Abstract
We previously demonstrated that 1-methyl-4-phenylpyridinium (MPP+) causes caspase-independent, non-apoptotic death of dopaminergic (DA) neuronal cells. Here, we specifically examined whether change of glucose concentration in culture medium may play a role for determining cell death modes of DA neurons following MPP+ treatment. By incubating MN9D cells in medium containing varying concentrations of glucose (5~35 mM), we found that cells underwent a distinct cell death as determined by morphological and biochemical criteria. At 5~10 mM glucose concentration (low glucose levels), MPP+ induced typical of the apoptotic dell death accompanied with caspase activation and DNA fragmentation as well as cell shrinkage. In contrast, MN9D cells cultivated in medium containing more than 17.5 mM (high glucose levels) did not demonstrate any of these changes. Subsequently, we observed that MPP+ at low glucose levels but not high glucose levels led to ROS generation and subsequent JNK activation. Therefore, MPP+-induced cell death only at low glucose levels was significantly ameliorated following co-treatment with ROS scavenger, caspase inhibitor or JNK inhibitor. We basically confirmed the quite similar pattern of cell death in primary cultures of DA neurons. Taken together, our results suggest that a biochemically distinct cell death mode is recruited by MPP+ depending on extracellular glucose levels.
Keywords: MPP+, Parkinson's disease, Glucose, caspase, reactive oxygen species, JNK
INTRODUCTION
Parkinson's disease (PD) is one of major neurodegenerative disorders and characterized by a progressive demise of DA neurons in the substantia nigra pars compacta. Although etiology of the PD is not clearly understood, several plausible mechanisms that may contribute to the selective cell death of the dopaminergic neurons have been proposed. These include the impairment of mitochondrial function, overproduction of reactive oxygen species (ROS), inflammation and excitotoxicity [1]. Although recent advances have been made in defining molecular and cellular events underlying the pathogenesis of PD, evidence is accumulating that either favors or argues against apoptosis [2,3,4,5,6,7,8,9,10]. For example, several reports have demonstrated that morphological and biochemical apoptosis may reflect the critical mechanism underlying dopaminergic neuronal death. In contrast, several other studies still conflict with regard to the mode of cell death. Indeed, we previously demonstrated that prototypic DA neurotoxins recruit a distinct cell death pathway in primary cultures of cortical or DA neurons as well as MN9D DA neuronal cell line [11,12]. More specifically, we provided evidence supporting a notion that 6-hydroxydopamine induces caspase-dependent apoptosis along with a surge of ROS and MAPK activation. In contrast, no obvious signs of apoptotic cell death were observed in MPP+-treated neuronal cell. In this study, we therefore attempted to experimentally address the question of whether and how glucose levels in the medium may play a critical role for determining a specific cell death pathway using MN9D cells and primary cultured DA neurons. Based on our present data, we propose a plausible scenario indicating that MPP+ can induce at least two different cell death pathways depending on levels of extracellular glucose: either apoptosis or non-apoptotic cell death.
MATERIALS AND METHODS
Cell culture and drug treatment
MN9D cells were cultivated on the poly-D-Lysine (25 µg/ml PDL: Sigma Chemical Co., St. Louis, MO, USA)-coated p-100 plate in Dulbecco's modified Eagle's medium (DMEM: Sigma) supplemented with heat inactivated 10% fetal bovine serum (FBS: BioWhittaker, Walkersville, MD, USA) in an atmosphere of 10% CO2 at 37℃. Prior to drug treatment, culture medium was switched to serum-free, chemically-defined N2 supplement [13] containing the predetermined concentrations of D-glucose (Sigma, 5-35 mM) plus 200 µM MPP+. If necessary, cells were co-treated with Boc-aspartyl(OMe)-fluoromethylketone (BAF; Enzyme Systems Products, Dublin, CA, USA), N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (zVAD; Enzyme Systems Products), N-acetylcystein (NAC, Sigma), or SP600125 (Cell Signaling; Boston, MA, USA).
Immunoblot analysis
Following drug treatment, cells were washed twice with ice cold PBS and dissolved in a buffer containing 50 mM Tris, pH 8.0, 2 mM EDTA, 1% triton X-100, 2 mM phenymethylsulfonylfluoride (PMSF), and 50 µg/ml aprotinin (all from Sigma). Lysates were homogenized in a Dounce homogenizer on ice, followed by centrifugation at 13,000×g for 30 min at 4℃. Protein concentrations of the supernatant were measured using Bio-Rad protein assay kit (Herculus, CA, USA). Approximately 50 µg of protein was separated on the 12.5% SDS-polyacrylamide gel and blotted onto pre-wet polyvinylidene difluoride (PVDF; Bio-Rad) membrane. The membrane was blocked with Tris-buffered saline (TBS) containing 5% nonfat milk for 60 min at RT, followed by incubation at 4℃ with the indicated primary antibodies. These include a rabbit polyclonal anti-activated caspase 3 antibody (1: 1,000; Cell Signaling), a rabbit polyclonal anti-activated caspase 9 antibody (1: 1,000; Cell Signaling), a rabbit polyclonal anti-phospho-JNK antibody (1: 1,000; Cell Signaling), a rabbit polyclonal anti-JNK antibody (1: 1,000; Cell Signaling), a rabbit polyclonal anti-phospho-p38 antibody (1: 1,000; Cell Signaling), a rabbit polyclonal anti-p38 antibody (1: 1,000; Cell Signaling). Blots were further incubated with an appropriated secondary antibody conjugated with horse radish peroxidase for 60 min at RT. Signals were visualized using Enhanced Chemiluminescence (ECL) kit (Amersham Phamarcia, San Diego, CA, USA).
