Abstract
Histopathologic examination of the testis is the most sensitive means to detect effects on spermatogenesis; however, the complexity of testicular histology, interrelatedness of cell types within the testis, and long duration of spermatogenesis can make assessment of a testicular toxicant challenging. A thorough understanding of the histology and morphologic manifestations of response to injury is critical to successfully identify a testicular effect and to begin to understand the underlying mechanism of action. The basic patterns of response to xenobiotic-induced injury to the testis and epididymis are detailed and discussed.
Keywords: testis, epididymis, toxicity, histopathology, rat, mouse, dog, cynomolgus monkey
Abbreviations
- BTB
blood-testis barrier
- EGME
ethylene glycol monomethyl ether
- SC
Sertoli cells
- MEHP
mono-(2-ethylhexyl) phthalate
- 2
5 HD, 2, 5 hexanedione
- STF
seminiferous tubular fluid
- LH
luteinizing hormone
- GnRH
gonadotropin releasing hormone
- FSH
follicle stimulating hormone
- LC
Leydig cells
- DHT
dihydrotestosterone
- PTMC
peritubular myoid cells
Introduction
Histopathology is generally recognized as the most reliable and sensitive means of detecting effects on spermatogenesis.1,2 A toxicologic pathologist generally recognizes histopathologic changes in a tissue or cell type that has been affected by administration of a compound. The morphologic changes observed can frequently provide insight into the mechanism of action of the compound on the tissue impacted. In the case of male reproductive toxicologic histopathology, the complexity of the histology, interrelatedness of cell types within the testis, and long duration of spermatogenesis can make assessment of a testicular toxicant challenging. A thorough understanding of the histology and morphologic manifestations of response to injury is critical to successfully identify a testicular effect and to begin to understand the underlying mechanism of action. The basic patterns of response to xenobiotic-induced injury to the testis and epididymis are detailed and discussed.
Manifestations of toxicity on germ cells
Germ cell injury as a primary morphologic event is a common manifestation following administration of cytotoxic compounds. Spermatogonia, as the mitotic component of spermatogenesis and the main germ cell type not protected by the blood-testis barrier (BTB), are the most vulnerable to toxic effects.3 An important point is that the stem cell spermatogonia divide infrequently and are less sensitive to cytotoxicity compared to spermatogonia that have entered the proliferative and developmental pool; thus, effects of toxic compounds on the testis may be reversible following cessation of compound exposure through seminiferous epithelial reconstitution from surviving stem cell spermatogonia. However, there is variability across different classes of toxic compounds, stem cell proliferation is slow, and in humans it may take months to years for sperm production to recover.4
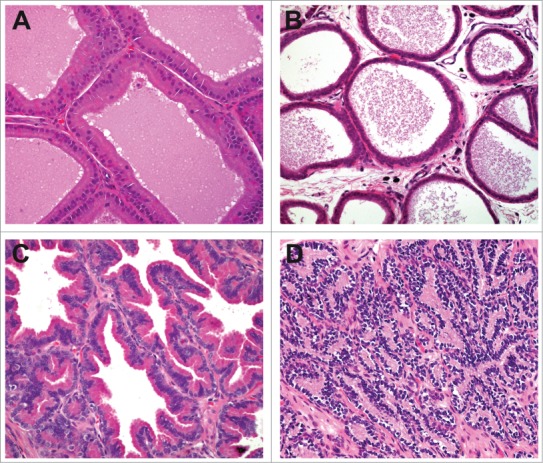
Apoptotic spermatogonia appear as cells having hyalinized, hypereosinophilic, condensed cytoplasm and indistinct nuclei, occasionally surrounded by a clear space as the cells shrink (also see Murphy and Richburg, this issue). Morphologic changes due to effects of toxins on spermatogonia are not distinguishable from the spontaneous physiologic attrition of germ cells5,6 resulting from inherent apoptotic machinery that expedites removal of defective cells and prevents germ cell populations exceeding feasible Sertoli cell support; however, physiologic apoptosis of germ cells tends to be limited in number and stage-specific (e.g. spermatogonia of stage XII in rat). In the absence of confirmatory evidence of the apoptotic process (e.g., TUNEL staining for DNA fragmentation or immunohistochemistry for apoptotic effector proteins) pathologists often conservatively refer to the morphologic change as ‘degeneration’. Since by nature the apoptotic process is rapid and inconspicuous, apoptotic spermatogonia may appear in small numbers, but compounded loss of spermatogonia can lead to elimination of the basal-most layer of the seminiferous epithelium, which is often first detected as loss of preleptotene spermatocytes in stage VII tubular profiles (Figs. 1 and 2).
Figure 1.
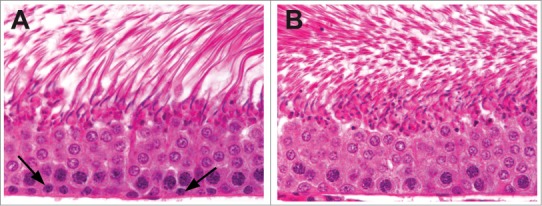
Stage VII tubules from a control rat (A) and a rat given an antimitotic agent for 2 weeks (B). Preleptotene spermatocytes are present adjacent to the basement membrane (arrows) in the control rat and are absent following mitotic inhibition.
Figure 2.
Stage V tubules from a control dog (A and C) and a dog given an antimitotic agent for 2 weeks (B and D). Preleptotene spermatocytes are present adjacent to the basement membrane (arrows) in the control dog and are absent following mitotic inhibition.
With prolonged toxic compound exposure (i.e. for rat, multiples of the 2-week duration of the spermatogenic epithelial cycle) successive populations of post-spermatogonial developing germ cells (and the corresponding layers of seminiferous epithelium) are eliminated through the process termed ‘maturation depletion’ (Figs. 3 and 4). Chemotherapeutic agents, compounds altering the balance of endogenous apoptotic and anti-apoptotic proteins (e.g. p53, Fas, and Bcl systems),7-9 and compounds interfering with growth factors supporting spermatogonia (e.g., stem cell factor or its receptor c-kit)10 can all cause spermatogonial death and loss across multiple species.11–13(also see Murphy and Richburg, this issue)
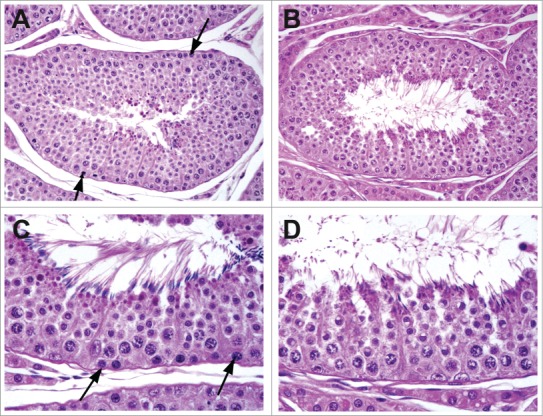
Figure 3.
Stage VII tubule from a rat demonstrating maturation depletion. Administration for 4 weeks of a compound causing spermatogonial loss has resulted in depletion of spermatocytes, preleptotene spermatocytes, and spermatogonia.
Figure 4.
Testes from a dog given a spermatogonial toxicant for 13 weeks at low (A) and high (B) magnification. Tubules depleted of all germ cells due to progressive maturation depletion following loss of spermatogonia and prepachytene spermatocytes.
Toxic agents may impact germ cells other than spermatogonia, but in most cases must circumvent the protection of the BTB in order to do so, as early spermatocytes are moved inside the BTB. Sequential examination of rat testes following administration of doxorubicin demonstrates degenerate/apoptotic meiotic spermatocytes as early as 6 hours and before the detection of apoptotic spermatogonia.12 Degeneration of spermatocytes during meiotic division leads to a stage specific lesion (Figs. 5 and 6) with subsequent depletion. Ethylene glycolmono methyl ether (EGME) is another agent known to induce degeneration of mature spermatocytes at the time of meiotic division.14 Whereas spermatogonial degeneration is almost certainly a primary germ cell effect, the requisite penetration of the BTB suggests an additional, possibly primary effect on Sertoli cells (the primary component of the BTB) when spermatocytes are affected. Sertoli cells (SC), however, are themselves strikingly resistant to death by apoptosis or necrosis, typically being the only cell type remaining in end-stage seminiferous tubules. Notable SC toxicants, including 2,5 hexanedione and mono-(2-ethylhexyl) phthalate (MEHP), also cause germ cell apoptosis, occurring by means of up-regulation of the Fas ligand by the Sertoli cell stimulating the Fas receptor on spermatocytes.15 Prolonged exposures to compounds toxic to spermatocytes can cause maturation depletion (Fig. 7) resulting in loss of successively more mature germ cell layers (round and elongating spermatids), similar to the situation with spermatogonial toxicants.
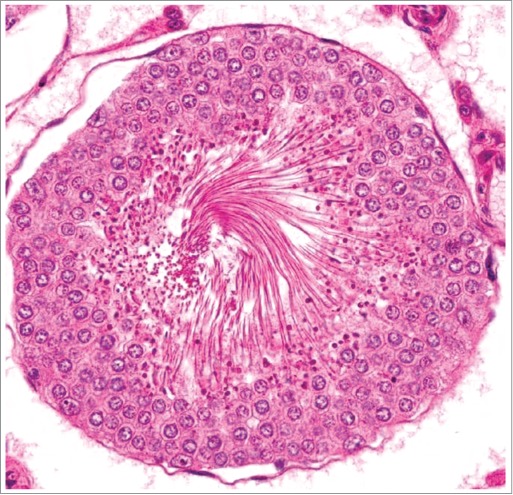
Figure 5.
