Abstract
Phosphoinositide 3-kinases (PI3Ks) are among the most frequently activated signaling pathways in cancer. In chronic lymphocytic leukemia (CLL), signals from the microenvironment are critical for expansion of the malignant B cells, and cause constitutive activation of PI3Ks. CXCR4 is a key receptor for CLL cell migration and adhesion to marrow stromal cells (MSCs). Because of the importance of CXCR4 and PI3Ks for CLL-microenvironment cross-talk, we investigated the activity of novel, isoform-selective PI3K inhibitors that target different isoforms of the p110-kDa subunit. Inhibition with p110α inhibitors (PIK-90 and PI-103) resulted in a significant reduction of chemotaxis and actin polymerization to CXCL12 and reduced migration beneath MSC (pseudoemperipolesis). Western blot and reverse phase protein array analyses consistently demonstrated that PIK-90 and PI-103 inhibited phosphorylation of Akt and S6, whereas p110δ or p110β/p110δ inhibitors were less effective. In suspension and MSC cocultures, PI-103 and PIK-90 were potent inducers of CLL cell apoptosis. Moreover, these p110α inhibitors enhanced the cytotoxicity of fludarabine and reversed the protective effect of MSC on fludarabine-induced apoptosis. Collectively, our data demonstrate that p110α inhibitors antagonize stromal cell-derived migration, survival, and drug-resistance signals and therefore provide a rational to explore the therapeutic activity of these promising agents in CLL.
Introduction
Chronic lymphocytic leukemia (CLL), the most prevalent form of adult leukemia in Western countries, is characterized by the progressive accumulation of phenotypically mature, monoclonal B lymphocytes in the peripheral blood, lymph nodes, and bone marrow. These long-lived CLL B cells are mostly arrested in the G0/G1 phase of the cell cycle and display features consistent with a defect in programmed cell death (apoptosis), such as overexpression of Bcl-2-family proteins.1,2 Despite their apparent longevity in vivo, CLL cells undergo spontaneous apoptosis in vitro, once removed from their in vivo microenvironment and placed into suspension culture without supportive stromal cells.3,4 Spontaneous apoptosis can be prevented by coculture with various stromal cells, such as marrow stromal cells (MSCs), follicular dendritic cells, or nurse-like cells.4–8 This prosurvival effect of stromal cells is largely dependent on direct cell contact between CLL and stromal cells.4,5,9 Chemokine secretion by stromal cells and expression of corresponding chemokine receptors on leukemia cells play a critical role in directional migration (chemotaxis) and adhesion of leukemia cells to MSCs, both in vitro10 and in vivo.11
CXCL12, previously called stromal cell–derived factor–1, is a chemokine constitutively secreted by MSCs that attracts and confines CLL cells to stromal cells via its cognate receptor CXCR4 expressed at high levels on CLL cells.10,12 This mechanism is shared with normal hematopoietic stem cells that require this receptor for homing to stromal niches in the marrow.13,14 Besides its activity on adhesion and migration of CLL cells,10 which is partially dependent on PI3K activation,15 CXCL12 also has a direct prosurvival effect on CLL cells.8,16 Once they engage in adhesion to stromal cells, CLL cells become resistant to the cytotoxic effects of drugs commonly used to treat CLL patients, such as fludarabine17 or corticosteroids.4 This primary drug resistance mechanism, also called cell adhesion–mediated drug resistance,18 may account for minimal residual disease in tissue compartments such as the marrow and relapses commonly seen in treatment of CLL patients.19–21
We previously demonstrated that CXCR4 antagonists can partially resensitize CLL cells to cytotoxic drugs in cocultures with MSCs,17 a finding that is currently pursued in clinical trials in leukemia patients,22 using the small molecule CXCR4 antagonist AMD3100 (now called Plerixafor). However, from our previous work17 and other studies,23,24 it is also apparent that targeting of CXCR4 only partially overcomes stromal cell–mediated drug resistance; therefore, other CLL-microenvironment interactions may represent alternative therapeutic targets.
Phosphoinositide 3-kinases (PI3Ks) are among the most commonly activated signaling pathways in human cancers.25–27 In freshly isolated CLL cells, PI3Ks are constitutive activated,28 and CLL patients with unmutated immunoglobulin variable heavy chain genes, which generally display a more aggressive clinicalcourse than variable heavy chain-mutated patients, show overexpression of PI3K by real-time quantitative polymerase chain reaction.29 Furthermore, growth and survival signals from the microenvironment, such as adhesion to MSCs,9 CXCR4 activation,15 and B-cell receptor (BCR) activation,30 cause PI3K activation in CLL cells. Therefore, we investigated the activity of isoform-selective PI3K inhibitors using a panel of novel isoform-selective PI3K inhibitors that target different isoforms of the p110 subunit.
Therapeutic targeting of PI3K has been decelerated until recently because of the lack of specific inhibitors that possess sufficient activity, specificity, and bioavailability. The prototype PI3K inhibitors wortmannin and LY294002 are pan-specific PI3K inhibitors that sensitize human cancer cells to chemotherapy and radiation in vitro and in vivo31 but lack substrate specificity and show toxicity in animal studies,32 precluding their clinical development. However, over the past few years, we have witnessed a rapid expansion of information about new small molecules that target the PI3K family.33–35
In response to cell stimulation by various growth factors and chemokines, PI3Ks phosphorylate phosphatidylinositol lipids at the D-3 position of the inositol ring, catalyzing the production of phosphatidylinositol-3,4,5-trisphosphate (PIP3), which then sets in motion a coordinated set of events leading to cell growth, migration, and survival.25,36 The PI3Ks have been classified into 3 groups according to their structure and substrate specificity. Class IA isoforms couple to tyrosine kinases and consist of a p110 catalytic subunit (p110α, p110β, or p110γ), which is bound to one of 5 distinct p85 regulatory subunits, linking PI3K activity to the receptor tyrosine kinases (class Ia) or G protein–coupled receptors (class Ib).33 These heterodimers can be recruited either directly to cell-surface receptors, for instance, growth factor receptors, or indirectly by adaptor molecules, such as Shc, Grb2, or IRS-1.37 The p110α and p110β isoforms are ubiquitous, whereas p110γ is predominantly expressed in leukocytes.38,39 P110δ was originally identified in leukocytes but is also expressed in other cell types, including breast tissue and melanocytes.40
The serine-threonine protein kinase Akt (also protein kinase B) is activated as a result of PI3K activity and thus mediates many of the downstream effects of PI3K and consequently plays an important role in both normal and pathologic signaling through the PI3K pathway. Activation of Akt involves membrane binding as well as phosphorylation. Akt requires PIP3 to be translocated to the cell membrane.41 After PIP3 binding and positioning in the cell membrane, full activation of Akt requires phosphorylation on Ser473 in the hydrophobic motif by the mTOR complex 2 (mTORC2) and on Thr308 in the activation loop by the phosphoinositide-dependent kinase 1.38,42 Further downstream, Akt phosphorylates a variety of substrates involved in the regulation of key cellular functions. Because of the importance of PI3Ks in transducing a variety of external, microenvironment-derived migration, growth, and survival signals, we tested new, isoform-selective PI3K inhibitors for activity on CLL cell migration to CXCL12, stromal cell interactions, and stromal cell-mediated drug resistance.
Methods
Reagents and antibodies
All reagents were purchased from Sigma-Aldrich (St Louis, MO) unless otherwise stated. Trypsin/ethylenediaminetetraacetic acid solution was obtained from Invitrogen (Carlsbad, CA), synthetic human CXCL12 (stromal cell–derived factor–1α) from Upstate Biotechnology (Charlottesville, VA), and Ly294002 (phosphatidylinositol 3-kinase inhibitor) from Calbiochem (San Diego, CA). The isoform-selective PI3K inhibitors PIK-90, PI-103, TGX115, ZK75, and IC87114 were synthesized after published patent specifications as described,43 prepared as 20-mM stocks in dimethyl sulfoxide and stored in aliquots at −20°C before use. The structures and key characteristics of these inhibitors have previously been detailed.33,35 The isoform-selective PI3K inhibitors were used at 1- and 10-μM concentrations for the various assays because we previously established that these inhibitors display activity in cellular systems at concentrations between 0.1 and 10 μM.43,44 Moreover, pharmacokinetic analysis revealed that concentrations of greater than 1-μM concentrations of these drugs (PI103) can be achieved in vivo.45
If not otherwise stated, all primary antibodies were bought from Cell Signaling Technology (Danvers, MA). Secondary anti–mouse IgM (μ)-peroxidase antibody was purchased from Roche Diagnostics (Mannheim, Germany) and secondary enhanced chemiluminescence peroxidase-labeled anti–rabbit antibody from Jackson ImmunoResearch Laboratories (West Grove, PA). The dephosphorylated fludarabine nucleoside arabinosyl-2-fluoroadenine (F-ara-A) was purchased from Sigma-Aldrich.
