Abstract
The survival rate for childhood cancer is steadily improving, and the current estimate for the prevalence of childhood cancer survivors in the United States is 420,000. With this encouraging trend and the aging of this population, there is an ever-increasing responsibility to identify adult survivors of childhood cancer with adverse health outcomes related to cancer treatment across the span of their lives. To accomplish this, large cohort studies have been developed to follow survivors longitudinally. Compared to siblings, survivors have a higher cumulative incidence of morbidity and mortality, and this gap in incidence only widens with age. One of the most significant late toxicities in survivors is late onset cardiotoxicity, largely due to anthracycline and chest-directed radiation exposure. Survivors also have an increased prevalence of traditional cardiovascular risk factors as they age, which potentiates the risk for major cardiac events. Prevention is essential. Minimizing anthracycline dose exposure in pediatric cancer patients is a primary method of cardioprotection. Dexrazoxane and enalapril have also been studied as primary (pre-exposure) and secondary (post-exposure) cardioprotecant agents, respectively. Additionally, the Children’s Oncology Group has published exposure-driven, risk-based screening guidelines for long-term follow-up, which may be a cost-effective way to identify subclinical cardiac disease before progression to clinical presentation. Ongoing research is needed to determine the most effective diagnostic modality for screening (e.g. echocardiography), and the most effective intervention strategies to improve long-term outcomes.
Keywords: Anthracycline, cardiomyopathy, cardioprotection, intervention, screening
Introduction
The five-year survival rate for childhood cancer has been improving for decades and recently surpassed 80%, according to data from the Surveillance Epidemiology and End Results program within the United States National Cancer Institute.1 These data also report the prevalence of childhood cancer in the United States at over 379,100 as of January 2010. Assuming constant rates of incidence and diagnosis of childhood cancers, the current estimate for the prevalence of childhood cancer survivors in the United States is greater than 420,000.2 As a greater percentage of children diagnosed with cancer survive into adulthood, there is an ever-increasing responsibility to monitor these survivors for late adverse health outcomes related to their cancer treatment.
To determine the prevalence of, and risk factors for these late effects of cancer treatment, large cohort studies have been developed to follow survivors longitudinally. The largest study of this kind in the United States is the Childhood Cancer Survivor Study, a population of more than 14,000 adults treated for a childhood cancer between 1970 and 1986.3, 4 Recent expansion of the cohort now includes 10,000 additional survivors diagnosed between 1987 and 1999. Continued follow-up allows for late, treatment-related cardiotoxicities to be identified in this aging survivor population, in which there is often a long latency period prior to the clinical onset of adverse cardiac health outcomes. Similar long-term cohorts have been developed via national population-based healthcare registries in several countries, however, often without the benefit of direct access to specific treatment exposure data. The data from longitudinal cohort studies like the Childhood Cancer Survivor Study and national registries provides the essential data on the incidence of and risk factors for late onset cardiotoxicity as can be used to identify better methods of screening and intervention.5
The increasing risk of treatment-related cardiac outcomes in aging survivors
Even after children treated for cancer have transitioned into adulthood, the long-term impact of treatment is substantial. Yeh et al. modeled mortality risk among a simulated cohort of five-year survivors, based on a representative Childhood Cancer Survivor Study population, and predicted a loss in life expectancy of greater than ten years, compared to the general population.6 More recent data in this cohort (14,359 survivors analyzed) showed that this shortened lifespan is coupled with an inflated burden of chronic health outcomes. Compared to siblings, survivors in the Childhood Cancer Survivor Study cohort had a higher cumulative incidence of severe, life threatening, or fatal organ-specific health conditions that continued to increase with age.7 In this respect, these data showed that a 24 year-old survivor of childhood cancer has the same cumulative incidence of severe and life threatening health events as a 50 year-old sibling.
The disparity in the incidence of severe, life-threatening, or fatal health conditions between survivors and siblings does not resolve with time, even though the general population ages and acquires health problems; on the contrary, the gap between survivors and siblings actually widens with age.7 Notably, this same study by Armstrong et al. demonstrated that even middle-aged survivors with no previous history of severe, life-threatening, or fatal events move along an escalated trajectory for developing such conditions, compared to siblings. For example, even beyond the age of 35 years survivors had a hazard ratio of 10.9 (95% CI, 4.5 to 26.0) for developing heart failure, compared to siblings.7 This increased rate of development of negative health outcomes among aging survivors is important for defining risk accurately.
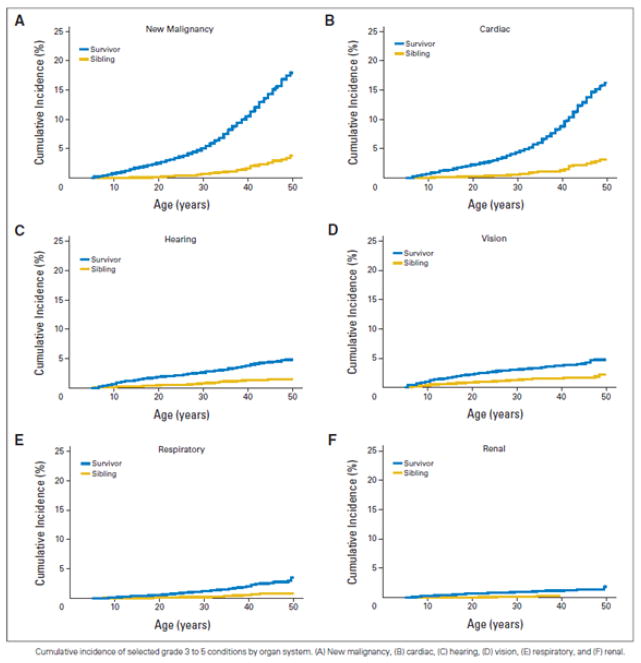
Of all the late toxicities tracked in childhood cancer survivors, major cardiac events and the development of malignant neoplasms are the most common.7 In addition, there continues to be a striking increase in the cumulative incidence of these health outcomes for survivors beyond age 35 years relative to other health outcomes (Figure 1). This finding is consistent with previous Childhood Cancer Survivor Study analyses of late mortality, in which subsequent neoplasms and major cardiac events were the most common cause of death after recurrence of primary cancer (standardized mortality ratios of 15.2 [95% CI, 13.9 to 16.6] and 7.0 [95% CI, 5.9 to 8.2] for subsequent malignancies and cardiac events, respectively, compared to an age and sex-matched comparison population).8
Figure 1. Cumulative incidence of grade 3 to 5 conditions in childhood cancer survivors.