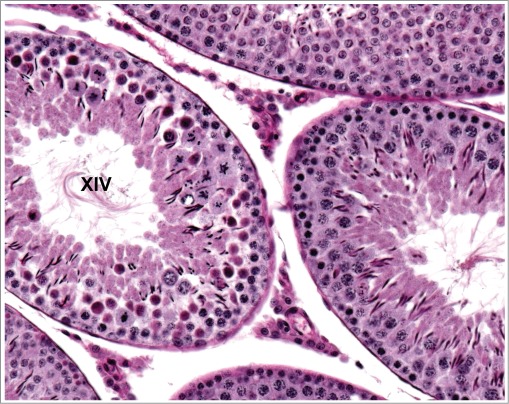
Stage XIV tubule from a rat. Numerous spermatocytes undergoing meiotic division in this stage have hypereosinophilic cytoplasm indicating degeneration. The underlying spermatocytes and overlying elongating spermatids are normal.
Figure 6.
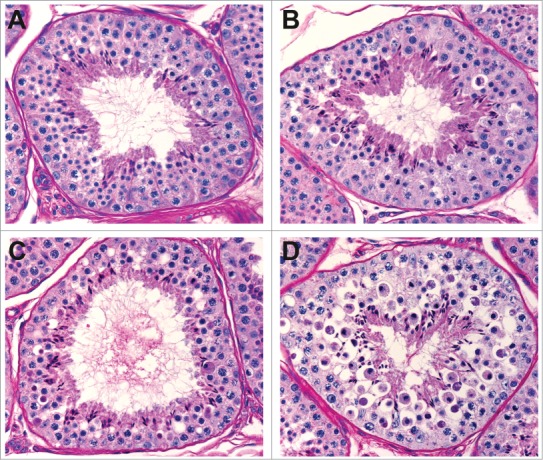
Stage XII-I tubules from cynomolgus monkeys given vehicle (A), low dose (B), mid dose (C), and high dose (D) of a compound causing degeneration of spermatocytes during meiotic division.
Figure 7.
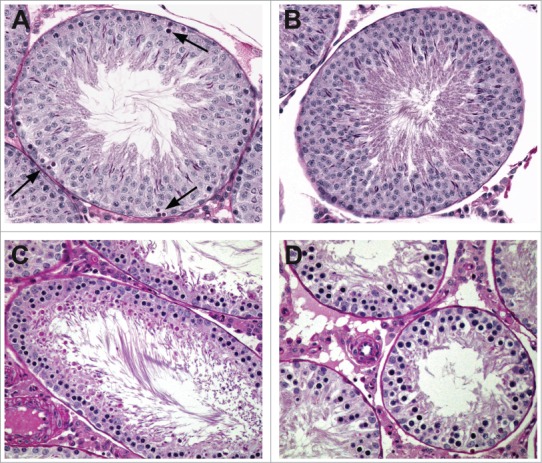
Pattern of cell depletion with daily dosing of a spermatocyte toxicant in the rat. (A) Stage I tubule with degeneration of pachytene spermatocytes (arrows) following a single dose. (B) Stage I tubule with depletion of pachytene spermatocytes following one week of dosing. (C) Stage VIII tubule with depletion of pachytene spermatocytes and round spermatids following 2 weeks of dosing. (D) After 4 weeks of dosing only prepachytene spermatocytes remain.
Another classic example of germ cell degeneration/apoptosis is the morphologic consequence of testosterone deprivation in the rat testis. Degeneration of stage VII/VIII pachytene spermatocytes and round spermatids, in addition to spermatid retention are early indicators of intratesticular testosterone depletion (Fig. 8)(also see Weinbauer, this issue). This hormonal effect involves the SC, as it is generally accepted that androgen receptors are not expressed in germ cells, but have their highest expression in Sertoli cells of stage VII/VIII,16,17 near the time of release of mature spermatids into the tubular lumen (spermiation). Decreased androgen levels can interfere with this process, resulting in spermatid retention (also see O’Donnell, this issue). Androgen receptors are also present on Leydig cells and peritubular myoid cells, which may contribute to the morphologic effects of testosterone depletion.
Figure 8.
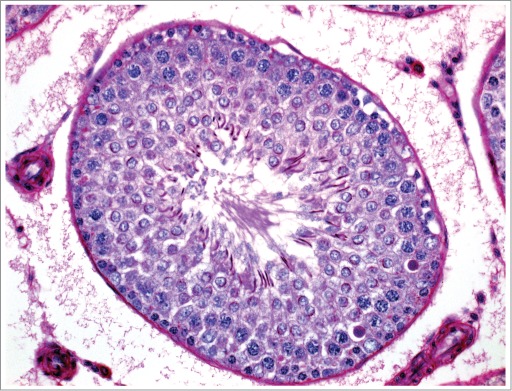
Stage VII tubule from a rat given exogenous testosterone. The increased negative feedback has led to decreased gonadotrophins and caused Leydig cell atrophy and decreased intratesticular androgen levels resulting in pachytene spermatocyte degeneration.
Sertoli cell injury
Injury to SCs is typically manifested by loss of function18-20 rather than death and loss of SCs themselves. The many roles that SCs play in spermatogenesis are reflected in the variety of morphologic manifestations associated with SC toxicity. As already noted, impaired SC function often manifests as germ cell degeneration and loss.
Sertoli cells are instrumental in the dramatic morphologic changes associated with maturation of elongating spermatids (spermiogenesis) and their ultimate release (spermiation) into the tubular lumen by the end of Stage VII/VIII (rat)(see O’Donnell, this issue). Membrane modifications on the SC (ectoplasmic specialization) and spermatid (tubulobulbar complex) provide an extensive surface area for cross-communication. Degeneration of elongating spermatids (variably presenting as nuclear condensation, irregularity, or fragmentation or cytoplasmic vacuolation) may reflect SC toxicity or direct germ cell injury following penetration of the compound through the BTB (Figs. 9 and 10).21 Delayed acrosomal maturation observed with administration of the Sertoli cell toxicant carbendazim resembles loss of synchrony within cell associations.22
Figure 9.
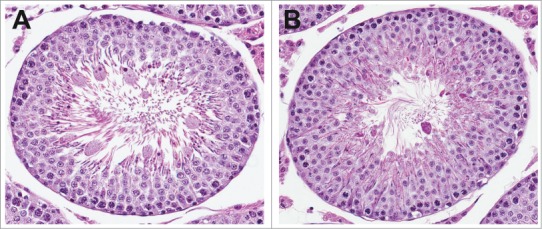
Degeneration of elongated spermatids in the rat. (A) Stage VII tubule with enlarged elongated spermatids. (B) Stage V tubule with irregular, hypereosinophilic elongated spermatids indicating degeneration.
Figure 10.
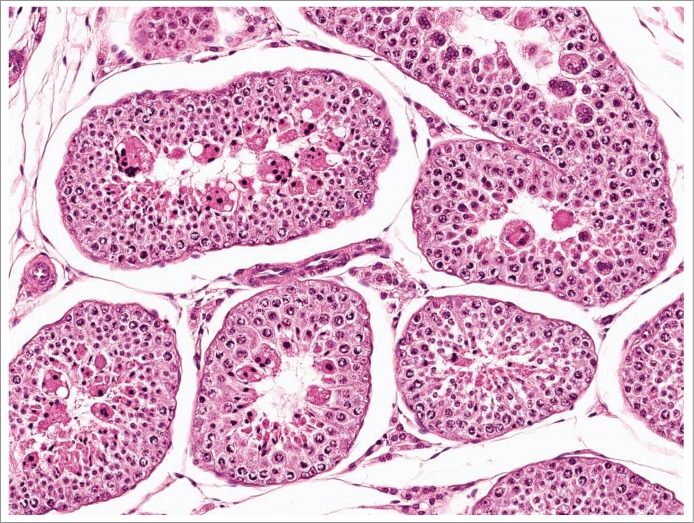
Degeneration of elongated spermatids in the dog.
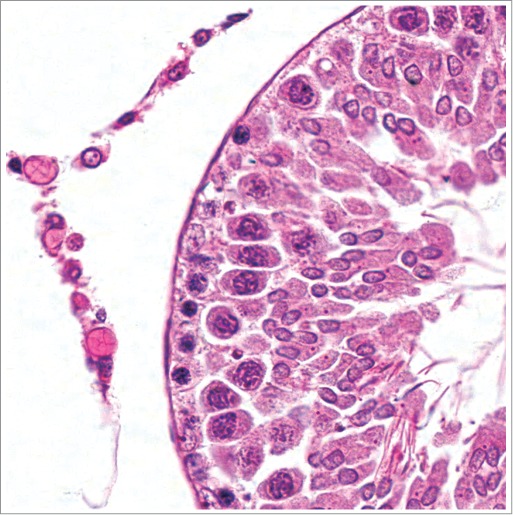
Spermiation requires step 19 spermatids to be located at the lumen with the removal of superfluous cytoplasmic remnants in the form of residual bodies with subsequent resorption of residual bodies by Sertoli cells beginning in stage VIII. Failure of step 19 spermatids to be located and properly oriented at the lumen (Fig. 11) and abnormally large or malformed residual bodies, possibly with delayed resorption, are occasionally observed as evidence of diminished SC function (Fig. 12) (also see O’Donnell, this issue). The large residual bodies normally present in mice should not be confused with abnormal residual bodies.