Cells and cell-culture conditions
After informed consent, acquired in accordance with the Declaration of Helsinki and Institutional Review Board approval, peripheral blood samples were obtained either from patients diagnosed with CLL according to clinical and immunophenotypic criteria at the Leukemia Department, University of Texas M. D. Anderson Cancer Center, or from healthy volunteers. Patients' characteristics are summarized in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Peripheral blood mononuclear cells (PBMCs) were isolated via density gradient centrifugation over Ficoll Paque (GE Healthcare, Little Chalfont, United Kingdom). Cells were used fresh or viably frozen in fetal calf serum (FCS) plus 10% dimethyl sulfoxide for storage in liquid nitrogen. Immediately after isolation or thawing, CLL B cells were resuspended at 3 to 6 × 106 cells/mL in RPMI 1640 (HyClone Laboratories, Logan, UT) with 10% FCS (heat-inactivated) and 1% penicillin-streptomycin (Invitrogen) at 37°C in a humidified atmosphere containing 5% carbon dioxide. The murine marrow stromal cell line M2-10B4 was purchased from ATCC (Manassas, VA).
Chemotaxis assay
The migration of PI3K inhibitor–pretreated CLL B cells in response to CXCL12 was evaluated using 24-well Corning chemotaxis chambers (Corning Life Sciences, Acton, MA) as described.10 In brief, transwell chambers with polycarbonate inserts with a 5-μm pore size were used, and CLL cells (5 × 106 in 100 μL) were added to the top chamber, whereas CXCL12 was added to the lower wells. The filter inserts were then placed into the wells and incubated at 37°C. Assay medium (RPMI with 0.5% bovine serum albumin [BSA]) without CXCL12 was used as baseline control. After 3 hours, cells that transmigrated into the lower chamber were collected and counted by flow cytometry (FACSCalibur) at high flow for 20 seconds. A 1:20 dilution of input cells was counted under the same conditions. Results are shown as migration index (mean ± SEM), which represents the ratio between cells that migrated to the lower chamber in the presence of CXCL12 and cells that migrated in response to medium alone. Pretreated CLL cells were incubated with the PI3K inhibitors or with vehicle (untreated controls) for 2 hours at 37°C.
Pseudoemperipolesis assay (in vitro migration of CLL B cells beneath stromal cells)
The migration of CLL cells beneath MSCs was observed and quantified as previously described.10 Briefly, the day before performing the assay, M2-10B4 stromal cells in RPMI supplemented with 10% FCS and antibiotics were seeded in collagen-coated 24-well plates at a concentration of 1.5 × 105 cells per well. After overnight culture, the confluence of the stromal cell layer was controlled by phase-contrast microscopy, and then untreated and pretreated CLL B cells were added into each well to a final concentration of 5 × 106 CLL cells per well. Pretreatment of the CLL cells was performed as described in “Reagents and antibodies.” The plates were incubated at 37°C for 5 hours, and after that nonmigrated cells were removed by vigorously washing of each well for 5 times with RPMI medium. Before detaching the migrated cells containing stromal cell layer for 1 minute with prewarmed (37°C) trypsin/ethylenediaminetetraacetic acid solution, the complete removal of nonmigrated cells and the integrity of the stromal cell layer were checked via phase-contrast microscopy. The detached cells were immediately suspended in 200-μL RPMI with 10% FCS for counting by flow cytometry. To exclude stromal cells from the counts, a lymphocyte gate was set using the different relative size and granularity (forward scatter, side scatter), and cell counting then was performed at high flow for 20 seconds.
Actin polymerization assay
Actin polymerization was performed as described.10 Briefly, 1.5 × 106 CLL cells, pretreated with PI3K inhibitors as described in “Reagents and antibodies,” were suspended in RPMI medium with 0.5% BSA and incubated with 200 ng/mL CXCL12 at 37°C for various amounts of time. At the indicated time points, 400 μL of the cell suspension was added to 100-μL staining solution (4 × 10−7 mol/L fluorescein isothiocyanate–labeled phalloidin, 0.5 mg/mL 1-α-lysophosphatidylcholin, and 8% formaldehyde, all Sigma-Aldrich) in phosphate-buffered saline. The analyses of the fixed cells were performed by flow cytometry on a FACSCalibur. All time points are plotted relative to the mean fluorescence intensity of the same sample before the chemokine was added.
Measurement of cell viability
To determine the viability of CLL B cells, 200-μL cells were removed from the wells of a 24-well plate at the indicated time points and incubated for 15 minutes in fluorescence-activated cell sorter buffer (RPMI + 0.5% BSA) containing 40 nM 3,3′-dihexyloxacarbocyanine iodide (DiOC6) and 10 μg/mL propidium iodide (PI), as described.8 Within 30 minutes, the cells were then analyzed by flow cytometry on a FACSCalibur (BD Biosciences, San Jose, CA). Viable cells show high DiOC6 and low PI fluorescence, whereas apoptotic cells have low DiOC6 and PI fluorescence; necrotic cells are characterized by low DiOC6 and high PI fluorescence (Figure 7A). We also cultured normal PBMCs under the same conditions, with or without the various PI3K inhibitors, fludarabine, and with or without stromal cell support, and their viability was also determined by staining with DiOC6 and PI.
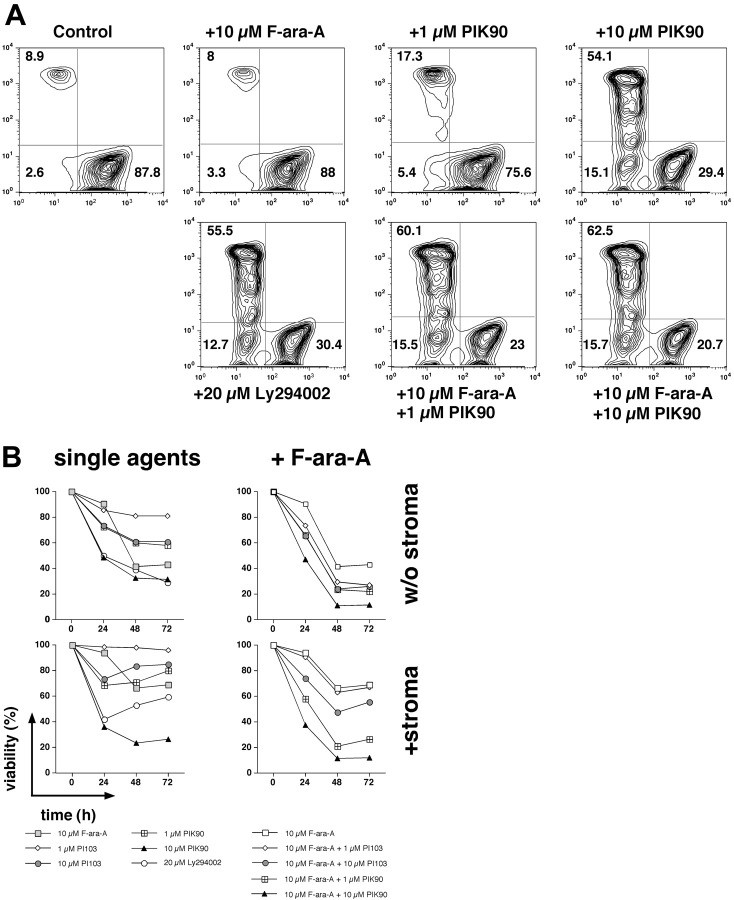
Figure 7.
