Abstract
Malignant neoplasms arising from epithelial cells are called carcinomas. Such cancers are diagnosed in about one in three humans in ‘developed’ countries, with the most common sites affected being lung, breast, prostate, colon, ovary and pancreas. By contrast, carcinomas are said to be rare in captive chimpanzees, which share more than 99% protein sequence homology with humans (and possibly in other related ‘great apes’—bonobos, gorillas and orangutans). Simple ascertainment bias is an unlikely explanation, as these nonhuman hominids are recipients of excellent veterinary care in research facilities and zoos, and are typically subjected to necropsies when they die. In keeping with this notion, benign tumours and cancers that are less common in humans are well documented in this population. In this brief overview, we discuss other possible explanations for the reported rarity of carcinomas in our closest evolutionary cousins, including inadequacy of numbers surveyed, differences in life expectancy, diet, genetic susceptibility, immune responses or their microbiomes, and other potential environmental factors. We conclude that while relative carcinoma risk is a likely difference between humans and chimpanzees (and possibly other ‘great apes’), a more systematic survey of available data is required for validation of this claim.
Keywords: carcinomas, hominids, evolution, epidemiology, primates
1. What are carcinomas?
During embryogenesis, the three germ layers (endoderm, ectoderm, mesoderm) differentiate into epithelial and nonepithelial cells, which eventually form differentiated tissues and organs [1]. Epithelial cells arise from stem cells and often line body surfaces that interact directly with the environment [2]. The type of epithelium reflects location and function. For example, the squamous epithelium of the skin epidermis, oropharynx and uterine cervix is stratified, impervious and protective against shear forces. By contrast, the epithelial lining of the lumen of the gastrointestinal tract and bronchial airways, and the ducts of pancreas, breast and prostate are columnar or cuboidal (arranged in a single column), with basal nuclei and abundant cytoplasm, and can have secretory or absorptive functions. Epithelial cells are typically attached to underlying connective tissue by a basement membrane, and the underlying stroma includes blood vessels, lymphatic vessels, haematopoietic cells, stromal fibroblasts, extracellular matrix, neuronal structures, smooth muscle and adipose tissue [1].
Neoplasia is the term given to the new growth of cells proliferating without regard to stop signals, with attendant new blood vessels, forming a tumour mass (neoplasm) [3]. If the growing neoplasm within an epithelial layer stays restricted by a basement membrane and does not invade that barrier, it is designated as a benign tumour, which grows locally and may have physical effects, or occasionally secrete bioactive molecules. Benign neoplasms arising from columnar epithelia that have not breached the basement membrane are termed adenomas (or adenomatous polyps, if they protrude into the lumen of a hollow organ).
However, once tumour cells undergo multiple genetic changes [4,5] and breach and infiltrate through the basement membrane, the neoplasm becomes invasive and malignant, and the cells use proteases and glycosidases, which allow breakdown of the extracellular matrix, reaching and invading blood vessels [6]. Together with many other complex genetic, epigenetic and biochemical changes, this then starts the process of metastatic spread. A malignant neoplasm originating from epithelial cells is called a carcinoma. Squamous carcinomas arise from squamous epithelium but the majority of malignancies in humans are adenocarcinomas, arising from ductal epithelia [7,8].
Carcinomas are easily diagnosed by their typical histological appearance. As an example, figure 1 shows an adenocarcinoma of the colon arising adjacent to normal colon epithelium. The normal epithelial cells are columnar with basal small nuclei, and are also secreting mucin, which is stored within the goblet cells (seen as empty vacuoles remaining after processing of the tissue). The neoplastic malignant cells have large pleomorphic nuclei, some with prominent nucleoli, some in mitotic phase of proliferation and some have invaded beyond the basement membrane (arrows).
Figure 1.
