Abstract
AIM: To determine the incidence of and the risk factors for cytomegalovirus (CMV) symptomatic infection and end-organ disease after autologous stem cell transplantation (ASCT).
METHODS: A total of 327 consecutive non CD34+ selected autografts performed from the Hematology and Stem Cell Transplantation Unit of Regina Elena National Cancer Institute of Rome (Italy) in the period comprised between January 2003 to January 2015, were reviewed. Over the 327 autografts, 201 were performed in patients with multiple myeloma, whereas the remaining 126 in patients affected by non-Hodgkin’s lymphoma and Hodgkin’s lymphoma. The patients who underwent an ASCT for an acute leukemia (n = 20) in the same period were excluded from this analysis. CMV DNA load in the blood has been determined by polymerase-chain reaction in the case of a clinical suspicion of reactivation, therefore, no routine monitoring strategy was adopted. In the presence of signs and symptoms of CMV reactivation an antiviral treatment was performed.
RESULTS: Overall, 36 patients (11%) required a specific antiviral treatment for a symptomatic CMV reactivation (n = 32) or an end-organ disease (n = 4). We observed 20 and 16 cases of CMV reactivation among lymphoma (16%) and myeloma patients (8%), respectively. Among cases of end-organ disease, 3 were diagnosed as interstitial pneumonia and one remaining case as hemorrhagic enteritis. All cases of CMV reactivation were observed in IgG seropositive patients, with no documented cases of primary CMV infection. All patients were treated with a specific antiviral therapy, with a global rate of hospitalization of 55%; four patients received intravenous immunoglobulins. Transplant-related mortality was significantly higher in patients who experienced a CMV reactivation (8.4% ± 4.7% vs 1.7% ± 0.8%; P = 0.047). In univariate analysis, a pre-transplant HBcIgG seropositivity, a diagnosis of T-cell non-Hodgkin’s lymphoma and higher median age at transplant were significantly associated with the risk of developing a clinically relevant CMV infection requiring specific antiviral therapy (P < 0.001, P = 0.042 and P = 0.004, respectively). In multivariate analysis, only a pre-transplant HBcIgG seropositivity (OR = 8.928, 95%CI: 1.991-33.321; P = 0.023) and a diagnosis of T-cell non-Hodgkin’s lymphoma (OR = 4.739, 95%CI: 1.511-11.112; P = 0.042) proved to be independent predictors of a post-transplant clinically relevant CMV reactivation.
CONCLUSION: A symptomatic CMV infection can occur in about 11% of adult patients with lymphoma or myeloma undergoing ASCT. A pre-transplant HBcIgG seropositivity and a diagnosis of T-cell non-Hodgkin’s lymphoma should be considered as independent predictor factors of CMV reactivation.
Keywords: Cytomegalovirus, Autologous hematopoietic stem cell transplantation, Lymphoma, Myeloma, HBcIgG seropositivity, Transplant-related mortality
Core tip: Data about cytomegalovirus (CMV) reactivation in autologous hematopoietic stem cell transplantation (ASCT) are limited. We performed a retrospective observational study on 327 autografts consecutively performed for lymphoma (n = 126) or myeloma (n = 201) patients in our Institution. Aim of the study was to determine the incidence of and the risk factors for CMV symptomatic infection and/or end-organ disease, defined according to published recommendations, and the impact on Transplant-Related Mortality. Our data show that a symptomatic CMV infection can occur in about 11% of adult patients with lymphoma or myeloma undergoing ASCT. Most of cases of CMV reactivation are easily manageable but it can be a potentially life-threatening complication. As for risk factors, a pre-transplant HBcIgG seropositivity and a diagnosis of T-cell non-Hodgkin’s lymphoma should be considered as independent risk factors for CMV reactivation after ASCT.
