Abstract
Upper non-variceal gastrointestinal bleeding is a condition that requires immediate medical intervention and has a high associated mortality rate (exceeding 10%). The vast majority of upper gastrointestinal bleeding cases are due to peptic ulcers. Helicobacter pylori infection, non-steroidal anti-inflammatory drugs and aspirin are the main risk factors for peptic ulcer disease. Endoscopic therapy has generally been recommended as the first-line treatment for upper gastrointestinal bleeding as it has been shown to reduce recurrent bleeding, the need for surgery and mortality. Early endoscopy (within 24 h of hospital admission) has a greater impact than delayed endoscopy on the length of hospital stay and requirement for blood transfusion. This paper aims to review and compare the efficacy of the types of endoscopic hemostasis most commonly used to control non-variceal gastrointestinal bleeding by pooling data from the literature.
Keywords: Upper gastrointestinal bleeding, Non-variceal bleeding, Endoscopic hemostasis, Endoscopic therapy
Core tip: Review and comparison the efficacy of the most commonly used types of endoscopic hemostasis for the control of non-variceal gastrointestinal bleeding in clinical practice by pooling data from the literature.
INTRODUCTION
Acute upper gastrointestinal bleeding (UGIB) is a common medical entity for which endoscopy has become the primary diagnostic and therapeutic technique. Endoscopy performed in patients with UGIB corresponds with a reduction in required blood transfusions and length of intensive care unit/total hospital stay[1]. Upper endoscopy is required for most patients with UGIB and should be performed within 24 h of hospital admission after adequate prior fluid resuscitation[2]. The key to improving outcomes is the proper initial management of individuals who present with UGIB. In most clinical conditions, the vast majority (80%-90%) of episodes of acute upper gastrointestinal bleeding are secondary to non-variceal origin. This review addresses different endoscopic techniques of hemostasis that are used to treat acute upper gastrointestinal bleeding of non-variceal origin (NVUGIB) in the world practice.
EPIDEMIOLOGY
UGIB is mostly non-variceal in origin and still remains one of the most common challenges encountered by surgeons, gastroenterologists and endoscopists in a daily clinical setting. The incidence rate of non-variceal UGIB ranges from 50 to 150 per 100000 adults/year[3]. In spite of major advances in the approaches used to manage non-variceal UGIB over the past 2 decades, including the peptic ulcer bleeding prevention, the optimal use of endoscopic therapy, as well as the use of adjuvant high-dose proton pump inhibitors (PPIs) to eradicate Helicobacter pylori, it is still associated with considerable morbidity, mortality, and health care costs. The most common non-variceal bleeding etiologies include gastroduodenal peptic ulcer (20%-50%), gastroduodenal erosions (8%-15%), Mallory-Weiss tears (8%-15%), erosive esophagitis (5%-15%), arterio-venous malformations/GAVE (5%); several other conditions [e.g., Dieulafoy’s lesion, upper gastrointestinal (GI) tract malignancy] make up the remaining causes[4-7]. Peptic ulcer disease still remains the most common cause of acute NVUGIB and accounts for at least 50% of cases. Ulcers with signs of active spurting (Forrest class IA) or oozing blood (Forrest class IB) and ulcers with a nonbleeding visible vessel (Forrest IIA) are at high risk of recurrent bleeding when only medical therapy is used. Thus, endoscopic hemostasis is required for patients with high-risk stigmata [IA, IB] or a visible vessel in an ulcer niche [IIA]. Clean-based ulcers (Forrest class III) or flat pigmented spots in the ulcer bed (Forrest class IIC) are low-risk lesions that only rebleed in 4% to 13% of cases and can therefore be treated with pharmacotherapy alone and considered for outpatient management[8,9]. Ulcers with adherent clots (Forrest class IIB) have an intermediate risk of rebleeding (approximately 25%) that depends on the underlying lesion. For that reason, clot removal should be performed with vigorous irrigation and manipulation with an endoscope, forceps, or snare. In patients suffering from peptic ulcer disease, duodenal ulcer bleeding appears more frequently than from gastric ulcers[10]. A Blatchford score or pre-endoscopic Rockall score (based on age, comorbidity, and the presence or absence of hemodynamic instability) should be used to stratify risk and determine which patients require prompt endoscopy or, conversely, to determine suitability for early discharge (Table 1). The Blatchford score, a validated risk-stratification tool based solely on clinical and laboratory variables, is used to predict the need for endoscopic intervention in patients with acute upper GI hemorrhage. A higher score indicates a higher likelihood of needing endoscopic intervention (score ranges from 0 to 23). The clinical Rockall score (i.e., the score obtained before endoscopy is performed) is calculated solely on the basis of clinical variables at the time of patient presentation. The complete Rockall score makes use of both clinical and endoscopic criteria to assess patient risk of re-bleeding and mortality. Rockall score ranges from 0 to 11 points, with higher scores indicating a higher risk for a poor outcome (Table 2)[11,12].