Cumulative incidence of selected grade 3 to 5 conditions by organ system. (A) New malignancy, (B) Cardiac, (C) hearing, (D) vision, (E) respiratory, and (F) renal.
The longitudinal follow-up of survivorship cohorts has shed light on late major cardiac outcomes, specifically those due to cardiotoxicity from anthracycline and chest-directed radiation exposure. Mulrooney et al. demonstrated that, compared to siblings, survivors in the Childhood Cancer Survivor Study were at a significantly increased risk for congestive heart failure (Hazard Ratio [HR] 5.9, 95% Confidence Interval [CI] 3.4 to 9.6), myocardial infarction (HR 5.0, 95% CI 2.3 to 10.4), pericardial disease (HR 6.3, 95% CI 3.3 to 11.9), and valvular abnormalities (HR 4.8, 95% CI 3.0 to 7.6), even after adjustment for demographic characteristics and smoking status (Table 1).9 In this analysis, 14,358 survivors and 3,899 siblings completed baseline questionnaires that addressed cardiac complications. An adjusted multivariable model evaluated the independent effect of various risk factors on each cardiac outcome (Table 2). A statistically significant association was found between radiotherapy dose and the risk for all major cardiac events assessed. Anthracycline exposure > 250 mg/m2 was independently associated with an increased risk for congestive heart failure, pericardial disease, and valvular abnormalities.
Table 1.
Hazard ratios and 95% confidence intervals of reported cardiac conditions in childhood cancer survivors, by diagnosis*
| Congestive heart failure | Myocardial infarction | Pericardial disease | Valvular abnormalities | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| All diagnoses | 5.9 | 3.4–9.6 | <0.001 | 5.0 | 2.3–10.4 | <0.001 | 6.3 | 3.3–11.9 | <0.001 | 4.8 | 3.0–7.6 | <0.001 |
| Leukemia | 4.2 | 2.3–7.4 | <0.001 | 3.3 | 1.2–8.6 | 0.018 | 2.6 | 1.2–5.5 | 0.012 | 2.6 | 1.3–4.9 | 0.004 |
| Brain tumor | 2.2 | 1.0–4.7 | 0.039 | 6.1 | 2.3–16.2 | <0.001 | 2.9 | 1.2–6.8 | 0.014 | 2.2 | 1.0–4.9 | 0.052† |
| Hodgkin lymphoma | 6.8 | 3.9–11.7 | <0.001 | 12.2 | 5.2–28.2 | <0.001 | 10.4 | 5.4–19.9 | <0.001 | 10.5 | 6.1–17.9 | <0.001 |
| Non-Hodgkin lymphoma | 5.1 | 2.6–10.0 | <0.001 | 2.9 | 0.9–9.6 | 0.085 | 4.7 | 2.1–10.7 | <0.001 | 5.4 | 2.7–10.8 | <0.001 |
| Kidney tumour | 4.9 | 2.4–10.0 | <0.001 | ‡ | ‡ | — | 2.4 | 0.8–6.9 | 0.12† | 3.6 | 1.6–8.4 | 0.003 |
| Neuroblastoma | 4.1 | 1.7–9.7 | 0.002 | 11.1 | 3.3–36.9 | <0.001 | 5.1 | 1.9–14.0 | 0.002 | 7.7 | 3.6–16.5 | <0.001 |
| Sarcoma | 4.6 | 2.4–8.8 | <0.001 | 3.6 | 1.2–11.0 | 0.026 | 5.1 | 2.4–11.0 | <0.001 | 2.2 | 1.0–4.9 | 0.050† |
| Bone cancer | 6.5 | 3.6–12.0 | <0.001 | 4.2 | 1.5–11.8 | 0.007 | 4.9 | 2.3–10.5 | <0.001 | 4.4 | 2.3–8.5 | <0.001 |
CI = confidence interval
HR = hazard ratio
Adjusted for gender, race, household income, education, and tobacco use.
Not significant at P=0.05.
Unable to estimate.
Table 2.
