Abstract
Sensing mechanisms for nutrients, in particular dietary fat, operate in the mouth, brain, and gastrointestinal tract and play a key role in regulating feeding behavior and energy balance. Critical to these regulatory mechanisms are the specialized receptors present on taste buds on the tongue, on neurons in specialized centers in the brain, and on epithelial and enteroendocrine cells in the intestinal mucosa. These receptors recognize nutrients and respond by inducing intracellular signals that trigger release of bioactive compounds that influence other organs and help coordinate the response to the meal. Components of dietary fat that are recognized by these receptors are the long-chain fatty acids that act as ligands for 2 G protein-coupled receptors, GPR40 and GPR120, and the fatty acid (FA) translocase/CD36. Recent evidence that emphasizes the important role of CD36 in orosensory, intestinal, and neuronal sensing of FAs under physiologic conditions is highlighted in the review. How this role intersects with that of GPR120 and GPR40 in the regulation of food preference and energy balance is briefly discussed.
Keywords: fat taste, chylomicron, secretin, cholecystokinin, calcium, cAMP, OEA
Introduction
The homeostatic mechanisms that regulate energy balance and body weight involve a complex network of nutrient-sensing pathways that begin with food ingestion. Taste receptors localized on the taste bud cells (TBCs)6 of the lingual epithelium are activated by tastants (salt, sour, sweet, bitter, umami, and fatty) and transmit central signals via sensory afferent nerve fibers. This initiates signaling mechanisms that trigger the cephalic phase of digestion, which prepares the organism for nutrient absorption and utilization and contributes to the regulation of satiation. Remarkably, the chemosensory taste receptors present on the tongue are also found in the gastrointestinal tract. The idea that the gastrointestinal tract functions as a chemosensory organ was first suggested by Bayliss and Starling in 1902 (1). Later, Fujita and Kobayashi (2) proposed the intestinal sensor cell theory that described existence of specialized nutrient-sensing bipolar cells in the intestinal epithelium, including enterocytes and enteroendocrine cells (EECs). The EECs that were subsequently shown to be the primary chemosensorsy cells are strategically stacked between enterocytes that line the epithelium and have cytoplasmic projections that extend to the lumen that enable direct access to luminal contents. On activation the EECs mobilize secretory granules localized in the basolateral portion of the cell to release bioactive compounds that can relay signals via endocrine and/or neural pathways. More than 20 different EECs are reported, and, although they constitute <1% of the epithelial population, they are the largest endocrine organ that releases an array of hormones, peptides, and bioactive amines in response to luminal nutrients (3–5). The primary stimuli for the release of gut peptides are lipids, although glucose produced from polysaccharides and disaccharides by the action of intestinal digestive enzymes and products of protein hydrolysis, peptones, and amino acids also stimulate release of gut peptides, including glucagon-like peptides (GLP-1, GLP-2), and cholecystokinin via different mechanisms (6).
The components of dietary fat that are sensed by TBCs and gut EECs are long-chain FAs (LCFAs) released from TGs by lipases in the mouth (lingual lipase) or intestinal (pancreatic lipase) lumen. LCFAs of chain length >12 carbons are considered the most potent stimulators; however, SCFAs and medium-chain FAs are also capable of eliciting appreciable chemosensory responses (7, 8). Sensing of LCFAs is mediated by the presence of membrane receptors that include a number of G protein-coupled receptors (GPRs) and the FA translocase CD36. The GPRs are a large family of trans-membrane receptors that can initiate activation of signal transduction pathways on interaction with FAs and other ligands. In the gut, members of this class have varied distribution and specificity for FA ligands that lead to release of different EEC products, reviewed in (5). For example, GPR40 and GPR120 respond to medium-chain FAs and LCFAs (9, 10), whereas GPR41 and GPR43 are stimulated by SCFAs. GPR41 and GPR43 were identified in peptide YY-positive rat endocrine cells and in mucosal mast cells that secrete serotonin in the distal small intestine and colon. GPR119 is expressed on rodent and human L and K cells, and its ligand was identified as the endogenous FA product, oleoylethanolamide (11), which stimulates release of GLP-1 and gastric inhibitory peptide (GIP) (12, 13). GPR40 is linked to incretin secretion and colocalizes with secreting K and L cells that secrete GIP and GLP-1 located in the proximal and distal small intestine, respectively (9). GPR120 is abundantly expressed in human and mouse intestine and in the in vitro EEC model, STC-1 cells, and its stimulation was shown to promote secretion of GLP-1 (7). Although GPR40 and GPR120, which bind LCFAs, were initially implicated in orosensory fat taste perception by TBCs, recent reports suggest their influence on fat taste perception is indirect. As discussed in the next sections, GPR40 is not expressed in TBCs, and GPR120, although presence in these cells, is poorly responsive to FAs (14, 15). Both GPR40 and GPR120 are involved in incretin secretion and as a result influence operation of the ileal brake, a reflex response in which luminal contents of the ileum elicit a feedback mechanism to delay gastric emptying and thereby reduce nutrient delivery to distal segments (16).