MTT assay
The rate of cell viability was assessed by a colorimetric measurement of 3-[4,5-dimethythioazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT: Sigma) reduction assay [14]. Briefly, cells at various time points were incubated with 1 µg/ml of MTT solution for 2 hr and then further incubated with 20% SDS in 50% dimethylformamide (DMF: Sigma) for 24 hr. The optical density of the dissolved formazan grains within the cell were measured at 570 nm. Values of each treatment were calculated as a percentage over the untreated control (100% survival).
DNA fragmentation assay
Following drug treatment, MN9D cells on P-100 plate were washed twice with ice cold PBS and subjected to lysis in a buffer containing 500 µl 1% Triton X-100, 50 mM Tris buffer, PH7.4, and 2 mM EDTA for 30 min on ice. After microcentrifugation at 4℃ for 15 min, the supernatants were subjected to phenol/chloroform extraction and ethanol precipitation. Residual RNA contaminants were digested by incubation with DNase-free RNAse A. The resulting samples were loaded onto 1.2% agarose gel and subsequently electrophoresed at 50V for 4 hr. After staining with ethidium bromide, DNA gel was visualized on a UV-transilluminator and photographed using Polaroid 667 film.
Measurement of ROS
Levels of ROS in the MN9D cell were measured with ROS-sensitive fluorescent dyes: dihydroethidium and 2, 7-dichlorofluorescin diacetate (DCF: Molecular Probes, Eugene, OR, USA). Following drug treatment, MN9D cells were washed twice with a saline containing 144 mM NaCl, 10 mM HEPES, 2 mM CaCl2, 1 mM MgCl2, 1 mM KCl, and 10 mM D-glucose. Cells were loaded with 1 µM dihydroethidium and incubated at 37℃ for 15 min. The cells were then washed twice with a saline and photographed under a fluorescence microscope (Carl Zeiss, Zena, Germany). To improve the permeability of DCF dye, cells were loaded with 5 µM DCF dye containing 0.1% pluronic F-127 (Molecular Probes) for 30 min prior to MPP+ treatment.
Primary cultures of DA neurons
To prepare primary cultures of DA neurons, the ventral mesencephalon was isolated from the 14d gestation Sprague Dawley rat embryo (Daehan; Daejon, Korea) as described previously [12]. The dissected tissues were incubated with 0.01% trypsin in HBSS for 10 min at 37℃ and triturated with a constricted Pasteur pipette. The dissociated cells were plated at 1.0×105 cells per 8-mm diameter Aclar embedded film (Electron Microscopy Sciences, Fort Washington, PA, USA) pre-coated with 100 µg/ml poly-D-lysine (Sigma) and 4 µg/ml laminin (Invitrogen, San Diego, CA, USA) in DMEM containing 2 mM glutamine plus varying concentrations of glucose.
Immunocytochemistry
For a double staining of tyrosine hydroxylase (TH; a rate-limiting enzyme of dopamine biosynthesis) and activated caspase 3, DA neurons exposed to 3 µM MPP+ for 48 hr were fixed with 4% paraformaldehyde for 20 min at RT and blocked for 1 hr in PBS containing 5% normal goat serum and 0.1% Triton X-100 at 37℃ overnight. Primary antibodies used were a mouse monoclonal anti-TH antibody (1: 7500; Pel-Freez, Rogers, AR, USA) and a rabbit polyclonal anti activated caspase-3 antibody (1: 200; Cell Signaling). After washing with PBS, the DA neurons were incubated with Alexa Flour 568-conjugated goat anti-rabbit IgG and Alexa Fluor 488-conjugated goat anti-mouse IgG (1: 200; Molecular Probes) at RT for 1 hr. Cells were then examined under an Axiocam Digital Camera (Carl Zeiss). To determine the number of the surviving TH-positive neurons after MPP+ treatment in the presence or absence of various inhibitors, DA neurons were fixed, blocked, and incubated with a monoclonal anti-TH as described above. After washing with PBS, cells were incubated with a biotinylated goat anti-mouse Ig G at RT for 1 hr, followed by peroxidase-conjugated avidin-biotin complex (1: 200; Vector Laboratories, Burlingame, CA, USA) for 30 min at RT. Staining was visualized by applying 3, 3'-diaminibenzidine (DAB, substrate for peroxidase; Vector Laboratories). Manual counts from each condition present in each 8 mm Aclar embedding film were made of all of the TH-positive cells having neurites twice the length of the soma [12]. A total of 300~400 TH positive neurons were counted in the untreated control.
