Abstract
Background: Creatine synthesis from guanidinoacetate consumes ∼50% of s-adenosylmethionine (SAM)–derived methyl groups, accounting for an equivalent proportion of s-adenosylhomocysteine (SAH) and total homocysteine (tHcys) synthesis. Dietary creatine inhibits the synthesis of guanidinoacetate, thereby lowering plasma tHcys in rats.
Objective: We tested the hypotheses that creatine supplementation lowers plasma guanidinoacetate, increases blood SAM, lowers blood SAH, and lowers plasma tHcys.
Methods: Bangladeshi adults were randomly assigned to receive 1 of 4 treatments for 12 wk: placebo (n = 101), 3 g/d creatine (Cr; n = 101), 400 μg/d folic acid (FA; n = 153), or 3 g/d creatine plus 400 μg/d folic acid (Cr+FA; n = 103). The outcomes of plasma guanidinoacetate and tHcys, as well as whole blood SAM and SAH, were analyzed at baseline and week 12 by HPLC. Treatment effects of creatine supplementation were examined with the use of the group comparisons of Cr vs. placebo and Cr+FA vs. FA.
Results: Plasma guanidinoacetate declined by 10.6% (95% CI: 4.9, 15.9) in the Cr group while increasing nonsignificantly in the placebo group (3.7%; 95% CI: −0.8, 8.5) (Pgroup difference = 0.0002). Similarly, plasma guanidinoacetate declined by 9.0% (95% CI: 3.4, 14.2) in the Cr+FA group while increasing in the FA group (7.0%; 95% CI: 2.0, 12.2) (Pgroup difference < 0.0001). Plasma tHcys declined by 23.4% (95% CI: 19.5, 27.1) and 21.0% (95% CI: 16.4, 25.2) in the FA and Cr+FA groups, respectively (Pgroup difference = 0.41), with no significant changes in the placebo or Cr groups (Pgroup difference = 0.35). A decrease in guanidinoacetate over time was associated with a decrease in tHcys over time in the Cr+FA group (β = 0.30; 95% CI: 0.17, 0.43; P < 0.0001).
Conclusions: Our findings indicate that whereas creatine supplementation downregulates endogenous creatine synthesis, this may not on average lower plasma tHcys in humans. However, tHcys did decrease in those participants who experienced a decline in plasma guanidinoacetate while receiving creatine plus folic acid supplementation. This trial was registered at clinicaltrials.gov as NCT01050556.
Keywords: creatine, guanidinoacetate, homocysteine, folic acid, folate, s-adenosylmethionine, RCT, Bangladesh, hyperhomocysteinemia
Introduction
Folate deficiency and hyperhomocysteinemia are widely prevalent among adults in Bangladesh. In a cross-sectional study of 1650 Bangladeshi adults, 39% of women and 57% of men were found to be folate deficient (plasma folate <9 nmol/L), whereas 26% of women and 63% of men were found to have hyperhomocysteinemia [defined as plasma total homocysteine (tHcys)8 ≥10.4 μmol/L for women and ≥11.4 μmol/L for men] (1). Hyperhomocysteinemia has been associated with an increased risk of cardiovascular events, stroke, and cognitive disorders (2–4).
Folate deficiency is a common cause of hyperhomocysteinemia: 5-methyltetrahydrofolate (5-mTHF) donates a methyl group for the remethylation of homocysteine to methionine, and also inhibits glycine N-methyltransferase (GNMT), a major consumer of s-adenosylmethionine (SAM) and source of s-adenosylhomocysteine (SAH) (5) (Figure 1). Hyperhomocysteinemia can also arise from causes other than folate deficiency, including vitamin B-12 deficiency (6), renal disease (7), and genetic polymorphisms that impair homocysteine metabolism (8). Whereas much attention has been given to interventions influencing homocysteine removal, such as folic acid and vitamin B-12 supplementation, little focus has been placed on interventions that could downregulate homocysteine synthesis.
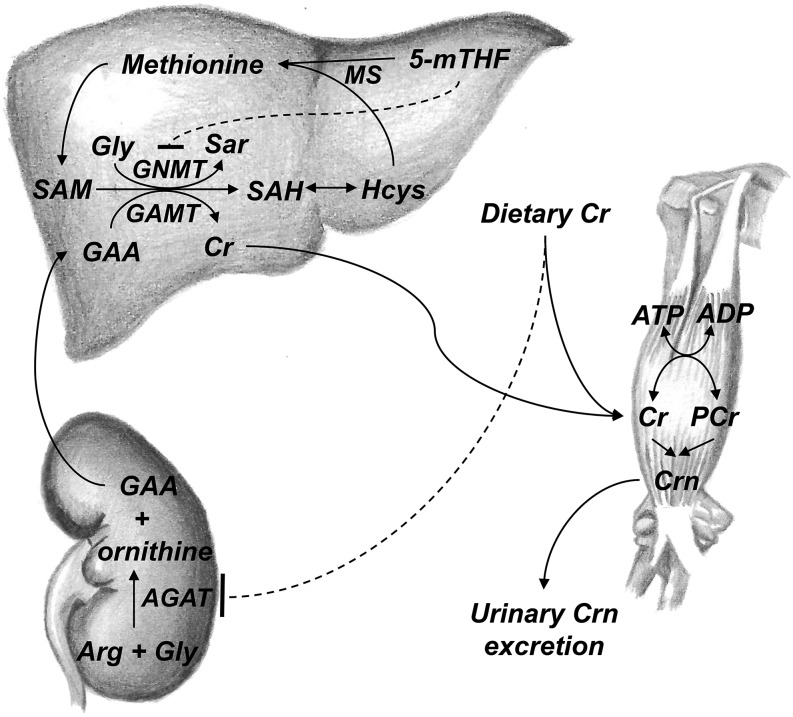
FIGURE 1.
Overview of Cr metabolism and the methionine cycle. In the first, rate-limiting step of Cr biosynthesis, GAA is formed from arginine and glycine by AGAT (30); in the rat, this reaction occurs primarily in the kidney (36). Dietary Cr (primarily from meat) leads to pretranslational inhibition of AGAT (12), thereby inhibiting endogenous Cr biosynthesis. GAA is transported to the liver, where it is methylated by GAMT to generate Cr, with SAM as the methyl donor (30). The byproduct of this methylation reaction (and others) is SAH. SAH is hydrolyzed to generate Hcys. Hcys can be remethylated to methionine by MS, with 5-mTHF as the methyl donor, or it can be directed to the transsulfuration pathway through which it is ultimately catabolized. 5-mTHF also reduces Hcys by inhibition of GNMT, a major consumer of SAM (5). Cr, whether derived from endogenous biosynthesis or dietary sources, is transported to tissues with high energy requirements such as the skeletal muscle, heart, and brain, where it is phosphorylated to PCr (30). PCr is used for the regeneration of ATP during intensive exercise. Cr and PCr are converted nonenzymatically at a constant rate to Crn, which is then excreted in the urine (30). AGAT, arginine:glycine amidinotransferase; Cr, creatine; Crn, creatinine; GAA, guanidinoacetate; GAMT, guanidinoacetate methyltransferase; GNMT, glycine N-methyltransferase; Hcys, homocysteine; MS, methionine synthase; PCr, phosphorylcreatine; SAH, s-adenosylhomocysteine; SAM, s-adenosylmethionine; Sar, sarcosine; 5-mTHF, 5-methyltetrahydrofolate. Drawings by Brandilyn A Peters; reproduced with permission.
