Abstract
Naturally occurring cancers in non-laboratory species have great potential in helping to decipher the often complex causes of neoplasia. Wild animal models could add substantially to our understanding of carcinogenesis, particularly of genetic and environmental interactions, but they are currently underutilized. Studying neoplasia in wild animals is difficult and especially challenging in marine mammals owing to their inaccessibility, lack of exposure history, and ethical, logistical and legal limits on experimentation. Despite this, California sea lions (Zalophus californianus) offer an opportunity to investigate risk factors for neoplasia development that have implications for terrestrial mammals and humans who share much of their environment and diet. A relatively accessible California sea lion population on the west coast of the USA has a high prevalence of urogenital carcinoma and is regularly sampled during veterinary care in wildlife rehabilitation centres. Collaborative studies have revealed that genotype, persistent organic pollutants and a herpesvirus are all associated with this cancer. This paper reviews research to date on the epidemiology and pathogenesis of urogenital carcinoma in this species, and presents the California sea lion as an important and currently underexploited wild animal model of carcinogenesis.
Keywords: California sea lion, urogenital carcinoma, genetics, contaminants, herpesvirus
1. Introduction
The use of laboratory animal models in the study of cancer has provided enormous insight into carcinogenesis [1] and remains integral to studying the biochemical and physiological processes involved in the onset and development of disease [2]. However, extrapolating findings from laboratory models to other species, including humans, is challenging [3]. These models are often generated in inbred mice strains in which the complex pleiotropic interactions between multiple genes and the environment cannot easily be replicated. It has therefore been suggested that naturally occurring cancers in non-laboratory species, such as domestic dogs, could provide solutions and bridge some of the gaps between natural disease and translational medicine [4]. While dogs are becoming of increasing interest as useful models for a variety of human cancers such as osteosarcoma, gastrointestinal stromal tumours and prostate cancer [5,6], the utility of other species as models is being increasingly explored, including lower order species such as fish and invertebrates [7]. Thus, in line with current thinking, this paper discusses the potential that study of a naturally occurring cancer in a non-laboratory species, the California sea lion (Zalophus californianus, CSL), could provide insights into the complex interactions among genes, infection and the environment that lead to carcinogenesis. This is extremely difficult to replicate in an experimental model. The value of the use of the CSL over species such as the domestic dog as a model for carcinogenesis lies in its natural exposure to environmental contaminants in a diet shared by humans, as well as in its uncontrolled reproduction allowing for natural selection of genes that may protect against neoplasia but are as yet uncharacterized, and in the practical considerations of ease of repeated sampling and availability of large samples sizes for case control studies. The knowledge to be gained from studying disease processes in wildlife for their wider implications beyond the specific veterinary, ecological or evolutionary questions of interest has so far been limited to just a few examples [8], but clearly studies on genetically diverse animals in their natural environment has great potential that should be explored further.
Neoplasia has been reported in a range of marine mammal species worldwide [9], with the most consistently highest prevalence over time being a specific carcinoma in the CSL that has been studied in some detail as it was first recognized in the 1980s [10]. Despite the challenges involved in investigating disease in a species that spends much of its life at sea, sick animals that strand and are admitted to The Marine Mammal Center in California have provided an invaluable resource for the study of this disease. This has enabled investigators to explore the disease process and its potential causes for many years. While these studies have been driven by veterinary health and welfare concerns for individual animals, it has become increasing clear that this model may also provide invaluable insights to the broader fields of veterinary and human oncology [11].
2. Metastatic carcinoma of urogenital origin in the California sea lion
California sea lions are large carnivorous mammals ranging along the west coast of North America from Baja California, Mexico in the south to British Columbia in the north [12]. They belong to the suborder Pinnipedia, which includes the seals, fur seals and walruses which all shared a common ancestor with dogs approximately 45 Mya [13]. CSL spend periods on land and are subsequently more accessible as a study species than many other marine mammals. Furthermore, approximately 1000 animals strand dead along beaches each year and are examined and sampled at specialized wildlife rehabilitation facilities, offering an opportunity for sampling both carcinoma cases and control animals which strand for other reasons [14]. In the context of a new model species for cancer, CSL exhibit a high prevalence of metastatic carcinoma of urogenital origin (UGC) with 26% of adult CSL examined post-mortem at The Marine Mammal Center, California (TMMC), during a 15-year period (1998–2012) being affected (FMD Gulland 2014, personal observation). This was an increase from an 18% prevalence noted in animals admitted between 1979 and 1994 [10]. Although the true prevalence of carcinoma in CSLs is likely to be lower than this, as only the sicker animals are likely to strand and the overall wild population is over 180 000 animals [15], this is still a very significant number of animals and cause of death in this population.
