Abstract
BACKGROUND
Elevated urinary albumin-creatinine ratio (UACR) and decreased estimated glomerular filtration rate (eGFR) predict all-cause mortality, but whether these markers of kidney damage and function do so in adults with obstructive lung function (OLF) is unclear. The objective of this study was to examine the associations between UACR and eGFR and all-cause mortality in adults with OLF.
METHODS
Data of 5,711 US adults aged 40 to 79 years, including 1,390 adults with any OLF who participated in the National Health and Nutrition Examination Survey III (1988–1994), were analyzed. Mortality follow-up was conducted through 2006.
RESULTS
During the median follow-up of 13.7 years, 650 adults with OLF died. After maximal adjustment, mean levels of UACR were higher in adults with moderate-severe OLF (7.5 mg/g; 95% CI, 6.7–8.5) than in adults with normal pulmonary function (6.2 mg/g; 95% CI, 5.8–6.6) (P = .003) and mild OLF (6.2 mg/g; 95% CI, 5.5–6.9) (P = .014). Adjusted mean levels of eGFR were lower in adults with moderate-severe OLF (87.6 mL/min/1.73 m2; < 95% CI, 86.0–89.1) than in adults with normal lung function (89.6 mL/min/1.73 m2; < 95% CI, 88.9–90.3) (P = .015). Among adults with OLF, hazard ratios for all-cause mortality increased as levels of UACR, modeled as categorical or continuous variables, increased (maximally adjusted hazard ratio for quintile 5 vs 1: 2.23; 95% CI, 1.56–3.18). eGFR, modeled as a continuous variable but not as quintiles, was significantly associated with mortality.
CONCLUSIONS
UACR and eGFR, in continuous form, were associated with all-cause mortality among US adults with OLF.
Chronic lower respiratory disease of which COPD represents the majority rose to the third leading cause of death in the United States in 2008.1 Compared with adults with normal lung function, those with COPD have an elevated rate of mortality2,3 The reasons for this elevated mortality rate are varied and include complications of the disease itself such as respiratory exacerbations, high levels of comorbidities,4 and a more pronounced cardiovascular risk profile, which increases cardiovascular mortality.5 An improved understanding of the predictors of mortality among adults with COPD could eventually translate into improved risk assessment.
Increased albuminuria and reduced glomerular filtration rate (GFR) have been associated with mortality6–8 The prevalence of stage 1 to 4 chronic kidney disease (CKD) based on measurements of albuminuria and estimated GFR (eGFR) is estimated at 11.5% in the United States.9 The question of whether these kidney measures constitute risk markers or factors remains unsettled, and interventions directed at improving levels of these measures could help to resolve this uncertainty.10 Some data have shown that therapy aimed at the renin-angiotensin system improves urinary albumin excretion and reduces cardiovascular events.11
Limited data suggest that patients with COPD are more likely to manifest albuminuria than those without COPD.12–15 If so, urinary albumin-creatinine ratio (UACR) may help to explain increased mortality in patients with COPD. However, whether UACR and GFR are associated with mortality among people with COPD appears not to have been investigated. Therefore, the primary objective of the present study was to examine the association of levels of UACR and eGFR with all-cause mortality among US adults with obstructive lung function (OLF). A secondary objective was to compare levels of UACR and eGFR between adults with normal and OLF.
Materials and Methods
This investigation was conducted using data from the Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality Study.16 The original NHANES III took place from 1988 through 1994.17 To assemble a sample of participants who were representative of the civilian noninstitutionalized population in the United States, selection was performed by using a complex sampling design (stratified multistage probability design). After offering their informed consent, participants were interviewed in their homes. Those attending an examination in the mobile examination center completed additional questionnaires, underwent a series of examinations, and provided blood and urine specimens. The interview and examination response rates were 86% and 78%, respectively. Because the present analysis used public-use data, the study was exempt from human subjects review.
Mortality follow-up of the original NHANES III attendees was conducted through 2006. Deaths were identified through a probabilistic match of participants’ information with National Death Index death certificate records. Participants for whom no match was made were assumed to be alive at the end of the follow-up period.
A detailed account of the procedures used to conduct spirometry can be found elsewhere.18 No postbronchodilator testing was performed. Equations published by Hankinson and colleagues19 were used to calculate predicted FEV1 and FVC. Categories of OLF included mild OLF (FEV1/FVC < 0.70 and FEV1 ≥ 80%), moderate OLF (FEV1/FVC < 0.70 and FEV1 50 to < 80% predicted), and severe OLF (FEV1/FVC < 0.70 and FEV1 < 50% predicted). Participants with a FEV1/FVC ≥ 0.70 and FVC ≥ 80% predicted were considered as having normal lung function. Among participants who did not have OLF, those with restrictive lung function as FEV1 /FVC ≥ 0.70 and FVC < 80% predicted were excluded from analyses. Because of limited numbers of participants with OLF, those with mild, moderate, or severe COPD were combined for the mortality analyses. For analyses examining potential differences in levels of UACR and eGFR between participants with normal lung function and OLF, the latter were divided into those with mild and moderate-severe OLF.
UACR was calculated from urinary albumin that was measured using a fluorescent immunoassay on a Sequoia-Turner Fluoremeter (Sequoia-Turner Corporation/Abbott) and urinary creatinine that was measured from the rate of color formation on a Beckman Synchron AS/ASTRA clinical analyzer (Beckman Instruments Inc). The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equations after a correction was made to concentrations of creatinine.9,20 For some analyses, UACR was grouped as < 30 mg/g, 30 to < 300 mg/g, and ≥ 300 mg/g and eGFR as < 60, 60 to < 90, and ≥ 90 mL/min/1.73 m2.21 In addition, eGFR was divided into seven categories: < 45, 45 to < 60, 60 to < 75, 75 to < 90, 90 to < 95, 95 to < 105, and ≥ 105 mL/min/1.73 m2.6
Covariates included age, sex, self-reported race or ethnicity (white, black, and other), educational level (< 12, 12, and > 12 years), leisure-time physical activity, alcohol use, smoking status, BMI, systolic BP, high-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, C-reactive protein, diabetes, and histories of myocardial infarction and stroke.