PI3K inhibitors sensitize CLL cells to fludarabine. CLL B cells were cultured with or without marrow stromal cells and treated with PI3K inhibitors alone (single agents) or a combination of PI3K inhibitors and fludarabine (+F-ara-A). (A) Contour plots of viable (bottom right quadrant), apoptotic (bottom left quadrant), and dead (top left quadrant) CLL cells from a representative patient, cultured on marrow stromal cells, and treated with the reagents indicated above or below each of the plots. Next to each of these cell populations, the relative proportion of viable, apoptotic, and dead cells is displayed. Pretreatment of CLL cells with the PI3-K inhibitors Ly 294002, PI-103, and PIK-90 enhanced the cytotoxicity of fludarabine and also partially reversed the protective effect of stromal cells on fludarabine-induced apoptosis. In this case, F-ara-A treatment alone did not affect CLL viability, and treatment with PIK-90 at 1 μM reduced the viability to 75.6% from 87.8% in the controls. However, the combination of both drugs decreased the viability to 23%, suggesting synergism at this concentration. (B) This graph summarizes the effects of PI3K inhibitors alone and in combination with F-ara-A in cultures with or without marrow stromal cells in 10 different CLL cell samples and at 3 different time points. The viability of CLL cells was assessed at the time points indicated on the horizontal axis by staining with DiOC6 and PI. Each data point in panel B represents the mean (± SEM; n = 10) relative viability of treated CLL B cells compared with the untreated controls (100%).
Western blot analysis of Akt, pAkt, and β-actin
A total of 3 × 107 serum-starved CLL B cells were pretreated with the PI3K inhibitors for 2 hours and then stimulated with 200 ng/mL CXCL12 for 1 minute. After that, cells were lysed in ice-cold lysis buffer (Upstate Biotechnology) containing COMPLETE protease inhibitor cocktail tablets and PhosSTOP tablets (both Roche Diagnostics), 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, and the lysates were kept on ice for 30 minutes after a centrifugation at 14 000g for 30 minutes. The protein concentration of the supernatant was determined using Protein Assay solution (Bio-Rad, Hercules, CA) according to the manufacturer's protocol. A total of 30 μg total protein of each sample was denatured by boiling for 5 minutes in Laemmli sample buffer, separated on a 4% to 15% Tris-HCL gel (Bio-Rad), and finally transferred to nitrocellulose membranes (Millipore, Billerica, MA). The membranes were blocked with 5% nonfat dry milk in TBST (10 mM Tris-HCL, 150 mM NaCl, 0.5% Tween) and incubated with the indicated primary antibodies overnight at 4°C. After incubation with the horseradish peroxidase–conjugated secondary antibody, membranes were treated with a chemiluminescence reagent, enhanced chemiluminescence (GE Healthcare), and subsequently exposed to a radiographic film (Eastman Kodak, Rochester, NY).
Reverse-phase protein arrays
Reverse-phase protein arrays (RPPAs) were performed as described.46 In brief, CLL B cells were pretreated with PI3K inhibitors and then stimulated with CXCL12 for 5 and for 30 minutes. The cells then were lysed in ice-cold lysis buffer as described for Western blot analysis. Cell lysates were normalized to a concentration of 4 μg/μL using bicinchonic acid and boiled with 1% sodium dodecyl sulfate, and the supernatants were manually diluted in 5 2-fold serial dilutions with lysis buffer. An Aushon Biosystems arrayer (Aushon Biosystems, Billerica, MA) created 6336 spot arrays on nitrocellulose-coated FAST slides (Whatman Schleicher and Schuell, Dassel, Germany) using the serial dilutions. Each slide was probed with a validated primary antibody, and the signal was amplified using a Dako North America–catalyzed system (Dako North America, Carpinteria, CA). A secondary antibody (anti–mouse or anti–rabbit) was used as an amplification starting point. The slides were scanned, analyzed, and quantified using Microvigene software (VigeneTech, Carlisle, MA) and the Supercurve software package, as described previously. To generate heat maps, Treeview (University of Glasgow) and X-cluster software were used.
Data analyses, statistics
Unless otherwise stated, results are shown as mean plus or minus SEM of at least 3 experiments each. For statistical comparison between groups, the Student paired t test or Bonferroni t test was used. Analyses were performed using Prism 5 for Mac (GraphPad Software, San Diego, CA) and Primer of Biostatistics software (McGraw Hill, Columbus, OH). Data collected via flow cytometry were analyzed using FlowJo (TreeStar, Ashland, OR). To determine whether the combination of F-ara-A and PIK90 or the combination of F-ara-A and PI103 had an additive or more than additive cytotoxic effect in CLL suspension cultures and/or MSC cocultures, we performed Wilcoxon rank sum tests. Briefly, we computed the differences in mean relative viabilities between the combination treatment and the 2 individual treatments (eg, difference = viability for F-ara-A in combination with PIK90 at 1 μM − [viability for F-ara-A alone + viability for PIK90 at 1 μM]/2). The hypothesis was that, if there were an additive effect, the expected difference would be 0. If there were synergism or antagonism, the difference would be significantly different from 0. There was synergism if the difference were significantly smaller than 0.
Results
CXCL12-induced CLL B-cell chemotaxis, actin polymerization, and pseudoemperipolesis require PI3K activation
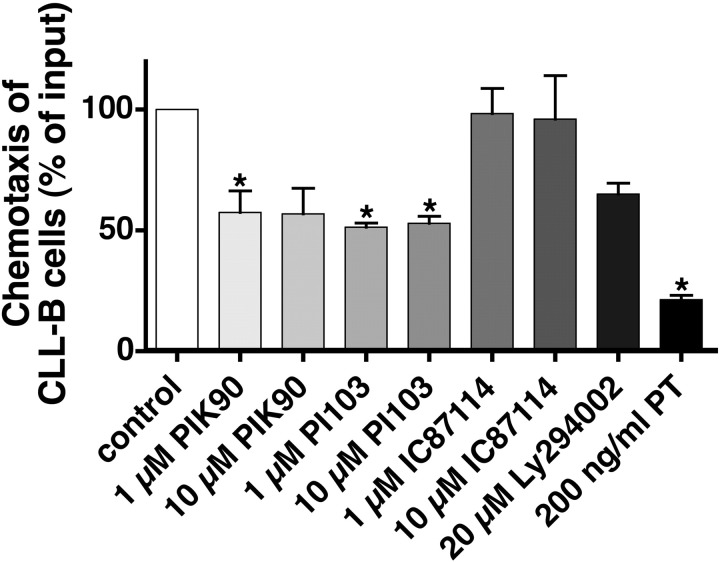
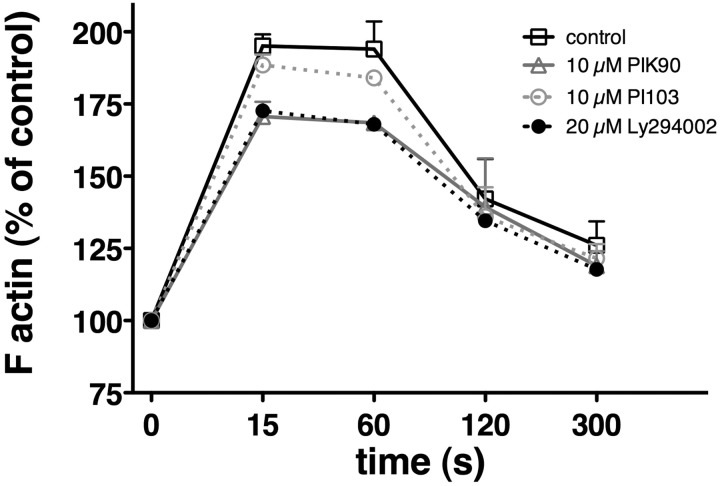
To determine the effects of new, isoform-selective PI3K inhibitors on CXCR4 responses in CLL cells, we examined the nonspecific PI3K inhibitor Ly294002 and the isoform-selective PI3K inhibitors PIK-90, PI-103, IC87114, and TGX115 in chemotaxis, pseudoemperipolesis, and actin polymerization assays, as these processes are well known to be regulated by activation of CXCR4 by its ligand CXCL12 in CLL B cells.15 As shown in Figure 1, the rather multitargeted p110α/p110γ inhibitors PI-103 and PIK-9043 inhibited chemotaxis to levels that were 57.8% plus or minus 8.9% (PIK-90) or 51.4% plus or minus 1.7% (PI-103) of controls at 1 μM (mean ± SEM; n = 5) and 56.8% plus or minus 10.6% (PIK-90) or 53% plus or minus 2.9% (PI-103) of controls at 10 μM (mean ± SEM; n = 5, P < .05). IC87114, which is highly selective for p110δ, and TGX115, also highly selective for p110β/p110δ, did not affect chemotaxis. Ly294002 displayed similar activity as the p110α inhibitors, whereas pertussis toxin, which interferes with the Gi component of adenylate cyclase, almost completely abrogated CLL cell chemotaxis. Consistent with the chemotaxis data, we found that PIK-90 and PI-103 inhibited pseudoemperipolesis to levels that were 74.2% plus or minus 12.9% (PIK-90) or 69.2% plus or minus 19.4% (PI-103) of controls (mean ± SEM; n = 4) at 1 μM and 57.9% plus or minus 13.7% (PIK-90) or 62.9% plus or minus 17% (PI-103) of controls at 10 μM (mean ± SEM; n = 4). These inhibitions were significant with P less than .05, except for the 1-μM concentrations. The phase-contrast photomicrographs displayed in Figure 2 illustrate the effect of pretreatment of CLL cells with 10 μM PIK-90, demonstrating a marked reduction of CLL cell migration into the stromal cell layer compared with untreated controls (Figure 2A vs Figure 2B). Cell migration in response to chemokines, such as CXCL12, requires actin polymerization, and we therefore analyzed the effects of PI3K inhibitors on this cellular response. As shown in Figure 3, CXCL12-induced actin polymerization was decreased to levels that peaked at 170.7% plus or minus 5.1% at 15 seconds with 10 μM PIK-90, compared with 188.5% plus or minus 3.6% (t = 15 seconds) for 10 μM PI-103 and 195.1% plus or minus 4.1% (t = 15 seconds) for the untreated controls (mean ± SEM; n = 4 for each condition). This was a significant inhibition for PIK-90 (P = .01), but not for PI-103 (P = .27).