CD36 is a heavily glycosylated trans-membrane protein that was reported to be involved in cellular LCFA uptake on the basis of affinity labeling with reactive FA derivatives (17). In addition to LCFA CD36 recognizes a broad spectrum of ligands that include native and oxidized lipoproteins, thrombospondin-1, amyloid B, collagen, and malaria-infected erythrocytes (18, 19). CD36 has signal transduction ability triggered by binding of its ligands and mediated by partner kinases that interact with the C terminus of the protein. It is fairly ubiquitous, abundantly expressed in the heart, adipose tissue, skeletal muscle, taste buds, and small intestine. There is now a wealth of evidence to document a prominent role for gut CD36 in fat perception and absorption and in overall regulation of lipid metabolism (Figure 1), recently reviewed elsewhere (18). This review focuses on the role of CD36 in orosensory fat perception and in the chemosensory mechanisms operational in the gut that initiate signaling-mediated regulation of absorption and energy balance.
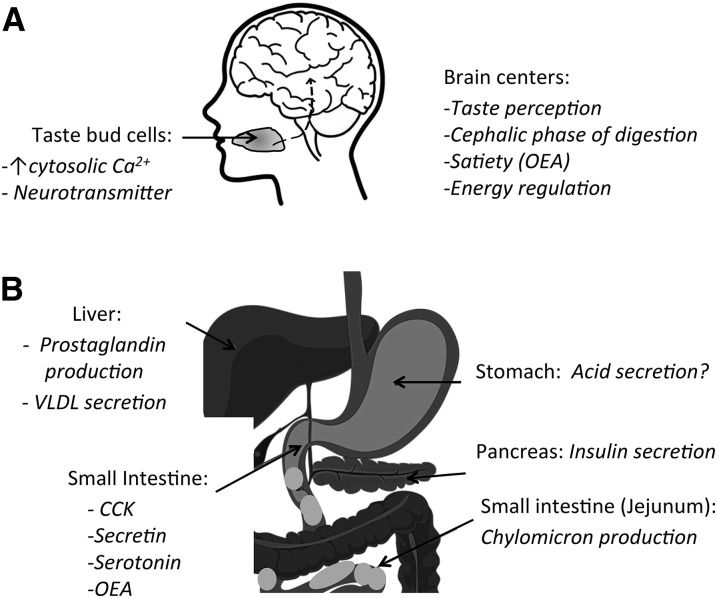
FIGURE 1.
CD36 regulation of multiple steps involved in the processing of a meal. A: Dietary fat is composed mainly of TGs, but the nutrient sensed by TBCs on the tongue is the FAs released from TG digestion by lingual lipase. The FAs interact with CD36 on TBCs in the circumvallate and fungiform papillae (back and sides of the tongue, respectively) to induce an increase in intracellular Ca2+. This ultimately results in neurotransmitter release and signal transmission to brain centers. In the brain, CD36 is important for calcium signaling by hypothalamic neurons, and its depletion prevents fat inhibition of food intake and results in abnormal glucose tolerance (20). B: In the liver, CD36 depletion reduces VLDL output, and this reflects in part increased production of prostaglandins, reviewed in (18). In the pancreas CD36 was shown to mediate the effect of long-chain FAs to induce insulin secretion. In the small intestine CD36 is expressed both on enterocytes and on enteroendocrine cells. It functions in facilitating chylomicron formation and mediating FA-induced release of CCK and secretin and the production of OEA from oleic acid, reviewed in (18). CCK, cholecystokinin; OEA, oleoylethanolamide; TBC, taste bud cell.
