Abstract
Amino acid deprivation promotes the inhibition of the mammalian target of rapamycin (mTOR) kinase and activation of the general control nonrepressed-2 (GCN2) kinase. Signaling pathways downstream of both kinases have been thought to independently induce autophagy. Here we demonstrate that these two amino acid sensing systems are linked. We show that inhibition of mTORC1 leads to activation of GCN2 and phosphorylation of the eukaryotic initiation factor 2α (eIF2α) in a mechanism dependent on the PP6C phosphatase. mTORC1 inhibition does not lead to autophagy in the absence of PP6C activity, GCN2, or eIF2α phosphorylation. PP6C mutations commonly found in melanoma blunt the formation of a PP6C-GCN2 complex and are rapidly degraded, but paradoxically stabilize the wild-type PP6C allele and increase eIF2α phosphorylation. These PP6C mutations associate with increased autophagy in vitro and in vivo. Thus we have determined that phosphorylation of eIF2α by mTORC1 inhibition is necessary for autophagy and describe a novel role for PP6C in this system, which may be dysregulated in melanoma.
Introduction
Intracellular amino acid deprivation results in the activation of cellular adaptive mechanisms. The two best established responses to amino acid deprivation, inhibition of the mammalian target of rapamycin (mTOR) kinase and phosphorylation of eukaryotic initiation factor 2α (eIF2α), are known to play key roles in the induction of macroautophagy, hereafter referred to as autophagy. During autophagy, organelles and misfolded/aggregated cytoplasmic proteins are targeted for lysosomal degradation, thus ridding the cells of potentially deleterious proteins and generating free amino acids in an attempt to maintain metabolic homeostasis. Because autophagy can provide a pro-survival mechanism for cells growing in a hostile tumor microenvironment, elucidating the pathways which regulate autophagy, and specifically identifying druggable targets such as kinases and phosphatases, could lead to novel therapies for cancer and other diseases in which cellular stress and autophagy play important roles.
mTOR is a serine/threonine protein kinase that exists as two complexes: mTORC1 which contains raptor, and mTORC2 which contains rictor (reviewed in (1)). mTORC1 inhibition by amino acid deprivation or treatment with the highly specific bacterial product rapamycin attenuates protein synthesis and suppresses cell growth via dephosphorylation of the eukaryotic initiation factor 4E binding protein 1 (4E-BP1). In contrast, the mechanism by which mTOR inhibition induces autophagy in mammalian cells is not entirely delineated. However, recent data suggest that, analogous to yeast, the suppression of mTORC1 activity permits ULK1 to form an autophagy initiation complex with ATG13 (2). The phosphorylation of eIF2α, which also occurs with amino acid deprivation as well as with endoplasmic reticulum (ER) and other cellular stresses, similarly attenuates global protein synthesis (reviewed in (3)). However, eIF2α phosphorylation also paradoxically increases the translation of select mRNAs, including the transcription factor ATF-4 which transactivates the autophagy genes LC3B and ATG5 (4-7).
Remarkably, amino acid deprivation does not induce autophagy in the absence of eIF2α phosphorylation, despite the inhibition of mTOR (8, 9). This observation indicates that the inhibition of mTOR with amino acid deprivation is not sufficient to induce autophagy, and suggests that there could be coordination between these two amino acid sensing mechanisms. Indeed, beyond their independent roles in attenuating general protein synthesis, both TORC1 inhibition and eIF2α phosphorylation upregulate 4E-BP1 expression (10), and co-coordinately regulate the translation of select mRNAs (11). While crosstalk of the mTOR and eIF2α signaling pathways has not been demonstrated in mammalian cells, in S. cerevisiae TORC1 inhibition leads to the activation of the Suppressor of Initiation of Transcription 4 (SIT4) phosphatase, which dephosphorylates and activates the general control nonrepressed 2 (GCN2) eIF2α kinase (12). GCN2 is also activated by TORC1 inhibition in S. pombe (13). The mammalian homologue for SIT4 is the serine/threonine protein phosphatase 6 catalytic subunit (PP6C), which in contrast to other members of the mammalian PP2A-like phosphatases, rescues SIT4 deletion in S. cerevisiae and S. pombe (14, 15). Consistent with its specificity, PP6C, but not PP2A or PP4, can individually associate with three regulatory proteins, PP6R1, PP6R2, and PP6R3, which bridge the phosphatase to substrate proteins (16). Interestingly, two independent groups recently identified driver PP6C mutations in approximately 10% of all melanomas, although the biological consequences of these mutations are unknown (17, 18).
Despite the similarities between SIT4 and PP6C, the role of PP6C in mTORC induced eIF2α phosphorylation has not been established. We thus investigated whether mTOR inhibition contributes to eIF2α phosphorylation, and if this potential relationship affects the induction of autophagy. We specifically addressed whether PP6C could play a role in linking these two amino acid sensing mechanisms, and whether this signaling pathway is affected by the recently identified PP6C mutations found in melanoma.
Results
mTOR inhibition leads to eIF2α phosphorylation
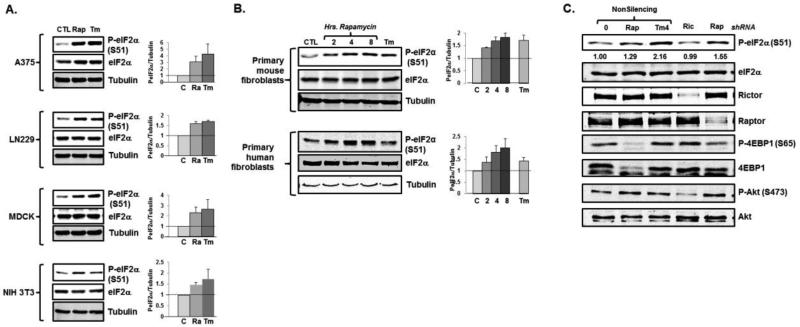
To investigate the relationship between eIF2α phosphorylation and mTOR inhibition, we first treated a variety of transformed cell lines with the mTORC1 inhibitor rapamycin. We noted a robust phosphorylation of eIF2α with rapamycin treatment, similar to that seen when cells were treated with the N-linked glycosylation inhibitor tunicamycin, a well-described inducer of ER stress and activator of the eIF2α kinase PERK (Fig 1A). Rapamycin treatment also led to a time-dependent increase in eIF2α phosphorylation in primary mouse and human fibroblasts (Fig 1B). As we and others have previously noted, the extent and kinetics of tunicamycin-induced eIF2α phosphorylation varied amongst cell lines (19), as did rapamycin-induced eIF2α phosphorylation. However, in most cases the extent of rapamycin treated was similar to the extent of eIF2α phosphorylation noted with ER stress. To confirm that mTORC1 inhibition was responsible for the induction of eIF2α phosphorylation, we depleted the osteosarcoma cell line, U2OS, of a necessary component of TORC1 (raptor) or, as a control, TORC2 (rictor) activity and assessed eIF2α phosphorylation. As expected, depletion of rictor had a profound effect on AKT phosphorylation, while both raptor depletion and treatment with the mTORC1 inhibitor rapamycin decreased 4EBP1 phosphorylation (Fig 1C). Raptor depletion and rapamycin treatment also led to phosphorylation of eIF2α, whereas rictor depletion had no effect (Fig 1C). Together, these findings suggest that the phosphorylation of eIF2α is a general response to mTORC1 inhibition.
Fig 1.
mTORC1 inhibition induces eIF2α phosphorylation. A. Cell lines were treated with 100nM rapamycin (Rap) or tunicamycin (Tm) for six hours and eIF2α phosphorylation was assessed by immunoblot. Representative blots are displayed and quantitations reflect average and standard error (N=3 biological replicates). B. Primary MEFs and BJhTERT cells were treated with rapamycin for the indicated times or tunicamycin and eIF2α phosphorylation was assessed. Quantitation reflects average and standard error (N=3 biological replicates). C. U2OS cells were treated with rapamycin, tunicamycin, or depleted of rictor or raptor, and eIF2α phosphorylation was assessed. Representative blots are displayed and quantitation reflects average values (N=2 biological replicates).