Statistical analyses
The data were expressed as the means±S.D. or the means±S. E.M. Significance between groups was determined by one-way analysis of variance (ANOVA) and the Tukey's post hoc test using GraphPad Prism 6. Values of **p<0.01 or *p<0.05 were considered statistically significant.
RESULTS AND DISCUSSION
A distinct cell death mode is detected after MPP+ treatment in MN9D cells cultivated in varying concentrations of glucose
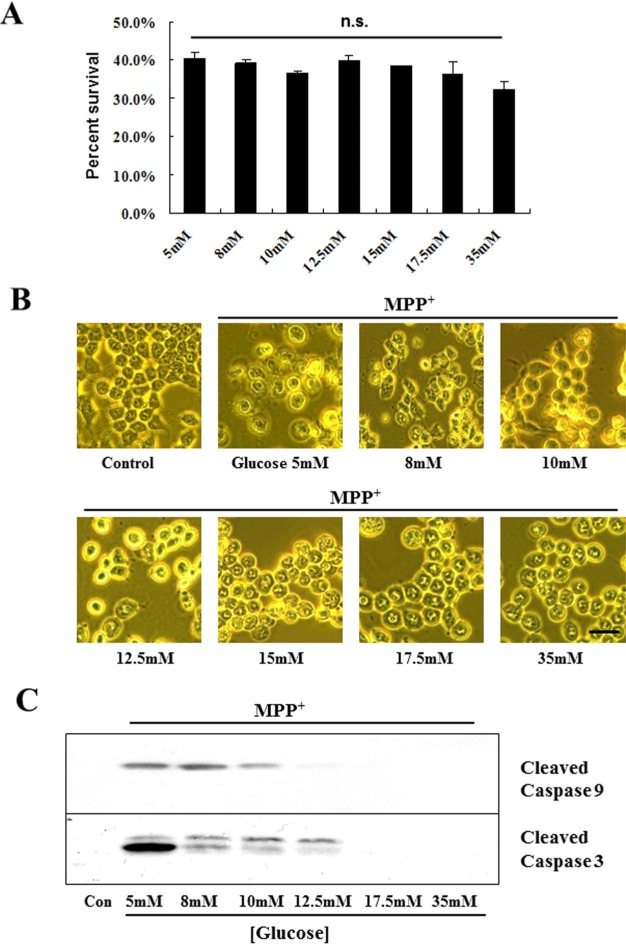
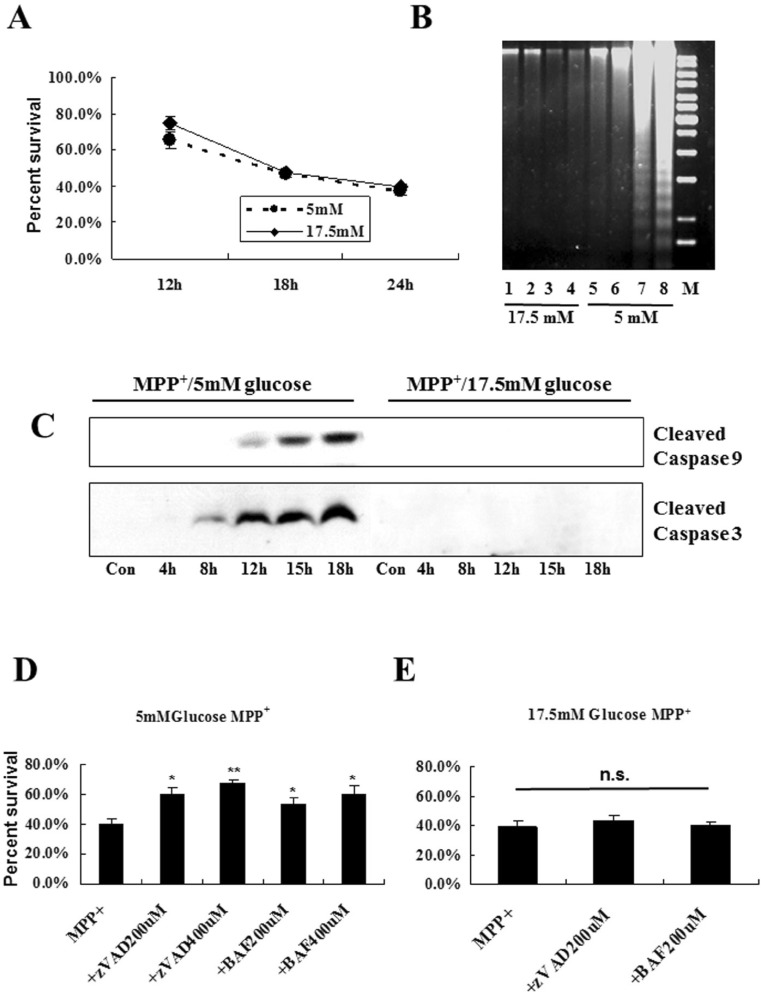
To examine whether change of extracellular D-glucose concentrations may contribute to inducing a distinct cell death mode after MPP+ treatment, MN9D cells were incubated in N2 culture medium containing varying concentrations of D-glucose (5~35 mM) plus 200 µM MPP+. As shown in Fig. 1A, MPP+ caused a quite similar rate of cell death regardless of extracellular concentration of glucose. However, we observed that morphology of dying cells was quite differed and it depended on extracellular glucose levels. At lower concentrations of glucose (5~12.5 mM), for example, MPP+ led to typical of apoptotic cell death accompanying with shrinkage of cell body and a phase-bright appearance (Fig. 1B). At higher concentrations of glucose (15~35 mM), vacuolelike structures and flat appearance were apparent in cells after treatment with MPP+. Immunoblot analysis clearly indicated that activation of both caspase-9 and -3 was only detected in cells cultivated in lower concentrations of glucose (5~12.5 mM) following exposure to MPP+ (Fig. 1C). This is quite contrary to our previous study [11,12] demonstrating that no obvious signs of apoptosis were detected in MN9D cells and primary cultured DA neurons following exposure to MPP+. Since these cells were cultivated in culture medium containing 17.5 mM or higher, we further attempted to confirm that MN9D cells indeed die of apoptosis at lower concentrations of glucose. Therefore, we selected two doses of extracellular glucose (5 mM vs 17.5 mM). Again, MPP+ caused a quite similar kinetic of cell death regardless of extracellular levels of glucose (Fig. 2A). DNA fragmentation assay showed that MPP+-induced DNA fragmentation was occurred in a time-dependent manner only in MN9D cells cultivated in media containing 5 mM glucose (Fig. 2B). Immunoblot analysis also demonstrated that a time-dependent appearance of activated capsase-9 and -3 is detected only in MN9D cells at 5 mM glucose after MPP+ treatment (Fig. 2C). Although activated caspase-3 appeared as double bands in most cases, a single band of the activated caspase-3 