CD36 Mediates Orosensory Perception of Fat
In the mouth, the FAs released from dietary TGs by lingual lipase interact with the FA receptors on TBCs. The FA receptors expressed in TBCs are GPR120 and CD36, but a primary role for CD36 in fat taste perception is suggested by recent studies (15, 23–29, 53). Although GPR40 was initially thought to be involved (21), several studies failed to detect its expression in TBCs (18). CD36 in TBCs functions at low FA concentrations to induce calcium signaling and neurotransmitter release, whereas GPR120 is poorly responsive even at high FA concentrations (15). In addition, the absence of GPR120 in the oral cavity is not associated with altered preference for oily and LCFA solutions on the basis of a combination of behavioral and functional studies (14). Interestingly, although not essential for oral fat preference, GPR40 and GPR120 were recently shown to play a critical role after ingestion in the oral stimulation of appetite by fat acting elsewhere in the gut. Compared with control mice, GPR40- and GPR120-double knockout mice consumed less of a flavored solution that was paired with intragastric oil infusions (22).
CD36 is present on the apical surface of circumvallate and foliate papillae in the tongue of rodents (23, 24) and humans (24, 25), and its deletion in mice eliminates fat taste perception and the early cephalic phase of digestion (24) (Figure 1). The cephalic phase triggered by fat on the tongue precedes fat ingestion and involves modest pancreatobiliary and intestinal TG secretions that serve to prime the body’s mechanisms that regulate fat absorption and satiety. Substantially blunted fat preference is also observed in mice with CD36 haploinsufficiency, heterozygous for CD36 deletion, and with CD36 expression that averaged 50% of controls (26).
The blunted fat preference of CD36-null mice can be reversed by experience, likely involving food-related cues other than taste perception. For example, although naive CD36-null mice do not display spontaneous preference for fat, they acquire this preference after repeated exposure to fat. However, the CD36-null mice continue to have lower intakes of high-oil emulsions, indicating persistence of a reduced acceptance of dietary lipid. The cause of the reduced intake might reflect postingestive effects related to altered fat handling or nutrient sensing by the small intestine (27).
LCFAs that bind to CD36 in TBCs induce a rise in cytosolic calcium that ultimately result in release of neurotransmitters (Figure 2), such as 5-hydroxytryptamine and noradrenalin, which are implicated in signaling fat perception to the central nervous system (28). The mechanism that mediates LCFAs that signal in taste buds involves the production of lipid mediators (Figure 2) such as inositol-1, 4, 5-triphosphate (IP3) and diacylglycerol that are generated by the hydrolysis of phosphatidylinositol-4,5-bisphosphate by the action of phospholipase C. IP3 binds to IP3 receptors on the endoplasmic reticulum (ER), leading to ER release of Ca2+ (28). Depletion of ER Ca2+ stores activates plasma membrane Ca2+ channels, called store-operated Ca2+ and consequently Ca2+ influx into the cell. Rapid increases in cytosolic Ca2+ in the TBCs associate with neurotransmitter release toward the afferent nerve fibers that transmit signals of fat taste perception to the brain, initiating the cephalic phase of digestion and appetite regulatory mechanisms.
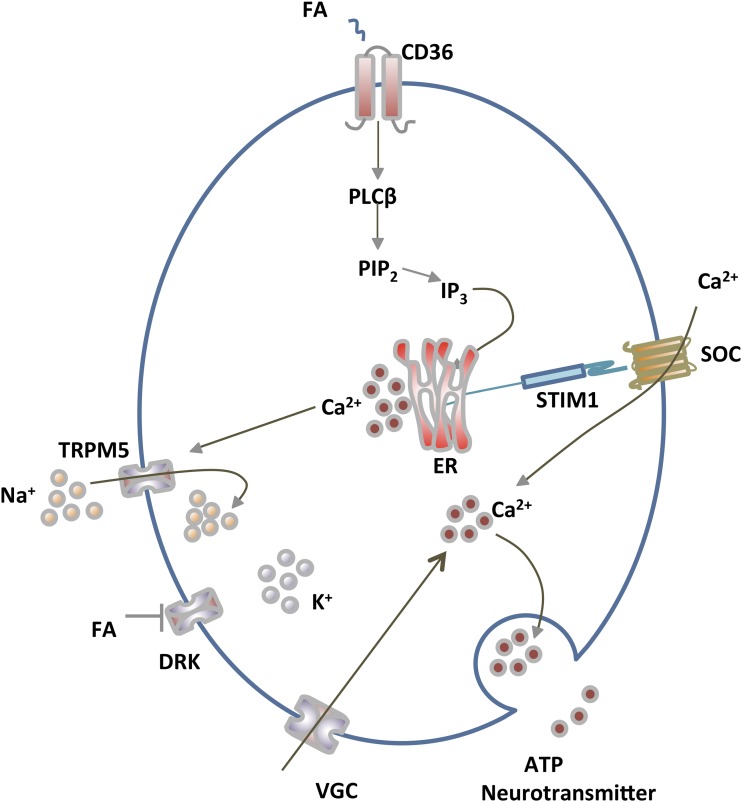
FIGURE 2.