Figure 1.
CLL B-cell chemotaxis toward CXCL12 is PIK-90 and PI-103 sensitive. CLL cells were preincubated for 120 minutes with the reagents that are displayed on the horizontal axis before being assayed for chemotaxis toward 200 ng/mL CXCL12. Each bar represents the mean (± SEM) relative chemotaxis of B CLL cells from 5 different patients compared with untreated controls (100%). *Both concentrations of PIK-90 and PI-103 as well as pertussis toxin, used as a positive control, significantly inhibited chemotaxis to CXCL12.
Figure 2.
Reduction of spontaneous migration of CLL cells beneath marrow stromal cells (pseudoemperipolesis) by PIK-90 and PI-103. CLL B cells were preincubated with PIK-90 or PI-103 and seeded onto confluent marrow stromal cell layers. After overnight incubation, the CLL cells that had not migrated into the stromal cell layer were vigorously washed off. Then, CLL B cells that migrated beneath the stromal cells were microphotographed (A) and quantified by flow cytometry (B). In phase-contrast, pseudoemperipolesis is characterized by the dark appearance of CLL cells that have migrated into the same focal plane as the stromal cells. Cells were imaged in medium using a phase-contrast microscope (Model ELWD 0.3; Nikon, Garden City, NY) with a 10×/0.25 NA objective lens. Images were captured with a Nikon D40 digital camera (Nikon, Tokyo, Japan) using Camera Control Pro software (Nikon Japan); when necessary, Adobe Photoshop 9.0 (Adobe Systems, San Jose, CA) was used for image processing. (A) Representative phase-contrast photomicrographs of pseudoemperipolesis of untreated CLL cells, labeled “control,” and, in comparison, reduced pseudoemperipolesis of the same CLL sample pretreated with 10 μM PIK-90 (bottom row). White bars in the left panels represent 100 μM (100× magnification); bars in the right panels, 50 μM (400× magnification); white filled arrow, a nonmigrated CLL cell; gray filled arrow, a migrated CLL cell; black filled arrow, a marrow stromal cell (framed). In panel B, each bar represents the mean (± SEM) relative number of migrated CLL cells from 4 different patients after pretreatment with the agents displayed on the horizontal axis, compared with the untreated controls. *Significant inhibition with P values less than .05.
Figure 3.
PI3K inhibitors PIK-90 and PI-103 reduce actin polymerization in response to CXCL12 stimulation. Intracellular F-actin was measured in CLL cells using fluorescein isothiocyanate–labeled phalloidin after adding 200 ng/mL CXCL12 at time 0. Results at the time points indicated on the horizontal axis are shown as percentage of intracellular F-actin relative to the value of F-actin before addition of CXCL12. Each data point represents the mean (± SEM) of 4 different patients. Pretreatment of CLL cells with Ly294002 and PIK-90 reduces intracellular F-actin to approximately 70% of controls, whereas PI-103 had only a minor effect with an approximate 10% reduction.
PIK-90 and PI-103 induce apoptosis in CLL B cells
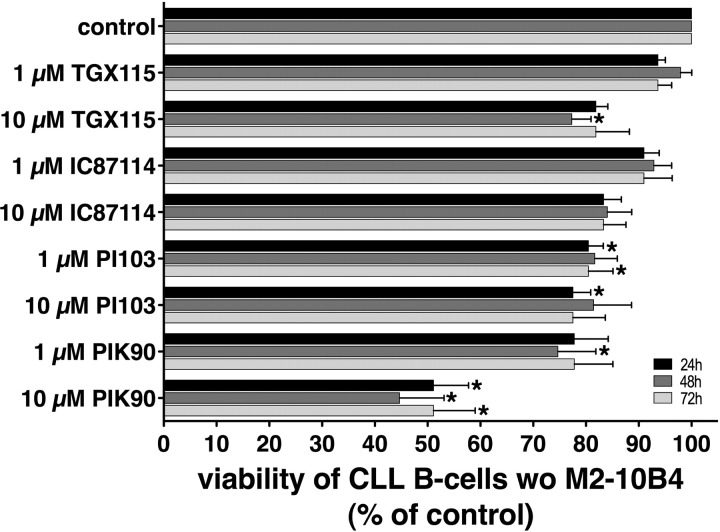
To determine the effects of the isoform-selective PI3K inhibitors on CLL cell viability, we incubated CLL cells from different patients with various concentrations of the inhibitors for 24, 48, and 72 hours. As shown in Figure 4, we found that the PI3K inhibitors PI-103 and PIK-90 revealed the strongest apoptosis-inducing effects at both concentrations and at all different time points. Using a concentration of 10 μM, PIK-90 reduced the viability of CLL cells to 51.1% plus or minus 6.6% at 24 hours, whereas 1 μM PIK-90 reduced the viability to 77.8% plus or minus 6.4%; 10 μM PI-103 reduced the viability of CLL cells to 77.5% plus or minus 3.4% and to 80.5% plus or minus 2.8% at 24 hours with 1 μM PI-103 (mean ± SEM; n = 7 for each condition). The asterisks in Figure 4 indicate which inhibitor and at what concentrations induced significant reduction in CLL viabilities with P less than .05. Using normal PBMCs from healthy donors, we found that the cytotoxicity of PI3K inhibitors was not restricted to CLL cells, but also evident in normal PBMCs. Generally, this cyctotoxic effect was less pronounced compared with CLL cells (Table S3).
Figure 4.
PI3K inhibitors induce apoptosis in CLL B cells. CLL cell viability was determined 24, 48, and 72 hours after addition of the PI3K inhibitors that are displayed on the vertical axis by staining with DiOC6 and PI. The results represent the mean (± SEM; n = 7) relative viability of PI3K inhibitor treated CLL B cells compared with untreated controls (100%). The strongest cytotoxic effects at 1 μM and 10 μM were obtained with PIK-90 and PI-103, whereas TGX115 and IC87114 were found to be less potent. *Significant decrease in viability (P < .05).