was detected depending on passages of culture. Consequently, co-treatment with one of two widely used pan-caspase inhibitor (zVAD and BAF) rescued cells from MPP+-induced death when it was cultivated at 5 mM glucose (Fig. 2D). In contrast, this protective effect was not detected in cells cultivated at 17.5 mM (Fig. 2E). Taken together, we clearly demonstrated that MPP+ can induce either caspase-dependent or caspase-independent cell death and this is largely depended on extracellular levels of glucose in the medium.
Fig. 1. MPP+-induced caspase activation is only seen in MN9D cells cultivated in lower levels of glucose. (A~C) MN9D cells were cultivated in serum-free, N2-supplemented medium containing 5~35 mM glucose and exposed to 200 µM MPP+ for 24 hr. (A) Cell viability was measured using MTT reduction assay. Values were expressed percent survival over the untreated control (100%). Data represent the mean±SEM from 3 independent cultures in triplicate. n.s. represents not significant. (B) Phase-contrast photomicrographs were taken under a Zeiss Axiovert 100 microscope. Scale bar represents 20 µm. (C) Approximately 50 µg proteins were separated on 12.5% SDS-PAGE and transferred to prewet PVDF membrane. Blots were immunolabeled with a rabbit polyclonal anti-cleaved caspase 9 or a rabbit polyclonal anti-cleaved caspase 3.
Fig. 2. A time-dependent increase of DNA fragmentation and caspase activity is detected in MN9D cells cultivated in 5 mM glucose but not in 17.5 mM glucose. MN9D cells cultivated in N2 medium containing either 5 mM or 17.5 mM glucose were exposed to 200 µM MPP+ for the indicated time periods. (A) MTT reduction assay was performed. Values were expressed percent survival over the untreated control (100%). Data represent the mean±SEM from 3 independent cultures in triplicate. No significant difference was measured between two groups. (B) Total DNA extracted from MN9D cells exposed to 200 µM MPP+ for 4 (lane 1, 5), 8 (lane 2, 6), 12 (lane 3, 7) and 18 hr (lane 4, 8) was separated on 1.2% agarose gel. Gels were stained with ethidium bromide and then photographed on a UV-transilluminator. (C) Immunoblot analysis for active forms of capsase-9 and caspase-3 was carried out as described above. (D, E) Cells cultivated either in (D) 5 mM glucose or (E) 17.5 mM glucose were exposed to 200 µM MPP+ for 24 hr in the presence or the absence of the indicated concentrations of zVAD or BAF. Viability was measured using MTT reduction assay and data represent the mean±SEM from 3 independent cultures in triplicate. **p<0.01; *p<0.05; n.s., not significant.