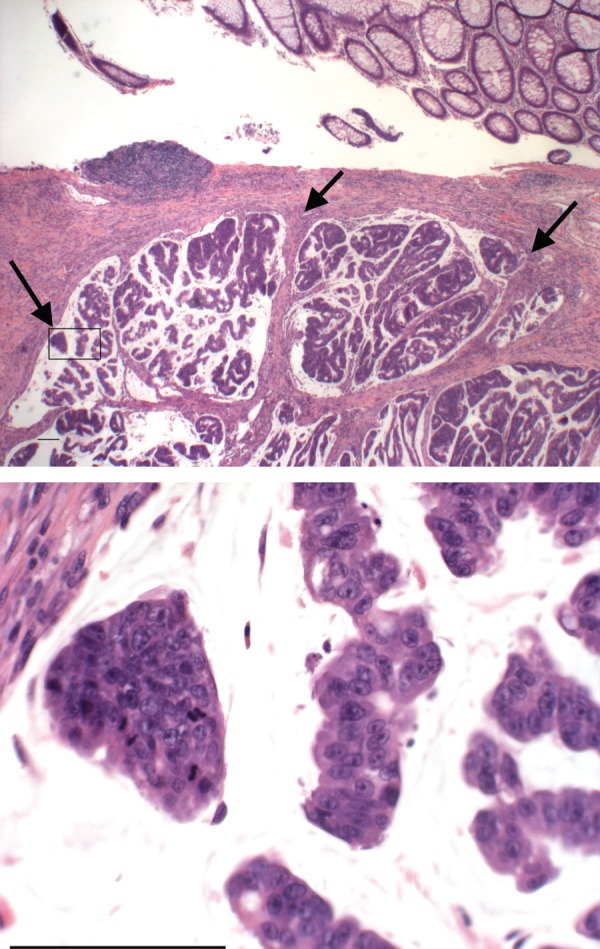
An example of the histological appearance of a human colon adenocarcinoma, along with adjacent normal colonic epithelium. The arrows point to sites of malignant cell invasion. See text for discussion. The area in the box on the low magnification image is enlarged in the lower panel to show mitoses, pleomorphic tumour nuclei and nucleoli. Scale bar, 100 µm. (Online version in colour.)
2. Adult humans have a high risk of developing carcinomas
Improvements in general nutrition and control of major infectious diseases and early inflammatory insults have resulted in a markedly increased human lifespan in many countries [9]. Accompanying this demographic transition has been a major increase in the lifetime risk of developing the types of carcinomas mentioned above [7,8]. While rare familial genetic variants contribute to a small fraction of these tumours in humans, the great majority are initiated by sporadic and spontaneous genetic mutations [3,10]. There is lifetime risk of about approximately 40% of males and approximately 30% of females being diagnosed with one of these tumours in so-called ‘developed’ countries, where these cancers now constitute a major proportion of overall causes of death [7,8].
3. ‘Great apes’, our closest evolutionary cousins
Abundant data now indicate that we humans are very closely related to other hominids including chimpanzees, bonobos, gorillas and orangutans (the so-called ‘great apes’, hereafter called nonhuman hominids, or NHHs) [11–13]. Indeed, humans are closer genetically to chimpanzees and bonobos than they are to gorillas and orangutans. And humans are closer genetically to the chimpanzee/bonobo clade than mice and rats are to each other [12,13]. Thus, it was reasonable to expect that chimpanzees would be good models for understanding human disease [14]. Prior to the recognition of their extreme cognitive similarities to humans and the resulting ethical concerns [15–18] large numbers of chimpanzees were thus placed in captivity, to be used for modelling of human diseases. While recent recommendations have markedly curtailed and almost eliminated the use of chimpanzees in biomedical research [17], there was already a considerable body of published and unpublished information regarding their disease profiles, particularly from facilities in the USA. Surprisingly, surveys of this existing information suggest that several common human diseases may be partially or completely unique to our species, and that the captive chimpanzee population may suffer from different profiles of pathology [19–22].
4. Extant literature suggests that carcinomas are uncommon in ‘great apes’
Among these apparent differences in disease incidence, one that has been emphasized in multiple reports is the rarity of occurrence of common human carcinomas in captive chimpanzees. Earlier surveys reporting on disease profiles and causes of death in captive chimpanzees from US facilities noted the rarity of these cancers [23–27]. A more recent thorough analysis [25] listed all neoplasms documented at two long-standing major US facilities from their inception many decades ago, through mid-2008. While the denominator (the total number of chimpanzees and life years at risk) was not made clear, there were only nine spontaneously arising epithelial malignancies reported over these many decades of observations of colonies that measured in the many hundreds. Moreover, even these cancers did not arise in the usual sites observed in humans, and were: one kidney carcinoma, one malignant carcinoma of the uterus, one basal-squamous carcinoma, one thyroid carcinoma, one adenocarcinoma of the parotid salivary gland, one nasopharyngeal carcinoma and three hepatocellular carcinomas (HCCs). There were a few additional cases of HCCs that were associated with experimental chronic hepatitis infection. No carcinomas of colon, breast, lung, prostate, stomach, pancreas or ovary were reported in this cohort [25]. Another recent review [28] indicates that the incidence of tumours in nonhuman primates does increase with age but that malignancies of the haematopoietic system are most frequent.
5. Simple ascertainment bias appears an unlikely explanation
In addition to ethical considerations, ‘great apes’ are charismatic animals, and also represent a very expensive and valuable long-term investment. Thus captive NHHs in research facilities and zoos are typically the recipients of excellent veterinary care and are often subjected to careful necropsies when they die. As a consequence, benign tumours and other neoplasms that are relatively uncommon in humans are routinely reported in this population (the above-mentioned review [25] documented 89 benign tumours). Overall, it thus seems unlikely that the apparent rarity of the common human carcinomas in NHHs is simply a matter of ascertainment bias. Notably, we are not just referring to microscopic disease, which is nowadays picked up by early detection methods in humans. Prior to the era of modern medicine, untreated carcinomas in humans used to manifest during an individual's lifetime with grossly evident or symptomatic primary or metastatic tumours. One would expect the same to happen in NHHs, who are not subjected to surveillance for microscopic disease. On the other hand, examination at post-mortem is of course biased towards internal organs.
