Abstract
Purpose
To examine the effect of body weight supported treadmill training (BWSTT) on gait and gross motor skill development in children (2–5 years old) with developmental delay who are ambulatory.
Methods
Twenty-four subjects (12 control, 12 BWSTT) were enrolled in this randomized control trial. All subjects continued to receive physical therapy. Subjects were tested at baseline, 4 weeks, 6 weeks, and at 6 weeks following completion of BWSTT. Outcomes were assessed using the 10 Meter Walk Test (10MWT) and Gross Motor Function Measure- D and E.
Results
Significant improvements were seen in gait velocity and gross motor skill attainment. With positive interactions in both the 10MWT and GMFM-E, the BWSTT group as compared to the control group demonstrated functional gains in gait velocity and gross motor skills, P = .033 and.017, respectively.
Conclusions
A 6-week high intensity BWSTT program can improve gait velocity and influence functional gains.
Keywords: body weight/physiology, child, female, gait/physiology, human, male, physical therapy/methods, randomized control trial, treadmill training
INTRODUCTION AND PURPOSE
Children with developmental delay (DD) make up 2–11% of the pediatric population worldwide.1–3 Developmental delay is a diagnosis given to children who fail to meet developmental milestones within an expected age range in 1 or more areas of development. Children with DD in the United States have been shown to use health care services under Medicaid more than children without DD.4 Indications that increased health care utilization in children with DD may continue past childhood have been found. Even minor disturbances in the development of movement can place children at risk for anxiety, attention disorders, delays in speech, and general DD.2 Cantell et al. reported that in a group of 15 year-old adolescents, 46% of those diagnosed with DD at 5 years of age were significantly different on both motor and perceptual tasks tested from their peers who were undiagnosed.3 The teenagers with persistent motor delays had fewer social hobbies and pastimes as well as decreased scholastic ambitions.3 Children with orthopedic problems related to DD such as joint laxity, inappropriate postural alignment, poor muscle tone, and poor gait and movement technique may experience future joint pain, further loss of muscle strength and flexibility, and poor cardiopulmonary fitness leading to further limitations in activity and participation and increasing health care costs.5 Children with motor impairments demonstrate reduced exploration of their environment and dependence in daily living skills which can greatly affect development in other areas including the psychosocial domain (i.e. self esteem).6 Deficits that persist into adulthood have been correlated with negative health outcomes including increased body fat and overall decreased physical activity.7 Physical therapists might ameliorate these negative effects of DD, and thus decrease future healthcare utilization, by providing intervention which effectively and efficiently assists children in obtaining optimal independent motor function. Traditionally, physical therapy (PT) for children with DD has been characterized by a “bottom up” technique employing interventions aimed at changing underlying deficits in order to improve task performance.5 More recently, physical therapists are exploring the use of “top down” methods employing a combination of cognitive processes and functional activities to improve a child’s strength and postural control while allowing the required practice of specific gross motor skills.5 This style of treatment is consistent with current theories of motor learning as well as with recommended practice for children with hypotonia, a characteristic common to children with DD.5,8 Current practice is rooted heavily in theory, thus little research exists that explored specific therapeutic practices in children with a primary diagnosis of DD. Physical therapists value efficiency and seek interventions that address multiple impairments in a safe, time-efficient, and clinically practical manner to make the best use of allotted time with their patients.9 Body weight supported treadmill training should be explored as a possible option that meet those criteria.
Body weight supported treadmill (BWSTT) training is a task-specific method of retraining or, in young children, developing gait. Body weight supported treadmill training employs the use of a suspended harness over a moving treadmill to unload a percentage of the user’s weight while providing a safe environment for repetitive stepping. This intervention provides the child with more sustained practice than can be provided over-ground, while affording the therapist greater ease for provision of tactile, verbal, and visual feedback. Additionally, BWSTT offers the child the chance to use feedback provided by both the therapist and the surface to vary performance accordingly and experience the consequences/rewards of that variability. For example, a child may take smaller steps and actually experience the sensation of a true fall, but in a safe environment. The child may then choose to increase step length and reap the benefits of more appropriate torque generation during the pre-swing phase of gait thus contributing to overall efficiency. By altering speed and incline, the therapist can create greater demand for and use of specific musculature. The pediatric literature regarding BWSTT has shown improved endurance10–11, gross motor function10,12–13,14–16, self-selected walking speed11–14,17–18, gait kinematics19, gait symmetry20, and improved safety and ability to navigate the environment following BWSTT in children with cerebral palsy, Down syndrome, and spinal cord injury.21
Often, despite obvious deficits related specifically to the diagnosis of Down syndrome (DS), straightforward comparisons between the clinical presentation of children with DS and children with DD can be made including delayed milestone achievement, increased joint laxity, low muscle tone, and poor exercise endurance. A modicum of high level (i.e. randomized control trials) evidence is available that suggests BWSTT will hasten the onset of walking as well as motor milestone achievement in children with DS.22–25 Additionally, the literature in the DS population has explored the effects between a generalized low intensity (LG) and a high intensity individualized (HI) BWSTT program in a series of studies using the same cohort. A series of studies in the DS population were performed and suggest that HI training in particular, facilitates significant improvements in stride length leading to a more mature gait pattern,22 earlier motor milestone achievement,24 increased stepping,24 and earlier onset of walking.22 The HI group also showed a significantly earlier acquirement of obstacle clearance technique.26 Additionally, 3D motion analysis revealed subjects in the HI group demonstrated more efficient energy storage and use during pre-swing with peak ankle plantar flexion occurring before toe-off and a subsequent longer duration of forward thigh swing after toe-off.19 Notably, the cohort used in this series of studies was non-ambulatory and receiving parent-directed BWSTT in the home.