Surge of ROS and ROS-mediated activation of MAPK are involved in MPP+-induced apoptotic cell death
Previously, we demonstrated that intracellular surge of calcium and its concurrent signaling is responsible for MPP+-induced cell death whereas ROS-mediated activation of MAPK significantly contributes to 6-OHDA-induced apoptotic cell death [15,16,17,18,19]. Therefore, we attempted to investigate whether generation of ROS at early phase of cell death and concurrent ROS-mediated MAPK signaling are involved and play an essential role in MPP+-induced apoptotic cell death. First, we measured levels of ROS using cell-permeable, ROS-sensitive fluorescent dyes: dihydroethidium (HET) and 2, 7-dichlorofluorescin diacetate (DCF) following treatment of MN9D cells with MPP+. Following MPP+ treatment, HET-positive cells appeared in a time-dependent manner when cells were cultivated at 5 mM glucose but not at 17.5 mM (Fig. 3A). Quantitative measurement indicated that levels of HET-sensitive ROS increased up to 2.5 fold in MN9D cells at 5 mM glucose. This increase was significantly blocked when cells were co-treated with an ROS scavenger, N-acetylcystein (NAC). When cells were stained with DCF, quite similar pattern of ROS surge was detected (Fig. 3B). Consequently, co-treatment of cells with NAC protected cells from MPP+-induced death when cells were cultivated only at 5 mM glucose (Fig. 3C).
Fig. 3. Surge of ROS is responsible for MPP+-induced death when cells are cultivated at 5 mM glucose but not at 17.5 mM glucose. (A, B) MN9D cells cultivated in N2 medium containing either 5 mM or 17.5 mM glucose were exposed to 200 µM MPP+ alone or in combination with I mM NAC for the indicated time periods. Cells were loaded with (A) 1 µM dihydroethdium or (B) 5 µM DCF and analyzed using fluorescence microscope equipped with a digital camera. Scale bar represents 20 µm. (C) After treatment with 200 µM MPP+ in the presence or the absence of 1 mM NAC for 24 hr, MTT reduction assay was performed to measure cell viability. Data represent the mean±SEM from 3 independent cultures in triplicate. *p<0.05; n.s., not significant.

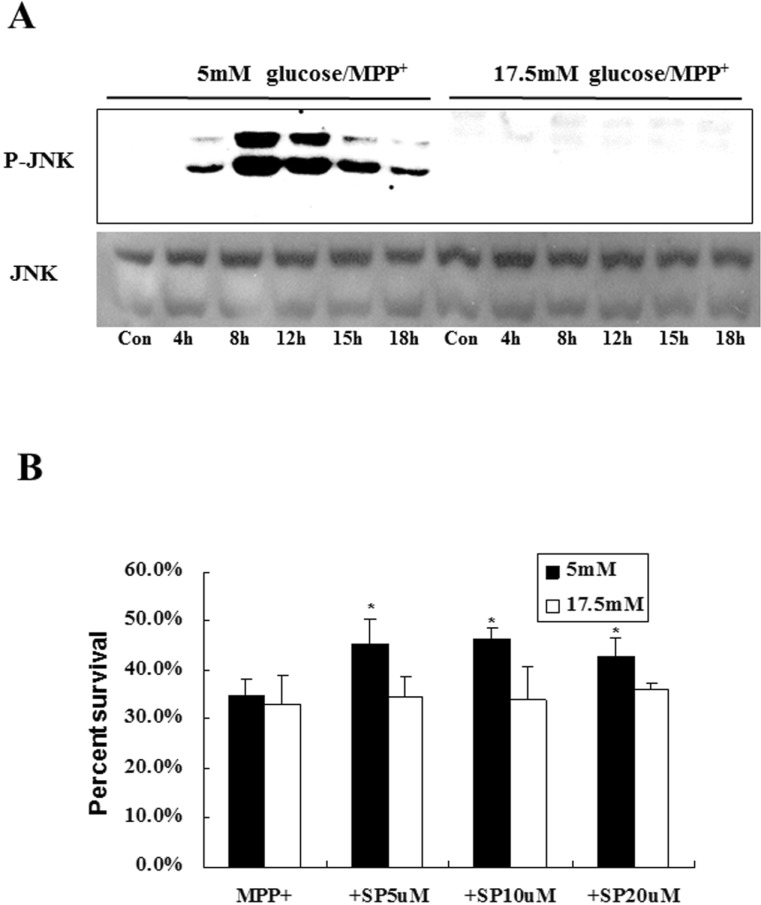
Next, we wondered whether MPP+-induced surge of ROS is linked to activation of MAPK. As shown in Fig. 4A, phospho-JNK was first seen at 4 hr after MPP+ treatment in cells cultivated at 5 mM glucose and reached at its maximum at 8 to 12 hr and decreased thereafter. Levels of JNK remained the same at all time periods tested. However, any discernible signs of MPP+-mediated JNK activation were not detected when cells were incubated at 17.5 mM glucose. Interestingly, phosphorylated forms of p38 MAPK were not detected at any conditions tested (data not shown). Consequently, co-treatment with SP600125 (a JNK-specific inhibitor) rescued cells from MPP+-induced death when cells were cultivated at 5 mM glucose (Fig. 4B). Again, co-treatment with p38 inhibitor (PD 169316) or ERK inhibitor (U0126) had no effect on cell viability (data not shown). In sum, we clearly demonstrated that MPP+ can induce apoptotic cell death via ROS generation and subsequent activation JNK when MN9D cells were incubated at 5 mM glucose.