Hazard ratios and 95% confidence intervals of reported cardiac conditions in childhood cancer survivors, by treatment
| Congestive heart failure | Myocardial infarction | Pericardial disease | Valvular abnormalities | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Gender | ||||||||||||
| Male | 1.0† | — | — | 1.0† | — | — | 1.0† | — | — | 1.0† | — | — |
| Female | 1.4 | 1.1–1.9 | 0.018 | 0.6 | 0.4–0.9 | 0.014 | 1.3 | 0.9–1.8 | 0.15 | 1.6 | 1.2–2.1 | 0.003 |
| Age at diagnosis | ||||||||||||
| 0–4 years | 3.9 | 2.1–7.3 | <0.001 | 1.0 | 0.4–3.0 | 0.96 | 1.8 | 0.9–3.8 | 0.13 | 2.7 | 1.4–5.3 | 0.004 |
| 5–9 years | 2.3 | 1.3–4.0 | 0.004 | 1.9 | 0.9–4.0 | 0.090 | 1.3 | 0.7–2.5 | 0.44 | 2.5 | 1.5–4.3 | 0.001 |
| 10–14 years | 1.2 | 0.8–1.9 | 0.37 | 0.8 | 0.4–1.5 | 0.49 | 0.8 | 0.5–1.3 | 0.41 | 1.5 | 1.0–2.2 | 0.050 |
| 15–20 years | 1.0† | — | — | 1.0† | — | — | 1.0† | — | — | 1.0† | — | — |
| Treatment era | ||||||||||||
| 1970–4 | 1.0† | — | — | 1.0† | — | — | 1.0† | — | — | 1.0† | — | — |
| 1975–9 | 1.1 | 0.7–1.7 | 0.60 | 2.1 | 1.2–3.8 | 0.010 | 1.5 | 1.0–2.4 | 0.078** | 1.4 | 1.0–2.0 | 0.090** |
| 1980–6 | 1.9 | 1.2–3.0 | 0.008 | 2.2 | 1.1–4.3 | 0.023 | 1.4 | 0.8–2.4 | 0.25 | 1.8 | 1.2–2.9 | 0.008 |
| Average cardiac radiation dose§ | ||||||||||||
| No cardiac radiation | 1.0† | — | — | 1.0† | — | — | 1.0† | — | — | 1.0† | — | — |
| <500 cGy | 0.9 | 0.6–1.4 | 0.75 | 0.7 | 0.4–1.4 | 0.36 | 0.7 | 0.4–1.1 | 0.11 | 0.6 | 0.4–1.0 | 0.063 |
| 500 to <1500 cGy | 1.3 | 0.7–2.5 | 0.43 | 0.6 | 0.1–2.5 | 0.45 | 1.9 | 0.9–3.9 | 0.077 | 1.4 | 0.7–2.9 | 0.40 |
| 1500 to <3500 cGy | 2.2 | 1.4–3.5 | <0.001 | 2.4 | 1.2–4.9 | 0.011 | 2.2 | 1.3–3.9 | 0.005 | 3.3 | 2.1–5.1 | <0.001 |
| ≥3500 cGy | 4.5 | 2.8–7.2 | <0.001 | 3.6 | 1.9–6.9 | <0.001 | 4.8 | 2.8–8.3 | <0.001 | 5.5 | 3.5–8.6 | <0.001 |
| Chemotherapy | ||||||||||||
| Anthracycline v none¶ | ||||||||||||
| <250 mg/m2 | 2.4 | 1.5–3.9 | <0.001 | 1.3 | 0.6–2.8 | 0.50 | 1.6 | 0.9–2.9 | 0.13 | 1.4 | 0.8–2.3 | 0.25 |
| ≥250 mg/m2 | 5.2 | 3.6–7.4 | <0.001 | 1.1 | 0.5–2.1 | 0.87 | 1.8 | 1.1–3.0 | 0.020 | 2.3 | 1.6–3.3 | <0.001 |
| Cisplatin v none | 1.7 | 0.9–2.9 | 0.062 | ‡ | ‡ | — | ‡ | ‡ | — | ‡ | ‡ | — |
| Vincristine v none | ‡ | ‡ | — | 0.7 | 0.4–1.1 | 0.081 | ‡ | ‡ | — | ‡ | ‡ | — |
| Bleomycin v none | ‡ | ‡ | — | ‡ | ‡ | — | 1.6 | 0.9–2.9 | 0.091 | ‡ | ‡ | — |
| Cyclophosphamide v none | ‡ | ‡ | — | ‡ | ‡ | — | 1.5 | 1.0–2.3 | 0.049** | ‡ | ‡ | — |
CI = confidence interval
HR = hazard ratio
Estimates adjusted for all variables in the table as well as race, household income, education, and tobacco use.
Reference group.
Not included in model.
Test for trend (P-value)-all outcomes (<0.001).
Test for trend (P-value)-congestive heart failure (<0.001), myocardial infarction (0.8), pericardial disease (0.03), valvular disease (<0.001).
Not significant at P=0.05
Similarly, an analysis of 1,362 5-year survivors (median age 29.1 years, range 5.2–54.2 years) of childhood cancer in the Netherlands confirmed that a higher anthracycline dose (hazard ratio = 1.7, 95% CI = 1.4 to 2.1, per 100 mg/m2) and a higher cardiac irradiation dose (hazard ratio = 1.8, 95% CI = 1.4 to 2.2, equivalent dose in 2-Gy fractions per 10 Gy) were associated with an increased risk of any cardiac event. Furthermore, the combined effect of anthracyclines and chest radiation was stronger than either exposure alone, demonstrated by a shorter induction period for developing a cardiac event in this population.10
Additional risk of chronic cardiac morbidity conferred by modifiable cardiovascular risk factors
As discussed above, there is substantial evidence to suggest that anthracyclines and chest radiation place survivors of childhood cancer at increased risk for late-onset cardiotoxicity. However, additional factors that should be considered in developing late cardiac outcomes as this population ages into adulthood are traditional, modifiable cardiovascular risk factors. A study by Lipshultz et al. showed that even survivors without a history of anthracycline exposure or cardiac irradiation had a higher proportion of certain cardiovascular risk factors (for example, elevated body mass index, insulin level, and non-high density lipoprotein cholesterol), compared to a sibling population.11
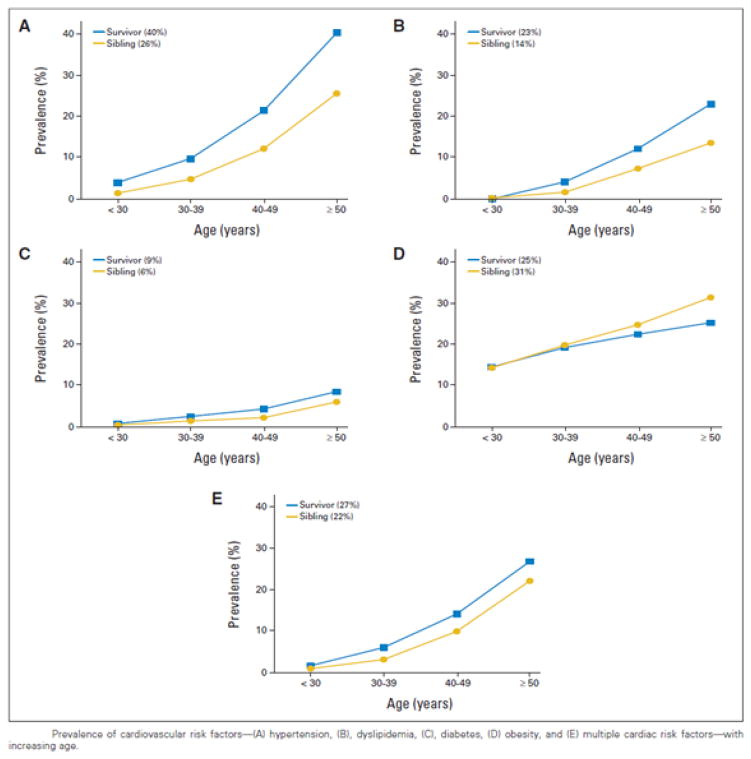
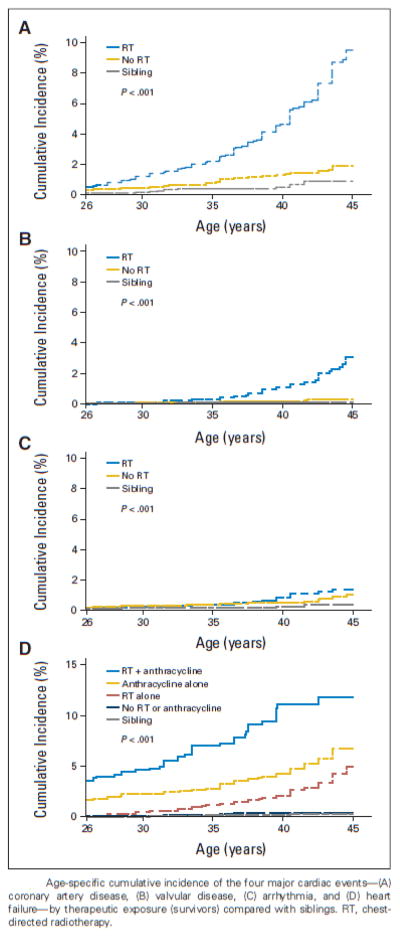