Though typically used to encourage development of independent walking in children as is the case in the literature focused on DS, BWSTT also has the potential to facilitate refinement of existing gait characteristics. Johnson et al. support the concept of refining performance with BWSTT through increased task specific practice with imposed demands.27 Female soccer players who received high-speed treadmill practice performed significantly better on the 40-yard sprint than a control group. There are currently no studies examining the use of BWSTT to refine gait in children with a primary diagnosis of DD who are ambulatory.
Treatment protocols for pediatric BWSTT vary widely, with statistically significant differences noted in outcomes including gait velocity and gross motor skill development with the use of high intensity, individualized protocols ranging from 2–5 sessions per week, 6–40 minutes per session, and 2–12 weeks in duration.28 A case series by Lowe et al. described the use of a 10 week, high intensity BWSTT in 3 children aged 2, 4, and 5 years old, with a diagnosis of DD.29 Statistically significant improvements were seen in Gross Motor Function Measure (GMFM) Section E after 4 weeks of training and in gait velocity following 6 weeks of training, with plateau in all variables after 6 weeks. Though not statistically significant, improvements were noted in GMFM Section D as well. Follow up testing 6 weeks after completion of the treadmill program revealed maintenance of these improvements in all 3 children.29
Children with DD are at risk for further delay in milestone achievement and without appropriate intervention for potential orthopedic concerns related to notable posture and gait deviations.3,5 By the age of 7 years, most children are walking with a mature gait representative of the pattern they will continue to use as adults.28 Furthermore, Dusing and Thorpe reported that after the age of 5 years no significant changes in normalized step length were noted.30 Therefore, the age group chosen for this study included children with developing gait patterns, aged 2 to 5 years, in which a training program could potentially have the greatest affect. Other than the case series performed by Lowe et al., no evidence has been reported for the use of BWSTT in children with a primary diagnosis of DD. The purpose of this study was to determine the effects of a 6-week, high intensity BWSTT program on gait velocity and gross motor skill development in a group of children (ages 2–5 years) with DD who were ambulatory.
METHODS
Subjects
The Institutional Review Board at the University of Central Arkansas approved this study. The investigator obtained informed consent from the parents of each child who participated. All subjects for this randomized control study met the following criteria: (1) between the ages of 2 and 5 years, (2) diagnosis of DD supported by a Z score of ≥ −1.5 on a standardized developmental test, (3) independently ambulating without assistive devices, and (4) current PT plan of care that did not include use of BWSTT. Subjects were excluded from participation if they met any of the following criteria: (1) were under consultation with a geneticist or neurologist, (2) had a known metabolic disorder, or (3) had a different primary diagnosis that resulted in DD. A total of 24 subjects (aged 2–5 years) with a primary diagnosis of DD enrolled in this study. Subjects were a sample of convenience drawn from children attending an inclusionary developmental preschool who were receiving PT. Subjects were randomized to the control or treatment group using a computer generated randomization chart. Each subject participated in all testing procedures. One subject was excluded following baseline testing based upon recommendation for neurological consultation. Two other subjects were excluded based upon baseline testing results that were 3 standard deviations above the values obtained from all other subjects resulting in these 2 subjects being outliers in all tests. Twenty-one subjects completed the study (12 subjects in the BWSTT group, 9 control subjects- see Table 1). The groups had a similar mean age and mean number of therapy minutes per week. One additional subject moved before follow-up data could be obtained but did complete the intervention portion in the control group.
Table 1.
Subject Characteristics
| Treatment (n = 12) | Control (n = 9) | |||
|---|---|---|---|---|
| Mean age months (range) | 36.67 (26–51 months) | 39.50 (27–48 months) | ||
| Gender % | 75% male | 25% female | 90% male | 10% female |
| Race % | ||||
| -White | 58.33% | 90% | ||
| -Black | 25% | 0% | ||
| -Other | 16.67% | 10% | ||
| Wearing orthoses % | 50% | 55.55% | ||
| Inserts 25% | SMO 25% | Inserts 22.22% | SMO 33.33% | |
Supramalleolar Orthosis (SMO)
Testing Procedures
Because 1 tester (7 years pediatric PT experience) was also the trainer for the BWSTT group, an additional tester (17 years pediatric PT experience) blinded to group was involved in all testing. Prior to initiation of testing for this study, 10 children aged 2 to 5 years with a diagnosis of DD were recruited for a separate reliability study. The testers established interrater reliability on each test by simultaneously scoring the same performance by each child. Intraclass correlation (ICC) demonstrated ICCs > .90 for each test.
Testing was performed at baseline, 4 weeks, 6 weeks, and 6 weeks post intervention, and consisted of measurements of self-selected gait velocity and developmental function using the Gross Motor Function Measure-88 (GMFM). To measure gait velocity, the subject was instructed to “walk like you usually walk, but don’t run” for a series of 3 passes over a 10-meter distance, always toward the testing therapist. Each trial was timed to the nearest 0.01 of a second using a digital stopwatch. Trials were averaged to determine average gait velocity. The test took place in a familiar hallway at the subjects’ preschool. Walking velocity is a valid and reliable measure of walking ability in children with and without disability.31–33 The GMFM is a standardized, criterion referenced clinical testing tool used to evaluate changes in gross motor function in children. The assessment is divided into 5 dimensions: (A) Lying and Rolling, (B) Sitting, (C) Crawling, (D) Standing, and (E) Walking, Running, and Jumping. Each dimension contains individual items scored on a 4-point Likert Scale. The GMFM has established reliability and validity in pediatric populations, specifically children with cerebral palsy and Down Syndrome.34–36 Though this test is not specifically valid or reliable for children with a primary diagnosis of DD, the GMFM was used only as a criterion assessment to compare each child’s performance across time in a pre-test/post-test manner. Dimensions D and E were chosen specifically to measure improvements in functional ambulation tasks. The test was administered in a familiar room of the subjects’ preschool. Some subjects wore bilateral inserts or supramalleolar orthoses (Table 1) and were able to wear them during testing.