INTRODUCTION
Cytomegalovirus (CMV) reactivation is not uncommon and could determine a CMV-related disease in immunocompromised patients. CMV disease may involve almost any organ, particularly lung and gastrointestinal tract. CMV reactivation and end-organ disease after allogeneic hematopoietic stem cell transplantation has been well studied[1]. On the contrary, hematologic patients treated with high-dose chemotherapy and who underwent autologous stem cell transplantation (ASCT) were historically considered to have a low risk of CMV reactivation or end-organ disease. Previous studies on lymphoma and myeloma patients suggested an incidence of CMV reactivations of about 30%-40% when CMV determination was based on polymerase-chain reaction (PCR)/antigenemia prospective surveillance and of 1%-13% when determinations were performed only on the basis of clinical suspicion of infection, with a infection-mortality rate that ranged between 0% and 100%[2-11]. The guidelines of the European Conference on Infections in Leukemia (ECIL), published in 2008, consider the routine monitoring of CMV unnecessary in patients undergoing ASCT because of the low risk progression from infection to disease, with the exception of patients receiving CD34- selected grafts and prior treatment with Fludarabine, Cladribine or Alemtuzumab, considering that this setting of patients presented a profound alteration of T-cell-mediated immunity functional status[12]. However, the recent large use of immunotherapeutic drugs for the treatment of lymphomas and the introduction of proteasome inhibitors in the treatment of myeloma has determined an increase of viral infections also outside allogeneic transplantation setting, as for ASCT. In the last years, some studies have been published by our and others groups in order to better characterize the incidence of and the risk factors for CMV infection in ASCT of both in lymphoma and myeloma patients[13-17]. However, considering the low number of patients studied and to the multicenter nature of some previous studies (potential bias for the heterogeneity of molecular virology laboratories and diagnostic strategies), data about this issue are not yet conclusive and needed to be validated. Based on these findings, the present study aimed to evaluate the risk factors for CMV symptomatic reactivation/end-organ disease and its impact in transplant-related mortality (TRM) in a large cohort of lymphoma and myeloma patients who underwent ASCT, under a unique and unchanged diagnostic strategy of this infection.
MATERIALS AND METHODS
Patients
A total of 327 consecutive non CD34+ selected autografts performed from the Hematology and Stem Cell Transplantation Unit of Regina Elena National Cancer Institute of Rome (Italy) in the period comprised between January 2003 to January 2015, were reviewed. Over the 327 autografts, 201 were performed in patients with multiple myeloma, whereas the remaining 126 in patients affected by non-Hodgkin’s lymphoma (NHL) and Hodgkin’s lymphoma. The patients who underwent an ASCT for an acute leukemia (n = 20) in the same period were excluded from this analysis. Patient characteristics at transplant are described in detail in Table 1. All patients were treated under a same anti-infectious and transfusional policy; in particular, all patients had received an antiviral prophylaxis with Valacyclovir and anti-Pneumocystiis prophylaxis with Cotrimoxazole given from the day of transplant until six months after and an anti-bacterial prophylaxis with Ciprofloxacin from the day of transplant until the resolution of severe neutropenia. All patients had signed an informed consent granting use of sensitive data for scientific purposes. The study has been approved by the institutional Ethical Committee.
Table 1.
Patient characteristics at transplant n (%)
| Median age (range) | 56 (18-72) |
| Sex, M/F | 198/129 |
| Diagnosis | |
| Multiple myeloma | 201 (61) |
| B-cell non-Hodgkin's lymphoma | 80 (25) |
| Hodgkin's lymphoma | 27 (8) |
| T-cell non-Hodgkin's lymphoma | 19 (6) |
| CMV IgG seropositivity1 | 304 (93) |
| HBcIgG serositivity | 46 (14) |
| HCVAb seropositivity1 | 5 (1.5) |
| Disease status | |
| Complete response | 205 (63) |
| Partial response | 114 (35) |
| Stable/progressive disease | 8 (2) |
| Prior chemotherapy lines | |
| 1 | 185 (57) |
| 2 | 120 (37) |
| ≥ 3 | 22 (6) |
| Prior fludarabine treatment | 5 (1.5) |
| Prior alemtuzumab treatment | 0 |
| Conditioning regimen | |
| BEAM or BEAM-like | 126 (39) |
| MEL200/MEL100 | 201 (61) |
| Median CD34+ infused cells × 106/kg (range) | 5.62 (2.36-28.48) |
Datum is missing in 2 patients. BEAM: Carmustine, Etoposide, Cytarabine, Melphalan; MEL200: Melphalan 200 mg/m2; MEL100: Melphalan 100 mg/m2; CMV: Cytomegalovirus.