CD36-mediated FA signaling for fat taste perception in TBCs. Long-chain FAs (mainly unsaturated) that bind to CD36 on TBCs activate phospholipase C to generate IP3, which interacts with its receptors on the ER to release Ca2+. ER Ca2+ depletion is sensed by STIM1, which activates membrane SOC influx. Gilbertson and Khan (28) provided evidence that the FAs also induce membrane depolarization by activating inward sodium flux via TRPM5 (and possibly inhibition of DRK-mediated potassium efflux), resulting in VGC influx. The sustained rise in cytosolic Ca2+ culminates in neurotransmitter release and relay of fat taste perception to brain centers (28). The 2 FA receptors identified on TBCs in rodents and humans are CD36 and GPR120. CD36 is the physiologically functional receptor (15). GPR120 might be important for release of GLP-1 and for amplifying signaling at high FA amounts. DRK, delayed rectifying K+ channel; ER, endoplasmic reticulum; GLP, glucagon-like peptide; GPR, G protein-coupled receptor; IP3, inositol-1,4,5-triphosphate; PIP2, phosphatidylinositol-4,5-bisphosphate; PLC, phospholipase C; SOC, store-operated Ca2+; STIM1, stromal interaction molecule 1; TBC, taste bud cell; TRMP5: transient receptor potential M5; VGC, voltage-gated Ca2+. Adapted from reference 18 with permission.
CD36 expression on TBCs is downregulated by dietary fat whereas that of GPR120 is not, and the reduction in CD36 is paralleled by a decrease of fat preference (26). LCFA-triggered increases in cytosolic Ca2+ are also blunted in CD36-expressing TBCs from obese compared with lean mice (15, 29), thereby altering the lipid-sensing mechanisms that mediate oral fat perception. This blunting reflects a reduction in total CD36 protein content in fungiform TBCs of obese mice together with diminution of CD36 amounts in rafts, the membrane domains that operate as signaling centers (15).
Role of CD36 in Intestinal FA Sensing
In the gut CD36 is abundant in the proximal small intestine, expressed on enterocytes (30, 31). Enterocyte CD36 contributes to LCFA and cholesterol uptake and to processes that optimize chylomicron formation. CD36 binds FA with high affinity, meaning at concentrations (low nanomolar) well below the high luminal FA concentrations that are likely to drive passive cellular entry of FA. Thus, in the gut CD36 is most relevant during the early stages of digestion when luminal FA amounts are low and when CD36-mediated signaling functions to initiate FA incorporation into TGs destined for chylomicrons. Lipids in the intestinal lumen induce CD36-dependent upregulation of key proteins of chylomicron formation, including the microsomal TG transfer protein and apoprotein B (32). In addition, CD36 is present in the ER prechylomicron transport vesicle complex and is important for prechylomicron transport vesicle exit from the ER to the Golgi where additional apoproteins and lipids are added to the particle (33). CD36-deficient mice have impaired chylomicron production with increased formation of smaller remnant-like chylomicron particles (34).
In addition to enterocytes, CD36 is present on EECs in the small intestinal epithelium (35). LCFAs released from fat digestion in the lumen influence secretion of a number of EEC peptides that affect the absorptive process and satiety. CD36 was identified on a subpopulation of secretin and cholecystokinin-positive EECs (35) and on serotonin-expressing cells (S Sundaresan, unpublished results, 2013). Secretin and cholecystokinin have prosatiety effects (36, 37) and participate in fat absorption. Secretin, released by S cells in the duodenum and jejunum (38, 39), stimulates water and bicarbonate secretion that serves to buffer the acidic chyme entering the duodenum (40). Cholecystokinin, secreted by I cells found in the proximal small intestine and ileum (41), is a key peptide involved in fat digestion that plays critical roles in gastric emptying, gallbladder contraction, pancreatic secretion, and intestinal motility (42). CD36−/− mice have significantly lower amounts of plasma secretin and cholecystokinin amounts in response to an oral lipid load, independent of gastric emptying effects. Studies in the EEC model STC-1 showed that the role of CD36 in release of the above-mentioned peptides is mediated via increases in intracellular cAMP and calcium, involving both protein kinase A-dependent and -independent mechanisms (35).
