Abstract
Background: Inaccuracies in energy intake (EI) measurement hinder identification of risk factors that predict weight gain and evaluation of obesity prevention and treatment interventions. Research has used objective measures of EI to identify underreporting correlates, producing mixed results, suggesting the need to examine novel potential correlates.
Objective: With the use of an objective measure of EI from doubly labeled water (DLW) this report examined multiple potential underreporting correlates.
Methods: Adolescents from 2 studies (study 1, n = 91; mean age: 18.4 ± 0.58 y; 100% female; study 2, n = 162; mean age: 15.2 ± 1.99 y; 82 female adolescents; 80 male adolescents) completed a DLW assessment of EI, a food-frequency questionnaire, and measures of perceived pressure for thinness, thin-ideal internalization, body dissatisfaction, dieting, food-cue reactivity, eating disorder symptoms, socioeconomic status, and neural response to food; BMI (in kg/m2) was measured over a 2-y follow-up.
Results: Elevated BMI correlated with underreported EI in study 1 (r = 0.26, P < 0.05) and study 2 (r = 0.20, P = 0.01), as did male sex in study 2 (r = 0.24, P < 0.01); the other survey measures did not. Underreporting correlated negatively (r = −0.29; uncorr P < 0.001) with responsivity of brain regions implicated in motor control to palatable food receipt and positively (r = 0.31; uncorr P < 0.001) with responsivity of a region implicated in taste processing to cues signaling impending milkshake receipt. Underreporting did not predict future change in BMI in either study.
Conclusions: Findings document marked underreporting and replicate evidence that BMI correlates positively with underreporting and extends this literature by revealing that several novel factors were unrelated to underreporting and further that neural responsivity to food correlated with underreporting, suggesting that adolescents who showed reduced responsivity in a motor control region to food receipt and elevated responsivity of gustatory regions to anticipated palatable food receipt showed greater underreporting. This trial was registered at clinicaltrials.gov as NCT00433680 and NCT02084836.
Keywords: energy intake, doubly labeled water, obesity, underreporting, weight gain
Introduction
Obesity increases risk of diabetes, heart disease, and various forms of cancer and is credited with 2.8 million deaths worldwide annually (1). It is therefore vital to elucidate risk factors that predict unhealthy weight gain and to identify prevention programs that reduce these risk factors and treatment interventions that reduce factors that maintain overeating. However, these pressing public health goals are hindered because it is difficult to accurately measure energy intake (EI)5. Most studies relied on self-report measures, such as 24-h dietary intake recalls and FFQs. Unfortunately, many individuals vastly underreport EI (2), and this underreporting varies systematically across research participants, violating the independence of errors assumption of many commonly used statistical tests. Underreporting of EI can result in inaccurate conclusions about the predictors of future weight gain and the identification of effective obesity prevention and treatment interventions (3).
Researchers have investigated correlates of dietary intake underreporting, with the hope that this will determine for whom self-reported EI is valid and inform the development of more accurate methods of measuring EI. Although several procedures are used to estimate underreporting of EI, the most accurate is to use doubly labeled water (DLW) estimates of EI. DLW uses isotopic tracers (oxygen-18 and deuterium [18O and D218O]) to assess total carbon dioxide production, which can be used to generate accurate estimates of habitual caloric intake (4), providing estimates of EI with only 7.8% SE of measurement (5). Studies that used this method defined underreporting as the ratio between self-reported EI with the use of dietary recall and DLW-estimated EI. The factors that emerged as significant correlates of underreporting in multiple studies were BMI (in kg/m2) (6–9), sex (10, 11), low income (6, 8), body dissatisfaction (8, 11), and dieting (9, 11). Although these findings have advanced knowledge of factors that correlate with underreporting, findings are inconsistent, suggesting the need to examine new potential correlates of underreporting. The present report sought to extend this literature by investigating a broad range of factors that might be theorized to correlate with DLW-assessed EI underreporting with the use of data from 2 samples. The factors examined include BMI, body dissatisfaction, thin-ideal internalization, perceived sociocultural pressure to be thin, reported dieting, eating disorder symptoms, food cue reactivity, socioeconomic status, and neural responsivity to palatable food receipt and anticipated receipt. For instance, elevated responsivity of reward, attention, and gustatory regions and lower responsivity of inhibitory regions to anticipated palatable food receipt may increase the risk of overeating, which could prompt individuals to minimize this propensity by underreporting caloric intake. In addition, we tested whether underreporting predicts future increases in BMI over a 2-y follow-up period, because it might be a proxy measure of a positive energy balance. To our knowledge, previous studies have not tested this latter hypothesis.