Figure 11.
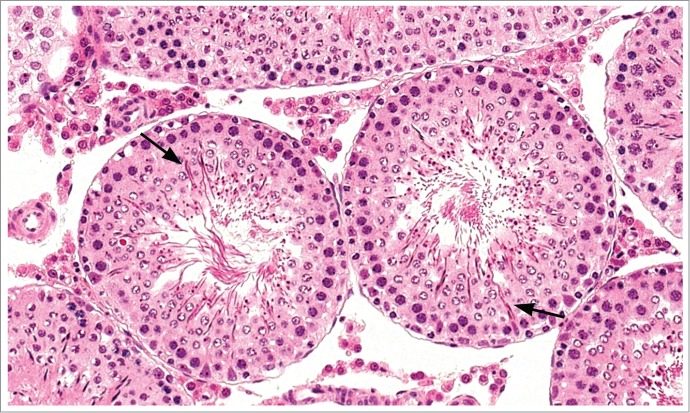
Testes from a rat. Stage VII-VIII tubules with step 19 spermatid heads deep with the tubular epithelium (arrows) instead of at the lumen.
Figure 12.
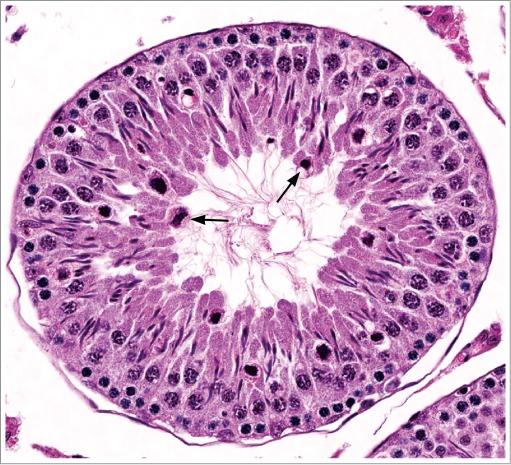
Testis from a rat given dibromoacetic acid, which has affected Sertoli cell function, manifesting as abnormally large residual bodies with delayed resorption (stage XII).
Impaired Sertoli cell function is often demonstrated in rats by the retention of mature step 19 spermatids. Retained spermatids may be found at the seminiferous tubular lumen edge beyond stage VIII (Fig. 13A) and/or resorbed into basilar Sertoli cell cytoplasm (Fig 13B) (abnormal at any stage).
Figure 13.
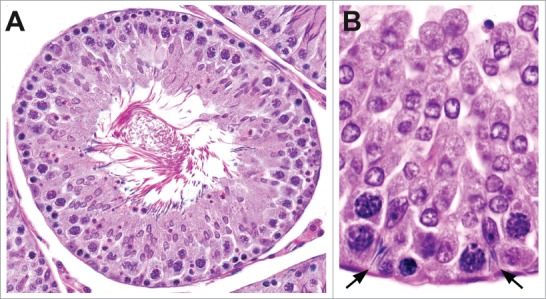
Spermatid retention in the rat. Retained step 19 spermatids can be present at the tubular lumen (A) or may be located deep within the seminiferous epithelium within Sertoli cells (B).
Sertoli cell cytoplasmic vacuolation (usually due to dilated endoplasmic reticulum), consisting of variably sized, discrete, clear, round structures predominantly located toward the basement membrane (Figs. 14–16), as seen following administration of 2,5 hexanedione (2,5 HD) or phthalate esters to rats, is an early change of Sertoli cells exposed to certain toxicants (see Johnson, this issue). In contrast, vacuolation of the seminiferous epithelium (Fig. 16) consisting of larger, non-membrane-bound spaces at various depths within the tubule results from germ cell loss, due to primary germ cell or SC effects. It is important to distinguish these 2 types of vacuolation from the occasionally observed vacuolation in control rats, which is often nonspecific.
Figure 14.
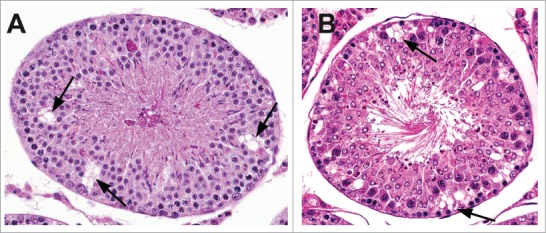
Vacuolation within the cytoplasm of Sertoli cells in the rat. Sertoli cell vacuolation (arrows) can be observed with other findings including degeneration (A) and/or spermatid retention (B).
Figure 15.
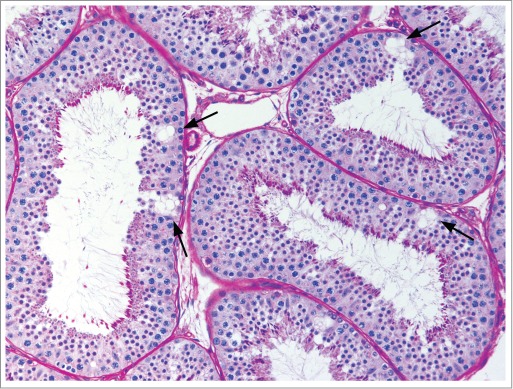
Vacuolation within the cytoplasm of Sertoli cells (arrows) in the cynomolgus monkey.
Figure 16.
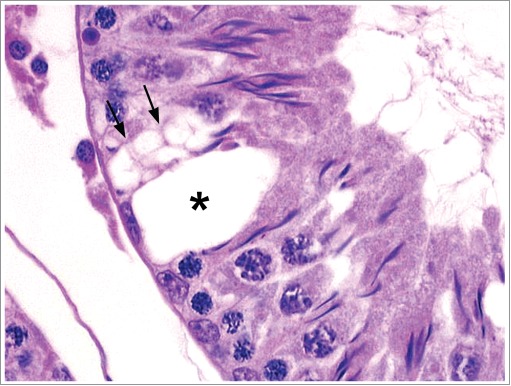
Vacuolation within a Stage XII tubules of a rat with a cluster of small vacuoles present within the cytoplasm of a Sertoli cell (arrows) and a single large vacuole (asterisk) likely present between Sertoli cells.
Exfoliation of germ cells is another indication of SC dysfunction. The SC toxicant carbendazim, a microtubule disruptor, causes loss of germ cell attachment to SC processes, with sloughing of germ cells (round spermatids, spermatocytes) into the tubular lumen and eventually into the excurrent ducts. Typically, the exfoliated cells lack morphologic changes of degeneration/apoptosis (Fig. 17). Some microtubule disruptors result in sloughing of SC processes along with germ cells into the lumen (see Johnson, this issue).
Figure 17.
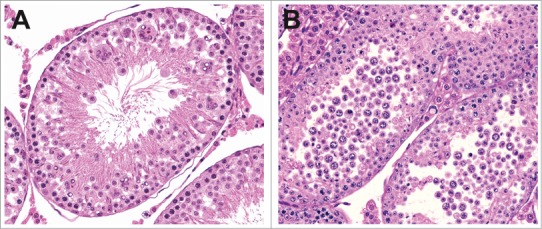
Exfoliation of germ cells in the rat. (A) Stage V tubule with exfoliation of germ cells and multinucleated giant cells. (B) Extensive exfoliation of germ cells.
With longer term exposures to Sertoli cell toxicants, disorganization of the seminiferous epithelium may occur with variable germ cell loss and irregularly arrayed germ cell layers (Fig. 18). Sertoli cell cytoplasm may become more apparent due to unmasking with germ cell loss and this may have a focal or segmental appearance (Fig 19A and B) or with more severe toxicity it may present as diffuse atrophy (Fig. 19C and D).
Figure 18.
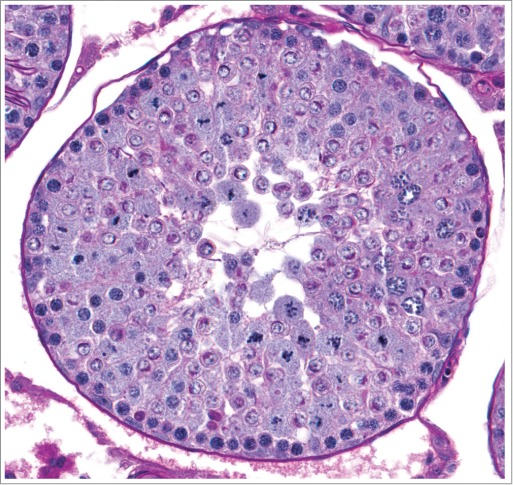
Rat testis with loss of normal cell associations including the presence of step 7 to 8 spermatids in tubules with spermatocytes undergoing meiotic division, spermatocytes present on the luminal side of round spermatids, and varying degrees of degeneration.
Figure 19.