Table 1.
Blatchford scoring: Admission risk markers and associated score component values[13]
| Admission risk marker | Score component value |
| Blood urea, mmol/L | |
| 6.5 to ≤ 8 | 2 |
| 8.0 to < 10.0 | 3 |
| 10.0 to < 25 | 4 |
| ≥ 25 | 6 |
| Hemoglobin for men, g/dL | |
| 12.0-13.0 | 1 |
| 10.0 to < 12.0 | 3 |
| < 10.0 | 6 |
| Hemoglobin for women, g/dL | |
| 10.0 to < 12.0 | 1 |
| 10 | 6 |
| Systolic blood pressure, mmHg | |
| 100-109 | 1 |
| 90-99 | 2 |
| < 90 | 3 |
| Other markers | |
| Pulse ≥ 100/min | 1 |
| Presentation with melena | 1 |
| Presentation with syncope | 2 |
| Hepatic disease | 2 |
| Cardiac failure | 2 |
Table 2.
Complete rockall risk scoring system for assessment after an episode of acute upper gastrointestinal bleeding[12]
| Variables | Score 0 | Score 1 | Score 2 | Score 3 |
| Age | Younger than 60 yr | 60-79 yr | 80 yr or older | - |
| Shock symptoms, systolic blood pressure, heart rate | Shock absent, blood pressure 100 mmHg or greater, heart rate 100 bpm or greater | Tachycardia, blood pressure 100 mmHg or greater, heart rate 100 bpm or greater | Hypotension, blood pressure less than 100 mmHg | - |
| Comorbidities | No major comorbidity | - | Heart failure, coronary artery disease, any major comorbidity | Renal failure, liver failure, disseminated malignancy |
| Endoscopic diagnosis | Mallory-Weiss tear or no lesion identified, and no stigmata of recent hemorrhage | All other diagnoses | Malignancy of upper GI tract | - |
| Stigmata of recent hemorrhage | Low-risk | - | High-risk | - |
Low-risk stigmata of bleeding: Clean base ulcer, pigmented spots; High-risk stigmata of bleeding: Adherent clot, visible or spurting vessel, active bleeding; Bpm: Beats per minute; GI: Gastrointestinal.
ENDOSCOPIC MANAGEMENT
The aim of therapeutic endoscopy is to stop any ongoing bleeding and to prevent rebleeding. Cooper et al[13] studied the effectiveness of performing an early endoscopy within the first 24 h of an acute UGIB episode and found it to be associated with reductions in the length of hospital stay, the rate of recurrent bleeding, and the need for emergent surgical intervention. According to the 2010 international consensus on non-variceal upper gastrointestinal bleeding, early endoscopy (within 24 h of presentation) is appropriate for most patients with UGIB[2]. In cases of rebleeding, a second attempt at endoscopic therapy is recommended to reduce the need for surgery. In patients who have undergone failed endoscopic therapy, surgery should be considered. Despite adequate initial endoscopic therapy, recurrent UGIB can occur in up to 24% of high-risk patients. The use of PPI therapy in addition to endoscopic therapy reduces the rate of recurrent bleeding to approximately 10%. Patients with recurrent bleeding generally respond favorably to repeated endoscopic therapy. Routine second-look endoscopy, defined as a planned endoscopy performed within 24 h of the initial endoscopy, is not recommended. In cases where the initial endoscopy failed to identify the source (e.g., because of a large clot in the stomach) or if there are concerns that inadequate therapy was delivered, second-look endoscopy may be appropriate (Table 3).
Table 3.
Recommendations of the american society for gastrointestinal endoscopy concerning upper gastrointestinal bleeding management[38]
| We recommend that patients with UGIB be adequately resuscitated before endoscopy |
| We recommend antisecretory therapy with PPIs for patients with bleeding caused by peptic ulcers or in those with suspected peptic ulcer bleeding awaiting endoscopy |
| We suggest prokinetic agents in patients with a high probability of having fresh blood or a clot in the stomach when undergoing endoscopy |
| We recommend endoscopy to diagnose the etiology of acute UGIB. The timing of endoscopy should depend on clinical factors. Urgent endoscopy (within 24 h of presentation) is recommended for patients with a history of malignancy or cirrhosis, presentation with hematemesis, and signs of hypovolemia including hypotension, tachycardia and shock, and a hemoglobin < 8 g/dL |
| We recommend endoscopic therapy for peptic ulcers with high-risk stigmata (active spurting, visible vessel). The management of PUD with an adherent clot is controversial. Recommended endoscopic treatment modalities include injection (sclerosants, thrombin, fibrin, or cyanoacrylate glue), cautery, and mechanical therapies |
| We recommend against epinephrine injection alone for peptic ulcer bleeding. If epinephrine injection is performed, it should be combined with a second endoscopic treatment modality (e.g., cautery or clips) |
| We recommend that patients with low-risk lesions be considered for outpatient management |
| We recommend against routine second-look endoscopy in patients who have received adequate endoscopic therapy |
| We recommend repeat endoscopy for patients with evidence of recurrent bleeding |
UGIB: Upper gastrointestinal bleeding; PPIs: Proton pump inhibitors; PUD: Peptic ulcer disease.