Methods
Study 1
Participants in study 1 were a randomly selected subsample from a large obesity prevention trial that targeted young women with weight concerns. Participants were 91 female late adolescents aged 18–20 y (mean age: 18.4 y ± 0.58; mean BMI: 23.7 ± 4.07; 90% white, 2% American Indian or Alaska Native, 3% Asian, and 5% did not report). Exclusion criteria included participants who had diabetes, conditions that required supplemental oxygen, or pregnancy. Participants provided data during 6 visits to the laboratory as follows: baseline, 2 wk, 4 wk, 6 mo, 1 y, and 2 y after baseline. Participants were also required to avoid traveling >322 km from the study site in the 2 wk between the second and third visit to the laboratory because regional variation in the amount of oxygen-18 and deuteruim in water would have introduced error variance. Participants provided written informed consent, and research was conducted according to the ethical standards required by this institutional review board-approved study.
Study 2
Participants in study 2 were recruited with advertisements and flyers to participate in a study that examined how the brain responds to food and potential causes of overeating. Participants were 162 adolescents between the ages of 14 and 17 y old (82 female adolescents, 80 male adolescents; mean age: 15.2 ± 1.99 y; mean BMI: 20.8 ± 1.91; 84% white, 5% American Indian or Alaska Native; 2% Asian, 3% black or African American, 0.5% Native Hawaiian or Pacific Islander, 0.5% other or mixed racial heritage, and 5% did not report). Exclusion criteria were a BMI <18 or >25, pregnancy, head injury with loss of consciousness, substantial cognitive impairment, major psychiatric disorders in the past year, more than weekly use of psychoactive substances (including nicotine and alcohol), or psychotropic medications. Participants provided data during 4 visits to the laboratory at baseline and 2 wk, 1 y, and 2 y after baseline. Participants were required to avoid traveling >322 km from the study site in the 2 wk between the second and third visit to the laboratory. Participants and their parents provided written informed consent, and research was conducted according to the ethical standards required by this institutional review board-approved study.
Measures (study 1 and study 2)
Body mass.
BMI was used to reflect height-adjusted adiposity. After removal of shoes and coats, height was measured to the nearest millimeter with the use of a stadiometer, and weight was assessed to the nearest 0.1 kg with the use of a digital scale. Two measures of height and weight were obtained and averaged at each visit. BMI correlates with direct measures of total body fat such as DXA (r = 0.80–0.90) and with health measures, including blood pressure, adverse lipoprotein profiles, atherosclerotic lesions, serum insulin concentrations, and diabetes mellitus in adolescent samples (12).
Objective measure of energy intake.
DLW was used to estimate EI over a 2-wk period. DLW was administered immediately after subjects tested negatively for pregnancy (if applicable). Doses were 1.6–2.0 g H218O (10 atom percent)/kg estimated total body water. Spot urine samples were collected immediately before DLW was administered and 1, 3, and 4 h after dosing. Two weeks later, 2 additional spot urine samples were collected at the same time of day as 3- and 4-h postdosing samples. No samples were the first void of the day. Energy expenditure (EE) was calculated with the use of equation A6 (4), dilution space ratios (13), and the modified Weir’s equation (14), as previously described (15). EI per day was calculated from the sum of EE from DLW and the estimated change in body energy stores from serial body weight measurements performed at baseline and 2 wk after dosing. This figure was divided by the number of days between baseline and 2 wk after the test to calculate the daily source of energy substrates from weight loss or storage of excess EI as weight gain (16). The equation used for each participant was as follows: EI = EE + [(2-wk weight − baseline weight) × 7800)]/(2-wk date − baseline date). The 7800 kcal/kg is an estimate of the energy density of adipose tissue (17).
Self-reported energy intake.
An adapted version of the Block Food-Frequency Questionnaire (BFFQ) (18) assessed frequency of consumption of specific food types over the past 2 wk. Participants were given a definition of a medium portion and asked to indicate the frequency of consumption over the previous 2-wk period. BFFQ values correlated (r = 0.57) with 4-d food record estimates for total EI and most nutrients (18) and showed 2-wk test-retest reliability (mean r = 0.69) (19).
Underreporting of caloric intake.
Underreporting was calculated as the difference between objectively measured EI, as estimated with the use of DLW, and self-reported caloric intake, measured with the BFFQ, such that higher scores represent greater underreporting of caloric intake.
Dieting.
The Dutch Restrained Eating Scale (20) assesses dietary behaviors designed to produce weight loss and weight maintenance (sample item: Do you deliberately eat less in order not to become too heavy?). The Dutch Restrained Eating Scale has shown internal consistency (α = 0.95), 2-wk test-retest reliability (r = 0.82), convergent validity with self-reported caloric intake (but not objectively measured caloric intake), predictive validity for bulimic symptom onset, and sensitivity to detecting intervention effects (20, 21) (α = 0.92 at baseline).
Eating disorder.