In addition to CD36, GPR40 is expressed on cholecystokinin-secreting EECs in the proximal intestine and is shown to mediate FA-induced cholecystokinin release (43). Thus, CD36 and GPR40 appear to contribute the main part of FA-induced cholecystokinin release during fat absorption. Both GPR40 and GPR120 mediate release of the incretins, GLP-1 and GIP, in response to FAs (9, 44, 45), whereas CD36 does not appear to be directly involved in this process. However, in the CD36-null mouse, secretion of both GIP and GLP-1 is enhanced as a result of the delayed fat absorption (S Sundaresan, F Nassir, and NA Abumrad, unpublished results, 2015) and of more dietary fat that reaches GPR40 and GPR120 in the ileum where both receptors are well expressed (9, 46). Abundance of CD36 in the proximal intestine is consistent with its involvement in fat absorption, which occurs predominantly in the jejunum and with its mediation of FA-induced release of secretin and cholecystokinin, peptides that play a role in facilitating fat absorption in the upper intestine. Overall, FA-induced signaling in the small intestine whether mediated by CD36, GPR40, or GPR120 would contribute to fat-induced effects on satiety by stimulating release of intestinal peptides such as cholecystokinin (GPR40, CD36), secretin (CD36), and the incretins (GPR40 and GPR120). Further research is needed on how high-fat intake influences the function of these receptors on EECs and how this affects the EEC response to fat intake.
In addition to secretion of gut peptides, CD36 is important for production by enterocytes of the proximal small intestine of the bioactive lipid messenger oleoylethanolamide, which is generated from internalized dietary oleic acid (47). Oleoylethanolamide mediates fat-induced satiety via the afferent sensory fibers of the vagus nerve. Generation of oleoylethanolamide from dietary oleic acid and fat-induced satiety are abrogated in CD36-deficient mice. Satiety effects of oleoylethanolamide are attributed to its activation of PPAR-α (47) and to its induction of GLP-1 release as observed in intestinal L cell models, including murine GLUTag, human (h) NCI-H716, and primary fetal rat intestinal L cell consequent to its interaction with GPR119 (13).
Future Studies: FA Sensing by CD36, Food Intake, and Obesity
The previous sections described how dietary LCFAs induce CD36-mediated signaling for release of neurotransmitters that mediate fat taste perception, the cephalic phase of digestion, and the secretion of various intestinal factors (cholecystokinin, secretin, oleoylethanolamide) that influence the digestive process and satiety. CD36 signaling might also directly influence neuronal activity in particular brain centers. A growing body of evidence, since the first report by Oomura et al. (48) that neurons can sense FAs, has established the importance of brain FA sensing in the regulation of food intake (49, 50). Recently, Le Foll et al. (20, 51) reported that CD36 contributes to FA sensing by ventromedial hypothalamic neurons and that CD36-depletion in the ventromedial hypothalamus of rats prevents fat-induced suppression of food intake, increases subcutaneous fat deposition, and associates with abnormal glucose tolerance. The role of CD36 in hypothalamic FA sensing and regulation of energy balance is a promising area of future investigation.
Emerging evidence supports the concept that obesity associates with disruption of nutrient sensing that reduces activity of brain reward systems, resulting in compensatory overeating behavior (52). This disruption could reflect at least in part abnormal function of FA receptors. Humans that carry common genetic variants that associate with reduced CD36 amounts have several fold lower thresholds for sensing oleic acid, indicating reduced sensitivity to fat taste perception (53). How the reduced sensitivity affects fat intake is difficult to predict on the basis of the data available so far, but there is some preliminary evidence that it might induce more fat consumption by increasing the fat satiation threshold. When individuals with a genetic variant that reduces CD36 amounts were exposed to salad dressing samples, which contain fat concentrations well above detection thresholds, they reported liking more added fats (54). Furthermore, recent findings show that obese individuals have lower fat detection thresholds, and this diminished orosensitivity associates with higher fat consumption on the basis of analysis of dietary habits in addition to abnormalities of the cephalic response (55), suggesting that abnormalities of CD36 function might also occur in obese humans. As already mentioned obese mice have reduced oral fat sensitivity (15, 29) associated with reduced TBC CD36 protein amounts (15).
