Abstract
Coccidioidomycosis (CM), a serious life-threatening fungal infection endemic to arid regions of the western United States and Mexico, can be challenging to diagnose in a timely manner. Commercially developed enzyme immunoassays (EIAs) (from Meridian Biosciences and Immuno-Mycologics [IMMY]) have provided faster, simpler means for serodiagnosis; however, independent evaluations have questioned EIA specificity, particularly IgM-positive/IgG-negative results. This study was conducted to evaluate EIA specificity among persons residing in Puerto Rico (n = 534), where CM is not endemic (who were not likely to have been exposed to Coccidioides spp.), compared to blood bank donors residing in Arizona (n = 1,218), where CM is endemic. Upon comparing serum reactivity between Puerto Rico and Arizona, the Meridian EIA showed a significant difference in IgG reactivity (0.37% versus 3.6%; P < 0.001) but not IgM reactivity (3.4% versus 2.4%; P = 0.31). No IgM-/IgG-reactive sera were detected among sera from Puerto Rico, compared to 7 (0.57%) sera from Arizona. Similar results were observed using the IMMY EIA, although significantly (P = 0.03) fewer IgM-reactive sera from Arizona were observed, compared to the Meridian EIA. EIA-reactive sera were also evaluated by immunodiffusion before and after 3- to 4-fold concentration of the sera. These results demonstrate that elevated IgG EIA reactivity is present in sera from healthy individuals in regions of endemicity and that IgM EIA reactivity observed in sera from individuals residing outside regions of endemicity is most likely nonspecific. Other criteria, including clinical and microbiological evaluations, should be taken into account when interpreting results from surveillance studies and other reporting measures.
INTRODUCTION
Coccidioidomycosis (CM) is a serious life-threatening fungal infection endemic to arid regions of the western United States and Mexico (1, 2). Providing timely accurate diagnoses is important, particularly for high-risk patients who are immunosuppressed or of East Asian or African American ancestry (3, 4). Serodiagnosis is an important tool for the diagnosis of CM. However, the current serological gold standards of complement fixation (CF) and immunodiffusion (ID) are tedious and time-consuming and require technical expertise of which few laboratories are capable. Due to its high throughput, ease of use, and increased sensitivity, enzyme immunoassay (EIA) is a diagnostic method that can be performed in smaller and less sophisticated laboratories that are closer to patients. As a result, EIA has become widely used for the diagnosis and surveillance of CM.
A commercially available EIA (Premier Coccidioides EIA; Meridian Biosciences, Cincinnati, OH) for the separate detection of both IgG and IgM antibodies against Coccidioides has been in use for over 15 years. Initial reports described the assay sensitivity and specificity for the detection of IgG as 92% and 97% and those for the detection of IgM as 74% and 96%, respectively. Combining the results from the two assays provided the best sensitivity (97%), with a minimal reduction in specificity (94%) (5, 6). The early detection of IgM is considered an important marker for determination of infection and initiation of treatment (7). Recent reports have questioned the specificity of the assay, particularly with IgM-positive/IgG-negative results ranging from 0% to 18% (3, 8, 9), with one study reporting a false-positive rate of 82% (10). It has been speculated that the different specificities observed with this assay may reflect the population being evaluated, since specificity increases with increased confidence in the diagnosis prior to testing (3, 8).
A major limitation of the previous independent specificity assessments of the EIA was that those studies were performed using sera collected from regions in which CM is endemic. Since approximately 60% of infections are asymptomatic (11), antibody titers observed in patients living in regions in which the disease is endemic may be the result of a current infection (asymptomatic or symptomatic), a past infection, or a potential false-positive reaction. To eliminate the effect of exposure in a region of endemicity, this study assessed the specificity of EIA using sera collected from persons outside a region of endemicity, who likely were never exposed to Coccidioides spp. The sera were evaluated for IgG and IgM reactivity using the Meridian EIA and a recently available EIA (Omega Coccidioides antibody enzyme immunoassay; Immuno-Mycologics [IMMY], Norman, OK). For comparison, sera from blood bank donors living within a region in which the disease is endemic were also tested, to assess assay specificity in the region of endemicity.
MATERIALS AND METHODS
Serum specimens.