The Eating Disorder Diagnostic Interview (21) assessed eating disorder symptoms. Items that assessed symptoms in the past month were summed to form an overall symptom composite. This composite has shown internal consistency (α = 0.92), 1-wk test-retest reliability (r = 0.90), inter-rater agreement (intraclass correlation coefficient r = 0.93), sensitivity to detecting effects of eating disorder prevention and treatment interventions, and predictive validity for future onset of depression (21, 22).
Parental education.
Parental education, as a proxy for socioeconomic status, was assessed. Participants were asked to provide the highest level of education completed by each parent. The mean of both parents’ reports were calculated to create the parental education score.
Additional study 1 measures
Food cue reactivity.
The 18-item Power of Food Scale (23) assessed individual differences in appetitive responsiveness to food and food cues. This scale shows good internal consistency (α = 0.93) and test-retest reliability over a 4-mo period (r = 0.80) (24).
Body dissatisfaction.
Items from the Satisfaction and Dissatisfaction with Body Parts Scale (25) assessed dissatisfaction with 9 body parts. The scale has shown internal consistency (α = 0.94), 3-wk test-retest reliability (r = 0.90), predictive validity for bulimic symptom onset, and sensitivity to detecting intervention effects (26) (α = 0.91 at baseline).
Thin-ideal internalization.
The Ideal-Body Stereotype Scale-Revised assessed thin-ideal internalization (26). The scale has shown internal consistency (α = 0.91), 2-wk test-retest reliability (r = 0.80), predictive validity for bulimic symptom onset, and sensitivity to detecting intervention effects (26) (α = 0.78 at baseline).
Sociocultural pressure to be thin.
Perceived pressure from family, peers, dating partners, and the media to be thin was assessed with the Perceived Sociocultural Pressure Scale (27). This scale has shown internal consistency (α = 0.88), 2-wk test-retest reliability (r = 0.93), and predictive validity for future onset of bulimic symptoms (28) (α = 0.85 at baseline).
Additional study 2 measures
Food reward paradigm.
The food reward paradigm assessed response to receipt of a palatable milkshake and anticipated receipt of the milkshake. Stimuli were 2 images (glasses of milkshake and water) that signaled (cued) impending delivery of either 0.5 mL chocolate milkshake or tasteless solution, respectively. On 40% of the trials the taste was not delivered after the cue to allow investigation of the neural response to anticipation of a taste that was not confounded with actual receipt of the taste (unpaired trials). There were 30 repeats of both milkshake receipt and tasteless solution receipt and 20 repeats of both the unpaired milkshake cue and the unpaired tasteless solution cue. Tastes were delivered with programmable syringe pumps. Syringes filled with milkshake and tasteless solution were connected via Tygon tubing to a manifold that fit into the mouths of participants and delivered the taste to a consistent tongue segment. Participants were instructed to swallow when they saw the swallow cue.
fMRI statistical analyses.
A detailed description of the fMRI data acquisition and preprocessing are provided elsewhere (29). To identify brain regions activated in response to palatable food intake, BOLD response was contrasted during receipt of milkshake vs. tasteless solution (milkshake receipt > tasteless solution receipt). The arrival of a taste in the mouth was considered to be receipt. To identify brain regions activated in response to anticipated receipt, BOLD response during presentation of the unpaired cue signaling impending delivery of the milkshake was contrasted with response during presentation of the unpaired cue signaling impending delivery of the tasteless solution (milkshake cue > tasteless cue). Contrast images were constructed for each participant. Random-effects analyses were used to account for within-subject variance. Individual’s statistical parametric maps of the above-mentioned contrasts were regressed on the EI underreporting score. Self-reported hunger levels were included as a covariate.
We determined the thresholds by way of Monte Carlo simulations of random noise distribution with the use of the AlphaSim module of Analysis of Functional NeuroImaging (Cox) (30). The inherent smoothness was calculated from the residual mean squares with the use of the 3dFWHM module of Analysis of Functional NeuroImaging (30). The mean gray matter mask of the whole brain was derived from the sample with the use of Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra segmentation in Statistical Parametric Mapping (Welcome Department of Imaging Neuroscience), following standard methods in Voxel-Based Morphometry (31). Once segmented, mean gray matter was resliced to 3 mm3 and binarized with the use of the image calculator function in Statistical Parametric Mapping at the level of i1 (binary selector vector) >0.03. This mask was used in the Monte Carlo simulations and applied at the second level for all analyses to reduce the number of voxels in white matter tested, because any peaks in white matter would be considered artifact findings. The resulting threshold of P < 0.001 with a cluster (k) ≥12 was considered corrected for multiple comparisons across the whole brain. Effect sizes were derived from the Z values (Z/√N). We confirmed that influential outliers did not drive effects.