6. Inadequacy of numbers surveyed
All told, there are probably only a few thousand NHHs that have been carefully studied in captivity over the past century. Thus, no reliable comments can be made about diseases that have the relative frequency of less than 1 per 100 in the human population. However, many of human carcinomas we have mentioned have an incidence rate well above that threshold. Thus, while a systematic re-analysis is needed, it seems likely that the population surveyed should have been sufficient to pick up these tumours, if they were indeed occurring. On the other hand, when larger populations of monkeys have been surveyed, the occurrence of carcinomas is more evident [26,28–30]. But even in a large series of baboons surveyed for squamous cell carcinomas, only 13 cases were found in more than 3000 animals in captivity [31].
7. Differences in life expectancy and population demographics
As mentioned earlier, most human carcinomas occur later in the lifespan. Thus, given the fact that the NHHs appear to age more rapidly and die earlier [32], one possible explanation is that they simply do not live long enough to experience the same carcinoma risk that humans do. However, with progressive improvements in veterinary care, these animals can now live into the fourth or even early fifth decade of life, and a few survive to the age of 60 [32]. Thus, one might have expected to see a few cases of adenocarcinomas of the colon, breast, ovary or pancreas, which do occur in humans before the age of 50. But any conclusions must remain speculative until there is a systematic survey of the entire population demographics of captive chimpanzees and other ‘great apes’ from all facilities that have routinely performed complete necropsies. Of course, this question is impractical to address for NHHs that live in the wild, because they die earlier, and also because necropsies are hardly ever possible in such cases. Necropsies performed on African and Asian sanctuary NHHs may be more detailed, but their accuracy would depend on the time of recovery of the bodies after death, as post-mortem autolysis would be rapid under tropical conditions.
8. Dietary factors
Dietary factors such as soluble fibre protection from colon cancer [33] and a red meat consumption risk for multiple carcinomas [34–40] must also be taken into consideration. Primate facilities have typically used commercially prepared blended foods (‘extruded pellets’ or ‘biscuits’, composed of many by-products of human food production), supplemented by fresh produce. However, a systematic survey of the dietary composition experienced by captive NHHs is lacking. Moreover, dietary requirements and gastrointestinal anatomy and physiology for each NHH are markedly different, so comparisons must be done carefully. Also, it is likely that dietary regimes at various facilities have been a moving target over time. Regardless, it seems unlikely that the dietary composition of captive NHHs is markedly different from that experienced by humans. With regard to one particular risk factor for human carcinomas, our recent work suggests that it is human-specific. In this instance, the nonhuman sialic acid Neu5Gc is metabolically incorporated from red meats (lamb, pork and beef) into human epithelia and endothelia, and therein interacts with circulating anti-Neu5Gc antibodies [39]. Our evidence suggests that the resulting chronic inflammation may contribute to cancer progression [39,40]. Even if the diet of NHHs happens to contain lots of Neu5Gc, this risk factor would not apply to the great apes as they all already express Neu5Gc naturally [41]. Regardless, the impact of red meat consumption on human carcinoma risk is modest [37,38], and this factor cannot by itself account for the overall difference.
9. Lifestyle and environmental factors
While the common squamous cell carcinomas in humans are typically associated with definitive environmental risk factors (smoking for lung cancer, ultraviolet radiation for skin cancer, papilloma virus infection for cervical cancer, etc.), the adenocarcinomas associated with internal organs do not have such clear-cut associations with environmental factors (other than a few examples such as red meat consumption). Captive chimpanzees likely also experience somewhat similar exposure to potential carcinogens in the air, water and food. With regard to breast cancer risk, it is worth noting that systematic vasectomy of males has long been used to curtail fertility in almost all current chimpanzee research and retirement facilities [17,42]. Thus, there are many living captive adult female chimpanzees that have never experienced pregnancy, live births or lactation. While these lifestyle changes constitute well-known risk factors for breast cancer in humans [43], there have been no reported cases of breast cancer in any chimpanzee to date. Again what is needed is a systematic survey of the population at potential risk, taking into account the number of years that have passed since the vasectomy policies were instituted.
