Abstract
Objectives To identify children demonstrating “good” sleep health in a sample of urban children with persistent asthma; to compare sociocontextual, asthma clinical characteristics, and sleep behaviors in children with “good” versus “poor” sleep health; and to examine protective effects of family-based health behaviors on sleep health. Methods Participants were 249 Black (33%), Latino (51%) and non-Latino White (16%) children with asthma, ages 7–9 years, and their primary caregivers. Results 32 percent of children had “good” sleep health. Well-controlled asthma and better lung function were more likely in this group. In the context of urban risks, sleep hygiene appeared to be a protective factor associated with better sleep quality. The protective effect of asthma management functioned differently by ethnic group. Conclusions This study identifies protective processes that may guard against urban risks to optimize sleep health in children with asthma. Intervention programs can be tailored to consider specific supports that enhance sleep health in this high-risk group.
Keywords: asthma, children, disparities, health behavior, race/ethnicity, resilience, sleep
Introduction
Urban Minority Children With Asthma: At Risk for Asthma Morbidity and Poor Sleep
Urban minority children with asthma are at greater risk for poor asthma outcomes (Centers for Disease Control and Prevention, 2007), particularly Black children, and Latino children from Puerto Rican and Dominican backgrounds (Lara, Akinbami, Flores, & Morgenstern, 2006). More ethnic minority children and families reside in urban areas, and these groups are exposed to poverty, environmental triggers (Kattan et al., 1997), acculturative stress, discrimination (Koinis Mitchell et al., 2007), and language and medication barriers (McQuaid et al., 2012), which affect asthma morbidity (Canino et al., 2006; Koinis Mitchell et al., 2007; McQuaid et al., 2012). Poorly controlled asthma can negatively affect all aspects of children’s daily functioning and behaviors, including sleep (Boergers & Koinis Mitchell, 2010).
Sleep Health Plays an Important Role in Children’s Functioning and Health
A child can achieve good sleep health when s/he obtains undisturbed, restorative sleep of sufficient duration according to developmental level (Buysse, 2014). Buysse (2014) has defined good sleep health as a multidimensional pattern of sleep-wakefulness characterized by subjective satisfaction, appropriate timing, adequate duration, high efficiency, and sustained alertness during waking hours. For the purposes of this article, given many studies with pediatric samples have not assessed sleep using multiple indicators, we focus on those including one or more of these sleep quality indicators to provide support for our study questions.
Good sleep health is essential for children’s optimal daytime functioning and physical health (Beebe, 2011; Dewald, Meijer, Oort, Kerkhof, & Bogels, 2010; Frederiksen, Rhodes, & Reddy, 2004; Hart, Palermo, & Rosen, 2005; McNeil, Doucet, & Chaput, 2013; Sadeh, Gruber, & Raviv, 2002). Children who have a chronic illness with a variable symptom presentation, particularly those not adherent to their treatment regimen, are at risk for poor sleep (Boergers & Koinis Mitchell, 2010). For example, poor asthma (increased symptoms and poor lung function) and poor quality sleep (e.g., sleep efficiency measured via actigraphy) is associated with difficulties with schoolwork and greater school absences (Koinis Mitchell et al., 2014).
Poor quality sleep also contributes to illness-related symptoms in children in other disease groups, such as juvenile rheumatoid arthritis (Bloom et al., 2002). Although, in general, there are few studies focusing on sleep in urban and ethnically diverse children, shorter sleep duration and inconsistent bedtimes have been found in children from urban and low income settings (Sheares et al., 2013; Spilsbury et al., 2004). This is of concern, given short sleep duration can have deleterious effects on children’s concurrent and future health outcomes (e.g., diabetes, obesity; Chaput, Brunet, & Tremblay, 2006; McNeil et al., 2013; Nixon et al., 2008). Further, risks associated with urban poverty (e.g., family stress affecting bedtime routines, crowded housing) can affect children’s sleep environment (noise, sleep disruptions; see Boergers & Koinis Mitchell, 2010) and sleep behaviors (e.g., inconsistent sleep/wake times; Spilsbury et al., 2004). Healthy minority children also are found to have short sleep duration and patterns (e.g., later bedtimes; LeBourgeois, Giannotti, Cortesi, Wolfson, & Harsh, 2005). Given urban children with chronic illnesses are prone to poor sleep, it is important to better understand what key factors related to illness and sleep serve as future intervention targets in this high-risk group.
Poor Sleep Health in Urban Children With Asthma
Children with asthma may wake often during the night when their asthma is poorly controlled (e.g., Daniel, Boergers, Kopel, & Koinis-Mitchell, 2012; Fagnano, Bayer, Isensee, Hernandez, & Halterman, 2011; Strunk, Sternberg, Bacharier, & Szefler, 2002). Nocturnal symptoms occur often with persistent levels of asthma and can disrupt children’s sleep continuity (e.g., Fitzpatrick et al., 1991; Lewis, Fagnano, Koehler, & Halterman, 2014). Cross-sectional studies have shown associations between reports of missed sleep related to symptoms and increased daytime sleepiness (Calhoun et al., 2011; Janson et al., 1996), poorer school performance, and more school absences (e.g., Diette, Markson, Skinner, Nguen, & Algatt-Bergstrom, 2000). Results from a few small studies using polysomnography also have shown that adults with asthma have longer sleep onset latency, than healthy controls (Crenshaw & Edinger, 1999).
Poor sleep also may affect the occurrence of asthma symptoms, although research to date is sparse and on small samples (Ballard, 1999). For example, in a small experimental study of adults with asthma, acute sleep restriction increased bronchoconstriction (Catterall et al., 1986). Thus, asthma can affect sleep, and sleep curtailment may influence nocturnal asthma (Ranjbaran, Keefer, Stepanski, Farhadi, & Keshavarzian, 2007).
Multilevel factors (pathophysiological, environmental, illness, and family-related) can contribute to the link between nocturnal asthma and sleep disruption (see review, Koinis Mitchell, Craig, Esteban, & Klein, 2012; Koinis Mitchell et al., 2015). Briefly, suboptimal controller medication adherence (Garrison, Lozano, & Christakis, 2011; McQuaid et al., 2012), and increased exposure to home environmental triggers (Morgan et al., 2004), pollen counts, and sleep posture, can facilitate mucus production and inflammation, symptoms, and awakenings (Martin & Banks-Schlegel, 1998; Meijer et al., 1995).
Good Sleep Health for Urban Children With Asthma
Although the risks contributing to poor sleep in urban children with asthma are well-described (Boergers & Koinis Mitchell, 2010; Koinis Mitchell, Craig, et al., 2012), our previous work emphasizes the importance also of studying characteristics of urban children and families who are functioning well in the face of heightened risk (Koinis Mitchell, McQuaid, et al., 2012). Identifying protective factors offers targets for culturally tailored interventions to meet the needs of specific ethnic groups, by incorporating characteristics that families already use and draw upon to manage an illness successfully (e.g., utilizing family connections to support children’s effective asthma management; Koinis Mitchell, McQuaid, et al., 2012). We have applied a strength-based approach to identify protective factors associated with optimal asthma outcomes, despite the presence of risks related to urban poverty (Koinis Mitchell, McQuaid, et al., 2012; Koinis Mitchell, Murdock, & McQuaid, 2004). Such approaches also have been applied in studies of other chronic diseases, such as diabetes (Hilliard, Harris, & Weissberg-Benchell, 2012) and sickle cell disease (Simon, Barakat, Patterson, & Dampier, 2009).
The definitions of resilience, risk, and protective factors involved in this strength-based, theoretical approach have been detailed elsewhere (Luthar, Cicchetti, & Becker, 2000), and these descriptions help to minimize the incorrect use of the terms. Briefly, resilience is defined as “a dynamic process encompassing positive adaptation within the context of significant adversity” (p. 543, Luthar et al., 2000). Resilience is demonstrated when positive outcomes occur in the presence of one or more risks (Hauser, Allen, & Golden, 2006; Luthar & Brown, 2007). Resilient-based outcomes (e.g., psychosocial competence) are achieved when protective factor(s) (e.g., self-competence, social support) minimize the effect of risk factors to contribute to a more optimal outcome (Deb & Arora, 2008; Luthar et al., 2000; Luthar & Goldstein, 2004; Masten, 2001). This framework has been used to study urban minority families of children with asthma (e.g., Koinis Mitchell, McQuaid, et al., 2012; Koinis Mitchell & Murdock, 2005), with a focus on protective factors across individual (e.g., child adaptability), family/cultural (e.g., family connectedness), and illness-related (e.g., child asthma management self-efficacy) levels that minimize asthma morbidity in the context of cumulative urban and asthma-related risks.
The Current Study
We apply this strength-based framework to understand what constitutes “good” sleep health in this high-risk group. We also focus on family-level protective factors, given childhood asthma is managed in the context of the family (e.g., McQuaid, Walders, Kopel, Fritz, & Klinnert, 2005), and children’s sleep health is most effectively supported by developmentally appropriate, healthy and consistent family sleep practices (e.g., consistent family bedtime routines, family support and monitoring of minimal bedtime distractions) (Mindell, Meltzer, Carskadon, & Chervin, 2009; Morgenthaler et al., 2006). Two potential illness and sleep-related processes particularly relevant for improving sleep health for children with asthma include family asthma management and sleep hygiene. Effective asthma management strategies (identification of symptoms, appropriate trigger control, consistent medication use) are necessary to minimize asthma morbidity (McQuaid et al., 2009; National Heart Lung and Blood Institute, 2007). An important indicator in the assessment of asthma control and severity is the extent to which nighttime asthma symptoms promote nocturnal awakenings (National Heart Lung and Blood Institute, 2007). Given that nocturnal awakenings related to asthma can disrupt sleep, effective asthma management strategies, particularly those strategies most relevant to optimal sleep (e.g., use of rescue inhaler in response to nighttime symptoms, trigger control), become an important modifiable target for intervention for urban children with asthma.