The analyses included men and nonpregnant women aged 40 to 79 years who had a spirometric examination in the mobile examination center, reproducible FEV1 and FVC results, and a complete set of data. UACR was transformed to the −0.1 power to better approximate a normal distribution, and mean levels were back-transformed. Using the direct method, the projected year 2000 US population for adults aged 40 to 79 years was used to calculate age-adjusted mortality rates. Proportional hazards analysis was used to estimate hazard ratios (HRs) for UACR and eGFR modeled as quintiles or other categories and continuous variables and all-cause mortality. For analyses limited to participants with OLF, the distributions of UACR and eGFR among participants with OLF were used to derive quintiles. Results were generated with the statistical programs SAS (SAS Institute Inc) and SUDAAN (RTI International).
Results
Of the 8,486 men and nonpregnant women aged 40 to 79 years who visited the mobile examination center at baseline, 7,827 had a value for FEV1 and FVC. After retaining participants with acceptable examinations, 7,050 remained (4,870 with normal lung function, 621 with restrictive lung function who were excluded, and 1,559 with any OLF). Of the 6,429 participants with normal lung function or OLF, data to calculate the UACR and eGFR were available for 6,335 and 6,420 participants, respectively. After excluding records with missing values for covariates, the final analytic sample included 4,321 participants with normal lung function and 1,390 participants with any OLF (730 with mild, 543 with moderate, and 117 with severe OLF) who had a complete set of data for all the study variables. Participants who were excluded differed on most characteristics from those who were included (e-Table 1).
Lung Function Status and UACR and eGFR
Adjusted mean transformed levels of UACR were higher in adults with moderate-severe OLF than in adults with either normal pulmonary function (P < .001) or mild OLF (P < .001) (Table 1). However, adjusted mean levels of eGFR were similar among the groups except in models 3 to 5 involving comparisons of adults with normal lung function and moderate-severe OLF (P < .05).
TABLE 1.
Least-Square Adjusted Mean Concentrations of UACR Among Adults Aged 40 to 79 y by Pulmonary Function Status, NHANES III, 1988–1994
| Modela | NLF (n = 4,321) | Mild OLF (n = 730) | Moderate/Severe/Very Severe OLF (n = 660) |
P Values | ||
|---|---|---|---|---|---|---|
| NLF vs Mild OLF |
NLF vs Mod+ OLF |
Mild OLF vs Mod+ OLF |
||||
| UACR, b mg/g | ||||||
| 1 | 6.0 (5.6, 6.5) | 5.9 (5.3, 6.6) | 8.6 (7.7, 9.6) | .677 | < .001 | < .001 |
| 2 | 6.0 (5.6, 6.5) | 6.1 (5.5, 6.8) | 8.6 (7.7, 9.6) | .842 | < .001 | < .001 |
| 3 | 6.1 (5.7, 6.6) | 6.0 (5.4, 6.7) | 8.1 (7.2, 9.1) | .856 | < .001 | .001 |
| 4 | 6.2 (5.8, 6.6) | 6.0 (5.4, 6.7) | 7.7 (6.9, 8.7) | .636 | < .001 | .001 |
| 5 | 6.2 (5.8, 6.6) | 6.2 (5.5, 6.9) | 7.5 (6.7, 8.5) | .997 | .003 | .014 |
| GFR, mL/min/1.73 m2 | ||||||
| 1 | 89.5 (88.7, 90.2) | 89.0 (87.1, 90.9) | 88.3 (86.8, 89.9) | .657 | .197 | .587 |
| 2 | 89.4 (88.7, 90.1) | 89.6 (87.7, 91.4) | 88.5 (87.0, 90.0) | .856 | .280 | .372 |
| 3 | 89.6 (88.8, 90.3) | 89.3 (87.4, 91.2) | 87.7 (86.2, 89.2) | .768 | .023 | .177 |
| 4 | 89.6 (88.9, 90.3) | 89.1 (87.2, 90.9) | 87.5 (86.1, 89.0) | .612 | .011 | .164 |
| 5 | 89.6 (88.9, 90.3) | 89.1 (87.3, 90.9) | 87.6 (86.0, 89.1) | .619 | .015 | .171 |
| 6 | 91.0 (90.0, 92.0) | 85.0 (83.3, 86.6) | 83.3 (81.4, 85.1) | <.001 | <.001 | .174 |
GFR = glomerular filtration rate; Mod + = moderate/severe/very severe; NHANES = National Health and Nutrition Examination Survey; NLF = normal lung function; OLF = obstructive lung function; UACR = urinary albumin-creatinine ratio.
Model 1 is adjusted for age. Model 2 is adjusted for variables in model 1 plus sex, race or ethnicity, and education. Model 3 is adjusted for variables in model 2 plus smoking status, alcohol use, and leisure-time physical activity. Model 4 is adjusted for variables in model 3 plus systolic BP, high-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, BMI, and C-reactive protein. Model 5 is adjusted for variables in model 4 plus health status, diabetes, history of myocardial infarction, and history of stroke. Model 6 is adjusted for variables in model 5 minus age, sex, and race or ethnicity.
Back-transformed means.
UACR and eGFR and Mortality Among Participants
With regard to UACR and eGFR, and mortality among participants with any OLF, the demographic breakdown of the sample with OLF was as follows: 849 men, 541 women, 857 whites, 284 blacks, 214 Mexican Americans, and 35 of another race or ethnicity. The mean and median ages were 60.5 years and 62.0 years, respectively.
During the follow-up period (mean and median follow-ups were 12.5 and 13.7 years, respectively), 650 (40.6%) of the 1,390 participants with any OLF died. Significant differences in age, educational status, sex, systolic BP, FEV1, FEV1 /FVC, smoking status, health status, and diabetes status by vital status were evident (Table 2). Decedents had a significantly higher age-adjusted UACR than survivors, but the mean age-adjusted eGFR was similar between the two groups.
TABLE 2.