10. Potential differences in immunosurveillance and/or immunostimulation
It is now clear that surveillance by cells of the immune system serves to eliminate many early tumours [44]. On the other hand, many of the same immune responses can facilitate the growth of established tumours by virtue of contributing to local chronic inflammation [45–47]. While this ‘double-edged sword’ role of the immune system in cancer is well recognized, it has only recently been appreciated how narrow the quantitative gap between the two mechanisms might be [48,49]. Siglecs are sialic-acid binding immunoglobulin-like lectins expressed on immune cells, playing multiple roles in human disease, including cancer [50]. In this regard, we have noted that the relative lack of Siglecs on human immune cells compared with those from the chimpanzee might contribute to increased immunoreactivity of some cell types [51,52]. While purely speculative, it is possible that this difference in inflammatory potential could also contribute to the increased risk of tumour progression in humans. More extensive surveys of the differences in responses of immune cells in whole blood samples suggest that the situation may be more complex [53].
11. Genetic susceptibility
As mentioned in §7, the rare inherited genetic variants that increase human cancer risk cannot account for the apparent difference in rates of sporadic adenocarcinomas. Attempts have therefore been made to look for genetic features unique to humans as a species that could contribute to increased risk. A comparative analysis of a defined set of 333 orthologous cancer genes in humans and chimpanzees showed a high degree of conservation [54]. However, a detailed analysis detected small differences in certain tumour suppressor genes, which might potentially influence the differences in cancer susceptibility [54]. Given the important role of telomere caps at chromosomal ends in modulating cell survival, ageing and cancer [55,56], it was reasonable to look for a difference in humans. Paradoxical to the finding of extended lifespan in humans [32,57], telomere lengths were found to be greater in nonhuman primates than in humans, and telomere shortening rates were not apparently different between humans and chimpanzees [58,59]. Given the importance of genome damage and DNA repair in cancer, it is also interesting that one study indicated that humans and chimpanzees differ in their cellular response to DNA damage and noncoding sequence elements of DNA repair-associated genes, with evidence for accelerated evolution in some promoter regions and introns [60]. Yet another theory considers the finding that expression of genes involved in programmed cell death of brain neurons is different between humans and chimpanzees, and predicts a reduced level of neuronal apoptosis in humans [61]. This pattern of expression is evidently maintained in other human organs, suggesting that cellular apoptosis may be generally reduced in humans relative to chimpanzees. Assuming that a decreased rate of neuronal apoptosis may have been important for increased cognitive ability in humans, this evolutionary process could have coincidently resulted in an increased risk of cancer and other diseases associated with reduced apoptotic functions [61]. A related mystery arises from the recent report indicating that the lifetime risk of cancers of many different human tissue types is strongly correlated with the total number of divisions of the normal self-renewing cells maintaining that tissue's homeostasis, i.e. the suggestion that the majority of cancers result from ‘bad luck’—random mutations arising during DNA replication in normal, noncancerous stem cells [10]. If this line of reasoning is correct, it suggests that there may be something very different about rates of stem renewal and/or apoptosis in humans versus other hominids. Considering another possibility, comparative epigenomic studies have observed differing patterns of DNA methylation in the brain between humans and chimpanzees [62,63]. While interpretation was cautioned because DNA methylation changes also vary with age, it is possible that epigenomic changes required for modulating brain gene expression during human evolution could have secondarily affected the impact of the non-neural epigenome in modulating cancer progression. Overall, while these or other potential genetic differences may contribute to the apparent discrepancy in carcinoma risk, there is no single leading candidate mechanism.
12. Infectious agents
It is well known that chronic infections can contribute to the incidence and progression of various cancers [45–47,64]. In this regard, a classic example is the high frequency of HCC in humans who have suffered long-term chronic infection with the hepatitis B or C viruses [65]. While the literature is limited, the impression is that HCCs following long-term hepatitis infections in chimpanzees are rather rare [25,66]. What are needed are systematic and complete data on how many chimpanzees in total have been chronically infected with HBV or HCV, their years at risk following infection, and the number of cases of HCCs that have since occurred in this population. Another striking difference between humans and NHHs is the lack of long-term endemic infectious retroviral infections in human populations [67–69]—setting aside the recent introduction of HIV and HTLV viruses into the human population from NHH sources [67,70,71]. As chronic retroviral infections are associated with increased risk of cancer, in general, this fact also appears counterintuitive.
13. Impact of the microbiome
It is now well established that the complex communities of microbes that normally live in different sites of the body (the microbiome) can have a very strong influence on local inflammation, food metabolism, as well as on the processing of bioactive molecules such as carcinogens. It also seems likely that components of the microbiota can promote chronic inflammation and tumour development via complex interactions with the innate and adaptive immune systems [72]. Given that many epithelial surfaces are in contact with the exterior world, it is not surprising that these locations feature the most extensive microbiomes. Assuming differences between human and NHH microbiomes [73–75], it is reasonable to speculate that this factor contributes to the relative risk of carcinoma development. In this regard, another consideration is the increasing use of antibiotics in humans, which could cause significant effects on the composition of the microbiome, and secondary effects on various biological processes [76,77]. As part of any survey, it would be interesting to compare the pattern and frequency of antibiotic usage between humans and captive NHHs.