mTORC1 regulated autophagy depends on eIF2α phosphorylation
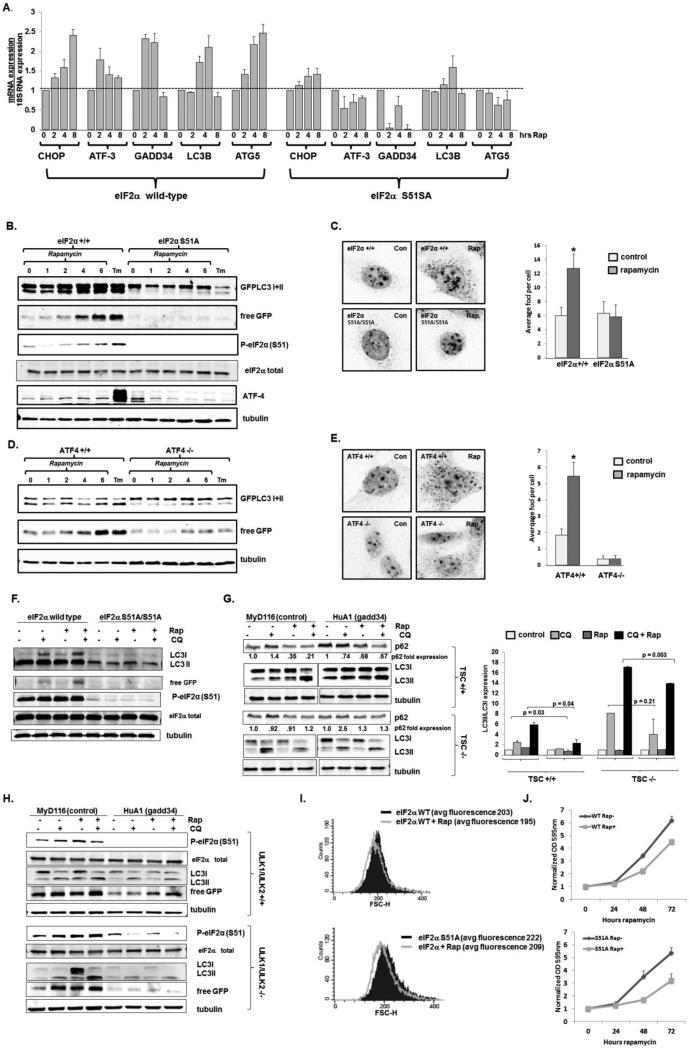
To determine the biological consequences of eIF2α phosphorylation resulting from mTORC1 inhibition, we examined the effect of rapamycin in cells with an intact eIF2α and in cells where both eIF2α alleles had been replaced with an eIF2α that cannot be phosphorylated (eIF2α S51A/S51A) (20). We noted that multiple ATF-4 downstream transcriptional targets (ATF-3, CHOP, the CHOP target gadd34) and transcripts important for the induction of autophagy (LC3B and ATG5 (4, 6, 7)) were upregulated in rapamycin treated eIF2α wild-type cells and not in eIF2α S51A/S51A cells (Fig 2A), despite a minimal upregulation of ATF-4 protein with rapamycin treatment (Fig 2B).
Fig 2.
eIF2α phosphorylation is necessary for rapamycin induced autophagy. A. Wild-type and eIF2α S51A MEFs were treated with 100 nM rapamycin (Rap) for the indicated times, and the expression of ATF-4 transcriptional targets were assessed by real time PCR. Average and standard error are shown (N=3 biological replicates). B. Wild-type and eIF2α S51A/S51A MEFs stably expressing GFP-LC3 were treated with 300nM rapamycin for the indicated times (hours) or tunicamycin for 24 hours. Cell lysates were then immunoblotted for GFP and phosphorylated eIF2α (S51). A representative blot from two biologically replicated experiments is displayed. C. Wild-type and eIF2α S51A MEFs were treated with 300nM rapamycin for 8 hours and endogenous LC3 foci were visualized (left panel) and quantitated (right panel). p values determined by Wilcoxon Rank Sum test; N=3 biological replicates with greater than 200 cells counted in each experiment. D. Wild-type and ATF4 knockout MEFs stably expressing GFPLC3 were treated with 300nM rapamycin for the indicated times (hours) or tunicamycin for 24 hours and the lysates were immunoblotted for GFP. A representative blot from two biologically replicated experiments is displayed. E. Wild-type and ATF-4 knockout MEFs were treated with 300nM rapamycin for 8 hours and endogenous LC3 foci were visualized and quantified from 200 cells. p values determined by Wilcoxon Rank Sum test; N=3 biological replicates with greater than 200 cells counted in each experiment. F. eIF2α wild-type and S51A/S51A cells were treated with 100 nM rapamycin in the absence or presence or 60 μM chloroquine (CQ), or CQ alone, and immunoblots were performed. Total eIF2α serves as a loading control. G. TSC wild-type (top) and deficient (bottom) MEFs, expressing either gadd34 or a control, were treated with 100 nM rapamycin in the absence or presence or 60 μM chloroquine (CQ), or CQ alone, and immunoblots were performed. A representative blot is displayed, with average p62 expression (left) and LC3II/LCI ratios (right) displayed (N=2-3 biological replicates). p values calculated by Students T test. H. ULK1/2 wildtype (top) and deficient (bottom) MEFs, expressing either gadd34 or a control, were treated with 100 nM rapamycin in the absence or presence or 60 μM chloroquine (CQ), or CQ alone, and immunoblots were performed. A representative blot from two biologically replicated experiments is displayed. I. Wild-type and eIF2α S51A MEFs were treated with 300nM rapamycin for 72 hours and cell size was measured by forward scatter flow cytometric analysis. Average fluoresence of replicate experiments (N=2 biological replicates) is shown J. Wild-type and eIF2α S51A MEFs were treated with 300nM rapamycin for the times indicated and proliferation was determined by crystal violet staining and OD measurement with average and standard error displayed (N= 3 biological replicates).
We then assessed autophagy, a well-established consequence of mTORC1 inhibition, using several complementary standard assays including the formation of the faster migrating LC3II from LC3I, the formation of free GFP from a LC3-GFP fusion protein that occurs in autophagosomes, and the migration of endogenous LC3 from a diffuse cytoplasmic distribution to punctate intracellular foci (21). Remarkably, we noted that while rapamycin induced eIF2α phosphorylation and autophagy in wild-type mouse embryo fibroblasts (MEFs) (documented by increased LC3I to LC3II conversion, free GFP generation, and endogenous LC3 foci), autophagy was not induced in rapamycin-treated eIF2α S51A/S51A cells (Fig 2B, 2C). Confirming the importance of ATF-4 in mTORC1 regulation of autophagy, there was minimal LC3I to LC3II conversion, free GFP generation, or endogenous LC3 foci induced in rapamycin treated ATF4 deficient MEFs, compared to (Fig 2D, 2E). Autophagy was blunted in rapamycin treated eIF2α S51A/S51A cells even in the presence of an inhibitor of lysosomal acidification (chloroquine), indicating that eIF2α phosphorylation is necessary for the induction of autophagy, and does not simply suppress the clearance of autophagosomes. (Fig 2F). Consistent with these findings, expression of an eIF2α phosphatase, gadd34, dramatically decreased both p62 degradation and increased endogenous LC3II to LC3I conversion in rapamycin treated MEFs, in contrast to the expression of a kinase dead control (22) (Fig 2G). When mTOR is constitutively active, due to the absence of a functional tuberous sclerosis complex (TSC), rapamcyin did not induce autophagy, confirming that suppression of mTOR activity is necessary for rapamycin's regulation of autophagy (Fig 2F). The expression of gadd34 in TSC2−/− MEFs still decreased autophagic flux in chloroquine treated cells, although to a lesser degree than TSC2 wild-type MEFs, indicating that even with hyperactive mTOR, suppressing eIF2α phosphorylation can diminish basal autophagy.
Canonical regulation of autophagy by mTORC1 occurs through the phosphorylation of uncoordinated family member (unc)-51–like kinase 1 and 2 (ULK1 and ULK2), the mammalian homologs of the autophagy essential yeast ATG1 gene. Although the ULK1/2 complex is required for autophagy induction in response to amino acid deprivation, autophagy is potently induced by glucose deprivation and ammonia, and more mildly with rapamycin treatment in ULK1/2 deficient cells, suggesting that autophagy regulation and mTOR signaling include a ULK independent pathway (21, 23-25). We therefore investigated whether mTORC1 regulation of autophagy by eIF2α phosphorylation was independent of ULK1/2 activity. Similar to what we noted in TSC2 wild-type MEFs (Fig 2G), dephosphorylation of eIF2α by expression of the gadd34 phosphatase markedly suppressed autophagy induction in response to rapamycin treatment in ULK1/2 wild-type MEFs, although mild autophagic flux was still present as revealed by an increase in LC3I→LC3II conversion and free GFP generation with concomitant treatment with rapamycin and chloroquine (Fig 2H top panel). As previously reported (24, 25), ULK1/2 deficiency only modestly blunted basal autophagy, as demonstrated by continued LC3I→LC3II conversion and free GFP generation with chloroquine treatment in these cells (Fig 2H bottom panel). In addition, rapamycin treatment also led to an induction of autophagy even in the absence of ULK1/2, as demonstrated by free GFP generation and LC31→LC3II generation with rapamycin and rapamycin/chlroquine treatments. Both basal autophagy and rapamycin induced autophagy was dramatically diminished by blunting eIF2α phosphorylation in ULK1/2 deficient cells, confirming the existence of mTORC1 regulated but ULK1/2 independent autophagy pathways.