When possible, to minimize fatigue, all testing occurred mid-morning following breakfast and prior to any outdoor recess time or other scheduled therapies. The children did not receive PT or BWSTT on baseline or any other testing days. The test administration took less than 1 hour for each child. Rest periods were allowed as needed between tests. Both groups were tested at baseline, upon completion of 4 and 6 weeks of treadmill training by the training group, and at 6 weeks following withdrawal of the intervention.
Treadmill Training
All subjects continued their usual PT sessions consisting of therapeutic activities to promote functional stability and mobility, exercises focused on developing balance and coordination, and core and proximal strengthening activities. All PT sessions were provided by pediatric physical therapists in the same facility. In addition to their usual therapy, all subjects in the BWSTT group received up to 3 additional BWSTT sessions of up to 15 minutes each per week, for 6 weeks. The LiteGait® (Mobility Research; Tempe, Arizona) gait training device placed over the GaitKeeper™ (Mobility Research; Tempe, Arizona) treadmill was used for all training sessions (Figure 1). The BWSTT sessions took place at the subjects’ developmental preschool and were supervised by the primary investigator. The subjects began the study walking at speeds ranging from 1.2–1.8mph (0.54–0.80m/s), treadmill inclination at a grade of 0–1, for 8–11.3 minutes. All subjects began treadmill training at or near their baseline self-selected walking speed. The objective was to begin walking on the treadmill as close to the baseline self-selected walking speed as possible without increasing the presence of gait deviations and without increasing the child’s anxiety while on the treadmill. Treadmill speed was increased within each session as the subject tolerated, and subsequent sessions were initiated at the maximum speed achieved during the previous session. Over the course of 6 weeks, the speed, inclination, and time on the treadmill was increased gradually as each child tolerated to 1.8–2.4mph (0.80–1.07 m/s), grade of 1–3, and 15 minutes respectively. These changes were made based upon the subject’s ability to walk at the given body weight support, speed, incline, and time on the treadmill without increasing gait deviations. Additionally, the subjects practiced ambulation with their upper extremities free to practice reciprocal arm swing. All handrail attachments were removed to prevent weight bearing through the upper extremities and encourage reciprocal arm swing.
Figure 1.
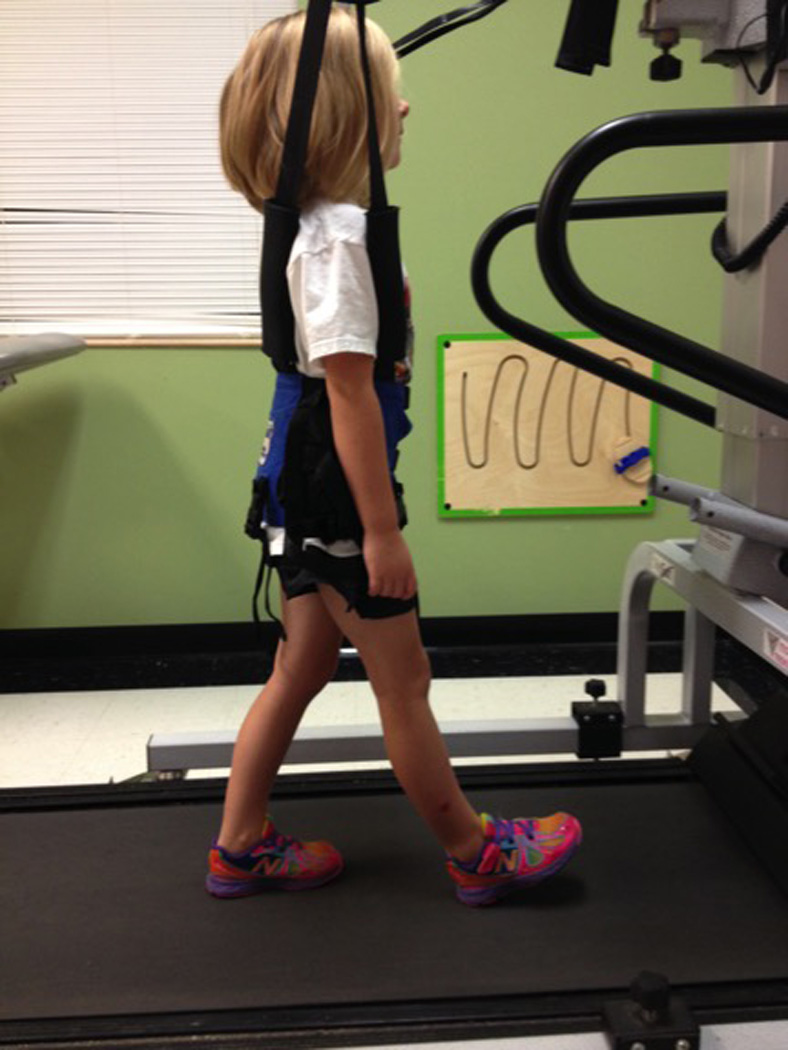
The investigator chose to make changes in the parameters that would allow continued appropriate gait practice while building on the previous session’s achievements. If changes were made that promoted the presence of increased gait deviations during practice, those changes were retracted and more appropriate parameters put in place. This use of clinical judgment is consistent with the pediatric literature which provides varied protocols for BWSTT based upon a number of factors including time available, patient availability, patient presentation and performance, and facility or equipment restrictions.36
The investigator provided tactile feedback as indicated to correct gait deviations present while walking on the treadmill. Gradually, tactile cueing was decreased and replaced with active subject movement correction with verbal or visual cueing such as bandwidth feedback using chalk lines on the treadmill belt. Additionally, body weight support (BWS) was determined using the BiSym™ Scale (Mobility Research; Tempe, Arizona) that measures the number of pounds the overhead yoke is supporting. This number was converted into a percentage of the child’s body weight, which was measured at baseline. As tolerated, BWS was decreased with each subject achieving safe treadmill ambulation (with or without therapist facilitation) to achieve 0% BWS (though the harness remained in place for safety measures). The subjects were required to be present for at least 1 session per week to continue in the program. The mean number of BWSTT sessions throughout the 6-week period was 13.92 with a range of 10–15 sessions. No adverse events occurred for any subject.