Criteria for diagnosis of CMV symptomatic infection and end-organ disease
The criteria were based on published recommendations[12,18-20]. According to local policy and published guidelines[12], CMV DNAemia was determined only upon clinical suspicion of post-transplant reactivation, therefore no routine monitoring CMV strategy was adopted. Clinical suspicion criteria for check CMV DNAemia were defined as follows: presence of fever (temperature > 38 °C) and overt clinical signs of bone marrow suppression, and in the absence of concomitant bacterial, viral (i.e., HHV-6, EBV, parvovirus B19) or fungal co-infections (as demonstrated by clinical examination, thoracic computerized tomography, and repeated cultures from blood and urine). Bone marrow suppression was defined as a delay of neutrophilis and/or platelet recovery from ASCT (absence of complete neutrophilis and platelets recovery after 14 and 21 d from transplantation, respectively) or a drop in neutrophilis and/or platelet count after recovery (absolute count of neutrophilis or platelets < 1000/mcL or 100000/mcL, respectively, or a decrease of at least 30% of the counts in two consecutive determinations). CMV symptomatic infection was defined as a documented CMV DNAemia, confirmed by two consecutive determinations, in presence of clinical suspicion criteria of reactivation. CMV end-organ disease was defined by the presence of signs consistent with CMV infection, as determined by a combination of imaging and clinical and histopathological/molecular evaluations. In particular, CMV gastrointestinal disease was defined by the presence of a combination of clinical symptoms from the upper or lower gastrointestinal tract, findings of macroscopic mucosal lesions on endoscopy, and demonstration of the presence of CMV inclusion bodies in the tissue biopsy, further confirmed by positive immunohistochemical staining of CMV antigens in tissue sections of the gastrointestinal tract. CMV pneumonia was defined by the presence of clinical (hypoxemia) and radiological signs of interstitial pulmonary disease combined with the detection of high viral loads of CMV by quantitative PCR in bronchoalveolar lavage fluid confirmed by detection of CMV by direct immunostaining of alveolar cells[18,20]. Lung tissue biopsies to demonstrate the presence of CMV inclusion bodies in the tissue biopsy, were not performed considering the high risk of complications derived from a pulmonary biopsy in patients with a severe respiratory distress and a great hemorrhagic risk. In the presence of signs and symptoms of CMV reactivation, as above specified, an antiviral treatment was performed. The choice of antiviral agent to use for symptomatic reactivation treatment (Ganciclovir, Valganciclovir, Foscarnet sodium) was based on clinical features of the patients at the time of reactivation.
Quantification of CMV DNA
Automated nucleic acid sample preparation systems NucliSENSeasyMAG® (BioMerieux, Durham, United States) has been used for DNA extraction from plasma, according to the manufacturer’s instructions. Amplification for detection and quantification of viral DNA has been performed using commercially available real-time PCR assays (Affigene® CMV Trender diagnostic assay), according to the manufacturer’s instructions (Cepheid AB, Bromma, Sweden) on a Mx3000P® System (Stratagene, La Jolla, CA, United States) until August 2013 then the analogous Geneproof CMV PCR kit (Czech Republic) on SLAN® Real-Time PCR Detection System (Shanghai Hongshi Medical Technology Co., Ltd). The limit of detection was 88 copies/mL in both kit.
Statistical analysis
Data were analyzed by Statistical Package of Social Sciences software (SPSS, version 17.0, Chicago, United States). Univariate analysis was performed in order to identify risk factors for clinically relevant CMV infection requiring specific treatment by using χ2 test (Fisher or Pearson) and analysis of variance for categorical and quantitative variables, respectively. Two-sided P-values below 0.05 were considered to be statistically significant for the multivariate analysis. In case of two or more significant variables with reciprocal competitive effect, only the variable statistically more significant or clinically more relevant was included in the final model. Binary logistic regression model was used to analyze associations between significant baseline characteristics and the occurrence of CMV infection. Enter and remove limits were 0.05 and 0.1, respectively. TRM was estimated with the cumulative incidence method considering dead for relapse or other not transplant-related causes as competing risks. The curves of various subgroups were compared using Gray’s test.