Although more research is required to characterize the causes of the increased propensity for cardiovascular risk factors in survivors, a Childhood Cancer Survivor Study analysis by Armstrong et al. has given strong evidence for the additional risk that they contribute to the development of cardiac disease in treatment-exposed patients. In this study, 10,724 5-year survivors were evaluated longitudinally for the development of traditional cardiovascular risk factors (Figure 2) and subsequent development of major cardiac events (Figure 3).12 The cumulative prevalence of all cardiovascular risk factors increased with age in survivors and was greater in survivors than siblings, with the exception of obesity. The cumulative incidence of all major cardiac events was greater in survivors than in siblings, and cumulative incidence was also associated with exposure to cardiotoxic therapies, consistent with previous findings from Mulrooney et al., now with an additional ten years of follow-up. Importantly, compared to survivors with no anthracycline exposure and no hypertension, the relative risk for congestive heart failure was 8.3 (95% CI, 4.4 to 15.6) among survivors with anthracycline exposure but no hypertension and 34.1 (95% CI, 17.7 to 65.6) among survivors with hypertension but no previous anthracycline exposure. However, the risk among patients with both anthracycline exposure and hypertension was 88.5 (95% CI, 45.2 to 161.8, Table 3). Instead of a simple additive effect, hypertension potentiated the risk of heart failure in patients previously exposed to anthracyclines. A similar potentiation effect was also seen in patients exposed both to chest irradiation and various combinations of cardiovascular risk factors. Lastly, the risk for developing each of the four cardiac events increased with an increasing number of cardiovascular risk factors.12
Figure 2. Prevalence of cardiovascular risk factors in childhood cancer survivors with increasing age.
Prevalence of cardiovascular risk factors—(A) hypertension, (B), dyslipidemia, (C), diabetes, (D) obesity, and (E) multiple cardiac risk factors—with increasing age.
Figure 3. Age-specific cumulative incidence of the four major cardiac events by therapeutic exposure in childhood cancer survivors, compared with siblings.
Age-specific cumulative incidence of the four major cardiac events—(A) coronary artery disease, (B) valvular disease, (C) arrhythmia, and (D) heart failure—by therapeutic exposure (survivors) compared with siblings. RT, chest-directed radiotherapy.
Table 3.
Treatment-specific rate ratios for Grade 3 to 5 Cardiac Events According to Treatment Exposure and Cardiovascular Disease Risk Factor Status
| Chest-Directed Radiotherapy |
Anthracycline Chemotherapy |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coronary Artery Disease |
Heart Failure | Valvular Disease | Arrhythmia | Heart Failure | ||||||||
| Cardiovascular Risk Factor |
Treatment Exposure Present |
Risk Factor Present |
RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI |
| Hypertension † | No | No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| No | Yes | 8.7 | 4.8–15.8 | 12.2 | 7.4–20.2 | 8.1 | 1.6–40.8 | 9.3 | 3.8–23.0 | 34.1 | 17.7–65.6 | |
| Yes | No | 5.3 | 3.2–8.7 | 3.2 | 1.9–5.2 | 10.1 | 2.9–35.6 | 2.9 | 1.2–7.0 | 8.3 | 4.4–15.6 | |
| Yes | Yes | 37.2 | 22.2–62.3 | 55.8 | 35.1–88.7 | 106.8 | 31.1–366.9 | 18.5 | 7.4–46.2 | 85.5 | 45.2–161.8 | |
| Relative excess risk due to interaction** | 24.2 | 11.8–39.7 | 41.4 | 24.1–7.8 | 89.6 | 32.6–5.0 | 7.3 | −4.7–24.8 | 44.5 | 17.2–106.1 | ||
| Dyslipidemia† | No | No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| No | Yes | 5.0 | 2.4–10.3 | 3.5 | 1.7–7.3 | 2.7 | 0.3–23.6 | 0.0 | 0.0–1.3 | 2.3 | 1.1–4.8 | |
| Yes | No | 4.6 | 3.0–6.9 | 4.3 | 3.0–6.1 | 12.3 | 4.7–32.1 | 1.8 | 0.9–3.6 | 4.3 | 3.0–6.2 | |
| Yes | Yes | 25.0 | 15.2–41.3 | 7.0 | 3.5–13.8 | 33.8 | 11.3–101.0 | 6.9 | 2.8–17.2 | 8.9 | 4.6–17.4 | |
| Relative excess risk due to interaction** | 16.4 | 7.9–29.8 | 0.1 | −4.8–5.4 | 19.8 | 3.0–109.8 | 6.1 | 1.6–14.5 | 3.3 | −2.2–10.6 | ||
| Diabetes† | No | No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| No | Yes | 5.2 | 2.2–12.5 | 0.6 | 0.1–4.7 | 6.4 | 0.7–55.3 | 1.7 | 0.2–12.8 | 2.6 | 1.0–7.4 | |
| Yes | No | 5.1 | 3.5–7.5 | 3.6 | 2.6–5.1 | 14.4 | 5.6–37.0 | 2.4 | 1.2–4.6 | 4.2 | 3.0–6.1 | |
| Yes | Yes | 20.1 | 10.6–38.4 | 13.5 | 6.9–26.6 | 36.4 | 9.5–138.8 | 9.4 | 2.7–32.4 | 10.8 | 4.9–23.9 | |
| Relative excess risk due to interaction** | 10.8 | 0.0–28.6 | 10.2 | 2.9–22.8 | 16.6 | −19.3–123.0 | 6.3 | −3.8–21.4 | 4.9 | −2.9–17.2 | ||