Statistical Analysis
The data met the criteria for normality of distribution of scores as determined by the Kolmogorov-Smirnov Test. All data were non-significant (P>.05) and additionally, the data created normal probability plots when plotted against the expected value from the normal distribution. Because the assumptions for normality were not violated, the data were treated as continuous with assumed equal intervals and parametric tests were used to analyze average gait velocity and GMFM Dimensions D and E raw scores. A mixed between-within-subjects analysis of variance was conducted to assess the effect of the 2 interventions (PT plus BWSTT versus PT only) on subjects’ average gait velocity and GMFM scores across the 3 time test points (baseline, 4 weeks, and 6 weeks). Additionally, a mixed between-within-subjects analysis of variance was used to compare 6 week and follow up scores (taken 6 weeks post-intervention) for each variable to investigate maintenance of observed effects. Calculation of all descriptive statistics and parametric tests were performed using the SPSS™ PASW Statistics 17.0 Package (International Business Machines; Armonk, New York).
RESULTS
A total of 21 subjects completed this study. One subject was excluded from statistical analysis of gait velocity because his baseline velocity was at the mean for gait velocity of hispeers his age without disability (Figure 2).28 Another subject was excluded from each of the analyses of GMFM D and E because the child’s raw scores at baseline approached the maximum possible score, thus creating a ceiling effect (Figure 2). Upon removal of these 2 outliers, the groups’ raw scores were not statistically different at baseline. The subjects in this study presented with self-selected walking speeds observed to be slower than the children in their preschool that were typically developing (TD) as well as speeds reported in the literature.30,38 The gait velocities obtained were compared with the velocities obtained by Dusing and Thorpe who examined gait in 438 children ages 1–10 that were TD.30 When compared to the children who were TD in the study by Dusing and Thorpe, 75% of subjects in our study ambulated at a baseline velocity below the mean for their age groups (Table 2).30 Two subjects whose baseline velocities were within normal limits were noted to walk pitched forward and slightly elevated on their toes, likely due to their inability to “keep up with” their forward center of mass. For these subjects, this “normal” velocity represented poor postural control with the inability to use eccentric control and stay upright.
Figure 2.
Table 2.
Comparative Baseline Self Selected Walking Speed
(original data in cm/sec adapted to m/sec)
| Subject | Age Group | Baseline m/sec | Normative Means30 (± SD) |
|---|---|---|---|
| 1 | 24–35 months | .74 | .93±.23 |
| 2 | .84 | ||
| 3 | .89 | ||
| 9 | .86 | ||
| 14 | .58 | ||
| 15 | 1.00 | ||
| 16 | .58 | ||
| 17 | .60 | ||
| 20 | .71 | ||
| 21 | .80 | ||
| 4 | 36–47 months | 1.44* | .99±.22 |
| 7 | 1.13 | ||
| 8 | 1.00 | ||
| 11 | .79 | ||
| 13 | .96 | ||
| 18 | .81 | ||
| 19 | .70 | ||
| 23 | .83 | ||
| 24 | .87 | ||
| 5 | 48–59 months | 1.20 | 1.14±.24 |
| 10 | 1.25 |
denotes exclusion from data analysis based upon outlier status for this test or for multiple tests
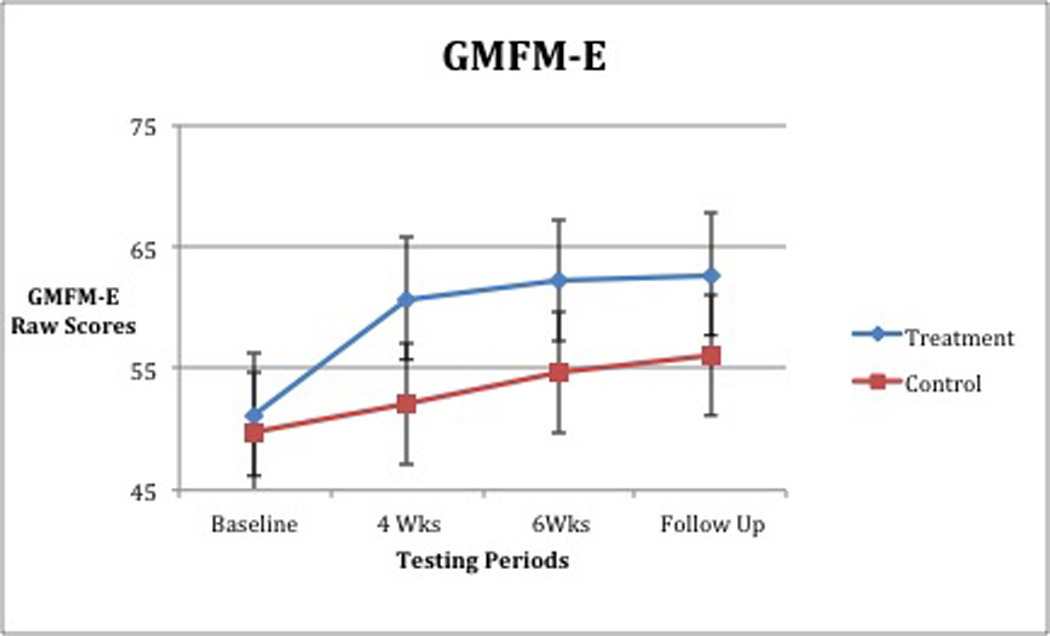
When analyzed, walking velocity revealed a significant interaction between group and time, (Wilks’slambda =0.67 F [2,17] =4.198, p =0.033, partial eta squared = 0.33; Table 3, Figure 3). For GMFM-D (activities related to standing), no significant interaction between group and time was found, (Wilks’slambda = 0.75, F [2,17] = 2.875, p = 0.08, partial eta squared = 0.25; Table 4, Figure 4). A significant main effect for time was found, (Wilks’slambda = 0.24, F [2,17] = 27.73, p = <0.0005 partial eta squared = 0.77). The main effect comparing the 2 groups was also significant, (F [1,18] = 5.708, p = 0.028, partial eta squared = 0.24). For GMFM-E (activities related to walking, running, jumping), a significant interaction between group and time was observed, (Wilks’slambda = 0.62, F [2, 1c7] = 5.199, p = 0.017, partial eta squared = 0.38; Table 5, Figure 5). All improvements in average gait velocity and gross motor skills were maintained at 6 week follow up with P>.05 for all measures.