Sleep hygiene, the extent to which children and families practice consistent sleep behaviors and habits (e.g., consistent bedtime and wake time, removing disruptions in the child’s bedroom, eliminating caffeine before bedtime), has been found to influence sleep problems (Owens, Jones, & Nash, 2011) and self-reported sleep quality in children (LeBourgeois et al., 2005). Urban children may be at an increased risk for poor sleep hygiene, as poverty may increase exposure to risk factors that challenge families’ abilities to practice optimal sleep hygiene (Spilsbury et al., 2004). Sleep hygiene has been shown to have a protective effect on caregiver-reported sleep problems, in the context of asthma symptoms in urban children (Koinis Mitchell et al., 2015). To our knowledge, no published studies have examined the protective role of family-based health behaviors in the association between urban risks and sleep health measured through objective methods in urban children.
The current study involved two goals. We first sought to describe school-aged children (7–9 years) who have “good” sleep health in an urban sample of families from Black, Latino and non-Latino White (NLW) backgrounds, using objective methods and standard sleep quality indictors to characterize sleep health (Buysse, 2014). Using an approach applied in previous resilience research (Hauser et al., 2006), we then compared sociocontextual, and asthma clinical characteristics, as well as asthma-related and sleep behaviors in children with “good” versus “poor” sleep health, to identify risks, resources, and protective processes that may promote or challenge sleep health in this high-risk group. Asthma and sleep-related risk and protective factors were selected based on prior work, suggesting their relevance for optimal sleep and asthma in urban children (Koinis Mitchell, Craig, et al., 2012; Koinis Mitchell et al., 2015).
The second goal was to examine whether, in the entire sample, family-related asthma management and sleep hygiene behaviors served protective functions in their association with sleep health, in the context of urban risks. We expected higher levels of family asthma management and sleep hygiene would each exert a protective function on sleep health for all children, regardless of ethnic background. However, we expected these associations to be more robust in NLW children, given Latino and Black children face higher levels of asthma morbidity and more urban risks compared with their NLW peers (Koinis Mitchell et al., 2007).
Methods
Data for this study were collected from a larger study, Project Nocturnal Asthma and Performance in School, that assesses the co-occurrence of asthma and allergic rhinitis (AR) symptoms, sleep quality, and academic functioning in urban children (7–9 years of age), with persistent asthma (R01 HD057220, Koinis Mitchell, PI) across one academic year. The current study includes data from asthma participants who completed study participation in one of the first 4 years of the study.
Recruitment of participants occurred in the four largest and adjacent urban school districts in an urban Northeastern US city, in hospital-based, ambulatory pediatric clinics, and in a hospital-based asthma educational program. “Consent-to-contact” forms (signed by caregivers providing permission for study personnel to call the family) were distributed across recruitment settings. Research assistants screened families by phone to determine eligibility.
Eligibility criteria for the study required that the child’s age be between 7 and 9 years, child’s legal guardian is willing to participate, caregiver ethnicity is self-identified as Latino (Dominican or Puerto Rican), NLW, or Black, child attended public school in one of four targeted school districts, and that the child had physician-diagnosed asthma or breathing problems in the previous 12 months. Additionally at screening, each child met persistent asthma status, either by current prescription of an asthma controller medication, and/or by parent-reported recurrent daytime or nighttime symptoms, activity limitation, rescue medication use, or two or more oral steroid bursts in the previous 12 months (National Heart Lung and Blood Institute, 2007). Exclusionary criteria included moderate to severe cognitive impairment as determined by school placement, use of stimulant medication for attention deficit/hyperactivity disorder, another pulmonary or chronic health condition, or a diagnosed sleep disorder (e.g., restless leg syndrome) that would confound the primary hypotheses of the larger study.
Data included in the current study were collected during the fall/ early winter period of each study year (Figure 1). The initial study visit included the informed consent (parents) and assent (children) process, collection of demographic information, review of child’s prescribed medications, and a standardized interview about the family’s asthma management practices. The second session occurred at a hospital-based asthma and allergy clinic at least 2 weeks later. Asthma and AR diagnosis and severity, and allergy status were evaluated by the study clinicians, and asthma and AR medication use was confirmed. Immediately following this visit, children and their caregiver participated in a 4-week home-monitoring period in which they logged the child’s asthma symptoms twice daily in a diary, and during which the child wore an actigraph and used a handheld spirometer to assess sleep quality and lung function, respectively. Midway through the monitoring period, study staff conducted a home visit to download and review electronic sleep and lung function data and to administer the sleep hygiene measure. Staff implemented standard procedures to encourage protocol adherence and to insure the appropriate use of the electronic devices and daily diary completion.
Figure 1.
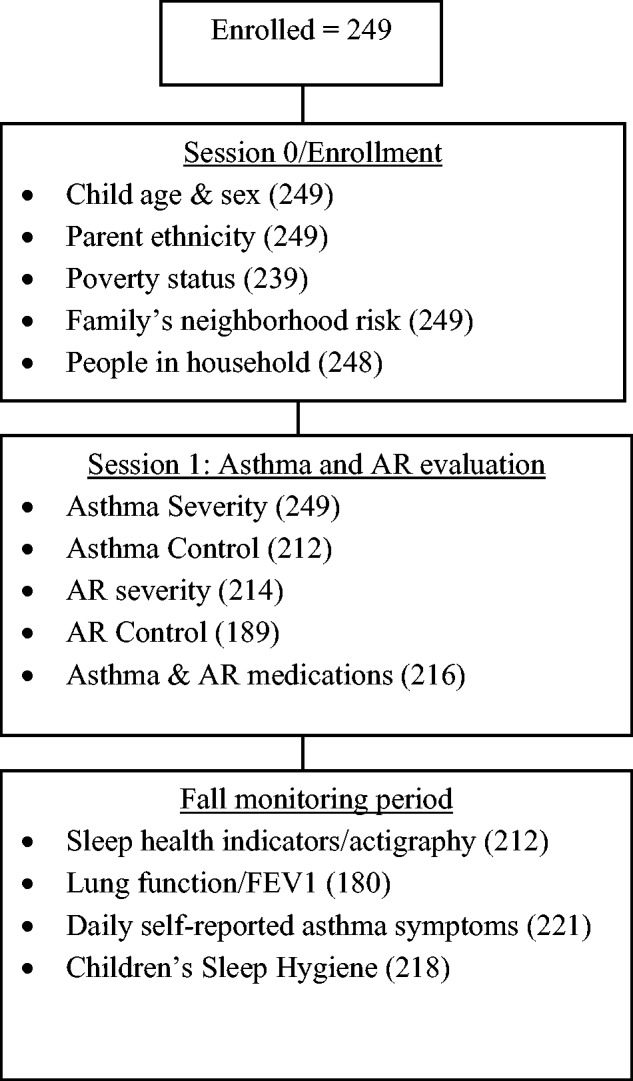
Data Collection Time Points and Number of Cases with Valid Data, by Assessment.
All research visits except the clinical evaluation were completed in families’ homes. Free transportation to the clinic visit was available. Assessments were administered verbally in English or Spanish according to participant preference. Standardized procedures were used for measure translation (Canino & Bravo, 1994). Compensation was provided at all visits. The study was approved by the local institutional review board.
Measures
Sociocontextual Risks: Demographic and Descriptive Information
Primary caregivers provided key demographic information (see Table I). Poverty status was determined by dividing the family’s annual income by the US federal per capita poverty threshold for a family of that size (Brooks-Gunn & Duncan, 1997; U.S. Department of Health and Human Services, 2005).
Table I.
