Abstract
Background: Dietary cocoa is an important source of flavonoids and is associated with favorable cardiovascular disease effects, such as improvements in vascular function and lipid profiles, in nondiabetic adults. Type 2 diabetes (T2D) is associated with adverse effects on postprandial serum glucose, lipids, inflammation, and vascular function.
Objective: We examined the hypothesis that cocoa reduces metabolic stress in obese T2D adults after a high-fat fast-food–style meal.
Methods: Adults with T2D [n = 18; age (mean ± SE): 56 ± 3 y; BMI (in kg/m2): 35.3 ± 2.0; 14 women; 4 men] were randomly assigned to receive cocoa beverage (960 mg total polyphenols; 480 mg flavanols) or flavanol-free placebo (110 mg total polyphenols; <0.1 mg flavanols) with a high-fat fast-food–style breakfast [766 kcal, 50 g fat (59% energy)] in a crossover trial. After an overnight fast (10–12 h), participants consumed the breakfast with cocoa or placebo, and blood sample collection [glucose, insulin, lipids, and high-sensitivity C-reactive protein (hsCRP)] and vascular measurements were conducted at 0.5, 1, 2, 4, and 6 h postprandially on each study day. Insulin resistance was evaluated by homeostasis model assessment.
Results: Over the 6-h study, and specifically at 1 and 4 h, cocoa increased HDL cholesterol vs. placebo (overall Δ: 1.5 ± 0.8 mg/dL; P ≤ 0.01) but had no effect on total and LDL cholesterol, triglycerides, glucose, and hsCRP. Cocoa increased serum insulin concentrations overall (Δ: 5.2 ± 3.2 mU/L; P < 0.05) and specifically at 4 h but had no overall effects on insulin resistance (except at 4 h, P < 0.05), systolic or diastolic blood pressure, or small artery elasticity. However, large artery elasticity was overall lower after cocoa vs. placebo (Δ: −1.6 ± 0.7 mL/mm Hg; P < 0.05), with the difference significant only at 2 h.
Conclusion: Acute cocoa supplementation showed no clear overall benefit in T2D patients after a high-fat fast-food–style meal challenge. Although HDL cholesterol and insulin remained higher throughout the 6-h postprandial period, an overall decrease in large artery elasticity was found after cocoa consumption. This trial was registered at clinicaltrials.gov as NCT01886989.
Keywords: type 2 diabetes, postprandial metabolism, dietary cocoa, HDL cholesterol, insulin
Introduction
Increasing evidence suggests that the postprandial (fed) state is a contributing factor to the development of atherosclerosis (1, 2). Given that most of the population in the United States with obesity and diabetes (3, 4) is in a postprandial state for most of the day, the postprandial state has become an increasingly important area of investigation. Endothelial function is altered early in diabetes. Postprandial hyperglycemia and hyperlipidemia may induce vascular stress, with diminished vasodilation and increased intimal thickening, in people with type 2 diabetes (T2D)9 (5–7). Several surrogate markers of endothelial dysfunction, especially the inflammatory cytokines and chemokines, are also elevated in response to postprandial metabolic stress in T2D (1, 8). These postprandial responses are aggravated by certain dietary macronutrients, especially refined carbohydrates and fats (1, 8–11) and fast-food–style meals (12, 13) and are reversed by the addition of antioxidant vitamins and dietary bioactive compounds, such as the polyphenolic flavonoids (14, 15). Studies that used high-fat or fast-food–style meal challenges in participants with the metabolic syndrome or T2D have revealed pronounced postprandial lipemia, glycemia, and inflammation, lasting up to 8 h, and these were associated with atherogenic effects and endothelial dysfunction (9, 10, 13). However, a few studies demonstrate the role of soluble fiber and vitamin therapy in attenuating meal-induced postprandial metabolic stress in T2D (14, 16, 17), and some studies show effects of red wine, berries, and fruit extracts in reversing postprandial metabolic stress, but these mostly involve nondiabetic adults (15). Thus, there is a substantial lack of studies that address the role of bioactive dietary compounds, especially the polyphenolic flavonoids, in the modulation of postprandial metabolism in diabetes.
Among the commonly consumed sources of bioactive compounds, cocoa is an important functional food with several medicinal health effects (18). Cocoa powder is a rich source of phytonutrients, especially the polyphenols, and they comprise 12–18% dry weight of unprocessed cocoa beans, although a smaller proportion of processed commercially available cocoa powder (19, 20). Cocoa or dark chocolate consumption improves cardiovascular disease (CVD) risk, especially endothelium-dependent vascular function and insulin sensitivity, based on recent epidemiologic and clinical studies (21, 22). In a few studies of healthy participants (23, 24) and smokers (25), acute supplementation with cocoa or its bioactive compound epicatechin was shown to improve postprandial lipemia and endothelial dysfunction. However, these studies in nondiabetic adults did not examine the role of cocoa in counteracting a high-fat or fast-food–type meal challenge; these foods are associated with weight gain, insulin resistance, and inflammation (12, 26, 27).
We hypothesized that dietary cocoa powder attenuates high-fat, fast-food meal-induced postprandial metabolic stress in people with T2D. To investigate this, we measured effects of cocoa vs. placebo on postprandial changes in serum glucose, insulin, conventional lipids, C-reactive protein (CRP), blood pressure, and arterial compliance in a randomized crossover trial.
Methods
Participants.
The study protocol was approved by the Institutional Review Boards of the Oklahoma University Health Sciences Center and the Oklahoma State University. Written informed consent was obtained from all enrolled participants. All study procedures were conducted at the Harold Hamm Diabetes Center at the Oklahoma University Health Sciences Center. Volunteers of either sex (n = 18) with elevated waist circumference (>89 cm for women or >102 cm for men) and with established and stable T2D for at least 5 y but not on insulin therapy were screened. T2D eligibility was confirmed according to the guidelines of the American Diabetes Association (28). Presence of other CVD risk factors, such as elevated blood pressure or serum lipids, did not preclude participation in the study. Potential recruits were excluded if they were ≤21 y of age; presented with preexisting conditions, such as cancer or coronary heart disease; or had abnormal liver, renal, or thyroid function or anemia on the basis of screening examinations. Potential recruits also were excluded if they were consuming antioxidants or fish oil supplements on a regular basis, were currently enrolled in a weight-loss program, were current smokers, were consuming alcohol on a regular basis (except social drinking, 1–2 drinks/wk), or were pregnant or lactating. Each participant’s regular oral medications were continued throughout the study.