In contrast to the requirement of eIF2α phosphorylation for rapamycin-induced autophagy, other well described mTORC1-regulated cellular functions including cell size (Fig 2H) and proliferation (Fig 2I) occurred in both wild-type MEFs and MEFs which cannot phosphorylate eIF2α. Together these data indicate that eIF2α phosphorylation is necessary for the autophagy noted with mTORC1 inhibition independent of ULK1/2 but dependent on ATF4, and in contrast to other mTOR regulated events autophagy may be uniquely affected by rapamycin's ability to phosphorylate eIF2α.
GCN2 is activated by mTORC1 inhibition
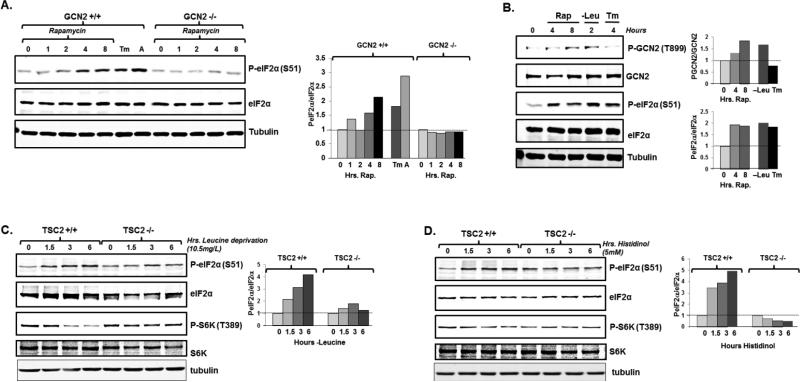
We next sought to identify the kinase(s) responsible for eIF2α phosphorylation in response to mTORC1 inhibition. While there are multiple eIF2α kinases, each responsive to specific cellular stresses, we first focused on GCN2 because of the documented relationship of mTOR inhibition to GCN2 activation in yeast (12, 13). GCN2 contains a region homologous to the histidyl-tRNA synthetase, which binds to uncharged tRNAs during periods of amino acid deprivation and triggers a conformational change of GCN2 that leads to auto-phosphorylation at T889 and GCN2 activation (26-28). We noted that rapamycin treatment did not promote eIF2α phosphorylation in GCN2 deficient MEFs (Fig 3A). In contrast, we still observed robust eIF2α phosphorylation in PERK deficient cells treated with rapamycin (Supplemental Fig 1A). In addition, ER stress was not induced by rapamycin treatment, as demonstrated by a lack of XBP1 splicing (Supplemental Fig 1B). We also noted that rapamycin treatment induced auto-phosphorylation of GCN2 T899 to a similar extent as did treatment with leucine deficient media (as expected, no GCN2 auto-phosphorylation was seen with tunicamycin treatment) (Fig 3B). Together these findings demonstrate that mTORC1 inhibition activates GCN2, and GCN2 is responsible for eIF2α phosphorylation.
Fig 3.
mTORC1 inhibition phosphorylates eIF2α through the GCN2 kinase. A. Wild-type and GCN2 knockout MEFs were treated with 300nM rapamycin for the indicated times (hours), tunicamycin for 12 hours, or amino acid deprivation for 12 hours. Cell lysates were then immunoblotted for GFP. A representative blot is displayed (left) and quantitation reflects average of two biological replicates (right) B. HeLa cells were treated with 300nM rapamycin for the indicated times, deprived of leucine for 2 hours, or treated with tunicamycin for 4 hours and cell lysates were then immunoblotted. A representative blot is displayed (left) and average quantitation reflects average of two biological replicates (right) C. Wild-type and TSC2 knockout MEFs were incubated in leucine deficient media (10.5mg/L) or D. histidinol for the indicated times and cell lysates were then immunoblotted. A representative blot is displayed (left) and average quantitation reflects average of two biological replicates (right)
We next determined the relative contribution of mTOR inhibition to eIF2α phosphorylation. The absence of TSC leads to constitutive activation of mTOR that is not suppressed by amino acid deprivation. Thus when these cells are deprived of amino acids, mTOR inhibition cannot contribute to GCN2 activation or eIF2α phosphorylation, as demonstrated by a failure to diminish S6K1 phosphorylation (Fig 3C). Consistent with previous reports that the loss of TSC trigger the ER stress response and PERK activation (29) TSC2−/− MEFs had increased baseline eIF2α phosphorylation compared to wild-type MEFs (Fig 3C). When cultured in media completely absent of leucine, eIF2α phosphorylation was similar between TSC2−/− MEFs and TSC2 +/+ MEFS (Supplemental Fig 2). However, in the presence of 10.5 mg/l leucine (10% of what is found in standard culture media), we noted that the time dependent increase of eIF2α phosphorylation seen in TSC2 +/+ MEFs was diminished in TSC2 −/− MEFs (Fig 3C). Similarly, treatment of cells with the histidinyl-tRNA synthetase inhibitor, histidinol, which induces His-tRNA deacylation to activate GCN2 and inhibit mTOR (30) led to decreased eIF2α phosphorylation in TSC2 −/− MEFs compared to identically treated TSC2 wild-type cells (Fig 3D). Together these data suggest that mTOR inhibition plays an important role in augmenting GCN2 activation and eIF2α phosphorylation during conditions of amino acid deprivation.
mTORC1 inhibition leads to eIF2α phosphorylation and autophagy via activation of PP6C
mTOR inhibition leads to a rapid dephosphorylation of its direct targets, including 4EBP1 and S6 Kinase, whereas the phosphorylation of eIF2α following mTOR inhibition takes longer (two to six hours, depending on the cell line (Fig 1-3)), arguing for a more complicated mechanism with multiple intermediaries. In S. cerevisiae, TORC1 inhibition activates the SIT4 phosphatase, and this leads to GCN2 serine 577 de-phosphorylation and GCN2 activation at modest concentrations of uncharged tRNAs (12, 31). While there is little peptide homology within this region between yeast and mammalian GCN2, we investigated whether the mammalian homologue of SIT4, PP6C, participates in the phosphorylation of eIF2α in response to mTORC1 inhibition.
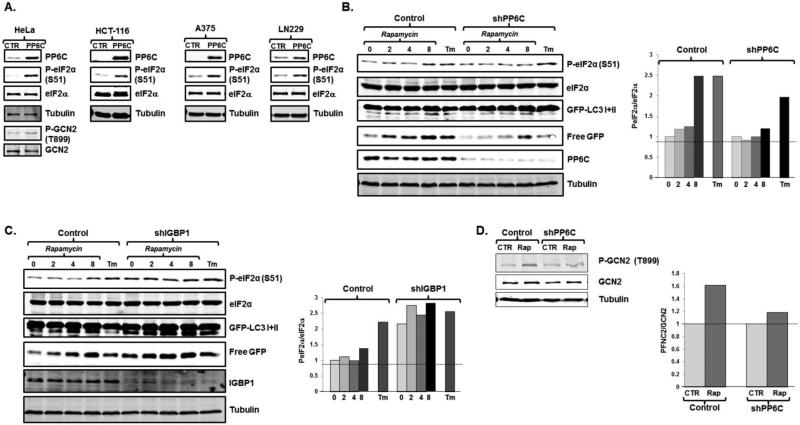
The over-expression of PP6C induced eIF2α phosphorylation in HeLa cells (Fig 4A). PP6C over-expression similarly induced eIF2α phosphorylation in a variety of cell lines derived from colon cancer, cervical cancer, melanoma, glioblastoma, and osteosarcoma (Figs 4A and Supplemental Fig 3A). As expected, in the presence of elevated PP6C expression, treatment of cells with rapamycin did not lead to further increases in eIF2α phosphorylation, although these cells were able to robustly phosphorylate eIF2α in response to tunicamycin and severe amino acid deprivation (Supplemental Fig 3A). Conversely, when we depleted cells of PP6C we noted that rapamycin treatment no longer led to eIF2α phosphorylation (Fig 4B). PP6C depletion had no effect on PERK-induced eIF2α phosphorylation, and no such effect was seen in PP2A depleted cells, suggesting there is specificity to the PP6C phosphatase (Fig 4B and supplemental Fig 3B). The depletion of PP6C not only led to a decrease in eIF2α phosphorylation with mTORC1 inhibition, but also a decrease in autophagy, as demonstrated by decreased LC3I to LC3II conversion and free GFP generation (Fig 4B).
Fig 4.