Age-Adjusted Baseline Means (SEs) and Percentages (SEs) of Study Variables Among Adults Aged 40 to 79 y by Mortality Status, NHANES III, 1988–1994
| Study Variables | Mortality Status (SE) | P Value | |
|---|---|---|---|
| Dead (n = 650) | Alive (n = 740) | ||
| Age, y | 66.8 (0.5) | 56.4 (0.6) | < .001 |
| Education, y | 11.6 (0.4) | 12.6 (0.2) | .029 |
| Number of drinks, /mo | 9.9 (1.8) | 11.5 (1.0) | .417 |
| Systolic BP, mm Hg | 131.4 (1.7) | 125.2 (0.8) | .003 |
| High-density lipoprotein cholesterol, mM | 1.2 (< 0.1) | 1.3 (< 0.1) | .107 |
| Non-high-density lipoprotein cholesterol, mM | 4.4 (0.2) | 4.3 (< 0.1) | .470 |
| BMI, kg/m2 | 25.8 (0.7) | 26.5 (0.3) | .380 |
| Albumin-creatinine ratio, a mg/g | 10.2 (1.0) | 6.0 (0.3) | < .001 |
| GFR, mL/min/1.73 m2 | 88.5 (1.6) | 88.7 (0.8) | .930 |
| FEV1, mL | 2386.0 (102.1) | 2619.4 (44.3) | .030 |
| FVC, mL | 3871.5 (124.0) | 4080.2 (58.8) | .124 |
| FEV1 /FVC | 0.61 (0.01) | 0.64 (< 0.01) | .001 |
| Men, % | 71.5 (3.4) | 56.6 (2.4) | < .001 |
| White, % | 84.0 (3.2) | 86.7 (1.1) | .390 |
| Current smoker, % | 58.9 (6.3) | 34.4 (2.2) | .001 |
| Moderate-vigorous leisure-time physical activity, % | 33.8 (4.1) | 41.6 (3.0) | .172 |
| Vitamin or mineral supplement use during past 30 d, % | 37.0 (5.8) | 47.3 (2.6) | .085 |
| C-reactive protein > 3 mg/L, % | 43.6 (5.4) | 33.0 (3.0) | .123 |
| ≥ Good health status, % | 75.3 (3.8) | 86.4 (1.6) | .006 |
| Diabetes, % | 9.7 (1.4) | 6.1 (0.9) | .045 |
| History of myocardial infarction, % | 8.2 (2.6) | 4.6 (1.2) | .209 |
| History of stroke, % | 5.5 (2.5) | 1.9 (0.7) | .172 |
See Table 1 legend for expansion of abbreviations.
Back-transformed means.
Adjusted HRs for all-cause mortality increased as quintile levels of UACR increased (Table 3). With maximal adjustment, the HR was 2.23 (95% CI, 1.56, 3.18). HRs for UACR as a transformed continuous variable were significant in all models, indicating that risk increased with increasing concentrations of UACR (Table 4). The apparent protective effect of UACR is due to the transformation. Squared terms for transformed UACR did not prove significant in any of the models.
Table 3.
HRs (95% Confidence Limits) for All-Cause Mortality in Function of Concentrations of Quintiles of UACR and GFR Among US Adults Aged 40 to 79 y With OLF, NHANES III, 1988–1994 to 2006
| Measures | Quintiles |
P Linear Trend |
||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| UACR | ||||||
| Approximate boundaries, mg/g | <4.0 | 4.0 to <6.1 | 6.1 to <8.9 | 8.9 to <17.9 | ≥ 17.9 | … |
| Unweighted No. of deaths | 75 | 94 | 105 | 166 | 210 | … |
| Unweighted No. at risk | 283 | 238 | 253 | 287 | 329 | … |
| Unadjusted rate per 1,000 person-y (SE) | 14.7 (2.2) | 24.5 (3.6) | 22.1 (2.8) | 47.7 (4.3) | 56.4 (5.3) | <.001 |
| Age-adjusted rate per 1,000 person-y (SE) | 13.7 (1.7) | 22.9 (3.2) | 18.0 (2.4) | 34.9 (3.1) | 40.2 (6.0) | <.001 |
| Modela | ||||||
| 1 | 1.00 | 1.52 (1.04, 2.22) | 1.11 (0.74, 1.68) | 1.98 (1.42, 2.75) | 2.39 (1.69, 3.38) | <.001 |
| 2 | 1.00 | 1.59 (1.09, 2.31) | 1.28 (0.85, 1.91) | 2.15 (1.57, 2.94) | 2.51 (1.76, 3.57) | <.001 |
| 3 | 1.00 | 1.60 (1.12, 2.30) | 1.35 (0.90, 2.03) | 2.21 (1.63, 2.99) | 2.56 (1.82, 3.61) | <.001 |
| 4 | 1.00 | 1.53 (1.06, 2.21) | 1.26 (0.83, 1.93) | 2.00 (1.48, 2.70) | 2.30 (1.61, 3.27) | <.001 |
| 5 | 1.00 | 1.54 (1.09, 2.18) | 1.24 (0.80, 1.91) | 1.95 (1.45, 2.62) | 2.25 (1.58, 3.19) | <.001 |
| 6 | 1.00 | 1.56 (1.09, 2.23) | 1.25 (0.81, 1.94) | 1.99 (1.47, 2.71) | 2.23 (1.56, 3.18) | <.001 |
| eGFR | ||||||
| Approximate boundaries, mL/min/1.73 m2 | <68.6 | 68.6 to <81.0 | 81.0 to <90.0 | 90.0 to < 100.0 | ≥ 100.0 | … |
| Unweighted No. of deaths | 184 | 126 | 157 | 113 | 70 | … |
| Unweighted No. at risk | 285 | 276 | 291 | 280 | 258 | … |
| Unadjusted rate per 1,000 person-y (SE) | 55.6 (5.6) | 30.3 (3.1) | 37.6 (3.7) | 26.7 (3.2) | 13.5 (2.9) | <.001 |
| Age-adjusted rate per 1,000 person-y (SE) | 30.2 (4.5) | 17.2 (1.9) | 23.6 (2.3) | 29.5 (4.4) | 23.3 (5.0) | .914 |
| Modela | ||||||
| 1 | 1.00 | 0.66 (0.48, 0.90) | 0.86 (0.63, 1.18) | 0.82 (0.56, 1.20) | 0.91 (0.50, 1.64) | .451 |
| 2 | 1.00 | 0.63 (0.46, 0.87) | 0.85 (0.62, 1.17) | 0.77 (0.53, 1.13) | 0.85 (0.47, 1.56) | .334 |
| 3 | 1.00 | 0.65 (0.47, 0.91) | 0.85 (0.63, 1.14) | 0.73 (0.50, 1.07) | 0.79 (0.46, 1.38) | .186 |
| 4 | 1.00 | 0.66 (0.47, 0.93) | 0.84 (0.63, 1.13) | 0.74 (0.51, 1.07) | 0.77 (0.44, 1.35) | .164 |
| 5 | 1.00 | 0.69 (0.49, 0.99) | 0.82 (0.61, 1.11) | 0.74 (0.52, 1.07) | 0.75 (0.42, 1.35) | .132 |
| 6 | 1.00 | 0.73 (0.51, 1.06) | 0.88 (0.65, 1.19) | 0.77 (0.54, 1.11) | 0.80 (0.45, 1.44) | .232 |
| 7 | 1.00 | 0.72 (0.53, 0.98) | 0.76 (0.56, 1.03) | 0.49 (0.35, 0.69) | 0.26 (0.15, 0.44) | <.001 |
eGFR = estimated glomerular filtration rate; HR = hazard ratio. See Table 1 legend for expansion of other abbreviations.