RESULTS
Clinical characteristics of patients at transplant are described in Table 1. The large majority of patients were seropositive for CMV IgG (304/327, 93%) and 46 (14%) were HBcIgG seropositive. Most of patients received un up-front ASCT (185/327, 57%) and 205 (63%) were transplanted in complete remission (CR). Median age at transplant was of 56 years (range: 18-72). Overall, 36 patients (11%) were treated with an antiviral therapy for a symptomatic CMV reactivation (n = 32) or an end-organ disease (n = 4). We observed 20 and 16 cases of CMV reactivation among lymphoma (16%) and myeloma patients (8%), respectively. The more relevant features of reactivation episodes are described in Table 2. Among cases of end-organ disease, 3 were diagnosed as interstitial pneumonia and one remaining case as hemorrhagic enteritis. We observed also three cases (8%) of extensive skin involvement by CMV infection, presenting as diffuse erythema not determined by others causes and promptly resolved after the begin of specific antiviral treatment. Median time from the transplant and the first detection of viral DNA in blood samples was of 33 d (range: 12-77). All cases of CMV reactivation were observed in IgG seropositive patients, with no documented cases of primary CMV infection. All patients were treated with a specific antiviral therapy (Table 2), with a global rate of hospitalization of 55%; four patients received intravenous immunoglobulins. The patients who experienced a symptomatic CMV reactivation presented a significant delay in neutrophilis and platelets recovery (P = 0.003 and P = 0.001, respectively). As for clinical outcome after antiviral treatment, 3 patients died, with a global mortality rate of 8%. However, we observe only one death directly related to CMV (respiratory distress caused by interstitial pneumonia), whereas in the others two cases, death was caused by gram negative septic shock. Figure 1 shows the cumulative incidence of 100-d TRM. As shown by the curves, TRM was significantly higher in patients who experienced a CMV reactivation (8.4% ± 4.7% vs 1.7% ± 0.8%; P = 0.047). A pre-transplant HBcIgG seropositivity, a diagnosis of T-cell NHL and an higher age at transplant were associated with the risk of post-transplant CMV reactivation, at univiariate analysis (P < 0.001, P = 0.042 and P = 0.004, respectively). All others baseline analyzed parameters, including sex, diagnosis (lymphoma vs myeloma), disease status at transplant, previous chemotherapy lines, conditioning regimes and median CD34+ infused cells, resulted not statistically significant (data not shown). In multivariate analysis, a pre-transplant HBcIgG seropositivity (OR = 8.928, 95%CI: 1.991-33.321; P = 0.023) and a diagnosis of T-cell NHL (OR = 4.739, 95%CI: 1.511-11.112; P = 0.042) were independent risk factors for a post-transplant CMV reactivation.
Table 2.
Clinical and laboratory features and outcome of cytomegalovirus reactivation episodes requiring specific antiviral treatment (36/327, 11%)
| Clinical and laboratory features | No. of cases |
| Fever (temperature > 38 °C persistent at least 60 min) | 36 (100%) |
| Signs of bone marrow suppression (delay of neutrophilis and/or platelet recovery or drop in neutrophilis and/or platelet count after recovery) | 35 (97%) |
| DNAemia positivity (PCR assay) | 36 (100%) |
| End-organ disease (according to published criteria) | 4 (11%) |
| Interstitial pneumonia | 3 |
| Enteritis | 1 |
| Median number of CMV copies at first detection (range)1 | 895 (188-10120) |
| Median day from transplant at first detection (range) | 33 (12-77) |
| Pre-transplant CMV IgG seropositivity | 36 (100%) |
| Outcome | |
| Treatment2 | |
| Ganciclovir | 8 |
| Foscarnet sodium | 16 |
| Valganciclovir | 12 |
| Immunoglobulins | 4 |
| Need of hospital admission | 20 (55%) |
| Hematological recovery, median (range)3 | |
| Neutrophilis > 500/mcL | 14 (10-25) |
| Platelets > 20000/mcL | 20 (11-88) |
| Alive | 33 (92%) |
| Dead (48, 62, 89 d from transplant) | 3 (8%) |
Limit of detection of PCR testing: 88 copies/mL;
Foscarnet sodium dosage: 60 mg/kg twice daily for 14 d, than 60 mg/kg per day for subsequent 5 d weekly for 2 wk); Ganciclovir dosage: 5 mg/kg twice daily for 14 d, than 5 mg/kg per day for subsequent 5 d weekly for 2 wk; Valganciclovir dosage: 900 mg twice daily for 14 d, than 900 mg/d for subsequent 5 d weekly for 2 wk;
The occurrence of a symptomatic CMV reactivation after ASCT, requiring antiviral treatment, leads to a delay in neutrophilis and platelets recovery (P = 0.003 and P = 0.001 respectively). ASCT: Autologous hematopoietic stem cell transplantation; CMV: Cytomegalovirus.
Figure 1.