Sociocontextual, Asthma Clinical Characteristics, and Family-Based Behaviors of Participants
| Characteristics of sample | Sample | Latino | Black | Non-Latino White | Ethnic group differencesa | Effect sizesb |
|---|---|---|---|---|---|---|
| n | 249 | 127 | 83 | 39 | – | – |
| Sociocontextual characteristics | ||||||
| Child age in years, M (95% CI) | 8.3 (7.9–8.5) | 8.3 (8.1–8.5) | 8.3 (8.1–8.5) | 8.2 (7.9–8.5) | F(2,246) < 1.0 | = .01 |
| Male (%) | 53 | 51 | 60 | 39 | χ2 = 5.1 | ϕc = .14 |
| Caregiver race/ethnicity (%) | ||||||
| Black | 33 | – | – | – | – | – |
| Latino | 51 | – | – | – | – | – |
| Non-Latino White | 16 | – | – | – | – | – |
| At/below poverty threshold (%) | 69 | 81 | 64 | 38 | χ2 = 26.1** | ϕc = .33 |
| Neighborhood Risk Index, M (95% CI) | 5.1 (4.8–5.3) | 5.6 (5.3–5.9) | 5.3 (4.9–5.8) | 3.0 (2.2–3.8) | F(2,242) = 26.2** | = .17 |
| Total number of people in household, M (95% CI) | 4.6 (4.4–4.8) | 4.7 (4.4–5.0) | 4.5 (4.1–5.0) | 4.4 (4.0–4.9) | F(2,245) < 1.0 | = .00 |
| Asthma clinical characteristics | ||||||
| Asthma severity (%) | χ2 = 6.6 | ϕc = .12 | ||||
| Mild persistent | 55 | 57 | 50 | 63 | ||
| Moderate persistent | 32 | 32 | 30 | 34 | ||
| Severe | 13 | 11 | 20 | 3 | ||
| Asthma poorly controlled (%) | 42 | 35 | 53 | 38 | χ2 = 5.6 | ϕc = .16 |
| Allergic rhinitis severity (%) | χ2 = 1.8 | ϕc = .06 | ||||
| Mild | 21 | 19 | 21 | 27 | ||
| Moderate | 58 | 59 | 55 | 59 | ||
| Severe | 22 | 22 | 24 | 15 | ||
| Rhinitis symptoms poorly controlled (%) | 38 | 39 | 42 | 24 | χ2 = 2.7 | ϕc = .12 |
| Asthma medications (%) | ||||||
| Any controller medication | 80 | 79 | 84 | 74 | χ2 = 1.5 | ϕc = .08 |
| Inhaled corticosteroid | 72 | 72 | 77 | 63 | χ2 = 2.4 | ϕc = .10 |
| Medication adherence (FAMSSc), M (95% CI) | 5.4 (5.1–5.6) | 5.5 (5.2–5.8) | 5.1 (4.7–5.5) | 5.4 (4.7–6.0) | F(2,223) = 1.1 | = .01 |
| Allergic rhinitis medications (%) | ||||||
| First-generation antihistamine | 18 | 19 | 19 | 11 | χ2 = 1.1 | ϕc = .07 |
| Family-level asthma and sleep behaviors | ||||||
| Family asthma management, M (95% CI) | 4.8 (4.6–4.9) | 4.9 (4.7–5.1) | 4.7 (4.4–5.0) | 4.6 (4.2–5.0) | F(2,226) = 1.5 | = .00 |
| Children’s sleep hygiene,d M (95% CI) | 4.5 (4.4–4.6) | 4.3 (4.3–4.5) | 4.5 (4.3–4.6) | 4.7 (4.5–4.9) | F(2,215) = 3.4* | = .02 |
| Sleep health indicators | ||||||
| Sleep efficiency (%), M (95% CI) | 86.5 (86.1–87.1) | 86.9 (86.2–87.6) | 85.8 (84.9–86.6) | 87.1 (85.9–88.3) | F(2,209) = 2.6 | = .02 |
| Sleep duration (hours), M (95% CI) | 9.3 (9.2–9.3) | 9.1 (9.0–9.2) | 9.3 (9.1–9.4) | 9.6 (9.4–9.8) | F(2,209) = 9.6** | = .08 |
| Mean number of awakenings per night, M (95% CI) | 5.4 (5.0–5.7) | 5.2 (4.7–5.7) | 5.6 (5.0–6.2) | 5.2 (4.4–6.0) | F(2,211) < 1.0 | = .01 |
aAnalyses of variance effect sizes are partial omega squared (); chi square effect sizes are Cramer’s Phi (ϕc).
bIn all statistically significant ethnic group analyses, the NLW group significantly differed from the other groups.
cFamily Asthma Management System Scale. Scores range from 1 to 9. Higher ratings indicate better management on each domain and the total score.
dSleep hygiene total score is the mean across items ranging from 1 to 6. Higher scores indicate better hygiene.
*p < .05; **p < .01; ***p < .001.
Neighborhood Risks
A neighborhood risk score was computed based on participant home address, which was geocoded for census block group membership. Data from the 2010 census characterizing the block groups were used to identify presence of each of eight risk factors: per capita income, less than high school education, unemployment, racial/ethnic minority status, vacant housing, small housing, poverty status, and non-English speaking. Each participant could receive a risk score from 0 to 8, based on the number of risk characteristics identified. This variable has been used in our previous pediatric asthma disparities research and it has been shown to correlate with asthma outcomes in urban children (Canino et al., 2009; 2012).
Clinical Characteristics: Asthma and AR Diagnosis and Severity Classification
The clinic study visit consisted of a medical history and physical examination, allergy testing, and pulmonary function testing. Confirmation of asthma and classification of severity were made by a study clinician using standard NHLBI EPR-3 guidelines (National Heart Lung and Blood Institute, 2007). Current asthma and allergy medication use was documented, and caregivers provided ratings indicating how often their child missed doses of daily controller medications on a scale from 1 (misses doses all of the time) to 5 (never misses doses). AR status and severity level were evaluated using standard clinical guidelines (Bender & Milgrom, 2004; Bousquet et al., 2008) and study procedures that are detailed elsewhere (Esteban et al., 2014). Two hundred and seventy-seven children with asthma enrolled in the study, of which 249 had persistent asthma. The 28 enrollees omitted owing to intermittent asthma status did not differ from those retained by sex, age, poverty level, or racial/ethnic background.
Physician Query
Child participants’ primary care providers and (when applicable) asthma/allergy specialists completed a checklist detailing the date of child’s last office visit and previous and current medical status. The query was used by the study clinician to evaluate the child’s current asthma and allergy status (Esteban et al., 2014; Koinis Mitchell et al., 2015).
Asthma Control
Parents and children completed the Asthma Control Test (Liu et al., 2007), a well-validated questionnaire of asthma-related impairment. Using standardized procedures (Nathan et al., 2004), we dichotomized scoring using a total cutoff score of 19; those below were considered to have poor asthma control, and those above to have well controlled asthma. Internal consistency in our sample was moderate (Cronbach’s α = .70).
AR Control
Parents completed the Rhinitis Control Assessment Test (Schatz et al., 2010), a well-validated questionnaire that assesses rhinitis disease control in children. Respondents rate on a 5-point scale the frequency of common symptoms and symptom control (e.g., nasal congestion) in the previous week. A dichotomous variable (well/not well controlled) also was computed for descriptive purposes as in our previous work (Koinis Mitchell et al., 2015), using a cutoff value of 21 (Schatz et al., 2010). Sample internal consistency was good (Cronbach’s α = .78).
Daily Self-Reported Asthma Symptoms
Families completed a diary twice daily to document the child’s breathing problems during the night or day using standard procedures (Esteban et al., 2014; Koinis Mitchell et al., 2015). Diary-reported asthma symptoms were summarized as the proportion of monitored days during the fall monitoring period during which any breathing problems were noted (Koinis Mitchell et al., 2015).
Lung Function: FEV1 Percent Predicted
Children’s lung function was measured twice daily by a hand-held computerized spirometer (Jaeger AM2; VIASYS Healthcare; Yorba Lina, CA). The best of three FEV1 % predicted values per trial were retained. A series of data cleaning and reduction steps were employed (Koinis Mitchell et al., 2009). Data were downloaded at the home during the mid-point and end of the monitoring period. Parents and children were oriented to the proper use of the device as well as how to conduct spirometry, using standard procedures (Koinis Mitchell et al., 2009). Participants were instructed to complete three “blows” before any asthma or allergy medications in the morning and at night.
Sleep Health Indicators
Participants wore an actigraph on their non-dominant wrist for the monitoring period (MiniMitter Company, Bend, OR, USA). One-minute epochs were estimated as sleep or wakefulness with Actiware-Sleep V 2.53 software using activity levels produced in the surrounding 2-minute interval (medium sensitivity). This algorithm was applied to portions of the record identified as sleep through a combination of diary reports and actigraph event markers set by participants at “lights-off” and “lights-on.” In comparison with polysomography, this algorithm shows high overall epoch-by-epoch agreement and is excellent in detecting sleep (sensitivity = 94%); however, it overestimates wake during the sleep period (specificity = 69%) (Meltzer, Walsh, Traylor, & Westin, 2012). To inform scoring of sleep data, families recorded in the aforementioned daily diary instances when the child was sick with an illness other than asthma, plus morning wake times and evening bedtimes, and times when the actigraph was not worn (Acebo et al., 1999). Standard scoring rules were then applied to each sleep episode (Acebo et al., 2005). Episodes were excluded when (1) the actigraph was off for all/part of the sleep period, (2) the concurrent diary report was not available, or (3) there was a diary-reported illness other than asthma that could have affected sleep on a given night. Actigraphy data were not available for 37 children due to protocol nonadherence (n = 34) or device loss/technical failure (n = 3). There were no differences between those with and without sleep data on demographic (sex, age, ethnicity, poverty) or clinical (asthma and AR severity) characteristics. Actigraphy data were available for 212 children, with an average of 18 scorable nights (SD = 8, range = 2–40).
Three sleep quality variables were computed as the aggregate across the monitoring period, specifically, sleep efficiency, defined as the % time asleep/total time in bed; sleep duration, defined as total time between evening sleep onset and morning waking, and mean # of awakenings per night of at least 3 minutes in duration.
Sleep Health Categories
To address our first question, we identified children who qualified for “good” sleep health using two methods. We identified cut-points using two sleep quality indicators from our actigraphy measurement: sleep efficiency and sleep duration (Buysse, 2014). We based our cut-points on results from studies that have used actigraphy both with healthy children and urban samples. Published norms for this age range are 90–95% (Aronen, Paavonen, Soininen, & Fjallberg, 2001; Scholle et al., 2011). For sleep duration, guidelines recommend that children between the ages of 7 and 9 years should sleep 10.5–11 hr per night (Ferber, 1996; Mindell & Owens, 2003). Further, children in this age-group who sleep fewer than 9 hr per night have been found to be at an increased risk for poor health outcomes (Paavonen et al., 2009). The other method we used for developing cut-points involved examining sample means for sleep efficiency (87%) and duration (9 hours). We set lower thresholds for “good” sleep health on both indicators than what is suggested for healthy children of this age-group, given the urban context of our sample and the persistent nature of their asthma. Of note is that only 33 (16%) of children in our sample met the criterion for healthy sleep efficiency (90%) based on previous studies, and no children met both this and the criterion of at least 10.5 hr sleep duration based on recommended guidelines. A categorical variable was created to characterize children as having “good” and poor sleep health. Good sleepers were defined as having (1) a mean sleep efficiency score from the actigraphy data ≥87% and (2) mean sleep duration of ≥9 hr per night.