Table 3.
10 Meter Walk Test: Means (m/s) and Standard Deviations
| N | Group | Baseline | 4 Weeks | 6 Weeks | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| 11 | Treatment | 0.86 | 0.20 | 1.01 | 0.17 | 1.08 | 0.19 |
| 9 | Control | 0.86 | 0.19 | 0.85 | 0.15 | 0.83 | 0.20 |
| 20 | Total | 0.86 | 0.19 | 0.94 | 0.18 | 0.97 | 0.13 |
Figure 3.
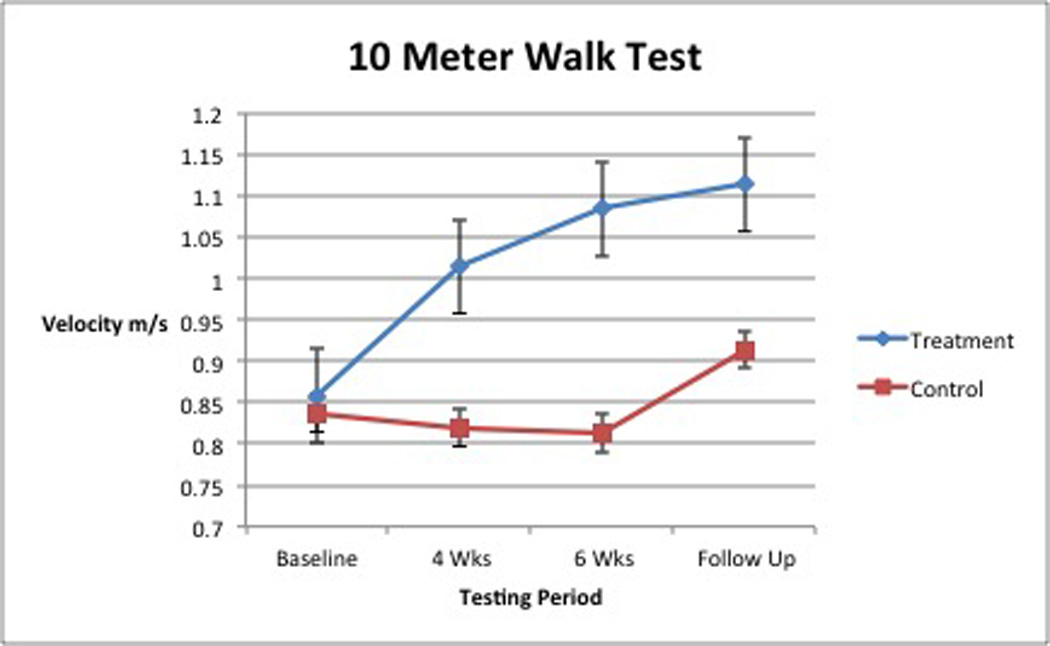
Table 4.
Gross Motor Function Measure- D: Means (raw scores) and Standard Deviations
| N | Group | Baseline | 4 Weeks | 6 Weeks | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| 12 | Treatment | 32.17 | 2.95 | 34.92 | 2.02 | 36.58 | 2.02 |
| 8 | Control | 30.75 | 2.49 | 32.75 | 2.66 | 33.25 | 2.19 |
| 20 | Total | 31.60 | 2.80 | 34.05 | 2.48 | 35.25 | 2.63 |
Figure 4.
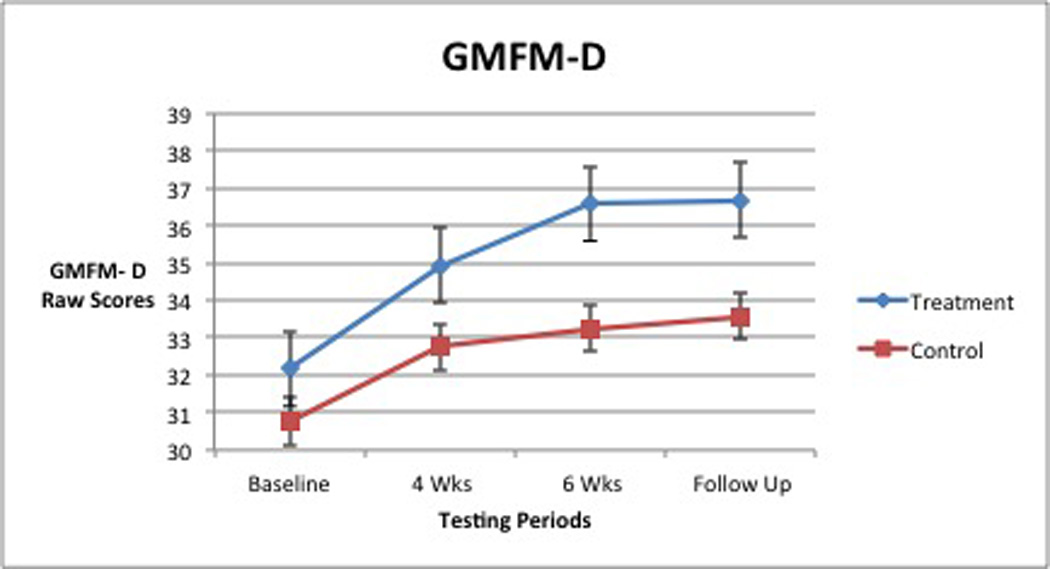
Table 5.