Creatine is a nitrogenous organic acid that occurs naturally in food; rich sources include meat and fish. In humans, creatine requirements are fulfilled through dietary sources and/or endogenous synthesis (9). Creatine synthesis from guanidinoacetate, a reaction catalyzed by guanidinoacetate methyltransferase (GAMT), consumes roughly 50% of all SAM-derived methyl groups (10, 11), accounting for an equivalent proportion of all SAH and homocysteine synthesis (Figure 1).
Dietary creatine intake inhibits synthesis of guanidinoacetate in the rat kidney by pretranslational inhibition of arginine:glycine amidinotransferase (AGAT) (12) (Figure 1). Studies in rats have demonstrated that plasma tHcys concentrations can be lowered by creatine supplementation in the diet, which reduces methylation demand (13–16). These studies indicate that creatine supplementation has the potential to decrease the synthesis of homocysteine. In humans, however, creatine supplementation has not been sufficiently examined as a homocysteine-lowering agent. Two small placebo-controlled studies in healthy young adults found no significant effect from creatine supplementation on plasma tHcys (17, 18). In 2004, a 4 wk study of healthy volunteers (n = 16) found a significantly greater decrease in plasma tHcys in participants receiving creatine plus multivitamins than in those receiving multivitamins alone (19). These studies were limited by small sample sizes and the use of healthy study populations without hyperhomocysteinemia. With the use of data from a large randomized, controlled trial testing folic acid and creatine as therapeutic approaches to lower blood arsenic in Bangladesh (20), we focus here on a prespecified secondary outcome of the trial, plasma tHcys, to test the hypothesis that creatine supplementation lowers plasma tHcys in humans.
Methods
Study participants.
The Folic Acid and Creatine Trial has been described elsewhere in detail (20). Briefly, participants for this trial were selected from the Health Effects of Arsenic Longitudinal Study, a prospective cohort study that, at the time of the Folic Acid and Creatine Trial, had recruited >20,000 adults living within a 25 km2 region in Araihazar, Bangladesh (21). Eligible participants in the Health Effects of Arsenic Longitudinal Study cohort were married adults between the ages of 20 and 65 who had been drinking from their current well for at least 3 y. For inclusion in the current study, participants were randomly selected from cohort participants who had been drinking from a household well having water arsenic >50 μg/L, the Bangladeshi limit for arsenic in drinking water, for at least 1 y. Pregnant women; individuals taking nutritional supplements; individuals with protein in their urine; and individuals with known renal disease, diabetes, or gastrointestinal or other health problems were excluded from the study. Informed consent was obtained by our Bangladeshi field staff physicians. Ethical approval was obtained from the Institutional Review Board of Columbia Presbyterian Medical Center and the Bangladesh Medical Research Council.
Study design and fieldwork.
A total of 622 participants were recruited for the study and were randomly assigned to 1 of 5 treatments; the analyses in this paper concentrate on those randomly assigned to receive either placebo (n = 104), 3 g/d creatine (Cr; n = 104), 400 μg/d folic acid (FA; n = 156), or 3 g/d creatine + 400 μg/d folic acid (Cr+FA; n = 104) (folic acid and creatine supplements supplied by Douglas Laboratories). Participants randomly selected to receive 800 μg/d folic acid were excluded from this analysis because there was no comparable creatine group. The creatine dosage is based on the estimations of Brosnan et al. (22) that a 20- to 39-y-old 70 kg man loses ∼14.6 mmol creatine/d (∼1.9 g/d), with the mean creatine losses for women 80% of that of men; we therefore concluded that 3 g creatine/d should be sufficient to downregulate endogenous creatine synthesis. This dose is also considered safe by the European Food Safety Authority (23). The folic acid dosage is based on the US RDA for adults (24).
This was a randomized, double-blind, placebo-controlled trial. Blocked randomization within gender strata was used to ensure balance in the treatment groups. Within each block, 2 persons were randomly assigned to receive placebo, 2 to receive Cr, 3 to receive FA, and 2 to receive Cr+FA, for an allocation ratio of 1:1:1.5:1. Within each block, the order of treatments was randomly permutated by a statistician at Columbia University. A pharmacist in Bangladesh distributed barcode-labeled pill bottles to field staff sequentially as they enrolled participants; bottles were distributed in the order of the random assignment list generated at Columbia University. Five experienced teams, each having one interviewer and one physician (all specifically trained for this study) worked simultaneously to recruit and follow study participants through house-to-house visits. These teams were responsible for all visits, at which blood and urine samples were collected. All participants received a household-level arsenic removal water filter (READ-F filter, Brota Services International) at baseline for the provision of low arsenic (<10 μg/L) water. Participants received and retained 2 bottles of pills at enrollment: one bottle contained folate pills or folate-matched placebo pills, and the second bottle contained creatine or creatine-matched placebo pills. Village health workers either witnessed or inquired about compliance on a daily basis. With the exception of 2 data management specialists (one in Bangladesh and one in the United States) who assigned letters (A, B, C, etc.) to treatments, all study investigators, fieldwork teams, village health workers, lab technicians, and study participants were blinded to study treatments for the entire duration of the study through the use of blind-labeled pill bottles and participant identification barcodes on all pill bottles and biological samples. Study enrollment began in December 2009 and follow-up was completed in May 2011.
A total of 10 participants were dropped from these groups over the course of the study for various reasons, including adverse events [n = 3; 1 in the placebo group (abdominal cramps), 1 in the FA group (hypertension), and 1 in the Cr group (severe vertigo)], pregnancy (n = 3; 1 each in the Cr, FA, and Cr+FA groups), missing plasma samples (n = 2; 1 each in the placebo group and Cr group), and dropout (n = 2; 1 each in the placebo group and FA group). The total sample size for this analysis by treatment group was as follows: placebo group (n = 101), FA group (n = 153), Cr group (n = 101), and Cr+FA group (n = 103).
Plasma tHcys was the primary outcome of interest for this analysis. Secondary outcomes of interest were plasma creatine + creatinine (Cr+Crn) and guanidinoacetate, blood SAM and SAH, and plasma total cysteine (tCys). Variables in the tables are listed in the order of the hypothesized biological response to the creatine intervention (plasma Cr+Crn, plasma guanidinoacetate, blood SAM, blood SAH, plasma tHcys, and plasma tCys). Venous blood samples were collected at baseline and week 12 for the measurement of primary and secondary outcomes.
Sample collection and handling.
Blood samples were obtained by venipuncture at the time of recruitment and after the 12 wk intervention. Blood was collected into EDTA evacuated tubes and placed in IsoRack cool packs (Brinkmann Instruments) designed to maintain samples at 4°C for 6 h. Within 4 h, samples were transported in coolers to our local laboratory, situated at our field clinic in Araihazar. Samples were centrifuged at 3000 × g for 10 min at 4°C, and plasma was separated from cells. Aliquots of plasma and whole blood were stored at −80°C and shipped, frozen on dry ice, to Columbia University for analysis.