Family-Based Behaviors: Family Asthma Management
The Family Asthma Management System Scale (FAMSS; Klinnert, 1997; McQuaid et al., 2005) is a semistructured interview covering multiple domains of asthma management, including symptom recognition, response to symptoms, medication adherence, and environmental trigger control. Trained interviewers rated participants on each domain, using a scale from 1 (poor) to 9 (optimal); a total score is the mean across domains. The FAMSS has been used in cross-sectional and treatment outcome studies, and has been validated with minority samples (Celano, Klinnert, Holsey, & McQuaid, 2011) and in English and Spanish (Canino et al., 2009). The domains and the total score correlate with objectively measured controller medication adherence (McQuaid et al., 2005). Internal consistency has been very good in prior studies (Cronbach’s α = .84, McQuaid et al., 2005) and in the current sample (Cronbach’s α = .76).
Children’s Sleep Hygiene Scale
This 22-item measure examines parent report of sleep hygiene (Harsh, Easley, & LeBourgeois, 2002), with items covering behaviors relating to hygiene and behaviors affecting sleep initiation and maintenance. Responses are on a 6-point scale, from never to always. Higher scores indicate better hygiene. The scale has acceptable internal consistency when used with school-aged children (Cronbach’s α = 0.76; Harsh et al., 2002) and in our sample (Cronbach’s α = .70).
Analysis Plan
We first evaluated the proportion of children in the sample exhibiting “good” sleep quality, defined as those with a mean sleep efficiency of >87% and who typically slept at least 9 hr per night during the monitoring period. Cases that did not meet both criteria were classified as having “poor” sleep quality. We then sought to assess the extent to which sociocontextual risk factors (e.g., poverty status, neighborhood risk, environmental trigger control), asthma indicators (e.g., asthma severity, asthma control lung function), and family-based, asthma-related, and sleep behaviors (family asthma management and sleep hygiene) differed as a function of good/poor sleep health, using analyses of variance.
We then assessed whether family asthma management or sleep hygiene functioned as protective factors in their association with sleep quality indicators (efficiency, duration, wakings) in the face of urban risks using a standard approach involving testing the moderating role of each process (Luthar et al., 2000). This allows for identification of both resource (i.e., indicated by a main effect) and protective factors (i.e., indicated by an interaction effect).
Pearson’s correlations and chi square tests were also used to evaluate child age, sex, asthma, and AR severity as potential covariates in associations with protective factors and sleep health variables. Family-level poverty and primary caregiver education level (Table I) are related to components of the risk index development and thus were not assessed as potential covariates. Given our plan involved testing the moderational models proposed by ethnic group, we did not include ethnicity as a covariate; however, we note ethnic differences in poverty, severity, and in each of the predictor and outcome variables studied (Table I). Using hierarchical regression analyses and adjusting for covariates as appropriate, the separate contribution of each protective process (e.g., asthma management, sleep hygiene) on each sleep health indicator was tested. Specifically, neighborhood risk (geocode) was entered first, to control for level of risk. Next entered was the potential protective process (i.e., the family asthma management total score or the sleep hygiene total score, indicating a main effect or that the process was a “resource factor”), followed by the mean-centered interaction term of risk and that protective factor in the final step. Lastly, we probed significant interaction effects (e.g., did the levels of protective process differ for individuals under low, medium, or high levels of neighborhood risk?; Koinis Mitchell, McQuaid, et al., 2012; Koinis Mitchell et al., 2004; Luthar et al., 2000). Analyses were conducted for the overall sample and stratified by ethnic group.
Before analyses, variables found to violate assumptions of normality were transformed using appropriate procedures (Tabachnick & Fidell, 1996). Offending variables were Neighborhood Risk and Diary-Reported Symptoms, both of which remained significantly non-normally distributed after data transformation. In analyses examining group differences (i.e., ethnic group and sleep health categories), results did not change based on the use of the transformed or untransformed variables. Hence, to facilitate interpretation of analyses by group, we retained these variables in their original form for all analyses. An alpha level of p < .05 was used for all statistical tests, which were conducted with SPSS Version 20. Effect sizes for analyses of variance were expressed as partial omega squared (), interpreted as small (0.01), medium (0.06), or large (0.14; Cohen & Williamson, 1998). Chi square effect sizes are expressed as Cramer’s Phi (ϕc), the interpretation of which is akin to a point-biserial correlation. R2-adjusted are presented for multiple regression results.
Results
Preliminary Analyses
Table I details sociocontextual and clinical characteristics of 249 participants with persistent asthma, with a mean age of 8.3 years (SD = 0.90) and 53% male. Forty-two percent of children had poorly controlled asthma. There were no differences in sleep efficiency or duration by asthma severity. There was a trend for those with severe asthma to have more night awakenings (M = 6.2, SD = 3) relative to those with mild persistent (M = 5.0, SD = 2.2) and moderate persistent (M = 5.7, SD = 2.8) asthma (F(2, 196) = 3.0, p = .05, = .02).
Ethnic Differences in Participant Characteristics
A smaller proportion of NLWs (38%) were below the poverty threshold, compared with Black (64%) and Latino (81%) participants (χ2 = 26.1, p < .001, ϕc = .33). NLW participants had the lowest levels of neighborhood risk (M = 3, SD = 2.3) relative to Blacks (M = 5.3, SD = 2.0) and Latinos (M = 5.6, SD = 1.7; (F(2, 242) = 26.2, p < .001, = .17). Modest but significant group differences also emerged for the sleep hygiene total score, specifically, scores were highest in NLWs (M = 4.7, SD = 0.6) compared with Blacks (M = 4.5, SD = 0.6) and Latinos (M = 4.4, SD = 0.6; F(2, 216) = 3.4, p < .05, = .02). No ethnic group differences were found for asthma severity or for family asthma management.
Participant Characteristics by Good/Poor Sleep Health Categories
We first examined the proportion of our sample characterized as having good or poor sleep health by our study definitions. Over two-thirds of participants (68%) were classified as having poor sleep health. We next evaluated whether there were differences in sociocontextual, asthma clinical characteristics, and family-based behaviors by sleep health category (Table II). While no sociocontextual variables differed by group, there was a trend for race/ethnicity, with 47% of NLWs being classified into the good sleep group, versus 31% of Latinos and 25% of Blacks (χ2 = 4.7, p = .07, ϕc = .15). Of the asthma indicators, lung function was higher in participants with good sleep health (M FEV1 = 87% predicted, SD = 12%) relative to those with poor sleep health (M = 81%, SD = 13%; F(1, 171) = 6.3, p < .05, = .03). Asthma control differed across groups (χ2 = 5.7, p < .05, ϕc = .17), specifically 46% of those with poor sleep health had poor asthma control, relative to 27% of those achieving good sleep health.
Table II.
Sociocontextual, Asthma Clinical Characteristics, Family-Based Behaviors by and Across Sleep Health
| Characteristics of sample | Sample | Good sleep health | Poor sleep health | Sleep group differences | Effect sizesa |
|---|---|---|---|---|---|
| Cases with valid sleep data, n (%) | 212 | 67 (32) | 145 (68) | – | – |
| Sociocontextual factors | |||||
| At/below poverty (%) | 69 (63–76) | 67 (55–79) | 70 (62–78) | F(1,202) < 1.0 | = .00 |
| Neighborhood risk index, M (95% CI) | 5.1 (4.8–5.4) | 5.0 (4.5–5.5) | 5.1 (4.8–5.5) | F(1,209) < 1.0 | = .00 |
| Ethnicity (%) | χ2 = 4.7† | ϕc = .15 | |||
| Latino | – | 31 | 69 | ||
| Black | – | 25 | 75 | ||
| NLW | – | 47 | 53 | ||
| Caregiver education in years, M (95% CI) | 12.2 (11.9–12.5) | 12.1 (11.5–12.7) | 12.2 (11.8–12.6) | F(1,209) < 1.0 | = .00 |
| Number of people in household, M (95% CI) | 4.7 (4.4–4.9) | 4.9 (4.4–5.3) | 4.6 (4.3–4.9) | F(1,210) = 1.1 | = .00 |
| FAMSS environmental control, M (95% CI) | 3.9 (3.6–4.3) | 3.8 (3.2–4.5) | 4.0 (3.5–4.4) | F(1,197) < 1.0 | = .01 |
| Smoke exposure (%) | χ2 = .13 | ϕc = .03 | |||
| None | 61 | 62 | 61 | ||
| Intermittent | 13 | 12 | 14 | ||
| Regular | 25 | 26 | 68 | ||
| Asthma indicators | |||||
| Asthma severity (%) | χ2 = 5.1† | ϕc = .02 | |||
| Mild persistent | 55 | 67 | 50 | ||
| Moderate persistent | 31 | 24 | 35 | ||
| Severe | 14 | 10 | 16 | ||
| Diary-reported asthma symptoms, % monitored days (95% CI) | 13 (11–16) | 11 (7–14) | 14 (11–17) | F(1,208) = 2.3 | = .01 |
| FEV1 % predicted, M (95% CI) | 83.2 (81.2–85.1) | 86.5 (83.2–89.8) | 81.4 (79.1–83.8) | F(1,171) = 6.3* | = .03 |
| Poor asthma control (%) | 40 | 27 | 46 | χ2 = 5.7* | = .17 |
| Prescribed asthma controller medication (%) | 80 | 76 | 81 | χ2 = .5 | ϕc = .05 |
| Medication adherence, M (95% CI) | 5.3 (5.1–5.6) | 5.4 (4.9–5.9) | 5.3 (5.0–5.6) | F(1,193) < 1.0 | = .00 |
| Asthma and sleep behaviors | |||||
| Family asthma management, M (95% CI) | 4.8 (4.7–5.0) | 4.7 (4.4–5.0) | 4.9 (4.7–5.0) | F(1,195) < 1.0 | = .00 |
| Sleep hygiene total score, M (95% CI) | 4.5 (4.4–4.6) | 4.6 (4.4–4.7) | 4.5(4.4–4.5) | F(1,207) = 2.6 | = .01 |
aAnalyses of variance effect sizes are partial omega squared (); chi square effect sizes are Cramer’s Phi (ϕc).