Study design.
Participants completed a randomized, double-blind, crossover study in which they made 2 separate visits to the study site, arriving in a fasting state (10–12 h). Each visit lasted ∼6–7 h. The 2 study days were separated by a 1-wk washout phase (Supplemental Figure 1). On each study day, blood draws, blood pressure, and vascular measurements were conducted in the fasting state (baseline), then again at 30 (0.5 h), 60 (1 h), 120 (2 h), 240 (4 h), and 360 min (6 h) postprandial time points, starting from the time of completion of the breakfast meal and beverage intake. After the fasting blood draw and measurements of blood pressure and arterial elasticity, participants were administered a high-fat fast-food–style breakfast with the cocoa or placebo beverage. The breakfast was prepared at the clinic, and all participants underwent supervised consumption of the test meals. Participants were asked to refrain from alcohol and caffeine for 24 h and from polyphenol-containing foods, such as berries, tea, red wine, soy products, citrus juices, nuts, chocolate, and cocoa-containing products, or dietary supplements of these food extracts for 48 h before each study visit. Otherwise, participants were asked to maintain their usual diet, medications, and lifestyle during the entire course of the study. Three-day food records (2 weekdays and 1 weekend day) were collected at baseline before the start of the study, and nutrient and food group intakes were analyzed with Nutritionist Pro version 3.2 (Axxya Systems).
Interventions.
The nutritional composition of the cocoa and placebo powder is summarized in Table 1. Cocoa powder had substantially higher total polyphenols and flavanols per serving than the flavanol-free placebo powder. The total polyphenol content was determined by the Folin-Ciocalteu assay as described by Singleton et al. (29), and the total flavanol content was determined by the 4-dimethylaminocinnamaldehyde assay as described by Payne et al. (30). In addition to the cocoa, the powder contained the following ingredients: sunflower oil, corn syrup solids, and ≤2% food additives (carrageenan, vanillin, salt, sodium caseinate, aspartame, dipotassium phosphate, monoglycerides, diglycerides, acesulfame potassium, soy lecithin, and silicon dioxide). The composition of the placebo powder mix was similar to that of the cocoa mix, except the cocoa was replaced with milk protein isolate. The fast-food–style breakfast meal consisted of 2 scrambled eggs (no added fat), hash brown potatoes (70 g), 2 buttermilk biscuits, butter (15 g), and a sausage patty (57 g). The breakfast meal provided a total of 766 kcal, 50 g total fat (25 g saturated fats, 12 g monounsaturated fats, 13 g polyunsaturated fats; 59% energy), 50 g carbohydrates (26% energy), 30 g proteins (16% energy), 465 mg cholesterol, and 2.4 g dietary fiber. All meal ingredients were purchased from a local grocery store and prepared in the metabolic kitchen at the clinic each morning on the 2 trial days. The cocoa and placebo beverage mixes were reconstituted in warm water and were provided to the participants in closed lid cups with the breakfast meal for supervised consumption.
TABLE 1.
Composition of cocoa and placebo powders administered in the postprandial study in participants with type 2 diabetes1
| Composition | Cocoa | Placebo |
| Serving size, g | 20 | 12 |
| Energy, kcal | 67 | 66 |
| Fat, g | 4.7 | 5.0 |
| Protein, g | 2.7 | 1.1 |
| Carbohydrates, g | 9.6 | 4.0 |
| Carbohydrate:protein | 3.6:1 | 3.6:1 |
| Fiber, g | 4.4 | 0.1 |
| Total polyphenols, mg | 960 | 110 |
| Total flavanols, mg | 480 | <0.1 |
| Proanthocyanidins 1–10, mg | 201 | <0.001 |
| Epicatechins, mg | 40 | 0 |
| Catechins, mg | 18 | 0 |
| Theobromine, mg | 220 | 0 |
| Caffeine, mg | 21 | 0 |
Source of the powders: The Hershey Company (Hershey, PA).
Anthropometrics and vascular measurements.
Body weight (±0.05 kg) was measured on a calibrated scale, and height (±0.05 cm) was measured with the use of a stadiometer. Waist circumference (±0.05 cm) was measured at the superior iliac crest. On each day of the trial, systolic and diastolic blood pressures and arterial compliance were measured in a quiet, temperature-controlled laboratory after an overnight fast and before blood sampling. With the subject supine, radial arterial waveforms were recorded for 30 s. The pressure transducer amplifier system was connected to a specially designed device (Model CR-2000; Hypertension Diagnostics Inc.). This technique was previously validated (31, 32) and was performed with a simple noninvasive radial pulse wave recording and computer analysis of the diastolic decay. This method provided separate assessment of the large artery or capacitive compliance and small artery reflective or oscillatory compliance. These measures were performed at baseline and at 0.5, 1, 2, 4, and 6 h postprandially.
Biochemical variables.
Freshly drawn blood samples were sent to the Oklahoma University Medical Center Laboratory for analysis of serum glucose, insulin, lipid profiles (total cholesterol, TGs, LDL cholesterol, and HDL cholesterol) and high-sensitivity CRP (hsCRP). Analyses for glucose, insulin, and lipids were conducted with the use of automated diagnostic equipment (Abbott Architect Instruments) by enzymatic colorimetric methods that used commercially available kits according to manufacturer’s protocols. hsCRP was assayed by ultrasensitive nephelometry (Dade Behring). Serum glycated hemoglobin was analyzed with the use of a DCA 2000+ Analyzer (Bayer). Insulin resistance was evaluated by HOMA-IR and was calculated as follows: [fasting insulin (mU/L) × fasting glucose (mmol/L)]/22.5 (33).
Statistical analysis.