Results
Study 1
Baseline characteristics for both study 1 and study 2 are shown in Table 1. The mean underreporting score was 1270 ± 666 kcal/d (range: −174 to 2683 kcal/d). The correlations between underreporting, BMI, dieting, body dissatisfaction, thin-ideal internalization, parental education, eating disorder symptoms, perceived sociocultural pressure to be thin, and food cue responsivity are shown in Table 2. Underreporting showed a significant positive correlation with baseline BMI (r = 0.26, P < 0.05), wherein those with higher BMIs showed greater underreporting. None of the other factors showed significant relations with underreporting (r = −0.22 to 0.10). The BFFQ used in study 1 and study 2 showed only a weak correlation with DLW EI (r = 0.14) (3).
TABLE 1.
Baseline subject characteristics and behavioral measures for female adolescents with weight concerns (study 1) and male and female adolescents (study 2)1
| Study 1 (n = 91) | Study 2 (n = 162) | |
| Characteristics | ||
| Age, y | 18.4 ± 0.58 | 15.2 ± 1.99 |
| BMI, kg/m2 | 23.7 ± 4.07 | 20.8 ± 1.91 |
| Reported energy intake, kcal/d | 1226 ± 420 | 1884 ± 858 |
| DLW energy intake, kcal/d | 2524 ± 588 | 2563 ± 774 |
| Underreporting,2 kcal/d | 1270 ± 666 | 667 ± 1061 |
| Behavioral measures | ||
| Dieting3 | 2.85 ± 0.803 | 1.60 ± 0.631 |
| Parental education4 | 4.74 ± 0.95 | 4.21 ± 1.04 |
| Eating disorder symptoms5 | 11.4 ± 13.2 | 2.40 ± 1.36 |
| Thin-ideal internalization6 | 3.71 ± 0.467 | |
| Sociocultural pressure7 | 2.54 ± 0.775 | |
| Food cue reactivity8 | 2.44 ± 0.802 | |
| Body dissatisfaction9 | 3.35 ± 0.665 |
Values are means ± SDs. DLW, doubly labeled water.
Calculated as (DLW energy intake − reported energy intake).
Assessed with the use of the Dutch Restrained Eating Scale (20), higher scores indicate greater dieting.
Anchored by 1 for grade school graduate through 6 for advanced degree.
Assessed with the Eating Disorder Diagnostic Interview (21); higher scores indicate greater disordered eating symptoms.
Assessed with the Ideal-Body Stereotype Scale-Revised (26).
Assessed with the Perceived Sociocultural Pressure Scale (27).
Assessed with the Power of Food Scale (23).
Assessed with the Body Parts Scale (25) that assesses dissatisfaction of 9 body parts.
TABLE 2.
Correlations between underreporting of caloric intake and predictive factors in female adolescents with weight concerns (study 1)1
| Study 1 | BMI | Dieting | Body dissatisfaction | Thin-ideal internalization | Parental education | Eating disorder symptoms | Sociocultural pressure | Food cue reactivity | Underreporting |
| BMI (kg/m2) | −0.02 | 0.22* | −0.19 | 0.01 | −0.01 | −0.03 | 0.02 | 0.26* | |
| Dieting2 | 0.32** | 0.28* | 0.20 | 0.47*** | 0.41*** | 0.19 | 0.01 | ||
| Body dissatisfaction3 | 0.23* | 0.16 | 0.41*** | 0.22* | 0.01 | 0.06 | |||
| Thin-ideal internalization4 | 0.22* | 0.22* | 0.48*** | 0.12 | −0.11 | ||||
| Parental education5 | −0.38*** | −0.09 | 0.10 | −0.07 | |||||
| Eating disorder symptoms6 | −0.38*** | 0.03 | 0.10 | ||||||
| Sociocultural pressure7 | 0.26* | −0.19 | |||||||
| Food cue reactivity8 | −0.02 | ||||||||
| Underreporting9 |
n = 91. *, **, ***Correlations between underreporting and predictive factors: *P < 0.05; **P < 0.001; ***P < 0.001. Underreporting showed a significant positive correlation with baseline BMI (r = 0.26, P < 0.05), wherein those with higher BMIs showed greater underreporting. DLW, doubly labeled water.
Assessed with the Dutch Restrained Eating Scale (20); higher scores indicate greater dieting.
Assessed with the Body Parts Scale (25) that assessed dissatisfaction of 9 body parts.
Assessed with the Ideal-Body Stereotype Scale-Revised (26).
Anchored by 1 for grade school graduate through 6 for advanced degree.
Assessed with the use of the Eating Disorder Diagnostic Interview (21); higher scores indicate greater disordered eating symptoms.
Assessed with the use of the Perceived Sociocultural Pressure Scale (27).
Assessed with the use of the Power of Food Scale (23).
Calculated as (DLW energy intake − reported energy intake).
Regression models indicated that degree of underreporting of EI was not significantly related to future increases in BMI over the 2-y follow-up.