†p < .08; *p < .05; **p < .01; ***p < .001.
Identification of Resources/Protective Factors Associated With Good Sleep Quality in the Context of Neighborhood Risk
As a preliminary step, Pearson’s correlations were examined between demographic variables and clinical characteristics (child sex, age, asthma, and AR severity) and the proposed protective factors (family asthma management and sleep hygiene total scores) and sleep quality indicators, to identify potential covariates for moderation analyses. Variables correlated both with a potential protective factor and a sleep health indicator were controlled in subsequent analyses when warranted. AR severity was correlated with the sleep hygiene score (r = −.14, p < .05), sleep efficiency (r = −.23), and number of night awakenings (r = .22, p values < .01). In analyses by ethnicity, asthma severity was associated with the family asthma management score (r = .36, p < .05) and number of night awakenings (r = .35, p < .05) in NLWs. AR and asthma severity were thus treated as covariates in specific models.
Family-Related Behaviors: Family Asthma Management
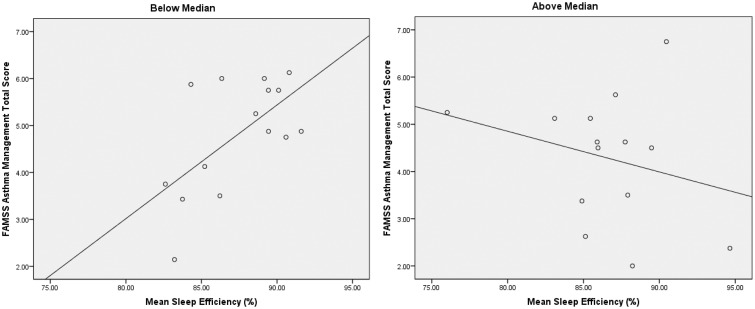
In analyses examining family asthma management, this process did not emerge as a resource or protective factor in the sample as a whole or in the Latino and Black subsamples. In the NLW group, family asthma management emerged as a significant protective factor, as evidence by a significant interaction with neighborhood risk in models examining sleep efficiency (β = −.44, p < .05, R2 adjusted = .12) and night awakenings (controlling for asthma severity, β = .51, p < .01, R2 adjusted = .36). In the model assessing sleep efficiency, post hoc probing was undertaken by dichotomizing neighborhood risk by median split and then conducting follow-up regression analyses examining the relationship between asthma management and sleep efficiency at lower and higher levels of risk. In participants below the risk median, there was a strong positive relationship between asthma management and sleep efficiency (β = .62, p < .05, R2 adjusted = .33), whereas there was a weaker, nonsignificant relationship in participants above the median (β = −.27, ns), Figure 2. In the model assessing awakenings, a similar pattern emerged from post hoc tests of the significant interaction between asthma management and neighborhood risk (Table III).
Figure 2.
Family Asthma Management and Sleep Efficiency by Neighborhood Risk (Median Split)—Non-Latino White Participants.
Table III.
Neighborhood Risk, Family-Based Resource/Protective Factors, and Sleep Health Outcomesa
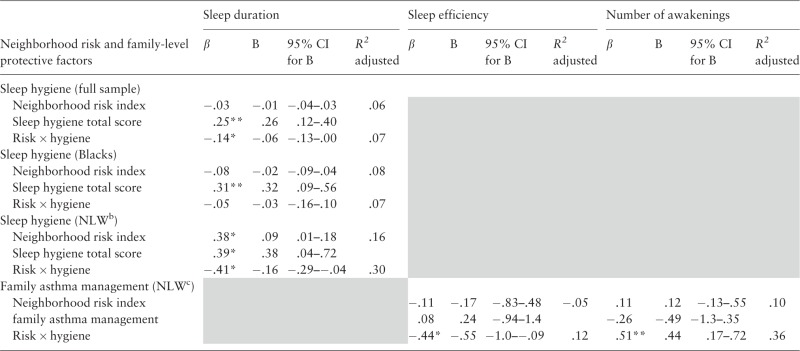 |
aResults for models with nonsignificant results are not shown.
bNon-Latino White.
cAsthma severity was a covariate in the model assessing mean number of nightly awakenings and in the NLW group.
*p < .05; **p < .01.
Sleep Hygiene
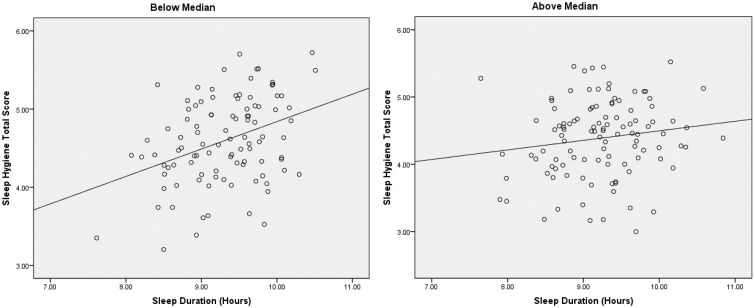
Results of regression analyses appear in Table III. In the full sample, sleep hygiene exerted a resource function in its relationship with sleep duration, as evidenced by a significant main effect (β = .25, p < .001; R2 adjusted = .06). There also was support for sleep hygiene as a protective factor, indicated by a significant interaction with neighborhood risk on the last step (β = −.14, p < .05; R2 adjusted = .07). To further explore the interaction, neighborhood risk was dichotomized by median split, and follow-up regression analyses were conducted examining sleep hygiene and sleep duration at lower and higher levels of risk (Figure 3). For participants below the median, better sleep hygiene was related to longer sleep duration (β = .37, p < .001; R2 adjusted = .12), whereas no relationship emerged in the group with risk scores above the median (β = .15; R2 adjusted = .01).
Figure 3.
Sleep Hygiene and Sleep Duration by Neighborhood Risk (Median Split).
In analyses stratified by ethnic group, NLWs evidenced the same protective and resource relationships between sleep hygiene and sleep duration as in the full sample (Table III). In the Black group, there was a significant main effect for sleep hygiene in its relationship with sleep duration (β = .31, p < .01, R2 adjusted = .08). The interaction in this model was not significant (β = −.05), suggesting that level of neighborhood risk did not impact the relationship for Black participants. There were no significant main effects or interactions in the Latino group.
Discussion
The current study applies a resilience-based approach to characterize urban children with asthma who demonstrate “good” sleep health in the context of persistent asthma and urban risks. We applied two methods used in resilience research to characterize those who demonstrate an optimal outcome, and to identify risk and protective factors that may be associated with optimal outcomes in children. Identifying which children do well and how in the face of risks are fundamental questions to pursue in studies applying a resilience-based, theoretical approach (Hauser et al., 2006; Luthar et al., 2000; Masten, 2001). Thirty-two percent of urban children with asthma in our sample qualified for good sleep health based on our study criteria. It is noteworthy that eligibility for inclusion in our larger study was not based on sleep but on persistent asthma and urban status. In light of this, the fact that a third of participants met both criteria for good sleep health suggests that some urban families implement healthy sleep behaviors, even in the context of urban poverty and managing a chronic illness.
Following guidelines set by Hauser and colleagues (2006), we then described the sociocontextual and asthma clinical characteristics, as well as the family-based behaviors relevant to asthma and sleep that distinguished good versus poor sleepers. This is a useful first-step in understanding which factors may function as risk and resources for specific outcomes in high-risk groups (Hauser et al., 2006). Results from these efforts suggest that the likelihood of children meeting criteria for good sleep health in our sample based on potential differences in financial resources was low, as sociocontextual factors did not differ in the two groups. Further, children who demonstrated good sleep health were more likely to have better lung function and better asthma control than their peers who had poorer sleep health.
Our findings also suggest that, even when controlling for risks, sleep hygiene exerted a protective effect on sleep quality in our entire sample. Thus, even in the context of multiple urban risks, developmentally appropriate and effective sleep hygiene practices were associated with more optimal sleep health as measured by objective methods. This is in line with results from intervention studies showing that more effective sleep hygiene improves sleep quality in children (Tan, Healey, Gray, & Galland, 2012).
Although family-level poverty did not differ in the groups of children who demonstrated good versus poor sleep health, it is unclear the extent to which the child’s sleep environment differed (multiple children in one room, etc.). The association between sleep hygiene and more optimal sleep health appeared to be stronger in the context of lower levels of neighborhood risks. In our future work, we will identify which specific family sleep practices are supportive of more effective sleep hygiene behaviors to apply this information to structure tailored sleep interventions for this group. For example, it would be useful to identify which strategies help some families administer bedtime routines and bedtimes consistently, even in the midst of everyday stress. Better asthma control was also demonstrated in the “good” sleepers of this sample, although sleep hygiene did not differ in children who demonstrated good versus poor control. It may be that maintaining better asthma control allows children and families to concentrate on better sleep hygiene practices.
Contrary to our expectations, in the entire sample, more effective family asthma management strategies did not exert a protective effect on sleep quality, in the context of risks. Consistent with our prediction, however, higher levels of family asthma management were associated with more optimal sleep quality in children of NLW backgrounds, despite the presence of risks. It is important to note that there were no differences in how families managed asthma by ethnic group. On average, families across the sample exhibited less than optimal asthma management strategies (the average score of our sample was 4.8 on a 9-point scale with higher scores indicating better management). Scores were also less than optimal (and consistent across ethnic groups) on this measure’s asthma medication adherence domain. This suggests that in our sample, there may have been limited variability in the family asthma management scores, which may have affected the protective effect of family asthma management on sleep quality. Further, families from NLW backgrounds had lower community-level risk scores, relative to the ethnic minority families in the sample, which may have played a role in why family asthma management exerted a protective effect on sleep in the NLW children of our sample. In light of previous research showing how caregivers’ perceptions of neighborhood risks may challenge families’ abilities to manage asthma effectively (Coutinho, McQuaid, & Koinis-Mitchell, 2013; Everhart et al., 2011), our community-level multiple risk indicator may have captured risks that were beyond some families’ abilities to control.