14. Conclusion and perspectives
Considering all available evidence, it is not possible to definitively conclude that humans as a species are at higher risk of developing carcinomas, compared with our closest evolutionary relatives. To paraphrase Carl Sagan, absence of definitive evidence is not definitive evidence of absence. While the existing data make it likely that there is a major difference, a more systematic survey of all facilities that have cared for chimpanzees and other NHHs and carried out good necropsies is needed. While this had been the initial hope [15,16,18], it now seems unlikely that we will get much in-depth information from chimpanzee retirement facilities, which still await funding, and other resources to perform complete and thorough necropsies for comparative analyses. Collaboration with veterinarians working with in situ NHHs (free ranging and sanctuaries) could also yield some information about this comparison. However, funding and resources to achieve this goal are again limiting. The situation is particularly unfortunate: these naturally ageing populations are valuable and ephemeral sources for important demographic comparisons with humans, adding key insights into the genesis of some of the most common diseases of humans, and those of our closest evolutionary relatives.
Acknowledgements
We acknowledge the many investigators and workers who have cared for and studied the nonhuman primates discussed in this work. We also appreciate comments from Pascal Gagneux, and inspiration derived from the intellectual activities in the UCSD/Salk Center for Academic Research and Training in Anthropogeny (CARTA).
Funding statement
The authors are supported by National Cancer Institute grant no. R01CA38701.
Conflict of interests
The authors are cofounders of SiaMab Therapeutics Inc., a biotechnology company interested in the roles of sialic acids in cancer biology and therapeutics.
References
- 1.Alberts A, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P. 2015. Molecular biology of the cell, 6th edn New York, NY: Garland Science, Taylor and Francis Group. [Google Scholar]
- 2.Blanpain C, Fuchs E. 2014. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 344, 1242281 ( 10.1126/science.1242281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinberg RA. 2014. The biology of cancer, 2nd edn New York, NY: Garland Publishing. [Google Scholar]
- 4.Fearon ER. 2011. Molecular genetics of colorectal cancer. Annu. Rev. Pathol. 6, 479–507. ( 10.1146/annurev-pathol-011110-130235) [DOI] [PubMed] [Google Scholar]
- 5.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LAJ, Kinzler KW. 2013. Cancer genome landscapes. Science 339, 1546–1558. ( 10.1126/science.1235122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. ( 10.1016/j.cell.2011.02.013) [DOI] [PubMed] [Google Scholar]
- 7.Smith RA, Manassaram-Baptiste D, Brooks D, Cokkinides V, Doroshenk M, Saslow D, Wender RC, Brawley OW. 2014. Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 64, 30–51. ( 10.3322/caac.21212) [DOI] [PubMed] [Google Scholar]
- 8.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. 2014. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 64, 252–271. ( 10.3322/caac.21235) [DOI] [PubMed] [Google Scholar]
- 9.Finch CE. 2010. Evolution in health and medicine Sackler colloquium: evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc. Natl Acad. Sci. USA 107(Suppl. 1), 1718–1724. ( 10.1073/pnas.0909606106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomasetti C, Vogelstein B. 2015. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347, 78–81. ( 10.1126/science.1260825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodman M, Grossman LI, Wildman DE. 2005. Moving primate genomics beyond the chimpanzee genome. Trends Genet. 9, 511–517. ( 10.1016/j.tig.2005.06.012) [DOI] [PubMed] [Google Scholar]
- 12.Sims GE, Jun SR, Wu GA, Kim SH. 2009. Whole-genome phylogeny of mammals: evolutionary information in genic and nongenic regions. Proc. Natl Acad. Sci. USA 106, 17 077–17 082. ( 10.1073/pnas.0909377106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enard W. 2012. Functional primate genomics—leveraging the medical potential. J. Mol. Med. (Berl.) 90, 471–480. ( 10.1007/s00109-012-0901-4) [DOI] [PubMed] [Google Scholar]
- 14.Olson MV, Varki A. 2004. Genomics. The chimpanzee genome—a bittersweet celebration. Science 305, 191–192. ( 10.1126/science.1100975) [DOI] [PubMed] [Google Scholar]
- 15.Gagneux P, Moore JJ, Varki A. 2005. The ethics of research on great apes. Nature 437, 27–29. ( 10.1038/437027a) [DOI] [PubMed] [Google Scholar]