CD36 signaling in the small intestine facilitates release of satiety signals (e.g., cholecystokinin, oleoylethanolamide, secretin) that act in the brain to affect feeding behavior. FA sensing in the small intestine is attenuated in subjects with reduced oral FA sensitivity or after high-fat feeding (56). Obesity alters the intestinal cholecystokinin response to food, and administration of nondegradable cholecystokinin to high fat-fed mice improves the metabolic phenotype (57). High-fat diet-induced obesity also suppresses production of gut oleoylethanolamide which results in diminished activity of the brain reward dopaminergic circuits, a diminution thought to trigger compensatory overeating. The reduced dopaminergic activity is restored by oleoylethanolamide administration (58). Together these findings suggest that the alteration of cellular CD36 amount and localization that were shown to accompany high-fat feeding in TBCs (15) if they occur in other cells where CD36 signaling is active (enterocytes, EECs, neurons) would be predicted to alter nutrient signaling, disturbing the normal gut-brain crosstalk that regulates food intake and energy homeostasis.
In humans, CD36 variants are linked to the risk of the metabolic syndrome, and genetic variants that result in reduced protein expression in monocytes (59) associate with diminished oral FA sensitivity (53), but their potential to alter the cephalic phase of digestion or activity of brain reward centers is not unexplored. Total CD36 deficiency (∼2–3% in populations of Asian and African ancestry) alters chylomicron production in humans as in rodents (60). Chylomicron formation is facilitated by CD36 signaling, and humans with partial or total CD36 deficiency might display alteration of other gut lipid-sensing mechanisms. Additional investigation of the role of CD36 and GPRs in the pathways underlying gut-brain communication in health and disease could provide novel strategies to prevent or alleviate metabolic pathologies.
Acknowledgments
SS wrote the review; SS and NAA edited and revised the review. Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: EEC, enteroendocrine cell; ER, endoplasmic reticulum; GIP, gastric inhibitory peptide; GLP, glucagon-like peptide; GPR, G-protein coupled receptor; IP3, inositol-1, 4, 5-triphosphate; LCFA, long-chain FA; TBC, taste bud cell.
References
- 1.Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol 1902;28:325–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujita T, Kobayashi S. Structure and function of gut endocrine cells. Int Rev Cytol Suppl 1977; (6):187–233. [PubMed] [Google Scholar]
- 3.DiPatrizio NV, Piomelli D. Intestinal lipid-derived signals that sense dietary fat. J Clin Invest 2015;125:891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Côté CD, Zadeh-Tahmasebi M, Rasmussen BA, Duca FA, Lam TK. Hormonal signaling in the gut. J Biol Chem 2014;289:11642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abumrad NA, Davidson NO. Role of the gut in lipid homeostasis. Physiol Rev 2012;92:1061–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol 1999;277:G922–8. [DOI] [PubMed] [Google Scholar]
- 7.Hara T, Kashihara D, Ichimura A, Kimura I, Tsujimoto G, Hirasawa A. Role of free fatty acid receptors in the regulation of energy metabolism. Biochim Biophys Acta 2014;1841:1292–300. [DOI] [PubMed] [Google Scholar]
- 8.Janssen S, Depoortere I. Nutrient sensing in the gut: new roads to therapeutics?. Trends Endocrinol Metab 2013;24:92–100. [DOI] [PubMed] [Google Scholar]
- 9.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 2008;57:2280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyauchi S, Hirasawa A, Ichimura A, Hara T, Tsujimoto G. New frontiers in gut nutrient sensor research: free fatty acid sensing in the gastrointestinal tract. J Pharmacol Sci 2010;112:19–24. [DOI] [PubMed] [Google Scholar]
- 11.Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab 2006;3:167–75. [DOI] [PubMed] [Google Scholar]
- 12.Chu ZL, Carroll C, Alfonso J, Gutierrez V, He H, Lucman A, Pedraza M, Mondala H, Gao H, Bagnol D, et al. A role for intestinal endocrine cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucagon-like Peptide-1 and glucose-dependent insulinotropic Peptide release. Endocrinology 2008;149:2038–47. [DOI] [PubMed] [Google Scholar]