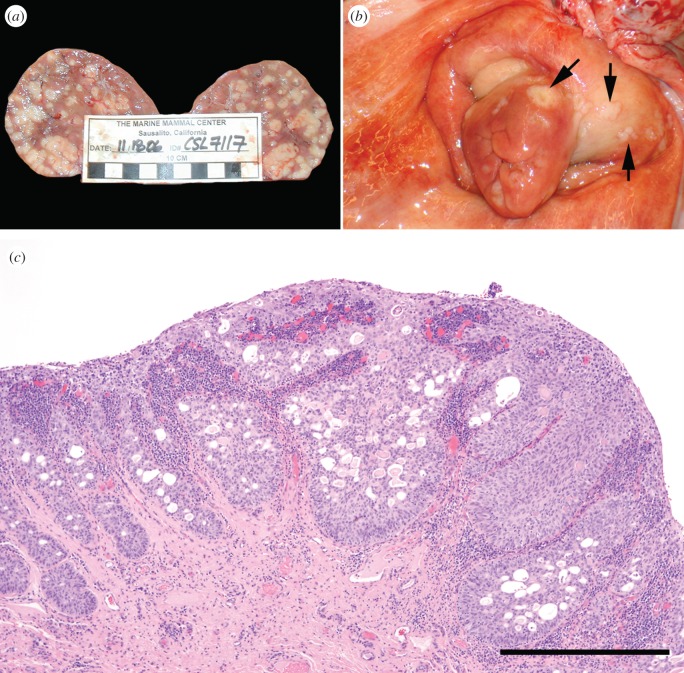
Urogenital carcinoma in CSL affects sub-adult and adult animals of both sexes with a mean age of approximately 8 years old [10,16]. The cancer typically originates in the genital tract and metastasizes to a number of sites including the abdominal and pelvic lymph nodes, kidney and urinary bladder as well as more distant sites, such as the liver, lungs and spleen (figure 1a). Initial work suggested that the origin of the tumour was the urinary tract, based on histological findings of presumed transitional cells [10], however, later work identified intraepithelial neoplasia (IEN) in the cervix and vagina of females and the penis, prepuce and urethra of males (figure 1b) [17]. IEN lesions also occur in humans with cervical cancer where they are classified according to the cervical intraepithelial neoplasia (CIN) grading system and range from CIN I (mild) to CIN III (carcinoma in situ) [18–20]. In the CSL, histopathological lesions have been described as low-grade intraepithelial (LGIL) lesions, which correspond to CIN I, and high-grade intraepithelial (HGIL) lesions, corresponding to CIN II and III (figure 1c). IEN can be found in animals without metastases, however, once invasion of neoplastic cells past the basement membrane is noted, metastasis is typically widespread [21].
Figure 1.
(a) Kidney from a California sea lion with metastatic urogenital carcinoma. There are numerous pale tan masses scattered throughout the parenchyma. (b) Cervix from a California sea lion with urogenital carcinoma. Note the raised, irregular pale yellow masses on the external cervix (arrows). (c) Haematoxylin and eosin stained section of cervix from a California sea lion with high-grade intraepithelial neoplasia. Scale bar, 500 µm. (Online version in colour.)
3. Aetiology of urogenital carcinoma in the California sea lion
To date, four main areas of potential aetiological importance have been explored based on risk factors identified in other species and other types of cancer: hormone receptor expression, genetic factors, contaminant exposure and infectious disease. However, further work is clearly necessary to fully understand the development of this complex condition which will likely include as yet unknown factors. In the light of this, the Sea Lion Cancer Consortium (SLiCC) was established in 2010 (http://www.smru.st-andrews.ac.uk/slicc/) to coordinate and foster research into the disease.
(a). Variation in hormone receptor and p53 expression
To increase characterization of the CSL carcinoma and guide further research into its aetiology, hormonal receptor expression was explored owing to location of the tumour in the reproductive tract. Using immunohistochemistry (IHC), the expression of the progesterone receptor (PR) and the oestrogen receptor alpha (ER-α) was studied in tumours and affected and unaffected genital epithelium [21]. Although the PR expression showed no difference, ER-α expression was significantly reduced in affected tissue [21]. ER-α and PR have previously been identified as prognostic markers in assessing breast cancer in humans with loss of expression of ER-α and/or PR indicating poor prognosis [22,23]. In human cervical cancer, a study had similar results to those in sea lions with loss of ER expression but increased PR expression in IEN [24]. Studies in other animals have identified similar associations. Increased ER-α and PR expression were found to be correlated with a better prognosis in canine mammary tumours [25,26]. In rabbits with uterine adenocarcinoma, however, expression of ER-α and PR did not reflect prognosis, despite varying hormone receptor expression playing a role in the development of two different types of tumours, papillary or tubular [27]. The discrepancies identified suggest that the usefulness of these receptors as markers may be species and/or tumour type dependent. Hormone cofactors could play a role in either neoplastic transformation or proliferation of neoplastic cells in CSL [21].
The reduction in ER-α identified in the tumour-affected tissue in the CSL coincided with increased Ki67 (cell proliferation) index and p53 expression [21]. The protein p53 is the product of expression of the gene TP53, a known tumour suppressor gene [28]. Increased expression of this gene is recognized in a variety of cancers in humans and is also suggested as a possible prognostic marker in non-small cell lung cancer alongside Ki67 expression [29]. Similarly, p53 overexpression has been identified as a prognostic marker in canine mammary cancer [30]. The increased labelling of p53 in neoplastic tissues in the CSL suggests that it may be involved in UGC pathogenesis [21].