Currently, the efficacy and safety of endoscopic hemostasis rely on the identification of lesions that are suitable for endoscopic therapy, the selection of the appropriate hemostatic devices, attention to technique, and prompt recognition and management of procedure-related adverse events. The suitable technique should be chosen based on the appearance of the bleeding focus and the related risk for persistent or recurrent bleeding.
The traditional endoscopic modalities are injection, mechanical therapy, and thermal approaches. Injection agents include saline, dilute epinephrine, sclerosing agents (ethanolamine, polidocanol, absolute alcohol, and sodium tetradecyl sulfate), and tissue adhesives (cyanoacrylate, thrombin, and fibrin glue). Mechanical therapy offers endoscopic clips and band ligation. Thermal devices deliver electrical current (through direct contact or via an inert gas plasma) or heat to the target tissue. Moreover, a few new technologies have emerged, such as hemostatic powders.
INJECTION TREATMENT
Injection needles consist of an outer sheath (plastic, Teflon, or stainless steel) and an inner hollow-core needle (19-25 gauge)[14]. Using a handle on the end of the needle sheath, the operator can retract the needle into the sheath for safe passage through the working channel of the endoscope. When the catheter is placed near the target tissue, the needle is extended a preset distance out of the end of the sheath, and a syringe attached to the handle is used to inject liquid agents into the target tissue. Dilute epinephrine in saline (1:10000) is applied with an injection needle in 0.5-1.0 mL boluses to the four quadrants around the high-risk stigmata or to the base of the active bleeding site and then in the middle of it, up to a total of 10 mL[15,16]. Some practitioners prefer to use absolute alcohol in much smaller volumes (1-2 mL in 0.1 mL aliquots) or combinations of epinephrine and alcohol or sclerosants, which are used for the treatment of varices. Epinephrine injection therapy promotes initial hemostasis through a combination of vasoconstriction, compression (local tamponade), and platelet activation, but this effect declines after 20 min. If epinephrine injection is performed, it should usually be combined with a second endoscopic treatment modality (e.g., electrocautery or clips)[17]. If epinephrine is used alone, there is a significant risk of rebleeding. This can be reduced by injecting large volumes, as high as 30 mL, which are associated with no clearly described cardiologic adverse events, and the rebleeding rate decreases linearly with the injected volume[18,19]. Dilute epinephrine injection is inferior at preventing rebleeding and surgery when compared with bipolar electrocoagulation, clips, or fibrin glue[20]. Other injected substances, such as sclerosing agents (e.g., polidocanol, ethanolamine, and ethanol), have similar efficacy but more side effects, including transmural necrosis or perforation[21]. Another class of injectable agents are tissue adhesives, including cyanoacrylate glues, thrombin and fibrin, which are used to create a primary seal at the site of bleeding by inducing thrombosis through direct tissue injury. However, they may also evoke tissue necrosis and, hence, the limit for injected volumes is less than 1 mL. Cyanoacrylate (n-butyl-2-cyanoacrylate, Histoacryl; Braun, Germany) is a liquid tissue adhesive that consists of monomers that rapidly polymerize (creating long and strong chains) in an exothermic reaction after contact with hydroxide ions[22]. Cyanoacrylate is widely used for the management of bleeding esophageal and gastric varices, but it is not recommended for acute non-variceal upper gastrointestinal bleeding. However, in difficult-to-arrest non-variceal bleeding, it could be a useful and safe therapeutic tool.
MECHANICAL THERAPY
Mechanical therapy refers to the use of a device that causes physical tamponade at a bleeding site[15]. It includes endoscopic clips and band ligation (Figures 1 and 2). Metal clips are particularly useful for small bleeding ulcers (i.e., Dieulafoy lesions), for Mallory-Weiss tears, and for large, visible vessels. Endoscopic clips are deployed over a bleeding site (e.g., visible vessel) and typically slough off within days to weeks after placement. Endoscopic clips function by mechanical compression of the bleeding vessel and theoretically cause less tissue injury than cautery methods. Band ligation is widely used in variceal bleeding. However, it has also been found to be effective in treating bleeding Dieulafoy’s lesions[23].