Model 1 is adjusted for age and COPD severity. Model 2 is adjusted for variables in model 1 plus sex, race or ethnicity, and education. Model 3 is adjusted for variables in model 2 plus smoking status, alcohol use, and leisure-time physical activity. Model 4 is adjusted for variables in model 3 plus systolic BP, high-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, BMI, and C-reactive protein. Model 5 is adjusted for variables in model 4 plus health status, diabetes, history of myocardial infarction, and history of stroke. Model 6 is adjusted for variables in model 5 plus GFR or UACR. Model 7 is adjusted for variables in model 6 minus age, sex, and race or ethnicity
TABLE 4.
HRs for All-Cause Mortality in Function of Concentrations of UACR and eGFR as Continuous Variables Among US Adults Aged 40 to 79 y With OLF, NHANES III, 1988–1994 to 2006
| Modela | HR (95% CI) | P Value |
|---|---|---|
| UACR, b per transformed mg/g | ||
| 1 | 0.03 (0.01, 0.10) | < .001 |
| 2 | 0.03 (0.01, 0.10) | < .001 |
| 3 | 0.03 (0.01, 0.09) | < .001 |
| 4 | 0.04 (0.01, 0.13) | < .001 |
| 5 | 0.05 (0.02, 0.14) | < .001 |
| 6 | 0.06 (0.02, 0.17) | < .001 |
| eGFR, per 10 mL/min/1.73 m2 | ||
| 1 | 0.93 (0.87, 1.00) | .040 |
| 2 | 0.93 (0.87, 0.99) | .027 |
| 3 | 0.91 (0.86, 0.97) | .007 |
| 4 | 0.92 (0.86, 0.98) | .007 |
| 5 | 0.92 (0.86, 0.97) | .006 |
| 6 | 0.93 (0.88, 0.99) | .025 |
See Table 1 and 3 legends for expansion of abbreviations.
Model 1 is adjusted for age and severity of OLF. Model 2 is adjusted for variables in model 1 plus sex, race or ethnicity, and education. Model 3 is adjusted for variables in model 2 plus smoking status, alcohol use, and leisure-time physical activity. Model 4 is adjusted for variables in model 3 plus systolic BP, high-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, BMI, and C-reactive protein. Model 5 is adjusted for variables in model 4 plus health status, diabetes, history of myocardial infarction, and history of stroke. Model 6 is adjusted for variables in model 5 plus glomerular filtration rate.
Transformed to the −0.1 power
The interaction between diabetes status and UACR status was significant (P = .018). Among participants without diabetes, the HRs were 1.17 (95% CI, 0.86, 1.59) for participants with a UACR of 30 to < 300 mg/g and 0.85 (95% CI, 0.31, 2.35) for participants with a UACR of ≥ 300 mg/g. Among participants with diabetes, the HRs were 1.95 (95% CI, 1.31, 2.92) for participants with a UACR of 30 to < 300 mg/g and 5.67 (95% CI, 1.77, 18.16) for participants with a UACR of ≥ 300 mg/g.
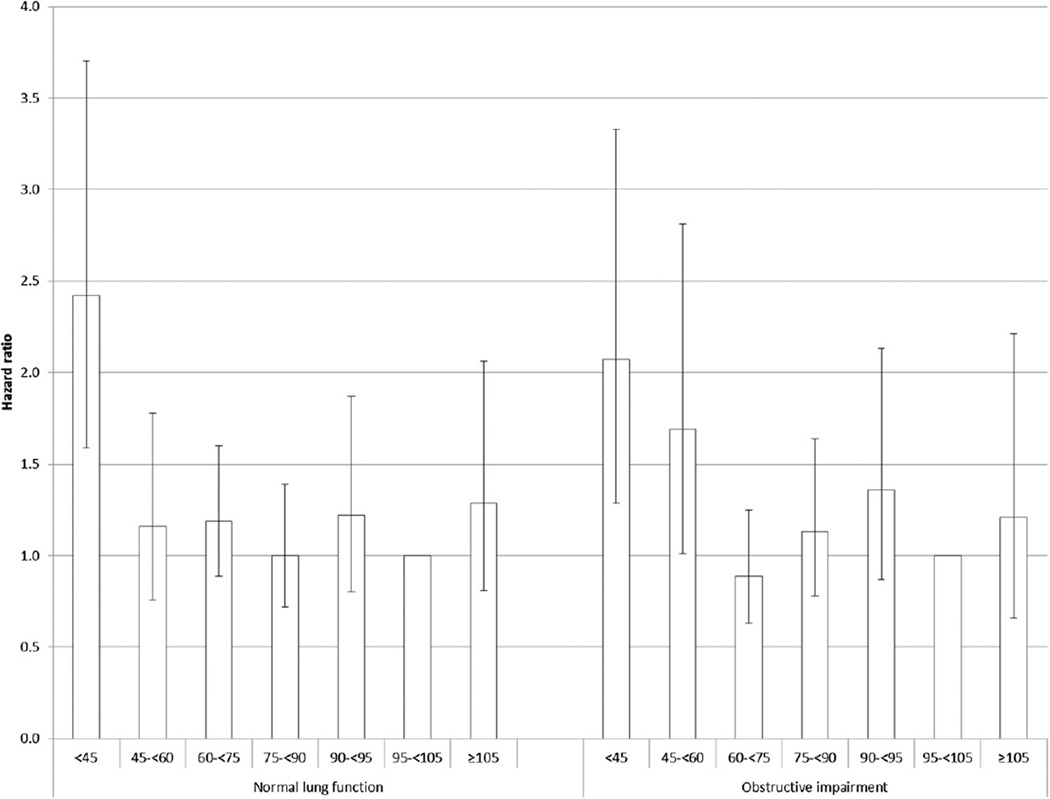
The results for eGFR were less straightforward. The adjusted HR for all-cause mortality was lowest in quintile 2 of eGFR (Table 3). When eGFR was divided into seven categories, elevated HRs among adults with OLF were noted for those with an eGFR of < 45 mL/min/1.73 m2(adjusted HR= 2.07; 95% CI, 1.29, 3.33) and 45 to < 60 mL/min/1.73 m2(adjusted HR = 1.69, 95% CI, 1.01, 2.81) when compared with adults with an eGFR of 95 to < 105 mL/min/1.73 m2. Although the tests for linear trend using the median values of the eGFR quintiles did not prove significant, the P values for eGFR modeled as a continuous variable were significant indicating that risk decreased as eGFR increased (Table 4). Squared terms for eGFR to examine a possible nonlinearity of the association were not significant.