For each outcome, change from baseline (i.e., the fasting, preintervention time point) was calculated for each time point. Data are presented as means ± SEMs for serum glucose, insulin, HOMA-IR, lipid profiles, hsCRP, blood pressure, and arterial compliance. For this crossover design, between-intervention comparisons were performed at each time point with the use of generalized estimating equation analyses to account for the repeated measures in the same individual. Between-intervention comparisons across all time points, that is, for the entire 6-h time period, were performed with the use of generalized estimating equation analyses (with results indicated as overall P) with the intervention effect being fitted after the (nuisance) time effect. In addition, the AUC (0–360 min) was calculated for each subject and intervention. Differences between intervention and placebo were analyzed with the use of paired t tests. Data analyses were conducted with the use of IBM SPSS Statistics version 20.0 (IBM Corp.). Results corresponding to P < 0.05 are described as significant for the purposes of discussion.
Results
Baseline characteristics.
All participants had clinically diagnosed T2D (28), with a large proportion taking oral hypoglycemic agents (83%) and antihypertensive medications (84%) (Table 2). All were obese [BMI (in kg/m2): >30]. At baseline, all participants had hyperglycemia (>126 mg/dL) and elevated systolic and diastolic blood pressures, whereas fasting lipid profiles were mostly within optimal ranges. Baseline dietary analyses revealed low intake of fruits and vegetables and high intake of total fats and fat calories (37%).
TABLE 2.
Baseline characteristics of the study participants1
| Variable | Value |
| n | 18 |
| Age, y | 56 ± 3.2 |
| Sex (n), M/F | 4/14 |
| Weight, kg | 100 ± 12 |
| BMI, kg/m2 | 35.3 ± 2.0 |
| Waist circumference, cm | 114 ± 4.57 |
| Serum glucose, mg/dL | 136 ± 16 |
| Serum insulin, mU/L | 14.8 ± 2.6 |
| Insulin resistance (HOMA-IR) | 3.5 ± 0.9 |
| Serum HbA1c, % | 8.2 ± 0.6 |
| Serum total cholesterol, mg/dL | 188 ± 11 |
| Serum LDL cholesterol, mg/dL | 112 ± 11 |
| Serum HDL cholesterol, mg/dL | 46 ± 2.5 |
| Serum LDL:HDL cholesterol | 2.54 ± 0.24 |
| Serum TGs, mg/dL | 140 ± 13 |
| Serum hsCRP, mg/L | 5.3 ± 1.2 |
| Systolic blood pressure, mm Hg | 144 ± 5.5 |
| Diastolic blood pressure, mm Hg | 85 ± 3.0 |
| Small artery elasticity index, mL/mm Hg × 100 | 5.6 ± 3.6 |
| Large artery elasticity index, mL/mm Hg × 10 | 17 ± 7.4 |
| Medication/supplement use, n (%) | |
| Insulin | 0 (0) |
| Oral hypoglycemic agents | 15 (83) |
| Statins/fibrates | 5 (28) |
| CCBs | 3 (17) |
| ACEIs/ARBs | 10 (56) |
| Diuretics | 2 (11) |
| Aspirin | 6 (33) |
| Multivitamins/minerals | 6 (33) |
| Botanical supplements | 2 (11) |
| Macronutrient and food intake | |
| Energy, kcal/d | 2216 ± 198 |
| Carbohydrates, g/d | 263 ± 38 |
| Total fats, g/d | 92 ± 8 |
| Protein, g/d | 94 ± 9 |
| Fiber, g/d | 30 ± 9 |
| Fruit servings, g/d | 144 ± 52 |
| Vegetable servings, g/d | 160 ± 35 |
Values are means ± SEMs unless otherwise indicated. ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker; CCB, calcium channel blocker; HbA1c, glycated hemoglobin; hsCRP, high-sensitivity C-reactive protein.
Postprandial glucose, insulin, and insulin resistance.
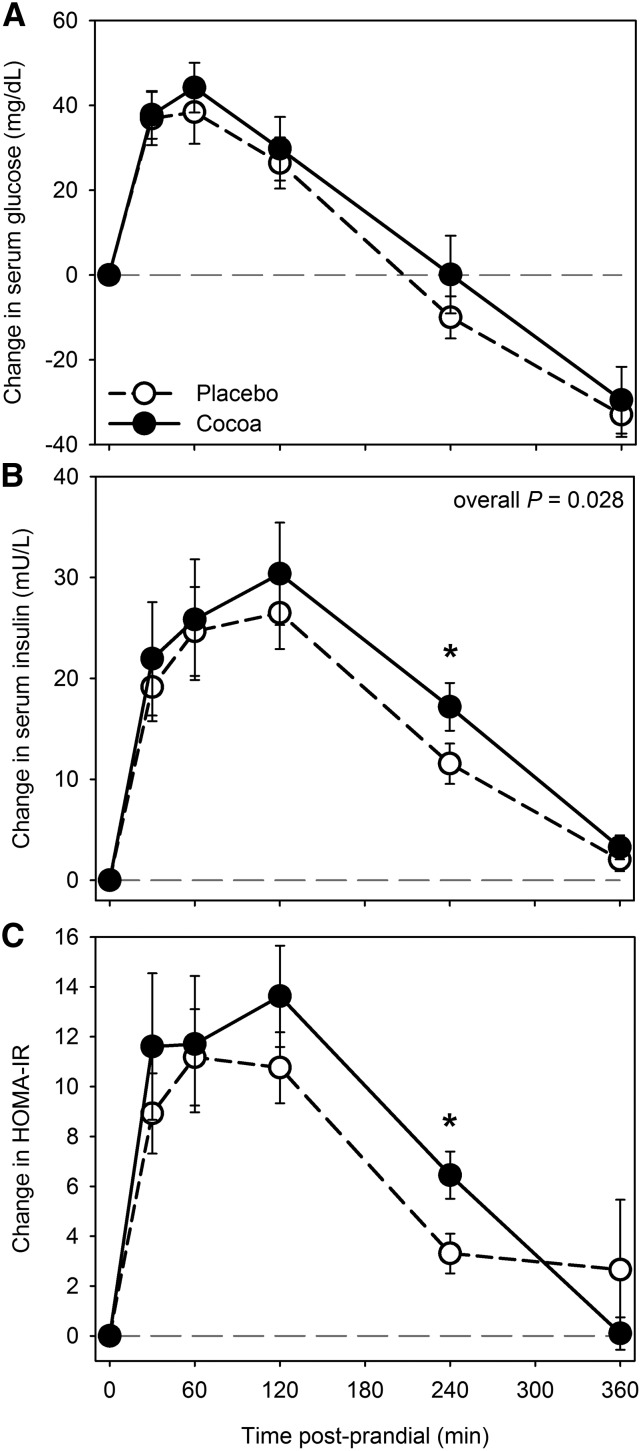
Changes in glucose concentrations were not significantly different after cocoa compared with placebo intervention over the 6-h postprandial phase compared with baseline (0 h) (Figure 1A). In addition, the mean AUC (0–360 min) of serum glucose responses did not show any significant difference after cocoa vs. placebo intervention. Overall changes in insulin during the entire 6-h postprandial phase were significantly higher after cocoa (overall P = 0.028) than after placebo, but overall changes in HOMA-IR did not differ between the 2 interventions (overall P = 0.24) (Figure 1B, C). However, both insulin and insulin resistance (HOMA-IR) were increased 4 h after cocoa vs. placebo (P < 0.01; Figure 1B, C). Mean AUC (0–360 min) for serum insulin was not significantly different at any time point, whereas AUC (0–240 min) for HOMA-IR remained significantly higher at 4 h after cocoa than after placebo (P = 0.026) (data not shown).