(b). Genetic factors
Genetic factors have been explored in CSL with UGC including diversity of the major histocompatibility class II locus and the potential effect of inbreeding. In the CSL, the presence of a specific MHC class II locus (Zaca-DRB.A) was associated with the presence of UGC [31]. The DRB family of genes in the CSL consists of eight loci designated Zaca-DRB.A to Zaca-DRB.H [31,32] and varying combinations of these have been identified. However, the presence of the Zaca-DRB.A locus in any combination was significantly associated with the presence of UGC [31]. The importance of MHC loci has additionally been noted in Devil facial tumour disease (DFTD), a transmissible invasive facial tumour of Tasmanian Devils (Sarcophilus harrisii), typified by large scale chromosome aberrations [33]. Loss or lack of MHC diversity is postulated to contribute to the successful spread of the disease due to reduced effectiveness of the immune response [34].
A further genetic association with UGC was identified in the CSL indicating the potential effect of inbreeding on susceptibility to cancer [35]. The study used microsatellite markers to evaluate variation in the susceptibility to a number of diseases using a measure of heterozygosity called ‘internal relatedness’ (IR). Interestingly, the disease group with the highest IR score was animals with cancer [35]. Further analysis found that the strength of the association was driven by a particular microsatellite in the animals with neoplasia, namely Pv11 (KT Acevedo-Whitehouse 2009, unpublished data). Recent work involving the genotyping of 383 adult and sub-adult CSL as part of a case–control study identified that animals homozygous at the Pv11 locus were almost twice as likely to suffer from UGC as those heterozygous at that locus [11].
Comparative genomic studies, taking advantage of the synteny between the CSL and dog genomes, placed the Pv11 microsatellite within a gene called heparanase 2 (HPSE2) [11]. In humans, the protein heparanase 2 (HPA2) encoded by HPSE2 has been associated with numerous cancers [36–39]. Initial studies in the CSL did identify mRNA expression of HPSE2 in female lower genital tract tissue both in animals with UGC and those without, but did not find a difference according to disease state or between different Pv11 genotypes [11]. However, IHC studies labelled the HPA2 protein in animals of a single homozygous Pv11 genotype, but only if they had suffered from UGC. This strongly implied that the HPSE2 gene and Pv11 microsatellite were linked, suggesting that HPSE2 may be the first cancer gene identified in a wildlife species [11]. However, uncertainty exists surrounding the role of HPA2, especially in its relationship to the inhibition or promotion of neoplasia, presenting the question of whether this gene has oncogenic activity or is in fact a tumour suppressor gene. The location of the Pv11 microsatellite within the HSPE2 gene of the CSL, as well as its link with homozygosity and UGC, offers another avenue of investigation for the characterization of HPA2 function and supports further investigation into the wider involvement of HPSE2 in mammalian cancers.
(c). Environmental contaminants
Environmental contaminants, particularly those of the polycyclic aromatic hydrocarbon (PAH) and organochlorine (OC) groups of compounds, have been implicated in the aetiology of various cancers in humans [40–42], in laboratory animal models [43] and in wildlife [44–46]. These ubiquitous persistent pollutants, found in both the terrestrial and marine environments, have arisen through the widespread use of industrial chemicals such as the polychlorinated biphenyls (found in capacitors and transformers) and pesticides such as DDT. While the production and use of some of these compounds has been largely banned in the USA since the 1980s, their persistent and lipophilic nature means they will continue to be present at sometimes very high levels in the environment and thus continue to be a concern for human health [47]. For marine mammals they have particular significance as due to their high affinity for lipids they are biomagnified through the food chain and are stored in the blubber to be remobilized when fat stores are used for energy [48].
Environmental studies have identified OC and PAH pollutants in coastal waters around California [49–51]. In particular, OCs have been found in high levels in the Southern California Bight adjacent to the Channel Islands where CSLs breed and pup [50]. The OCs, particularly the DDTs, are also found at relatively high levels in CSL blubber compared to levels in other marine mammals, and cross the placenta to expose developing fetal tissues to these compounds [52–54]. They are also transferred to the offspring during lactation as their highly lipophilic nature mean they are found at high levels in the milk. The levels of OCs in the blubber of CSLs diagnosed with UGC were found to be higher than in non-cancer animals [55]. However, confounding factors such as the effect of changing body condition on blubber lipid dynamics confuse the association, as blubber OC levels increase when blubber lipid is lost during chronic disease such as cancer. PAH adducts have been identified in the liver of CSLs, however, as yet a link with neoplasia has not been established [56].
(d). Infectious disease
To date, two infectious agents have been associated with UGC in the CSL: Otarine herpes virus 1 (OtHV-1), a gammaherpesvirus [16,17,57] and beta (β) haemolytic Streptococcus [58]. The latter bacteria is common in chronic inflammatory lesions in CSLs, so may not play a significant role in carcinoma aetiology, although in humans certain cervical bacteria have been associated with presence of CIN [59]. OtHV-1 is a gammaherpesvirus in the genus Rhadinovirus and is phylogenetically related to human herpevirus-8 (HHV-8), an oncogenic herpesvirus and the aetiological agent of Kaposi's sarcoma [17,57,60]. In one study, OtHV-1 was present in all animals suffering from UGC and was more common in tissues of the lower genital tract [16,57]. It was also present at a lower prevalence in non-cancer sea lions, with prevalence increasing at sexual maturity [16]. This finding, coupled with the higher prevalence of OtHV-1 in the genital tract, led the authors to suggest that the virus is sexually transmitted and that there was similarity between the epidemiology of OtHV-1 infection in CSL and HHV-8 in humans [61]. Studies into the epidemiology of HHV-8 have suggested that sexual activity plays an important role in the transmission of the virus [62], particularly in the case of AIDS-associated Kaposi's sarcoma [63,64].