Moreover, asthma severity differed by ethnic group in our sample; children from Latino and Black backgrounds had more severe levels of persistent asthma than NLW children. This suggests that a higher frequency of symptoms experienced in Latino and Black families may have induced more frequent wakings, as demonstrated by actigraphy. It may be that there are specific aspects of asthma management that are more important for nocturnal asthma symptoms, and this may have a bearing on specific aspects of sleep quality. For example, differences in access and use of asthma medications by ethnic groups have been shown, and minority children tend to have less optimal medication adherence (McQuaid et al., 2012). It is possible that there are differences in nighttime asthma management practices that may be relevant to nocturnal symptoms and sleep, and more closely tied to sleep quality (e.g., is the rescue inhaler ready and available for use if the child is symptomatic).
Study Limitations and Suggestions for Future Research
Several limitations of this study should be considered and addressed in future research. First, the thresholds for defining sleep quality in this sample were, in part, based on the average sleep duration and quality indicators for this sample, so they may not be generalizable to all groups. Although our work contributes to what is currently known about sleep quality in urban children with asthma using a daily, objective measurement, we did not include a comprehensive assessment of sleep health as suggested by Buysse (2014). Our future work will include additional assessments of sleep including sleep satisfaction, bedtime/wake times, and alertness during waking hours. Finally, although we identified a proportion of children with good quality sleep, it is important to examine how they fare in terms of other aspects of functioning, such as academic performance.
Moreover, although we collected daily measurements of sleep and asthma, we used summary variables (means across the monitoring period) for each indicator. Therefore, the cross-sectional design of this study poses limitations for testing important causal associations. There are other processes across multiple levels (biological, environmental, family/cultural, illness-related) that may affect sleep quality, which were not assessed in this study. As noted earlier, future work should assess risk and protective processes closely linked to nighttime sleep, such as risks for increasing nocturnal asthma (e.g., environmental triggers in the child’s bedroom and exposure to secondhand tobacco smoke in the household, rescue medication use and availability, medication adherence). Family and cultural beliefs about sleep behaviors, and specific aspects of the sleep environment (sleep location, noise) that may support effective sleep hygiene and quality need to be further assessed in this group.
Participating families of this study were a convenience sample, which could have introduced sample bias on the study results. Although we considered the role of AR control on study questions, other co-morbid conditions of asthma need to be considered, which may be relevant to children’s sleep, such as sleep disordered breathing and obesity (Desager, Nelen, Weyler, & De Backer, 2005; Stingone, Ramirez, Svensson, & Claudio, 2011). In our sample, 46% of the child participants were at risk for sleep disordered breathing by caregiver report (Redline, Tichler, Hans, Tosteson, & Strohl, 1997), and 40% were overweight or obese; investigation of these co-morbidities on sleep quality will be important. We also recognize that some medications for AR cause sleepiness; 16% of our participants were taking these medications, by caregiver report. Future research needs to track adherence to both daily and as needed asthma and AR medications by objective methods, and their potential side effects, to examine their effects on sleep quality in this group.
Given children with asthma, particularly those living in urban contexts, are at risk for poor sleep, our findings underscore the importance of sleep as a potential modifiable target of intervention for this high-risk group. Results also suggest that family-based interventions integrating both asthma management and sleep hygiene strategies may be useful for improving sleep in urban children. Specifically, asthma management strategies relevant to optimal sleep (e.g., better trigger control in the child’s bedroom, availability and use of rescue inhaler if needed before and during bedtime) may improve both day-to-day lung function and overall asthma control, two important asthma indicators relevant to optimal sleep health in this group, as indicated by our study’s results. Sleep hygiene strategies should involve what is in the families’ abilities to modify and administer consistently, with consideration of asthma, family, and urban stressors. Similar strength-based approaches can be applied to other pediatric samples to identify risk and protective processes associated with sleep, given much of the literature still applies risk-based approaches in the examination of children’s sleep outcomes. Pediatric research that applies resilience-based approaches to study sleep provides an opportunity to describe the positive characteristics of high-risk families, and can demonstrate that despite challenges, better quality sleep can be promoted in children.
Funding
This work was supported by The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health & Human Development (R01 HD057220 to D.K.M). This article is dedicated to the work and memory of Stuart T. Hauser, MD, PhD, whose profound contribution to the study of resilience in pediatric populations is integrated in our research and clinical work with urban families on a daily basis.
Conflicts of interest: None declared.
References
- Acebo C., Sadeh A., Seifer R., Tzischinsky O., Hafer A., Carskadon M. A. (2005). Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep , 28, 1568–1577. [DOI] [PubMed] [Google Scholar]
- Acebo C., Sadeh A., Seifer R., Tzischinsky O., Wolfson A., Hafer A., Carskadon M. (1999). Estimating sleep patterns with activity monitoring in children and adolescents: How many nights are necessary for reliable measures? Sleep , 22, 95–103. [DOI] [PubMed] [Google Scholar]
- Aronen E. T., Paavonen E. J., Soininen M., Fjallberg M. (2001). Associations of age and gender with activity and sleep. Acta Paediatrica , 90, 222–224. [DOI] [PubMed] [Google Scholar]
- Ballard R. D. (1999). Sleep, respiratory physiology, and nocturnal asthma. Chronobiology International , 16, 565–580. [DOI] [PubMed] [Google Scholar]
- Beebe D. W. (2011). Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatric Clinics of North America , 58, 649–665. doi: 10.1016/j.pcl.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender B., Milgrom H. (2004). Comparison of the effects of fluticasone propionate aqueous nasal spary and loratadine on daytime altertness and performance in children with seasonal allergic rhinitis. Annals of Allergy, Asthma, and Immunology , 92, 344–349. [DOI] [PubMed] [Google Scholar]
- Bloom B. J., Owens J. A., McGuinn M., Nobile C., Schaeffer L., Alario A. J. (2002). Sleep and its relationship to pain, dysfunction, and disease activity in juvenile rheumatoid arthritis. The Journal of Rheumatology , 29, 169–173. [PubMed] [Google Scholar]
- Boergers J., Koinis Mitchell D. (2010). Sleep and culture in children with medical conditions. Journal of Pediatric Psychology , 35, 915–926. doi: jsq016 [pii] 10.1093/jpepsy/jsq016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet J., Khaltaev N., Cruz A. A., Denburg J., Fokkens W. J., Togias A., Zuberbier T., Baena-Cagnani C. E., Canonica G. W., van Weel C., Agache I., Aït-Khaled N., Bachert C., Blaiss M. S., Bonini S., Boulet L. P., Bousquet P. J., Camargos P., Carlsen K. H., Chen Y., Custovic A., Dahl R., Demoly P., Douagui H., Durham S. R., van Wijk R. G., Kalayci O., Kaliner M. A., Kim Y. Y., Kowalski M. L., Kuna P., Le L. T., Lemiere C., Li J., Lockey R. F., Mavale-Manuel S., Meltzer E. O., Mohammad Y., Mullol J., Naclerio R., O'Hehir R. E., Ohta K., Ouedraogo S., Palkonen S., Papadopoulos N., Passalacqua G., Pawankar R., Popov T. A., Rabe K. F., Rosado-Pinto J., Scadding G. K., Simons F. E., Toskala E., Valovirta E., van Cauwenberge P., Wang D. Y., Wickman M., Yawn B. P., Yorgancioglu A., Yusuf O. M., Zar H., Annesi-Maesano I., Bateman E. D., Ben Kheder A., Boakye D. A., Bouchard J., Burney P., Busse W. W., Chan-Yeung M., Chavannes N. H., Chuchalin A., Dolen W. K., Emuzyte R., Grouse L., Humbert M., Jackson C., Johnston S. L., Keith P. K., Kemp J. P., Klossek J. M., Larenas-Linnemann D., Lipworth B., Malo J. L., Marshall G. D., Naspitz C., Nekam K., Niggemann B., Nizankowska-Mogilnicka E., Okamoto Y., Orru M. P., Potter P., Price D., Stoloff S. W., Vandenplas O., Viegi G., Williams D. (2008). Allergic Rhinitis and its Impact on Asthma (ARIA). (2008) update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy, 63(Suppl 86), pp. 8–160. doi: ALL1620 [pii] 10.1111/j.1398-9995.2007.01620.x [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J., Duncan G. J. (1997). The effects of poverty on children. The Future of Children , 7, 55–71. [PubMed] [Google Scholar]
- Buysse D. J. (2014). Sleep health: Can we define it? Does it matter? Sleep , 37, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun S. L., Vgontzas A. N., Fernandez-Mendoza J., Mayes S. D., Tsaoussoglou M., Basta M., Bixler E. O. (2011). Prevalence and risk factors of excessive daytime sleepiness in a community sample of young children: The role of obesity, asthma, anxiety/depression, and sleep. Sleep , 34, 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canino G., Bravo M. (1994). The adaptation and testing of diagnostic and outcome measures for cross-cultural research. International Review of Psychiatry , 6, 281–286. [Google Scholar]