Comparison of Associations for UACR and eGFR and Mortality
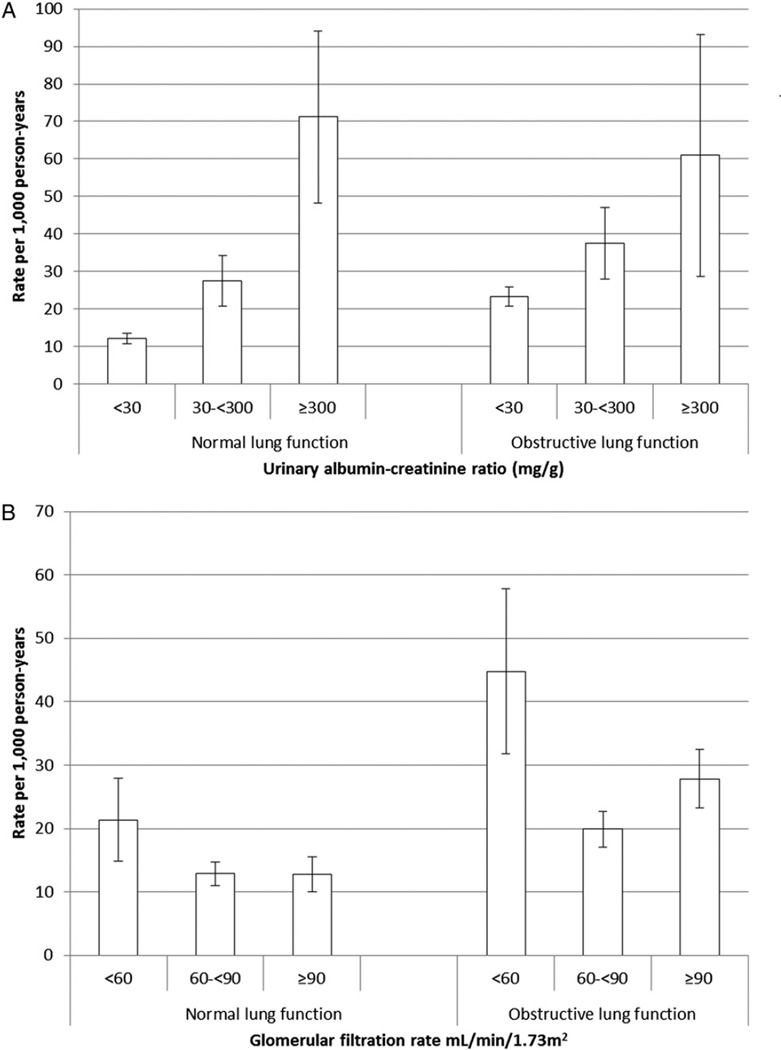
Associations for UACR and eGFR and mortality between participants with normal and OLF were compared. For the analyses that follow, UACR was categorized into < 30, 30 to < 300, and ≥ 300 mg/g and eGFR was categorized into < 60, 60 to < 90, and ≥ 90 mL/min/1.73 m2. Of the 4,321 participants with normal lung function, 900 died. The age-adjusted rates for all-cause mortality increased with increasing levels of UACR (Fig 1). For adults with UACRs < 30 mg/g, the age-adjusted rate was significantly higher among adults with OLF than adults with normal lung function (P < .001). For adults with UACRs of 30 to < 300 mg/g and ≥ 300 mg/g, however, rates did not differ significantly (P = .097, P = .612, respectively).
Figure 1.
A–B, Age-adjusted all-cause mortality rates associated with levels of urinary albumin-creatinine ratio (A) and estimated glomerular filtration rate (B) among adults aged 40 to 79 y with normal and obstructive lung function, National Health and Nutrition Examination Survey III Linked Mortality Study from 1988–1994 to 2006.
For each category of eGFR, the age-adjusted mortality rates among participants with OLF were significantly higher than among those with normal lung function (P = .001, P <.001, P <.001, respectively) (Fig 1). For both adults with OLF and normal lung function, the age-adjusted mortality rate was highest in the lowest category of eGFR.