Sera (n = 534) from individuals who were living in a region in which CM is not endemic were obtained from the serum collection at the Centers for Disease Control and Prevention, Dengue Branch, in San Juan, Puerto Rico. These 534 patients did not reside outside Puerto Rico and had not traveled outside Puerto Rico for at least 14 days prior to serum collection. These sera were negative for antibodies to the dengue virus. Sera (n = 1,218) from individuals who were living in a region in which CM is endemic but who were not known to be infected with Coccidioides spp. were obtained from routine blood bank donations at a single blood bank in northwest Phoenix, Arizona.
Enzyme immunoassays.
Premier Coccidioides enzyme immunoassay (Meridian Biosciences, Cincinnati, OH) and Omega Coccidioides antibody enzyme immunoassay (IMMY, Norman, OK) were used to test for the presence of IgM and IgG antibodies to Coccidioides sp. The enzyme immunoassays (EIAs) were performed according to the instructions provided by each manufacturer. Results from the Meridian Biosciences Premier assay were interpreted using the optical density at 450 nm (OD450) values from the spectrophotometric readings. OD values of <0.150 were considered negative, OD values of 0.150 to 0.199 were considered indeterminate, and OD values of ≥0.200 were considered positive for the presence of IgM or IgG antibodies. Results from the IMMY Omega assay were evaluated using an EIA index, which was calculated by dividing the OD450 value of the patient's serum by the OD value of a calibrator serum sample provided with the assay kit. EIA index values of <1.0 were considered negative, EIA index values of 1.0 to <1.5 were considered indeterminate, and EIA index values of ≥1.5 were considered positive for the presence of IgM or IgG antibodies. All sera were tested with both the Meridian EIA and the IMMY EIA.
Immunodiffusion assays.
Sera that were reactive with either Coccidioides EIA were further evaluated for antibody reactivity using immunodiffusion. A microimmunodiffusion assay for the detection of antibodies against the Coccidioides complement fixation antigen (IDCF) was performed using CDC-prepared reagents and procedures described previously (12). An immunodiffusion assay for the detection of antibodies against the Coccidioides tube precipitin antigen (IDTP) was performed using commercially prepared reagents and precast Cleargel agar plates (IMMY, Norman, OK), according to the manufacturer’s instructions. Immunodiffusion plates were incubated in a humidified chamber at ambient temperature, and results were assessed at 48 h or as indicated in the manufacturer's instructions. EIA-reactive sera were also concentrated, as described below, and evaluated by immunodiffusion using commercially available IDCF and IDTP reagents and precast Cleargel agar plates (IMMY). Because histoplasmosis is endemic in Puerto Rico, EIA-reactive sera from Puerto Rico were also evaluated for antibodies to Histoplasma capsulatum, using the CDC microimmunodiffusion assay, to rule out the possibility of cross-reactivity of Histoplasma capsulatum-specific antibodies in the EIA.
Concentration of serum.
Aliquots of patient sera were concentrated to enhance the sensitivity of antibody testing by immunodiffusion, using an Amicon Ultra 2-ml centrifugal filter concentrator kit (EMD Millipore, Billerica, MA). Briefly, the filter device containing 200 to 400 μl serum was placed in a Sorvall RC5C Plus centrifuge (Sorvall/Thermo Fisher Scientific, Waltham, MA) with an SM-24 fixed-angle rotor and was centrifuged at 2,930 × g for 2.0 to 2.5 h at 18°C. The resulting serum concentrate was retrieved by inverting the concentration device onto a conical collection tube (provided in the kit) and centrifuging for 2 min at 1,000 × g. This procedure resulted in approximately 3- to 4-fold concentration from the original volume.
Statistics.
Fisher's exact test (two-tailed) was used to compare the levels of antibody reactivity in sera obtained from regions of endemicity and nonendemicity and for comparisons between EIAs.
RESULTS
Meridian EIA reactivity.