- Canino G., Garro A., Alvarez M. M., Colon-Semidey A., Esteban C., Fritz G., Koinis-Mitchell D., Kopel S. J., Ortega A. N., Seifer R., McQuaid E. L. (2012). Factors associated with disparities in emergency department use among Latino children with asthma. Annals Allergy Asthma Immunol , 108, 266–270. doi: S1081-1206(12)00074-9 [pii] 10.1016/j.anai.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canino G., Koinis-Mitchell D., Ortega A. N., McQuaid E. L., Fritz G. K., Alegria M. (2006). Asthma disparities in the prevalence, morbidity, and treatment of Latino children. Social Science and Medicine , 63, 2926–2937. doi: S0277-9536(06)00380-7 [pii] 10.1016/j.socscimed.2006.07.017 [DOI] [PubMed] [Google Scholar]
- Canino G., McQuaid E. L., Alvarez M., Colon A., Esteban C., Febo V., Klein R. B., Mitchell D. K., Kopel S. J., Montealegre F., Ortega A. N., Rodriguez-Santana J., Seifer R., Fritz G. K. (2009). Issues and methods in disparities research: The Rhode Island-Puerto Rico asthma center. Pediatric Pulmonology , 44, 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall J. R., Rhind G. B., Stewart I. C., Whyte K. F., Shapiro C. M., Douglas N. J. (1986). Effect of sleep deprivation on overnight bronchoconstriction in nocturnal asthma. Thorax , 41, 676–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celano M. P., Klinnert M. D., Holsey C. N., McQuaid E. L. (2011). Validity of the family asthma management system scale with an urban African-American sample. Journal of Pediatric Psychology , 36, 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2007). National Center for Health Statistics . Asthma Prevalence, Health Care Use and Mortality: United States from www.cdc.gov/nchs/products/pubs/pubd/hestats/ashtma03-05/asthma03-05.htm
- Chaput J. P., Brunet M., Tremblay A. (2006). Relationship between short sleeping hours and childhood overweight/obesity: Results from the ‘Quebec en Forme’ Project. International Journal of Obesity (London) , 30, 1080–1085. doi: 10.1038/sj.ijo.0803291 [DOI] [PubMed] [Google Scholar]
- Cohen S., Williamson G. M. (1998). In Spacapan S., Oskamp S. (Eds), Perceieved stress in a probability sample of the United States (pp. 31–67). Thousand Oaks, CA: The Social Psychology of Health Sage. [Google Scholar]
- Coutinho M. T., McQuaid E. L., Koinis-Mitchell D. (2013). Contextual and cultural risks and their association with family asthma management in urban children. Journal of Child Health Care , 17(2), 138–152. [DOI] [PubMed] [Google Scholar]
- Crenshaw M. C., Edinger J. D. (1999). Slow-wave sleep and waking cognitive performance among older adults with and without insomnia complaints. Physiology and Behavior , 66(3), 485–492. doi: S0031-9384(98)00316-3 [pii] [DOI] [PubMed] [Google Scholar]
- Daniel L. C., Boergers J., Kopel S. J., Koinis-Mitchell D. (2012). Missed sleep and asthma morbidity in urban children. Annals of Allergy, Asthma and Immunology , 109, 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb A., Arora M. (2008). Resilience in children and adolescents: An overview. Psychological Studies , 53, 114–121. [Google Scholar]
- Desager K. N., Nelen V., Weyler J. J., De Backer W. A. (2005). Sleep disturbance and daytime symptoms in wheezing school-aged children. Journal of Sleep Research , 14, 77–82. doi: JSR432 [pii] 10.1111/j.1365-2869.2004.00432.x [DOI] [PubMed] [Google Scholar]
- Dewald J. F., Meijer A. M., Oort F. J., Kerkhof G. A., Bogels S. M. (2010). The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep Medicine Reviews , 14, 179–189. doi: S1087-0792(09)00100-2 [pii] 10.1016/j.smrv.2009.10.004 [DOI] [PubMed] [Google Scholar]
- Diette G. B., Markson L., Skinner E. A., Nguen T. T., Algatt-Bergstrom P. (2000). Nocturnal asthma in children affects school attendance, school performance and parents' work attendance. Archives of Pediatric Adolescent Medicine , 154, 923–928. [DOI] [PubMed] [Google Scholar]
- Esteban C. A., Klein R. B., Kopel S. J., McQauid E. L., Fritz G. K., Seifer R., York D., Golova N., Jandasek B., Koinis Mitchell D. (2014). Under-diagnosed and undertreated allergic rhinitis in urban school-age children with asthma. Pediatric Allergy Immunology and Pulmonology , 27, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everhart R. S., Kopel S., McQuaid E. L., Salcedo L., York D., Potter C., Koinis-Mitchell D. (2011). Differences in environmental control and asthma outcomes among urban Latino, African American, and non-Latino white families. Pediatric Allergy, Immunology, and Pulmonology , 24, 165–169. doi: 10.1089/ped.2011.0081 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagnano M., Bayer A. L., Isensee C. A., Hernandez T., Halterman J. S. (2011). Nocturnal asthma symptoms and poor sleep quality among urban school children with asthma. Academic Pediatrics , 11, 493–499. doi: S1876-2859(11)00136-7 [pii] 10.1016/j.acap.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber R. (1996). Childhood sleep disorders. Neurologic Clinics , 14, 493–511. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick M. F., Engleman H., Whyte K. F., Deary I. J., Shapiro C. M., Douglas N. J. (1991). Morbidity in nocturnal asthma: Sleep quality and daytime cognitive performance. Thorax , 46, 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen K., Rhodes J., Reddy R. (2004). Sleepless in Chicago: Tracking the effects of adolescent sleep loss during the middle school years. Child Development , 75, 84–95. [DOI] [PubMed] [Google Scholar]
- Garrison M. M., Lozano P., Christakis D. A. (2011). Controller medication use and sleep problems in pediatric asthma: A longitudinal case-crossover analysis. Archives of Pediatrics and Adolescent Medicine , 165, 826–830. doi: 165/9/826 [pii] 10.1001/archpediatrics.2011.139 [DOI] [PubMed] [Google Scholar]
- Harsh J. R., Easley A., LeBourgeois M. K. (2002). A measure of children's sleep hygiene. Sleep , 25, A316–A317. [Google Scholar]
- Hart C. N., Palermo T. M., Rosen C. L. (2005). Health-related quality of life among children presenting to a pediatric sleep disorders clinic. Behavioral Sleep Medicine , 3, 4–17. [DOI] [PubMed] [Google Scholar]
- Hauser S. T., Allen J. P., Golden E. (2006). Out of the woods: Tales of resilient teens. Cambridge, MA: Harvard University Press. [Google Scholar]
- Hilliard M. E., Harris M. A., Weissberg-Benchell J. (2012). Diabetes resilience: A model of risk and protection in type 1 diabetes. Current Diabetes Report , 12(6), 739–748. [DOI] [PubMed] [Google Scholar]
- Janson C., De Backer W., Gislason T., Plaschke P., Bjornsson E., Hetta J., Kristbjarnarson H., Vermeire P., Boman G. (1996). Increased prevalence of sleep disturbances and daytime sleepiness in subjects with bronchial asthma: A population study of young adults in three European countries. European Respiratory Journal , 9, 2132–2138. [DOI] [PubMed] [Google Scholar]
- Kattan M., Mitchell H., Eggleston P., Gergen P., Crain E., Redline S., Weiss K., Evans R., III, Kaslow R., Kercsmar C., Leickly F., Malveaux F., Wedner H. J. (1997). Characteristics of inner-city children with asthma: The National Cooperative Inner-City Asthma Study. Pediatric Pulmonology , 24, 253–262. doi: 10.1002/(SICI)1099-0496(199710)24:4<253::AID-PPUL4>3.0.CO;2-L [pii] [DOI] [PubMed] [Google Scholar]
- Klinnert M. D. (1997). Psychosocial influences on asthma among inner-city children. Pediatric Pulmonology , 24, 234–236. [DOI] [PubMed] [Google Scholar]
- Koinis Mitchell D., Kopel S. J., Esteban C. E., McQuaid E. L., Seifer R., Fritz G. K., Klein R. B. (2014). Disparities in Asthma and school outcomes in urban children: Is sleep a contributing factor? Paper presented at the American Thoracic Society International Conference San Diego, CA. http://dx.doi.org/10.1164/ajrccm-conference.2014.189.1_ MeetingAbstracts.A5078 [Google Scholar]
- Koinis Mitchell D., Craig T., Esteban C. A., Klein R. B. (2012). Sleep and allergic disease: A summary of the literature and future directions for research. Journal of Allergy and Clinical Immunology , 130, 1275–1281. doi: 10.1016/j.jaci.2012.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koinis Mitchell D., Kopel S. J., Boergers J., Ramos K., LeBourgeois M., McQuaid E. L., Esteban C. A., Seifer R., Fritz G. K., Klein R. (2015). Asthma, allergic rhinitis, and sleep problems in urban children. Journal of Clinical Sleep Medicine, 11, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koinis Mitchell D., McQuaid E. L., Jandasek B., Kopel S. J., Seifer R., Klein R. B., Potter C., Fritz G. K. (2012). Identifying individual, cultural and asthma-related risk and protective factors associated with resilient asthma outcomes in urban children and families. Journal of Pediatric Psychology , 37, 424–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koinis Mitchell D., McQuaid E. L., Kopel S., Nassau J., Klein R. B., Feldman J., Wamboldt M. Z., Fritz G. K. (2009). Symptom perception in children with asthma: Cognitive and psychological factors. Health Psychology , 28, 226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koinis Mitchell D., McQuaid E. L., Seifer R., Kopel S. J., Esteban C., Canino G., Garcia-Coll C., Klein R., Fritz G. K. (2007). Multiple urban and asthma-related risks and their association with asthma morbidity in children. Journal of Pediatric Psychology , 32, 582–595. doi: jsl050 [pii] 10.1093/jpepsy/jsl050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koinis Mitchell D., Murdock K. K. (2005). Identifying risk and resource factors in children with asthma from urban settings: The context-health-development model. Journal of Asthma , 42, 425–436. [DOI] [PubMed] [Google Scholar]