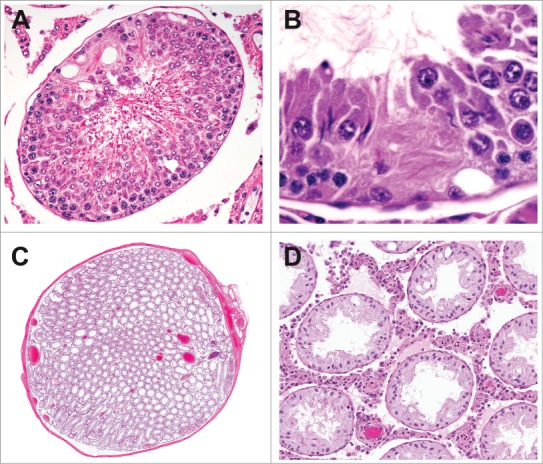
Tubular atrophy in the rat can range from focal or segmental (A and B) to diffuse (C and D) with a reduction in organ weight.
Sertoli cells normally produce seminiferous tubular fluid (STF). Altered functionality associated with SC toxicant administration may lead to increases or decreases in tubular diameter associated with changes in STF production.
Sertoli cells are responsible for maintaining the separation of individual germ cells within the cohorts of clones resulting from serial mitotic divisions of a single antecedent spermatogonium. Multinucleated germ cells (most often round spermatids, occasionally spermatocytes) result from failed integrity of the intercellular bridges serving as partitions (Figs. 20 and 21) (see O’Donnell, this issue).
Figure 20.
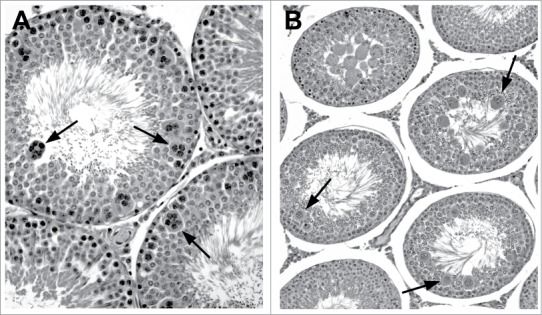
Multinucleated germs cells in the rat. (A) Stage VII tubules with numerous multinucleated pachytene spermatocytes (arrows). (B) Stage VIII/IX tubule with degeneration of round spermatids and formation of multinucleate aggregates (arrows).
Figure 21.
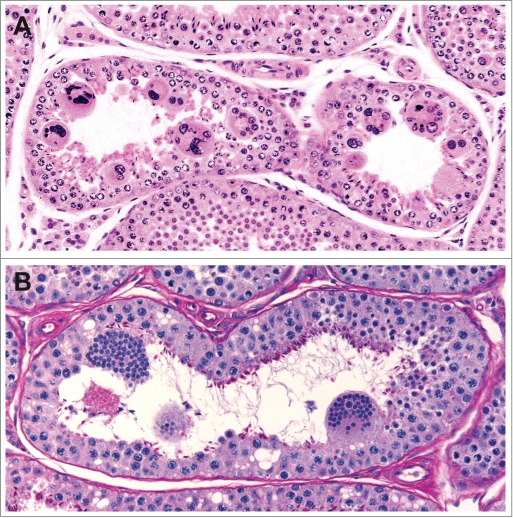
Multinucleated germ cells in the dog (A) and cynomolgus monkey (B).
Loss of BTB integrity may occur with SC toxicity. With BTB breakdown, normally sequestered ‘foreign’ antigens are exposed to the immune apparatus and inflammation may ensue.
Hormonal effects
Histopathologic manifestations of hormonal disturbances can vary markedly among species (see Weinbauer, this issue). Endocrine effects can be precipitated by mechanisms at the central (hypothalamus/pituitary) or peripheral (testis/Leydig cell) level, involving a hormone or its receptor or its metabolism/clearance, an upstream releasing hormone or its receptor, a transport protein, or factors involved in feedback loops. Although species generally share similar factors and mechanisms in endocrine regulation, species peculiarities may alter the morphologic response to an endocrine modification. The significant differences in hormonal mechanisms in rats and other laboratory animals compared to humans affect the relevance of the respective species in male reproductive toxicologic safety assessment.
As previously described, specific morphologic changes in rat testes (including spermatid retention and degeneration of spermatocytes and round spermatids in stage VII/VIII [Fig 22]) are associated with decreased intratesticular testosterone levels, which are normally approximately 50 times higher than serum testosterone levels. Such changes may be triggered by exogenous testosterone23,24 resulting in negative feedback to hypothalamus and pituitary gland with subsequent decreased luteinizing hormone (LH) release and decreased Leydig cell production of testosterone. The same morphologic manifestations in testis may occur following direct cytotoxic actions on Leydig cells (e.g. with ethylene dimethane sulfate)25 and with gonadotropin releasing hormone (GnRH) antagonism.26 Following more severe or chronic reductions in androgens, there is a more generalized reduction in cellularity with a loss of spermatids (Fig. 23). Excessive intratesticular estradiol in rats also causes spermatid retention.27
Figure 22.
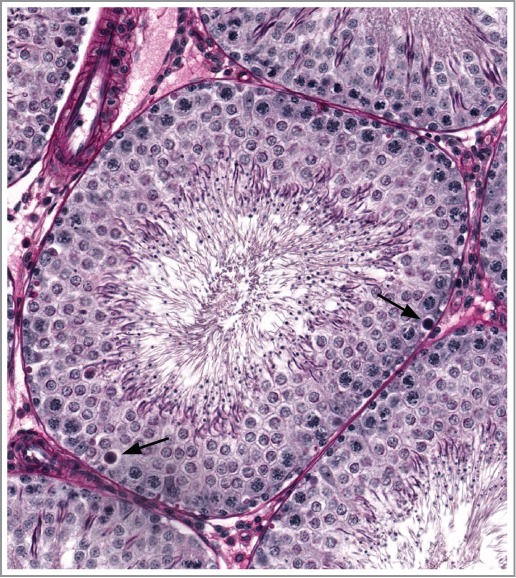
Stage VII tubule from a rat demonstrating numerous degenerate spermatocytes (arrows) resulting from slightly reduced testosterone.
Figure 23.
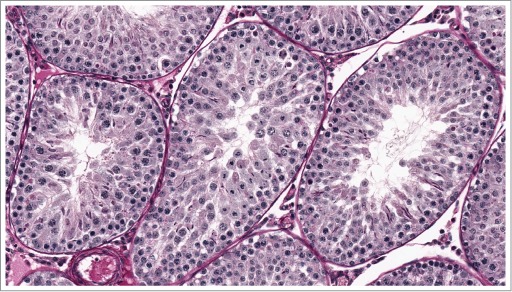
Rat with severely reduced testosterone demonstrating generalized loss of elongating spermatids.
In the dog and nonhuman primate, the histologic changes in the testis secondary to decreases in androgens lack the stage specificity seen in the rat and are often most pronounced when coupled with centrally mediated decreases in gonadotropins. In the dog, testosterone depletion has been induced by administration of neurokinin receptor antagonists28,29 or by immunization of dogs against luteinizing hormone releasing hormone30 with histopathologic changes of germ cell sloughing and depletion (Fig. 24). Despite comparable neurokinin receptor antagonist exposures in dogs and rats for each compound, rats did not manifest testicular morphologic changes or altered hormonal levels, illustrating different roles for neurokinin receptors in endocrine regulation between species. Cynomolgus monkeys also differ from rats in their response to testosterone deficiency, due to follicle stimulating hormone (FSH) having a more prominent role in supporting spermatogenesis.31-33 Decreases in gonadotropins and testosterone in the cynomolgus monkey lead to a more generalized depletion of germ cells with variably present spermatid retention.34 (also see Weinbauer, this issue)
Figure 24.
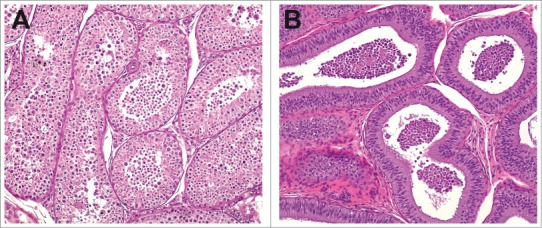
Testis (A) and epididymis (B) with abundant exfoliated germ cells from a dog with centrally mediated reductions in androgens.
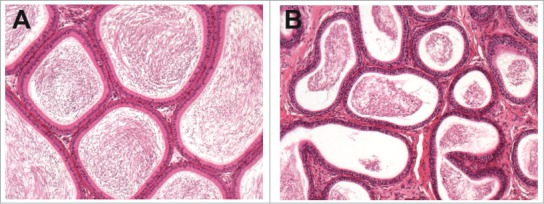
Changes related to decreases in androgens are often most readily identified in the accessory sex organs as a decrease in weight, secretory content, and/or epithelial height (Fig. 25).
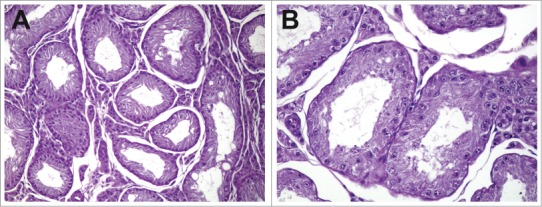
Figure 25.
Prostate from a control rat (A) and dog (C). Prostate from a rat (B) and dog (D) with severely reduced androgen levels.