Rapamycin leads to eIF2α phosphorylation and autophagy through activation of PP6C. A. HCT-116 and HeLa cells stably expressing a control retroviral vector or a vector expressing PP6C were assessed for GCN2 and/or eIF2α phosphorylation. Representative blots are displayed (N=2 biological replicates). B. U2OS cells stably expressing either a control, or shPP6C lentivirus were treated with 300nM rapamycin for the times indicated (hours) or tunicamycin for 12 hours. Protein lysates were then immunoblotted for phosphorylated eIF2α and other noted proteins. A representative blot is displayed (left) and quantitation reflects average of two biological replicates (right). C. HeLa cells stably expressing either a control, or shIGBP1 lentivirus were treated with 300nM rapamycin for the times indicated (hours) or tunicamycin for 12 hours. Protein lysates were then immunoblotted, with a representative blot (left) and quantitation reflective of the average of two biological replicates (right). D. Scramble (con) and PP6C depleted cells were treated with rapamycin for 6 hours and phosphorylated and total GCN2 levels were assessed. A representative blot is displayed (left) and quantitation reflects average of two biological replicates (right).
The activity of PP6C and other members of the PP2A family are inhibited by IGBP1/α4 (32, 33). Indeed, in S. cerevisiae TOR inhibition activates SIT4 by dissociating SIT4 from the yeast homologue of IGBP1/α4, TAP42 (12, 33, 34). When PP6C activity was increased via the depletion of IGBP1/α4, we found that basal eIF2α phosphorylation was increased compared to control cells and was minimally responsive to rapamycin treatment (Fig 4C). Consistent with the importance of GCN2 activation and eIF2α phosphorylation in mTORC1 regulated autophagy, autophagy was increased in rapamycin treated cells depleted of IGBP1/α4, as demonstrated by increased LC3I to LC3II conversion and free GFP generation (Fig 4C). Conversely, IGBP1/α4 over-expression, which diminishes PP6C activity, blunted baseline eIF2α phosphorylation as well as eIF2α phosphorylation in response to rapamycin treatment, though not in response to complete amino acid deprivation or tunicamycin treatment (Supplemental Fig 3C). In PP6C depleted cells, rapamycin did not lead to a significant increase in GCN2 phosphorylation (Fig 4D), congruent with our genetic data that GCN2 plays a crucial role in regulating eIF2α phosphorylation in response to mTORC1 suppression (Fig 3A). Together, these data suggest that PP6C activity is necessary for mTORC1 regulated eIF2α phosphorylation and autophagy.
PP6C, PP6Rs, and GCN2 form a complex that is necessary for eIF2α phosphorylation
The PP6C regulatory subunits, PP6R1, PP6R2 and PP6R3 bridge PP6C to its substrates. Some substrates only bind to a specific subunit, whereas other substrates are more promiscuous (16, 35). We found that endogenous PP6R1, PP6R2, and PP6R2 were each able to immunoprecipitate both endogenous GCN2 and endogenous PP6C, in contrast to a non-relevant antibody which immunoprecipitated neither, suggesting that all three regulatory subunits can participate in a GCN2/PP6C complex (Fig 5A). Depletion of PP6R1, PP6R2, or PP6R3 led to a partial decrease in basal eIF2α phosphorylation which correlated with the efficiency of knock-down, confirming the functional overlap between these regulatory subunits (Fig 5B). The co-depletion of all three subunits led to a decrease in basal (Fig 5C) and rapamycin induced (Fig 5D) eIF2α phosphorylation. These observations confirm the role of PP6C in phosphorylating eIF2α, and suggest that a PP6C-GCN2 complex may be responsible.
Fig 5.
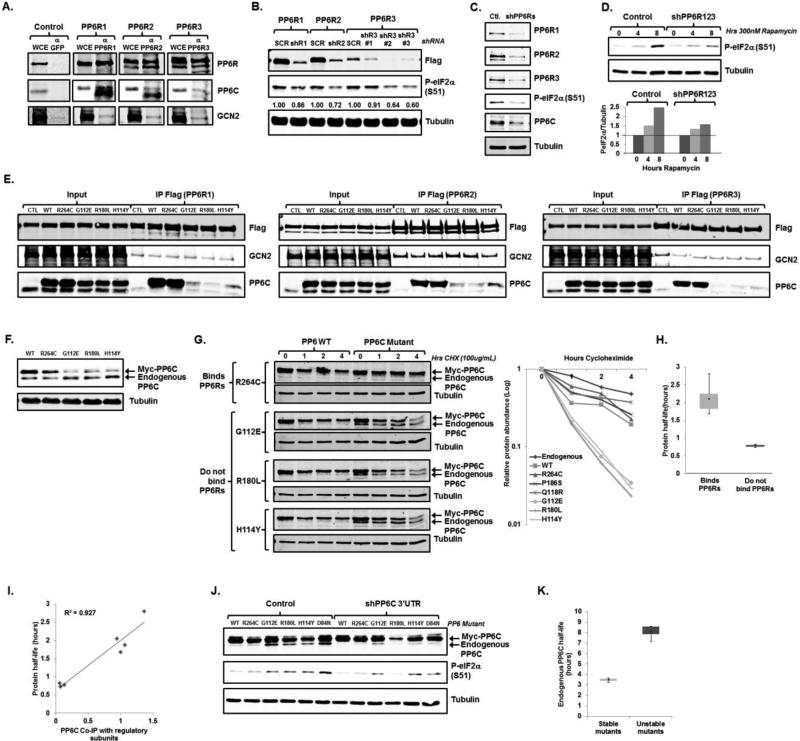
PP6C, PP6Rs, and GCN2 form a complex that is necessary eIF2α phosphorylation and is dissociated with PP6C mutants found in melanoma. A. HeLa cells were harvested and lysates were immunoprecipitated with protein G beads conjugated to antibodies targeting GFP, PP6R1, PP6R2, or PP6R3. Whole cell extract input (WCE) and immunoprecipitated samples were then immunoblotted for endogenous PP6Rs, GCN2, and PP6C. B. Lysates from U2OS cells stably expressing Flag-tagged PP6R1, PP6R2, or PP6R3 and stably expressing a scramble (SCR) or corresponding shRNA were immunoblotted. A representative blot is displayed with average phosphorylated eIF2α/total eIF2α expression quantitated (N=2 biological replicates). C. Lysates from U2OS cells stably expressing either a control or three shRNAs targeting PP6R1, PP6R2, and PP6R3 were immunoblotted for PP6C, phosphorylated eIF2α, and other noted proteins. A representative blots is displayed (N=2 biological replicates). D. U2OS cells stably expressing either a control or three shRNAs targeting PP6R1, PP6R2, and PP6R3 were treated with 300nM rapamycin for the times indicated and cell lysates were immunoblotted. A representative blot is displayed (top) with average phosphorylated eIF2α/total eIF2α expression quantitated (bottom) (N=2 biological replicates). E. 293T cells were co-transfected with vector expressing a Myc-tagged PP6C mutant and a vector expressing Flag-PP6R1, Flag-PP6R2, or Flag-PP6R3. Lysates were immunoprecipitated with sepharose beads conjugated to an anti-Flag antibody, and lysates were then immunoblotted. Representative blots are displayed (N=2 biological replicates). F. Lysates from HCT-116 cells stably expressing a Myc-tagged PP6C mutant or were immunoblotted. A representative blots is displayed (N=2 biological replicates). G. U2OS cells stably expressing a Myc-tagged PP6C expression retrovirus were treated with 100μg/mL cycloheximide for the times indicated. Cell lysates were then immunoblotted for PP6C. A representative blots is displayed (left) with expression of exogenous PP6C graphed as a function of time (right) from 2 biological replicates). H. Dot-plot of the half-life of mutants that bind to the PP6Rs (N=5) versus mutants that do not bind (N=4) with standard error displayed. I. Half-lives were calculated for each PP6C mutant and correlated with its ability to bind to the PP6Rs with the Pearson correlation coefficient (R2) is displayed (N=8). J. Lysates from U2OS cells stably expressing a Myc-tagged PP6C mutant or D84N catalytic mutant in addition to a control or shPP6C (targeting the 3’UTR) lentivirus were immunoblotted for PP6C and phosphorylate eIF2α. A representative blot is displayed (top) with average phosphorylated eIF2α/total eIF2α expression quantitated (bottom) (N=2 biological replicates). K. Dot-plot of the half-life of endogenous PP6C in U2OS cells expressing stable (N=4) or unstable (N=4) PP6C mutants.