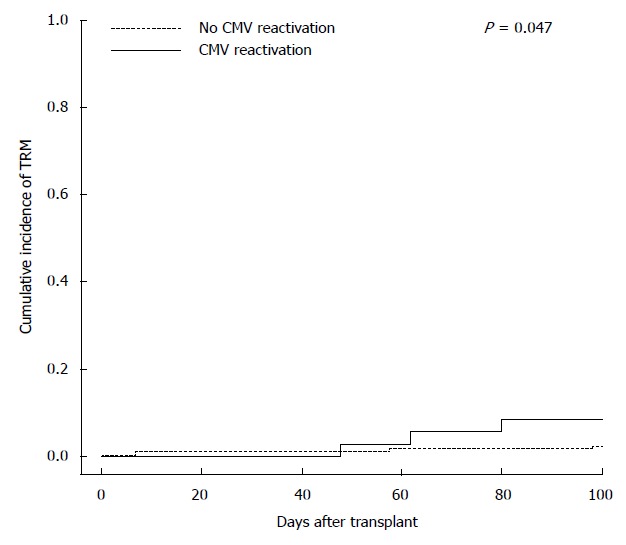
Cumulative incidence of 100-d transplant-related-mortality according to occurrence or not of a cytomegalovirus symptomatic reactivation/end-organ disease (8.4% ± 4.7% vs 1.7% ± 0.8%; P = 0.047). TRM: transplant-related-mortality; CMV: Cytomegalovirus.
DISCUSSION
CMV reactivation can be a relevant cause of morbidity following ASCT in adult lymphoma and myeloma patients. From our survey, 36 over 327 patients (11%) were treated for a post-transplant symptomatic CMV reactivation. Moreover, we observed 4 cases of end-organ disease (1%; 3 cases of interstitial pneumonia and 1 case of hemorrhagic enteritis). Cumulative incidence of TRM was significantly affected by the occurrence of a symptomatic CMV reactivation (5-fold risen, Figure 1), although only one death was directly attributable to CMV (respiratory distress caused by interstitial pneumonia), whereas the remaining two were caused by a gram negative bacterial co-infection, indirectly favored by the graft failure consequent to CMV reactivation. The global incidence of CMV reactivation and of end-organ disease observed in our study were substantially similar to our previously reports[14,15,17], but also to the others published studies in which it has been used a same diagnostic strategy of CMV reactivation[5,7-10,21]. Data about the global incidence of CMV reactivation from our present study are particularly relevant because obtained in a single institution under a unique and unchanged strategy of diagnosis of this infection. Most of cases of CMV reactivation were easily manageable, particularly in myeloma patients and about one third of cases (12/36, 33%; Table 2) were treated with oral Valganciclovir. However, considering that the occurrence of a symptomatic CMV reactivation had lead to a delay of neutrophilis and platelets recovery or to a graft failure, most of cases were treated with intravenous Foscarnet sodium, with a global rate of hospital re-admission of about 50% (Table 2). Our data confirm that lymphoma and myeloma patients who underwent an ASCT from CD34- non selected cells and not receiving Fludarabine and Alemtuzumab prior transplant are at low risk of CMV reactivation and a CMV end-organ disease is a rare event in this setting. However, it is an important cause of morbidity and, despite often easily manageable, CMV reactivation is also capable to affect TRM, as direct or indirect action. In our opinion, in this setting of patients, a prospective monitoring of PCR (surveillance strategy) is not recommended in all patients (according to ECIL guidelines[12]), but clinicians should be aware of this potentially severe complication, especially in the presence of post-transplant unexplained fever and drop in neutrophilis and platelets count. In this study, pre-transplant HBcIgG seropositivity is an independent factor able to predict the occurrence of a post-transplant CMV reactivation (Table 3). This datum, obtained in a larger number of lymphoma and myeloma patients and in a single-center setting, confirms our previous published results in lymphoma patients[14]. HBcIgG is a marker of occult hepatitis B virus (HBV) infection carrier. HBV positive patients could be considered as patients at risk for CMV reactivation[22,23]. The role of a HBV latent co-infection as independent factor for CMV reactivation observed in our study has a physiopathologic rationale, considering that interactions among some different viruses have been demonstrated to have a role in the pathogenesis of infections, through mechanisms of cross-permissiveness mediated by the immune system[14,24-26]. In fact, the mechanisms of virus-virus interaction is common and crucial to understanding pathogenesis of viral infections; we hypothesized that HBV is capable of favoring a CMV co-infection through direct interaction of viral molecules, but also trough acting on cell-mediate immune system[26]. However, contrasting data are recently obtained in allogeneic hematopoietic stem cell transplantation by Lin and collaborators, that suggest that the underlying HBV infection in donors or recipients before transplant does not increase the risk of CMV infection and end-organ disease[27]. Moreover, our data suggest for the first time that also a diagnosis of T-cell NHL seems to be an independent risk factor for post-transplant CMV reactivation in ASCT (Table 3). Although obtained on a small number of patients, also this datum is not surprising if we consider that CMV reactivation is associated with the presence of dysfunctional antigen-specific CD8+ cells[28] and that T-cell-mediated immunity plays a crucial role in the control of latent CMV infection. In this point of view, we could hypothesize that the impaired T-cell function observed in T-cell NHL is a favoring factor for post-transplant reactivation of CMV in autografted patients. In conclusion, from our study in adult lymphoma and myeloma patients undergoing ASCT, three issues may be addressed: (1) The incidence of CMV reactivation and end-organ disease are about of 11% and 1%, respectively. The occurrence of a CMV symptomatic reactivation is often easily manageable but is able to affect directly or indirectly the cumulative incidence of TRM (5-fold risen); (2) Our data confirm in a larger cohort of patients that a pre-transplant HBcIgG seropositivity is an independent risk factor for post-transplant CMV reactivation; and (3) With the caution due to limited number of patients, our data suggest for the first time that T-cell lymphoma patients could be also considered at high risk for post-transplant symptomatic CMV reactivation.