FIGURE 1.
Changes from baseline (fasting) in serum glucose (A), insulin (B), and HOMA-IR (C) after cocoa vs. placebo intervention with a high-fat meal in obese participants with type 2 diabetes. Data are means ± SEMs, n = 18. *Different from placebo, P < 0.05. Overall P, derived from general estimating equation, is for difference between change after cocoa vs. placebo intervention across the entire 6-h period, accounting for the variation at each time point.
Postprandial lipid profiles.
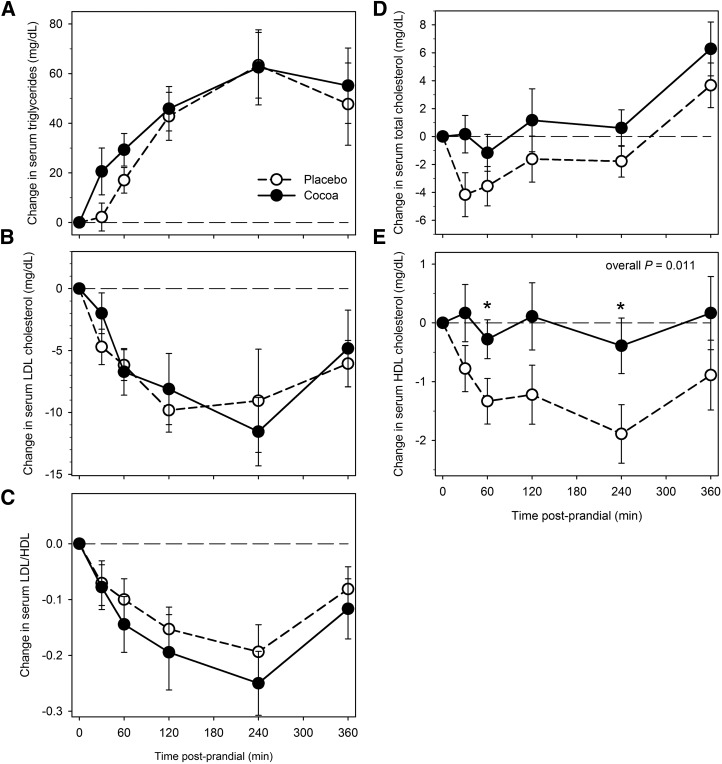
Postprandial serum TGs, total and LDL cholesterol, and the LDL cholesterol:HDL cholesterol ratio did not significantly differ after cocoa vs. placebo intervention (Figure 2A–D). No differences were observed in AUC values. However, postprandial HDL cholesterol remained higher after cocoa than after placebo throughout the 6-h postprandial period (overall P = 0.011), with significant differences at 1 and 4 h into the study (P ≤ 0.01; Figure 2E). Mean AUC (0–360 min) of serum HDL cholesterol was significantly higher after cocoa than after placebo intervention (P < 0.02) (data not shown).
FIGURE 2.
Changes from baseline (fasting) in serum TGs (A), LDL cholesterol (B), LDL:HDL cholesterol ratio (C), total cholesterol (D), and HDL cholesterol (E) after cocoa vs. placebo intervention with a high-fat meal in obese participants with type 2 diabetes. Data are means ± SEMs, n = 18. *Different from placebo, P < 0.05. Overall P, derived from general estimating equation, is for difference between change after cocoa vs. placebo intervention across the entire 6-h period, accounting for the variation at each time point.
Postprandial CRP.
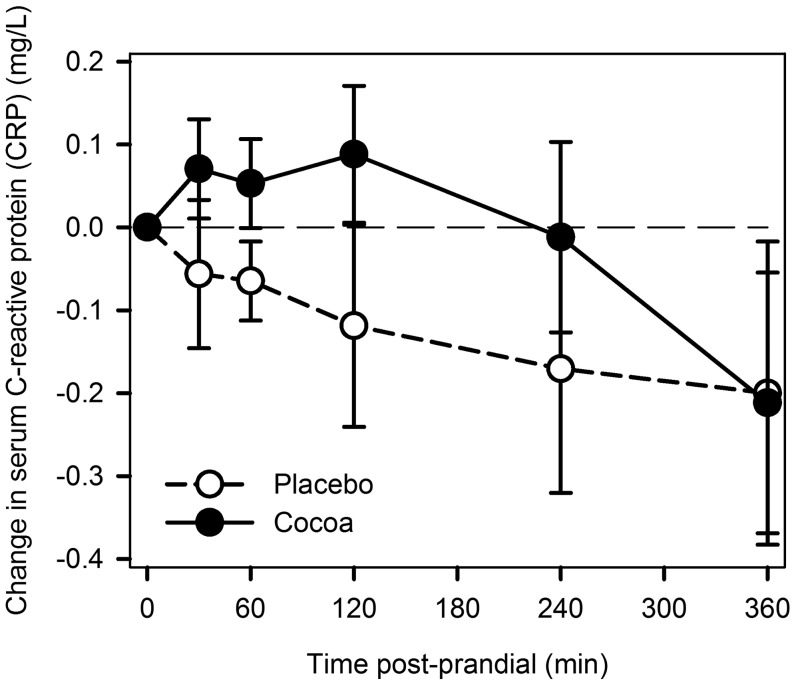
No significant changes after cocoa or placebo were noted for CRP over the 6-h postprandial period (Figure 3). No difference was observed in the AUC.