Plasma Cr+Crn and guanidinoacetate.
Plasma Cr+Crn and guanidinoacetate were measured by HPLC with fluorescence detection according to the method described by Carducci et al. (25). We used an Inertsil ODS-3 3 μm HPLC column 4.6 × 100mm (GL Sciences) and excitation and emission wavelengths of 335 and 435 nm, respectively. Intra- and interassay CVs for Cr+Crn were 7% and 9%, respectively, and for guanidinoacetate were 8% and 9%, respectively.
Plasma homocysteine and cysteine concentrations.
Plasma tHcys and tCys concentrations were measured by HPLC with fluorescence detection according to the method described by Pfeiffer et al. (26). Intra- and interassay CVs for tHcys were 5% and 7%, respectively, and for tCys were 4% and 13%, respectively.
Whole blood SAM and SAH.
SAM and SAH were measured in whole blood by HPLC according to the method described by Poirier et al. (27), the only difference being that blood (400 μL) was added to tubes containing 200 μL 0.1 mol/L sodium acetate, pH 6, and 160 μL 40% trichloroacetic acid in the field, and were then vortexed and frozen at −80°C. Sample processing was then completed at Columbia University. Intra- and interassay CVs for SAM were 2% and 6%, respectively, and for SAH were 10% and 26%, respectively.
Plasma folate and vitamin B-12.
Plasma folate and vitamin B-12 were measured by radioimmunoassay (SimulTRAC-SNB Vitamin B-12/Folate RIA kit; MP Biomedicals). Intra- and interassay CVs for folate were 5% and 13%, respectively, and for vitamin B-12 were 6% and 17%, respectively.
Sample size considerations.
Blood SAM and SAH could not be assessed for some participants because of insufficient blood volume. Therefore our sample sizes for SAM and SAH outcomes are somewhat reduced. In addition, we excluded plasma Cr+Crn and guanidinoacetate data from 1 d on which the positive control did not run properly. Finally, in some samples, there was insufficient plasma volume to measure Cr+Crn and guanidinoacetate. To address these sample issues, we present results with the use of the available sample for each outcome individually, and compare these with the results from the analysis with the use of participants with data available for all 6 outcome variables of interest. For the Cr+Crn and guanidinoacetate variables, we have n = 130 in the FA group and n = 102 in the Cr+FA group. For the SAM variable, we have n = 76 in the placebo group, n = 89 in the Cr group, n = 139 in the FA group, and n = 94 in the Cr+FA group. For the SAH variable and for the analysis with complete data, there are n = 68 in the placebo group, n = 86 in the Cr group, n = 110 in the FA group, and n = 84 in the Cr+FA group.
Statistical analyses.
The primary group comparisons of interest were Cr vs. placebo and Cr+FA vs. FA, in order to determine the treatment effects of creatine supplementation, with and without folic acid. The comparison of FA vs. placebo was not of interest, given that the tHcys-lowering effects of folic acid are already well established. Descriptive statistics were calculated by treatment group to describe the sample characteristics. Treatment group differences were detected by using the chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. We applied ln transformations to the outcomes of Cr+Crn, guanidinoacetate, SAM, SAH, tHcys, and tCys in order to stabilize the variance of the outcomes and reduce the impact of extreme values in parametric analyses. For all outcomes, we calculated the geometric mean (antilog-transformed mean of the ln of an outcome, e.g., emean[ln(Hcys0)]) and 95% CIs of each outcome at baseline and week 12 by treatment group. To describe the change in an outcome over 12 wk, we calculated geometric mean ratios and 95% CIs by treatment group. These geometric mean ratios are equivalent to the antilog-transformed mean within-person change in the ln-transformed variable (e.g., emean[ln(Hcys12)−ln(Hcys0)]), and are also equivalent to the ratio of the geometric means at week 12 and week 0 (e.g., geometricmeanHcys12/geometricmeanHcys0). We then used the ratio of the geometric means to determine the percentage change in the geometric mean of an outcome over the 12 wk, with the use of the following equation: percent change = (geometric mean ratio – 1) × 100. We used paired t tests to detect within-person change over time in the ln-transformed outcomes within each treatment group. To detect the treatment effects of creatine supplementation, we compared the within-person change in the ln-transformed outcomes between the Cr and placebo groups, and between the Cr+FA and FA groups, with the use of 2-sample t tests.
A subset of participants had blood SAM and SAH available at either baseline or week 12, but not at both time points; in order to include all available data for these outcomes, we conducted a sensitivity analysis in which we used linear models with repeated measures for the outcomes of ln(SAM) and ln(SAH) to examine change over time by treatment groups. Generalized estimating equations, which use all available data and account for within-subject correlations in the repeated measures, were used to estimate the regression parameters. The models include indicator variables for treatment groups (Cr vs. placebo or Cr+FA vs. FA), for time (week 12 vs. baseline), and for treatment × time interaction. The model estimate for the interaction term indicates the treatment group difference in mean change of the outcome over 12 wk.
A decrease in guanidinoacetate over time in a Cr or Cr+FA participant suggests that endogenous creatine synthesis was downregulated in that participant; therefore, we used linear regression models with within-person change in ln-transformed guanidinoacetate over time [Δln(guanidinoacetate)] as a predictor of within-person change in ln-transformed variables over time [Δln(SAM), Δln(SAH), Δln(tHcys), and Δln(tCys)]. The models were adjusted for baseline ln(guanidinoacetate) and the ln-transformed baseline of the outcome, and the analysis was conducted separately by treatment group. We used the Wald test to detect treatment group differences (Cr vs. placebo and Cr+FA vs. FA) in the covariate-adjusted regression coefficient for the effect of Δln(guanidinoacetate) on the outcome variables.
All analyses were performed as intent to treat, and were carried out with the use of SAS 9.2 or R (version 3.0.2). All statistical tests were 2-sided with a significance level of 0.05.
Results
The demographic and biochemical characteristics of the study participants by treatment group at baseline are presented in Table 1. By design, the study enrolled approximately equal numbers of men and women. The mean age of the study participants was 38 y, and on average study participants had low BMI and a low level of education. In addition, there was a substantial proportion of smokers (26%) and betel nut chewers (24%) and a high prevalence of folate deficiency (20%) and hyperhomocysteinemia (53%) in the study population. There were no meaningful between-group differences in baseline characteristics. The participants with complete outcome data (n = 348) had lower creatinine-adjusted urinary arsenic (P = 0.03), slightly higher plasma folate (P = 0.06), and lower plasma cysteine (P = 0.06) at baseline than participants with at least one missing outcome (n = 110) (Supplemental Table 1).
TABLE 1.