4. Urogenital carcinoma in the California sea lion exhibits similarities to cervical cancer in women
Cancer of cervical origin is a major cause of morbidity and mortality in humans [65] and work undertaken on UGC in CSL has revealed some interesting parallels between the diseases. In the wild, CSLs can live up to 20 years, however, UGC affects animals with a mean age of 8 years. Therefore, it is not a disease typical of old age [10], mirroring human cervical carcinoma, which predominately occurs in adult but not necessarily aged women [16,66,67]. Worldwide cervical cancer in women is strongly associated with papillomavirus 16 and 18 (HPV16 and 18) which has influenced vaccine development [65,68,69]. Papillomaviruses surprisingly have not been detected to date in CSL UGC; however, the potential involvement of an oncogenic herpesvirus (OtHV-1) in the aetiology of the disease in is an area of continued investigation (FMD Gulland 2014, personal communication).
Identification of an MHC class II locus of importance in the development of the UGC in the CSL is reported [31]; likewise, in humans certain MHC class II alleles are associated with an increased risk of cervical cancer [70]. A genetic basis for the disease has additionally been investigated in CSL in relation to inbreeding, where UGC is noted to be more common in inbred individuals [35,71]. Inbreeding in human populations is recognized among certain ethnicities; however, an increase in cervical cancer is not evident [72], suggesting that the influence of other risk factors such as exposure to HPV and lifestyle are important. The recent work identifying homozygosity at the Pv11 microsatellite as a risk factor for disease in CSL subsequently identified HPSE2 as a potential gene of interest. Increased labelling of HPA2 with increasing severity of disease is noted in human cervical tissues [73]. Preliminary studies in tissues from CSL identified differential labelling of HPA2 according to genotype but did not find an association between quantity of labelling and severity of disease [11].
The clear parallels that exist between the disease in humans and CSLs, especially in age of occurrence, histomorphology, association with viral infection and MHC class II alleles, suggest that the California sea lion may, perhaps surprisingly, be a suitable model for the disease in humans. The potential role of organochlorines in the aetiology also emphasize the value of the CSL as a natural model for neoplasia over domestic animals such as the dog that are fed artificial diets and not exposed to the environmental contaminants marine mammals and humans are.
5. Conclusion
The work undertaken to date on CSL UGC supports a multi-factorial aetiology of the disease, and offers avenues for further research into the interactions and potential synergistic effects of these factors, at the whole animal, the cellular and the molecular levels. Investigating diseases in wildlife involves many difficulties not encountered using other approaches, yet the parallels that exist between diseases in humans and other animals show it to be an area highly worthy of further exploration. The ability to carry out well designed, robust case control studies that can elucidate the environmental risk factors as well as taking a molecular and genetic approach, such as has been the case in the study of UGC in CSLs, may provide a comprehensive picture of the carcinogenic process not possible with standard in vitro, ex vivo or in vivo laboratory methodologies. The value of using a wild marine mammal over other model animals such as domestic dogs is that these wild animals are naturally exposed to a mix of environmental contaminants in their diet that domesticated species are not. Furthermore, breeding in wild CSL is uncontrolled, so natural selection for genes that may be important in protection from neoplasia but are as yet unknown may occur, making CSLs a useful model for exploring genetic factors in cancer development. Finally, as CSLs are not pet animals, it is possible to design case control studies with repeated sampling that have robust epidemiological designs. Thus, studying naturally occurring neoplasms in tractable wildlife models presents us with new opportunities that will undoubtedly produce equally unexpected findings. Such opportunities will also offer exciting new collaborations between human and veterinary oncologists.
Acknowledgements
We thank all members of the Sea Lion Cancer Consortium, the staff and volunteers of The Marine Mammal Center, and the National Marine Fisheries Service for assistance in all aspects of the study of sea lion cancer.
Ethical statement
All research on California sea lions referred to in this manuscript was performed under accordance with the Marine Mammal Protection Act 1972 and the US Animal Welfare Act. Research was conducted under MMPA Permit No. 932-1905/MA-009526.
Data accessibility
The data published in this study is available through the British Oceanographic Data Centre.
Authors' contributions
Manuscript writing and data compilation H.M.B., F.M.D.G., J.A.H., K.M.C., A.J.H.; histology K.M.C.
Competing interests
We have no competing interests.
Funding statement
F.M.D.G. was supported by The Marine Mammal Center, with research funds from the NMFS John Prescott grant program. J.A.H. was supported by a Biotechnology and Biological Sciences Research Council Institute Strategic Program on Livestock Viral Diseases awarded to The Pirbright Institute. A.J.H. and H.M.B. were supported by funding from the Natural Environment Research Council.