FIGURE 3.
Changes from baseline (fasting) in serum C-reactive protein after cocoa vs. placebo intervention with a high-fat meal in obese participants with type 2 diabetes. Data are means ± SEMs, n = 18.
Postprandial blood pressure and artery elasticity.
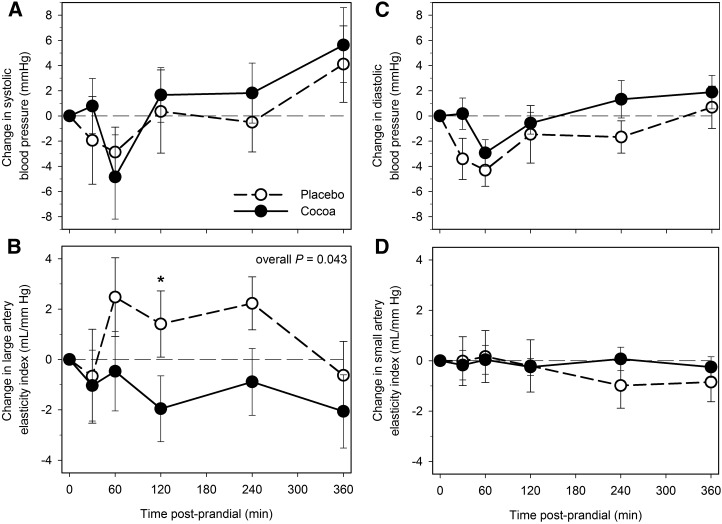
Postprandial changes in systolic and diastolic blood pressures did not differ between cocoa and placebo intervention at any time point (Figure 4A, C). A general decrease was found in the large artery elasticity after cocoa vs. placebo (overall P = 0.043), significant at 2 h (P < 0.05; Figure 4B), but with no differences in small artery elasticity (Figure 4D).
FIGURE 4.
Changes from baseline (fasting) in systolic blood pressure (A), large artery elasticity index (B), diastolic blood pressure (C), and small artery elasticity index (D) after cocoa vs. placebo intervention with a high-fat meal in obese participants with type 2 diabetes. Data are means ± SEMs, n = 18. *Different from placebo, P < 0.05. Overall P, derived from general estimating equation, is for difference between change after cocoa vs. placebo intervention across the entire 6-h period, accounting for the variation at each time point.
Discussion
Cocoa and its principal flavonoid, epicatechin, have displayed antidiabetic effects in laboratory-based studies (34, 35) and were shown to improve insulin resistance in clinical studies conducted mostly in nondiabetic participants (36). However, in postprandial studies of acute cocoa or dark chocolate supplementation no significant differences were noted in postprandial glycemia and insulin concentrations after an oral fat load in healthy volunteers (23) or in volunteers with smoking habits (25) or T2D (37). In our study, although postprandial glucose showed no differences after cocoa vs. placebo interventions, cocoa intervention elicited a higher insulin response and revealed insulin resistance (HOMA-IR) in participants with T2D. Our findings are consistent with previously reported studies that showed higher postprandial insulin after consumption of cocoa products in healthy nondiabetic participants (38, 39) and in male participants with T2D (40). Specific insulinogenic amino acids in cocoa (41) or greater cephalic phase insulin release in response to chocolate confectioneries (42) were proposed to explain these observations. However, in a postprandial study in participants with type 1 diabetes, no differences in glucose and insulin responses were found after a chocolate cake-substitution meal vs. a standard meal (43). Thus, cocoa supplementation may counteract postprandial hyperglycemia by inducing insulin secretion and concomitant insulin resistance in the presence of a high-fat meal as observed in our study. Further studies are needed to address the long-term implications of these repeated episodes in diabetes.
Evidence of effects of cocoa and chocolate on blood lipid regulation comes from experimental animal models of atherosclerosis (44, 45) and from clinical trials in nondiabetic participants in which they showed a significant reduction in total and/or LDL cholesterol, marginal increases or no effects on HDL cholesterol, and no effects on TGs (36, 46). In a postprandial study in healthy volunteers reported by Westphal and Luley (23), acute cocoa supplementation after an oral fat load (100 mL whipping cream with 33 g total fats) showed significant improvements in lipemia-induced endothelial dysfunction. However, the study showed no effects of cocoa on postprandial lipids (23). In contrast, acute supplementation of epicatchin, the principal bioactive cocoa flavonoid, in overweight adults showed significant decreases in postprandial TGs and increased fat oxidation after an oral challenge with a commercial nutritional supplement (meal replacement drink, 237 mL, 6 g total fats) (24). In our postprandial study, HDL cholesterol after cocoa remained higher throughout the postprandial phase than concentrations after placebo, whereas no notable effects were observed in the case of total and LDL cholesterol or TGs. The magnitude of difference in HDL cholesterol in our study is comparable with the overall increase in HDL due to cocoa intervention in a meta-analysis of clinical trials (36). The effects of chronic cocoa intake in raising HDL cholesterol in healthy participants were identified many years ago by Kris-Etherton and Mustad (47) and were explained by mechanisms such as the increased expression and secretion of apoAI and increased production of mature form sterol regulatory element binding proteins by cocoa polyphenols in hepatocytes (48). Thus, on the basis of this evidence, cocoa may offer some protection against postprandial dyslipidemia after a high-fat meal challenge. This needs further investigation in larger trials of participants with diabetes.