| Obesity† | No | No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| No | Yes | 1.4 | 0.7–2.6 | 1.4 | 0.8–2.5 | 0.8 | 0.1–6.6 | 0.7 | 0.2–2.5 | 2.0 | 1.1–3.6 | |
| Yes | No | 4.6 | 3.1–7.0 | 4.1 | 2.8–5.9 | 10.4 | 4.0–27.3 | 2.0 | 1.0–4.0 | 5.0 | 3.3–7.4 | |
| Yes | Yes | 9.3 | 5.6–15.5 | 5.7 | 3.3–10.1 | 23.8 | 8.3–68.3 | 5.5 | 2.2–13.5 | 5.4 | 3.0–9.8 | |
| Relative excess risk due to interaction** | 4.3 | 0.9–8.7 | 1.3 | −1.7–4.6 | 13.6 | 0.2–66.4 | 3.8 | 0.1–8.6 | −0.6 | −3.9–2.6 | ||
| Multiple (≥2) risk factors including Hypertension† | No | No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| No | Yes | 7.9 | 4.1–15.1 | 5.2 | 2.7–9.9 | 7.4 | 1.3–41.1 | 1.5 | 0.3–6.7 | 8.7 | 4.8–15.5 | |
| Yes | No | 5.0 | 3.3–7.7 | 3.7 | 2.6–5.4 | 13.4 | 4.6–38.9 | 2.0 | 1.0–4.0 | 4.9 | 3.3–7.3 | |
| Yes | Yes | 39.8 | 23.9–66.3 | 26.3 | 15.7–43.9 | 80.7 | 25.7–253.8 | 11.1 | 4.4–27.7 | 24.5 | 13.7–43.6 | |
| Relative excess risk due to interaction** | 27.9 | 14.6–51.0 | 18.3 | 7.6–37.4 | 60.9 | 18.0–487.0 | 8.6 | 1.7–21.7 | 11.9 | 0.3–29.6 | ||
| Multiple (≥2) risk factors excluding Hypertension† | No | No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| No | Yes | 0.0 | 0.0–2.5 | 0.0 | 0.0–2.6 | 0.0 | 0.0–22.6 | 0.0 | 0.0–7.5 | 0.0 | 0.0–2.3 | |
| Yes | No | 4.9 | 3.4–7.0 | 4.0 | 2.9–5.6 | 13.0 | 5.4–31.1 | 2.6 | 1.4–4.8 | 4.3 | 3.0–6.1 | |
| Yes | Yes | 3.0 | 0.4–21.6 | 0.0 | 0.0–4.6 | 0.0 | 0.0–36.6 | 0.0 | 0.0–11.6 | 0.0 | 0.0–5.2 | |
| Relative excess risk due to interaction** | −0.9 | −5.4–5.9 | −3.0 | −4.5–2.0 | −12.0 | −45.4–5.7 | −1.6 | −3.5–0.5 | −3.3 | −5.1–2.1 | ||
CI = confidence interval
RR = rate ratio
All models adjusted for age, household income, and education, which were all time dependent variables, and sex, race, smoking, chest-directed radiotherapy and anthracycline exposure.
Relative excess risk due to interaction: A term statistically significantly greater than zero indicates that interaction between treatment and cardiovascular risk factor is more than additive. Bold indicates statistically significant relative excess risk due to interaction
The results were based on a single model that included the entire study population.
Similarly, Armenian et al. demonstrated the “two-hit” phenomenon identified in the study by Armstrong et al. 12 (cardiotoxic therapeutic exposure, followed by the development of traditional cardiovascular risk factors as survivors age) in an analysis of 1,885 patients treated with hematopoietic cell transplantation for a hematologic malignancy.13 A higher cumulative incidence of cardiovascular disease was associated with a higher number of cardiovascular risk factors: no factors, 4.7% (± 1.0%); one factor, 7.0% (±1.1%); and multiple factors, 11.2% (±1.8%). The cumulative incidence of cardiovascular disease was especially high in patients with multiple cardiovascular risk factors in conjunction with cardiotoxic exposure: 15.0% (±2.5%), v. 2.6% (±0.1%) in patients with <2 factors and no cardiotoxic exposure. The risk factors included were those in the previous study, except body mass index; the cardiovascular diseases evaluated were myocardial infarction, symptomatic coronary artery stenosis, stroke, and congestive heart failure.13
While these data clarify the risk for a population exposed to cardiotoxic therapies and traditional cardiovascular risk factors, they do not provide patient-specific risk (i.e. risk calculator) that could potentially be more useful in a clinical setting. Using this same population from the Childhood Cancer Survival Study, Chow et al. developed clinical models to predict the risk of heart failure in individual survivors soon after completion of therapy up to age 40 years. Age, chemotherapy exposures, and radiation exposures were explored to find the most influential predictors for developing heart failure. These risk factors were converted to integer risk scores and summed into three risk groups (low, moderate, high) such that each group was distinct from the sibling group and the immediate lower survivor group in its risk for developing heart failure. Validation of these-risk based scores among three external survivor cohorts confirmed similar cumulative incidences of heart failure between equivalent risk groups in different cohorts. Even the simplest model (a yes/no score for each exposure) segregated survivors into distinct risk groups. Information on a survivor’s burden of risk factors may be easily input to determine the individual risk for developing heart failure using the CCSS-CHF Risk Calculator that can be found at ____@stjude.org.