Table 3.
Risk factors for the occurrence of cytomegalovirus symptomatic reactivation
| Variables |
Univariate analysis |
Multivariate analysis |
|||
| Occurrence of symptomatic infection or end-organ disease | P | OR (95%CI) | P | ||
| HBcIgG | Positive | 16/46 (34%) | < 0.001 | 8.928 (1.991-33.321) | 0.023 |
| Negative | 20/281 (7%) | ||||
| Diagnosis | MM | 16/201 (8%) | 0.095 | 1.841 (1.058-5.633) | 0.125 |
| Lymphoma | 20/126 (16%) | ||||
| Diagnosis of T-cell NHL | Yes | 6/19 (31%) | 0.022 | 4.739 (1.511-11.112) | 0.042 |
| No | 30/308 (10%) | ||||
| Median age at transplant | Years (range) | 60 (35-71) vs 52 (18-72) | 0.004 | 2.922 (1.273-6.295) | 0.088 |
MM: Multiple myeloma; NHL: Non-Hodgkin’s lymphoma.
COMMENTS
Background
The introduction of novel immunosoppressive drugs in the treatment of hematologic malignancies had lead to an increase of interest for cytomegalovirus (CMV) infection also in setting different to allogeneic transplant. The authors reviewed 327 autografts performed in their institution with the aim to determine the incidence of and the risk factors for CMV symptomatic infection and end-organ disease after autologous stem cell transplantation.
Research frontiers
The search of risk factors of CMV reactivation in this setting of patients could permit to individuate patients that could beneficiate of a surveillance diagnostic strategy of CMV reactivation, and also of a pre-emptive therapy.
Innovations and breakthroughs
This study validated our previous results and for the first time highlighted the role of a diagnosis of T-cell non-Hodgkin’s lymphoma as risk factor for post-transplant CMV reactivation.
Applications
The findings found in this study could be used by clinicians to decide in which patients they could adopt a surveillance diagnostic strategy of CMV reactivation in autologous hematopoietic stem cell transplantation.
Terminology
Post-transplant CMV symptomatic infection is defined as a documented CMV DNAemia, confirmed by two consecutive determinations, in presence of clinical suspicion criteria of reactivation (e.g., graft failure or drop in neutrophilis and platelets values, fever not explained).
Peer-review
The authors have performed a good study, the manuscript is interesting.
Footnotes
Institutional review board statement: The study was approved by the institutional Ethical Committee without a formal document, considering that all patients had signed an informed consent granting use of sensitive data for scientific purposes at time of admission in our Institute.
Informed consent statement: All the patients had signed an informed consent granting use of sensitive data for scientific purposes at time of admission in our Institute.