Evidence of the anti-inflammatory benefits of cocoa was supported predominantly by studies of animal models and in vitro and ex vivo experiments (49), although a review of clinical trials in nondiabetic participants has shown conflicting data on selected biomarkers of inflammation (50). In most of these clinical studies, CRP was unaffected, whereas other inflammatory biomarkers, such as cytokines and chemokines, were shown to be modulated by cocoa intervention (50). In our postprandial study, CRP revealed no significant changes after cocoa vs. placebo in diabetic participants. Consistent with this, acute supplementation of tea and berry polyphenols also showed no effect on postprandial CRP in participants with CVD or its risk factors (51, 52).
The health benefits of cocoa in improving vascular function, especially in lowering blood pressure and arterial stiffness and increasing vasodilation, have received great emphasis in a substantial number of clinical trials (36). Vascular benefits of acute cocoa supplementation were also reported in postprandial studies of healthy volunteers (23, 53, 54) and in volunteers with T2D (37). However, in contrast to these positive findings, in the present study that used a fast-food–style meal in T2D patients, no such effects were observed. Methodologic differences, such as flow-mediated dilation vs. pulse wave velocity in assessing vascular reactivity, study design (acute with or without meal challenge vs. chronic; type of meal), and differences in study participants must be taken into consideration when assessing effects of cocoa on vascular function. In addition, acute food intake was associated with variability of vascular reactivity in the early postprandial phase (55); thus, our observed changes in arterial elasticity within 2 h of the meal challenge may be of limited clinical implications.
Our study has limitations, specifically the small sample size, the absence of a nondiabetic group for comparison, and the lack of analyses of biomarkers of inflammation and oxidative stress, that may be modulated by cocoa in the postprandial phase. In addition, we used just one type of test meal, albeit an important one, and did not include interventions with low-fat or standard breakfast meals to assess effects of meal composition. Our study involves an acute intervention with cocoa, as opposed to a priming phase in which short-term polyphenol supplementation before an acute meal challenge could lead to favorable postprandial responses as was shown previously (56). Finally, our participants with T2D on blood glucose management with the use of diet, exercise, and oral hypoglycemic agents may not be generalizable to diabetic participants with advanced vascular complications or those on insulin therapy.
In conclusion, our postprandial study in obese participants with T2D revealed that cocoa supplementation increased postprandial insulin but with no effects on glucose and raised HDL cholesterol but with no effects on total or LDL cholesterol or TGs compared with the placebo intervention. Finally, no notable effects were observed on the inflammatory marker CRP and measures of blood pressure and vascular function. Thus, in contrast to the existing body of evidence that supports substantial health benefits of cocoa in short- and long-term studies, we report modest benefits of acute cocoa supplementation in diabetes after a high-fat meal challenge. This difference may be explained by our use of an acute fat overload as the primary meal challenge that may blunt any secondary effects of cocoa beverage, especially in the presence of CVD risk factors such as obesity, hypertension, and diabetes. The consumption of high-fat fast foods is common and increasing, and diabetes becomes ever more prevalent. Further investigations are warranted to determine whether cocoa supplementation, acute or long term, has a role in the prevention of vascular complications of diabetes.
Acknowledgments
We thank Alecia Bryant for providing assistance in dietary analyses. AB, NMB, and TJL designed the research, analyzed the data, and wrote the paper; AB, MJL, and DF conducted the research and performed laboratory analyses; CEA performed statistical analyses; and AB had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CRP, C-reactive protein; CVD, cardiovascular disease; hsCRP, high-sensitivity C-reactive protein; T2D, type 2 diabetes.
References
- 1.Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, Marfella R, Giugliano D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol 2002;39:1145–50. [DOI] [PubMed] [Google Scholar]
- 2.Karpe F, Steiner G, Uffelman K, Olivecrona T, Hamsten A. Postprandial lipoproteins and progression of coronary atherosclerosis. Atherosclerosis 1994;106:83–97. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307:491–7. [DOI] [PubMed] [Google Scholar]
- 4.Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, Daviglus ML, Lloyd-Jones DM. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003–2008. Circulation 2012;125:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teno S, Uto Y, Nagashima H, Endoh Y, Iwamoto Y, Omori Y, Takizawa T. Association of postprandial hypertriglyceridemia and carotid intima-media thickness in patients with type 2 diabetes. Diabetes Care 2000;23:1401–6. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Liu W, Huang R, Zhang X. Postchallenge plasma glucose excursions, carotid intima-media thickness, and risk factors for atherosclerosis in Chinese population with type 2 diabetes. Atherosclerosis 2010;210:302–6. [DOI] [PubMed] [Google Scholar]
- 7.Shige H, Ishikawa T, Suzukawa M, Ito T, Nakajima K, Higashi K, Ayaori M, Tabata S, Ohsuzu F, Nakamura H. Endothelium-dependent flow-mediated vasodilation in the postprandial state in type 2 diabetes mellitus. Am J Cardiol 1999;84:1272–4, A9. [DOI] [PubMed] [Google Scholar]