Gross Motor Function Measure- Section E: Means (raw scores) and Standard Deviations
| N | Group | Baseline | 4 Weeks | 6 Weeks | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| 12 | Treatment | 51.18 | 8.68 | 60.73 | 6.39 | 62.27 | 4.67 |
| 8 | Control | 49.78 | 9.60 | 52.11 | 10.01 | 54.67 | 8.91 |
| 20 | Total | 50.55 | 8.89 | 56.85 | 9.01 | 58.85 | 7.75 |
Figure 5.
DISCUSSION
Consistent with the findings of the pilot case series by Lowe et al.,29 subjects in the current study who received BWSTT exhibited statistically significant improvements in self selected average gait velocity. While all subjects exhibited statistically significant gains in their GMFM-E scores, these gains were greater for subjects in the BWSTT group.
The results from gait velocity testing suggest that the BWSTT group made significant improvements. The significant interaction between group and time suggests that assignment to the intervention group did matter over time. As Figure 3 shows, the BWSTT group showed improvement over time while the control group did not. This clinically meaningful change in mean gait velocity (0.20 m/s) allows a child previously walking at the gait velocity expected of a2 year old with TD to exhibit velocities of a 4 to 5 year old child with TD.30
The change in gait velocity supports the idea that task-specific training, such as walking on a treadmill at enforced parameters, translates to improvements in that task.5 The harness allows this practice to occur in a more optimal bio-mechanical alignment. Therefore, training speed and varying surface inclination while supporting a small percentage of body weight could contribute to improved efficiency of gait and improve endurance. Task specific improvements and refinement of existing skills were also found in a recent study examining a 6-week, high speed treadmill (HST) program in high school aged, female soccer players.27 The subjects were divided into 3 groups: a HST groups with BWS, a HST group without BWS, and a control group that did not receive HST training. All groups continued typical soccer practice and game schedules. The 2 HST groups showed statistically significant improvements in 40-yard sprint times when compared to the control group with the BWS group reporting 58% fewer overuse injuries as well. These athletes who are capable of running independently, gained speed and efficiency in an established skill through BWSTT. Thus, BWSTT can be used to enhance existing motor skills, as seen in the current study through gait velocity improvements in children who were ambulatory.
The mean GMFM-D scores of the groups were significantly different irrespective of time. Likewise, a significant improvement in scores was seen regardless of group or testing time. These findings suggest that PT, a constant intervention for both groups, along with time and body system maturation may have assisted in acquisition of and improvement in gross motor skills related to standing (Figure 4). A positive interaction for group and time for GMFM-E scores was also seen. The BWSTT group showed both greater and more rapid improvements (Figure 5), but because both groups improved, the difference between groups was not significant. Upon withdrawal of BWSTT, but with continued PT intervention, achieved gains in GMFM-E scores were maintained at the 6-week follow-up. Our findings are consistent with those seen in children with DS, (a group similar in muscle tone, skill development, gait speed, and onset of ambulation), who exhibited improved scores on pediatric developmental motor tests such as the GMFM,24–25 and improved gait velocity,22 following BWSTT.
Both statistically significant and clinically meaningful improvements were observed during the study. Clinically meaningful improvements in the subjects in this study were noted through clinician observation. The 2 children in the intervention group that walked pitched forward on their toes improved both the velocity of their gait as well as other characteristics, including increased use of heel-toe gait and increased reciprocal arm swing. In addition to improved GMFM scores, the therapists treating subjects in the BWSTT group reported expedited achievement of PT objectives and improved performance of strengthening activities during PT sessions after 3 to 5 weeks of BWSTT. Two subjects from the BWSTT group demonstrated enough improvement to warrant reduction in treatment time. None of the control subjects exhibited changes that warranted such reductions in therapy time. As this study has shown, there are potentially beneficial effects of pediatric PT alone that are observable even within a short amount of time (4 to 6 weeks). Our results show that BWSTT, in addition to appropriate PT treatment, can accelerate acquisition of gross motor skills potentially leading to an earlier discharge from services.
Limitations
Certain limitations were present in this study that could prevent generalization of these findings to the population of children with developmental delay.
This study included an unequal ratio of males to females across a range of ages from 2 to 5 years. A higher percentage of female subjects across both groups would allow better generalization to the population with DD. This sample, however, may be representative of a potential difference related to the diagnosis of DD between the genders and could further be indicative of potential gender specific expectations regarding boys and girls as they relate to recommendation for evaluation and diagnosis of DD. This area of diagnosis of primary DD and referral for services needs more exploration. Additionally, more subjects in the 4- to 5-year-old population would better allow generalization to this age group within the population of DD. Also, the ethnic make-up of the experimental group and the control group was not equally dispersed. This could affect the ability to generalize these findings to children of all ethnicities; however, though not equally dispersed, the experimental group did have representation from each ethnicity.
Though 1 tester was blinded to group allocation, the other was not. The un-blinded tester was necessary as a familiar face to the subjects. The 2 testers, with established interrater reliability, scored the subjects concurrently to provide accountability and to decrease rater bias. Additionally, the un-blinded tester served as a trainer for the BWSTT group. A true-blinded design would be optimal.
Further research in this area is needed to determine if the gains made during this time period continue for greater than 6 weeks after completion of the treatment. Additionally, studies are needed to investigate the effectiveness of BWSTT as a treatment to hasten the onset of ambulation in children with a primary diagnosis of DD. A double-blinded study examining the effects of BWSTT on additional parameters of gross motor development including balance, coordination, and additional gait characteristics is needed. Additionally, a formal ecological assessment utilizing parent and teacher report and measuring participation would be optimal.