Leydig cell changes
Leydig cells (LC) as the producers of testosterone are important in the mechanisms and manifestations of hormonal disruption. Species differences in endocrine feedback loops and in regulation of LC hormone receptor expression result in different morphologic responses to compounds causing endocrine modulation.
Different mechanisms in negative feedback for GnRH release between dog and rat are represented in varying responses in LCs to administration of aromatase inhibitors.35 Aromatase catalyzes the change from androgens to estrogens. Due to the importance of estrogens as the major negative feedback stimulus in dogs (as in primates), both steroidal and non-steroidal aromatase inhibitors administered to dogs result in LC hypertrophy/hyperplasia. In contrast, rats depend on testosterone and dihydrotestosterone for negative feedback to the hypothalamus. Rats administered a steroidal aromatase inhibitor manifested LC atrophy (Fig. 26) due to the negative feedback caused by the xenobiotic, whereas a non-steroidal aromatase inhibitor had no effect on rats. Leydig cell atrophy is associated with decreased testosterone production and therefore, in rodents, is frequently seen in conjunction with the characteristic morphologic changes in the seminiferous epithelium due to testosterone withdrawal.
Figure 26.
Leydig cell atrophy in a rat. Leydig cells (left) have scant cytoplasm. This stage X tubule has retained step 19 spermatids (lower right) as a manifestation of local testosterone depletion.
Prolactin has a distinctive endocrine regulatory role in the rat compared to other species. Prolactin stimulates the expression of LH receptors in rat Leydig cells making them more responsive. Dopamine inhibits prolactin secretion from the pituitary gland. Dopamine agonists, therefore, inhibit prolactin release, thereby decreasing Leydig cell responsiveness, decreasing testosterone production, and increasing LH with consequent LC hypertrophy/hyperplasia (Fig. 27) and increased incidence of LC tumors (Fig. 27) in chronic studies.36 This tumorigenic effect of dopamine agonists is peculiar to the rat and considered not to be relevant to human.37 Leydig cell hyperplasia and tumors in mice are usually associated with estrogenic stimuli.38,39
Figure 27.
Leydig cell adenoma accompanied by tubular atrophy in a rat.
Few compounds are associated with necrosis of Leydig cells, the most notable one being ethylene dimethane sulfate.25,40
Epididymal changes
The excurrent ducts (rete testis, efferent ducts, epididymis, and vas deferens) conduct the outflow of mature spermatids while supporting maturation into fertile spermatozoa. Fluid resorption, protein synthesis, secretory activity, and hormonal modulation occur within specific portions of the epididymis. Disruption of these processes can adversely impact fertility (also see Kempinas and Klinefelter, this issue).
Testosterone within the STF is delivered to the epididymis where it is converted by 5‑α reductase to dihydrotestosterone (DHT), which has greater affinity for the androgen receptor and is thus more potent than testosterone. Any compound causing decreased testicular testosterone results in decreased epididymal weight and atrophy (Fig. 28) of the luminal epithelium. Testosterone deficiency also manifests as single cell necrosis (apoptosis) of the epididymal epithelium (Figs. 29-31), cribriform change associated with epithelial cell loss, and/or decreased tubular diameter. Antagonists of the androgen receptor cause similar changes to the epididymis. Inhibitors of 5-α reductase, such as finasteride, cause more subtle epididymal changes, only detected morphometrically.41
Figure 28.
Epididymides from a control rat (A) and from a rat with markedly reduced androgen levels demonstrating epithelial atrophy and reduced luminal diameter (B).
Figure 29.
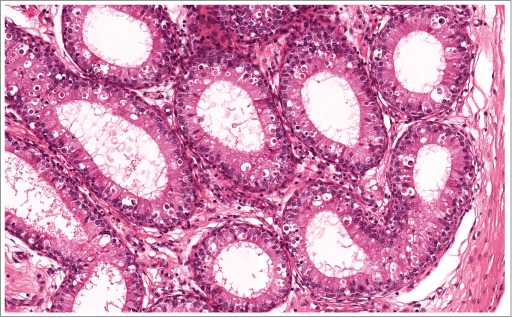
Initial segment of the epididymis from a rat with reduced androgen levels demonstrating increased epithelial apoptosis.
Figure 30.
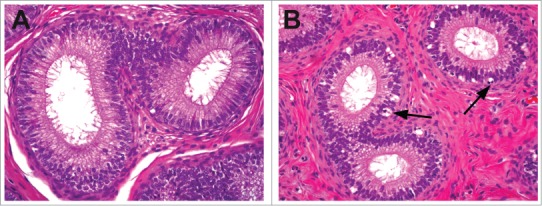
Epididymides from a control dog (A) and from a dog with decreased androgen levels demonstrating epithelial atrophy and increased apoptosis (arrows).
The anatomic arrangement of rat efferent ducts (multiple conduits converging on a single outflow tract) predisposes them to occlusion by sloughed debris. Various compounds causing dilation in rat excurrent ducts followed by spermatoceles, sperm granuloma formation, and retrograde fluid pressure and seminiferous tubular atrophy have been described (Figs. 32 and 33).42-44 (also see Hess, this issue). Dogs, having blind-ended structures within the efferent ductular arrangement, are predisposed to development of sperm granulomas, but less prone to occlusion.45 The epididymis is also a frequent target of compounds inducing phospholipidosis, resulting in epithelial cell cytoplasmic vacuolation (Figs. 34 and 35).46
Figure 32.
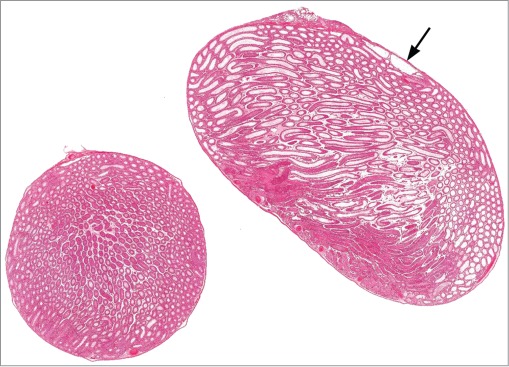
Cross and longitudinal sections of right and left testes from a rat. The cross section is normal and the longitudinal section has diffuse dilation of tubules and rete testis (arrow) due to outflow obstruction.
Fig 33.
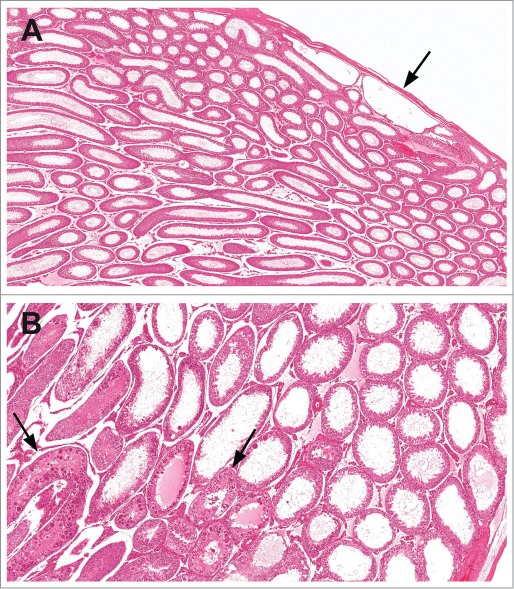
Rat testes with dilation of the rete testis (arrow) and tubular dilation (A) and tubular dilation with secondary degeneration (arrows) of the seminiferous tubules (B).
Figure 34.
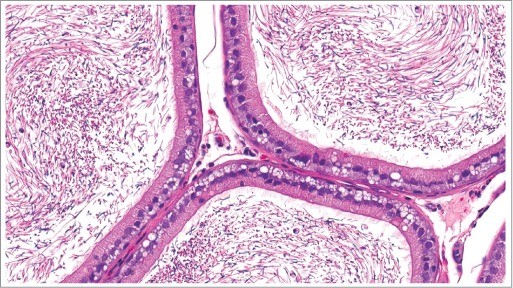
Epithelial vacuolation within the epididymis of a rat.
Figure 35.
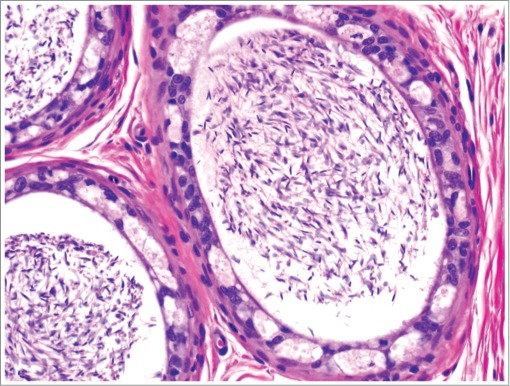
Epithelial vacuolation within the epididymis of a dog.
Much like the Sertoli cell, cellular junctions between epididymal epithelial cells maintain sequestration of the foreign antigens present on spermatozoa from the immune system. Compromise of this barrier function, often due to ischemia of the vulnerable caput epididymis, can result in inflammation and granuloma formation (Fig. 36).20 (also see Gregory and Cyr, this issue)
Figure 31.