- 8.Esposito K, Nappo F, Giugliano F, Di Palo C, Ciotola M, Barbieri M, Paolisso G, Giugliano D. Meal modulation of circulating interleukin 18 and adiponectin concentrations in healthy subjects and in patients with type 2 diabetes mellitus. Am J Clin Nutr 2003;78:1135–40. [DOI] [PubMed] [Google Scholar]
- 9.Thomsen C, Storm H, Holst JJ, Hermansen K. Differential effects of saturated and monounsaturated fats on postprandial lipemia and glucagon-like peptide 1 responses in patients with type 2 diabetes. Am J Clin Nutr 2003;77:605–11. [DOI] [PubMed] [Google Scholar]
- 10.Mortensen LS, Hartvigsen ML, Brader LJ, Astrup A, Schrezenmeir J, Holst JJ, Thomsen C, Hermansen K. Differential effects of protein quality on postprandial lipemia in response to a fat-rich meal in type 2 diabetes: comparison of whey, casein, gluten, and cod protein. Am J Clin Nutr 2009;90:41–8. [DOI] [PubMed] [Google Scholar]
- 11.Bozzetto L, De Natale C, Di Capua L, Della Corte G, Patti L, Maione S, Riccardi G, Rivellese AA, Annuzzi G. The association of hs-CRP with fasting and postprandial plasma lipids in patients with type 2 diabetes is disrupted by dietary monounsaturated fatty acids. Acta Diabetol 2013;50:273–6. [DOI] [PubMed] [Google Scholar]
- 12.Carroll MF, Schade DS. Timing of antioxidant vitamin ingestion alters postprandial proatherogenic serum markers. Circulation 2003;108:24–31. [DOI] [PubMed] [Google Scholar]
- 13.Devaraj S, Wang-Polagruto J, Polagruto J, Keen CL, Jialal I. High-fat, energy-dense, fast-food-style breakfast results in an increase in oxidative stress in metabolic syndrome. Metabolism 2008;57:867–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neri S, Calvagno S, Mauceri B, Misseri M, Tsami A, Vecchio C, Mastrosimone G, Di Pino A, Maiorca D, Judica A, et al. Effects of antioxidants on postprandial oxidative stress and endothelial dysfunction in subjects with impaired glucose tolerance and type 2 diabetes. Eur J Nutr 2010;49:409–16. [DOI] [PubMed] [Google Scholar]
- 15.Burton-Freeman B. Postprandial metabolic events and fruit-derived phenolics: a review of the science. Br J Nutr 2010;104 Suppl 3:S1–14. [DOI] [PubMed] [Google Scholar]
- 16.Anderson RA, Evans LM, Ellis GR, Khan N, Morris K, Jackson SK, Rees A, Lewis MJ, Frenneaux MP. Prolonged deterioration of endothelial dysfunction in response to postprandial lipaemia is attenuated by vitamin C in type 2 diabetes. Diabet Med 2006;23:258–64. [DOI] [PubMed] [Google Scholar]
- 17.Clark CA, Gardiner J, McBurney MI, Anderson S, Weatherspoon LJ, Henry DN, Hord NG. Effects of breakfast meal composition on second meal metabolic responses in adults with type 2 diabetes mellitus. Eur J Clin Nutr 2006;60:1122–9. [DOI] [PubMed] [Google Scholar]
- 18.Dillinger TL, Barriga P, Escarcega S, Jimenez M, Salazar Lowe D, Grivetti LE. Food of the gods: cure for humanity? A cultural history of the medicinal and ritual use of chocolate. J Nutr 2000;130:2057S–72S. [DOI] [PubMed] [Google Scholar]
- 19.Oracz J, Zyzelewicz D, Nebesny E. The content of polyphenolic compounds in cocoa beans (Theobroma cacao L.), depending on variety, growing region and processing operations: a review. Crit Rev Food Sci Nutr 2015;55:1176–92. [DOI] [PubMed] [Google Scholar]
- 20.Miller KB, Hurst WJ, Payne MJ, Stuart DA, Apgar J, Sweigart DS, Ou B. Impact of alkalization on the antioxidant and flavanol content of commercial cocoa powders. J Agric Food Chem 2008;56:8527–33. [DOI] [PubMed] [Google Scholar]
- 21.Arranz S, Valderas-Martinez P, Chiva-Blanch G, Casas R, Urpi-Sarda M, Lamuela-Raventos RM, Estruch R. Cardioprotective effects of cocoa: clinical evidence from randomized clinical intervention trials in humans. Mol Nutr Food Res 2013;57:936–47. [DOI] [PubMed] [Google Scholar]
- 22.Blumberg JB, Ding EL, Dixon R, Pasinetti GM, Villarreal F. The science of cocoa flavanols: bioavailability, emerging evidence, and proposed mechanisms. Adv Nutr 2014;5:547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westphal S, Luley C. Flavanol-rich cocoa ameliorates lipemia-induced endothelial dysfunction. Heart Vessels 2011;26:511–5. [DOI] [PubMed] [Google Scholar]
- 24.Gutiérrez-Salmeán G, Ortiz-Vilchis P, Vacaseydel CM, Rubio-Gayosso I, Meaney E, Villarreal F, Ramirez-Sánchez I, Ceballos G. Acute effects of an oral supplement of (-)-epicatechin on postprandial fat and carbohydrate metabolism in normal and overweight subjects. Food Funct 2014;5:521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heiss C, Kleinbongard P, Dejam A, Perre S, Schroeter H, Sies H, Kelm M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol 2005;46:1276–83. [DOI] [PubMed] [Google Scholar]
- 26.Pereira MA, Kartashov AI, Ebbeling CB, Van Horn L, Slattery ML, Jacobs DR Jr, Ludwig DS. Fast-food habits, weight gain, and insulin resistance (the CARDIA study): 15-year prospective analysis. Lancet 2005;365:36–42. [DOI] [PubMed] [Google Scholar]
- 27.Schröder H, Fito M, Covas MI; REGICOR investigators. Association of fast food consumption with energy intake, diet quality, body mass index and the risk of obesity in a representative Mediterranean population. Br J Nutr 2007;98:1274–80. [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care 2012;35 Suppl 1:S11–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singleton VL, Rossi JA Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 1965;16:144. [Google Scholar]
- 30.Payne MJ, Hurst WJ, Stuart DA, Ou B, Fan E, Ji H, Kou Y. Determination of total procyanidins in selected chocolate and confectionery products using DMAC. J AOAC Int 2010;93:89–96. [PubMed] [Google Scholar]