PIK-90 and PI-103 inhibit AktSer473 phosphorylation
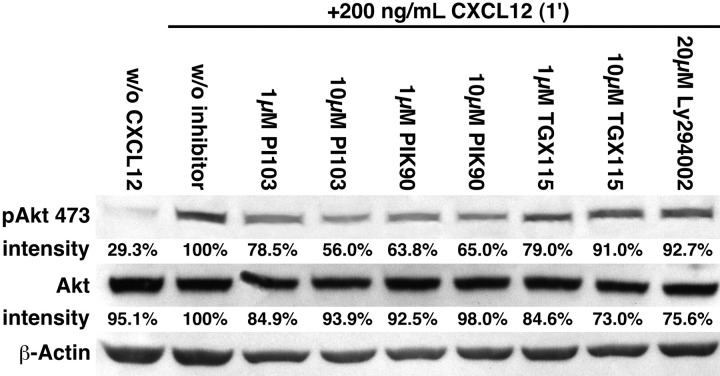
CXCL-12 induced activation of the PI3-K effector Akt, and the effects of the isoform-specific PI3K inhibitors were assayed by Western blotting. CXCL12 stimulation resulted in the phosphorylation of Akt, especially at Ser473, whereas Thr308 revealed a significantly weaker phosphorylation (data not shown). A total of 1 and 10 μM PIK-90 reduced the phospho-AktSer473 to 63.8% and 65%, and PI-103 reduced the phospho-AktSer473 to 56% or 78.5% of controls, as shown in Figure 5.
Figure 5.
Activation of Akt after stimulation with CXCL12 is inhibited by PIK-90 and PI-103. After preincubation with the PI3K inhibitors that are indicated above the bands, CLL B cells were stimulated with CXCL12 for 1 minute and then lysed. The phosphorylated forms of Akt (p473; p308 not shown) were visualized by Western blot, and the band intensities were analyzed using the Software LabImage 1D. The relative band intensities compared with the band intensity of the control sample (+CXCL12, no PI3K inhibitor, 100%) are indicated below each band. PIK-90 and PI-103 reduced the p473 phosphorylation of Akt by approximately 50% compared with the control. In contrast, the inhibitory effects of TGX115 and Ly294002 on CXCL12-induced Akt phosphorylation were minor. The figure shows data from 1 representative patient sample of 3 patient samples with similar results.
PIK-90 and PI-103 inhibit signaling through AKT and S6 ribosomal proteins and up-regulate apoptosis-related proteins
We next used a sensitive high-throughput RPPA approach to assay PI3K inhibitor-induced changes in the phosphorylation status of proteins that are involved in PI3K-AKT signaling and to analyze the expression of apoptosis-related proteins. The advantage of RPPA is that it allows a more comprehensive interrogation of signaling events than does Western blotting. As shown in Figure 6A, pretreatment of CLL cells with PIK-90 and PI-103 resulted in decreased levels of phosphorylated Akt (especially at Ser473), of phosphorylated serine/threonine protein kinase glycogen synthase kinase 3 (at Ser21/9- PIK90 only), and of phosphorylated S6 ribosomal protein (at Ser235/236 and Ser240/244). A total of 1 μM PIK-90 significantly reduced phosphorylation of Akt at Ser473 to 80% plus or minus 2% and phosphorylation of S6 at Ser235/236 to 77% plus or minus 8% of the levels in untreated controls after stimulation with CXCL12. PIK-90 at 1 μM also produced a trend to decreased phosphorylation of glycogen synthase kinase 3 at Ser21/9 (to 85.2% ± 10% of the level in untreated controls) and to increased cleavage of caspase 7 (to 114% ± 12% of the level in untreated controls). A total of 1 μM PI-103 reduced phosphorylation of Akt at Ser473 to 72% plus or minus 3% and of S6 ribosomal protein at Ser236/236 to 83% plus or minus 8% along with a trend to increased cleavage of PARP to 114% plus or minus 16% of the level in untreated controls (mean ± SEM; n = 5 for all data). The heat map in Figure 6B shows the individual results from 5 different patients in terms of phosphorylation of the aforementioned PI3K-AKT signaling proteins and of cleavage of the apoptosis-related proteins caspase 7 and PARP after treatment with 2 different concentrations of the PI3K inhibitors PIK-90 and PI-103. The heat map demonstrates a general increase in cleavage of caspase 7 and of PARP after treatment with the PI3K inhibitors shown along with a general decrease in phosphorylation of Akt (at Thr308 and Ser473) and of S6 (at Ser235/236 and Ser240/244) in comparison with untreated controls. S6 phosphorylation was most consistently down-regulated in the 5 patients after treatment with PIK90.
Figure 6.

Inhibition of PI3K-AKT signaling and up-regulation of apoptosis-related proteins by PIK-90 and PI-103. (A) PIK-90 and PI-103 reduce CXCL12-induced phosphorylation of Akt (especially at Ser473) and of its downstream effector S6 ribosomal protein (at Ser235/236 and Ser240/244) and produce a trend to increased cleavage of the apoptosis-related proteins caspase 7 and its target poly-(ADP-ribose) polymerase (PARP). To generate the bar graphs, (phospho)protein expression of B CLL cells from 5 different patients was quantified with RPPA, and the quantification data were then corrected for loading and normalized to controls that were stimulated with CXCL12 but not preincubated with PI3K inhibitors. Each bar represents the mean (± SEM; n = 5) relative quantity of the (phospho)proteins that are displayed on the right vertical axis after pretreatment with the PI3K inhibitors that are indicated on the left vertical axis and stimulation of all samples with CXCL12 for 5 minutes. *Significant differences from the control samples. (B) Heat map showing decreases in phosphorylation of PI3K-AKT signaling proteins and increases of the apoptosis-related proteins cleaved caspase 7 and cleaved PARP in 5 different CLL patients after treatment with PI3K inhibitors. To generate this heat map, protein expression was quantified with RPPA, and the quantification data were corrected for loading and normalized to respective control samples from each patient who were stimulated with CXCL12 but not pretreated with PI3K inhibitors. Each square represents expression levels of the respective proteins indicated on the vertical axis for one individual CLL sample. The PI3K inhibitors and their respective concentrations are displayed on the top horizontal axis, whereas the bottom horizontal axis indicates that all samples were stimulated for 5 minutes with CXCL12. Shades of red and green indicate up- or down-regulation of a given (phospho)protein, respectively, in comparison with the levels in untreated control cells from each patient (the latter were normalized to a black color in each case).