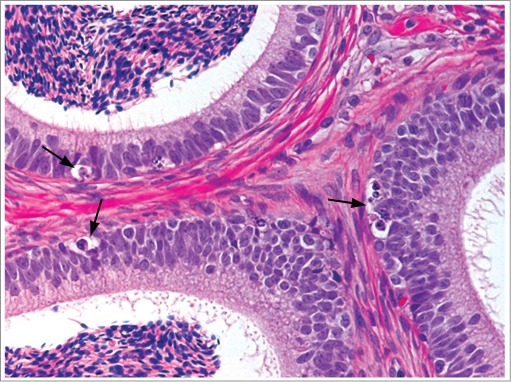
Epididymis from a cynomolgus monkey with increased epithelial apoptosis (arrows).
Figure 36.
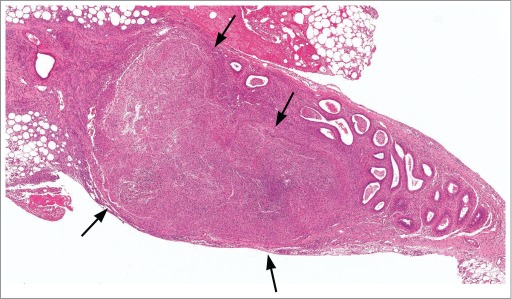
Sperm granuloma (arrows) within the epididymis of a rat. This granuloma was large enough to be documented as a macroscopic observation at necropsy.
Testicular toxicity of any cause can lead to secondary changes within the epididymis including increased cellular debris/sloughed germ cells, decreased luminal content, and/or cribriform change (Figs. 37 and 38). Increases in the number of sloughed germ cells can be an early indicator of testicular toxicity, but often the secondary changes in the epididymis become more pronounced as the duration and/or severity of the testicular toxicity increases.
Figure 37.
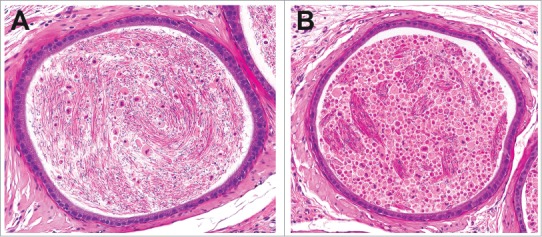
Rat epididymides with a mild increase in cellular debris (A) compared to a marked increase (B). Note that in minimal to mild examples, there is a mix of normal appearing sperm with increased cellular debris, while in more severe examples the lumen is filled with sloughed degenerate germ cells and cellular debris.
Figure 38.
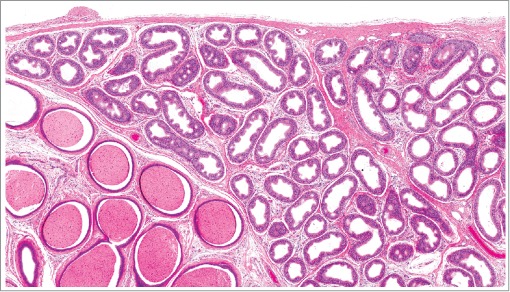
Epididymis from a rat with decreased luminal contents and focal infoldings of the epithelium with pseudoglandular structures termed cribriform change.
Fluid disturbances
Tubular fluid dynamics are influenced by the production of STF by SCs, the propagation of luminal contents by contraction of peritubular myoid cells (PTMC), the resorption of fluid within efferent ducts and epididymides, and any circumstances resulting in obstruction of the excurrent path.
As noted previously, impaired SC function may affect STF production, resulting in alteration of the inner and/or outer seminiferous tubular diameter. STF production is androgen-dependent and influenced by numbers of germ cells (particularly elongating spermatids). Fluid accumulation can result in increased testis weight, but may be transient as the increased intratubular pressure leads to germ cells loss resulting in decreased testis weight and tubular atrophy (see Hess, this issue).
Contraction of PTMC is stimulated by endothelin-1 activity.47,48 Although the precise mechanism for testicular degeneration associated with endothelin receptor antagonists has not yet been elucidated, it is plausible that altered fluid dynamics associated with PTMC impairment may contribute; vascular effects have also been considered. Necrosis of PTMC has been reported following administration of the histamine antagonist cimetidine to rats, with subsequent changes consistent with SC injury.49,50 PTMC provide essential support to SCs and decreased SC function may be secondary to PTMC injury. The interrelatedness of testicular cellular components blurs the discernment of which morphologic effects are primary versus secondary.
A serotonin receptor agonist administered to rats caused vasoconstriction with subsequent seminiferous tubular, rete, and efferent duct distention.51
Several compounds (ethylene dimethane sulfate, α-chlorhydrin, dinitrobenzene, cadmium, and carbendazim) have been implicated in obstruction of efferent ducts due to epithelial hyperplasia, sperm stasis, or occlusion by sloughed cells, with subsequent inflammation (Figs. 39 and 40) and testicular tubular degenerative effects.52 Failed excurrent ductular fluid resorption in mice lacking the estrogen receptor53 suggests estrogen modulatory compounds could result in similar fluid disturbances.
Figure 39.
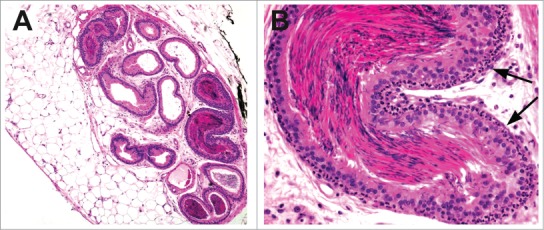
Efferent ducts from a rat with sperm stasis and a neutrophilic, peritubular inflammatory cell infiltrate (arrows).
Figure 40.
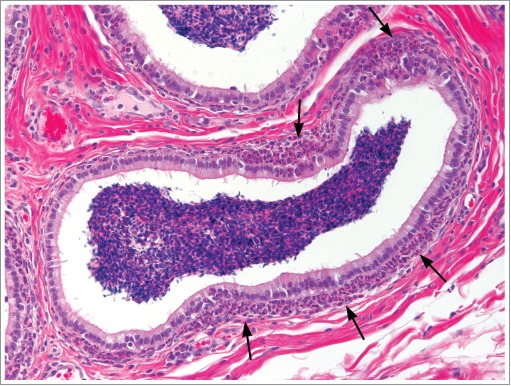
Efferent ducts from a cynomolgus monkey with sperm stasis and a neutrophilic, peritubular inflammatory cell infiltrate (arrows).
Vascular effects
Ischemia due to vascular compromise results in coagulative necrosis of testicular components, similar to severe the testicular vascular compromise occurring spontaneously with testicular torsion (Fig. 41). Cadmium is the best known testicular endothelial cell toxicant causing necrosis (in contrast to apoptosis) of germ cells and stromal cells, including SCs, LCs, PTMCs and interstitial vasculature.54 Sequelae of ischemic necrosis include inflammation, fibrosis and complete tubular atrophy. Focal ischemic necrosis has been seen following high doses of human chorionic gonadotropin with effects mediated by local production of vasoactive prostaglandins (Fig. 42).55 Less severe vascular effects due to vasoactive compounds such as serotonin, histamine and epinephrine cause less extensive effects impacting primarily germ cells and leading to patchy tubular atrophy (Fig. 43).
Figure 41.
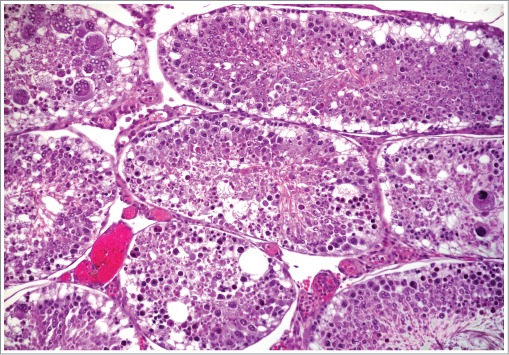
Testis from a rat with marked necrosis of the seminiferous tubules.
Figure 42.
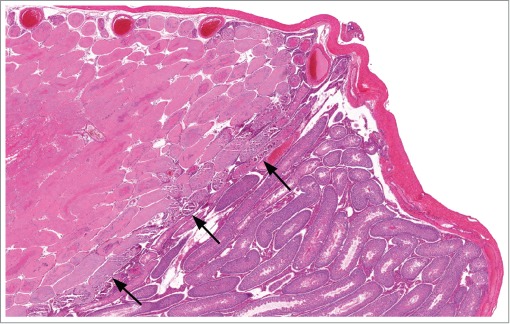
Testis from a rat with focal ischemic necrosis of frontal region of testis. Note the distinct line of demarcation between normal and abnormal tissue (arrows).
Figure 43.
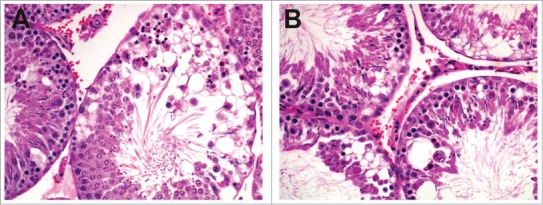
Rat testes demonstrating multifocal ischemic necrosis of germ cells (A) with secondary loss of germ cells (B). Note the interstitial hemorrhage.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Dianne Creasy for providing images and Beverly Maleeff for preparation of the figures.