- 31.Cohn JN, Finkelstein S, McVeigh G, Morgan D, LeMay L, Robinson J, Mock J. Noninvasive pulse wave analysis for the early detection of vascular disease. Hypertension 1995;26:503–8. [DOI] [PubMed] [Google Scholar]
- 32.Zimlichman R, Shargorodsky M, Boaz M, Duprez D, Rahn KH, Rizzoni D, Payeras AC, Hamm C, McVeigh G. Determination of arterial compliance using blood pressure waveform analysis with the CR-2000 system: Reliability, repeatability, and establishment of normal values for healthy European population–the seven European sites study (SESS). Am J Hypertens 2005;18:65–71. [DOI] [PubMed] [Google Scholar]
- 33.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–95. [DOI] [PubMed] [Google Scholar]
- 34.Martin MÁ, Fernández-Millán E, Ramos S, Bravo L, Goya L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Mol Nutr Food Res 2014;58:447–56. [DOI] [PubMed] [Google Scholar]
- 35.Cordero-Herrera I, Martin MA, Bravo L, Goya L, Ramos S. Cocoa flavonoids improve insulin signalling and modulate glucose production via AKT and AMPK in HepG2 cells. Mol Nutr Food Res 2013;57:974–85. [DOI] [PubMed] [Google Scholar]
- 36.Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr 2012;95:740–51. [DOI] [PubMed] [Google Scholar]
- 37.Balzer J, Rassaf T, Heiss C, Kleinbongard P, Lauer T, Merx M, Heussen N, Gross HB, Keen CL, Schroeter H, et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J Am Coll Cardiol 2008;51:2141–9. [DOI] [PubMed] [Google Scholar]
- 38.Brand-Miller J, Holt SH, de Jong V, Petocz P. Cocoa powder increases postprandial insulinemia in lean young adults. J Nutr 2003;133:3149–52. [DOI] [PubMed] [Google Scholar]
- 39.Miller JB, Pang E, Broomhead L. The glycaemic index of foods containing sugars: comparison of foods with naturally-occurring v. added sugars. Br J Nutr 1995;73:613–23. [DOI] [PubMed] [Google Scholar]
- 40.Gee JM, Cooke D, Gorick S, Wortley GM, Greenwood RH, Zumbe A, Johnson IT. Effects of conventional sucrose-based, fructose-based and isomalt-based chocolates on postprandial metabolism in non-insulin-dependent diabetics. Eur J Clin Nutr 1991;45:561–6. [PubMed] [Google Scholar]
- 41.van Loon LJ, Saris WH, Verhagen H, Wagenmakers AJ. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am J Clin Nutr 2000;72:96–105. [DOI] [PubMed] [Google Scholar]
- 42.Teff KL, Mattes RD, Engelman K, Mattern J. Cephalic-phase insulin in obese and normal-weight men: relation to postprandial insulin. Metabolism 1993;42:1600–8. [DOI] [PubMed] [Google Scholar]
- 43.Peters AL, Davidson MB, Eisenberg K. Effect of isocaloric substitution of chocolate cake for potato in type I diabetic patients. Diabetes Care 1990;13:888–92. [DOI] [PubMed] [Google Scholar]
- 44.Kurosawa T, Itoh F, Nozaki A, Nakano Y, Katsuda S, Osakabe N, Tsubone H, Kondo K, Itakura H. Suppressive effects of cacao liquor polyphenols (CLP) on LDL oxidation and the development of atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic rabbits. Atherosclerosis 2005;179:237–46. [DOI] [PubMed] [Google Scholar]
- 45.Osakabe N, Yamagishi M. Procyanidins in Theobroma cacao reduce plasma cholesterol levels in high cholesterol-fed rats. J Clin Biochem Nutr 2009;45:131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tokede OA, Gaziano JM, Djousse L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr 2011;65:879–86. [DOI] [PubMed] [Google Scholar]
- 47.Kris-Etherton PM, Mustad VA. Chocolate feeding studies: a novel approach for evaluating the plasma lipid effects of stearic acid. Am J Clin Nutr 1994;60:1029S–36S. [DOI] [PubMed] [Google Scholar]
- 48.Yasuda A, Natsume M, Osakabe N, Kawahata K, Koga J. Cacao polyphenols influence the regulation of apolipoprotein in HepG2 and Caco2 cells. J Agric Food Chem 2011;59:1470–6. [DOI] [PubMed] [Google Scholar]
- 49.Selmi C, Cocchi CA, Lanfredini M, Keen CL, Gershwin ME. Chocolate at heart: the anti-inflammatory impact of cocoa flavanols. Mol Nutr Food Res 2008;52:1340–8. [DOI] [PubMed] [Google Scholar]
- 50.Khan N, Khymenets O, Urpi-Sarda M, Tulipani S, Garcia-Aloy M, Monagas M, Mora-Cubillos X, Llorach R, Andres-Lacueva C. Cocoa polyphenols and inflammatory markers of cardiovascular disease. Nutrients 2014;6:844–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koutelidakis AE, Rallidis L, Koniari K, Panagiotakos D, Komaitis M, Zampelas A, Anastasiou-Nana M, Kapsokefalou M. Effect of green tea on postprandial antioxidant capacity, serum lipids, C-reactive protein and glucose levels in patients with coronary artery disease. Eur J Nutr 2014;53:479–86. [DOI] [PubMed] [Google Scholar]
- 52.Sardo CL, Kitzmiller JP, Apseloff G, Harris RB, Roe DJD, Stoner GD, Jacobs ET. An open-label randomized crossover trial of lyophilized black raspberries on postprandial inflammation in older overweight males: a pilot study. Am J Ther 2013. Aug 26 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 53.Grassi D, Desideri G, Necozione S, Ruggieri F, Blumberg JB, Stornello M, Ferri C. Protective effects of flavanol-rich dark chocolate on endothelial function and wave reflection during acute hyperglycemia. Hypertension 2012;60:827–32. [DOI] [PubMed] [Google Scholar]
- 54.Faridi Z, Njike VY, Dutta S, Ali A, Katz DL. Acute dark chocolate and cocoa ingestion and endothelial function: a randomized controlled crossover trial. Am J Clin Nutr 2008;88:58–63. [DOI] [PubMed] [Google Scholar]
- 55.Ahuja KD, Robertson IK, Ball MJ. Acute effects of food on postprandial blood pressure and measures of arterial stiffness in healthy humans. Am J Clin Nutr 2009;90:298–303. [DOI] [PubMed] [Google Scholar]
- 56.Ellis CL, Edirisinghe I, Kappagoda T, Burton-Freeman B. Attenuation of meal-induced inflammatory and thrombotic responses in overweight men and women after 6-week daily strawberry (Fragaria) intake. A randomized placebo-controlled trial. J Atheroscler Thromb 2011;18:318–27. [DOI] [PubMed] [Google Scholar]