Figure 1.
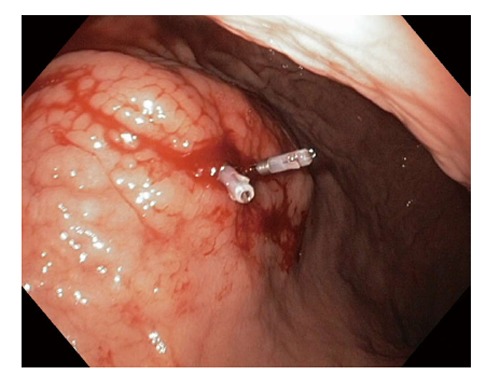
Endoscopic clips.
Figure 2.
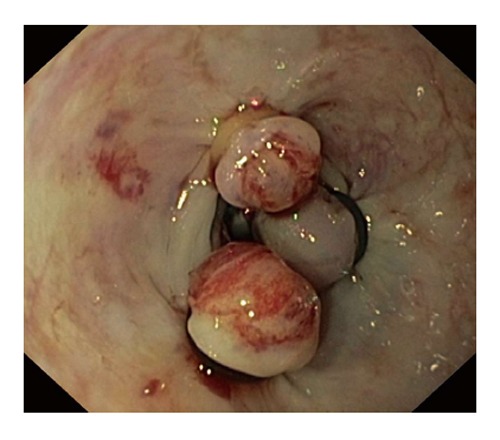
Endoscopic band ligation.
The Over-The-Scope Clip (OTSC; Ovesco, Tübingen, Germany) is a modern endoscopic clipping device designed for tissue approximation. It has been used for the closure of fistulas and perforations. OTSC consists of a nitinol clip mounted on an applicator cap that is affixed to the tip of the endoscope. The deployed clip captures and closes tissue suctioned into the applicator cap, thus compressing the lesions until healing. Studies on animal models and limited data from clinical use support the efficacy of OTSC for the treatment of GI bleeding, and a number of small case series have shown effective hemostasis resulting from the use of OTSC in patients for whom epinephrine injection or standard clip placement failed[24]. The OTSC is now available on the market and gives the physician a tool for the immediate management of complications, such as deep-wall lesions, difficult bleeding or perforations.
THERMAL THERAPY
Thermal therapies include electrocautery probes (monopolar, bipolar or multipolar) and heater probes, which are referred to as contact thermal modalities, and argon plasma coagulation (APC) and laser phototherapy, which are known as noncontact techniques. Bipolar and multipolar probes provide constant bipolar electrocoagulation, which is assumed to be safer than monopolar diathermy (which produces an unpredictable depth of damage and a higher risk of perforation). A foot pedal controls the delivery of energy. The power output is in watts (W). Maximum power settings are dependent on the generator used but usually do not exceed 50 W. A standard setting is 20 W.
A heater probe provides constant heat at 250 °C, which is released by a diode in the probe tip and directly transferred to tissue to affect coagulation. Contact treatment devices share some common principles. All can be applied tangentially but are better used face-on, if possible. When the vessel is actively bleeding, direct probe pressure on the vessel or feeding vessel will reduce the flow and increase the effectiveness of treatment. Mechanically pressuring the probe tip directly to the bleeding site, combined with heat or electrical current to coagulate blood vessels, is a process known as “captive coagulation”. The bipolar and heater probes incorporate a flushing water jet, which helps to prevent sticking.
Argon plasma coagulation, which is performed without tissue contact, uses the electrical conductivity of argon gas (Figure 3). The argon, passed down an electrode catheter and energized via an intelligent-circuitry electrosurgical unit and patient plate, ionizes to produce a local plasma arc. The produced heating effect is inherently superficial (2-3 mm at most, unless the current is applied in the same place for many seconds). Therefore, APC is used to treat superficial mucosal lesions, such as vascular malformations and gastric antral vascular ectasias. The APC probe should be positioned 2-10 mm from the lesion and the argon gas flow should be 1.5-2 L/min at a power of 40-50 W[25,26].
Figure 3.
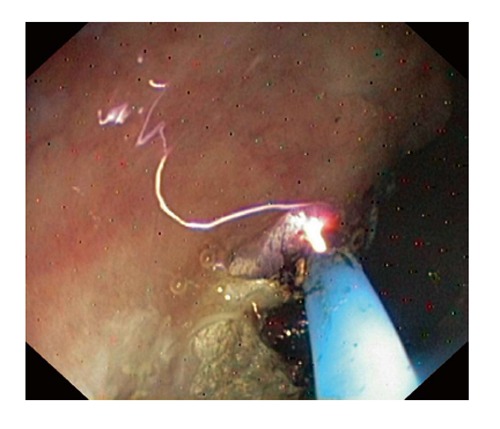
Argon plasma coagulation.