- 13.Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes 2009;58:1058–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ancel D, Bernard A, Subramaniam S, Hirasawa A, Tsujimoto G, Hashimoto T, Passilly-Degrace P, Khan NA, Besnard P. The oral lipid sensor GPR120 is not indispensable for the orosensory detection of dietary lipids in mice. J Lipid Res 2015;56:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozdener MH, Subramaniam S, Sundaresan S, Sery O, Hashimoto T, Asakawa Y, Besnard P, Abumrad NA, Khan NA. CD36- and GPR120-mediated Ca(2)(+) signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology 2014;146:995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Depoortere I. Taste receptors of the gut: emerging roles in health and disease. Gut 2014;63:179–90. [DOI] [PubMed] [Google Scholar]
- 17.Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem 1993;268:17665–8. [PubMed] [Google Scholar]
- 18.Pepino MY, Kuda O, Samovski D, Abumrad NA. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu Rev Nutr 2014;34:281–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal 2009;2:re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Foll C, Dunn-Meynell AA, Levin BE. Role of FAT/CD36 in fatty acid sensing, energy, and glucose homeostasis regulation in DIO and DR rats. Am J Physiol Regul Integr Comp Physiol 2015;308:R188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci 2010;30:8376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sclafani A, Zukerman S, Ackroff K. GPR40 and GPR120 fatty acid sensors are critical for postoral but not oral mediation of fat preferences in the mouse. Am J Physiol Regul Integr Comp Physiol 2013;305:R1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuwatari T, Kawada T, Tsuruta M, Hiraoka T, Iwanaga T, Sugimoto E, Fushiki T. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett 1997;414:461–4. [DOI] [PubMed] [Google Scholar]
- 24.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest 2005;115:3177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simons PJ, Kummer JA, Luiken JJ, Boon L. Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae. Acta Histochem 2011;113:839–43. [DOI] [PubMed] [Google Scholar]
- 26.Martin C, Passilly-Degrace P, Gaillard D, Merlin JF, Chevrot M, Besnard P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One 2011;6:e24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol 2007;293:R1823–32. [DOI] [PubMed] [Google Scholar]
- 28.Gilbertson TA, Khan NA. Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog Lipid Res 2014;53:82–92. [DOI] [PubMed] [Google Scholar]
- 29.Chevrot M, Bernard A, Ancel D, Buttet M, Martin C, Abdoul-Azize S, Merlin JF, Poirier H, Niot I, Khan NA, et al. Obesity alters the gustatory perception of lipids in the mouse: plausible involvement of lingual CD36. J Lipid Res 2013;54:2485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lobo MV, Huerta L, Ruiz-Velasco N, Teixeiro E, de la Cueva P, Celdran A, Martin-Hidalgo A, Vega MA, Bragado R. Localization of the lipid receptors CD36 and CLA-1/SR-BI in the human gastrointestinal tract: towards the identification of receptors mediating the intestinal absorption of dietary lipids. J Histochem Cytochem 2001;49:1253–60. [DOI] [PubMed] [Google Scholar]
- 31.Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem 2007;282:19493–501. [DOI] [PubMed] [Google Scholar]
- 32.Tran TT, Poirier H, Clement L, Nassir F, Pelsers MM, Petit V, Degrace P, Monnot MC, Glatz JF, Abumrad NA, et al. Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. J Biol Chem 2011;286:25201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddiqi S, Saleem U, Abumrad NA, Davidson NO, Storch J, Siddiqi SA, Mansbach CM 2nd. A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. J Lipid Res 2010;51:1918–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology 2006;131:1197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundaresan S, Shahid R, Riehl TE, Chandra R, Nassir F, Stenson WF, Liddle RA, Abumrad NA. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin. FASEB J 2013;27:1191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng CY, Chu JY, Chow BK. Central and peripheral administration of secretin inhibits food intake in mice through the activation of the melanocortin system. Neuropsychopharmacology 2011;36:459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dockray GJ. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes 2012;19:8–12. [DOI] [PubMed] [Google Scholar]
- 38.Lam IP, Lee LT, Choi HS, Chow BK. Localization of small heterodimer partner (SHP) and secretin in mouse duodenal cells. Ann N Y Acad Sci 2006;1070:371–5. [DOI] [PubMed] [Google Scholar]