References
- 1.Workman P, et al. 2010. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 102, 1555–1577. ( 10.1038/sj.bjc.6605642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu S, Yang M, Nam KT. 2014. Mouse models of gastric carcinogenesis. J. Gastric Cancer 14, 67–86. ( 10.5230/jgc.2014.14.2.67) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mak IW, Evaniew N, Ghert M. 2014. Lost in translation: animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 6, 114–118. [PMC free article] [PubMed] [Google Scholar]
- 4.Rowell JL, McCarthy DO, Alvarez CE. 2011. Dog models of naturally occurring cancer. Trends Mol. Med. 17, 380–388. ( 10.1016/j.molmed.2011.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langdon SP. 2012. Animal modeling of cancer pathology and studying tumor response to therapy. Curr. Drug Targets 13, 1535–1547. ( 10.2174/138945012803530152) [DOI] [PubMed] [Google Scholar]
- 6.Pinho SS, Carvalho S, Cabral J, Reis CA, Gartner F. 2012. Canine tumors: a spontaneous animal model of human carcinogenesis. Transl. Res. 159, 165–172. ( 10.1016/j.trsl.2011.11.005) [DOI] [PubMed] [Google Scholar]
- 7.Kelley ML, Winge P, Heaney JD, Stephens RE, Farell JH, Van Beneden RJ, Reinisch CL, Lesser MP, Walker CW. 2001. Expression of homologues for p53 and p73 in the softshell clam (Mya arenaria), a naturally-occurring model for human cancer. Oncogene 20, 748–758. ( 10.1038/sj.onc.1204144) [DOI] [PubMed] [Google Scholar]
- 8.van der Schalie WH, et al. 1999. Animals as sentinels of human health hazards of environmental chemicals. Environ. Health Perspect. 107, 309–315. ( 10.1289/ehp.99107309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman SJ, Smith SA. 2006. Marine mammal neoplasia: a review. Vet. Pathol. 43, 865–880. ( 10.1354/vp.43-6-865) [DOI] [PubMed] [Google Scholar]
- 10.Gulland FM, Trupkiewicz JG, Spraker TR, Lowenstine LJ. 1996. Metastatic carcinoma of probable transitional cell origin in 66 free-living California sea lions (Zalophus californianus), 1979 to 1994. J. Wildl. Dis. 32, 250–258. ( 10.7589/0090-3558-32.2.250) [DOI] [PubMed] [Google Scholar]
- 11.Browning HM, Acevedo-Whitehouse K, Gulland FMD, Hall AJ, Finlayson J, Dagleish MP, Billington KJ, Colegrove K, Hammond JA. 2014. Evidence for a genetic basis of urogenital carcinoma in the wild California sea lion. Proc. R. Soc. B 281, 20140240 ( 10.1098/rspb.2014.0240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heath CB, Perrin WR. 2009. C: California, Galapagos, and Japanese sea lions Zalophus californianus, Z. wollebaeki, and Z. japonicus. In Encyclopedia of marine mammals (eds Perrin WF, Würsig B, Thewissen JGM.), pp. 170–176, 2nd edn. London, UK: Elsevier. [Google Scholar]
- 13.Arnason U, Gullberg A, Janke A, Kullberg M, Lehman N, Petrov EA, Vainola R. 2006. Pinniped phylogeny and a new hypothesis for their origin and dispersal. Mol. Phylogenet. Evol. 41, 345–354. ( 10.1016/j.ympev.2006.05.022) [DOI] [PubMed] [Google Scholar]
- 14.Greig DJ, Gulland FMD, Kreuder C. 2005. A decade of live California Sea Lion (Zalophus californianus) strandings along the Central California Coast: causes and trends, 1991–2000. Aquat. Mamm. 31, 11–22. ( 10.1578/AM.31.1.2005.11) [DOI] [Google Scholar]
- 15.Gulland FM. 1999. Stranded seals: important sentinels. J. Am. Vet. Med. Assoc. 214, 1191–1192. [PubMed] [Google Scholar]
- 16.Buckles EL, et al. 2006. Otarine herpesvirus-1, not papillomavirus, is associated with endemic tumours in California sea lions (Zalophus californianus). J. Comp. Pathol. 135, 183–189. ( 10.1016/j.jcpa.2006.06.007) [DOI] [PubMed] [Google Scholar]
- 17.Lipscomb TP, Scott DP, Garber RL, Krafft AE, Tsai MM, Lichy JH, Taubenberger JK, Schulman FY, Gulland FM. 2000. Common metastatic carcinoma of California sea lions (Zalophus californianus): evidence of genital origin and association with novel gammaherpesvirus. Vet. Pathol. 37, 609–617. ( 10.1354/vp.37-6-609) [DOI] [PubMed] [Google Scholar]
- 18.Crum CP. 2005. The female genital tract, ch. 22, 7th edn. Philadelphia, PA: Elsevier Saunders. [Google Scholar]
- 19.Herbert A, Bergeron C, Wiener H, Schenck U, Klinkhamer P, Bulten J, Arbyn M. 2007. European guidelines for quality assurance in cervical cancer screening: recommendations for cervical cytology terminology. Cytopathology 18, 213–219. ( 10.1111/j.1365-2303.2007.00469.x) [DOI] [PubMed] [Google Scholar]