Study 2
The mean underreporting score was 668 ± 1062 kcal/d (range: −2435 to 3324 kcal/d). The correlations between underreporting, BMI, dieting, parental education, eating pathology, and sex are shown in Table 3. Underreporting was significantly correlated with baseline BMI (r = 0.20, P = 0.01) and sex (r = 0.24, P < 0.01), indicating that participants with higher baseline BMI underreport more than participants with a lower BMI, and that male adolescents underreport more than female adolescents. None of the other self-reported factors were significantly correlated with underreporting (r = 0.03–0.14).
TABLE 3.
Correlations between underreporting of caloric intake and predictive factors in male and female adolescents (study 2)1
| Study 2 | BMI | Dieting | Parental education | Eating disorder symptoms | Sex | Underreporting |
| BMI | 0.27* | −0.11 | 0.06 | −0.06 | 0.20** | |
| Dieting2 | 0.11 | 0.32*** | −0.34*** | 0.03 | ||
| Parental education3 | −0.05 | 0.05 | 0.11 | |||
| Eating disorder symptoms4 | −0.17* | 0.14 | ||||
| Sex | 0.24** | |||||
| Underreporting5 |
n = 162. *, **, ***Correlations between underreporting and predictive factors: *P < 0.05; **P < 0.01; ***P < 0.001. Underreporting was significantly correlated with baseline BMI (r = 0.20, P = 0.01) and sex (r = 0.24, P < 0.01), indicating that participants with higher baseline BMI underreport more than participants with a lower BMI, and that male participants underreport more than female participants. DLW, doubly labeled water.
Assessed with the Dutch Restrained Eating Scale (20); higher scores indicate greater dieting.
Anchored by 1 for grade school graduate through 6 for advanced degree.
Assessed with the Eating Disorder Diagnostic Interview (21); higher scores indicate greater disordered eating symptoms.
Calculated as (DLW energy intake − reported energy intake).
Underreporting was negatively correlated (r = −0.29) with activation in a region in the left anterior cerebellar lobe (Montreal Neurological Institute coordinates: −15, −55, −26, Z = 3.64, k = 18) in response to milkshake receipt (relative to tasteless receipt). Further, underreporting was positively correlated (r = 0.31) with activation in the left pons (Montreal Neurological Institute coordinates: −6, −34, −47, Z = 3.89, k = 17) in response to anticipated receipt of milkshake (relative to anticipated receipt of water).
Regression models indicated that degree of underreporting of EI was not significantly related to future increases in BMI over the 2-y follow-up.
Discussion
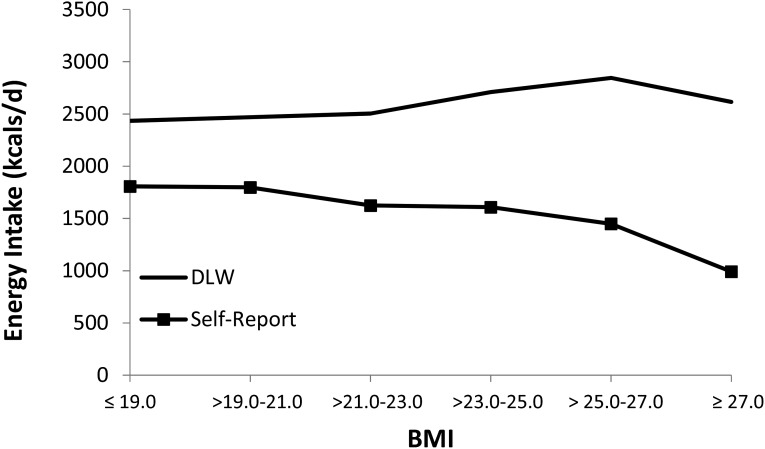
In both studies we found that baseline BMI was significantly correlated with degree of underreporting of EI. This relation apparently explains why underreporting was greater in study 1 (mean underreported intake: 1270 kcal/d), which had a mean BMI of 23.7, than in study 2 (mean underreported intake: 658 kcal/d), which had a mean BMI of 20.8. The relation between elevated BMI and underreported EI replicates findings from previous studies that used DLW to calculate underreporting (6, 32, 33). Our results also converge with previous findings from studies that did not use an objective measure of EI to validate self-reported intake (34–37). The relation between reported EI and DLW was estimated EI as a function of BMI (Figure 1), combining data from the 2 samples examined in the present study. As expected, DLW EI generally increased with increasing BMI scores. However, self-reported EI actually decreased as BMI increased, creating greater underreporting of EI for participants with an elevated BMI. Perhaps the most remarkable aspect of these results is that, whereas lean participants report ∼66% of the calories they consume, the most overweight participants report only ∼20% of the calories they consume. Critically, the fact that the degree of underreporting scales with BMI means that the assumption of independent errors is violated, implying that it may not be appropriate to use ordinary least-squares analyses (e.g., regression or ANOVA models) when examining self-reported caloric intake.