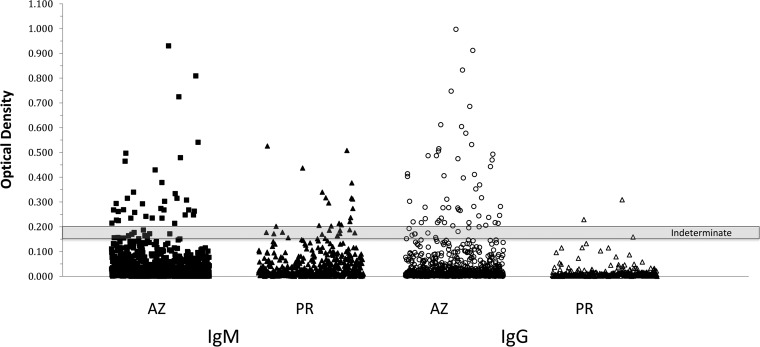
Figure 1 depicts the serum reactivity determined using the Meridian EIA, particularly the difference in the ranges of reactivities observed with sera from Puerto Rico versus sera from Arizona. Twenty sera (3.7%) from Puerto Rico, in which the disease is not endemic, were reactive in the EIA, of which 18 (3.4%) were reactive for IgM and 2 (0.37%) were reactive for IgG. In contrast, 80 (6.6%) of 1,218 sera from Arizona (where the disease is endemic) were reactive in the EIA, of which 29 (2.4%) were reactive for IgM and 44 (3.6%) were reactive for IgG. Overall, the percentage of EIA-reactive sera from Puerto Rico was significantly lower than that observed with sera from Arizona (3.7% versus 6.6%; P = 0.022) (Table 1). While the proportions of IgM-reactive sera were not statistically different for the samples from Arizona versus Puerto Rico (2.4% versus 3.4%; P = 0.31), the percentage of IgG-reactive sera from Puerto Rico was significantly lower (0.37% versus 3.6%; P < 0.001) (Table 1). In addition, seven double IgM-/IgG-reactive sera were identified among the Arizona samples, but no double reactive sera were detected among the Puerto Rico specimens. The percentages of indeterminate results were comparable for the two groups.
FIG 1.
Levels of anti-Coccidioides IgM (filled symbols) and IgG (open symbols) measured by the Meridian EIA in 1,218 sera obtained from Arizona (AZ) and 534 sera obtained from Puerto Rico (PR). Shaded area, optical density range for indeterminate results. Sera with results above and below the indeterminate range are considered positive and negative, respectively, for antibodies against Coccidioides.
TABLE 1.
Coccidioides EIA-reactive sera from regions of endemicity and nonendemicity
| EIA type and result | No. (%) of seraa |
|||||
|---|---|---|---|---|---|---|
| Puerto Rico (n = 534) |
Arizona (n = 1,218) |
|||||
| Positive | Indeterminate | Negative | Positive | Indeterminate | Negative | |
| Meridian EIA | ||||||
| IgM reactive | 18 (3.4) | 13 (2.4) | 503 (94.2) | 29 (2.4) | 15 (1.2) | 1,174 (96.4) |
| IgG reactive | 2 (0.37) | 1 (0.19) | 531 (99.4) | 44 (3.6)b | 10 (0.8) | 1,164 (95.6) |
| IgM and IgG reactive | 0 | 0 | 534 (100) | 7 (0.57) | 0 | 1,121 (99.4) |
| Total | 20 (3.7) | 14 (2.6) | 500 (93.6) | 80 (6.6)c | 35 (2.9) | 1,103 (90.6) |
| IMMY EIA | ||||||
| IgM reactive | 8 (1.5) | 36 (6.7) | 490 (91.8) | 14 (1.1) | 56 (4.6) | 1,148 (94.2) |
| IgG reactive | 4 (0.75) | 11 (2.1) | 519 (97.2) | 39 (3.2)d | 79 (6.5) | 1,100 (90.3) |
| IgM and IgG reactive | 0 | 0 | 534 (100) | 3 (0.25) | 0 | 1,215 (99.7) |
| Total | 12 (2.2) | 47 (8.8) | 475 (89.6) | 56 (4.6)c | 135 (11.1) | 1,027 (84.3) |
Positive, EIA reactive; indeterminate, EIA indeterminate; negative, EIA nonreactive.
Significant difference from sera from Puerto Rico, P < 0.001, Fisher's exact test.
Significant difference from sera from Puerto Rico, P = 0.02, Fisher's exact test.
Significant difference from sera from Puerto Rico, P = 0.002, Fisher's exact test.
ID results for Meridian EIA-reactive sera.
None of the 20 EIA-reactive sera from Puerto Rico was ID reactive prior to serum concentration, but 2 of 20 sera (both IgM reactive) were IDTP reactive after concentration (data not shown). All EIA-reactive sera from Puerto Rico were also negative for immunoprecipitating antibodies to H. capsulatum. Of 80 EIA-reactive sera from Arizona, 13 (16%) were ID reactive, which increased to 52 (65%) after concentration (Table 2).