Fig. 4. JNK activation plays a role for determining MPP+-induced death when cells are cultivated at 5 mM glucose but not at 17.5 mM glucose. (A) MN9D cells cultivated in N2 medium containing either 5 mM or 17.5 mM glucose were exposed to 200 µM MPP+ for the indicated time periods. Immunoblot analysis was carried out using anti-phosphorylated forms of JNK. Anti-JNK was used as a loading control. (B) MN9D cells were treated with 200 µM MPP+ alone or in combination with the indicated concentrations of SP600125 (SP) for 24 hr. Cell viability was measured by a MTT reduction assay. Data represent the mean±SEM from 3 independent cultures in triplicate. *p<0.05. No significant difference was measured in MN9D cells cultivated in 17.5 mM glucose.
MPP+-induced caspase activation is triggered when primary cultures of DA neurons were cultivated at lower levels of glucose
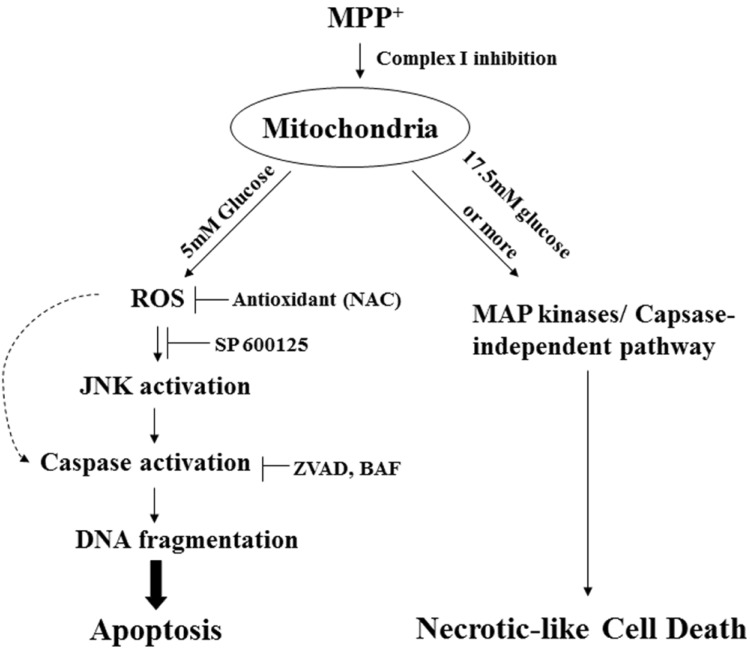
Finally, we chose primary cultures of DA neurons to firmly establish a correlation between levels of extracellular glucose and cell death mode. As we previously demonstrated [12], we prepared primary cultured DA neurons from the ventral mesencephalon of E14 rat embryos. We empirically first tested ranges of glucose concentrations in the medium that did not cause any detectable signs of cell death without drug treatment. We found that DA neurons were healthy when they are cultivated in medium containing 5~33.3 mM glucose for up to 10 days (data not shown). Double immunofluorescent staining showed that, following MPP+ treatment, active forms of caspase-3 appeared in TH-positive neurons when cells were cultivated at 5 mM glucose but not at higher concentrations of glucose (e.g., 20 mM or 33.3 mM glucose; Fig. 5A). Quantitative measurement assay showed that approximately 6.6% of TH-positive cells showed the active forms of caspase-3 in cells at 5 mM glucose (Fig. 5B). As shown in Fig. 6A, neurites from TH-positive cells were retracted and fragmented after MPP+ treatment. However, retraction and/or fragmentation of neurites were less obvious in cells co-treated with NAC (ROS scavenger), zVAD (a pan-caspase inhibitor) or SP600125 (JNK inhibitor). Indeed, numbers of TH-positive cells having neurites twice the length of the soma were increased when DA neurons were cultivated in the presence of one of these inhibitors (Fig. 6B). Taken all together, we showed an interesting scenario in which MPP+ can induces either ROS-mediated, caspase-activated apoptosis or caspase-independent cell death (Fig. 7). We have reported that MPP+ induces a distinct form of cell death that quite resembles necrosis in morphology [11]. In addition, we have demonstrated that MPP+-induced cell death cannot be rescued by a pan-caspase inhibitor [12]. In these previous reports, cells were cultivated in a medium containing higher levels of glucose (17.5 mM or higher). Several contradicting reports from other laboratories have indicated that ROS is involved in MPP+-induced cell death [20,21,22]. Our present results explain why and how cells die of either ROS-mediated, caspase-dependent pathway or -independent cell death pathway. To our knowledge, it is not known whether glucose level may be linked to DA neuronal death in patients with PD. Based on our present results, however, we propose that determination of cell death mode seems to be relied on levels of glucose in extracellular milieu. Therefore, our data explain how both caspase-dependent or -independent cell death are detected in postmortem brains of patient with PD.