FIGURE 1.
Combined mean self-reported and DLW-measured EI for female adolescents with weight concerns (study 1, n = 91) and for male and female adolescents (study 2, n = 162) as a function of BMI. Combined data from study 1 and study 2 shows the graphical representation of the relation between reported EI, DLW, and BMI. DLW-determined EI generally increased with increasing BMI scores. Self-reported EI decreased as BMI increased, creating greater underreporting of EI for participants with an elevated BMI. DLW, doubly labeled water; EI, energy intake.
In study 2 we also found that sex was significantly related to underreporting, with male adolescents underreporting their intake significantly more than female adolescents. The male participants in our study underreported their intake by a mean of 924 kcal/d, whereas the female participants underreported by a mean of 418 kcal/d. Evidence that male participants underreport EI to a greater degree than female participants was found previously in another study that used DLW to validate self-reported intake (38), but it does not line up with other studies that found that female participants underreport more than male participants per DLW estimates of EI (9, 11). Male participants in our sample self-reported greater mean EI than female participants (2054 kcal/d vs. 1719 kcal/d, respectively). Previous research has shown that underreporting increases as intake increases (39), which may explain why underreporting appears greater for male participants than for female participants in the present data. This could be because the more food an individual consumes, the more difficult it is to accurately report consumption, or because of social pressure to not overeat.
In study 2, we examined whether neural response to receipt and anticipated receipt of palatable food (chocolate milkshake) was significantly related to underreporting. We found a significant negative effect for activation in the left anterior cerebellar lobe in response to milkshake receipt and a positive effect for activation in the left pons in response to anticipated receipt of milkshake. The significant negative response in the cerebellum to milkshake receipt, which encodes procedural memory, motor control, and coordination, may imply that participants with less recruitment of motor control regions in response to tastes of palatable foods tend to overeat and consequently underreport intake to a greater degree. The significant positive response in the pons region of the brain to anticipated palatable food receipt is also noteworthy, given that the pons mainly encodes sensory information, including taste. This finding suggests that participants who have greater activation in a taste-encoding region in response to anticipated milkshake receipt may overeat and show consequent underreporting of intake more than participants who do not experience as much neural response in this gustatory region. These effects are novel, because no previous studies have examined whether neural response to palatable food correlates with underreporting of caloric intake.
This report also examined whether other self-reported measures correlated with underreporting in our samples; however, none of these correlations were significant. Although previous studies that used DLW to validate self-reported intake have found significant correlations with dieting (8, 9) and body dissatisfaction (8), these effects did not replicate in the present study. Note that the past studies reported large effects for both dieting (mean r = 0.60) and body dissatisfaction (r = 0.91), given that we did not replicate these findings, yet this may be because the past studies involved adults, whereas we studied adolescents. None of our other measures, including thin-ideal internalization, parental education, sociocultural pressure to be thin, or food cue reactivity, were significantly related to underreporting in our samples, and, to the best of our knowledge, this was the first study to examine whether these factors were related to underreporting.
We also tested the novel hypothesis that degree of underreporting of caloric intake might predict future weight gain, because this variable may identify individuals who are in a positive energy balance. However, degree of underreporting of EI did not predict future increases in BMI in either study.
It is important to consider the limitations of the present studies. First, the sample for both studies included individuals within a narrow age range and from limited ethnic and racial backgrounds, and study 1 comprised individuals with weight concerns, so results should be generalized to other populations with caution. Second, the present study used only one measure of self-reported intake (the BFFQ) to determine degree of underreporting. It might be useful for future studies seeking to identify correlates of underreporting to include additional self-report measures (e.g., 24-h dietary recalls). Third, we were not powered to detect small effects in either of our samples, which should be taken into consideration when interpreting our findings.
In conclusion, our results replicated findings that elevated BMI was significantly related to underreporting, and they provided evidence that among adolescents male teenagers underreport EI more than female teenagers, presumably because male teenagers reported greater EI overall, and underreporting increases as intake increases. Critically, combined data from our 2 samples graphically illustrated that the degree of underreporting increased with increasing BMI, implying that self-reported caloric intake violates a key assumption of commonly used ordinary least-squares analyses (independence of errors), such as Pearson’s product moment correlations, ANOVA, and regression analyses. We were unable to replicate that underreporting of caloric intake was related to dieting and body dissatisfaction measures, potentially because this relation does not hold in adolescent samples. Collectively, these results imply that underreporting of caloric intake is greatest for those individuals with the largest positive energy imbalance, suggesting that underreporting may be primarily rooted in social desirability factors. The magnitude of underreporting suggests that food-frequency measures should not be used to assess caloric intake in research studies, because they account for only a small amount of variance in actual caloric intake. Finally, the fMRI results show that underreporting may be related to deficits in motor control and elevated sensitivity of brain regions that encode taste. The evidence that neural response to receipt and anticipated receipt of palatable food predict degree of underreporting provides an important new direction for future research.