- 39.Polak JM, Coulling I, Bloom S, Pearse AG. Immunofluorescent localization of secretin and enteroglucagon in human intestinal mucosa. Scand J Gastroenterol 1971;6:739–44. [DOI] [PubMed] [Google Scholar]
- 40.Chey WY, Chang TM. Secretin, 100 years later. J Gastroenterol 2003;38:1025–35. [DOI] [PubMed] [Google Scholar]
- 41.Liddle RA. Regulation of cholecystokinin secretion in humans. J Gastroenterol 2000;35:181–7. [DOI] [PubMed] [Google Scholar]
- 42.Berna MJ, Jensen RT. Role of CCK/gastrin receptors in gastrointestinal/metabolic diseases and results of human studies using gastrin/CCK receptor agonists/antagonists in these diseases. Curr Top Med Chem 2007;7:1211–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liou AP, Lu X, Sei Y, Zhao X, Pechhold S, Carrero RJ, Raybould HE, Wank S. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology 2011;140:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 2005;11:90–4. [DOI] [PubMed] [Google Scholar]
- 45.Iwasaki K, Harada N, Sasaki K, Yamane S, Iida K, Suzuki K, Hamasaki A, Nasteska D, Shibue K, Joo E, et al. Free fatty acid receptor GPR120 is highly expressed in enteroendocrine K cells of the upper small intestine and has a critical role in GIP secretion after fat ingestion. Endocrinology 2015;156:837–46. [DOI] [PubMed] [Google Scholar]
- 46.Adachi T, Tanaka T, Takemoto K, Koshimizu TA, Hirasawa A, Tsujimoto G. Free fatty acids administered into the colon promote the secretion of glucagon-like peptide-1 and insulin. Biochem Biophys Res Commun 2006;340:332–7. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz GJ. Gut fat sensing in the negative feedback control of energy balance-recent advances. Physiol Behav 2011;104:621–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oomura Y, Nakamura T, Sugimori M, Yamada Y. Effect of free fatty acid on the rat lateral hypothalamic neurons. Physiol Behav 1975;14:483–6. [DOI] [PubMed] [Google Scholar]
- 49.Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat Neurosci 2005;8:579–84. [DOI] [PubMed] [Google Scholar]
- 50.Levin BE, Magnan C, Dunn-Meynell A, Le Foll C. Metabolic sensing and the brain: who, what, where, and how? Endocrinology 2011;152:2552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le Foll C, Dunn-Meynell A, Musatov S, Magnan C, Levin BE. FAT/CD36: a major regulator of neuronal fatty acid sensing and energy homeostasis in rats and mice. Diabetes 2013;62:2709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci 2011;15:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pepino MY, Love-Gregory L, Klein S, Abumrad NA. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res 2012;53:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keller KL, Liang LC, Sakimura J, May D, van Belle C, Breen C, Driggin E, Tepper BJ, Lanzano PC, Deng L, et al. Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity (Silver Spring) 2012;20:1066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chevrot M, Passilly-Degrace P, Ancel D, Bernard A, Enderli G, Gomes M, Robin I, Issanchou S, Verges B, Nicklaus S, et al. Obesity interferes with the orosensory detection of long-chain fatty acids in humans. Am J Clin Nutr 2014;99:975–83. [DOI] [PubMed] [Google Scholar]
- 56.Little TJ, Feinle-Bisset C. Effects of dietary fat on appetite and energy intake in health and obesity–oral and gastrointestinal sensory contributions. Physiol Behav 2011;104:613–20. [DOI] [PubMed] [Google Scholar]
- 57.Irwin N, Montgomery IA, O'Harte FP, Frizelle P, Flatt PR. Comparison of the independent and combined metabolic effects of subchronic modulation of CCK and GIP receptor action in obesity-related diabetes. Int J Obes (London) 2013;37:1058–63. [DOI] [PubMed] [Google Scholar]
- 58.Tellez LA, Medina S, Han W, Ferreira JG, Licona-Limon P, Ren X, Lam TT, Schwartz GJ, de Araujo IE. A gut lipid messenger links excess dietary fat to dopamine deficiency. Science 2013;341:800–2. [DOI] [PubMed] [Google Scholar]
- 59.Love-Gregory L, Sherva R, Schappe T, Qi JS, McCrea J, Klein S, Connelly MA, Abumrad NA. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum Mol Genet 2011;20:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masuda D, Hirano K, Oku H, Sandoval JC, Kawase R, Yuasa-Kawase M, Yamashita Y, Takada M, Tsubakio-Yamamoto K, Tochino Y, et al. Chylomicron remnants are increased in the postprandial state in CD36 deficiency. J Lipid Res 2009;50:999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]