- 20.Buckley CH, Butler EB, Fox H. 1982. Cervical intraepithelial neoplasia. J. Clin. Pathol. 35, 1–13. ( 10.1136/jcp.35.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colegrove KM, Gulland FM, Naydan DK, Lowenstine LJ. 2009. Tumor morphology and immunohistochemical expression of estrogen receptor, progesterone receptor, p53, and Ki67 in urogenital carcinomas of California sea lions (Zalophus californianus). Vet. Pathol. 46, 642–655. ( 10.1354/vp.08-VP-0214-C-FL) [DOI] [PubMed] [Google Scholar]
- 22.Murphy LC, Watson P. 2002. Steroid receptors in human breast tumorigenesis and breast cancer progression. Biomed. Pharmacother. 56, 65–77. ( 10.1016/S0753-3322(01)00157-3) [DOI] [PubMed] [Google Scholar]
- 23.Vollenweider-Zerargui L, Barrelet L, Wong Y, Lemarchand-Beraud T, Gomez F. 1986. The predictive value of estrogen and progesterone receptors' concentrations on the clinical behavior of breast cancer in women. Clinical correlation on 547 patients. Cancer 57, 1171–1180. () [DOI] [PubMed] [Google Scholar]
- 24.Nikolaou M, Koumoundourou D, Ravazoula P, Papadopoulou M, Michail G, Decavalas G. 2014. An immunohistochemical analysis of sex-steroid receptors, tumor suppressor gene p53 and Ki-67 in the normal and neoplastic uterine cervix squamous epithelium. Med. Pregl. 67, 202–207. ( 10.2298/MPNS1408202N) [DOI] [PubMed] [Google Scholar]
- 25.Nieto A, Pena L, Perez-Alenza MD, Sanchez MA, Flores JM, Castano M. 2000. Immunohistologic detection of estrogen receptor alpha in canine mammary tumors: clinical and pathologic associations and prognostic significance. Vet. Pathol. 37, 239–247. ( 10.1354/vp.37-3-239) [DOI] [PubMed] [Google Scholar]
- 26.Mariotti F, Giacomo R, Subeide M. 2013. Immunohistochemical evaluation of ovarian hormonal receptors in canine mammary tumors. Open J. Vet. Med. 3, 104–110. ( 10.4236/ojvm.2013.32017) [DOI] [Google Scholar]
- 27.Asakawa MG, Goldschmidt MH, Une Y, Nomura Y. 2008. The immunohistochemical evaluation of estrogen receptor-alpha and progesterone receptors of normal, hyperplastic, and neoplastic endometrium in 88 pet rabbits. Vet. Pathol. 45, 217–225. ( 10.1354/vp.45-2-217) [DOI] [PubMed] [Google Scholar]
- 28.Adkinson LR, Brown MD. 2007. Elsevier's integrated genetics, pp. 63–89. Philadelphia, PA: Mosby/Elsevier. [Google Scholar]
- 29.Ciancio N, Galasso MG, Campisi R, Bivona L, Migliore M, Di Maria GU. 2012. Prognostic value of p53 and Ki67 expression in fiberoptic bronchial biopsies of patients with non small cell lung cancer. Multidiscip. Respir. Med. 7, 29 ( 10.1186/2049-6958-7-29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CH, Kim WH, Lim JH, Kang MS, Kim DY, Kweon OK. 2004. Mutation and overexpression of p53 as a prognostic factor in canine mammary tumors. J. Vet. Sci. 5, 63–69. [PubMed] [Google Scholar]
- 31.Bowen L, Aldridge BM, Delong R, Melin S, Buckles EL, Gulland F, Lowenstine LJ, Stott JL, Johnson ML. 2005. An immunogenetic basis for the high prevalence of urogenital cancer in a free-ranging population of California sea lions (Zalophus californianus). Immunogenetics 56, 846–848. ( 10.1007/s00251-004-0757-z) [DOI] [PubMed] [Google Scholar]
- 32.Bowen L, Aldridge BM, Gulland F, Van Bonn W, DeLong R, Melin S, Lowenstine LJ, Stott JL, Johnson ML. 2004. Class II multiformity generated by variable MHC-DRB region configurations in the California sea lion (Zalophus californianus). Immunogenetics 56, 12–27. ( 10.1007/s00251-004-0655-4) [DOI] [PubMed] [Google Scholar]
- 33.Murchison EP. 2009. Clonally transmissible cancers in dogs and Tasmanian devils. Oncogene 27, S19–S30. ( 10.1038/onc.2009.350) [DOI] [PubMed] [Google Scholar]
- 34.Siddle HV, Kreiss A, Eldridge MD, Noonan E, Clarke CJ, Pyecroft S, Woods GM, Belov K. 2007. Transmission of a fatal clonal tumor by biting occurs due to depleted MHC diversity in a threatened carnivorous marsupial. Proc. Natl Acad. Sci. USA 104, 16 221–16 226. ( 10.1073/pnas.0704580104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acevedo-Whitehouse K, Gulland F, Greig D, Amos W. 2003. Inbreeding: disease susceptibility in California sea lions. Nature 422, 35 ( 10.1038/422035a) [DOI] [PubMed] [Google Scholar]
- 36.Levy-Adam F, et al. 2010. Heparanase 2 interacts with heparan sulfate with high affinity and inhibits heparanase activity. J. Biol. Chem. 285, 28 010–28 019. ( 10.1074/jbc.M110.116384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Moura JP, Jr, Nicolau SM, Stavale JN, da Silva Pinhal MA, de Matos LL, Baracat EC, de Lima GR. 2009. Heparanase-2 expression in normal ovarian epithelium and in benign and malignant ovarian tumors. Int. J. Gynecol. Cancer 19, 1494–1500. ( 10.1111/IGC.0b013e3181a834a2) [DOI] [PubMed] [Google Scholar]