Baseline characteristics of the Bangladeshi adults participating in a folic acid and creatine randomized, controlled trial1
| Placebo (n = 101) | Cr (n = 101) | FA (n = 153) | Cr+FA (n = 103) | |
| Age, y | 37.9 ± 7.3 | 38.3 ± 8.2 | 39.0 ± 8.0 | 38.0 ± 7.7 |
| Men | 50.5 | 50.5 | 50.3 | 50.5 |
| Education, y | 3.5 ± 3.7 | 3.3 ± 3.6 | 3.3 ± 3.6 | 3.9 ± 4.1 |
| BMI,2 kg/m2 | 20.4 ± 3.1 | 20.0 ± 3.0 | 19.5 ± 2.3 | 19.5 ± 2.5 |
| Ever smoked3 | 24.8 | 28.7 | 23.8 | 30.1 |
| Ever used betel nut3 | 27.7 | 24.8 | 23.8 | 20.4 |
| Water arsenic, μg/L | 158 ± 126 | 159 ± 125 | 149 ± 118 | 167 ± 147 |
| Urinary arsenic, μg/L | 139 ± 137 | 180 ± 212 | 160 ± 164 | 178 ± 155 |
| Urinary arsenic, μg/g creatinine | 305 ± 202 | 328 ± 253 | 340 ± 325 | 312 ± 164 |
| Urinary creatinine, mg/dL | 49.2 ± 35.0 | 57.3 ± 36.7 | 57.7 ± 45.2 | 62.4 ± 47.2 |
| Plasma folate, nmol/L | 16.7 ± 17.3 | 16.0 ± 7.9 | 16.7 ± 14.2 | 15.4 ± 8.7 |
| Folate deficient (<9 nmol/L) | 21.6 | 13.9 | 23.5 | 20.4 |
| Plasma vitamin B-12, pmol/L | 226 ± 97.4 | 256 ± 141 | 246 ± 131 | 237 ± 121 |
| Plasma Cr+Crn,4 μmol/L | 81.3 ± 23.8 | 77.3 ± 23.0 | 82.0 ± 31.1 | 81.3 ± 28.5 |
| Plasma GAA,4 μmol/L | 2.05 ± 0.66 | 1.95 ± 0.57 | 1.89 ± 0.74 | 1.98 ± 0.67 |
| Blood SAM,5 μmol/L | 2.23 ± 0.81 | 2.10 ± 0.72 | 2.19 ± 0.66 | 2.07 ± 0.64 |
| Blood SAH,6 μmol/L | 0.24 ± 0.12 | 0.25 ± 0.13 | 0.23 ± 0.11 | 0.25 ± 0.13 |
| Plasma tHcys, μmol/L | 13.9 ± 10.8 | 12.4 ± 5.5 | 13.6 ± 8.8 | 12.8 ± 5.6 |
| Hyperhomocysteinemia7 | 54.9 | 50.5 | 52.3 | 56.3 |
| Plasma tCys, μmol/L | 222 ± 41.9 | 220 ± 46.4 | 220 ± 43.5 | 218 ± 46.6 |
Values are means ± SDs or percentages. Cr, 3 g/d creatine group; Cr+FA, 3 g/d creatine plus 400 μg/d folic acid group; Cr+Crn, creatine plus creatinine; FA, 400 μg/d folic acid group; GAA, guanidinoacetate; SAH, s-adenosylhomocysteine; SAM, s-adenosylmethionine; tCys, total cysteine; tHcys, total homocysteine.
Reduced sample size (placebo, n = 100; Cr, n = 98; FA, n = 150; Cr+FA, n = 102).
Reduced sample size (FA, n = 151).
Reduced sample size (FA, n = 130; Cr+FA, n = 102).
Reduced sample size (placebo, n = 76; Cr, n = 89; FA, n = 139; Cr+FA, n = 94).
Reduced sample size (placebo, n = 68; Cr, n = 86; FA, n = 127; Cr+FA, n = 84).
Defined as plasma total homocysteine ≥10.4 μmol/L for women and ≥11.4 μmol/L for men.
The changes in plasma Cr+Crn and guanidinoacetate, blood SAM and SAH, and plasma tHcys and tCys by treatment group over the 12 wk of the trial are shown in Table 2. There was a significant within-person increase in plasma Cr+Crn in the Cr group (P < 0.0001) and Cr+FA group (P < 0.0001), but not in the placebo group (P = 0.22) or the FA group (P = 0.67), with significant group differences between the Cr and placebo groups (P < 0.0001) and the Cr+FA and FA groups (P < 0.0001). This indicates high compliance with supplementation regimens. Plasma guanidinoacetate declined by 10.6% (P = 0.0005) in the Cr group and 9.0% (P = 0.002) in the Cr+FA group, and increased nonsignificantly in the placebo group (P = 0.11) and significantly in the FA group (P = 0.006) (Table 2; Figure 2). The within-person change in plasma guanidinoacetate differed significantly between the Cr and placebo groups (P = 0.0002) and between the Cr+FA and FA groups (P < 0.0001). The increase in guanidinoacetate in the FA group was not significantly different from that in the placebo group (P = 0.35).
TABLE 2.
Baseline and week 12 geometric means and percentage change in the geometric mean from baseline by treatment group for outcomes measured in Bangladeshi adults participating in a folic acid and creatine randomized, controlled trial1
| Placebo | Cr | P(Cr vs. placebo)2 | FA | Cr+FA | P(Cr+FA vs. FA)2 | |
| Plasma Cr+Crn | n = 101 | n = 101 | n = 130 | n = 102 | ||
| Baseline mean (95% CI) | 78 (74, 83) | 74 (70, 79) | 0.21 | 77 (73, 82) | 77 (72, 82) | 0.91 |
| Week 12 mean (95% CI) | 76 (72, 80) | 123 (113, 133) | <0.0001 | 78 (74, 82) | 124 (115, 132) | <0.0001 |
| % change (95% CI) | −2.7 (−6.9, 1.7) | 65.5 (51.1, 81.2) | <0.0001 | 1.2 (−4.1, 6.7) | 60.9 (49.8, 72.8) | <0.0001 |
| Plasma GAA | n = 101 | n = 101 | n = 130 | n = 102 | ||
| Baseline mean (95% CI) | 1.9 (1.8, 2.1) | 1.9 (1.8, 2.0) | 0.38 | 1.8 (1.7, 1.9) | 1.9 (1.8, 2.0) | 0.28 |
| Week 12 mean (95% CI) | 2.0 (1.9, 2.1) | 1.7 (1.6, 1.8) | <0.0001 | 1.9 (1.8, 2.0) | 1.7 (1.6, 1.8) | 0.01 |
| % change (95% CI) | 3.7 (−0.8, 8.5) | −10.6 (−15.9, −4.9) | 0.0002 | 7.0 (2.0, 12.2) | −9.0 (−14.2, −3.4) | <0.0001 |
| Blood SAM | n = 76 | n = 89 | n = 139 | n = 94 | ||
| Baseline mean (95% CI) | 2.1 (2.0, 2.3) | 2.0 (1.9, 2.1) | 0.19 | 2.1 (2.0, 2.2) | 2.0 (1.9, 2.1) | 0.15 |
| Week 12 mean (95% CI) | 2.1 (2.0, 2.3) | 2.1 (1.9, 2.2) | 0.66 | 2.1 (2.0, 2.2) | 2.0 (1.9, 2.1) | 0.11 |
| % change (95% CI) | −0.8 (−5.9, 4.5) | 3.1 (−2.8, 9.5) | 0.33 | 1.1 (−3.3, 5.6) | 0.6 (−4.5, 6.0) | 0.90 |
| Blood SAH | n = 68 | n = 86 | n = 127 | n = 84 | ||
| Baseline mean (95% CI) | 0.21 (0.19, 0.24) | 0.22 (0.19, 0.25) | 0.84 | 0.21 (0.19, 0.23) | 0.22 (0.19, 0.25) | 0.43 |
| Week 12 mean (95% CI) | 0.21 (0.18, 0.24) | 0.22 (0.20, 0.25) | 0.42 | 0.22 (0.20, 0.24) | 0.24 (0.22, 0.27) | 0.17 |
| % change (95% CI) | −2.1 (−13.6, 10.9) | 3.5 (−10.6, 19.7) | 0.57 | 4.7 (−5.1, 15.5) | 8.9 (-5.0, 24.9) | 0.64 |