CONCLUSIONS
The results of this study suggest that BWSTT is a safe, efficient, and clinically practical intervention for children with DD who are ambulatory. A high intensity protocol of BWSTT in conjunction with typical PT treatment may be used to assist children with DD to increase their self-selected gait velocity and assist them in improving their functional gross motor skills in 6 weeks or less. With continued PT intervention, these improvements may be maintained up to 6 weeks following completion of the BWSTT program.
Acknowledgments
The authors thank the families who generously allowed us to work with their children and Pediatrics Plus Therapy Services, Inc. for their support during this study. Additionally, the authors would like to thank Nick Garza for his assistance during the data collection portion of this study.
Grant Support: NIGMS IDEA Program award P30 GM110702
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest.
References
- 1.Sacker A, Quigley MA, Kelley YJ. Breastfeeding and developmental delay: findings from the millennium cohort study. Pediatrics. 2006;118(3):682–689. doi: 10.1542/peds.2005-3141. [DOI] [PubMed] [Google Scholar]
- 2.Caniato RN, Stich HL, Baune BT. Increasing prevalence of motor impairments in preschool children from 1997–2009: results of the Bavarian pre-school morbidity survey. International Research Journals. 2011;2:1409–1416. [Google Scholar]
- 3.Cantell MH, Smyth MM, Ahonen TP. Clumsiness in adolescence: educational, motor, and social outcomes of motor delay detected at 5 years. Adapted Physical Activity Quarterly. 1994;11:115–129. [Google Scholar]
- 4.Majnemer A. Benefits of Early Intervention for Children With Developmental Disabilities. Seminars In Pediatric Neurology. 1998;5(1):62–69. doi: 10.1016/s1071-9091(98)80020-x. [DOI] [PubMed] [Google Scholar]
- 5.Campbell SK, Palisano RJ, Orlin MN. Physical Therapy for Children. 4th Edition. St. Louis, MO: Elsevier Saunders; 2012. pp. 175–204. [Google Scholar]
- 6.Gallaher MM, Christakis DA, Connell FA. Healthcare use by children diagnosed as having developmental delay. Archives of Pediatric Adolescent Medicine. 2002;156:146–151. doi: 10.1001/archpedi.156.3.246. [DOI] [PubMed] [Google Scholar]
- 7.Bethell C, Peck C, Abrams M, Halfon N, Sareen H, Collins KS. Partnering with parents to promote the healthy development of young children enrolled in Medicaid: results from a survey assessing the quality of preventative and developmental services for young children enrolled in Medicaid in three states. [Accessed September 15, 2012];The Common Wealth Foundation. 2002 www.cmwf.org. [Google Scholar]
- 8.Shumway-Cook A, Woolacott MH. Motor Control: Theory and Practical Applications. 2nd Edition. Baltimore, MD: Lippincott Williams & Wilson; 2001. p. 20. [Google Scholar]
- 9.LaForme Fiss A, Effgen SK. Use of groups in pediatric physical therapy: survey of current practices. Pediatric Physical Therapy. 2007;19:154–159. doi: 10.1097/pep.0b013e31804a57d3. [DOI] [PubMed] [Google Scholar]
- 10.Smania N, Bonetti P, Gandolfi M, Cosentino A, Waldner A, Hesse S, Werner C, Bisoffi G, Geroin C, Munari D. Improved gait after repetitive locomotor training in children with cerebral palsy. American Journal of Physical Medicine and Rehabilitation. 2011;90:137–149. doi: 10.1097/PHM.0b013e318201741e. [DOI] [PubMed] [Google Scholar]
- 11.Hornby TG, Zemon DH, Campbell D. Robotic –assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Physical Therapy. 2005;85:52–66. [PubMed] [Google Scholar]
- 12.Borggraefe I, Meyer-Heim A, Kumar A, et al. Improved gait parameters after robotic-assisted locomotor treadmill therapy in a 6-year-old child with cerebral palsy. Movement Disorders. 2008;23:280–283. doi: 10.1002/mds.21802. [DOI] [PubMed] [Google Scholar]
- 13.Meyer-Heim A, Borggraefe I, Ammann-Reiffer C, et al. Feasibility of robotic-assisted locomotor training in children with central gait impairment. Developmental Medicine and Child Neurology. 2007;49:900–906. doi: 10.1111/j.1469-8749.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- 14.Chan NNC, Smith AW, Lo SK. Efficacy of neuromuscular electrical stimulation in improving ankle kinetics during walking in children with cerebral palsy. Hong Kong Physiotherapy Journal. 2004;22:50–56. [Google Scholar]
- 15.Chernig RJ, Liu CF, Lau TW, et al. Effect of treadmill training with body weight support on gait and gross motor function in children with spastic cerebral palsy. American Journal of Physical Medicine and Rehabilitation. 2007;86:548–555. doi: 10.1097/PHM.0b013e31806dc302. [DOI] [PubMed] [Google Scholar]
- 16.Day JA, Fox EJ, Lowe J, et al. Locomotor training with partial body weight support on a treadmill in a nonambulatory child with spastic tetraplegic cerebral palsy: a case report. Pediatric Physical Therapy. 2004;16:106–113. doi: 10.1097/01.PEP.0000127569.83372.C8. [DOI] [PubMed] [Google Scholar]
- 17.Blundell SW, Shepherd RB, Dean CM, et al. Functional strength training in cerebral palsy: a pilot study of a group circuit training class for children aged 4–8 years. Clinical Rehabilitation. 2003;17:48–57. doi: 10.1191/0269215503cr584oa. [DOI] [PubMed] [Google Scholar]