Conflict-of-interest statement: No disclosures.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 9, 2015
First decision: June 3, 2015
Article in press: July 8, 2015
P- Reviewer: Kim KM, Kita K, Rodrigo L S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
References
- 1.Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect Dis Clin North Am. 2010;24:319–337. doi: 10.1016/j.idc.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Lin PC, Lee MY, Lin JT, Hsiao LT, Chen PM, Chiou TJ. Virus reactivation in high-risk non-Hodgkin’s lymphoma patients after autologous CD34+ -selected peripheral blood progenitor cell transplantation. Int J Hematol. 2008;87:434–439. doi: 10.1007/s12185-008-0053-z. [DOI] [PubMed] [Google Scholar]
- 3.Rossini F, Terruzzi E, Cammarota S, Morini F, Fumagalli M, Verga L, Elli E, Verga M, Miccolis I, Parma M, et al. Cytomegalovirus infection after autologous stem cell transplantation: incidence and outcome in a group of patients undergoing a surveillance program. Transpl Infect Dis. 2005;7:122–125. doi: 10.1111/j.1399-3062.2005.000111.x. [DOI] [PubMed] [Google Scholar]
- 4.Fassas AB, Bolaños-Meade J, Buddharaju LN, Rapoport A, Cottler-Fox M, Chen T, Lovchik JC, Cross A, Tricot G. Cytomegalovirus infection and non-neutropenic fever after autologous stem cell transplantation: high rates of reactivation in patients with multiple myeloma and lymphoma. Br J Haematol. 2001;112:237–241. doi: 10.1046/j.1365-2141.2001.02487.x. [DOI] [PubMed] [Google Scholar]
- 5.Boeckh M, Stevens-Ayers T, Bowden RA. Cytomegalovirus pp65 antigenemia after autologous marrow and peripheral blood stem cell transplantation. J Infect Dis. 1996;174:907–912. doi: 10.1093/infdis/174.5.907. [DOI] [PubMed] [Google Scholar]
- 6.Hebart H, Schröder A, Löffler J, Klingebiel T, Martin H, Wassmann B, Gerneth F, Rabenau H, Jahn G, Kanz L, et al. Cytomegalovirus monitoring by polymerase chain reaction of whole blood samples from patients undergoing autologous bone marrow or peripheral blood progenitor cell transplantation. J Infect Dis. 1997;175:1490–1493. doi: 10.1086/516484. [DOI] [PubMed] [Google Scholar]
- 7.Bilgrami S, Aslanzadeh J, Feingold JM, Bona RD, Clive J, Dorsky D, Edwards RL, Tutschka PJ. Cytomegalovirus viremia, viruria and disease after autologous peripheral blood stem cell transplantation: no need for surveillance. Bone Marrow Transplant. 1999;24:69–73. doi: 10.1038/sj.bmt.1701827. [DOI] [PubMed] [Google Scholar]
- 8.Ng AP, Worth L, Chen L, Seymour JF, Prince HM, Slavin M, Thursky K. Cytomegalovirus DNAemia and disease: incidence, natural history and management in settings other than allogeneic stem cell transplantation. Haematologica. 2005;90:1672–1679. [PubMed] [Google Scholar]
- 9.Han XY. Epidemiologic analysis of reactivated cytomegalovirus antigenemia in patients with cancer. J Clin Microbiol. 2007;45:1126–1132. doi: 10.1128/JCM.01670-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmberg LA, Boeckh M, Hooper H, Leisenring W, Rowley S, Heimfeld S, Press O, Maloney DG, McSweeney P, Corey L, et al. Increased incidence of cytomegalovirus disease after autologous CD34-selected peripheral blood stem cell transplantation. Blood. 1999;94:4029–4035. [PubMed] [Google Scholar]
- 11.Offidani M, Corvatta L, Olivieri A, Rupoli S, Frayfer J, Mele A, Manso E, Montanari M, Centurioni R, Leoni P. Infectious complications after autologous peripheral blood progenitor cell transplantation followed by G-CSF. Bone Marrow Transplant. 1999;24:1079–1087. doi: 10.1038/sj.bmt.1702033. [DOI] [PubMed] [Google Scholar]
- 12.Ljungman P, de la Camara R, Cordonnier C, Einsele H, Engelhard D, Reusser P, Styczynski J, Ward K. Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant. 2008;42:227–240. doi: 10.1038/bmt.2008.162. [DOI] [PubMed] [Google Scholar]
- 13.Lee MY, Chiou TJ, Hsiao LT, Yang MH, Lin PC, Poh SB, Yen CC, Liu JH, Teng HW, Chao TC, et al. Rituximab therapy increased post-transplant cytomegalovirus complications in Non-Hodgkin’s lymphoma patients receiving autologous hematopoietic stem cell transplantation. Ann Hematol. 2008;87:285–289. doi: 10.1007/s00277-007-0397-0. [DOI] [PubMed] [Google Scholar]