- 16.Brent L. 2004. Solutions for research chimpanzees. Lab. Anim. (NY) 33, 37–43. [DOI] [PubMed] [Google Scholar]
- 17.US Institute of Medicine and National Research Council Committee on the Use of Chimpanzees in Biomedical and Behavioral Research 2011. Chimpanzees in biomedical and behavioral research: assessing the necessity (eds BM Altevogt, DE Pankevich, MK Shelton-Davenport, JP Kahn) Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 18.de Waal FB. 2012. Research chimpanzees may get a break. PLoS Biol. 10, e1001291 ( 10.1371/journal.pbio.1001291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varki N, et al. 2009. Heart disease is common in humans and chimpanzees, but is caused by different pathological processes. Evol. Appl. 2, 101–112. ( 10.1111/j.1752-4571.2008.00064.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finch CE, Austad SN. 2014. Commentary: is Alzheimer's disease uniquely human? Neurobiol. Aging 36, 553–555. ( 10.1016/j.neurobiolaging.2014.10.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varki NM, Strobert E, Dick EJ, Benirschke K, Varki A. 2011. Biomedical differences between human and nonhuman hominids: potential roles for uniquely human aspects of sialic acid biology. Annu. Rev. Pathol. 6, 365–393. ( 10.1146/annurev-pathol-011110-130315) [DOI] [PubMed] [Google Scholar]
- 22.Varki A. 2012. Nothing in medicine makes sense, except in the light of evolution. J. Mol. Med. (Berl.) 90, 481–494. ( 10.1007/s00109-012-0900-5) [DOI] [PubMed] [Google Scholar]
- 23.McClure HM. 1973. Tumors in nonhuman primates: observations during a six-year period in the Yerkes primate center colony. Am. J Phys. Anthropol. 38, 425–429. ( 10.1002/ajpa.1330380243) [DOI] [PubMed] [Google Scholar]
- 24.Beniashvili DS. 1989. An overview of the world literature on spontaneous tumors in nonhuman primates. J. Med. Primatol. 18, 423–437. [PubMed] [Google Scholar]
- 25.Brown SL, Anderson DC, Dick EJJ, Guardado-Mendoza R, Garcia AP, Hubbard GB. 2009. Neoplasia in the chimpanzee (Pan spp.). J. Med. Primatol. 38, 137–144. ( 10.1111/j.1600-0684.2008.00321.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller AD. 2012. Neoplasia and proliferative disorders of nonhuman primates. In Nonhuman primates in biomedical research (eds Abee CR, Mansfield K, Tardif SD, Morris T.), ch. 6, 2nd edn Amsterdam, The Netherlands: Academic Press. [Google Scholar]
- 27.Lowenstine LJ. 2003. A primer of primate pathology: lesions and nonlesions. Toxicol. Pathol. 31(Suppl), 92–102. ( 10.1080/01926230390177668) [DOI] [PubMed] [Google Scholar]
- 28.Lapin BA, Yakovleva LA. 2014. Spontaneous and experimental malignancies in non-human primates. J. Med. Primatol. 43, 100–110. ( 10.1111/jmp.12098) [DOI] [PubMed] [Google Scholar]
- 29.Remick AK, Van Wettere AJ, Williams CV. 2009. Neoplasia in prosimians: case series from a captive prosimian population and literature review. Vet. Pathol. 46, 746–772. ( 10.1354/vp.08-VP-0154-R-FL) [DOI] [PubMed] [Google Scholar]
- 30.Simmons HA, Mattison JA. 2011. The incidence of spontaneous neoplasia in two populations of captive rhesus macaques (Macaca mulatta). Antioxid. Redox Signal. 14, 221–227. ( 10.1089/ars.2010.3311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haddad JL, Dick EJJ, Guardado-Mendoza R, Hubbard GB. 2009. Spontaneous squamous cell carcinomas in 13 baboons, a first report in a spider monkey, and a review of the non-human primate literature. J. Med. Primatol. 38, 175–186. ( 10.1111/j.1600-0684.2009.00338.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawkes K, Smith KR, Robson SL. 2009. Mortality and fertility rates in humans and chimpanzees: how within-species variation complicates cross-species comparisons. Am. J. Hum. Biol. 21, 578–586. ( 10.1002/ajhb.20890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson AJ, Collins PD. 2011. Colon cancer: a civilization disorder. Dig. Dis. 29, 222–228. ( 10.1159/000323926) [DOI] [PubMed] [Google Scholar]
- 34.Fraser GE. 1999. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am. J. Clin. Nutr. 70, 532S–538S. [DOI] [PubMed] [Google Scholar]
- 35.Key TJ, et al. 1999. Mortality in vegetarians and nonvegetarians: detailed findings from a collaborative analysis of 5 prospective studies. Am. J. Clin. Nutr. 70, 516S–524S. [DOI] [PubMed] [Google Scholar]
- 36.Wiseman M. 2008. The second world cancer research fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc. Nutr. Soc. 67, 253–256. ( 10.1017/S002966510800712X) [DOI] [PubMed] [Google Scholar]