Laser phototherapy uses an Nd:YAG laser to create hemostasis by generating heat to induce direct vessel coagulation. This is a noncontact thermal method. It is not as effective as captive coagulation because it lacks the use of compression to create a tamponade effect[27]. An additional deterrent to its use is expense.
To perform laser coagulation, the area near the vessel is first injected with epinephrine to reduce blood flow (reducing the heat-sink effect). Then, the laser is applied around the vessel, producing a wall of edema. Caution must be taken to avoid drilling into the vessel with the laser, which can cause increased bleeding.
TOPICAL HEMOSTATIC AGENTS
Topical hemostatic agents are new tools used in endoscopic hemostasis. Three different powders are available: Hemospray (Cook Medical, Winston-Salem, NC, United States), Ankaferd BloodStopper, and EndoClot (EndoClot Plus Inc., Santa Clara, CA, United States)[28].
Hemospray (TC-325), a novel proprietary inorganic powder, has recently been approved in Canada for the management of NVUGIB[29]. The powder is administered through a 10- or 7-French catheter via a CO2-pressurised canister. It achieves hemostasis by adhering to the bleeding site, leading to mechanical tamponade and, by concentrating and activating platelets and coagulation factors, promoting thrombus formation. Its ability to cover large areas with multiple bleeding points makes it a suitable choice for hemorrhagic gastritis, gastric antral vascular ectasia, radiation-induced mucosal injury and malignancy-related bleeding[29]. Other advantages include ease of use, the lack of need for precise lesion targeting and access to lesions in difficult locations.
Hemostatic sprays derived from plants have also been invented. Clinical use of these agents for endoscopic hemostasis is currently limited to the off-label use of ankaferd blood stopper (ABS) (Ankaferd Health Products Ltd., Istanbul, Turkey), a mixture of extracts from several plants that is approved in Turkey for the topical treatment of dental and postsurgical external bleeding. ABS is delivered through the working channel of the scope using a spray catheter.
The EndoClot Polysaccharide Hemostatic System (EndoClot Plus Inc., Santa Clara, CA, United States) is the latest available hemostatic powder. It consists of starch, which explains its availability in European countries, Australia, Malaysia, and Turkey, despite a lack of rigorous scientific evidence for its efficacy. The effectiveness of the powder at controlling and preventing bleeding related to endoscopic mucosal resection has been recently described[28].
PRE-ENDOSCOPY PHARMACOLOGIC THERAPY
Prokinetic agents, such as intravenously administered erythromycin or metoclopramide, should be considered for use 30 min prior to endoscopy to improve visibility[30]. Intravenous prokinetic agents, when administered 20 to 120 min before endoscopy in patients with acute UGIB, decrease the need for a repeat endoscopy to determine the site and cause of bleeding. However, their use has not demonstrated any benefit to other clinical parameters, such as transfusion requirement, length of hospital stay, or need for surgery.
Proton pump inhibitor (PPI) therapy is another pharmacologic intervention that should be considered in patients suspected to have UGIB (e.g., pantoprazole 80 mg bolus followed by 8 mg/h continuous drip or 40 mg intravenously every 12 h). The infusion is continued for 48-72 h. The relative efficacy of PPIs may be due to their superior ability to maintain gastric pH at a level above 6.0, thereby protecting ulcer clots from fibrinolysis. Multiple analyses have shown that applying PPI therapy before a procedure significantly reduced the rate of high-risk stigmata that are identified by endoscopy and the need for endoscopic therapy. Therefore, intravenous PPI therapy is recommended for patients who are suspected of having acute NVUGIB.