Fig. 5. MPP+-induced caspase activity is detected in primary cultures of DA neurons cultivated in 5.5 mM glucose. Primary cultures of DA neurons were prepared from the mesencephalon of ED 14 embryos. At 5 DIV, cells cultivated in the indicated concentrations of glucose were exposed to 3 µM MPP+ for 48 hr. (A) Cultures were subjected to a double immunofluorescent localization of tyrosine hydroxylase (TH) and active caspase 3, and photographed as described in Materials and Methods. Scale bar represents 50 µm. (B) Among TH-positive cells (300~400 cells) from two representative culture, means of percentage of the cells having a caspase-3 activity was determined.
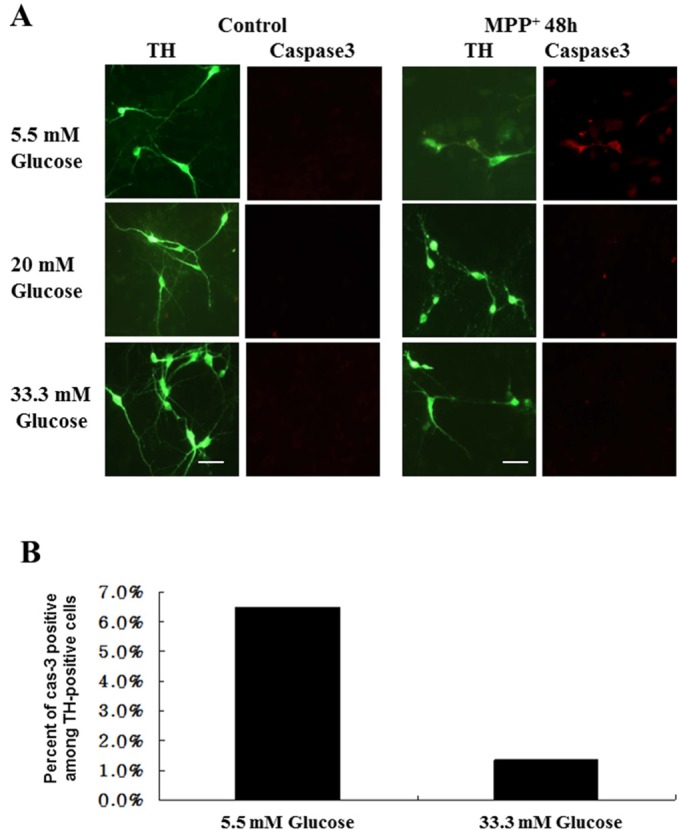
Fig. 6. Inhibition of ROS generation, caspase activity and JNK activation all rescues primary cultured DA neurons from MPP+-induced death. (A) At 5 DIV, primary cultures of DA neurons cells cultivated in 5.5 mM glucose were exposed to 3 µM MPP+ for 48 hr in the presence or the absence of 1 mM NAC, 200 µM zVAD or 20 µM SP600125. Cells were then subjected to DAB staining for TH-positive cells and photographed. Scale bar represents 200 µm. (B) Manual counts were made of all of the TH-positive cells having neurites twice the length of the soma. A total of approximately 300~400 TH positive neurons were counted and expressed as a percentage over the untreated control (100%). Data represent the mean±SD from 3 independent cultures. No significant difference was measured in DA neurons cultivated in 33.3 mM glucose.
Fig. 7. A schematic model for two distinct cell death modes recruited in dopaminergic neuronal cells after exposure to MPP+.
ACKNOWLEDGMENT
This work was supported from the Ministry for Health, Welfare and Family Affairs (A111382).