PIK-90 and PI-103 display a more than additive cytotoxic effect in combination with fludarabine and reverse the protective effect of stromal cells
Inhibitors of the PI3K-AKT pathway combined with conventional cytotoxic drugs may provide a more effective strategy to enhance treatment responses compared with either agent by itself.47 Because PIK-90 and PI-103 were the most potent PI3K inhibitors in our migration and stromal cell–adhesion assays and induced significant apoptosis as single agents in suspension cultures (Figure 4), we subsequently tested these PI3K inhibitors alone and in combination with fludarabine in CLL-MSC cocultures. Figure 7A shows a representative analysis of CLL cells cultured with M2-10B4 cells and treated with 10 μM F-ara-A and different concentrations of PIK-90 for 24 hours. Although in this case the addition of 10 μM F-ara-A alone did not lead to a significant decrease in viability (87.8% for controls, and 88% after treatment with 10 μM F-ara-A), 1 μM PIK-90 caused a decreased in viability to approximately 75.6%, and the addition of both compounds together decreased the viability of the B CLL cells to only 23%. A total of 10 μM PIK-90 alone also reduced the viability to 29.4%, whereas the combination of both compounds resulted in an additional decrease in viability to 20.7%. Figure 7B compares the viability of CLL B cells from 10 different patients cultured with or without M2-10B4 cells and treated with 10 μM F-ara-A or PI3K inhibitors alone, or a combination of both agents at different time points (24, 48, 72 hours). Pretreatment of CLL cells with the PI3-K inhibitors Ly 294002, PI-103, and PIK-90 enhanced the cytotoxicity of fludarabine (compare right column to left column) and also partially reversed the protective effect of stromal cells on fludarabine-induced apoptosis (compare top row with bottom row). For example, the relative viability of CLL cells cultured on MSC treated with 10 μM F-ara-A was 93.8% plus or minus 2.4% at 24 hours, 66.3% plus or minus 11% at 48 hours, and 68.9% plus or minus 7.6% at 72 compared with untreated controls. For comparison, treatment with 1 μM PIK-90 decreased the viability to 68.3% plus or minus 3.7% at 24 hours, 70.6% plus or minus 5.9% at 48 hours, and 79.8% plus or minus 6% at 72 hours. However, the combination of both 10 μM F-ara-A and 1 μM PIK-90 decreased the viability to 57.7% plus or minus 5.4% at 24 hours, 21% plus or minus 6.3% at 48 hours, and 26.5% plus or minus 5.3% at 72 hours (mean ± SEM; n = 10 for all data points; Figure 7B bottom right diagram). We used the Wilcoxon rank sum test to determine that there was an additive or more than additive effect for the combination treatment of PI3K inhibitors and F-ara-A. We found that both PIK90 and PI103 displayed a more than additive cytotoxic effect when combined with F-ara-A, both in suspension and CLL cell cocultures with MSCs. Moreover, this synergistic effect was noticed for both concentrations of the PI3K inhibitors (10 and 1 μM). The data analysis for this experiment is shown in Tables S2A and S2B, which list the difference in viability for each of the combination treatments at 1 μM and at 10 μM for the PI3K inhibitors, as well as the result of Wilcoxon rank sum test, for the groups with and without M2, respectively.
Discussion
Interactions between CLL cells and the microenvironment are critical for maintenance and expansion of the neoplastic B-cell clone.6,19,48 These interactions occur in tissue compartments, such as the marrow and secondary lymphatic tissues, where CLL cells interact with a variety of nonmalignant accessory cells collectively referred to as stromal cells. These interactions provide growth and survival signals and also confer drug resistance and therefore may account for residual disease and relapses after conventional therapies.19,21 Therefore, increasing emphasis is being placed on therapeutically targeting the leukemia microenvironment. Targeting stroma-derived signaling pathways (such as the PI3K pathways) that control CLL cell survival and drug resistance is an attractive, new approach. Our data show that PI3K inhibitors can effectively antagonize migratory, survival, and drug-resistance signals derived from CXCL12 and CLL-MSC interactions. More specifically, we found that the class I PI3K inhibitors PIK-90 and PI-103 effectively inhibited CLL cell migration and signaling responses to CXCL12. Furthermore, PIK-90 and PI-103 inhibited CLL cell migration beneath stromal cells (Figure 2), S6, and Akt phosphorylation on Ser473 (Figures 5, 6), and induced CLL cell apoptosis in suspension (Figure 4) and MSC cocultures (Figure 7B). In addition, PIK-90 and PI-103 displayed a more than additive effect when used in combination with F-ara-A for induction of CLL cell apoptosis, and reversed stroma-mediated protection of CLL cells from fludarabine-induced apoptosis (Figure 7).
Wortmannin and Ly294002 previously have been reported to induce apoptosis in CLL cells28,49 and to sensitize CLL cells to cytotoxic drugs,50,51 but toxicities and poor pharmacokinetic properties have precluded their clinical development. Recently, novel PI3K inhibitors have been developed and their isoform specificities were profiled.33,35 This led to the demonstration that p110α plays a key role in insulin signaling using PIK-9043 and that combined inhibition of p110α and mTOR using PI-103 inhibits tumor cell proliferation in vitro and in vivo.44,52 Although PIK-90 and PI-103 generally are considered to be class I p110α inhibitors, PIK-90 also blocks p110γ and to a lesser extent p110δ, p110β, and mTOR (Table 1).35,53 PI-103 also has a broader activity among the p110 isoforms (Table 1). Compared with PIK-90 and PI-103, the highly selective p110δ inhibitor IC87114 and the highly selective p110β/p110δ inhibitor TGX115 had lesser, but reproducible, effects on CLL cell viability and Akt phosphorylation. This suggests that the prosurvival effect of PI3K activation is mediated through a combination of p110α and p110δ activation. Because PIK-90 and PI-103 can target both p110α and p110δ, these inhibitors display more activity than the inhibitors that target p110δ alone or p110β and p110δ. Previous studies have addressed the question of whether RNA interference and pharmacologic PI3K inhibition leads to an identical phenotype. Based on these studies, it is increasingly clear that siRNA and small-molecule approaches need not be aligned and that siRNA and small-molecule approaches to target PI3K isoforms display different phenotypes.54 More specifically, targeting the PI3K isoform p110β with a small molecule inhibitor blocked the activity of p110β, without affecting the content and activity of its regulatory partner, p85. In contrast, siRNA against p110β led to knockdown of this kinase, which resulted in a considerable alteration in detectable levels of p85. This was leading to tumor growth suppression with siRNA, but not with the small molecule inhibitor.44 These authors conclude that different forms of PI3K isoform inhibition (kinase knockdown vs kinase inhibition) can cause different “on target” activity, leading to different outcomes.
Table 1.
Specificity profiles of the PI3K inhibitors used in this study, based on IC50 values (μM)
| Name | p110α | p110β | p110γ | p110δ | Comment |
|---|---|---|---|---|---|
| Ly294002 | 9.3 | 2.9 | 38 | 6.0 | Pan-PI3K inhibitor |
| PIK-90 | 0.011 | 0.35 | 0.018 | 0.058 | Low mTOR activity, cell permeable |
| PI-103 | 0.008 | 0.088 | 0.15 | 0.048 | Targets PI3KC2β, mTORC2, DNA-PK, p235 |
| TGX115 | 61 | 0.13 | 100 | 0.63 | p110β- and δ-selective |
| IC87114 | 200 | 16 | 61 | 0.13 | Selective p110δ inhibitor, cell permeable |
Inhibition of CLL cell chemotaxis and other migration-related responses by PIK-90 and PI-103 reflects the importance of PI3K and its counterpart, the phosphatase and tensin homolog (PTEN) phosphatase, for regulation of G protein–coupled chemokine receptor signaling.55 When cells are placed in a gradient of chemoattractant, the balance between activation of PI3K and PTEN shifts toward an enrichment of PIP3 at the leading edge of the cell, resulting in the recruitment of adapter proteins for various downstream responses. As such, PI3K activation is considered a primary event in the signal transduction pathway that mediates directional sensing and cell polarization. However, chemical disruption of PI3K signaling by p110α inhibitors did not completely abolish chemotaxis (Figure 1), suggesting that PIP3-independent pathways are also involved in the regulation of chemotaxis, as suggested earlier.55,56
In cancer cells, constitutive PI3K activation is a common feature that can be caused through different mechanism. In several solid tumors, such as breast, colon, or endometrial cancers, PIK3CA, the gene encoding the PI3K catalytic subunit (p110α), is mutated and encodes mutant p110α proteins that constitutively activate its kinase activity and induce malignant growth in normal cells.57 Other mechanisms of PI3K activation in malignant cells are related to PTEN inactivation58 or to mutations in the p85 regulatory subunit.59 In contrast to solid tumors, where activating PI3K mutations are found in approximately 30% of the cases,58 PI3K mutations appear to be absent or extremely rare in cancers of the hematopoietic system, such as leukemias.57 No activating mutation in the PI3KCA or PI3KCD genes has been identified in acute myeloid leukemia and lymphoma patients.60–62
As such, the mechanisms leading to constitutive PI3K activation in patients with acute myeloid leukemia63 or CLL28,29,64 remain unclear. Recent studies and the findings presented in this paper suggest that constitutive PI3K activation in CLL cells originates from activating signals from the microenvironment, in particular contact-dependent signals from MSCs.9,65 On the molecular level, stromal cells in the microenvironment can activate PI3Ks in CLL cells via the CXCL12-CXCR4 axis, as demonstrated in this study, or by activating BCR signaling.30,66 Collectively, these data suggest that constitutive PI3K activation in CLL and other leukemias is induced by external signals from the microenvironment, rather than being intrinsic to the leukemia cells, and stromal cell–derived signals, such as activation of the CXCL12-CXCR4 axis, play a pivotal role in the stroma-CLL cross talk leading to PI3K activation.