EFFICACY AND COMPARATIVE ANALYSIS
Gastroduodenal peptic ulcers are by far the most common etiology of UGIB, accounting for 50% of admissions among patients with upper gastrointestinal hemorrhage[28]. Multiple meta-analyses evaluating endoscopic therapies for bleeding peptic ulcers have demonstrated that thermal devices, injectable agents other than epinephrine (i.e., sclerosants and thrombin/fibrin glue), and clips were all effective methods for achieving hemostasis in PUD, with no single modality being superior to the others. In particular, hemoclip placement, thermocoagulation (e.g., heater probe), and electrocoagulation (e.g., Gold probe, BICAP probe) all seem to be equivalent alternatives[20,31-34]. Dual combination therapy (i.e., epinephrine injection plus other injections or thermal or mechanical methods) was proven to be significantly superior to epinephrine injection alone, but displays no advantage over thermal or mechanical monotherapy. This means that epinephrine should no longer be applied as a monotherapy for treating lesions with high-risk stigmata and should only be used in combination with other methods as these combinations significantly reduce the risk of rebleeding and surgery. Prospective randomized trials have demonstrated that thermal therapy results in significant reductions in bleeding, blood transfusions, length of hospital stays, and the need for urgent surgery in patients with actively bleeding ulcers or nonbleeding ulcers with visible vessels[35]. A meta-analysis of randomized trials that evaluated rebleeding rates following injection, thermal therapy, clips, or combination therapy showed that clips were superior to thermal therapy[33]. The remaining causes of UGIB account for up to 50% of cases. For gastric antral vascular ectasia (GAVE), APC remains the most commonly reported modality that is usually performed over multiple endoscopic sessions. APC is associated with a decrease in transfusion requirements[36]. Mallory-Weiss tear bleeding usually spontaneously stops, with the rates of rebleeding from this etiology reaching up to 10%. Patients with active bleeding or oozing require endoscopic therapy. Bipolar electrocoagulation, epinephrine injection, clips, and band ligation have all been used successfully with no difference in immediate hemostasis or rebleeding. Endoscopic therapy is the first choice in bleeding Dieulafoy’s lesions and is usually performed via clipping or banding of the lesion[23]. Endoscopic clipping is superior to endoscopic injection and is comparable to thermocoagulation in securing hemostasis in bleeding peptic ulcers and Dieulafoy’s lesions[28]. Endoscopic hemostasis of bleeding upper GI tract tumors has proven to be less effective and to have higher rates of rebleeding. Various endoscopic treatment modalities have been described with no clear recommendations. Several studies have reported that cyanoacrylate was used for acute non-variceal gastrointestinal bleeding cessation[21]. Application of cyanoacrylate (by injection and/or spraying) is a safe and effective method for achieving immediate hemostasis when conventional endoscopic treatment has been unsuccessful. This technique is easy to perform and should be considered in cases of patients with difficult-to-arrest acute NVGIB. Recently, promising preliminary data have been reported following the use of the hemostatic powder TC-325 (Hemospray) for bleeding control from upper GI tract tumors[37].
CONCLUSION
Endoscopy is the mainstay for the modern management of NVUGIB. Ideally, endoscopy should be performed within 24 h of presentation, after adequate resuscitation has been performed. Many safe and effective devices are available for endoscopic hemostasis. Combination therapy using the injection of epinephrine plus another hemostatic technique is more effective than epinephrine alone. Hemospray is a new and promising endoscopic therapy. Patients with high-risk stigmata should receive continuous intravenous PPI administration for 72 h after endoscopy. After the acute phase, the underlying cause of the lesion should be verified and treated, when possible. The choice of therapy should remain at the discretion of the physician, based on the nature and position of the lesion, the availability and experience of the endoscopist and the previous endoscopic therapy that the patient has received.
Footnotes
P- Reviewer: Velayos B S- Editor: Ma YJ L- Editor: A E- Editor: Liu SQ
Conflict-of-interest statement: The authors have no conflict of interest related to the manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 24, 2015
First decision: June 2, 2015
Article in press: September 7, 2015
References
