Abstract
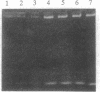
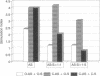
Phosphodiester oligodeoxynucleotides bearing a 5' cholesteryl (chol) modification bind to low density lipoprotein (LDL), apparently by partitioning the chol-modified oligonucleotides into the lipid layer. Both HL60 cells and primary mouse spleen T and B cells incubated with fluorescently labeled chol-modified oligonucleotide showed substantially increased cellular association by flow cytometry and increased internalization by confocal microscopy compared to an identical molecule not bearing the chol group. Cellular internalization of chol-modified oligonucleotide occurred at least partially through the LDL receptor; it was increased in mouse spleen cells by cell culture in lipoprotein-deficient medium and/or lovastatin, and it was decreased by culture in high serum medium. To determine whether chol-modified oligonucleotides are more potent antisense agents, we titered antisense unmodified phosphodiester and chol-modified oligonucleotides targeted against a mouse immunosuppressive protein. Murine spleen cells cultured with 20 microM phosphodiester antisense oligonucleotides had a 2-fold increase in RNA synthesis, indicating the expected lymphocyte activation. Antisense chol-modified oligonucleotides showed an 8-fold increase in relative potency: they caused a 2-fold increase in RNA synthesis at just 2.5 microM. The increased efficacy was blocked by heparin and was further increased by cell culture in 1% (vs. 10%) fetal bovine serum, suggesting that the effect may, at least in part, be mediated via the LDL receptor. Antisense chol-modified oligonucleotides are sequence specific and have increased potency as compared to unmodified oligonucleotides.
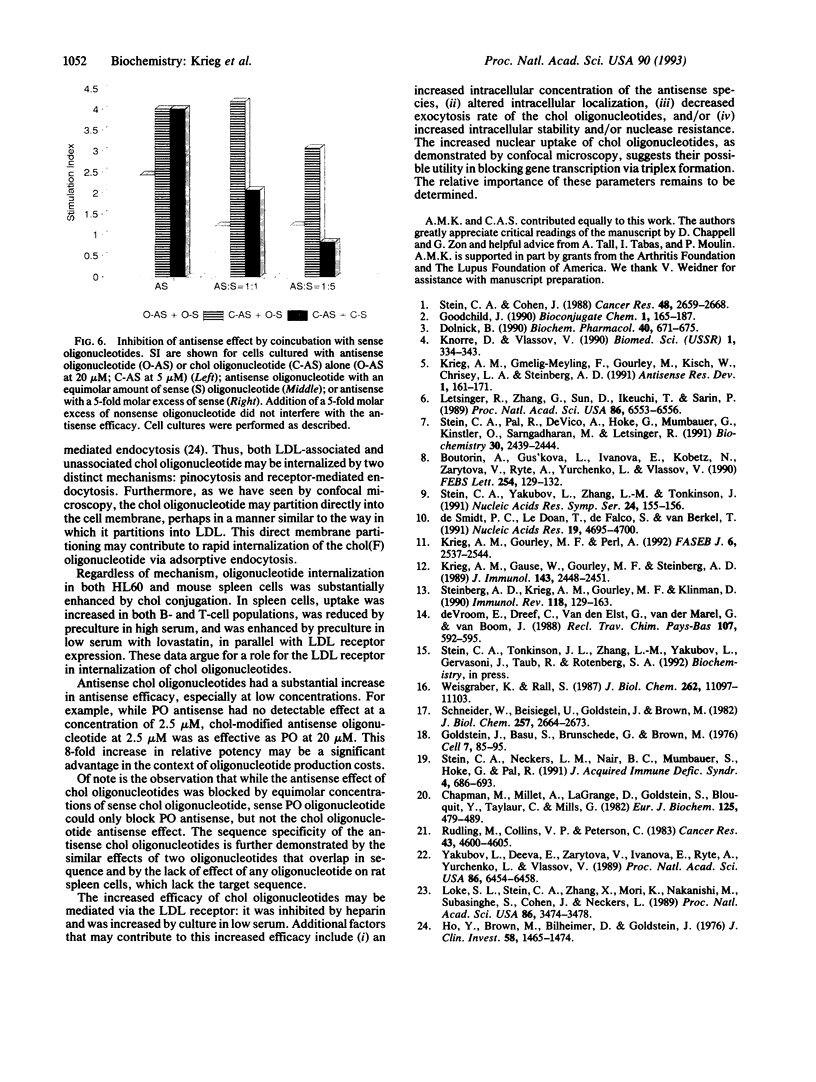
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boutorin A. S., Gus'kova L. V., Ivanova E. M., Kobetz N. D., Zarytova V. F., Ryte A. S., Yurchenko L. V., Vlassov V. V. Synthesis of alkylating oligonucleotide derivatives containing cholesterol or phenazinium residues at their 3'-terminus and their interaction with DNA within mammalian cells. FEBS Lett. 1989 Aug 28;254(1-2):129–132. doi: 10.1016/0014-5793(89)81023-3. [DOI] [PubMed] [Google Scholar]
- Chapman M. J., Millet A., Lagrange D., Goldstein S., Blouquit Y., Taylaur C. E., Mills G. L. The surface-exposed, trypsin-accessible segments of apolipoprotein B in the low-density lipoprotein of human serum. Fractionation and characterisation of the liberated peptides. Eur J Biochem. 1982 Jul;125(3):479–489. doi: 10.1111/j.1432-1033.1982.tb06708.x. [DOI] [PubMed] [Google Scholar]
- Dolnick B. J. Antisense agents in pharmacology. Biochem Pharmacol. 1990 Aug 15;40(4):671–675. doi: 10.1016/0006-2952(90)90300-a. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brunschede G. Y., Brown M. S. Release of low density lipoprotein from its cell surface receptor by sulfated glycosaminoglycans. Cell. 1976 Jan;7(1):85–95. doi: 10.1016/0092-8674(76)90258-0. [DOI] [PubMed] [Google Scholar]
- Goodchild J. Conjugates of oligonucleotides and modified oligonucleotides: a review of their synthesis and properties. Bioconjug Chem. 1990 May-Jun;1(3):165–187. doi: 10.1021/bc00003a001. [DOI] [PubMed] [Google Scholar]
- Ho Y. K., Brown S., Bilheimer D. W., Goldstein J. L. Regulation of low density lipoprotein receptor activity in freshly isolated human lymphocytes. J Clin Invest. 1976 Dec;58(6):1465–1474. doi: 10.1172/JCI108603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorre D. G., Vlassov V. V. Antisense oligonucleotide derivatives as gene-targeted drugs. Biomed Sci. 1990 Apr;1(4):334–343. [PubMed] [Google Scholar]