PP6C mutations found in melanoma can decrease PP6C-PP6R binding and PP6C stability
Recent unbiased searches for melanoma driver mutations by two independent groups have identified PP6C mutations in approximately 10% of all melanomas (17, 18). Up to 60% of the mutations found in PP6C cluster within a highly conserved region where PP6C is predicted to interface with PP6Rs (16, 17, 36). Because our studies demonstrate that PP6C binding to PP6R is necessary for eIF2α phosphorylation and PP6C expression, we further studied several of these mutants in order to gain insight into both PP6C biology and the molecular determinants of melanoma. Of the seven PP6C mutants found in melanoma examined, we identified three mutants with a marked diminished capacity to bind to PP6R1, PP6R2 and PP6R3 (Fig 5E and Supplemental 4A). As expected, these PP6C mutations did not interfere with PP6R binding to GCN2. The most frequent mutation found, PP6C R264C, did not alter PP6C binding to regulatory subunits, thus suggesting that distinct PP6C mutations may have distinct biochemical and molecular effects.
When stably expressed in several cell lines, we observed lower protein expression for those constructs that encoded PP6C mutants that were unable to bind to PP6Rs when compared to wild-type PP6C or PP6C mutations that retained the ability to bind to PP6Rs (Fig 5F and Supplemental Fig 4B). In addition, we noted decreased PP6C expression in cells depleted of PP6Rs (Fig 5C), and thus theorized that PP6C-PP6R interactions are required for PP6C stability. To test this hypothesis we assessed the half-life of PP6C protein generated endogenously and from wild-type and mutated PP6C constructs in transiently transfected HEK 293 cells, where similarly high expression of all constructs could be achieved. We determined that the half-lives of endogenous PP6C, over-expressed wild-type PP6C, as well as PP6C mutants that bound to PP6Rs averaged two to four hours, and were significantly greater than the half-lives of those PP6C mutants that do not bind to PP6Rs (average 0.8 hrs) (Fig 5G, 5H and supplemental 4C). In fact, there was a strong correlation between the ability of PP6C constructs to bind to PP6Rs to their half-lives (Fig 5I), indicating that PP6C mutants which do not bind to regulatory subunits are destabilized.
Non-stable PP6C mutants found in melanoma increase the stability of wild-type PP6C and induce eIF2α phosphorylation
We predicted that expression of those PP6C mutants which do not bind to PP6R would not promote, or might even blunt, eIF2α phosphorylation. Unexpectedly, however, we noted increased eIF2α phosphorylation in cells expressing mutated PP6C constructs that did not participate in the PP6R-GCN2 complex (Fig 5J). Remarkably, the ability of mutated PP6C to induce eIF2α phosphorylation was not only independent of its ability to bind PP6R, but also on its catalytic activity, as expression of the catalytically inactive D84N mutant (37) also led to eIF2α phosphorylation (Fig 5J). These seemingly contradictory findings were clarified when we observed that while the expression of wild-type PP6C and mutants that bound to PP6R led to diminished endogenous PP6C expression, the similar level of expression of PP6C mutants which did not bind to PP6Rs actually increased endogenous PP6C expression (Fig 5F, 5G). This increase in endogenous PP6C expression was associated with an increase in endogenous PP6C protein half-life from 3 hrs to 7 hrs in the presence of a PP6C mutant that does not bind PP6Rs, whereas endogenous PP6C stability was unchanged in the presence of a PP6C mutant that does bind to PP6Rs (Fig 5K). When we over-expressed PP6C mutants and at the same time depleted endogenous PP6C (via a shRNA directed against the 3’UTR of endogenous PP6C not contained in our constructs), eIF2α phosphorylation was diminished (Fig 5J). Thus, PP6C mutations that disrupt do not bind to PP6R and are destabilized, can promote the stability of the non-mutated PP6C allele, and lead to eIF2α phosphorylation.
Non-stable PP6C mutants found in melanoma induce autophagy in vitro and in vivo
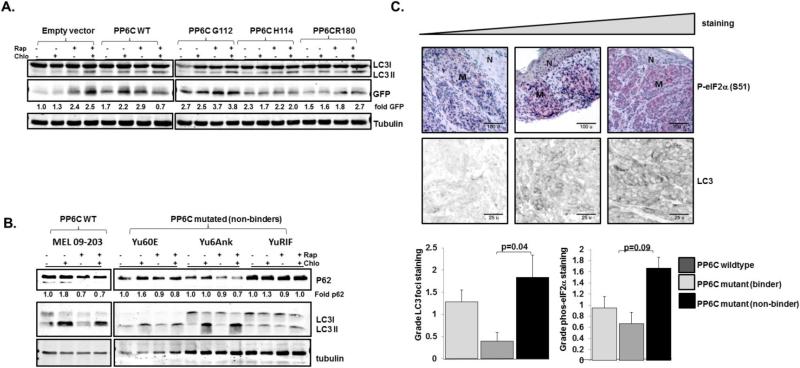
We next examined the effect of unstable PP6C mutants, which promote eIF2α phosphorylation despite an inability to bind to regulatory subunits, on autophagy. First we generated isogenic cell lines expressing a LC3-GFP fusion protein and containing either an empty vector, expressing wild-type PP6C, or expressing PP6C mutants which do not bind regulatory subunits. In accordance to our previous data (Fig 4), compared to empty vector the overexpression of wild type PP6C led to an increase in basal LC3I to LC3II conversion and free GFP expression, even in the presence of chloroquine, indicating increased autophagy induction. Cells expressing PP6C mutants unable to bind regulatory subunits also demonstrated increased basal levels of autophagy and, as expected, had a minimal response to rapamycin treatment (Fig 6A).
Fig 6.
Autophagy is increased with PP6C mutations that disrupt regulatory subunit binding. A. GFP-LC3 HCT116 cells expressing empty vector, wild-type PP6C, or mutated PP6C, were treated with vehicle, Rapamycin 100 nM for 6 hours, 60 μM Chloroquine (CQ) for 2 hours, or both and immunoblots for GFP and tubulin were performed. A representative blot is displayed with average GFP/tubulin quantitated (N=2 biological replicates). B. Primary cell cultures of wild-type and PP6C mutated melanoma tumors were treated as in A and immunbloted for LC3 and p62. A representative blot is displayed with average p62/tubulin quantitated (N=2 biological replicates). C. Melanoma tumors were genotyped for PP6C and stained for LC3 and blindly evaluated for percentage of cells with LC3 foci as described in the text. LC3 and phosphorylated eIF2α expression were assessed by IF and IHC respectively, and expression was graded as 0 (<25% cells with expression), 1 (25%-50% cells with expression), 2 (50%-75% of cells with expression) and 3 (>75% cells with expression. Representative images, including both melanoma (M) and normal skin (N) with increasing phosphorylated eIF2α and LC3 foci are displayed (top). Foci from tumors with wild-type PP6C (n=12), harboring PP6C mutations which bind to regulatory subunits (n=6) or PP6C mutations which do not bind to regulatory subunits (n=6) are shown. p values determined from Wilcoxon Ranks Sum Test.
We then assessed autophagy in primary short term cell cultures derived from tumors with wild-type PP6C, and from tumors which harbor PP6C mutations that do not bind regulatory subunits, with the caveat that these cells are not isogenic and carry multiple mutations, any of which may differentially affect autophagy (17, 18). As expected, basal autophagy and autophagic flux varied dramatically amongst these cell lines, as indicated by differing baseline LC3I/LC3II ratios and responses to the autophagy inhibition with chloroquine. Significantly, however, while the wild-type cell primary cells were responsive to rapamycin (demonstrating reductions in p62 and increases in LC3II/LC3I ratios), the PP6C mutant primary cell lines were not (Fig 6B), consistent with a model in which rapamycin-induced autophagy is at least partially dependent on PP6C.
In vivo autophagy in melanoma is likely not only reflective of a number of mutations affecting the autophagy machinery, but also of vascularization and other aspects of the tumor microenvironment. Despite these potential complicating features, we explored autophagy in paraffin embedded melanoma samples genotyped for PP6C (38). Six tumors with PP6C mutations which do not bind to PP6C regulatory subunits, six tumors with PP6C mutations which do not interfere with regulatory subunit binding, and 12 PP6C wild-type tumors were stained for LC3, and the number of cells with LC3 foci was assessed with indirect immunofluorescence. Those tumors with PP6C mutations disrupting regulatory subunit binding had, on average, a significantly greater number of LC3 foci compared to those tumors harboring mutant PP6Cs which bind to regulatory subunits (and presumably have alternative gain or loss of function activities (see discussion) (Fig 6C bottom left panel). Consistent with our model in which eIF2α phosphorylation plays a crucial role in PP6C mediated autophagy, eIF2α phosphorylation also trended higher in those tumors with PP6C mutations which do not bind to regulatory subunits (Fig 6C bottom right panel). Together, these studies, using three complementary approaches, which minimize the inherent weaknesses of each, demonstrate an association of PP6C mutations which do not bind regulatory subunits with increased autophagy.