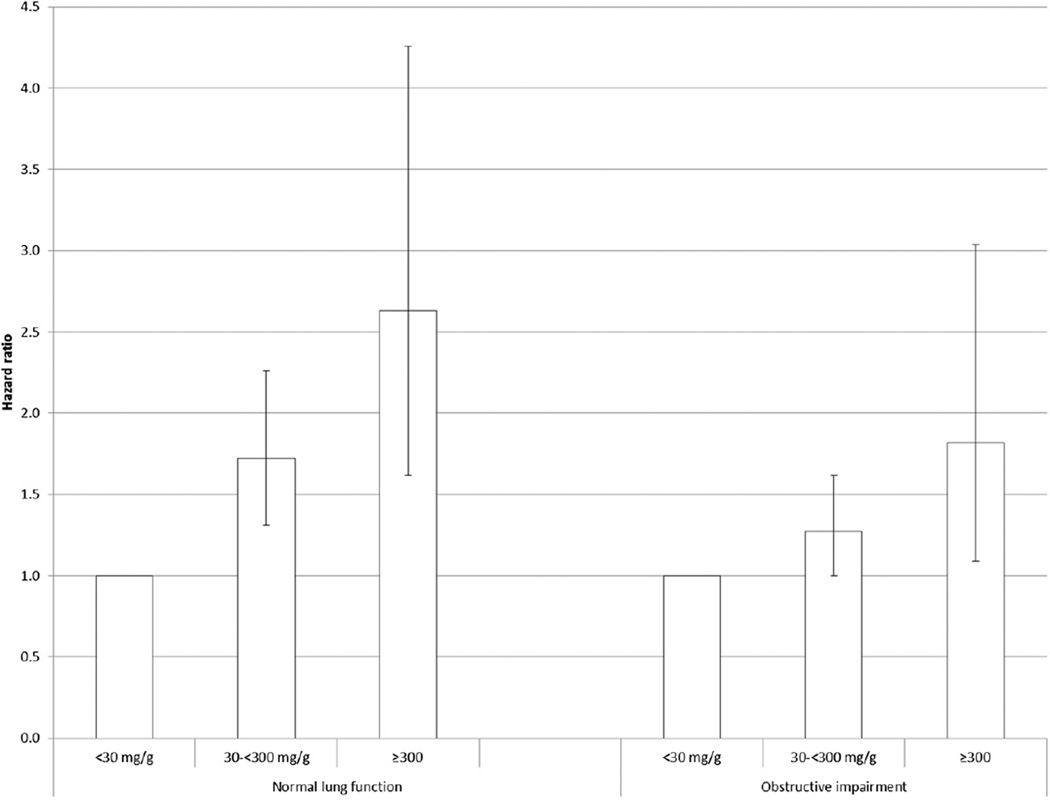
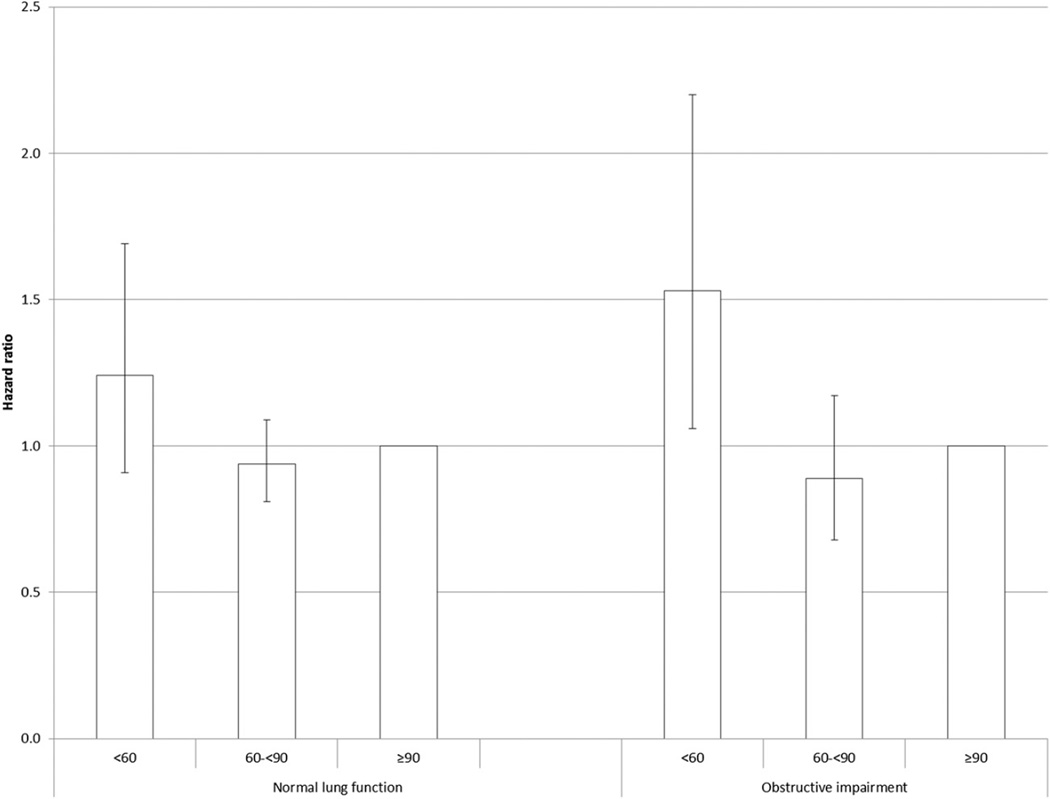
The adjusted HRs for all-cause mortality increased with increasing levels of UACR in both groups (Fig 2), and there was no statistical evidence that the associations differed (P interaction = .230; P interaction for continuous variable = .313). A significant association for all-cause mortality was noted in the lowest category of eGFR among adults with OLF but not among those with normal lung function (Fig 3), but there was no statistical support to suggest that the association between eGFR and all-cause mortality differed between adults with OLF and those with normal lung function (P interaction = .356; P interaction for continuous variable = .743). The interaction of OLF status and eGFR status modeled as seven categories was significant (P interaction = .028) (Fig 4).
Figure 2.
Hazard ratios (95% confidence limits) for all-cause mortality associated with urinary albumin-creatinine ratio among US adults aged 40 to 79 y with normal and obstructive lung function, National Health and Nutrition Examination Survey III Linked Mortality Study from 1988–1994 to 2006. Adjusted for age, sex, race or ethnicity, and education, smoking status, alcohol use, leisure-time physical activity, systolic BP, high-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, BMI, C-reactive protein, health status, diabetes, history of myocardial infarction, history of stroke, and estimated glomerular filtration rate.
Figure 3.
Hazard ratios (95% confidence limits) for all-cause mortality associated with estimated glomerular filtration rate (mL/min/1.73 m2) among US adults aged 40 to 79 y with normal and obstructive lung function, National Health and Nutrition Examination Survey III Linked Mortality Study from 1988–1994 to 2006. Adjusted for age, sex, race or ethnicity, and education, smoking status, alcohol use, leisure-time physical activity, systolic BP, high-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, BMI, C-reactive protein, health status, diabetes, history of myocardial infarction, history of stroke, and urinary albumin-creatinine ratio.