- Krieg A. M., Gause W. C., Gourley M. F., Steinberg A. D. A role for endogenous retroviral sequences in the regulation of lymphocyte activation. J Immunol. 1989 Oct 15;143(8):2448–2451. [PubMed] [Google Scholar]
- Krieg A. M., Gmelig-Meyling F., Gourley M. F., Kisch W. J., Chrisey L. A., Steinberg A. D. Uptake of oligodeoxyribonucleotides by lymphoid cells is heterogeneous and inducible. Antisense Res Dev. 1991 Summer;1(2):161–171. doi: 10.1089/ard.1991.1.161. [DOI] [PubMed] [Google Scholar]
- Krieg A. M., Gourley M. F., Perl A. Endogenous retroviruses: potential etiologic agents in autoimmunity. FASEB J. 1992 May;6(8):2537–2544. doi: 10.1096/fasebj.6.8.1592206. [DOI] [PubMed] [Google Scholar]
- Letsinger R. L., Zhang G. R., Sun D. K., Ikeuchi T., Sarin P. S. Cholesteryl-conjugated oligonucleotides: synthesis, properties, and activity as inhibitors of replication of human immunodeficiency virus in cell culture. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6553–6556. doi: 10.1073/pnas.86.17.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke S. L., Stein C. A., Zhang X. H., Mori K., Nakanishi M., Subasinghe C., Cohen J. S., Neckers L. M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudling M. J., Collins V. P., Peterson C. O. Delivery of aclacinomycin A to human glioma cells in vitro by the low-density lipoprotein pathway. Cancer Res. 1983 Oct;43(10):4600–4605. [PubMed] [Google Scholar]
- Schneider W. J., Beisiegel U., Goldstein J. L., Brown M. S. Purification of the low density lipoprotein receptor, an acidic glycoprotein of 164,000 molecular weight. J Biol Chem. 1982 Mar 10;257(5):2664–2673. [PubMed] [Google Scholar]
- Stein C. A., Cohen J. S. Oligodeoxynucleotides as inhibitors of gene expression: a review. Cancer Res. 1988 May 15;48(10):2659–2668. [PubMed] [Google Scholar]
- Stein C. A., Neckers L. M., Nair B. C., Mumbauer S., Hoke G., Pal R. Phosphorothioate oligodeoxycytidine interferes with binding of HIV-1 gp120 to CD4. J Acquir Immune Defic Syndr. 1991;4(7):686–693. [PubMed] [Google Scholar]
- Stein C. A., Pal R., DeVico A. L., Hoke G., Mumbauer S., Kinstler O., Sarngadharan M. G., Letsinger R. L. Mode of action of 5'-linked cholesteryl phosphorothioate oligodeoxynucleotides in inhibiting syncytia formation and infection by HIV-1 and HIV-2 in vitro. Biochemistry. 1991 Mar 5;30(9):2439–2444. doi: 10.1021/bi00223a020. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Yakubov L., Zhang L. M., Tonkinson J. Mode of uptake of 5'-cholesteryl-linked phosphodiester oligodeoxynucleotides in HL60 cells. Nucleic Acids Symp Ser. 1991;(24):155–156. [PubMed] [Google Scholar]
- Steinberg A. D., Krieg A. M., Gourley M. F., Klinman D. M. Theoretical and experimental approaches to generalized autoimmunity. Immunol Rev. 1990 Dec;118:129–163. doi: 10.1111/j.1600-065x.1990.tb00815.x. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H., Rall S. C., Jr Human apolipoprotein B-100 heparin-binding sites. J Biol Chem. 1987 Aug 15;262(23):11097–11103. [PubMed] [Google Scholar]
- Yakubov L. A., Deeva E. A., Zarytova V. F., Ivanova E. M., Ryte A. S., Yurchenko L. V., Vlassov V. V. Mechanism of oligonucleotide uptake by cells: involvement of specific receptors? Proc Natl Acad Sci U S A. 1989 Sep;86(17):6454–6458. doi: 10.1073/pnas.86.17.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smidt P. C., Le Doan T., de Falco S., van Berkel T. J. Association of antisense oligonucleotides with lipoproteins prolongs the plasma half-life and modifies the tissue distribution. Nucleic Acids Res. 1991 Sep 11;19(17):4695–4700. doi: 10.1093/nar/19.17.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]