- 18.Provost B, Dieruf K, Burtner PA, et al. Endurance and gait in children with cerebral palsy after intensive body weight-supported treadmill training. Pediatric Physical Therapy. 2007;19:2–10. doi: 10.1097/01.pep.0000249418.25913.a3. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Looper J, Ulrich DA, Angulo-Barosso RM. Effects of various treadmill interventions on the development of joint kinematics in infants with down syndrome. Physical Therapy. 2010;90(9):1265–1276. doi: 10.2522/ptj.20090281. [DOI] [PubMed] [Google Scholar]
- 20.Begnoche DM, Pitetti KH. Effects of traditional treatment and partial body weight treadmill training on motor skills of children with spastic cerebral palsy: a pilot study. Pediatric Physical Therapy. 2007;19:11–19. doi: 10.1097/01.pep.0000250023.06672.b6. [DOI] [PubMed] [Google Scholar]
- 21.Prosser LA. Locomotor training within an inpatient rehabilitation program after pediatric incomplete spinal cord injury. Physical Therapy. 2007;87:1224–1232. doi: 10.2522/ptj.20060252. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Looper J, Ulrich BD, Ulrich DA, Angulo-Barroso RM. Exploring effects of different treadmill interventions on walking onset and gait patterns in infants with Down Syndrome. Developmental Medicine and Child Neurology. 2007;49:839–845. doi: 10.1111/j.1469-8749.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- 23.Ulrich DA, Ulrich BD, Angulo-Kinzler RM, Yun J. Treadmill training of infants with down syndrome: evidence-based developmental outcomes. Pediatrics. 2001;108(5):1–7. doi: 10.1542/peds.108.5.e84. [DOI] [PubMed] [Google Scholar]
- 24.Ulrich DA, Meghann CL, Tiernan CW, Looper JE, Angulo-Barroso RM. Effects of intensity of treadmill training on developmental outcomes and stepping in infants with Down Syndrome: a randomized trial. Physical Therapy. 2008;88(1):114–122. doi: 10.2522/ptj.20070139. [DOI] [PubMed] [Google Scholar]
- 25.Looper J, Ulrich DA. Effect of treadmill training and supramalleolar orthosis use on motor skill development in infants with down syndrome: a randomized clinical trial. Physical Therapy. 2010;90(3):382–390. doi: 10.2522/ptj.20090021. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Ulrich DA, Looper J, Tiernan CW, Angulo-Barroso RM. Strategy adoption and locomotor adjustment in obstacle clearance of newly walking toddlers with down syndrome after different treadmill interventions. Experimental Brain Research. 2008;186:261–272. doi: 10.1007/s00221-007-1230-7. [DOI] [PubMed] [Google Scholar]
- 27.Johnson AW, Eastman CS, Feland JB, Mitchell UH, Mortensen BB, Eggett D. Effect of high-speed treadmill training with a body weight support system in a sport acceleration program with female soccer players. Journal of Strength and Conditioning Research. 2013;27(6):1496–1502. doi: 10.1519/JSC.0b013e31826cac04. [DOI] [PubMed] [Google Scholar]
- 28.Holm I, Tveter AT, Fredriksen PM, Vollestad N. A normative sample of gait and hopping on one leg parameters in children 7–12 years of age. Gait and Posture. 2009;29:317–321. doi: 10.1016/j.gaitpost.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Lowe L, Ferguson J, Yates C. Treadmill training with partial body weight support in ambulatory children with developmental delay. Combined Sections Meeting of the American Physical Therapy Association. 2012 doi: 10.1097/PEP.0000000000000172. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dusing S, Thorpe D. A normative sample of temporal and spatial gait parameters in children using the GAITRite® electronic walkway. Gait and Posture. 2007;25:135–139. doi: 10.1016/j.gaitpost.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Provost B, Dieruf K, Burtner PA, Phillips JP, Bernitsky-Beddignfield A, Sullivan KJ, Bowen CA, Toser L. Endurance and gait in children with cerebral palsy after intensive body weight-supported treadmill training. Pediatric Physical Therapy. 2007;19:2–10. doi: 10.1097/01.pep.0000249418.25913.a3. [DOI] [PubMed] [Google Scholar]
- 32.Boyd R, Fatone S, Rodda J, Olesch C, Starr R, Cullis E, Gallagher D, Carlin JB, Nattrass GR, Graham K. High- or low-technology measurements of energy expenditure in clinical gait analysis? Developmental Medicine & Child Neurology. 1999;41:676–682. doi: 10.1017/s0012162299001395. [DOI] [PubMed] [Google Scholar]
- 33.Wade DT. Measurement in neurological rehabilitation. Current Opinion In Neurology and Neurosurgery. 1992;5(5):682. [PubMed] [Google Scholar]
- 34.Bjornson KF, Graubert C, McLaughlin JF, et al. Test-retest reliability of the Gross motor Function Measure in children with cerebral palsy. Physical and Occupational Therapy in Pediatrics. 1998;18:25–28. [PubMed] [Google Scholar]
- 35.Bjornson KF, Graubert C, Buford V, Mclaughlin JF. Validity of the Gross Motor Function Measure. Pediatric Physical Therapy. 1998;10:43–47. [PubMed] [Google Scholar]
- 36.Russell DJ, Rosenbaum PL, Avery LM, et al. Gross Motor Function Measure (GMFM-66 and GMFM-88) User’s Manual. London: Mac Keith Press; 2002. [Google Scholar]
- 37.Damiano DL, DeJong SL. A systematic review of the effectiveness of treadmill training and body weight support in pediatric rehabilitation. Journal of Neurologic Physical Therapy. 2009;33:27–44. doi: 10.1097/NPT.0b013e31819800e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neuman DA. Kinesiology of the Musculoskeletal System: Foundations for Physical Rehabilitation. St. Louis, MO: Mosby; 2002. pp. 523–569. [Google Scholar]