Discussion
We have determined that the two best described amino acid sensing systems are linked in mammalian cells, and specifically that mTORC1 inhibition leads to eIF2α phosphorylation through activation of the eIF2α kinase GCN2. Our data demonstrate that PP6C plays a vital role in regulating GCN2 activation in response to mTORC1 inhibition, and augments eIF2α phosphorylation in response to moderate amino acid deprivation (Fig 7). This system thus appears to be homologous to the well described system in yeast (12, 13). In addition, we show that the robust activation of autophagy by mTORC1 inhibition depends on the cross-talk between these two pathways. Because the regulation of mTOR activity and eIF2α phosphorylation play vital roles in the cellular stress response, the identification of their interaction provides important insight into cellular adaptation in cancer and a variety of other physiological and pathological conditions.
Fig 7.
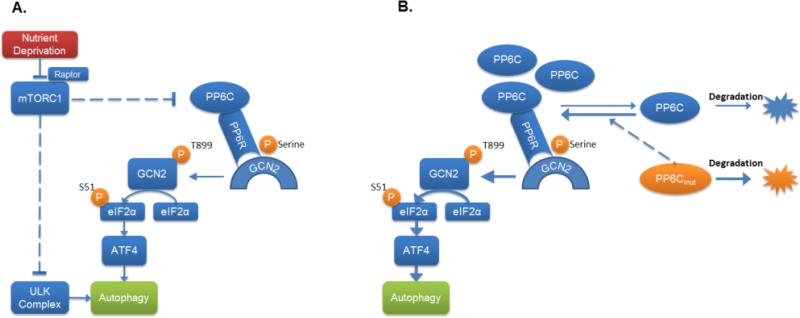
Proposed model of mTORC1 regulation of eIF2α phosphorylation through PP6C-mediated activation of GCN2. A. Under periods of nutrient deprivation, mTORC1 inhibition activates PP6C. PP6C then associates with GCN2 in a complex with a PP6 regulatory protein. PP6C subsequently dephosphorylates GCN2, promoting its activation. Activated GCN2 then leads to the phosphorylation of eIF2α and induction of autophagy. PP6C can also dissociate from the PP6 regulatory proteins, which decreases its stability. B. Several PP6C mutants found in melanoma are unable to bind to the PP6Rs and are rapidly degraded. This causes an increase in wild-type PP6C stability, sensitizing the cells autophagy induction in response to mTORC1 inhibition.
The inhibition of mTOR has been increasingly utilized in therapeutic strategies, and the induction of autophagy by mTOR inhibition may in part explain some of the failures of mTORC1 inhibitors as anti-cancer agents (39, 40). The recognition that mTORC1 inhibition-induced autophagy requires GCN2 activation and eIF2α phosphorylation may lead to strategies to inhibit mTOR without inducing autophagy. For example, the dual inhibition of mTORC1 and GCN2 might be one such strategy. An alternative approach to block mTORC1 regulated autophagy would be to interfere with GCN2 de-phosphorylation by PP6C. Because GCN2 and PP6C interact, it is likely that GCN2 is a direct PP6C substrate. In S. cerevisiae the dephosphorylation of S577 by SIT4 de-represses GCN2, enabling it to bind to uncharged amino acids with greater affinity, and autophosphorylate key threonines in its activation loop (12, 31). The GCN2 phosphorylation site(s) acted upon by PP6C are not yet known, and the expression of exogenous GCN2 (including serine to alanine GCN2 mutants) leads to the autoactivation of GCN2 rendering such experiments un-informative. Ultimately, however, identifying key phosphorylation sites on GCN2 that regulate GCN2's ability to be activated, and identifying the physical blue-print of PP6C-PP6R-GCN2 interactions, could provide a molecular target for autophagy manipulation.
We found that those PP6C mutations which disrupt PP6C-PP6R interactions are rapidly degraded and paradoxically stabilize wild-type PP6C. It is intriguing to consider that the effects of some PP6C mutations in melanoma may be due to their effects on increasing the expression of wild-type PP6C generated from the non-mutated allele. Although the majority of PP6C mutations reported in short term cultures from melanomas were predicted to be loss of function mutations (17, 18), in direct sequencing of PP6C from over 390 melanomas, we have found that many PP6C mutations are not associated with a loss of heterozygosity, and both PP6C mutated and non-mutated mRNA are found in single cell clones derived from PP6C mutated melanoma (38). Supporting the potential for some PP6C mutations to serve as activating mutations is the finding that PP6C is over-expressed in glioblastoma multiforme (41). The effect of mutated oncogenes on non-mutated alleles is becoming an increasingly recognized phenomenon. For example, it has recently been reported that oncogenic Ras increase the activation of wild-type Ras (42), although this mechanism has not previously been reported for unstable mutations.
While many of the PP6C mutations found in melanoma cluster within a highly conserved region where PP6C interfaces with PP6Rs, mutations are found throughout the PP6C gene. Indeed, we have found several mutations, including the common PP6C R264C mutation, do not disrupt binding. This raises the possibility that distinct mutations in PP6C may have separate biochemical, molecular, and phenotypic, implications, as has been noted for mutations in PTPN11, encoding the Shp2 phosphatase (43). In Noonan syndrome, mutations in this phosphatase are distributed throughout the coding region and result in hyperactivated or unregulated forms of this, due to an inability of the enzyme to maintain its auto-inhibited conformation. In contrast, in Leopard syndrome mutations occur in the catalytic core of the enzyme and produce catalytically impaired Shp2 variants. Both Noonan syndrome and Leopard syndrome are phenotypically similar, and it is unclear how mutations in the same gene, that result in biochemically opposite characteristics, result in similar genetic syndromes.
While our data demonstrate a critical role for PP6C in regulating autophagy, PP6C has also been reported to play a role in the degradation of IκBε in response to TNFα, de-phosphorylation of gamma H2AX after irradiation, and dephosphorylation Aurora Kinase A to regulate mitosis (35, 44-47). The impact of individual PP6C mutations on these pathways is not yet known. Indeed, in contrast to the gain of function activity unstable PP6C mutants demonstrate in the autophagy pathway, we and others have demonstrated that PP6C mutations that do not bind to regulatory subunits have a loss of function in dephosphorylating Aurora A Kinase (38, 48). Autophagy appears to play a dual role in cancer in general, and reports suggest that it may function as both a tumor suppressor as well as a serve in a pro-survival capacity (49-51). Further studies are required to better define the clinical, biological, and potentially therapeutic impact of PP6C mutations, GCN2 activation, eIF2α phosphorylation on autophagy and/or melanoma initiation and progression.
Experimental Procedures
Cell culture- Cells were cultured as previously described (52), and treated with 2.5μg/mL tunicamycin, 100-300 nM rapamycin, 60 μM Chloroquine, 10 μM histidinol, or depleted of amino acids by treating cells with low leucine media (10.5 mg/L) supplemented with 10% dialyzed fetal bovine serum. MEFs deficient of PERK, GCN2, TSC2 and eIF2α S51A/S51A cells and their wild-type controls have been previously described (20, 21, 53-55). For the protein stability experiments, cells were treated with 100μg/mL cycloheximide (CHX). Short term primary cultures from PP6C mutated melanoma were described (18) and obtained from the Yale Dermatology Cell Culture Facility.
Plasmids- The pLKO.1 shPP6C (TRC0000002764), shIGBP1 (TRC0000039966), shPP6R1 (TRC0000129845), shPP6R2 (TRC0000144656), and shPP6R3 (TRC0000159679) lentiviral vectors were obtained from Sigma. The gadd34 (haA1.pBabepu) and kinase dead control (Myd116.PFLAG.CMV2) (22) were obtained from Addgene. The pLKO.1 shRictor and shRaptor lentiviral vectors were the kind gift of R. Schneider. The GFP-LC3 fusion, the kind gift of G. Kroemer, was cloned into the retroviral vectors pQCXIN or pBabe. PP6R1, PP6R2 and PP6R3 were cloned into pCDNA. IGBP1/α4 was cloned into pLPC. PP6C was cloned into pQCXIN, pBABE-puro, and pLPC. Retroviruses were generated using standard retroviral generation and infection techniques (52, 56). 293T cells were transiently transfected by calcium phosphate precipitation.