- 37.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. 2009. Meat intake and mortality: a prospective study of over half a million people. Arch. Intern. Med. 169, 562–571. ( 10.1001/archinternmed.2009.6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. 2012. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch. Intern. Med. 172, 555–563. ( 10.1001/archinternmed.2011.2287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samraj AN, Laubli H, Varki N, Varki A. 2014. Involvement of a non-human sialic acid in human cancer. Front. Oncol. 4, 33 ( 10.3389/fonc.2014.00033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samraj AN, et al. 2014. A red meat-derived glycan promotes inflammation and cancer progression. Proc. Natl Acad. Sci. USA 112, 542–547. ( 10.1073/pnas.1417508112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muchmore EA, Diaz S, Varki A. 1998. A structural difference between the cell surfaces of humans and the great apes. Am. J. Phys. Anthropol. 107, 187–198. () [DOI] [PubMed] [Google Scholar]
- 42.Hoffman K, Howell S, Schwandt M, Fritz J. 2002. Vasectomy as a birth control modality for captive chimpanzees. Lab. Anim. (NY) 31, 45–48. ( 10.1038/5000184) [DOI] [PubMed] [Google Scholar]
- 43.Anderson KN, Schwab RB, Martinez ME. 2014. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res. Treat. 144, 1–10. ( 10.1007/s10549-014-2852-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schreiber RD, Old LJ, Smyth MJ. 2011. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 331, 1565–1570. ( 10.1126/science.1203486) [DOI] [PubMed] [Google Scholar]
- 45.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. 2013. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 13, 759–771. ( 10.1038/nrc3611) [DOI] [PubMed] [Google Scholar]
- 46.Mantovani A, Allavena P, Sica A, Balkwill F. 2008. Cancer-related inflammation. Nature 454, 436–444. ( 10.1038/nature07205) [DOI] [PubMed] [Google Scholar]
- 47.Coussens LM, Zitvogel L, Palucka AK. 2013. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science 339, 286–291. ( 10.1126/science.1232227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearce OM, Laubli H, Verhagen A, Secrest P, Zhang J, Varki NM, Crocker PR, Bui JD, Varki A. 2014. Inverse hormesis of cancer growth mediated by narrow ranges of tumor-directed antibodies. Proc. Natl Acad. Sci. USA 111, 5998–6003. ( 10.1073/pnas.1209067111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pearce OM, Laubli H, Bui J, Varki A. 2014. Hormesis in cancer immunology: does the quantity of an immune reactant matter? Oncoimmunology 3, e29312 ( 10.4161/onci.29312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laubli H, et al. 2014. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc. Natl Acad. Sci. USA 111, 14 211–14 216. ( 10.1073/pnas.1409580111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soto PC, Karris MY, Spina CA, Richman DD, Varki A. 2012. Cell-intrinsic mechanism involving Siglec-5 associated with divergent outcomes of HIV-1 infection in human and chimpanzee CD4 T cells. J. Mol. Med. (Berl.) 91, 261–270. ( 10.1007/s00109-012-0951-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Padler-Karavani V, et al. 2014. Rapid evolution of binding specificities and expression patterns of inhibitory CD33-related Siglecs in primates. FASEB J. 28, 1280–1293. ( 10.1096/fj.13-241497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barreiro LB, Marioni JC, Blekhman R, Stephens M, Gilad Y. 2010. Functional comparison of innate immune signaling pathways in primates. PLoS Genet. 6, e1001249 ( 10.1371/journal.pgen.1001249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puente XS, Velasco G, Gutierrez-Fernandez A, Bertranpetit J, King MC, Lopez-Otin C. 2006. Comparative analysis of cancer genes in the human and chimpanzee genomes. BMC Genom. 7, 15 ( 10.1186/1471-2164-7-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell 153, 1194–1217. ( 10.1016/j.cell.2013.05.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holohan B, Wright WE, Shay JW. 2014. Cell biology of disease: telomeropathies: an emerging spectrum disorder. J. Cell Biol. 205, 289–299. ( 10.1083/jcb.201401012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hawkes K. 2010. Colloquium paper: how grandmother effects plus individual variation in frailty shape fertility and mortality: guidance from human-chimpanzee comparisons. Proc. Natl Acad. Sci. USA 107(Suppl. 2), 8977–8984. ( 10.1073/pnas.0914627107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kakuo S, Asaoka K, Ide T. 1999. Human is a unique species among primates in terms of telomere length. Biochem. Biophys. Res. Commun. 263, 308–314. ( 10.1006/bbrc.1999.1385) [DOI] [PubMed] [Google Scholar]