- 38.Peretti T, Waisberg J, Mader AM, de Matos LL, da Costa RB, Conceicao GM, Lopes AC, Nader HB, Pinhal MA. 2008. Heparanase-2, syndecan-1, and extracellular matrix remodeling in colorectal carcinoma. Eur. J. Gastroenterol. Hepatol. 20, 756–765. ( 10.1097/MEG.0b013e3282fc2649) [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Xu S, Tan Q, Liu L. 2013. High expression of heparanase-2 is an independent prognostic parameter for favorable survival in gastric cancer patients. Cancer Epidemiol. 37, 1010–1013. ( 10.1016/j.canep.2013.09.012) [DOI] [PubMed] [Google Scholar]
- 40.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. 2007. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ. Health Perspect. 115, 1406–1414. ( 10.1289/ehp.10260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGlynn KA, et al. 2006. Serum concentrations of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (DDT) and 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and risk of primary liver cancer. J. Natl Cancer Inst. 98, 1005–1010. ( 10.1093/jnci/djj266) [DOI] [PubMed] [Google Scholar]
- 42.Freeman MD, Kohles SS. 2012. Plasma levels of polychlorinated biphenyls, non-Hodgkin lymphoma, and causation. J. Environ. Public Health 2012, 258981 ( 10.1155/2012/258981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vakonaki E, Androutsopoulos VP, Liesivuori J, Tsatsakis AM, Spandidos DA. 2013. Pesticides and oncogenic modulation. Toxicology 307, 42–45. ( 10.1016/j.tox.2013.01.008) [DOI] [PubMed] [Google Scholar]
- 44.Metcalfe C, Metcalfe T, Ray S, Paterson G, Koenig B. 1999. Polychlorinated biphenyls and organochlorine compounds in brain, liver and muscle of beluga whales (Delphinapterus leucas) from the Arctic and St. Lawrence estuary. Mar. Environ. Res. 47, 1–15. ( 10.1016/S0141-1136(98)00107-X) [DOI] [Google Scholar]
- 45.Martineau D, Lemberger K, Dallaire A, Labelle P, Lipscomb TP, Michel P, Mikaelian I. 2002. Cancer in wildlife, a case study: beluga from the St. Lawrence estuary, Quebec, Canada. Environ. Health Perspect. 110, 285–292. ( 10.1289/ehp.02110285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baumann PC. 1998. Epizootics of cancer in fish associated with genotoxins in sediment and water. Mutat. Res. 411, 227–233. ( 10.1016/S1383-5742(98)00015-5) [DOI] [PubMed] [Google Scholar]
- 47.Toft G. 2014. Persistent organochlorine pollutants and human reproductive health. Dan. Med. J. 61, B4967. [PubMed] [Google Scholar]
- 48.Hall AJ, Gulland FM, Ylitalo GM, Greig DJ, Lowenstine L. 2008. Changes in blubber contaminant concentrations in California sea lions (Zalophus californianus) associated with weight loss and gain during rehabilitation. Environ. Sci. Technol. 42, 4181–4187. ( 10.1021/es702685p) [DOI] [PubMed] [Google Scholar]
- 49.Oros DR, Ross JR, Spies RB, Mumley T. 2007. Polycyclic aromatic hydrocarbon (PAH) contamination in San Francisco Bay: a 10-year retrospective of monitoring in an urbanized estuary. Environ. Res. 105, 101–118. ( 10.1016/j.envres.2006.10.007) [DOI] [PubMed] [Google Scholar]
- 50.Zeng EY, Venkatesan MI. 1999. Dispersion of sediment DDTs in the coastal ocean off southern California. Sci. Total Environ. 229, 195–208. ( 10.1016/S0048-9697(99)00064-9) [DOI] [Google Scholar]
- 51.Schiff KC, Allen MJ, Zeng EY, Bay SM. 2000. Southern California. Mar. Pollut. Bull. 41, 76–93. ( 10.1016/S0025-326X(00)00103-X) [DOI] [Google Scholar]
- 52.Le Boeuf BJ, Bonnell ML. 1971. DDT in California sea lions. Nature 234, 108–110. ( 10.1038/234108a0) [DOI] [PubMed] [Google Scholar]
- 53.Le Boeuf BJ, Giesy JP, Kannan K, Kajiwara N, Tanabe S, Debier C. 2002. Organochloride pesticides in California sea lions revisited. BMC Ecol. 2, 11 ( 10.1186/1472-6785-2-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greig DJ, Ylitalo GM, Hall AJ, Fauquier DA, Gulland F. 2007. Transplacental transfer of organochlorines in California sea lions (Zalophus californianus). Environ. Toxicol. Chem. 26, 37–44. ( 10.1897/05-609R.1) [DOI] [PubMed] [Google Scholar]
- 55.Ylitalo GM, et al. 2005. The role of organochlorines in cancer-associated mortality in California sea lions (Zalophus californianus). Mar. Pollut. Bull. 50, 30–39. ( 10.1016/j.marpolbul.2004.08.005) [DOI] [PubMed] [Google Scholar]