References
- 1.Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 2.Hartley A, Stone JM, Heron C, Cooper JM, Schapira AH. Complex I inhibitors induce dose-dependent apoptosis in PC12 cells: relevance to Parkinson's disease. J Neurochem. 1994;63:1987–1990. doi: 10.1046/j.1471-4159.1994.63051987.x. [DOI] [PubMed] [Google Scholar]
- 3.Mochizuki H, Nakamura N, Nishi K, Mizuno Y. Apoptosis is induced by 1-methyl-4-phenylpyridinium ion (MPP+) in ventral mesencephalic-striatal co-culture in rat. Neurosci Lett. 1994;170:191–194. doi: 10.1016/0304-3940(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 4.Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- 5.Viswanath V, Wu Y, Boonplueang R, Chen S, Stevenson FF, Yantiri F, Yang L, Beal MF, Andersen JK. Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease. J Neurosci. 2001;21:9519–9528. doi: 10.1523/JNEUROSCI.21-24-09519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilsland J, Roy S, Xanthoudakis S, Nicholson DW, Han Y, Grimm E, Hefti F, Harper SJ. Caspase inhibitors attenuate 1-methyl-4-phenylpyridinium toxicity in primary cultures of mesencephalic dopaminergic neurons. J Neurosci. 2002;22:2637–2649. doi: 10.1523/JNEUROSCI.22-07-02637.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 8.Jeon BS, Jackson-Lewis V, Burke RE. 6-Hydroxydopamine lesion of the rat substantia nigra: time course and morphology of cell death. Neurodegeneration. 1995;4:131–137. doi: 10.1006/neur.1995.0016. [DOI] [PubMed] [Google Scholar]
- 9.Lotharius J, Dugan LL, O'Malley KL. Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. J Neurosci. 1999;19:1284–1293. doi: 10.1523/JNEUROSCI.19-04-01284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soldner F, Weller M, Haid S, Beinroth S, Miller SW, Wüllner U, Davis RE, Dichgans J, Klockgether T, Schulz JB. MPP+ inhibits proliferation of PC12 cells by a p21(WAF1/Cip1)-dependent pathway and induces cell death in cells lacking p21(WAF1/Cip1) Exp Cell Res. 1999;250:75–85. doi: 10.1006/excr.1999.4504. [DOI] [PubMed] [Google Scholar]
- 11.Choi WS, Yoon SY, Oh TH, Choi EJ, O'Malley KL, Oh YJ. Two distinct mechanisms are involved in 6-hydroxydopamine- and MPP+-induced dopaminergic neuronal cell death: role of caspases, ROS, and JNK. J Neurosci Res. 1999;57:86–94. doi: 10.1002/(SICI)1097-4547(19990701)57:1<86::AID-JNR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 12.Han BS, Hong HS, Choi WS, Markelonis GJ, Oh TH, Oh YJ. Caspase-dependent and -independent cell death pathways in primary cultures of mesencephalic dopaminergic neurons after neurotoxin treatment. J Neurosci. 2003;23:5069–5078. doi: 10.1523/JNEUROSCI.23-12-05069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 15.Choi WS, Eom DS, Han BS, Kim WK, Han BH, Choi EJ, Oh TH, Markelonis GJ, Cho JW, Oh YJ. Phosphorylation of p38 MAPK induced by oxidative stress is linked to activation of both caspase-8- and -9-mediated apoptotic pathways in dopaminergic neurons. J Biol Chem. 2004;279:20451–20460. doi: 10.1074/jbc.M311164200. [DOI] [PubMed] [Google Scholar]
- 16.Kim HE, Yoon SY, Lee JE, Choi WS, Jin BK, Oh TH, Markelonis GJ, Chun SY, Oh YJ. MPP(+) downregulates mitochondrially encoded gene transcripts and their activities in dopaminergic neuronal cells: protective role of Bcl-2. Biochem Biophys Res Commun. 2001;286:659–665. doi: 10.1006/bbrc.2001.5446. [DOI] [PubMed] [Google Scholar]
- 17.Choi WS, Canzoniero LM, Sensi SL, O'Malley KL, Gwag BJ, Sohn S, Kim JE, Oh TH, Lee EB, Oh YJ. Characterization of MPP(+)-induced cell death in a dopaminergic neuronal cell line: role of macromolecule synthesis, cytosolic calcium, caspase, and Bcl-2-related proteins. Exp Neurol. 1999;159:274–282. doi: 10.1006/exnr.1999.7133. [DOI] [PubMed] [Google Scholar]
- 18.Kim C, Yun N, Lee YM, Jeong JY, Baek JY, Song HY, Ju C, Youdim MB, Jin BK, Kim WK, Oh YJ. Gel-based protease proteomics for identifying the novel calpain substrates in dopaminergic neuronal cell. J Biol Chem. 2013;288:36717–36732. doi: 10.1074/jbc.M113.492876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang H, Han BS, Kim SJ, Oh YJ. Mechanisms to prevent caspase activation in rotenone-induced dopaminergic neurodegeneration: role of ATP depletion and procaspase-9 degradation. Apoptosis. 2012;17:449–462. doi: 10.1007/s10495-012-0699-0. [DOI] [PubMed] [Google Scholar]
- 20.Ali SF, David SN, Newport GD, Cadet JL, Slikker W., Jr MPTP-induced oxidative stress and neurotoxicity are age-dependent: evidence from measures of reactive oxygen species and striatal dopamine levels. Synapse. 1994;18:27–34. doi: 10.1002/syn.890180105. [DOI] [PubMed] [Google Scholar]
- 21.Smith TS, Bennett JP., Jr Mitochondrial toxins in models of neurodegenerative diseases. I: In vivo brain hydroxyl radical production during systemic MPTP treatment or following microdialysis infusion of methylpyridinium or azide ions. Brain Res. 1997;765:183–188. doi: 10.1016/s0006-8993(97)00429-0. [DOI] [PubMed] [Google Scholar]
- 22.Kalivendi SV, Kotamraju S, Cunningham S, Shang T, Hillard CJ, Kalyanaraman B. 1-Methyl-4-phenylpyridinium (MPP+)-induced apoptosis and mitochondrial oxidant generation: role of transferrin-receptor-dependent iron and hydrogen peroxide. Biochem J. 2003;371:151–164. doi: 10.1042/BJ20021525. [DOI] [PMC free article] [PubMed] [Google Scholar]