One may argue that signaling pathways activated by stromal cells could be redundant; therefore, blocking a single pathway, such as the PI3Ks, could be bypassed by alternative signaling routes. However, PI3Ks are involved in CXCR4-, BCR-, and other stromal cell–related signaling pathways, and are found to be overexpressed in the unmutated subgroup of CLL patients. These findings suggest that PI3Ks integrate multiple relevant signaling pathways in CLL cells. Moreover, our finding that the PIK inhibitors PIK-90 and PI-103 induce CLL cell apoptosis at low micromolar concentrations, even in the presence of MSCs, suggests that these drugs can turn off a critical survival pathway for CLL cells in stromal cell contact cultures. This is again highly relevant in the clinical context, where new drugs for CLL patients are needed to overcome stromal cell-mediated drug resistance in the marrow microenvironment to eradicate minimal residual disease.21 Another important concern regarding the therapeutic implications of our findings is the question of therapeutic specificity and safety of PIK inhibitors. Clearly, given the importance of PI3Ks in various tissues and biologic functions, PI3K inhibitors and particularly their long-term application could cause unwanted toxicities. On the other hand, pan-PI3K inhibitors are currently developed for cancer therapy, such as PI-103, and show encouraging preclinical toxicity data.35 Our finding that the PI3K inhibitors PIK-90 and PI-103 also induce cytotoxicity in normal blood mononuclear cells (Table S3) indicates that hematotoxicity and/or lymphocyte toxicity needs to be characterized and closely monitored in these initial clinical trials.
Collectively, our data demonstrate that PI3Ks play an important role in regulating interactions between CLL cells and MSCs via the CXCR4 receptor. The isoform-selective PI3K inhibitors PIK-90 and PI-103 induced CLL cell apoptosis in MSC cocultures, synergized with fludarabine in induction of CLL cell apoptosis, and reversed stromal cell–mediated resistance to fludarabine, providing a rationale to evaluate this class of PI3K inhibitors in CLL patients.
Acknowledgments
The authors thank Lianchun Xiao, Department of Biostatistics, University of Texas M. D. Anderson Cancer Center, for assistance with the data analysis.
This work was supported by a Kimmel Scholar Award by the Sidney Kimmel Foundation for Cancer Research (J.A.B.), an ASCO Career Development Award (J.A.B.), a Lauri Strauss Leukemia Foundation grant, and a CLL Global Research Foundation grant (J.A.B.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.N. performed experiments and data analysis and wrote the paper; M.H. and A.V.K. performed experiments and data analysis; B.T.H. performed the RPP arrays and RPPA analysis and reviewed the manuscript; Z.A.K. and K.M.S. helped with the experimental design and data analysis related to PI3K inhibitors and reviewed the manuscript; J.H. analyzed data regarding synergism between PI3K inhibitors and F-ara-A; W.G.W. and M.J.K. provided patient samples, analyzed data, and reviewed the manuscript; and J.A.B. designed the research, supervised the study, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan A. Burger, Department of Leukemia, Unit 428, University of Texas M. D. Anderson Cancer Center, PO Box 301402, Houston, TX 77230-1402; e-mail: jaburger@mdanderson.org.
References
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Reed JC. Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood. 2008;111:3322–3330. doi: 10.1182/blood-2007-09-078162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins RJ, Verschuer LA, Harmon BV, Prentice RL, Pope JH, Kerr JF. Spontaneous programmed death (apoptosis) of B-chronic lymphocytic leukaemia cells following their culture in vitro. Br J Haematol. 1989;71:343–350. doi: 10.1111/j.1365-2141.1989.tb04290.x. [DOI] [PubMed] [Google Scholar]
- 4.Panayiotidis P, Jones D, Ganeshaguru K, Foroni L, Hoffbrand AV. Human bone marrow stromal cells prevent apoptosis and support the survival of chronic lymphocytic leukaemia cells in vitro. Br J Haematol. 1996;92:97–103. doi: 10.1046/j.1365-2141.1996.00305.x. [DOI] [PubMed] [Google Scholar]
- 5.Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood. 1998;91:2387–2396. [PubMed] [Google Scholar]
- 6.Caligaris-Cappio F. Role of the microenvironment in chronic lymphocytic leukaemia. Br J Haematol. 2003;123:380–388. doi: 10.1046/j.1365-2141.2003.04679.x. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen IM, Kitada S, Leoni LM, et al. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood. 2002;100:1795–1801. [PubMed] [Google Scholar]
- 8.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell'Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–2663. [PubMed] [Google Scholar]
- 9.Edelmann J, Klein-Hitpass L, Carpinteiro A, et al. Bone marrow fibroblasts induce expression of PI3K/NF-kappaB pathway genes and a pro-angiogenic phenotype in CLL cells. Leuk Res. 2008;32:1565–1572. doi: 10.1016/j.leukres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94:3658–3667. [PubMed] [Google Scholar]
- 11.Sipkins DA, Wei X, Wu JW, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohle R, Failenschmid C, Bautz F, Kanz L. Overexpression of the chemokine receptor CXCR4 in B cell chronic lymphocytic leukemia is associated with increased functional response to stromal cell-derived factor-1 (SDF-1). Leukemia. 1999;13:1954–1959. doi: 10.1038/sj.leu.2401602. [DOI] [PubMed] [Google Scholar]
- 13.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 15.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94:3658–3667. [PubMed] [Google Scholar]
- 16.Nishio M, Endo T, Tsukada N, et al. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1alpha. Blood. 2005;106:1012–1020. doi: 10.1182/blood-2004-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burger M, Hartmann T, Krome M, et al. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106:1824–1830. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 18.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 19.Burger JA, Kipps TJ. Chemokine receptors and stromal cells in the homing and homeostasis of chronic lymphocytic leukemia B cells. Leuk Lymphoma. 2002;43:461–466. doi: 10.1080/10428190290011921. [DOI] [PubMed] [Google Scholar]
- 20.Burger JA, Burkle A. The CXCR4 chemokine receptor in acute and chronic leukaemia: a marrow homing receptor and potential therapeutic target. Br J Haematol. 2007;137:288–296. doi: 10.1111/j.1365-2141.2007.06590.x. [DOI] [PubMed] [Google Scholar]
- 21.Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14:2519–2526. doi: 10.1158/1078-0432.CCR-07-2223. [DOI] [PubMed] [Google Scholar]
- 22.Uy GL, Rettig MP, Ramirez P, Nervi B, Abboud CN, DiPersio JF. Kinetics of human and murine mobilization of acute myelogenous leukemia in response to AMD3100. Blood. 2007;110:265a. [Google Scholar]
- 23.Liesveld JL, Bechelli J, Rosell K, et al. Effects of AMD3100 on transmigration and survival of acute myelogenous leukemia cells. Leuk Res. 2007;31:1553–1563. doi: 10.1016/j.leukres.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng Z, Samudio IJ, Munsell M, et al. Inhibition of CXCR4 with the novel RCP168 peptide overcomes stroma-mediated chemoresistance in chronic and acute leukemias. Mol Cancer Ther. 2006;5:3113–3121. doi: 10.1158/1535-7163.MCT-06-0228. [DOI] [PubMed] [Google Scholar]