- 1.Chak A, Cooper GS, Lloyd LE, Kolz CS, Barnhart BA, Wong RC. Effectiveness of endoscopy in patients admitted to the intensive care unit with upper GI hemorrhage. Gastrointest Endosc. 2001;53:6–13. doi: 10.1067/mge.2001.108965. [DOI] [PubMed] [Google Scholar]
- 2.Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, Sinclair P; International Consensus Upper Gastrointestinal Bleeding Conference Group. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101–113. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 3.Rockall TA, Logan RF, Devlin HB, Northfield TC. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ. 1995;311:222–226. doi: 10.1136/bmj.311.6999.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Leerdam ME, Vreeburg EM, Rauws EA, Geraedts AA, Tijssen JG, Reitsma JB, Tytgat GN. Acute upper GI bleeding: did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol. 2003;98:1494–1499. doi: 10.1111/j.1572-0241.2003.07517.x. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert DA. Epidemiology of upper gastrointestinal bleeding. Gastrointest Endosc. 1990;36:S8–13. [PubMed] [Google Scholar]
- 6.Branicki FJ, Coleman SY, Fok PJ, Pritchett CJ, Fan ST, Lai EC, Mok FP, Cheung WL, Lau PW, Tuen HH. Bleeding peptic ulcer: a prospective evaluation of risk factors for rebleeding and mortality. World J Surg. 1990;14:262–269; discussion 269-270. doi: 10.1007/BF01664889. [DOI] [PubMed] [Google Scholar]
- 7.Barkun A, Bardou M, Marshall JK. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843–857. doi: 10.7326/0003-4819-139-10-200311180-00012. [DOI] [PubMed] [Google Scholar]
- 8.Holster IL, Kuipers EJ. Update on the endoscopic management of peptic ulcer bleeding. Curr Gastroenterol Rep. 2011;13:525–531. doi: 10.1007/s11894-011-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappell MS. Therapeutic endoscopy for acute upper gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2010;7:214–229. doi: 10.1038/nrgastro.2010.24. [DOI] [PubMed] [Google Scholar]
- 10.Holster IL, Kuipers EJ. Management of acute nonvariceal upper gastrointestinal bleeding: current policies and future perspectives. World J Gastroenterol. 2012;18:1202–1207. doi: 10.3748/wjg.v18.i11.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316–321. doi: 10.1136/gut.38.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356:1318–1321. doi: 10.1016/S0140-6736(00)02816-6. [DOI] [PubMed] [Google Scholar]
- 13.Cooper GS, Chak A, Way LE, Hammar PJ, Harper DL, Rosenthal GE. Early endoscopy in upper gastrointestinal hemorrhage: associations with recurrent bleeding, surgery, and length of hospital stay. Gastrointest Endosc. 1999;49:145–152. doi: 10.1016/s0016-5107(99)70478-5. [DOI] [PubMed] [Google Scholar]
- 14.Conway JD, Adler DG, Diehl DL, Farraye FA, Kantsevoy SV, Kaul V, Kethu SR, Kwon RS, Mamula P, Rodriguez SA, et al. Endoscopic hemostatic devices. Gastrointest Endosc. 2009;69:987–996. doi: 10.1016/j.gie.2008.12.251. [DOI] [PubMed] [Google Scholar]
- 15.Kovacs TO, Jensen DM. Endoscopic therapy for severe ulcer bleeding. Gastrointest Endosc Clin N Am. 2011;21:681–696. doi: 10.1016/j.giec.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo CC, Hsu PI, Lo GH, Lin CK, Chan HH, Tsai WL, Chen WC, Wu CJ, Yu HC, Cheng JS, et al. Comparison of hemostatic efficacy for epinephrine injection alone and injection combined with hemoclip therapy in treating high-risk bleeding ulcers. Gastrointest Endosc. 2006;63:767–773. doi: 10.1016/j.gie.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 17.Vergara M, Calvet X, Gisbert JP. Epinephrine injection versus epinephrine injection and a second endoscopic method in high risk bleeding ulcers. Cochrane Database Syst Rev. 2007;(2):CD005584. doi: 10.1002/14651858.CD005584.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Lin HJ, Hsieh YH, Tseng GY, Perng CL, Chang FY, Lee SD. A prospective, randomized trial of large- versus small-volume endoscopic injection of epinephrine for peptic ulcer bleeding. Gastrointest Endosc. 2002;55:615–619. doi: 10.1067/mge.2002.123271. [DOI] [PubMed] [Google Scholar]
- 19.Park CH, Lee SJ, Park JH, Park JH, Lee WS, Joo YE, Kim HS, Choi SK, Rew JS, Kim SJ. Optimal injection volume of epinephrine for endoscopic prevention of recurrent peptic ulcer bleeding. Gastrointest Endosc. 2004;60:875–880. doi: 10.1016/s0016-5107(04)02279-5. [DOI] [PubMed] [Google Scholar]
- 20.Laine L, McQuaid KR. Endoscopic therapy for bleeding ulcers: an evidence-based approach based on meta-analyses of randomized controlled trials. Clin Gastroenterol Hepatol. 2009;7:33–47; quiz 1-2. doi: 10.1016/j.cgh.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Scharnke W, Hust MH, Braun B, Schumm W. [Complete gastric wall necrosis after endoscopic sclerotherapy for a gastric ulcer with visible arterial stump] Dtsch Med Wochenschr. 1997;122:606–609. doi: 10.1055/s-2008-1047662. [DOI] [PubMed] [Google Scholar]
