical swine fever virus. Vaccine 19:1520–1525. doi: 10.1016/S0264-410X(00)00334-0. [DOI] [PubMed] [Google Scholar]
- 27.Leifer I, Lange E, Reimann I, Blome S, Juanola S, Duran JP, Beer M. 2009. Modified live marker vaccine candidate CP7_E2alf provides early onset of protection against lethal challenge infection with classical swine fever virus after both intramuscular and oral immunization. Vaccine 27:6522–6529. doi: 10.1016/j.vaccine.2009.08.057. [DOI] [PubMed] [Google Scholar]