- Koinis Mitchell D., Murdock K. M., McQuaid E. L. (2004). Risk and resilience in urban children with asthma: A conceptual model and exploratory study. Children's Health Care , 33, 275–298. [Google Scholar]
- Lara M., Akinbami L., Flores G., Morgenstern H. (2006). Heterogeneity of childhood asthma among Hispanic children: Puerto Rican children bear a disproportionate burden. Pediatrics , 117, 43–53. doi: 10.1542/peds.2004-1714 [DOI] [PubMed] [Google Scholar]
- LeBourgeois M., Giannotti F., Cortesi F., Wolfson A., Harsh J. (2005). The relationship between reported sleep quality and sleep hygiene in Itanlian and American adolescents. Pediatrics , 115, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P., Fagnano M., Koehler A., Halterman J. S. (2014). Racial disparities at the point of care for urban children with persistent asthma. Journal of Community Health , 39, 706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A. H., Zeiger R., Sorkness C. A., Mahr T., Ostrom N., Burgess S., Rosenzweig J. C., Manjunath R. (2007). Development and cross-sectional validation of the Childhood Asthma Control Test. Journal of Allergy and Clinical Immunology , 119, 817–825. [DOI] [PubMed] [Google Scholar]
- Luthar S. S., Brown P. J. (2007). Maximizing resilience through diverse levels of inquiry: Prevailing paradigms, possibilities, and priorities for the future. Development and Psychopathology , 19, 931–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthar S. S., Cicchetti D., Becker B. (2000). The construct of resilience: A critical evaluation and guidelines for future work. Child Development , 71, 543–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthar S. S., Goldstein A. (2004). Children's exposure to community violence: Implications for understanding risk and resilience. Journal of Clinical Child and Adolescent Psychology , 33, 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. J., Banks-Schlegel S. (1998). Chronobiology of asthma. American Journal of Respiratory and Critical Care Medicine , 158, 1002–1007. [DOI] [PubMed] [Google Scholar]
- Masten A. S. (2001). Ordinary magic: Resilience processes in development. American Psychologist , 56, 227–238. [DOI] [PubMed] [Google Scholar]
- McNeil J., Doucet E., Chaput J. P. (2013). Inadequate sleep as a contributor to obesity and type 2 diabetes. Canadian Journal of Diabetes , 37, 103–108. doi: 10.1016/j.jcjd.2013.02.060 [DOI] [PubMed] [Google Scholar]
- McQuaid E. L., Everhart R. S., Seifer R., Kopel S. J., Koinis Mitchell D., Klein R. B., Esteban C. A., Fritz G. K., Canino G. (2012). Medication adherence among Latino and non-Latino white children with asthma. Pediatrics , 129, e1404–e1410. doi: peds.2011-1391 [pii] 10.1542/peds.2011-1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid E. L., Vasquez J., Canino G., Fritz G. K., Ortega A. N., Colon A., Klein R. B., Kopel S. J., Koinis-Mitchell D., Esteban C. A., Seifer R. (2009). Beliefs and barriers to medication use in parents of Latino children with asthma. Pediatric Pulmonology , 44, 892–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid E. L., Walders N., Kopel S. J., Fritz G. K., Klinnert M. D. (2005). Pediatric asthma management in the family context: The Family Asthma Management System Scale. Journal of Pediatric Psychology , 30, 492–502. [DOI] [PubMed] [Google Scholar]
- Meijer G., Postma D., Wempe J., Gerristen J., Knol K., Van Aadleren W. (1995). Frequency of nocturnal symptoms in asthmatic children attending a hospital out-patient clinic. European Respiratory Journal , 8, 2076–2080. [DOI] [PubMed] [Google Scholar]
- Meltzer L. J., Walsh C. M., Traylor J., Westin A. M. (2012). Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep , 35, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell J. A., Meltzer L. J., Carskadon M. A., Chervin R. D. (2009). Developmental aspects of sleep hygiene: Findings from the 2004 National Sleep Foundation Sleep in America Poll. Sleep Medicine, 10, 771–779. [DOI] [PubMed] [Google Scholar]
- Mindell J. A., Owens J. A. (2003). A Clinical Guide to Pediatric Sleep: Diagnosis and Management of Sleep Problems. Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Morgan W. J., Crain E. F., Gruchalla R. S., O'Connor G. T., Kattan M., Evans R., III, Stout J., Malindzak G., Smartt E., Plaut M., Walter M., Vaughn B., Mitchell H.; Inner-City Asthma Study Group. (2004). Results of a home-based environmental intervention among urban children with asthma. New England Journal of Medicine , 351, 1068–1080. [DOI] [PubMed] [Google Scholar]
- Morgenthaler T. I., Owens J., Alessi C., Boehlecke B., Brown T. M., Coleman J., Jr., Friedman L., Kapur V. K., Lee-Chiong T., Pancer J., Swick T. J.; American Academy of Sleep Medicine. (2006). Practice parameters for behavioral treatment of bedtime problems and night wakings in infants and young children. Sleep , 29, 1277–1281. [PubMed] [Google Scholar]
- Nathan R. A., Sorkness C. A., Kosinski M., Schatz M., Li J. T., Marcus P., Murray J. J., Pendergraft T. B. (2004). Development of the asthma control test: A survey for assessing asthma control. Journal of Allergy and Clinical Immunology , 113, 59–65. [DOI] [PubMed] [Google Scholar]
- National Heart Lung and Blood Institute. (2007). Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health. [Google Scholar]
- Nixon G. M., Thompson J. M., Han D. Y., Becroft D. M., Clark P. M., Robinson E., Waldie K. E., Wild C. J., Black P. N., Mitchell E. A. (2008). Short sleep duration in middle childhood: Risk factors and consequences. Sleep , 31, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J. A., Jones C., Nash R. (2011). Caregivers' knowledge, behavior, and attitudes regarding healthy sleep in young children. Journal of Clinical Sleep Medicine , 15, 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavonen E. J., Raikkonen K., Lahti J., Komsi N., Heinonen K., Pesonen A. K., Järvenpää A. L., Strandberg T., Kajantie E., Porkka-Heiskanen T. (2009). Short sleep duration and behavioral symptoms of attention-deficit/hyperactivity disorder in healthy 7- to 8-year-old children. Pediatrics , 123, e857–e864. doi: 10.1542/peds.2008-2164 [DOI] [PubMed] [Google Scholar]
- Ranjbaran Z., Keefer L., Stepanski E., Farhadi A., Keshavarzian A. (2007). The relevance of sleep abnormalities to chronic inflammatory conditions. Inflammation Research , 56, 51–57. doi: 10.1007/s00011-006-6067-1 [DOI] [PubMed] [Google Scholar]
- Redline S., Tichler P. V., Hans M. G., Tosteson T. D., Strohl K. P. (1997). Risk factors for sleep-disordered breathing in children. American Journal of Respiratory and Critical Care Medicine , 155, 186–192. [DOI] [PubMed] [Google Scholar]
- Sadeh A., Gruber R., Raviv A. (2002). Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Development , 73, 405–417. [DOI] [PubMed] [Google Scholar]
- Schatz M., Meltzer E. O., Nathan R., Derebery M. J., Mintz M., Stanford R. H., Dalal A. A., Silvey M. J., Kosinski M. (2010). Psychometric validation of the rhinitis control assessment test: A brief patient-completed instrument for evaluating rhinitis symptom control. Annals of Allergy, Asthma and Immunology , 104, 118–124. doi: S1081-1206(09)00066-0 [pii] 10.1016/j.anai.2009.11.063 [DOI] [PubMed] [Google Scholar]
- Scholle S., Beyer U., Bernhard M., Eichholz S., Erler T., Graness P., Goldmann-Schnalke B., Heisch K., Kirchhoff F., Klementz K., Koch G., Kramer A., Schmidtlein C., Schneider B., Walther B., Wiater A., Scholle H. C. (2011). Normative values of polysomnographic parameters in childhood and adolescence: Quantitative sleep parameters. Sleep Medicine , 12, 542–549. doi: 10.1016/j.sleep.2010.11.011 [DOI] [PubMed] [Google Scholar]
- Sheares B. J., Kattan M., Leu C. S., Lamm C. I., Dorsey K. B., Evans D. (2013). Sleep problems in urban, minority, early-school-aged children more prevalent than previously recognized. Clinical Pediatrics , 52, 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon K., Barakat L. P., Patterson C. A., Dampier C. (2009). Symptoms of depression and anxiety in adolescents with sickle cell disease: The role of intrapersonal characteristics and stress processing variables. Child Psychiatry and Human Development , 40, 317–330. [DOI] [PubMed] [Google Scholar]
- Spilsbury J. C., Storfer-Isser A., Drotar D., Rosen C. L., Kirchner L. H., Benham H., Redline S. (2004). Sleep behavior in an urban U.S. sample of school-aged children. Archives of Pediatric and Adolescent Medicine , 158, 988–994. [DOI] [PubMed] [Google Scholar]
- Stingone J. A., Ramirez O. F., Svensson K., Claudio L. (2011). Prevalence, demographics, and health outcomes of comorbid asthma and overweight in urban children. Journal of Asthma , 48, 876–885. doi: 10.3109/02770903.2011.616615 [DOI] [PubMed] [Google Scholar]
- Strunk R. C., Sternberg A. L., Bacharier L. B., Szefler S. J. (2002). Nocturnal awakening caused by asthma in children with mild to moderate asthma in the childhood asthma management program. Journal of Asthma and Clinical Immunology , 110, 395–403. [DOI] [PubMed] [Google Scholar]
- Tabachnick B. G., Fidell L. S. (1996). Using Multivariate Statistics (3rd ed.). New York, NY: HarperCollins College Publishers. [Google Scholar]
- Tan E., Healey D., Gray A. R., Galland B. C. (2012). Sleep hygiene intervention for youth aged 10 to 18 years with problematic sleep: A before-after pilot study. BMC Pediatrics, 12, 189 doi: 10.1186/1471-2431-12-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2005). The 2005 HHS poverty guidelines. Washington, DC: U.S. Department of Health and Human Services. [Google Scholar]