Figure 4.
Hazard ratios (95% confidence limits) for all-cause mortality associated with detailed categories of estimated glomerular filtration rate (mL/min/1.73 m2) among US adults aged 40 to 79 y with normal and obstructive lung function, National Health and Nutrition Examination Survey III Linked Mortality Study from 1988–1994 to 2006. Adjusted for age, sex, race or ethnicity, and education, smoking status, alcohol use, leisure-time physical activity, systolic BP, high-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, BMI, C-reactive protein, health status, diabetes, history of myocardial infarction, history of stroke, and urinary albumin-creatinine ratio.
UACR and eGFR and Risk for Mortality Among Adults
Do UACR and eGFR account for the increased risk for mortality among adults with OLF compared with adults with normal lung function? Because previous research has shown that adults with COPD are at increased risk for death compared with those without COPD, I examined whether UACR and eGFR might account to some degree for this increased risk. In a model adjusted for the same set of confounders (with the exception of UACR) as model 5 in Table 3, the adjusted HR was 1.33 (95% CI, 1.13, 1.55) for adults with OLF compared with adults with normal lung function. After adding UACR and eGFR as continuous variables to the model, the adjusted HR remained unchanged at 1.34 (95% CI, 1.14, 1.56).
Discussion
The results of the present study show that adults with at least moderate OLF had higher adjusted mean levels of UACR than adults with normal lung function or mild OLF. However, adjusted mean levels of eGFR were similar among the three groups characterized by lung function status. Among adults with OLF, quintile levels of UACR were strongly associated with all-cause mortality. The association of levels of eGFR with all-cause mortality was less definitive, but the results suggested an inverse association.
Some data, mostly from small clinical samples, indicate that patients with COPD are more likely to have micro-albuminuria than comparison groups.12–15 The results from the present study now generalize these findings to the broad population. However, the clinical relevance of these differences, which were small, is uncertain. Additional studies with large population-based samples of adults with COPD are needed to build the evidence base of the links between COPD and CKD.
The evidence from cohort studies is generally convincing that albuminuria is directly and GFR is inversely associated with all-cause mortality.6,22 Furthermore, studies also noted that these findings apply to people with diabetes and hypertension.23,24 Whether these relationships also were applicable to people with COPD presented hitherto a gap in the knowledge base. The results from the present study now suggest that albu-minuria and to a lesser extent eGFR were associated with all-cause mortality in this group. The associations between eGFR and mortality were largely confined to levels below 75 mL/min/1.73 m2, a finding that is generally consistent with analyses undertaken by the Chronic Kidney Disease Consortium.6
Although the associations between markers of kidney damage and function seem well established, the interpretation of these associations seems unsettled in that albuminuria and GFR may be true risk factors for all-cause mortality or may be risk markers for other pathogenic processes or residual risk from untreated or inadequately treated risk factors. If albuminuria and GFR are true risk factors for mortality, a thorough understanding of the pathways through which these pathogenic factors increase mortality may lead to therapeutic targets that can delay death. Therapeutic interventions aimed at the renin-angiotensin-aldosterone system have been shown to be renal protective and also reduce morbidity and mortality.25 Nevertheless, the mechanisms through which CKD increases mortality remain ill defined.
CKD might represent a marker for suboptimal treatment of risk factors for cardiovascular disease, for duration and severity of vascular disease, or for unmeasured or unknown risk factors.22,26 Albuminuria is thought to reflect general vascular damage with increased permeability and endothelial dysfunction.27,28 This increased permeability may allow the transport of harmful molecules into the endothelial matrix, resulting in the initiation or amplification of disease processes. Therefore, albuminuria may signify a conveniently measured marker for other pathogenic processes. For example, screening for albuminuria has been proposed as a convenient approach to assess risk for cardiovascular disease or identify patients with undiagnosed cardiovascular risk factors.29–31
Among participants with OLF, the association of UACR with mortality was stronger among adults with diabetes than those without diabetes. Because of the limited sample size for these analyses, the results should be cautiously interpreted as evidenced in part by the wide confidence intervals for the HRs among adults with diabetes. Furthermore, the results of the present study are inconsistent with the results of a large meta-analysis that failed to find a difference in relative risks for the associations between UACR and mortality by diabetes status in general and high-risk cohorts.23 Consequently, the issue of diabetes as a potential effect modifier of the association between UACR and mortality among adults with OLF will require additional pursuit. If diabetes were indeed to amplify the risk associated with UACR on mortality among adults with OLF, identifying adults with both OLF and diabetes would be of considerable urgency to bring them under timely medical management for their conditions in the hope of mitigating excess risk for early death.
A systematic review noted considerable uncertainty about early referral strategies for patients with CKD.32 To stem the progression of CKD, current guidelines recommend a number of strategies including BP control, limiting protein intake in certain groups with CKD, glycemic control, limiting salt intake, engaging in recommended levels of physical activity, weight control, and smoking cessation.33 Furthermore, the current guidelines view all people with CKD as being at increased risk for cardiovascular disease and recommend that cardiovascular hygienic measures be implemented in these patients—smoking cessation, physical activity, weight control, lipid management, BP control, glucose control—and that patients be treated with antiplatelet agents.33 Given that adults with COPD are at increased risk for cardiovascular disease incidence and mortality and that cardiovascular disease mortality represents the largest component of all-cause mortality, these recommendations seem particularly pertinent for patients with COPD.
Several limitations of this study should be considered. The number of participants with OLF proved inadequate to undertake detailed analyses stratified by severity of OLF or other subgroups. Hence, future studies able to stratify by the level of severity will be helpful to better characterize the relationships between markers of kidney function and damage and mortality and other outcomes. No postbronchodilator spirometry was done, and consequently some percentage of participants characterized as having OLF had reversible OLF. Although the analyses included a substantial numbers of covariates, residual confounding because of failure to include relevant confounders is a possibility.
Conclusions
UACR was positively associated and eGFR, modeled as a continuous variable, was inversely associated with all-cause mortality among adults with OLF. Because of the dearth of studies addressing the potential associations of UACR and GFR with mortality in adults with COPD, additional studies with large numbers of adults with COPD are needed. The results of the present study suggest that the current Kidney Disease: Improving Global Outcomes guidelines have relevance for patients with COPD and evidence of impaired kidney function or damage.33
Supplementary Material
Acknowledgments
FUNDING/SUPPORT: The author has reported to CHEST that no funding was received for this study.