The interaction between modifiable cardiovascular risk factors and cardiotoxic therapy is particularly concerning because of the prevalence of these risk factors within the childhood cancer survivorship. In terms of population-level treatment exposure, anthracyclines are used in over 50% of childhood cancer treatments,14 and 24.2% of the current Childhood Cancer Survivor Study population received some form of chest-directed radiation during treatment.12 The high frequency of cardiovascular risk factors and cardiotoxic treatment exposure within the survivor population makes the compounded risk for heart failure and other cardiac events a phenomenon of utmost relevance.
Interventions to reduce risk of treatment-related cardiotoxicity
The interplay between cardiotoxic therapy, traditional cardiovascular risk factors, and cardiac events has changed the conversation about the rising field of cardio-oncology14 to include a broader discussion of cardiovascular oncology.15 The importance of the potentiation of cardiovascular risk with aging and accrual of traditional cardiovascular risk factors makes consideration of prevention essential. Moving forward, late cardiotoxicity in survivors may be prevented in two ways: at the time of treatment (primary prevention) and after exposure but before diagnosis of a major cardiac event (secondary prevention) in aging survivors.
Primary Prevention
Since it is well-established that anthracycline exposure confers a dose-dependent risk of cardiomyopathy on cancer survivors,16 minimizing the cumulative cardiotoxic dose, yet maintaining treatment efficacy, is a primary method of cardioprotection.17, 18 Of note, however, even low anthracycline doses (45 mg/m2) have been associated with significant cardiac abnormalities, so it is not clear that there is any safe dose of anthracyclines.16 A second form of primary cardioprotection may be conferred by the administration of dexrazoxane with every anthracycline dose. This concept was evaluated in a randomized control study of 205 children; administering dexrazoxane with anthracycline doses prevented statistically significant cardiac dysfunction at five-year follow-up, vs. anthracycline exposure alone, particularly in female patients.19
Secondary Prevention
Enalapril has been proposed as a possible cardioprotective agent in survivors who have already received anthracycline therapy. The first randomized control study evaluating its efficacy found that enalapril, with long-term follow-up, did not preserve or improve exercise performance in survivors exposed to anthracyclines compared to survivors who did not receive enalapril.20 However, treatment with enalapril did result in an early decrease in left ventricular end systolic wall stress.20 Additionally, a retrospective study by Lipshultz et al. found that the beneficial effects of enalapril on cardiac function were transient and may be largely related to changes in blood pressure rather than the primary defect, reduced ventricular wall thickness.21
One of the most effective forms of prevention, or at least delay in cardiotoxicity in long-term survivors may be routine risk-based screening. Unfortunately, survivors treated in a community care setting are less likely to receive the appropriate targeted screening (e.g. echocardiogram for cardiotoxicity) than survivors cared for in a cancer center facility.22 The Children’s Oncology Group, an international collaborative group for the treatment of childhood cancer, has released guidelines for long-term follow-up to assist all health care providers. Landier et al. employed these exposure-driven, risk-based screening guidelines and found that screening for asymptomatic cardiac conditions resulted in modest identification of asymptomatic survivors within a survivor cohort: Of all evaluable echocardiogram screenings performed (n=533), 6% were positive for left ventricular systolic dysfunction. This screening test was identified as intermediate yield (screening yield ≥ 1% to < 10%) for this study.23
Screening for cardiac dysfunction also yielded a significant level of new cardiac outcomes in a large population of at-risk survivors in a study by Hudson et al.24 Screening was performed upon enrollment into the St. Jude Lifetime Cohort Study (St. Jude LIFE), a lifetime cohort of adult survivors previously treated for cancer at St. Jude Children’s Research Hospital. Risk-based screening was employed as defined by Children’s Oncology Group guidelines. Of the 1,214 survivors within the St. Jude LIFE cohort exposed to anthracyclines, anthraquinones, and/or cardiac irradiation, the pre-St. Jude LIFE prevalence of diagnosed cardiomyopathy was 2.6 % (95% CI, 1.8% to 3.7%). After this same population was screened with echocardiogram, the total prevalence of cardiomyopathy increased to 6.2% (95% CI, 5.0% to 7.8%). In addition, the estimated cumulative prevalence of cardiomyopathy at 50 years of age increased from 10% to 21% after echocardiography screening. This identified that in this aging population a relatively large proportion of subclinical heart disease in this at-risk population had been previously undiagnosed, highlighting the importance of screening for late health outcomes known to be associated with specific cancer treatment modalities.24
In a subsequent study implementing the Children’s Oncology Group guidelines, Wong et al. evaluated financial costs and benefit of screening. In this study, computer-modeled populations were designed to mirror the Childhood Cancer Survivor Study cohort in demographic and treatment exposure characteristics. The study found that applying the Children’s Oncology Group screening guidelines for asymptomatic left ventricular dysfunction to this study population, followed by appropriate treatment, would increase life expectancy by 6.1 months and quality-adjusted life years by 1.6 months, compared to a modeled population of patients not screened for this cardiomyopathy.25 Likewise, the cumulative incidence of heart failure at 30 years after diagnosis was predicted to be reduced by 18% with screening and treatment.25 Although echocardiographic screening for asymptomatic left ventricular dysfunction according to the Children’s Oncology Group guidelines was found to be cost effective, the authors also explored more efficient screening models without compromising health benefits obtained by the current screening recommendations.25 Specifically, the frequency of screening was manipulated for each risk profile (a function of treatment exposure and age at diagnosis) to maximize quality-adjusted life years relative to the cost of screening and treatment. One permutation of screening frequencies was shown to provide 80% of the health benefits of the published guidelines, but at nearly half the incremental cost-effectiveness ratio. Therefore, it may be appropriate to continue screening survivors for cardiac dysfunction according to exposure-driven risk stratification while investigating screening strategies that further optimize the complex balance between prevention of disease and financial burden.25–27