- 59.Tackney J, Cawthon RM, Coxworth JE, Hawkes K. 2014. Blood cell telomere lengths and shortening rates of chimpanzee and human females. Am. J. Hum. Biol. 26, 452–460. ( 10.1002/ajhb.22538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weis E, Galetzka D, Herlyn H, Schneider E, Haaf T. 2008. Humans and chimpanzees differ in their cellular response to DNA damage and non-coding sequence elements of DNA repair-associated genes. Cytogenet. Genome Res. 122, 92–102. ( 10.1159/000163086) [DOI] [PubMed] [Google Scholar]
- 61.Arora G, Polavarapu N, McDonald JF. 2009. Did natural selection for increased cognitive ability in humans lead to an elevated risk of cancer? Med. Hypotheses 73, 453–456. ( 10.1016/j.mehy.2009.03.035) [DOI] [PubMed] [Google Scholar]
- 62.Enard W, Fassbender A, Model F, Adorjan P, Paabo S, Olek A. 2004. Differences in DNA methylation patterns between humans and chimpanzees. Curr. Biol. 14, R148–R149. ( 10.1016/S0960-9822(04)00072-7) [DOI] [PubMed] [Google Scholar]
- 63.Zeng J, Konopka G, Hunt BG, Preuss TM, Geschwind D, Yi SV. 2012. Divergent whole-genome methylation maps of human and chimpanzee brains reveal epigenetic basis of human regulatory evolution. Am. J. Hum. Genet. 91, 455–465. ( 10.1016/j.ajhg.2012.07.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ewald PW, Swain Ewald HA. 2012. Infection, mutation, and cancer evolution. J. Mol. Med. (Berl.) 90, 535–541. ( 10.1007/s00109-012-0891-2) [DOI] [PubMed] [Google Scholar]
- 65.Guidotti LG, Chisari FV. 2006. Immunobiology and pathogenesis of viral hepatitis. Annu. Rev. Pathol. 1, 23–61. ( 10.1146/annurev.pathol.1.110304.100230) [DOI] [PubMed] [Google Scholar]
- 66.Porter BF, Goens SD, Brasky KM, Hubbard GB. 2004. A case report of hepatocellular carcinoma and focal nodular hyperplasia with a myelolipoma in two chimpanzees and a review of spontaneous hepatobiliary tumors in non-human primates. J. Med. Primatol. 33, 38–47. ( 10.1111/j.1600-0684.2003.00048.x) [DOI] [PubMed] [Google Scholar]
- 67.Schweizer M, Turek R, Hahn H, Schliephake A, Netzer KO, Eder G, Reinhardt M, Rethwilm A, Neumann-Haefelin D. 1995. Markers of foamy virus infections in monkeys, apes, and accidentally infected humans: appropriate testing fails to confirm suspected foamy virus prevalence in humans. AIDS Res. Hum. Retroviruses 11, 161–170. ( 10.1089/aid.1995.11.161) [DOI] [PubMed] [Google Scholar]
- 68.Switzer WM, et al. 2005. Ancient co-speciation of simian foamy viruses and primates. Nature 434, 376–380. ( 10.1038/nature03341) [DOI] [PubMed] [Google Scholar]
- 69.Rua R, Betsem E, Calattini S, Saib A, Gessain A. 2012. Genetic characterization of simian foamy viruses infecting humans. J. Virol. 86, 13 350–13 359. ( 10.1128/JVI.01715-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharp PM, Hahn BH. 2010. The evolution of HIV-1 and the origin of AIDS. Phil. Trans. R. Soc. B 365, 2487–2494. ( 10.1098/rstb.2010.0031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keele BF. 2006. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 313, 523–526. ( 10.1126/science.1126531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gagliani N, Hu B, Huber S, Elinav E, Flavell RA. 2014. The fire within: microbes inflame tumors. Cell 157, 776–783. ( 10.1016/j.cell.2014.03.006) [DOI] [PubMed] [Google Scholar]
- 73.Degnan PH, Pusey AE, Lonsdorf EV, Goodall J, Wroblewski EE, Wilson ML, Rudicell RS, Hahn BH, Ochman H. 2012. Factors associated with the diversification of the gut microbial communities within chimpanzees from Gombe National Park. Proc. Natl Acad. Sci. USA 109, 13 034–13 039. ( 10.1073/pnas.1110994109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marcobal A, Southwick AM, Earle KA, Sonnenburg JL. 2013. A refined palate: Bacterial consumption of host glycans in the gut. Glycobiology 23, 1038–1046. ( 10.1093/glycob/cwt040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moeller AH, Peeters M, Ndjango JB, Li Y, Hahn BH, Ochman H. 2013. Sympatric chimpanzees and gorillas harbor convergent gut microbial communities. Genome Res. 23, 1715–1720. ( 10.1101/gr.154773.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cho I, et al. 2012. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626. ( 10.1038/nature11400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cox LM, et al. 2014. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158, 705–721. ( 10.1016/j.cell.2014.05.052) [DOI] [PMC free article] [PubMed] [Google Scholar]