TABLE 2.
Coccidioides ID reactivity of EIA-reactive sera from Arizona
| EIA type and result | No. (%) of reactive sera |
|||||||
|---|---|---|---|---|---|---|---|---|
| Unconcentrateda |
Concentratedb |
|||||||
| IDTP | IDCF | IDTP and IDCF | Total | IDTP | IDCF | IDTP and IDCF | Total | |
| Meridian EIA | ||||||||
| IgM reactive (n = 29) | 3 | 1 | 0 | 4 (14) | 12 | 1 | 3 | 16 (55) |
| IgG reactive (n = 44) | 4 | 1 | 0 | 5 (11) | 29 | 0 | 2 | 31 (70) |
| IgM and IgG reactive (n = 7) | 2 | 1 | 1 | 2 (57) | 1 | 0 | 4 | 5 (71) |
| Total (n = 80) | 9 | 3 | 1 | 13 (16) | 42 | 1 | 9 | 52 (65) |
| IMMY EIA | ||||||||
| IgM reactive (n = 14) | 0 | 2 | 0 | 2 (14) | 0 | 3 | 3 | 6 (43) |
| IgG reactive (n = 39) | 2 | 1 | 1 | 4 (10) | 2 | 4 | 3 | 9 (23) |
| IgM and IgG reactive (n = 3) | 1 | 0 | 0 | 1 (33) | 0 | 1 | 1 | 2 (66) |
| Total (n = 56) | 3 | 3 | 1 | 7 (13) | 2 | 8 | 7 | 17 (30) |
Sera reactive in ID assays prior to concentration.
Sera reactive in ID assays after 3- to 4-fold concentration.
IMMY EIA reactivity.
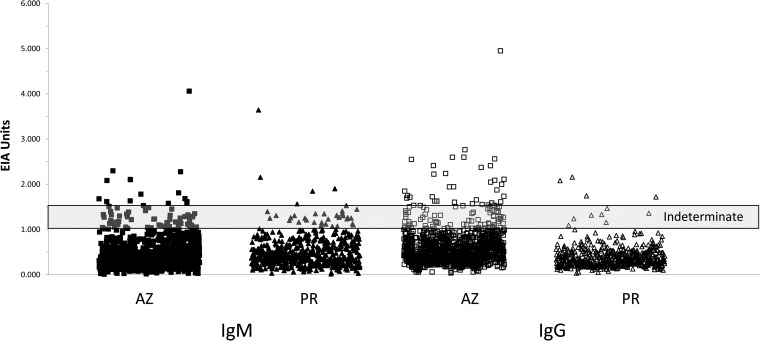
Results obtained using the IMMY EIA are presented in Fig. 2. Twelve sera (2.2%) from Puerto Rico were reactive, 8 (1.5%) for IgM and 4 (0.75%) for IgG (Table 1). In contrast, 56 (4.6%) of 1,218 sera from Arizona (where the disease is endemic) were reactive in the EIA, 14 (1.1%) for IgM and 39 (3.2%) for IgG (Table 1). Overall, the percentage of EIA-reactive sera from Puerto Rico was significantly lower than that observed with sera from Arizona (4.6% versus 2.2%; P = 0.02). While the proportions of IgM-reactive sera were not statistically different between Arizona and Puerto Rico (1.1% versus 1.5%; P = 0.7), the percentage of IgG-reactive sera from Puerto Rico was significantly lower (0.75% versus 3.2%; P = 0.002) (Table 1). No double IgM-/IgG-reactive sera were detected among the Puerto Rico specimens.
FIG 2.
Levels of anti-Coccidioides IgM (filled symbols) and IgG (open symbols) measured by the IMMY EIA in 1,218 sera obtained from Arizona (AZ) and 534 sera obtained from Puerto Rico (PR). Shaded area, EIA unit range for indeterminate results. Sera with results above and below the indeterminate range are considered positive and negative, respectively, for antibodies against Coccidioides.
ID results for IMMY EIA-reactive sera.
None of the 12 IMMY EIA-reactive sera from Puerto Rico, either unconcentrated or concentrated, were reactive in the ID assay (data not shown). Of 56 sera from Arizona that were reactive in the IMMY EIA, 7 (13%) were reactive in the ID assay when unconcentrated (Table 2) and 17 (30%) were reactive after concentration.