References
- 1. Ulbrich B, Palmer AK. Detection of effects on male reproduction-a literature survey. J Am Coll Toxicol 1995; 14: 293-327; http://dx.doi.org/ 10.3109/10915819509008703 [DOI] [Google Scholar]
- 2. Linder RE, Strader LF, Slott VL, Suarez JD. Endpoints of spermatotoxicity in the rat after short duration exposures to fourteen reproductive toxicants. Repro Toxicol 1992; 6: 491-505; http://dx.doi.org/ 10.1016/0890-6238(92)90034-Q [DOI] [PubMed] [Google Scholar]
- 3. Meistrich ML. Critical components of testicular function and sensitivity to disruption. Biol Reprod 1986; 34: 17-28; PMID:3955133; http://dx.doi.org/ 10.1095/biolreprod34.1.17 [DOI] [PubMed] [Google Scholar]
- 4. Roeser HP, Stocks AE, Smith AJ. Testicular damage due to cytotoxic drugs and recovery after cessation of therapy. Aust N Z J Med 1978; 8: 250-4; PMID:279319; http://dx.doi.org/ 10.1111/j.1445-5994.1978.tb04518.x [DOI] [PubMed] [Google Scholar]
- 5. Kerr JB. Spontaneous degeneration of germ cells in normal rat testis: assessment of cell types and frequency during the spermatogenetic cycle. J Reprod Fertil 1992; 95: 825-30; PMID:1404097; http://dx.doi.org/ 10.1530/jrf.0.0950825 [DOI] [PubMed] [Google Scholar]
- 6. Brinkworth MH, Weinbauer GF, Schlatt S, Nieschlag E. Identification of male germ cells undergoing apoptosis in male rats. J Reprod Fertil 1995; 105: 25-33; PMID:7490711; http://dx.doi.org/ 10.1530/jrf.0.1050025 [DOI] [PubMed] [Google Scholar]
- 7. Meehan T, Loveland KL, de Kretser D, Cory S, Print CG. Developmental regulation of the bcl-2 family during spermatogenesis: Insights into the sterility of bcl-w-/- male mice. Cell Death Diff 2001; 8: 225-33; http://dx.doi.org/ 10.1038/sj.cdd.4400799 [DOI] [PubMed] [Google Scholar]
- 8. Russell LD, Chiarini-Garcia H, Korsmeyer SJ, Knudson CM. Bax-dependent spermatogonia apoptosis is required for testicular development and spermatogenesis. Biol Reprod 2002; 66: 950-58; PMID:11906913; http://dx.doi.org/ 10.1095/biolreprod66.4.950 [DOI] [PubMed] [Google Scholar]
- 9. Yan W, Samson M, Jegou B, Toppari J. Bcl-w forms complexes with Bax and Bak, and elevated ratios of Bax/Bcl-w and Bak/Bcl-w correspond to spermatogonial and spermatocyte apoptosis in the testis. Mol Endocrinol 2000; 14: 682-99; PMID:10809232; http://dx.doi.org/ 10.1210/mend.14.5.0443 [DOI] [PubMed] [Google Scholar]
- 10. Nurmio M, Toppari J, Zaman F, Andersson A-M, Paranko J, Soeder O, Jahnukainen K. Inhibition of tyrosine kinases PDGFR and c-kit by imatinib mesylate interferes with postnatal testicular development in the rat. Internatl J Androl 2007; 30: 366-76; http://dx.doi.org/ 10.1111/j.1365-2605.2007.00755.x [DOI] [PubMed] [Google Scholar]
- 11. Blanco-Rodriguez J, Martinez-Garcia C. Apoptosis pattern elicited by several apoptotic agents on the seminiferous epithelium of the adult rat testis. J Androl 1998; 19: 487-97; PMID:9733152 [PubMed] [Google Scholar]
- 12. Shinoda K, Mitsumori K, Yashura K, Uneyama C, Onodera H, Hirose M, Uehara M. Doxorubicin induces male germ cell apoptosis in rats. Arch Toxicol 1999; 73: 274-81; PMID:10463394; http://dx.doi.org/ 10.1007/s002040050617 [DOI] [PubMed] [Google Scholar]
- 13. Boekelheide K. Mechanisms of toxic damage to spermatogenesis. J Natl Cancer Inst Monogr 2005; 34: 6-8; PMID:15784812 [DOI] [PubMed] [Google Scholar]
- 14. Foster PMD, Creasy DM, Foster JR, Thomas LV, Cook MW, Gangolli SD. Testicular toxicity of ethylene glycol monomethyl and monomethyl ethers in the rat. Toxicol Appl Pharmacol 1983; 69: 385-99; PMID:6879608; http://dx.doi.org/ 10.1016/0041-008X(83)90262-4 [DOI] [PubMed] [Google Scholar]
- 15. Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinol 1997; 138: 2081-88. [DOI] [PubMed] [Google Scholar]
- 16. Bremner WJ, Millar MR, Sharpe RM, Saunders PTK. Immunohistochemical localization of androgen receptors in the rat testis: Evidence for stage-dependent expression and regulation by androgens. Endocrinol 1994; 135: 1227-34. [DOI] [PubMed] [Google Scholar]
- 17. Sar M, Hall SH, Wilson EM, French FS, Clermont Y. Androgen regulation of Sertoli cells. In Russell LD, Griswold MD, eds. The Sertoli Cell 1990; 552-75, Cache River Press; Clearwater, FL, USA. [Google Scholar]
- 18. Moffitt JS, Bryant BH, Hall SJ, Boekelheide K. Dose-dependent effects of Sertoli cell toxicants 2,5-hexanedione, carbendazim, and mono-(2-ethylhexyl) phthalate in adult rat testis. Toxicol Pathol 2007; 35: 719-27; PMID:17763286; http://dx.doi.org/ 10.1080/01926230701481931 [DOI] [PubMed] [Google Scholar]
- 19. Hild SA, Reel JR, Dykstra MJ, Mann PC, Marshall GR. Acute adverse effects of the indenopyridine CDB-4022 on the ultrastructure of Sertoli cells, spermatocytes, and spermatids in rat testes: comparison to the known Sertoli cell toxicant Di-n-pentylphthalate (DPP). J Androl 2007; 28: 621-29; PMID:17409460; http://dx.doi.org/ 10.2164/jandrol.106.002295 [DOI] [PubMed] [Google Scholar]
- 20. Creasy. Pathogenesis of male reproductive toxicity. Toxicol Pathol 2001; 29: 64-76; PMID:11215686; http://dx.doi.org/ 10.1080/019262301301418865 [DOI] [PubMed] [Google Scholar]
- 21. Steinberger E, Sud BN. Specific effect of fluoroacetamide on spermiogenesis. Biol Reprod 1970; 2: 369-75; PMID:5527836; http://dx.doi.org/ 10.1095/biolreprod2.3.369 [DOI] [PubMed] [Google Scholar]
- 22. Nakai M, Toshimori K, Yoshinaga K, Nasu T, Hess RA. Carbendazim-induced abnormal development of the acrosome during early phases of spermiogenesis in the rat testis. Cell Tiss Res 1998; 294: 145-52; http://dx.doi.org/ 10.1007/s004410051164 [DOI] [PubMed] [Google Scholar]
- 23. Beardsley A, O’Donnell L. Characterization of normal spermiation and spermiation failure induced by hormone suppression in adult rats. Biol Reprod 2003; 68: 1299-307; PMID:12606480; http://dx.doi.org/ 10.1095/biolreprod.102.009811 [DOI] [PubMed] [Google Scholar]
- 24. Troiano L, Faustini Fustini M, Lovato E, Frasoldati A, Malorni W, Capri M, Grassilli E, Marrmam P, Franceschi C. Apoptosis and spermatogenesis: Evidence from an in vivo model of testosterone withdrawal in the adult rat. Biochem Biophys Res Comm 1994; 202: 1315-21; PMID:8060308; http://dx.doi.org/ 10.1006/bbrc.1994.2074 [DOI] [PubMed] [Google Scholar]
- 25. Bartlett JMS, Kerr JB, Sharpe RM. The effect of selective destruction and regeneration of rat Leydig cells on the intratesticular distribution of testosterone and morphology of the seminiferous epithelium. J Androl 1986; 7: 240-53; PMID:3745011 [DOI] [PubMed] [Google Scholar]
- 26. Hikim AP, Leung A, Swerdloff RS. Involvement of apoptosis in the induction of germ cell degeneration in adult rat after gonadotropin-releasing hormone antagonist treatment. Endocrinology 1995; 136: 2770-75; PMID:7750502 [DOI] [PubMed] [Google Scholar]
- 27. D’Souza R, Pathak S, Upadhyay R, Gaonkar R, D’Sousa S, Sonawane S, Gill-Sharma M, Balasinor NH. Disruption of tubulobulbar complex by high intratesticular estrogen leading to failed spermiation. Endocrinol 2009; 150: 1861-69; http://dx.doi.org/ 10.1210/en.2008-1232 [DOI] [PubMed] [Google Scholar]
- 28. Noritake K-I, Suzuki J, Matsuoka T, Makino T, Ohnishi H, Shimomura K, Uenoyama Y, Tsukamura H, Maeda K-I, Sanbuissho A. Testicular toxicity induced by a triple neurokinin receptor antagonist in male dogs. Reprod Toxicol 2011; 31: 440-46; PMID:21185367; http://dx.doi.org/ 10.1016/j.reprotox.2010.12.007 [DOI] [PubMed] [Google Scholar]