Echocardiographically measurable changes in myocardial deformation (strain imaging) may precede a reduction in left ventricular ejection fraction.28 Several recent studies have demonstrated the ability of modern echocardiographic technology to screen for these more subtle manifestations of long-term cardiotoxic damage before at-risk patients present clinically with heart failure. A recent meta-analysis of all myocardial deformation parameters used to predict subclinical cardiomyopathy found that the best measure of subclinical systolic dysfunction via two-dimensional speckle tracking echocardiography was global longitudinal strain.28 The utility of these subclinical parameters may be their ability to predict progression to cardiotoxicity and heart failure as has been demonstrated in adults with cancer treated with anthracycline therapy.28 However, evaluation of myocardial deformation in a large, aging population of survivors of childhood cancer has not occurred. Using more traditional echocardiography, two-dimensional echocardiography was shown to have limited diagnostic power compared to three-dimensional echocardiography and cardiac magnetic resonance imaging.29 However, further research will be required to develop an optimal battery of tests, employing both echocardiography and other modalities, that successfully monitors long-term cardiac function in survivors with exposure risk.30
In addition to understanding the best screening tests for each individual survivor, there is a growing body of research about the most effective methods (e.g. motivational interviewing) for improving screening rates. In a recent intervention trial within the Childhood Cancer Survivor Study cohort, survivors who were at risk for cardiotoxicity but had not undergone recent screening for cardiomyopathy were randomized to two intervention arms. The standard care group (n=234) received a personalized survivorship care plan with recommendations for follow-up, and the experimental group (n=238) received this care plan, as well as two telephone counseling sessions and follow-up letters from an advanced practice nurse at one and three weeks after receiving the care plan. Compared to the care plan group, the survivors who received information from advanced practice nurses were more likely to have completed screening for cardiomyopathy at one-year follow-up (relative risk, 2.31; 95% CI 1.74 to 3.07, p<0.0001). A systematic review of several studies, all of which outlined models of long-term follow-up, found that few conclusions could yet be drawn about the most effective models of intervention.31 The lack of clear answers may drive the need for continued research in this area.31
A second area of intervention is lifestyle modification to address traditional cardiovascular risk factors that increase risk for cardiac events. The metabolic syndrome, a known risk factor for cardiovascular disease,32 has also been associated with subclinical left ventricular myocardial dysfunction in both men and women.33 An increased risk for developing components of the metabolic syndrome has been frequently reported in childhood cancer survivors.34 A recent study verified this relationship, finding that the prevalence of the metabolic syndrome and its components in the analyzed sample (n=1598) was elevated compared to younger survivor cohorts but similar to a much older general adult population [Smith WA et al., Cancer, in press]. In this same study, survivors who did not meet the majority (≥4/7) of the healthy lifestyle habits prescribed by the World Cancer Research Fund and American Institute for Cancer Research were more likely to have the metabolic syndrome than those who did, with relative risks of 2.2 (95% CI, 1.6–3.0) and 2.4 (95% CI, 1.7–3.3) in men and women, respectively.
In light of recent research, lifestyle modification of diet and exercise may significantly improve cardiovascular health and prevent the metabolic syndrome from progressing to adverse cardiac outcomes. A cross-sectional study of 117 acute lymphoblastic leukemia survivors, 72% of which received anthracyclines, found that greater adherence to a Mediterranean diet pattern was associated with more favorable measures of waist circumference and blood pressure, as well as a lower likelihood of having the metabolic syndrome.35 Further research, including assessment of the nutritional intake of survivors, is needed to reach more definitive conclusions.35, 36 Similarly, clinical trials are needed to verify the safety and efficacy of exercise regimens in anthracycline-exposed survivors, and survivors in general.37–39 In a preliminary study of five childhood cancer survivors with anthracycline exposure, a twelve-week exercise prescription (86% compliance) improved body weight, body fat, peak oxygen consumption, and ejection fraction.37 One form of motivation, internet-based intervention, has been shown to encourage increased levels of physical activity in young adult survivors.40 It is important to investigate potential hurdles to lifestyle modification (e.g. elevated rates of fatigue in the survivor population) to optimize intervention strategies.41
In summary, risk-based screening and lifestyle modification may identify and potentially reduce the risk for many of the cardiovascular late effects identified in childhood cancer survivors with cardiotoxic exposure. These treatments place survivors at a higher risk for cardiac events and cardiovascular risk factors, compared to the general population. These risks remain elevated, and often increase further, with age. In addition, cardiotoxicity and cardiovascular risk factors confer compounded risk for developing chronic cardiac outcomes. Appropriate screening may detect subclinical disease before progression. Even better, modification of diet and exercise may be able to reverse or prevent elements of the metabolic syndrome, and ultimately cardiac dysfunction, enabling survivors who have already defeated cancer to control cardiotoxic risks and maintain healthy lives into middle and late adulthood.