Comparison of Meridian and IMMY assays.
Table 3 presents the EIA reactivity of individual sera in both the Meridian and IMMY assays and shows that few sera were reactive in both assays. Sera that were reactive in both assays displayed higher levels of EIA reactivity (data not shown).
TABLE 3.
Comparison of sera reactive in Meridian and IMMY assays
| Sample group and EIA reactivity | No. of reactive sera |
||
|---|---|---|---|
| Meridian | IMMY | Both | |
| Arizona | |||
| IgG | 44 | 39 | 5 |
| IgM | 29 | 14 | 2 |
| IgG and IgM | 7 | 3 | 1 |
| Total | 80 | 56 | 8 |
| Puerto Rico | |||
| IgG | 2 | 4 | 0 |
| IgM | 18 | 8 | 2 |
| IgG and IgM | 0 | 0 | 0 |
| Total | 20 | 12 | 2 |
No significant difference in EIA reactivity was observed between the Meridian and IMMY EIAs using the sera from Puerto Rico. In the sera from Arizona, the percentage of total reactivity (4.6% versus 6.6%; P = 0.04) and the percentage of IgM-reactive sera (1.1% versus 2.4%; P = 0.03) were significantly lower using the IMMY EIA versus the Meridian EIA; the Arizona IgG results obtained using the two assays were not statistically different (Table 1). In both the Arizona and Puerto Rico populations, greater numbers of indeterminate results were obtained using the IMMY EIA versus the Meridian EIA (P < 0.01). A small proportion of this difference may be due to the change of 10 Meridian EIA-reactive sera to IMMY indeterminate. However, the majority of IMMY-indeterminate sera were nonreactive in the Meridian EIA.
DISCUSSION
Serological testing is the most commonly employed laboratory approach for the diagnosis of primary CM infections. The current serological tests pose a significant public health challenge in determining the incidence of CM, due to potential problems with sensitivity and specificity. While testing of paired acute-phase and convalescent-phase sera enhances the diagnostic accuracy, incidence calculations are most often established from a single serological test result, since convalescent specimens are seldom available. Acknowledging this situation, in 2008 the Council of State and Territorial Epidemiologists (CSTE) case definition for a notifiable case of CM was modified from requiring a single IgM or rising IgG titer from two sera (http://wwwn.cdc.gov/nndss/conditions/coccidioidomycosis/case-definition/1996) to requiring IgM or IgG reactivity from only a single serum sample (http://wwwn.cdc.gov/nndss/conditions/coccidioidomycosis/case-definition/2008). In 2011, the CSTE case definition for a notifiable case of CM was further modified to remove the requirement for the demonstration of symptoms (http://wwwn.cdc.gov/nndss/conditions/coccidioidomycosis/case-definition/2011).
These actions have contributed to the current public health debate, which revolves around whether EIA results not confirmed by ID, particularly the IgM EIA result, are true-positive results due to the greater sensitivity of the EIA than of the ID test or whether these results are actually false-positive results, thereby overcounting CM cases. Some research groups have reported elevated rates of false positivity, especially among individuals with IgM-only positive results (3, 10). Others have reported that the predictive value of such tests depends on the prior probability of CM for a given patient (13). The current study addresses this question by assessing EIA results from individuals living outside a region of endemicity (Puerto Rico), who are not expected to have been exposed to Coccidioides spp., and comparing the results with those from individuals living in a region of endemicity (Arizona).
In this study, the overall reactivity in sera from Puerto Rico using the Meridian EIA was low (3.7%), comparing favorably with original evaluation reports for this EIA kit (5, 6, 9). The majority of the EIA-reactive sera from Puerto Rico (18 of 20 sera) were IgM reactive, with only 2 of 20 sera being IgG reactive and no sera being double IgM/IgG reactive. ID testing of the EIA-reactive sera from Puerto Rico also exhibited extremely small numbers of ID-reactive sera. Because the serum donors from the region of nonendemicity could not be exposed to Coccidioides in their natural environment and the probability that these people were exposed while traveling was also likely to be low, the reactivity observed with these sera was most likely nonspecific. A similar observation was noted for a series of patients from Missouri (where coccidioidomycosis is not endemic) who had no history of travel to a region in which the disease is endemic but displayed Coccidioides EIA IgM reactivity (14). These results may be complicated by the fact that the Meridian Coccidioides EIA has been observed to cross-react with sera from patients with histoplasmosis (assay package insert and unpublished data from the CDC), which is endemic in both Missouri and Puerto Rico. In our study, however, all Coccidioides EIA-reactive sera from Puerto Rico were negative for Histoplasma by immunodiffusion.