ABBREVIATIONS
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- GFR
glomerular filtration rate
- HR
hazard ratio
- NHANES
National Health and Nutrition Examination Survey
- OLF
obstructive lung function
- UACR
urinary albumin-creatinine ratio
Footnotes
Author contributions: E. S. F. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis. E. S. F. was responsible for the study concept and design, acquisition, analysis, and interpretation of data, and manuscript preparation.
Financial/nonfinancial disclosures: The author has reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: The work was performed at the Centers for Disease Control and Prevention. The findings and conclusions in this article are those of the author and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Additional information: The e-Table can be found in the Supplemental Materials section of the online article.
References
- 1.Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59(10):1–126. [PubMed] [Google Scholar]
- 2.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58(5):388–393. doi: 10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford ES, Mannino DM, Zhao G, Li C, Croft JB. Changes in mortality among US adults with COPD in two national cohorts recruited from 1971–1975 and 1988–1994. Chest. 2012;141(1):101–110. doi: 10.1378/chest.11-0472. [DOI] [PubMed] [Google Scholar]
- 4.Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest. 2005;128(4):2005–2011. doi: 10.1378/chest.128.4.2005. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988–1994 to 2007–2010. Chest. 2013;143(5):1395–1406. doi: 10.1378/chest.12-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsushita K, van der Velde M, Astor BC, et al. Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Velde M, Matsushita K, Coresh J, et al. Chronic Kidney Disease Prognosis Consortium. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 8.Astor BC, Matsushita K, Gansevoort RT, et al. Chronic Kidney Disease Prognosis Consortium. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Zeeuw D, Parving HH, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol. 2006;17(8):2100–2105. doi: 10.1681/ASN.2006050517. [DOI] [PubMed] [Google Scholar]
- 11.Asselbergs FW, Diercks GF, Hillege HL, et al. Prevention of Renal and Vascular Endstage Disease Intervention Trial (PREVEND IT) Investigators Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110(18):2809–2816. doi: 10.1161/01.CIR.0000146378.65439.7A. [DOI] [PubMed] [Google Scholar]
- 12.Kömürcüoğlu A, Kalenci S, Kalenci D, Kömürcüoğlu B, Tibet G. Microalbuminuria in chronic obstructive pulmonary disease. Monaldi Arch Chest Dis. 2003;59(4):269–272. [PubMed] [Google Scholar]
- 13.Polatli M, Cakir A, Cildag O, Bolaman AZ, Yenisey C, Yenicerioglu Y. Microalbuminuria, von Willebrand factor and fibrinogen levels as markers of the severity in COPD exacerbation. J Thromb Thrombolysis. 2008;26(2):97–102. doi: 10.1007/s11239-007-0073-1. [DOI] [PubMed] [Google Scholar]
- 14.Casanova C, de Torres JP, Navarro J, et al. Microalbuminuria and hypoxemia in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(8):1004–1010. doi: 10.1164/rccm.201003-0360OC. [DOI] [PubMed] [Google Scholar]
- 15.Bulcun E, Ekici M, Ekici A, Kisa U. Microalbuminuria in chronic obstructive pulmonary disease. COPD. 2013;10(2):186–192. doi: 10.3109/15412555.2012.735292. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. [Accessed January 7, 2011];NHANES III linked mortality file. CDC website. http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes3_linkage.htm.
- 17.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994;(32):1–407. [PubMed] [Google Scholar]
- 18.Westat, Inc. Third National Health and Nutrition Examination Survey. [Accessed July 24, 2013];Spirometry Procedure Manual. CDC website. www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/spiro.pdf.
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 20.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39(5):920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 22.Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 23.Fox CS, Matsushita K, Woodward M, et al. Chronic Kidney Disease Prognosis Consortium. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380(9854):1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahmoodi BK, Matsushita K, Woodward M, et al. Chronic Kidney Disease Prognosis Consortium. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet. 2012;380(9854):1649–1661. doi: 10.1016/S0140-6736(12)61272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holtkamp FA, de Zeeuw D, de Graeff PA, et al. Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J. 2011;32(12):1493–1499. doi: 10.1093/eurheartj/ehr017. [DOI] [PubMed] [Google Scholar]
- 26.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15(5):1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 27.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage The Steno hypothesis. Diabetologia. 1989;32(4):219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 28.Garg JP, Bakris GL. Microalbuminuria: marker of vascular dysfunction, risk factor for cardiovascular disease. Vasc Med. 2002;7(1):35–43. doi: 10.1191/1358863x02vm412ra. [DOI] [PubMed] [Google Scholar]
- 29.Atthobari J, Asselbergs FW, Boersma C, et al. PREVEND IT Study Group. Cost-effectiveness of screening for albuminuria with subsequent fosinopril treatment to prevent cardiovascular events: A pharmacoeconomic analysis linked to the prevention of renal and vascular endstage disease (PREVEND) study and the prevention of renal and vascular endstage disease intervention trial (PREVEND IT) Clin Ther. 2006;28(3):432–444. doi: 10.1016/j.clinthera.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Boersma C, Gansevoort RT, Pechlivanoglou P, et al. Prevention of Renal and Vascular End Stage Disease Study Group. Screen-and-treat strategies for albuminuria to prevent cardiovascular and renal disease: cost-effectiveness of nationwide and targeted interventions based on analysis of cohort data from the Netherlands. Clin Ther. 2010;32(6):1103–1121. doi: 10.1016/j.clinthera.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Özyilmaz A, Bakker SJ, de Zeeuw D, de Jong PE, Gansevoort RT PREVEND Study Group. Selection on albuminuria enhances the efficacy of screening for cardiovascular risk factors. Nephrol Dial Transplant. 2010;25(11):3560–3568. doi: 10.1093/ndt/gfq478. [DOI] [PubMed] [Google Scholar]
- 32.Black C, Sharma P, Scotland G, et al. Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol Assess. 2010;14(21):1–184. doi: 10.3310/hta14210. [DOI] [PubMed] [Google Scholar]
- 33.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3(1):1–150. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.