Acknowledgments
Funding: This work was supported by the National Cancer Institute (CA55727, G.T. Armstrong, Principal Investigator, Childhood Cancer Survivor Study). Support to St. Jude Children’s Research Hospital also provided by the Cancer Center Support (CORE) grant (CA21765, R. Gilbertson, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2011. National Cancer Institute; Bethesda, MD: 2014. http://seer.cancer.gov/csr/1975_2011/ [Google Scholar]
- 2.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–18. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–27. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steele JR, Wall M, Salkowski N, et al. Predictors of risk-based medical follow-up: a report from the Childhood Cancer Survivor Study. J Cancer Surviv. 2013;7:379–91. doi: 10.1007/s11764-013-0280-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeh JM, Nekhlyudov L, Goldie SJ, Mertens AC, Diller L. A model-based estimate of cumulative excess mortality in survivors of childhood cancer. Ann Intern Med. 2010;152:409–17. W131–8. doi: 10.1059/0003-4819-152-7-201004060-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–27. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–38. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Der Pal HJ, Van Dalen EC, Van Delden E, et al. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012;30:1429–37. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- 11.Lipshultz SE, Landy DC, Lopez-Mitnik G, et al. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol. 2012;30:1050–7. doi: 10.1200/JCO.2010.33.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–80. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armenian SH, Sun CL, Vase T, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120:4505–12. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102:14–25. doi: 10.1093/jnci/djp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prisco D, D’elios MM, Cenci C, Ciucciarelli L, Tamburini C. Cardiovascular oncology: a new discipline inside internal medicine? Intern Emerg Med. 2014;9:359–64. doi: 10.1007/s11739-014-1064-9. [DOI] [PubMed] [Google Scholar]
- 16.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–36. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 17.Harake D, Franco VI, Henkel JM, Miller TL, Lipshultz SE. Cardiotoxicity in childhood cancer survivors: strategies for prevention and management. Future Cardiol. 2012;8:647–70. doi: 10.2217/fca.12.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillenburg RF, Nathan P, Mertens L. Educational paper: decreasing the burden of cardiovascular disease in childhood cancer survivors: an update for the pediatrician. Eur J Pediatr. 2013;172:1149–60. doi: 10.1007/s00431-013-1931-9. [DOI] [PubMed] [Google Scholar]
- 19.Lipshultz SE, Scully RE, Lipsitz SR, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010;11:950–61. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silber JH, Cnaan A, Clark BJ, et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol. 2004;22:820–8. doi: 10.1200/JCO.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol. 2002;20:4517–22. doi: 10.1200/JCO.2002.12.102. [DOI] [PubMed] [Google Scholar]
- 22.Nathan PC, Ford JS, Henderson TO, et al. Health behaviors, medical care, and interventions to promote healthy living in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2363–73. doi: 10.1200/JCO.2008.21.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landier W, Armenian SH, Lee J, et al. Yield of Screening for Long-Term Complications Using the Children’s Oncology Group Long-Term Follow-Up Guidelines. J Clin Oncol. 2012;30:4401–8. doi: 10.1200/JCO.2012.43.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–81. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong FL, Bhatia S, Landier W, et al. Cost-Effectiveness of the Children’s Oncology Group Long-Term Follow-up Screening Guidelines for Childhood Cancer Survivors at Risk for Treatment-Related Heart Failure. Ann Intern Med. 2014;160:672–83. doi: 10.7326/M13-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh JM, Nohria A, Diller L. Routine echocardiography screening for asymptomatic left ventricular dysfunction in childhood cancer survivors: a model-based estimation of the clinical and economic effects. Ann Intern Med. 2014;160:661–71. doi: 10.7326/M13-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steingart RM, Liu JE, Oeffinger KC. Cost-effectiveness of screening for asymptomatic left ventricular dysfunction in childhood cancer survivors. Ann Intern Med. 2014;160:731–2. doi: 10.7326/M14-0823. [DOI] [PubMed] [Google Scholar]
- 28.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of Myocardial Strain Imaging by Echocardiography for the Early Detection of Cardiotoxicity in Patients During and After Cancer Chemotherapy: A Systematic Review. J Am Coll Cardiol. 2014;63:2751–68. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong GT, Plana JC, Zhang N, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–84. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz RG, Jain D, Storozynsky E. Traditional and novel methods to assess and prevent chemotherapy-related cardiac dysfunction noninvasively. J Nucl Cardiol. 2013;20:443–64. doi: 10.1007/s12350-013-9707-1. [DOI] [PubMed] [Google Scholar]
- 31.Heirs M, Suekarran S, Slack R, et al. A systematic review of models of care for the follow-up of childhood cancer survivors. Pediatr Blood Cancer. 2013;60:351–6. doi: 10.1002/pbc.24253. [DOI] [PubMed] [Google Scholar]
- 32.Van Waas M, Neggers SJ, Uitterlinden AG, et al. Treatment factors rather than genetic variation determine metabolic syndrome in childhood cancer survivors. Eur J Cancer. 2013;49:668–75. doi: 10.1016/j.ejca.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Voulgari C, Moyssakis I, Papazafiropoulou A, et al. The impact of metabolic syndrome on left ventricular myocardial performance. Diabetes Metab Res Rev. 2010;26:121–7. doi: 10.1002/dmrr.1063. [DOI] [PubMed] [Google Scholar]
- 34.Van Waas M, Neggers SJ, Van Der Lelij AJ, Pieters R, Van Den Heuvel-Eibrink MM. The metabolic syndrome in adult survivors of childhood cancer, a review. J Pediatr Hematol Oncol. 2010;32:171–9. doi: 10.1097/MPH.0b013e3181d419c3. [DOI] [PubMed] [Google Scholar]
- 35.Tonorezos ES, Robien K, Eshelman-Kent D, et al. Contribution of diet and physical activity to metabolic parameters among survivors of childhood leukemia. Cancer Causes Control. 2013;24:313–21. doi: 10.1007/s10552-012-0116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen J, Wakefield CE, Fleming CA, Gawthorne R, Tapsell LC, Cohn RJ. Dietary intake after treatment in child cancer survivors. Pediatr Blood Cancer. 2012;58:752–7. doi: 10.1002/pbc.23280. [DOI] [PubMed] [Google Scholar]
- 37.Smith WA, Ness KK, Joshi V, Hudson MM, Robison LL, Green DM. Exercise training in childhood cancer survivors with subclinical cardiomyopathy who were treated with anthracyclines. Pediatr Blood Cancer. 2013 doi: 10.1002/pbc.24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang TT, Ness KK. Exercise interventions in children with cancer: a review. Int J Pediatr. 2011;2011:461512. doi: 10.1155/2011/461512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braam KI, Van Der Torre P, Takken T, Veening MA, Van Dulmen-Den Broeder E, Kaspers GJ. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2013;4:CD008796. doi: 10.1002/14651858.CD008796.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Rabin C, Dunsiger S, Ness KK, Marcus BH. Internet-Based Physical Activity Intervention Targeting Young Adult Cancer Survivors. J Adolesc Young Adult Oncol. 2011;1:188–94. doi: 10.1089/jayao.2011.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badr H, Chandra J, Paxton RJ, et al. Health-related quality of life, lifestyle behaviors, and intervention preferences of survivors of childhood cancer. J Cancer Surviv. 2013;7:523–34. doi: 10.1007/s11764-013-0289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]