In contrast, the overall percentage of Coccidioides EIA-reactive sera from Arizona was significantly higher than that of sera from individuals in a region of nonendemicity. Almost 7% of sera from blood bank donors living in Arizona were reactive for IgG, IgM, or both, while the rest were negative. Unlike the results for sera from Puerto Rico, 44 of 80 (55%) of EIA-reactive sera from Arizona were reactive for IgG only; 7 of 80 (9%) were reactive for both IgG and IgM. The presence of antibodies detected by ID provides further evidence that the antibody reactivity observed in the Arizona population is, at least in part, specific. The antibodies in these sera were present at very low concentrations, as suggested by the 4-fold increase in number of ID-reactive sera after concentration. Interestingly, a large number of sera with EIA IgG reactivity were reactive with the IDTP antigen. This reactivity was noted by Gade et al. (15), who demonstrated that antibodies of the IgG isotype were able to bind to tube precipitin (TP) antigens. While ID may not be as sensitive as EIA for the detection of Coccidioides-specific antibodies, the assay is very specific (12). The relatively large number of individuals with EIA reactivity in Arizona is not surprising, since asymptomatic cases of CM are well documented (11) and antibodies resulting from previous infections are not unexpected; to our knowledge, however, these results provide the first formal assessment, using an EIA, of the background rates of seropositivity among healthy individuals in an area of endemicity.
Interestingly, while the rates of EIA IgG and ID reactivity were different between Puerto Rico and Arizona, the lack of significance in the rates of IgM-only reactivity between the two populations strengthens the argument that the IgM reactivity observed in sera from Puerto Rico and at least some of the sera from Arizona is nonspecific. Overall, the proportion of Meridian IgM reactivity was approximately 3%, which was lower than that observed in other studies (3, 10). These results indicate that, at least for healthy individuals, the rate of nonspecific IgM reactivity does not differ from the manufacturer's estimates; however, the proportion of IgM reactivity in the Arizona population that is nonspecific is not clear from these results. These results also do not address potential cross-reactivity with other pathogens, which needs to be further evaluated.
Like the Meridian EIA, the IMMY Omega Coccidioides EIA demonstrated significant differences in reactivity between the Puerto Rico and Arizona populations, with significantly fewer IgG-reactive sera in the Puerto Rico population and no difference in IgM reactivity. In comparison with the Meridian assay, the IMMY EIA displayed a significantly (P = 0.03) lower percentage of IgM-only reactivity in sera from Arizona. However, no significant difference was observed between the IMMY EIA and the Meridian EIA in IgM-only reactivity in sera from Puerto Rico. In addition, an increase in indeterminate results was observed using the IMMY EIA (P ≤ 0.01), most likely as a result of a broader indeterminate range, compared to the Meridian assay. The IMMY EIA is reported by the manufacturer to be 94.1% sensitive and 98.7% specific, compared to complement fixation; however, independent evaluations have not been reported. Further evaluation of the IMMY EIA using sera from confirmed cases of CM and related diseases is necessary to delineate the sensitivity and specificity of this assay.
Reactive sera did not always provide equivalent results in the two assays. Sera that were reactive in the Meridian EIA were often nonreactive or indeterminate in the IMMY EIA, and vice versa. This may be attributed to the differences in kit reagents, particularly the antigens used to coat the EIA microwells. Meridian EIA microwells are coated with a mixture of purified TP and CF antigens, placed in the same wells. The IMMY EIA uses a mixture of recombinant and native antigens in separate wells for IgM and IgG determinations. Thus, each serum sample may react differently depending on the antigens coating each well. Furthermore, these results address only reactivity in sera from patients who are not expected to have EIA reactivity. Further studies with sera from confirmed patients are needed for direct comparison of these assays.