Immunoblots and Immunoprecipitations - Immunoblots were done using fluorescent secondary antibodies and quantitated by Licor as previously described (56). Membranes were stained with antibodies directed against GFP (Cell Signaling, 2995), Tubulin (Sigma; T9026), phosphorylated eIF2α (Epitomics; 1090), eIF2α (Santa Cruz; SC-11386), ATF4 (Santa Cruz; SC-200), S6 kinase (Cell Signaling; 9202), phosphorylated S6 kinase (Cell Signaling; 9234), Rictor (Bethyl; A300-459A), Raptor (Millipore; 09-217), Akt (Cell Signaling; 9272), phosphorylated Akt (Cell Signaling; 9271), 4EBP1 (Cell Signaling; 9452), phosphorylated 4EBP1(Cell Signaling; 9456), GCN2 (Epitomics; 5417), phosphorylated GCN2 (Epitomics; 2425), PP2A (kind gift from C. Basilico), PP6C (Millipore; 07-1224), IGBP1 (Upstate; 05-930), PP6R1 (Bethyl; A300-968A), PP6R2 (Bethyl; A300-970A), PP6R3 (Bethyl; A300-972A), Flag-tag (Sigma; F3165), HA-tag (Santa Cruz; SC-7392), and Myc-tag (Cell Signaling; 2276). Blots were then washed in TBST and incubated in LI-COR IRDye-conjugated goat anti-rabbit (800nm) or goat anti-mouse (680nm) antibodies and imaged on the LI-COR Odyssey Infrared Imaging System. Immunoprecipitations were performed as previously described (57). 500-1000μg of whole cell extract was mixed with Protein G sepharose (GE Healthcare) as well as the immunoprecipitating antibody: PP6R1 (Bethyl; A300-968A), PP6R2 (Bethyl; A300-970A), or PP6R3 (Bethyl; A300-972A). For the Flag-tag immunoprecipitations, lysates were mixed with the Anti-Flag M2 affinity gel (Sigma; A2220). Samples were then incubated at 4°C with constant rotation over night. Beads were then washed 4 times with lysis buffer and then resuspended in 4× Laemmli buffer and boiled for 10 minutes at 95°C.
Microscopy and assessment of eIF2α phosphorylation and LC3 foci- cells were fixed with 3.7% paraformaldehyde for 20 minutes, stained with LC3 antibody (Novus; NB600-1384) and subsequently with a FITC-conjugated secondary antibody (Molecular Probes; F2765). Both these, and GFP foci were visualized with confocal microscopy as previously described (52). GFP-LC3 expressing cells containing three or more foci were counted as positive and quantified as a proportion of all cells within a given field. Endogenous LC3 foci were quantified using the ImageJ software. Image intensity thresholds were set to a constant value to include particles with a high signal compared to background. Regions of interest were drawn around each cell in a field and the number of foci were counted using the ‘analyze particles’ function. Paraffin embedded melanoma were obtained from the IRB approved NYU Interdisciplinary Melanoma Cooperative Group tumor bank. eIF2α immunohistochemistry and indirect immunofluorescence was performed as described (7, 56).
Supplementary Material
Summary.
mTOR inhibition leads to eIF2α phosphorylation and autophagy via the PP6C phosphatase
Acknowledgements
We gratefully acknowledge the kind gift of reagents from D. Ron, R. Kaufman, M. Sahin, D. Kwiatkowski, C. Koumenis, C. Thompson, G. Kroemer, and R. Schneider. This work was supported by T32GM066704 (J.W.), RO1DK081641 (L.B.G.) and the NYU Cancer Institute.
Footnotes
Supplementary Figures
Fig. S1. mTORC1 inhibition does not lead to phosphorylation of eIF2α through the unfolded protein response..
Fig. S2. eIF2α phosphorylation is induced by leucine deprivation.
Fig S3. PP6C and IGBP1 overexpression regulate rapamycin-induced eIF2α phosphorylation
Fig S4. PP6C binding to regulatory subunits affects PP6C stability.
The authors declare no competing interests.
References
- 1.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 4.Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, Bobrovnikova-Marjon E, Diehl JA, Ron D, Koumenis C. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29:2082–2096. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rzymski T, Milani M, Pike L, Buffa F, Mellor HR, Winchester L, Pires I, Hammond E, Ragoussis I, Harris AL. Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene. 2010;29:4424–4435. doi: 10.1038/onc.2010.191. [DOI] [PubMed] [Google Scholar]
- 6.Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, Lambin P, van der Kogel AJ, Koritzinsky M, Wouters BG. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120:127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wengrod J, Martin L, Wang D, Frischmeyer-Guerrerio P, Dietz HC, Gardner LB. The inhibition of nonsense mediated RNA decay activates autophagy. Mol Cell Biol. 2013 doi: 10.1128/MCB.00174-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talloczy Z, Jiang W, Virgin H. W. t., Leib DA, Scheuner D, Kaufman RJ, Eskelinen EL, Levine B. Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc Natl Acad Sci U S A. 2002;99:190–195. doi: 10.1073/pnas.012485299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi S, Ishihara H, Yamada T, Tamura A, Usui M, Tominaga R, Munakata Y, Satake C, Katagiri H, Tashiro F, Aburatani H, Tsukiyama-Kohara K, Miyazaki J, Sonenberg N, Oka Y. ATF4-mediated induction of 4E-BP1 contributes to pancreatic beta cell survival under endoplasmic reticulum stress. Cell Metab. 2008;7:269–276. doi: 10.1016/j.cmet.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Damgaard CK, Lykke-Andersen J. Translational coregulation of 5′TOP mRNAs by TIA-1 and TIAR. Genes Dev. 2011;25:2057–2068. doi: 10.1101/gad.17355911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherkasova VA, Hinnebusch AG. Translational control by TOR and TAP42 through dephosphorylation of eIF2alpha kinase GCN2. Genes Dev. 2003;17:859–872. doi: 10.1101/gad.1069003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valbuena N, Rozalen AE, Moreno S. Fission yeast TORC1 prevents eIF2alpha phosphorylation in response to nitrogen and amino acids via Gcn2 kinase. J Cell Sci. 2012;125:5955–5959. doi: 10.1242/jcs.105395. [DOI] [PubMed] [Google Scholar]
- 14.Bastians H, Ponstingl H. The novel human protein serine/threonine phosphatase 6 is a functional homologue of budding yeast Sit4p and fission yeast ppe1, which are involved in cell cycle regulation. J Cell Sci. 1996;109(Pt 12):2865–2874. doi: 10.1242/jcs.109.12.2865. [DOI] [PubMed] [Google Scholar]
- 15.Morales-Johansson H, Puria R, Brautigan DL, Cardenas ME. Human protein phosphatase PP6 regulatory subunits provide Sit4-dependent and rapamycin-sensitive sap function in Saccharomyces cerevisiae. PLoS ONE. 2009;4:e6331. doi: 10.1371/journal.pone.0006331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosing AS, Valerie NC, Dziegielewski J, Brautigan DL, Larner JM. PP6 regulatory subunit R1 is bidentate anchor for targeting protein phosphatase-6 to DNA-dependent protein kinase. J Biol Chem. 2012;287:9230–9239. doi: 10.1074/jbc.M111.333708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, Dicara D, Ramos AH, Lawrence MS, Cibulskis K, Sivachenko A, Voet D, Saksena G, Stransky N, Onofrio RC, Winckler W, Ardlie K, Wagle N, Wargo J, Chong K, Morton DL, Stemke-Hale K, Chen G, Noble M, Meyerson M, Ladbury JE, Davies MA, Gershenwald JE, Wagner SN, Hoon DS, Schadendorf D, Lander ES, Gabriel SB, Getz G, Garraway LA, Chin L. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, Cheng E, Davis MJ, Goh G, Choi M, Ariyan S, Narayan D, Dutton-Regester K, Capatana A, Holman EC, Bosenberg M, Sznol M, Kluger HM, Brash DE, Stern DF, Materin MA, Lo RS, Mane S, Ma S, Kidd KK, Hayward NK, Lifton RP, Schlessinger J, Boggon TJ, Halaban R. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012 doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin L, Kimball SR, Gardner LB. Regulation of the unfolded protein response by eif2bdelta isoforms. J Biol Chem. 2010;285:31944–31953. doi: 10.1074/jbc.M110.153148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 21.Cheong H, Lindsten T, Wu J, Lu C, Thompson CB. Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc Natl Acad Sci U S A. 2011;108:11121–11126. doi: 10.1073/pnas.1107969108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheong H, Wu J, Gonzales LK, Guttentag SH, Thompson CB, Lindsten T. Analysis of a lung defect in autophagy-deficient mouse strains. Autophagy. 2014;10:45–56. doi: 10.4161/auto.26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAlpine F, Williamson LE, Tooze SA, Chan EY. Regulation of nutrient-sensitive autophagy by uncoordinated 51-like kinases 1 and 2. Autophagy. 2013;9:361–373. doi: 10.4161/auto.23066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan EY, Kir S, Tooze SA. siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J Biol Chem. 2007;282:25464–25474. doi: 10.1074/jbc.M703663200. [DOI] [PubMed] [Google Scholar]