| Plasma tHcys | n = 101 | n = 101 | n = 153 | n = 103 | ||
| Baseline mean (95% CI) | 12.2 (11.2, 13.4) | 11.4 (10.5, 12.3) | 0.24 | 11.9 (11.1, 12.9) | 11.8 (10.9, 12.7) | 0.82 |
| Week 12 mean (95% CI) | 12.1 (11.1, 13.1) | 10.9 (10.1, 11.7) | 0.07 | 9.1 (8.7, 9.6) | 9.3 (8.8, 9.8) | 0.60 |
| % change (95% CI) | −1.3 (−5.4, 3.1) | −4.3 (−9.0, 0.7) | 0.35 | −23.4 (−27.1, −19.5) | −21.0 (−25.2, −16.4) | 0.41 |
| Plasma tCys | n = 101 | n = 101 | n = 153 | n = 103 | ||
| Baseline mean (95% CI) | 218 (210, 226) | 215 (207, 224) | 0.63 | 216 (210, 223) | 213 (204, 222) | 0.56 |
| Week 12 mean (95% CI) | 222 (215, 230) | 227 (219, 236) | 0.40 | 223 (217, 229) | 224 (216, 232) | 0.87 |
| % change (95% CI) | 2.1 (−0.4, 4.7) | 5.7 (2.6, 9.0) | 0.08 | 3.2 (1.1, 5.4) | 5.2 (2.5, 8.0) | 0.26 |
All geometric mean units are μmol/L. Percentage change defined as (geometric mean ratio – 1) × 100. Cr, 3 g/d creatine group; Cr+Crn, creatine plus creatinine; Cr+FA, 3 g/d creatine plus 400 μg/d folic acid group; FA, 400 μg/d folic acid group; GAA, guanidinoacetate; SAH, s-adenosylhomocysteine; SAM, s-adenosylmethionine; tCys, total cysteine; tHcys, total homocysteine.
From 2-sample t tests comparing ln-transformed outcomes or within-person change in ln-transformed outcomes between the placebo and Cr groups, or between the FA and Cr+FA groups.
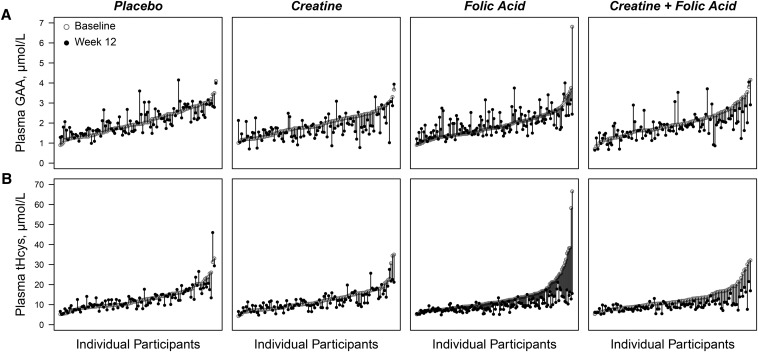
FIGURE 2.
Within-person changes in plasma GAA and tHcys from baseline to week 12 by treatment group in Bangladeshi adults participating in a folic acid and creatine randomized, controlled trial. Plots display the within-person change in plasma GAA (A) or plasma tHcys (B) by treatment group. Individual participants were sorted by baseline concentration on the x-axis. Rising and falling vertical bars indicate each participant’s increase or decrease, respectively, in GAA or tHcys from that participant’s baseline. One outlier was excluded from the tHcys placebo plot (baseline tHcys = 107 μmol/L, week 12 tHcys = 97 μmol/L) for better visual resolution on the y-axis. GAA, guanidinoacetate; tHcys, total homocysteine.
There were no significant changes in blood SAM or SAH in any group (all P ≥ 0.22) over the 12 wk, nor were there between-group differences in within-person change in blood SAM or SAH. Results were similar for the SAM and SAH outcomes when using all available data in repeated measures models with generalized estimating equations (data not shown).
Plasma tHcys declined by 4.3% (P = 0.09) in the Cr group, 23.4% (P < 0.0001) in the FA group, and 21% (P < 0.0001) in the Cr+FA group over the 12 wk intervention (Table 2; Figure 2). Plasma tHcys did not decline significantly in the placebo group (P = 0.55). The within-person decline in tHcys in the Cr group, although greater than that in the placebo group, was not significantly different from placebo (P = 0.35); likewise, the within-person decline in tHcys in the Cr+FA group was not significantly different from folic acid alone (P = 0.41).
Plasma tCys increased significantly in the groups receiving Cr (P = 0.0004), FA (P = 0.004), and Cr+FA (P = 0.0002) over the 12 wk intervention, while increasing nonsignificantly in the placebo group (P = 0.11). The within-person change in tCys did not differ significantly between the Cr and placebo groups (P = 0.08) or the Cr+FA and FA groups (P = 0.26). In addition, the change in tCys in the FA group did not differ significantly from the placebo group (P = 0.49). Results were similar for all outcomes in the complete data analysis.
A decrease in guanidinoacetate over time was associated with a decrease in tHcys (P < 0.0001) and tCys (P = 0.0001) over time in the Cr+FA group only (Table 3). The regression coefficient for the effect of Δguanidinoacetate on Δ tHcys differed between the Cr+FA and FA groups (P = 0.0003), whereas the regression coefficient for the effect of Δguanidinoacetate on ΔtCys did not differ significantly between the Cr+FA and FA groups (P = 0.12).
TABLE 3.
Association between Δln(GAA) and the outcomes of Δln(SAM), Δln(SAH), Δln(tHcys), and Δln(tCys) by treatment group in Bangladeshi adults participating in a folic acid and creatine randomized, controlled trial1
| β (95% CI ) |
β (95% CI) |
|||||
| Outcome | Placebo (n = 101) | Cr (n = 101) | P(Cr vs. placebo)2 | FA (n = 130) | Cr+FA (n = 102) | P(Cr+Fa vs. FA)2 |
| Δ ln(SAM)3 | 0.10 (−0.16, 0.36) | −0.09 (−0.28, 0.11) | 0.26 | −0.02 (−0.21, 0.18) | 0.02 (−0.14, 0.19) | 0.73 |
| Δ ln(SAH)4 | 0.55 (0.00, 1.10) | 0.07 (−0.35, 0.49) | 0.16 | −0.10 (−0.54, 0.34) | 0.31 (−0.03, 0.65) | 0.14 |
| Δ ln(tHcys) | 0.03 (−0.17, 0.23) | −0.12 (−0.28, 0.05) | 0.26 | −0.08 (−0.23, 0.08) | 0.30 (0.17, 0.43) | 0.0003 |
| Δ ln(tCys) | −0.03 (−0.14, 0.08) | −0.03 (−0.13, 0.07) | 0.98 | 0.07 (−0.02, 0.15) | 0.16 (0.08, 0.24) | 0.12 |
β (95% CI) is the estimated regression coefficient of Δln(GAA) from linear regression models. All models are adjusted for ln(baseline GAA) and ln(baseline outcome). Δ = ln(week 12) – ln(baseline). Cr, 3 g/d creatine group; Cr+FA, 3 g/d creatine plus 400 μg/d folic acid group; FA, 400 μg/d folic acid group; GAA, guanidinoacetate; SAH, s-adenosylhomocysteine; SAM, s-adenosylmethionine; tCys, total cysteine; tHcys, total homocysteine.