The observation that approximately 7% of sera from Arizona blood bank donors are EIA reactive provides insight into the extent of background reactivity, which may have important implications for surveillance studies or investigations of outbreaks of CM within regions in which the disease is endemic. EIA reactivity not only may indicate a current infection but also may detect remnant antibodies from a past infection. The public health impact of these results for evaluations of populations for CM is twofold. First, a proportion (∼3%) of the IgM reactivity in a given population may be nonspecific. Second, in a region in which the disease is endemic, 3.6% of IgG reactivity and approximately 7% of total EIA reactivity may not be the result of a current, active infection. With these results in mind, other criteria, including clinical and microbiological evaluation, should be taken into consideration when evaluating individuals for CM.
ACKNOWLEDGMENTS
The use of product names in this manuscript does not imply their endorsement by the U.S. Department of Health and Human Services. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC.
REFERENCES
- 1.Nguyen C, Barker BM, Hoover S, Nix DE, Ampel NM, Frelinger JA, Orbach MJ, Galgiani JN. 2013. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev 26:505–525. doi: 10.1128/CMR.00005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson G III, Stevens D, Clemons K, Fierer J, Johnson R, Sykes J, Rutherford G, Peterson M, Taylor J, Chaturvedi V. 2015. Call for a California coccidioidomycosis consortium to face the top ten challenges posed by a recalcitrant regional disease. Mycopathologia 179:1–9. doi: 10.1007/s11046-014-9816-7. [DOI] [PubMed] [Google Scholar]
- 3.Crum N, Lederman E, Stafford C, Parrish JS, Wallace M. 2004. Coccidioidomycosis: a descriptive survey of a reemerging disease: clinical characteristics and current controversies. Medicine (Baltimore) 83:149–175. doi: 10.1097/01.md.0000126762.91040.fd. [DOI] [PubMed] [Google Scholar]
- 4.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Johnson RH, Stevens DA, Williams PL. 2005. Coccidioidomycosis. Clin Infect Dis 41:1217–1223. doi: 10.1086/496991. [DOI] [PubMed] [Google Scholar]
- 5.Zartarian M, Peterson EM, de la Maza LM. 1997. Detection of antibodies to Coccidioides immitis by enzyme immunoassay. Am J Clin Pathol 107:148–153. [DOI] [PubMed] [Google Scholar]
- 6.Martins TB, Jaskowski TD, Mouritsen CL, Hill HR. 1995. Comparison of commercially available enzyme immunoassay with traditional serological tests for detection of antibodies to Coccidioides immitis. J Clin Microbiol 33:940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappagianis D, Zimmer BL. 1990. Serology of coccidioidomycosis. Clin Microbiol Rev 3:247–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blair JE, Currier JT. 2008. Significance of isolated positive IgM serologic results by enzyme immunoassay for coccidioidomycosis. Mycopathologia 166:77–82. doi: 10.1007/s11046-008-9129-9. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman L, Sekhon AS, Moledina N, Jalbert M, Pappagianis D. 1995. Comparative evaluation of commercial Premier EIA and microimmunodiffusion and complement fixation tests for Coccidioides immitis antibodies. J Clin Microbiol 33:618–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuberski T, Herrig J, Pappagianis D. 2010. False-positive IgM serology in coccidioidomycosis. J Clin Microbiol 48:2047–2049. doi: 10.1128/JCM.01843-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith CE, Beard RR. 1946. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health 36:1394–1402. doi: 10.2105/AJPH.36.12.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindsley MD, Warnock DW, Morrison CJ. 2006. Serological and molecular diagnosis of fungal infections, p 569–605. In Detrick B, Hamilton RG, Folds JD (ed), Manual of molecular and clinical laboratory immunology, 7th ed ASM Press, Washington, DC. [Google Scholar]
- 13.Blair J, Mendoza N, Force S, Chang Y-H, Grys T. 2013. Clinical specificity of the enzyme immunoassay test for coccidioidomycosis varies according to the reason for its performance. Clin Vaccine Immunol 20:95–98. doi: 10.1128/CVI.00531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turabelidze G, Aggu-Sher RK, Jahanpour E, Hinkle CJ. 2015. Coccidioidomycosis in a state where it is not known to be endemic — Missouri, 2004–2013. MMWR Morb Mortal Wkly Rep 64:636–639. [PMC free article] [PubMed] [Google Scholar]
- 15.Gade W, Ledman DW, Wethington R, Yi A. 1992. Serological responses to various Coccidioides antigen preparations in a new enzyme immunoassay. J Clin Microbiol 30:1907–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]