- 56.Colegrove K. 2008. Urogenital carcinoma in California sea lions (Zalophus californianus): molecular alterations and potential association with environmental contaminants. Davis, CA: University of California. [Google Scholar]
- 57.King DP, Hure MC, Goldstein T, Aldridge BM, Gulland FM, Saliki JT, Buckles EL, Lowenstine LJ, Stott JL. 2002. Otarine herpesvirus-1: a novel gammaherpesvirus associated with urogenital carcinoma in California sea lions (Zalophus californianus). Vet. Microbiol. 86, 131–137. ( 10.1016/S0378-1135(01)00497-7) [DOI] [PubMed] [Google Scholar]
- 58.Johnson S, Lowenstine L, Gulland F, Jang S, Imai D, Almy F, Delong R, Gardner I. 2006. Aerobic bacterial flora of the vagina and prepuce of California sea lions (Zalophus californianus) and investigation of associations with urogenital carcinoma. Vet. Microbiol. 114, 94–103. ( 10.1016/j.vetmic.2005.11.045) [DOI] [PubMed] [Google Scholar]
- 59.Guijon F, Paraskevas M, Rand F, Heywood E, Brunham R, McNicol P. 1992. Vaginal microbial flora as a cofactor in the pathogenesis of uterine cervical intraepithelial neoplasia. Int. J. Gynaecol. Obstet. 37, 185–191. ( 10.1016/0020-7292(92)90379-W) [DOI] [PubMed] [Google Scholar]
- 60.Damania B. 2004. Oncogenic gamma-herpesviruses: comparison of viral proteins involved in tumorigenesis. Nat. Rev. Microbiol. 2, 656–668. ( 10.1038/nrmicro958) [DOI] [PubMed] [Google Scholar]
- 61.Buckles EL, et al. 2007. Age-prevalence of Otarine Herpesvirus-1, a tumor-associated virus, and possibility of its sexual transmission in California sea lions. Vet. Microbiol. 120, 1–8. ( 10.1016/j.vetmic.2006.10.002) [DOI] [PubMed] [Google Scholar]
- 62.Monini P, de Lellis L, Fabris M, Rigolin F, Cassai E. 1996. Kaposi's sarcoma-associated herpesvirus DNA sequences in prostate tissue and human semen. N. Engl. J. Med. 334, 1168–1172. ( 10.1056/NEJM199605023341805) [DOI] [PubMed] [Google Scholar]
- 63.Kedes DH, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. 1996. The seroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat. Med. 2, 918–924. ( 10.1038/nm0896-918) [DOI] [PubMed] [Google Scholar]
- 64.Cai Q, Verma SC, Lu J, Robertson ES. 2010. Molecular biology of Kaposi's sarcoma-associated herpesvirus and related oncogenesis. Adv. Virus Res. 78, 87–142. ( 10.1016/B978-0-12-385032-4.00003-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, Shah KV, Meijer CJ. 2004. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int. J. Cancer 111, 278–285. ( 10.1002/ijc.20244) [DOI] [PubMed] [Google Scholar]
- 66.Gustafsson L, Ponten J, Bergstrom R, Adami HO. 1997. International incidence rates of invasive cervical cancer before cytological screening. Int. J. Cancer 71, 159–165. () [DOI] [PubMed] [Google Scholar]
- 67.Hemminki K, Li X, Mutanen P. 2001. Age-incidence relationships and time trends in cervical cancer in Sweden. Eur. J. Epidemiol. 17, 323–328. ( 10.1023/A:1012761717028) [DOI] [PubMed] [Google Scholar]
- 68.Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, Tortolero-Luna G, Kjaer SK, Munoz N. 2008. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 26(Suppl. 10), K1–K16. ( 10.1016/j.vaccine.2008.05.064) [DOI] [PubMed] [Google Scholar]
- 69.Schiffman MH, et al. 1993. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J. Natl Cancer Inst. 85, 958–964. ( 10.1093/jnci/85.12.958) [DOI] [PubMed] [Google Scholar]
- 70.Maciag PC, Schlecht NF, Souza PS, Franco EL, Villa LL, Petzl-Erler ML. 2000. Major histocompatibility complex class II polymorphisms and risk of cervical cancer and human papillomavirus infection in Brazilian women. Cancer Epidemiol. Biomarkers Prev. 9, 1183–1191. [PubMed] [Google Scholar]
- 71.Balloux F, Amos W, Coulson T. 2004. Does heterozygosity estimate inbreeding in real populations? Mol. Ecol. 13, 3021–3031. ( 10.1111/j.1365-294X.2004.02318.x) [DOI] [PubMed] [Google Scholar]
- 72.Denic S, Bener A. 2001. Consanguinity decreases risk of breast cancer--cervical cancer unaffected. Br. J. Cancer 85, 1675–1679. ( 10.1054/bjoc.2001.2131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marques RM, Focchi GR, Theodoro TR, Castelo A, Pinhal MA, Nicolau SM. 2012. The immunoexpression of heparanase 2 in normal epithelium, intraepithelial, and invasive squamous neoplasia of the cervix. J. Low Genit. Tract Dis. 16, 256–262. ( 10.1097/LGT.0b013e3182422c69) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data published in this study is available through the British Oceanographic Data Centre.