- 22.Kurek K, Baniukiewicz A, Swidnicka-Siergiejko A, Dąbrowski A. Application of cyanoacrylate in difficult-to-arrest acute non-variceal gastrointestinal bleeding. Wideochir Inne Tech Maloinwazyjne. 2014;9:489–493. doi: 10.5114/wiitm.2014.44169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alis H, Oner OZ, Kalayci MU, Dolay K, Kapan S, Soylu A, Aygun E. Is endoscopic band ligation superior to injection therapy for Dieulafoy lesion? Surg Endosc. 2009;23:1465–1469. doi: 10.1007/s00464-008-0255-8. [DOI] [PubMed] [Google Scholar]
- 24.Kirschniak A, Kratt T, Stüker D, Braun A, Schurr MO, Königsrainer A. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest Endosc. 2007;66:162–167. doi: 10.1016/j.gie.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 25.Wang HM, Hsu PI, Lo GH, Chen TA, Cheng LC, Chen WC, Lin CK, Yu HC, Chan HH, Tsai WL, et al. Comparison of hemostatic efficacy for argon plasma coagulation and distilled water injection in treating high-risk bleeding ulcers. J Clin Gastroenterol. 2009;43:941–945. doi: 10.1097/MCG.0b013e31819c3885. [DOI] [PubMed] [Google Scholar]
- 26.Karaman A, Baskol M, Gursoy S, Torun E, Yurci A, Ozel BD, Guven K, Ozbakir O, Yucesoy M. Epinephrine plus argon plasma or heater probe coagulation in ulcer bleeding. World J Gastroenterol. 2011;17:4109–4112. doi: 10.3748/wjg.v17.i36.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fallah MA, Prakash C, Edmundowicz S. Acute gastrointestinal bleeding. Med Clin North Am. 2000;84:1183–1208. doi: 10.1016/s0025-7125(05)70282-0. [DOI] [PubMed] [Google Scholar]
- 28.Jacques J, Legros R, Chaussade S, Sautereau D. Endoscopic haemostasis: an overview of procedures and clinical scenarios. Dig Liver Dis. 2014;46:766–776. doi: 10.1016/j.dld.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Sung JJ, Luo D, Wu JC, Ching JY, Chan FK, Lau JY, Mack S, Ducharme R, Okolo P, Canto M, et al. Early clinical experience of the safety and effectiveness of Hemospray in achieving hemostasis in patients with acute peptic ulcer bleeding. Endoscopy. 2011;43:291–295. doi: 10.1055/s-0030-1256311. [DOI] [PubMed] [Google Scholar]
- 30.Park T, Wassef W. Nonvariceal upper gastrointestinal bleeding. Curr Opin Gastroenterol. 2014;30:603–608. doi: 10.1097/MOG.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 31.Marmo R, Rotondano G, Piscopo R, Bianco MA, D’Angella R, Cipolletta L. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: a meta-analysis of controlled trials. Am J Gastroenterol. 2007;102:279–89; quiz 469. doi: 10.1111/j.1572-0241.2006.01023.x. [DOI] [PubMed] [Google Scholar]
- 32.Sung JJ, Tsoi KK, Lai LH, Wu JC, Lau JY. Endoscopic clipping versus injection and thermo-coagulation in the treatment of non-variceal upper gastrointestinal bleeding: a meta-analysis. Gut. 2007;56:1364–1373. doi: 10.1136/gut.2007.123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barkun AN, Martel M, Toubouti Y, Rahme E, Bardou M. Endoscopic hemostasis in peptic ulcer bleeding for patients with high-risk lesions: a series of meta-analyses. Gastrointest Endosc. 2009;69:786–799. doi: 10.1016/j.gie.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 34.Yuan Y, Wang C, Hunt RH. Endoscopic clipping for acute nonvariceal upper-GI bleeding: a meta-analysis and critical appraisal of randomized controlled trials. Gastrointest Endosc. 2008;68:339–351. doi: 10.1016/j.gie.2008.03.1122. [DOI] [PubMed] [Google Scholar]
- 35.Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345–60; quiz 361. doi: 10.1038/ajg.2011.480. [DOI] [PubMed] [Google Scholar]
- 36.Sebastian S, O’Morain CA, Buckley MJ. Review article: current therapeutic options for gastric antral vascular ectasia. Aliment Pharmacol Ther. 2003;18:157–165. doi: 10.1046/j.1365-2036.2003.01617.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen YI, Barkun AN, Soulellis C, Mayrand S, Ghali P. Use of the endoscopically applied hemostatic powder TC-325 in cancer-related upper GI hemorrhage: preliminary experience (with video) Gastrointest Endosc. 2012;75:1278–1281. doi: 10.1016/j.gie.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Hwang JH, Fisher DA, Ben-Menachem T, Chandrasekhara V, Chathadi K, Decker GA, Early DS, Evans JA, Fanelli RD, Foley K, Fukami N, Jain R, Jue TL, Khan KM, Lightdale J, Malpas PM, Maple JT, Pasha S, Saltzman J, Sharaf R, Shergill AK, Dominitz JA, Cash BD; Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. The role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest Endosc. 2012;75:1132–1138. doi: 10.1016/j.gie.2012.02.033. [DOI] [PubMed] [Google Scholar]