- 25.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 26.Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–929. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 27.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 28.Ringshausen I, Schneller F, Bogner C, et al. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cdelta. Blood. 2002;100:3741–3748. doi: 10.1182/blood-2002-02-0539. [DOI] [PubMed] [Google Scholar]
- 29.Kienle D, Benner A, Krober A, et al. Distinct gene expression patterns in chronic lymphocytic leukemia defined by usage of specific VH genes. Blood. 2006;107:2090–2093. doi: 10.1182/blood-2005-04-1483. [DOI] [PubMed] [Google Scholar]
- 30.Longo PG, Laurenti L, Gobessi S, Sica S, Leone G, Efremov DG. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111:846–855. doi: 10.1182/blood-2007-05-089037. [DOI] [PubMed] [Google Scholar]
- 31.Hu L, Hofmann J, Lu Y, Mills GB, Jaffe RB. Inhibition of phosphatidylinositol 3′-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 2002;62:1087–1092. [PubMed] [Google Scholar]
- 32.Gupta AK, Cerniglia GJ, Mick R, et al. Radiation sensitization of human cancer cells in vivo by inhibiting the activity of PI3K using LY294002. Int J Radiat Oncol Biol Phys. 2003;56:846–853. doi: 10.1016/s0360-3016(03)00214-1. [DOI] [PubMed] [Google Scholar]
- 33.Knight ZA, Shokat KM. Chemically targeting the PI3K family. Biochem Soc Trans. 2007;35:245–249. doi: 10.1042/BST0350245. [DOI] [PubMed] [Google Scholar]
- 34.Ruckle T, Schwarz MK, Rommel C. PI3Kgamma inhibition: towards an “aspirin of the 21st century?”. Nat Rev Drug Discov. 2006;5:903–918. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- 35.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta. 2008;1784:159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 37.Finan PM, Thomas MJ. PI 3-kinase inhibition: a therapeutic target for respiratory disease. Biochem Soc Trans. 2004;32:378–382. doi: 10.1042/bst0320378. [DOI] [PubMed] [Google Scholar]
- 38.Paez J, Sellers WR. PI3K/PTEN/AKT pathway: a critical mediator of oncogenic signaling. Cancer Treat Res. 2003;115:145–167. [PubMed] [Google Scholar]
- 39.Liu L, Puri KD, Penninger JM, Kubes P. Leukocyte PI3Kgamma and PI3Kdelta have temporally distinct roles for leukocyte recruitment in vivo. Blood. 2007;110:1191–1198. doi: 10.1182/blood-2006-11-060103. [DOI] [PubMed] [Google Scholar]
- 40.Vogt PK, Bader AG, Kang S. Phosphoinositide 3-kinase: from viral oncoprotein to drug target. Virology. 2006;344:131–138. doi: 10.1016/j.virol.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 41.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 42.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 43.Knight ZA, Gonzalez B, Feldman ME, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan QW, Knight ZA, Goldenberg DD, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raynaud FI, Eccles S, Clarke PA, et al. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 2007;67:5840–5850. doi: 10.1158/0008-5472.CAN-06-4615. [DOI] [PubMed] [Google Scholar]
- 46.Hennessy BT, Lu Y, Poradosu E, et al. Pharmacodynamic markers of perifosine efficacy. Clin Cancer Res. 2007;13:7421–7431. doi: 10.1158/1078-0432.CCR-07-0760. [DOI] [PubMed] [Google Scholar]
- 47.Lu Y, Wang H, Mills GB. Targeting PI3K-AKT pathway for cancer therapy. Rev Clin Exp Hematol. 2003;7:205–228. [PubMed] [Google Scholar]
- 48.Munk Pedersen I, Reed J. Microenvironmental interactions and survival of CLL B-cells. Leuk Lymphoma. 2004;45:2365–2372. doi: 10.1080/10428190412331272703. [DOI] [PubMed] [Google Scholar]
- 49.Barragan M, Bellosillo B, Campas C, Colomer D, Pons G, Gil J. Involvement of protein kinase C and phosphatidylinositol 3-kinase pathways in the survival of B-cell chronic lymphocytic leukemia cells. Blood. 2002;99:2969–2976. doi: 10.1182/blood.v99.8.2969. [DOI] [PubMed] [Google Scholar]
- 50.Christodoulopoulos G, Muller C, Salles B, Kazmi R, Panasci L. Potentiation of chlorambucil cytotoxicity in B-cell chronic lymphocytic leukemia by inhibition of DNA-dependent protein kinase activity using wortmannin. Cancer Res. 1998;58:1789–1792. [PubMed] [Google Scholar]
- 51.Deriano L, Guipaud O, Merle-Beral H, et al. Human chronic lymphocytic leukemia B cells can escape DNA damage-induced apoptosis through the nonhomologous end-joining DNA repair pathway. Blood. 2005;105:4776–4783. doi: 10.1182/blood-2004-07-2888. [DOI] [PubMed] [Google Scholar]
- 52.Chen JS, Zhou LJ, Entin-Meer M, et al. Characterization of structurally distinct, isoform-selective phosphoinositide 3′-kinase inhibitors in combination with radiation in the treatment of glioblastoma. Mol Cancer Ther. 2008;7:841–850. doi: 10.1158/1535-7163.MCT-07-0393. [DOI] [PubMed] [Google Scholar]
- 53.Knight ZA, Chiang GG, Alaimo PJ, et al. Isoform-specific phosphoinositide 3-kinase inhibitors from an arylmorpholine scaffold. Bioorg Med Chem. 2004;12:4749–4759. doi: 10.1016/j.bmc.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 54.Weiss WA, Taylor SS, Shokat KM. Recognizing and exploiting differences between RNAi and small-molecule inhibitors. Nat Chem Biol. 2007;3:739–744. doi: 10.1038/nchembio1207-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kolsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Comer FI, Parent CA. PI 3-kinases and PTEN: how opposites chemoattract. Cell. 2002;109:541–544. doi: 10.1016/s0092-8674(02)00765-1. [DOI] [PubMed] [Google Scholar]
- 57.Vogt PK, Kang S, Elsliger MA, Gymnopoulos M. Cancer-specific mutations in phosphatidylinositol 3-kinase. Trends Biochem Sci. 2007;32:342–349. doi: 10.1016/j.tibs.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Tamguney T, Stokoe D. New insights into PTEN. J Cell Sci. 2007;120:4071–4079. doi: 10.1242/jcs.015230. [DOI] [PubMed] [Google Scholar]
- 59.Huang CH, Mandelker D, Schmidt-Kittler O, et al. The structure of a human p110alpha/p85alpha complex elucidates the effects of oncogenic PI3Kalpha mutations. Science. 2007;318:1744–1748. doi: 10.1126/science.1150799. [DOI] [PubMed] [Google Scholar]
- 60.Cornillet-Lefebvre P, Cuccuini W, Bardet V, et al. Constitutive phosphoinositide 3-kinase activation in acute myeloid leukemia is not due to p110delta mutations. Leukemia. 2006;20:374–376. doi: 10.1038/sj.leu.2404054. [DOI] [PubMed] [Google Scholar]
- 61.Hummerdal P, Andersson P, Willander K, Linderholm M, Soderkvist P, Jonsson JI. Absence of hot spot mutations of the PIK3CA gene in acute myeloid leukaemia. Eur J Haematol. 2006;77:86–87. doi: 10.1111/j.0902-4441.2006.t01-1-EJH2605.x. [DOI] [PubMed] [Google Scholar]
- 62.Muller CI, Miller CW, Hofmann WK, et al. Rare mutations of the PIK3CA gene in malignancies of the hematopoietic system as well as endometrium, ovary, prostate and osteosarcomas, and discovery of a PIK3CA pseudogene. Leuk Res. 2007;31:27–32. doi: 10.1016/j.leukres.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 63.Tamburini J, Elie C, Bardet V, et al. Constitutive phosphoinositide 3-kinase/Akt activation represents a favorable prognostic factor in de novo acute myelogenous leukemia patients. Blood. 2007;110:1025–1028. doi: 10.1182/blood-2006-12-061283. [DOI] [PubMed] [Google Scholar]
- 64.Plate JM. PI3-kinase regulates survival of chronic lymphocytic leukemia B-cells by preventing caspase 8 activation. Leuk Lymphoma. 2004;45:1519–1529. doi: 10.1080/10428190410001683642. [DOI] [PubMed] [Google Scholar]
- 65.Cuni S, Perez-Aciego P, Perez-Chacon G, et al. A sustained activation of PI3K/NF-kappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia. 2004;18:1391–1400. doi: 10.1038/sj.leu.2403398. [DOI] [PubMed] [Google Scholar]
- 66.Bernal A, Pastore RD, Asgary Z, et al. Survival of leukemic B cells promoted by engagement of the antigen receptor. Blood. 2001;98:3050–3057. doi: 10.1182/blood.v98.10.3050. [DOI] [PubMed] [Google Scholar]