- 26.Romano PR, Garcia-Barrio MT, Zhang X, Wang Q, Taylor DR, Zhang F, Herring C, Mathews MB, Qin J, Hinnebusch AG. Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2alpha kinases PKR and GCN2. Mol Cell Biol. 1998;18:2282–2297. doi: 10.1128/mcb.18.4.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padyana AK, Qiu H, Roll-Mecak A, Hinnebusch AG, Burley SK. Structural basis for autoinhibition and mutational activation of eukaryotic initiation factor 2alpha protein kinase GCN2. J Biol Chem. 2005;280:29289–29299. doi: 10.1074/jbc.M504096200. [DOI] [PubMed] [Google Scholar]
- 28.Berlanga JJ, Santoyo J, De Haro C. Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2alpha kinase. Eur J Biochem. 1999;265:754–762. doi: 10.1046/j.1432-1327.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 29.Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–551. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimball SR, Do AN, Kutzler L, Cavener DR, Jefferson LS. Rapid turnover of the mTOR complex 1 (mTORC1) repressor REDD1 and activation of mTORC1 signaling following inhibition of protein synthesis. J Biol Chem. 2008;283:3465–3475. doi: 10.1074/jbc.M706643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Barrio M, Dong J, Cherkasova VA, Zhang X, Zhang F, Ufano S, Lai R, Qin J, Hinnebusch AG. Serine 577 is phosphorylated and negatively affects the tRNA binding and eIF2alpha kinase activities of GCN2. J Biol Chem. 2002;277:30675–30683. doi: 10.1074/jbc.M203187200. [DOI] [PubMed] [Google Scholar]
- 32.Kong M, Ditsworth D, Lindsten T, Thompson CB. Alpha4 is an essential regulator of PP2A phosphatase activity. Mol Cell. 2009;36:51–60. doi: 10.1016/j.molcel.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Peterson RT, Schreiber SL. Alpha 4 associates with protein phosphatases 2A, 4, and 6. Biochem Biophys Res Commun. 1998;247:827–832. doi: 10.1006/bbrc.1998.8792. [DOI] [PubMed] [Google Scholar]
- 34.Prickett TD, Brautigan DL. The alpha4 regulatory subunit exerts opposing allosteric effects on protein phosphatases PP6 and PP2A. J Biol Chem. 2006;281:30503–30511. doi: 10.1074/jbc.M601054200. [DOI] [PubMed] [Google Scholar]
- 35.Stefansson B, Brautigan DL. Protein phosphatase 6 subunit with conserved Sit4-associated protein domain targets IkappaBepsilon. J Biol Chem. 2006;281:22624–22634. doi: 10.1074/jbc.M601772200. [DOI] [PubMed] [Google Scholar]
- 36.Guergnon J, Derewenda U, Edelson JR, Brautigan DL. Mapping of protein phosphatase-6 association with its SAPS domain regulatory subunit using a model of helical repeats. BMC Biochem. 2009;10(24):1471–2091. doi: 10.1186/1471-2091-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kajino T, Ren H, Iemura S, Natsume T, Stefansson B, Brautigan DL, Matsumoto K, Ninomiya-Tsuji J. Protein phosphatase 6 down-regulates TAK1 kinase activation in the IL-1 signaling pathway. J Biol Chem. 2006;281:39891–39896. doi: 10.1074/jbc.M608155200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gold HL, Wengrod J, de Miera EV, Wang D, Fleming N, Sikkema L, Kirchhoff T, Hochman T, Goldberg JD, Osman I, Gardner LB. PP6C hotspot mutations in melanoma display sensitivity to Aurora kinase inhibition. Mol Cancer Res. 2014;12:433–439. doi: 10.1158/1541-7786.MCR-13-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loehberg CR, Strissel PL, Dittrich R, Strick R, Dittmer J, Dittmer A, Fabry B, Kalender WA, Koch T, Wachter DL, Groh N, Polier A, Brandt I, Lotz L, Hoffmann I, Koppitz F, Oeser S, Mueller A, Fasching PA, Lux MP, Beckmann MW, Schrauder MG. Akt and p53 are potential mediators of reduced mammary tumor growth by cloroquine and the mTOR inhibitor RAD001. Biochem Pharmacol. 2012;83:480–488. doi: 10.1016/j.bcp.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Xie X, White EP, Mehnert JM. Coordinate autophagy and mTOR pathway inhibition enhances cell death in melanoma. doi: 10.1371/journal.pone.0055096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen Y, Wang Y, Sheng K, Fei X, Guo Q, Larner J, Kong X, Qiu Y, Mi J. Serine/threonine protein phosphatase 6 modulates the radiation sensitivity of glioblastoma. Cell Death Dis. 2011;2:e241. doi: 10.1038/cddis.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeng HH, Taylor LJ, Bar-Sagi D. Sos-mediated cross-activation of wild-type Ras by oncogenic Ras is essential for tumorigenesis. Nat Commun. 3:1168. doi: 10.1038/ncomms2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kontaridis MI, Swanson KD, David FS, Barford D, Neel BG. PTPN11 (Shp2) mutations in LEOPARD syndrome have dominant negative, not activating, effects. J Biol Chem. 2006;281:6785–6792. doi: 10.1074/jbc.M513068200. [DOI] [PubMed] [Google Scholar]
- 44.Douglas P, Zhong J, Ye R, Moorhead GB, Xu X, Lees-Miller SP. Protein phosphatase 6 interacts with the DNA-dependent protein kinase catalytic subunit and dephosphorylates gamma-H2AX. Mol Cell Biol. 2010;30:1368–1381. doi: 10.1128/MCB.00741-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong J, Liao J, Liu X, Wang P, Liu J, Hou W, Zhu B, Yao L, Wang J, Li J, Stark JM, Xie Y, Xu X. Protein phosphatase PP6 is required for homology-directed repair of DNA double-strand breaks. Cell Cycle. 2011;10:1411–1419. doi: 10.4161/cc.10.9.15479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng K, Bastos RN, Barr FA, Gruneberg U. Protein phosphatase 6 regulates mitotic spindle formation by controlling the T-loop phosphorylation state of Aurora A bound to its activator TPX2. J Cell Biol. 2010;191:1315–1332. doi: 10.1083/jcb.201008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stefansson B, Brautigan DL. Protein phosphatase PP6 N terminal domain restricts G1 to S phase progression in human cancer cells. Cell Cycle. 2007;6:1386–1392. doi: 10.4161/cc.6.11.4276. [DOI] [PubMed] [Google Scholar]
- 48.Hammond D, Zeng K, Espert A, Bastos RN, Baron RD, Gruneberg U, Barr FA. Melanoma-associated mutations in protein phosphatase 6 cause chromosome instability and DNA damage due to dysregulated Aurora-A. J Cell Sci. 2013 doi: 10.1242/jcs.128397. [DOI] [PubMed] [Google Scholar]
- 49.Checinska A, Soengas MS. The gluttonous side of malignant melanoma: basic and clinical implications of macroautophagy. Pigment Cell Melanoma Res. 2011;24:1116–1132. doi: 10.1111/j.1755-148X.2011.00927.x. [DOI] [PubMed] [Google Scholar]
- 50.Ma XH, Piao S, Wang D, McAfee QW, Nathanson KL, Lum JJ, Li LZ, Amaravadi RK. Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clin Cancer Res. 2011;17:3478–3489. doi: 10.1158/1078-0432.CCR-10-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sivridis E, Giatromanolaki A, Liberis V, Koukourakis MI. Autophagy in endometrial carcinomas and prognostic relevance of ‘stone-like’ structures (SLS): what is destined for the atypical endometrial hyperplasia? Autophagy. 2011;7:74–82. doi: 10.4161/auto.7.1.13947. [DOI] [PubMed] [Google Scholar]
- 52.Gardner LB, Corn PG. Hypoxic regulation of mRNA expression. Cell Cycle. 2008;7:1916–1924. doi: 10.4161/cc.7.13.6203. [DOI] [PubMed] [Google Scholar]
- 53.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 54.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 55.Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ. Tsc2(+/−) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest. 1999;104:687–695. doi: 10.1172/JCI7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang D, Zavadil J, Martin L, Parisi F, Friedman E, Levy D, Harding H, Ron D, Gardner LB. Inhibition of nonsense-mediated RNA decay by the tumor microenvironment promotes tumorigenesis. Mol Cell Biol. 2011;31:3670–3680. doi: 10.1128/MCB.05704-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gardner LB, Li Q, Park MS, Flanagan WM, Semenza GL, Dang CV. Hypoxia inhibits G1/S transition through regulation of p27 expression. J Biol Chem. 2001;276:7919–7926. doi: 10.1074/jbc.M010189200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.