From Wald test for treatment group difference in covariate-adjusted regression coefficient.
Reduced sample size (placebo, n = 76; Cr, n = 89; FA, n = 118; Cr+FA, n = 93).
Reduced sample size (placebo, n = 68; Cr, n = 86; FA, n = 110; Cr+FA, n = 84).
Discussion
In this randomized, controlled trial of Bangladeshi adults, we hypothesized that creatine supplementation would downregulate endogenous creatine synthesis, thereby reducing methylation demand and resulting in increased blood SAM, decreased blood SAH, and decreased plasma tHcys. Treatment with Cr and Cr+FA significantly lowered plasma guanidinoacetate, which is consistent with pretranslational inhibition of AGAT in the kidney by dietary creatine (12, 28). However, although plasma tHcys was lowered in the Cr group, the mean within-person change was not significantly different from that in the placebo group. In addition, the mean within-person decrease in plasma tHcys in participants receiving Cr+FA was not different from that in participants receiving FA alone. Finally, Cr or Cr+FA supplementation did not change blood SAM or SAH. However, blood SAM and SAH may not be good proxies for SAM and SAH in the liver, which is the primary location of SAM synthesis (29) and the final step of endogenous creatine synthesis (30); we consider this to be likely, because folic acid supplementation also did not change blood SAM in our study, whereas folate is known to increase liver SAM in rats (31). To our knowledge, the current study is the only randomized, controlled trial to examine the effect of folic acid on whole blood SAM and SAH to date, although a small nonplacebo-controlled trial has also observed no effect of folic acid on blood SAM and SAH (32), supporting the questionable relevance of these biomarkers to hepatic concentrations. The lack of treatment effects on blood SAM and SAH in this study may be related to properties of SAM synthesis and metabolism in RBCs, which differ distinctly from liver. In RBCs, methionine adenosyltransferase (MAT; the enzyme which catalyzes SAM biosynthesis) is subject to product inhibition by SAM; this serves to maintain RBC SAM at a low, constant concentration (33). In contrast, the liver isoform of MAT is not subject to product inhibition by SAM (34). In addition, in contrast to the liver, where GAMT is highly expressed and consumes SAM for creatine biosynthesis, there are few methyltransferase enzymes with significant activity in intact human RBCs (35). These properties would imply that 1) folic acid would have a limited effect on RBC SAM because of product inhibition of RBC MAT, and 2) creatine would have a limited effect on RBC SAM because the GAMT reaction may not consume a large proportion of SAM in the RBC.
A decrease in plasma guanidinoacetate over time in participants in the Cr or Cr+FA groups suggests that the intervention was successful in those participants (i.e., downregulation of endogenous creatine synthesis). Because not all participants in the Cr or Cr+FA groups experienced a response to creatine supplementation, as measured by change in guanidinoacetate (Figure 2), we examined the effect of a within-person change in guanidinoacetate over time on within-person changes in other outcomes over time by treatment group. Interestingly, we observed that a decrease in guanidinoacetate over time in the Cr+FA group was associated with a decrease in tHcys over time, which differed significantly from the FA group. This finding supports an effect of decreased guanidinoacetate on tHcys synthesis, and suggests that the creatine plus folic acid intervention lowered tHcys in participants that experienced a decline in guanidinoacetate.
Studies in rats show a more substantial mean reduction in plasma tHcys after creatine supplementation (13–16). Creatine supplementation in these animal studies consisted of adding 0.4–2% creatine to the diet, which results in a higher daily dose of creatine than that used in our study. For example, rats fed 0.4% creatine in the diet [which decreases plasma guanidinoacetate by 72% (36) and plasma tHcys by 25% (16)] consumed ∼0.4 g/(kg body weight ⋅ d), which for a 70 kg person would be ∼28 g/d. In our study, 3 g/d creatine resulted in plasma guanidinoacetate declines of 9–11%; other studies in humans that employed higher creatine dosages achieved greater declines in guanidinoacetate. Plasma guanidinoacetate decreased by 33% in ∼18 y-old athletes receiving ∼22 g/d creatine for 7 d (18), and decreased by 20–30% in ∼19 y-old healthy volunteers who received 20 g/d creatine for 1 wk followed by 5 g/d Cr for 19 wk (37). Thus, a possible limitation is that the creatine dosage used in our study did not sufficiently inhibit AGAT, which might explain the lack of average treatment effects. The discrepancies between the current study and rat models could also relate to species differences in GAMT and AGAT; for example, different affinities of GAMT for guanidinoacetate, or different mechanisms of AGAT inhibition by creatine (38), might explain the lack of an average treatment effect on plasma tHcys in humans. However, to our knowledge, the kinetics of human GAMT and the inhibition of human AGAT by creatine have not been thoroughly investigated.
Several small studies in humans have observed no effect from creatine supplementation on plasma tHcys. In one study (n = 23), creatine supplementation (∼22 g/d for 7 d) to teenage athletes significantly lowered plasma guanidinoacetate compared with placebo; however, there was no effect on plasma tHcys or RBC SAM and SAH (18). Creatine supplementation also did not lower plasma tHcys in placebo-controlled trials of healthy young women (17), coronary artery disease patients (39), and chronic hemodialysis patients (40), and in a nonplacebo-controlled study of healthy men (41). A case study by Petr et al. (42) found that creatine (5 g/d for 30 d) substantially lowered plasma tHcys in a methylene tetrahydrofolate reductase (MTHFR) 677TT homozygote subject (n = 1), whereas tHcys tended to increase in 677CC+CT subjects (n = 9), although remaining within normal range. In contrast to the abovementioned studies, one trial in healthy humans (n = 16) receiving creatine (ranging from 2.1 to 5.5 g/d) for 4 wk did observe a decrease in plasma tHcys in comparison with placebo (19).