- 14.Marchesi F, Giannotti F, Avvisati G, Petti MC, Pimpinelli F, Paba P, Dessanti ML, Cerretti R, Tirindelli MC, Picardi A, et al. The potential role of pre-transplant HBcIgG seroposivity as predictor of clinically relevant cytomegalovirus infection in patients with lymphoma undergoing autologous hematopoietic stem cell transplantation: a study from the Rome Transplant Network. Am J Hematol. 2012;87:213–217. doi: 10.1002/ajh.22214. [DOI] [PubMed] [Google Scholar]
- 15.Marchesi F, Mengarelli A, Giannotti F, Tendas A, Anaclerico B, Porrini R, Picardi A, Cerchiara E, Dentamaro T, Chierichini A, et al. High incidence of post-transplant cytomegalovirus reactivations in myeloma patients undergoing autologous stem cell transplantation after treatment with bortezomib-based regimens: a survey from the Rome transplant network. Transpl Infect Dis. 2014;16:158–164. doi: 10.1111/tid.12162. [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Goulston C, Sanders S, Lampas M, Zangari M, Tricot G, Hanson KE. Cytomegalovirus reactivation following autologous peripheral blood stem cell transplantation for multiple myeloma in the era of novel chemotherapeutics and tandem transplantation. Biol Blood Marrow Transplant. 2012;18:1753–1758. doi: 10.1016/j.bbmt.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Marchesi F, Pimpinelli F, Dessanti ML, Gumenyuk S, Palombi F, Pisani F, Romano A, Spadea A, Maschio M, Ensoli F, et al. Evaluation of risk of symptomatic cytomegalovirus reactivation in myeloma patients treated with tandem autologous stem cell transplantation and novel agents: a single-institution study. Transpl Infect Dis. 2014;16:1032–1038. doi: 10.1111/tid.12309. [DOI] [PubMed] [Google Scholar]
- 18.Drew WL. Laboratory diagnosis of cytomegalovirus infection and disease in immunocompromised patients. Curr Opin Infect Dis. 2007;20:408–411. doi: 10.1097/QCO.0b013e32821f6010. [DOI] [PubMed] [Google Scholar]
- 19.Gor D, Sabin C, Prentice HG, Vyas N, Man S, Griffiths PD, Emery VC. Longitudinal fluctuations in cytomegalovirus load in bone marrow transplant patients: relationship between peak virus load, donor/recipient serostatus, acute GVHD and CMV disease. Bone Marrow Transplant. 1998;21:597–605. doi: 10.1038/sj.bmt.1701139. [DOI] [PubMed] [Google Scholar]
- 20.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 21.Boeckh M, Gooley TA, Reusser P, Buckner CD, Bowden RA. Failure of high-dose acyclovir to prevent cytomegalovirus disease after autologous marrow transplantation. J Infect Dis. 1995;172:939–943. doi: 10.1093/infdis/172.4.939. [DOI] [PubMed] [Google Scholar]
- 22.Bayram A, Ozkur A, Erkilic S. Prevalence of human cytomegalovirus co-infection in patients with chronic viral hepatitis B and C: a comparison of clinical and histological aspects. J Clin Virol. 2009;45:212–217. doi: 10.1016/j.jcv.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Lian Y, Wu W, Shi Y. [Preliminary study on relationship between different viral pathogenesis and disease prognosis in patients with severe viral hepatitis] Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1999;13:355–357. [PubMed] [Google Scholar]
- 24.Daar ES, Lynn H, Donfield S, Gomperts E, O’Brien SJ, Hilgartner MW, Hoots WK, Chernoff D, Arkin S, Wong WY, et al. Hepatitis C virus load is associated with human immunodeficiency virus type 1 disease progression in hemophiliacs. J Infect Dis. 2001;183:589–595. doi: 10.1086/318539. [DOI] [PubMed] [Google Scholar]
- 25.Cavanaugh VJ, Guidotti LG, Chisari FV. Inhibition of hepatitis B virus replication during adenovirus and cytomegalovirus infections in transgenic mice. J Virol. 1998;72:2630–2637. doi: 10.1128/jvi.72.4.2630-2637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DaPalma T, Doonan BP, Trager NM, Kasman LM. A systematic approach to virus-virus interactions. Virus Res. 2010;149:1–9. doi: 10.1016/j.virusres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu YC, Lu PL, Hsiao HH, Chang CS, Liu TC, Yang WC, Lin SF. Cytomegalovirus infection and disease after allogeneic hematopoietic stem cell transplantation: experience in a center with a high seroprevalence of both CMV and hepatitis B virus. Ann Hematol. 2012;91:587–595. doi: 10.1007/s00277-011-1351-8. [DOI] [PubMed] [Google Scholar]
- 28.Ozdemir E, St John LS, Gillespie G, Rowland-Jones S, Champlin RE, Molldrem JJ, Komanduri KV. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood. 2002;100:3690–3697. doi: 10.1182/blood-2002-05-1387. [DOI] [PubMed] [Google Scholar]