Acknowledgments
We thank C Nathan Marti, Jeff Gau, and Sonja Yokum for assistance with the statistical modeling and Scott Watrous, at the Lewis Center for Neuroimaging at the University of Oregon, for his contribution and assistance in imaging for this investigation. ES designed the research; ES and KSB conducted the research; ES analyzed the data; and ES, CAP, and KSB wrote the paper. ES had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BFFQ, Block Food-Frequency Questionnaire; DLW, doubly labeled water; EE, energy expenditure; EI, energy intake.
References
- 1.American Medical Association [Internet]. Chicago: The Association [updated 2013 Jun 18; cited 2014 Jul 14]. AMA adopts new policies on second day of voting at annual meeting. Available from: http://www.ama-assn.org/ama/pub/news/news/2013/2013–06–18-new-ama-policies-annual-meeting.page.
- 2.Goris AH, Meijer EP, Westerterp KR. Repeated measurement of habitual food intake increases under-reporting and induces selective under-reporting. Br J Nutr. 2001;85:629–34. [DOI] [PubMed] [Google Scholar]
- 3.Stice E, Durant S. Elevated objectively measured but not self-reported energy intake predicts future weight gain in adolescents. Appetite 2014;81:84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jequier E. Energy expenditure by doubly labeled water - validation in humans and proposed calculation. Am J Physiol 1986;250:R823–30. [DOI] [PubMed] [Google Scholar]
- 5.Schoeller DA, Hnilicka JM. Reliability of the doubly labeled water method for the measurement of total daily energy expenditure in free-living subjects. J Nutr 1996;126:348S–54S. [PubMed] [Google Scholar]
- 6.Johnson RK, Soultanakis RP, Matthews DE. Literacy and body fatness are associated with underreporting of energy intake in US low-income women using the multiple-pass 24-hour recall: a doubly labeled water study. J Am Diet Assoc 1998;98:1136–40. [DOI] [PubMed] [Google Scholar]
- 7.Scagliusi FB, Ferriolli E, Pfrimer K, Laureano C, Cunha CS, Gualano B, Lancha AH Jr. Underreporting of energy intake in Brazilian women varies according to dietary assessment: a cross-sectional study using doubly labeled water. J Am Diet Assoc 2008;108:2031–40. [DOI] [PubMed] [Google Scholar]
- 8.Scagliusi FB, Ferriolli E, Pfrimer K, Laureano C, Cunha CSF, Gualano B, Lancha AH. Characteristics of women who frequently under report their energy intake: a doubly labelled water study. Eur J Clin Nutr 2009;63:1192–9. [DOI] [PubMed] [Google Scholar]
- 9.Tooze JA, Subar AF, Thompson FE, Troiano R, Schatzkin A, Kipnis V. Psychosocial predictors of energy underreporting in a large doubly labeled water study. Am J Clin Nutr 2004;79:795–804. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RK, Goran MI, Poehlman ET. Correlates of over- and underreporting of energy intake in healthy older men and women. Am J Clin Nutr 1994;59:1286–90. [DOI] [PubMed] [Google Scholar]
- 11.Novotny JA, Rumpler WV, Riddick H, Hebert JR, Rhodes D, Judd JT, Briefel R. Personality characteristics as predictors of underreporting of energy intake on 24-hour dietary recall interviews. J Am Diet Assoc 2003;103:1146–51. [DOI] [PubMed] [Google Scholar]
- 12.Dietz WH, Robinson TN. Use of the body mass index (BMI) as a measure of overweight in children and adolescents. J Pediatr 1998;132:191–3. [DOI] [PubMed] [Google Scholar]
- 13.Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of h-2-labeled and o-18-labeled water in humans. Am J Physiol 1994;267:E585–90. [DOI] [PubMed] [Google Scholar]
- 14.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black AE, Prentice AM, Coward WA. Use of food quotients to predict respiratory quotients for the doubly-labeled water method of measuring energy-expenditure. Hum Nutr Clin Nutr 1986;40:381–91. [PubMed] [Google Scholar]
- 16.Forbes GB. Body fat content influences the body composition response to nutrition and exercise. In: Yasumura S, Wang J, Pierson RN, eds. In vivo body composition studies. New York: New York Academy of Sciences; 2000. p. 359–65. [DOI] [PubMed] [Google Scholar]
- 17.Poehlman ET, Melby CL, Badylak SF, Calles J. Aerobic fitness and resting energy expenditure in young adult males. Metabolism 1989;38:85–90. [DOI] [PubMed] [Google Scholar]
- 18.Block G, Subar AF. Estimates of nutrient intake from a food frequency questionnaire—the 1987 national-health interview survey. J Am Diet Assoc 1992;92:969–77. [PubMed] [Google Scholar]