In hindsight, the observation that creatine supplementation alone did not decrease plasma tHcys to a greater extent than placebo may not be surprising. Long-range allosteric interactions regulate intracellular SAM concentrations, and may diminish an increase in SAM resulting from decreased endogenous creatine synthesis. SAM inhibits MTHFR (43), resulting in decreased synthesis of 5-mTHF, an inhibitor of GNMT (5). GNMT utilizes SAM for the nonessential conversion of glycine to sarcosine (5), and serves as a major regulator of SAM concentrations (44). Therefore, an increase in SAM from creatine supplementation would result in inhibition of MTHFR, decreased synthesis of 5-mTHF, alleviated inhibition of GNMT, and ultimately, decreased SAM, increased SAH, and increased homocysteine. This regulatory interaction should be overcome with folic acid supplementation, which increases 5-mTHF and allows for continual inhibition of GNMT. Although we did not observe that creatine plus folic acid lowered plasma tHcys to a greater extent than folic acid alone on average, we did observe an association between decreasing guanidinoacetate and decreasing tHcys in the Cr+FA group. It is also possible that the folic acid dosage (400 μg/d) given with creatine was not sufficient to completely inhibit GNMT. An average homocysteine-lowering benefit from creatine supplementation may be difficult to observe, given that when one methyltransferase is downregulated, the flux through competing methyltransferase pathways (e.g., GNMT and phosphatidylethanolamine methyltransferase) are increased (10). For example, when flux through the GAMT pathway is set to zero in a mathematical model, flux through GNMT and phosphatidylethanolamine methyltransferase increases by ∼30% and 20%, respectively (MC Reed and HF Nijhout, Duke University, personal communication, 2015). These enzymes also produce SAH and homocysteine, which may offset the decrease in homocysteine from downregulated creatine synthesis.
We observed increases in plasma tCys in the Cr, FA, and Cr+FA groups over the 12 wk of the trial. It is possible that creatine and/or creatine plus folic acid may increase cysteine through increasing SAM; SAM is an allosteric activator of cystathionine-β-synthase (45), the first enzyme in the transsulfuration pathway through which homocysteine is converted to cysteine. However, given that the changes in tCys in the Cr or Cr+FA groups vs. placebo group or FA alone, respectively, did not reach statistical significance at P < 0.05, we are hesitant to draw a conclusion regarding an effect of creatine on plasma tCys.
In conclusion, on average, creatine or creatine plus folic acid supplementation did not decrease plasma tHcys to a greater extent than placebo or folic acid alone, respectively, in this large randomized, controlled trial of Bangladeshi adults. However, a decrease in guanidinoacetate in the Cr+FA group was significantly associated with a decrease in tHcys, indicating that participants responsive to the intervention in the Cr+FA group experienced a decline in tHcys. The generalizability of this finding to folate-fortified populations remains to be determined. Whereas creatine or creatine plus folic acid did not alter blood SAM concentrations, we cannot rule out the possibility that it may have increased hepatic SAM concentrations. Dietary creatine may have beneficial effects on SAM-dependent methylation independently of any benefits derived from a decrease in plasma tHcys. Thus, future studies should evaluate whether or not creatine or creatine plus folic acid supplementation influences the methylation of other substrates. The full implications of dietary creatine on methyl balance and health in humans warrants further investigation.
Acknowledgments
BAP and XL wrote the paper and analyzed the data; MVG designed the research; MNH and MVG directed field staff in Bangladesh; BAP, FP, ABS, HS, MNU, TI, VI, JHG, and MVG conducted the research; and BAP, MNH, XL, and MVG had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AGAT, arginine:glycine amidinotransferase; Cr, 3 g/d creatine group; Cr+Crn, plasma creatine plus creatinine; Cr+FA, 3 g/d creatine plus 400 μg/d folic acid group; FA, 400 μg/d folic acid group; GAMT, guanidinoacetate methyltransferase; GNMT, glycine N-methyltransferase; MTHFR, methylene tetrahydrofolate reductase; SAH, s-adenosylhomocysteine; SAM, s-adenosylmethionine; tCys, total cysteine; tHcys, total homocysteine; 5-mTHF, 5-methyltetrahydrofolate.
References
- 1.Gamble MV, Ahsan H, Liu X, Factor-Litvak P, Ilievski V, Slavkovich V, Parvez F, Graziano JH. Folate and cobalamin deficiencies and hyperhomocysteinemia in Bangladesh. Am J Clin Nutr 2005;81:1372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 2002;325:1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hankey GJ, Eikelboom JW. Homocysteine and vascular disease. Lancet 1999;354:407–13. [DOI] [PubMed] [Google Scholar]
- 4.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 2002;346:476–83. [DOI] [PubMed] [Google Scholar]
- 5.Wagner C, Briggs WT, Cook RJ. Inhibition of glycine n-methyltransferase activity by folate derivatives: Implications for regulation of methyl group metabolism. Biochem Biophys Res Commun 1985;127:746–52. [DOI] [PubMed] [Google Scholar]
- 6.Quinlivan EP, McPartlin J, McNulty H, Ward M, Strain JJ, Weir DG, Scott JM. Importance of both folic acid and vitamin B12 in reduction of risk of vascular disease. Lancet 2002;359:227–8. [DOI] [PubMed] [Google Scholar]
- 7.van Guldener C. Why is homocysteine elevated in renal failure and what can be expected from homocysteine-lowering? Nephrol Dial Transplant 2006;21:1161–6. [DOI] [PubMed] [Google Scholar]
- 8.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995;10:111–3. [DOI] [PubMed] [Google Scholar]
- 9.Brosnan JT, Brosnan ME. Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr 2007;27:241–61. [DOI] [PubMed] [Google Scholar]
- 10.Mudd SH, Brosnan JT, Brosnan ME, Jacobs RL, Stabler SP, Allen RH, Vance DE, Wagner C. Methyl balance and transmethylation fluxes in humans. Am J Clin Nutr 2007;85:19–25. [DOI] [PubMed] [Google Scholar]
- 11.Stead LM, Brosnan JT, Brosnan ME, Vance DE, Jacobs RL. Is it time to reevaluate methyl balance in humans? Am J Clin Nutr 2006;83:5–10. [DOI] [PubMed] [Google Scholar]
- 12.McGuire DM, Gross MD, Van Pilsum JF, Towle HC. Repression of rat kidney L-arginine:glycine amidinotransferase synthesis by creatine at a pretranslational level. J Biol Chem 1984;259:12034–8. [PubMed] [Google Scholar]
- 13.Taes YEC, Delanghe JR, De Vriese AS, Rombaut R, Van Camp J, Lameire NH. Creatine supplementation decreases homocysteine in an animal model of uremia. Kidney Int 2003;64:1331–7. [DOI] [PubMed] [Google Scholar]
- 14.Deminice R, Portari GV, Vannucchi H, Jordao AA. Effects of creatine supplementation on homocysteine levels and lipid peroxidation in rats. Br J Nutr 2009;102:110–6. [DOI] [PubMed] [Google Scholar]