- 29. Losco PE, Leach MW, Sinha D, Davis P, Schmahai TJ, Nomier A, Kakkar T, Reyderman L, Lynch ME. Administration of an antagonist of neurokinin receptors 1, 2, and 3 results in reproductive tract changes in beagle dogs, but not rats. Toxicol Pathol 2007; 35: 310-22; PMID:17366326; http://dx.doi.org/ 10.1080/01926230701198766 [DOI] [PubMed] [Google Scholar]
- 30. English HF, Schanbacher BD, Gross D, Walker MF, Falvo RE, Santen RJ. Animal model of isolated gonadotropin deficiency. J Androl 1983; 4: 240-47; PMID:6413470 [DOI] [PubMed] [Google Scholar]
- 31. Weinbauer GF, Schlatt S, Walter V, Nieschlag E. Testosterone-induced inhibition of spermatogenesis is more closely related to suppression of FSH than to testicular androgen levels in the cynomolgus monkey model (Macaca fascicularis). J Endocrinol 2001; 168: 25-38; PMID:11139767; http://dx.doi.org/ 10.1677/joe.0.1680025 [DOI] [PubMed] [Google Scholar]
- 32. Zhengwei Y, Wreford NG, Schlatt S, Weinbauer GF, Nieschlag E, McLachlan RI. Acute and specific impairment of spermatogonial development by GnRH antagonist-induced gonadotropin withdrawal in the adult macaque (Macaca fascicularis). J Reprod Fert 1998; 112: 139-47; http://dx.doi.org/ 10.1530/jrf.0.1120139 [DOI] [PubMed] [Google Scholar]
- 33. Narula A, Gu Y-Q, O'Donnell L, Stanton PG, Robertson DM, McLachlan RI, Bremner WJ. Variability in sperm suppression during testosterone administration to adult monkeys is related to follicle stimulating hormone suppression and not to intratesticular androgens. J Clin Endocrin Metab 2002; 87: 3399-406; http://dx.doi.org/ 10.1210/jcem.87.7.8681 [DOI] [PubMed] [Google Scholar]
- 34. O'Donnell L, Narula A, Balourdos G, Gu YQ, Wreford NG, Robertson DM, Bremner WJ, McLachlan RI. Impairment of spermatogonial development and spermiation after testosterone-induced gonadotropin suppression in adult monkeys (Macaca fascicularis). J Clin Endocrinol Metab 2001; 86: 1814-22; PMID:11297623; http://dx.doi.org/ 10.1210/jc.86.4.1814 [DOI] [PubMed] [Google Scholar]
- 35. Junker Walker U, Nogues V. Changes induced by treatment with aromatase inhibitors in testicular Leydig cells of rats and dogs. Exp Toxicol Pathol 1994; 46: 211-13; PMID:8000241; http://dx.doi.org/ 10.1016/S0940-2993(11)80083-7 [DOI] [PubMed] [Google Scholar]
- 36. Dirami G, Teerds KJ, Cooke BA. Effect of dopamine agonist on the development of Leydig cell hyperplasia in Sprague-Dawley rats. Toxicol Appl Pharmacol 1996; 141: 169-77; PMID:8917689 [DOI] [PubMed] [Google Scholar]
- 37. Alison RH, Capen CC, Prentice DE. Neoplastic lesion of questionable significance to humans. Toxicol Pathol 1994; 22: 179-86; PMID:7973365; http://dx.doi.org/ 10.1177/019262339402200211 [DOI] [PubMed] [Google Scholar]
- 38. Huseby RA. Demonstration of a direct carcinogenic effect of estradiol on Leydig cells of the mouse. Cancer Res 1980; 40: 1006-13; PMID:7357531 [PubMed] [Google Scholar]
- 39. Cook JC, Klinefelter GR, Hardisty JF, Sharpe RM, Foster PMD. Rodent Leydig cell tumorigenesis: a review of the physiology, pathology, mechanisms, and relevance to humans. Crit Rev Toxicol 1999; 29: 169-261; PMID:10213111; http://dx.doi.org/ 10.1080/10408449991349203 [DOI] [PubMed] [Google Scholar]
- 40. Morris AJ, Taylor MF, Morris ID. Leydig cell apoptosis in response to ethane dimethanesulphonate after both in vivo and in vitro treatment. J Androl 1997; 18: 274-80; PMID:9203055 [PubMed] [Google Scholar]
- 41. Garcia PV, Barbieri MF, Perobelli JE, Consonni SR, de Fatima Paccola Mesquita S, de Grava Kempinas W, Pereira LAV. Morphometric-stereological and functional epididymal alterations and a decrease in fertility in rats treated with finasteride and after a 30-day post-treatment recovery period. Fertil Steril 2012; 97: 1444-51; PMID:22521699; http://dx.doi.org/ 10.1016/j.fertnstert.2012.03.025 [DOI] [PubMed] [Google Scholar]
- 42. Heuser A, Mecklenburg L, Ockert D, Kohler M, Kemkowski J. Selective inhibition of PDE4 in Wistar rats can lead to dilatation in testis, efferent ducts, and epididymis and subsequent formation of sperm granulomas. Toxicol Pathol 2013; 41: 615-27; PMID:23197197; http://dx.doi.org/ 10.1177/0192623312463783 [DOI] [PubMed] [Google Scholar]
- 43. La DK, Creasy DM, Hess RA, Baxter E, Pereira M E, Johnson CA, Snook SS. Efferent duct toxicity with secondary testicular changes in rats following administration of a novel leukotriene A4 hydrolase inhibitor Toxicol Pathol 2012; 40: 705-14; PMID:22552396; http://dx.doi.org/ 10.1177/0192623312441412 [DOI] [PubMed] [Google Scholar]
- 44. Hess RA. Effects of environmental toxicants on the efferent ducts, epididymis and fertility. J Reprod Fertil Suppl 1998; 53: 247-59; PMID:10645284 [PubMed] [Google Scholar]
- 45. Foley GL, Bassily N, Hess RA. Intratubular spermatic granulomas of the canine efferent ductules. Toxicol Pathol 1995; 23: 731-34; http://dx.doi.org/ 10.1177/019262339502300612 [DOI] [PubMed] [Google Scholar]
- 46. Rudmann DG, McNerney ME, Vandereide SL, Schemmer JK, Eversole RR, Vonderfecht SL. Epididymal and systemic phospholipidosis in rats and dogs treated with the dopamine D3 selective antagonist PNU-177864. Toxicol Pathol 2004; 32: 326-32; PMID:15204974; http://dx.doi.org/ 10.1080/01926230490431754 [DOI] [PubMed] [Google Scholar]
- 47. Santiemma V, Belgiotti F, Magnanti M, Palleschi S, Silvestroni L, Fabbrini A. Endothelin-1 stimulates deoxyribonucleic acid synthesis and contraction in testicular peritubular myoid cells. Reprod Biol Endocrinol 1996; 54: 583-90; http://dx.doi.org/ 10.1095/biolreprod54.3.583 [DOI] [PubMed] [Google Scholar]
- 48. Tripiciano A, Filippini A, Giustiniani Q, Palombi F. Direct visualization of rat peritubular myoid cell contraction in response to endothelin. Biol Reprod 1996; 55: 25-31; PMID:8793054; http://dx.doi.org/ 10.1095/biolreprod55.1.25 [DOI] [PubMed] [Google Scholar]
- 49. Franca LR, Leal MC, Sasso-Cerri E, Vasconcelos A, Debeljuk L, Russell LD. Cimetidine (Tagamet) is a reproductive toxicant in male rats affecting peritubular cells. Biol Reprod 2000; 63: 1403-12; PMID:11058545; http://dx.doi.org/ 10.1095/biolreprod63.5.1403 [DOI] [PubMed] [Google Scholar]
- 50. Sasso-Cerri E, Cerri PS. Morphological evidences indicate that the interference of cimetidine on the peritubular components is responsible for detachment and apoptosis of Sertoli cells. Biol Reprod 2008; 6: 18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Piner J, Sutherland M, Millar M, Turner K, Newall D, Sharpe RM. Changes in vascular dynamics of the adult rat testis leading to transient accumulation of seminiferous tubule fluid after administration of a novel 5-hydroxytryptamine (5HT) agonist. Reprod Toxicol 2002; 16: 141-50; PMID:11955945; http://dx.doi.org/ 10.1016/S0890-6238(02)00008-4 [DOI] [PubMed] [Google Scholar]
- 52. Ilio KY, Hess RA. Structure and function of the ductuli efferentes: a review. Microsc Res Tech 1994; 29: 432-67; PMID:7873793; http://dx.doi.org/ 10.1002/jemt.1070290604 [DOI] [PubMed] [Google Scholar]
- 53. Hess RA, Bunick D, Lee K-H, Bahr J, Taylor JA, Korach KS, Lubahn DB. A role for oestrogens in the male reproductive system. Nature 1997; 390: 509-12; PMID:9393999; http://dx.doi.org/ 10.1038/37352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aoki A, Hoffer AP. Reexamination of the lesions in rat testis caused by cadmium. Biol Reprod 1978; 18: 579-91; PMID:207367; http://dx.doi.org/ 10.1095/biolreprod18.4.579 [DOI] [PubMed] [Google Scholar]
- 55. Chatani F. Possible mechanism for testicular focal necrosis induced by hCG in rats. J Toxicol Sci 2006; 31: 291-303; PMID:17077584; http://dx.doi.org/ 10.2131/jts.31.291 [DOI] [PubMed] [Google Scholar]