- 19.Klohe DM, Clarke KK, George CC, Milani TJ, Hanss-Nuss H, Freeland-Graves J. Relative validity and reliability of a food frequency questionnaire for a triethnic population of 1-year-old to 3-year-old children for low income families. J Am Diet Assoc 2005;105:727–34. [DOI] [PubMed] [Google Scholar]
- 20.Van Strien T, Frijters JE, Van Staveren WA, Defares PB, Deurenberg P. The predictive validity of the Dutch Restrained Eating Scale. Int J Eat Disord 1986;5:747–55. [Google Scholar]
- 21.Stice E, Rohde P, Gau J, Shaw H. An effectiveness trial of a dissonance-based eating disorder prevention program for high-risk adolescent girls. J Consult Clin Psychol 2009;77:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burton E, Stice E. Evaluation of a healthy-weight treatment program for bulimia nervosa: a preliminary randomized trial. Behav Res Ther 2006;44:1727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, Ochner CN, Coletta MC, Bellace D, Wallaert M, et al. The power of food scale. A new measure of the psychological influence of the food environment. Appetite 2009;53:114–8. [DOI] [PubMed] [Google Scholar]
- 24.Cappelleri JC, Bushmakin AG, Gerber RA, Leidy NK, Sexton CC, Karlsson J, Lowe MR. Evaluating the Power of Food Scale in obese subjects and a general sample of individuals: development and measurement properties. Int J Obes (Lond) 2009;33:913–22. [DOI] [PubMed] [Google Scholar]
- 25.Berscheid E, Walster E, Bohrnstedt G. The happy American body: A survey report. Psychol Today 1973;7:119–31. [Google Scholar]
- 26.Stice E, Marti CN, Spoor S, Presnell K, Shaw H. Dissonance and healthy weight eating disorder prevention programs: Long-term effects from a randomized efficacy trial. J Consult Clin Psychol 2008;76:329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stice E, Bearman SK. Body-image and eating disturbances prospectively predict increases in depressive symptoms in adolescent girls: a growth curve analysis. Dev Psychol 2001;37:597–607. [DOI] [PubMed] [Google Scholar]
- 28.Stice E, Presnell K, Spangler D. Risk factors for binge eating onset in adolescent girls: a 2 year prospective investigation. Health Psychol 2002;21:131–8. [PubMed] [Google Scholar]
- 29.Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci 2011;31:4360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward BD. Simultaneous inference for fMRI data [Internet]. 2000 [cited 2014 Nov 3]. Available from: http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf.
- 31.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 32.Platte P, Pirke KM, Wade SE, Trimborn P, Fichther MM. Physical activity, total energy expenditure, and food intake in grossly obese and normal weight women. Int J Eat Disord 1995;17:51–7. [DOI] [PubMed] [Google Scholar]
- 33.Prentice AM, Black AE, Coward WA, Davies HL, Goldberg GR, Murgatroyd PR, Ashford J, Sawyer M, Whitehead RG. High levels of energy expenditure in obese women. Br Med J (Clin Res Ed) 1986;292:983–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrison GG, Galal OM, Ibrahim N, Khorshid A, Stormer A, Leslie J, Saleh NT. Underreporting of food intake by dietary recall is not universal: a comparison of data from Egyptian and American women. J Nutr 2000;130:2049–54. [DOI] [PubMed] [Google Scholar]
- 35.Bazelmans C, Matthys C, De Henauw M, Dramaix M, Kornitzer GD, De Backer G, Levêque A. Predictors of misreporting in an elderly population: the ‘Quality of life after 65’ study. Public Health Nutr 2007;10:185–91. [DOI] [PubMed] [Google Scholar]
- 36.Gnardellis C, Boulou C, Trichopoulou A. Magnitude, determinants and impact of under-reporting of energy intake in a cohort study in Greece. Public Health Nutr 1998;1:131–7. [DOI] [PubMed] [Google Scholar]
- 37.Kretsch MJ, Fong AK, Green MW. Behavioral and body size correlates of energy intake underreporting by obese and normal-weight women. J Am Diet Assoc 1999;99:300–6. [DOI] [PubMed] [Google Scholar]
- 38.Seale JL, Rumpler WV. Comparison of energy measurements by diet records, energy intake balance, doubly labeled water and room calorimetry. Eur J Clin Nutr 1997;51:856–63. [DOI] [PubMed] [Google Scholar]
- 39.Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, Sharbaugh CO, Trabulsi J, Runswick S, Ballard-Barbash R, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol 2003;158:1–13. [DOI] [PubMed] [Google Scholar]