- 15.Deminice R, Vannucchi H, Simoes-Ambrosio LM, Jordao AA. Creatine supplementation reduces increased homocysteine concentration induced by acute exercise in rats. Eur J Appl Physiol 2011;111:2663–70. [DOI] [PubMed] [Google Scholar]
- 16.Stead LM, Au KP, Jacobs RL, Brosnan ME, Brosnan JT. Methylation demand and homocysteine metabolism: effects of dietary provision of creatine and guanidinoacetate. Am J Physiol Endocrinol Metab 2001;281:E1095–100. [DOI] [PubMed] [Google Scholar]
- 17.Steenge GR, Verhoef P, Greenhaff PL. The effect of creatine and resistance training on plasma homocysteine concentration in healthy volunteers. Arch Intern Med 2001;161:1455–6. [DOI] [PubMed] [Google Scholar]
- 18.Deminice R, Rosa FT, Franco GS, da Cunha SF, de Freitas EC, Jordao AA. Short-term creatine supplementation does not reduce increased homocysteine concentration induced by acute exercise in humans. Eur J Nutr 2014;6:1355–61. [DOI] [PubMed] [Google Scholar]
- 19.Korzun WJ. Oral creatine supplements lower plasma homocysteine concentrations in humans. Clinical laboratory science: journal of the American Society for Medical Technology 2004;17(2):102–6. [PubMed] [Google Scholar]
- 20.Peters BA, Hall MN, Liu X, Parvez F, Sanchez TR, van Geen A, Mey JL, Siddique AB, Shahriar H, Uddin MN, et al. Folic Acid and Creatine as Therapeutic Approaches to Lower Blood Arsenic: A Randomized Controlled Trial. Environ Health Perspect 2015 May 15 (Epub ahead of print, DOI: 10.1289/ehp.1409396). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahsan H, Chen Y, Parvez F, Argos M, Hussain AI, Momotaj H, Levy D, van Geen A, Howe G, Graziano J. Health Effects of Arsenic Longitudinal Study (HEALS): description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol 2006;16:191–205. [DOI] [PubMed] [Google Scholar]
- 22.Brosnan JT, da Silva RP, Brosnan ME. The metabolic burden of creatine synthesis. Amino Acids 2011;40:1325–31. [DOI] [PubMed] [Google Scholar]
- 23.European Food Safety Authority. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to creatine monohydrate for use in foods for particular nutritional uses. EFSA Journal 2004;36:1–6. [Google Scholar]
- 24.Institute of Medicine. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academy Press, 1998. [PubMed] [Google Scholar]
- 25.Carducci C, Birarelli M, Leuzzi V, Carducci C, Battini R, Cioni G, Antonozzi I. Guanidinoacetate and creatine plus creatinine assessment in physiologic fluids: an effective diagnostic tool for the biochemical diagnosis of arginine:glycine amidinotransferase and guanidinoacetate methyltransferase deficiencies. Clin Chem 2002;48:1772–8. [PubMed] [Google Scholar]
- 26.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem 1999;45:290–2. [PubMed] [Google Scholar]
- 27.Poirier LA, Wise CK, Delongchamp RR, Sinha R. Blood determinations of S-adenosylmethionine, S-adenosylhomocysteine, and homocysteine: correlations with diet. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2001;10(6):649–55. [PubMed] [Google Scholar]
- 28.Guthmiller P, Van Pilsum JF, Boen JR, McGuire DM. Cloning and sequencing of rat kidney L-arginine:glycine amidinotransferase. Studies on the mechanism of regulation by growth hormone and creatine. J Biol Chem 1994;269:17556–60. [PubMed] [Google Scholar]
- 29.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem 1990;1:228–37. [DOI] [PubMed] [Google Scholar]
- 30.Wyss M, Kaddurah-Daouk R. Creatine and Creatinine Metabolism. Physiol Rev 2000;80:1107–213. [DOI] [PubMed] [Google Scholar]
- 31.Miller JW, Nadeau MR, Smith J, Smith D, Selhub J. Folate-deficiency-induced homocysteinaemia in rats: disruption of S-adenosylmethionine’s co-ordinate regulation of homocysteine metabolism. Biochem J 1994;298:415–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stam F, Smulders YM, van Guldener C, Jakobs C, Stehouwer CD, de Meer K. Folic acid treatment increases homocysteine remethylation and methionine transmethylation in healthy subjects. Clinical science (London, England: 1979) 2005;108(5):449–56. [DOI] [PubMed] [Google Scholar]
- 33.Oden KL, Clarke S. S-adenosyl-L-methionine synthetase from human erythrocytes: role in the regulation of cellular S-adenosylmethionine levels. Biochemistry 1983;22:2978–86. [DOI] [PubMed] [Google Scholar]
- 34.Finkelstein JD. Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clinical chemistry and laboratory medicine: CCLM / FESCC 2007;45(12):1694–9. [DOI] [PubMed] [Google Scholar]
- 35.Barber JR, Morimoto BH, Brunauer LS, Clarke S. Metabolism of S-adenosyl-L-methionine in intact human erythrocytes. Biochim Biophys Acta 1986;886:361–72. [DOI] [PubMed] [Google Scholar]
- 36.Edison EE, Brosnan ME, Meyer C, Brosnan JT. Creatine synthesis: production of guanidinoacetate by the rat and human kidney in vivo. Am J Physiol Renal Physiol 2007;293:F1799–804. [DOI] [PubMed] [Google Scholar]
- 37.Derave W, Marescau B, Vanden Eede E, Eijnde BO, De Deyn PP, Hespel P. Plasma guanidino compounds are altered by oral creatine supplementation in healthy humans. Journal of applied physiology (Bethesda, Md: 1985) 2004;97(3):852–7. [DOI] [PubMed] [Google Scholar]
- 38.da Silva RP, Clow K, Brosnan JT, Brosnan ME. Synthesis of guanidinoacetate and creatine from amino acids by rat pancreas. Br J Nutr 2014;111:571–7. [DOI] [PubMed] [Google Scholar]
- 39.Jahangir E, Vita JA, Handy D, Holbrook M, Palmisano J, Beal R, Loscalzo J, Eberhardt RT. The effect of L-arginine and creatine on vascular function and homocysteine metabolism. Vascular medicine (London, England) 2009;14(3):239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taes YE, Delanghe JR, De Bacquer D, Langlois M, Stevens L, Geerolf I, Lameire NH, De Vriese AS. Creatine supplementation does not decrease total plasma homocysteine in chronic hemodialysis patients. Kidney Int 2004;66:2422–8. [DOI] [PubMed] [Google Scholar]
- 41.Moraes R, Van Bavel D, Moraes BS, Tibirica E. Effects of dietary creatine supplementation on systemic microvascular density and reactivity in healthy young adults. Nutr J 2014;13:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petr M, Steffl M, Kohlikova E. Effect of the MTHFR 677C/T polymorphism on homocysteinemia in response to creatine supplementation: a case study. Physiological research / Academia Scientiarum Bohemoslovaca 2013;62(6):721–9. [DOI] [PubMed] [Google Scholar]
- 43.Jencks DA, Mathews RG. Allosteric inhibition of methylenetetrahydrofolate reductase by adenosylmethionine. Effects of adenosylmethionine and NADPH on the equilibrium between active and inactive forms of the enzyme and on the kinetics of approach to equilibrium. J Biol Chem 1987;262:2485–93. [PubMed] [Google Scholar]
- 44.Luka Z, Mudd SH, Wagner C. Glycine N-methyltransferase and regulation of S-adenosylmethionine levels. J Biol Chem 2009;284:22507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finkelstein JD, Kyle WE, Martin JL, Pick AM. Activation of cystathionine synthase by adenosylmethionine and adenosylethionine. Biochem Biophys Res Commun 1975;66:81–7. [DOI] [PubMed] [Google Scholar]