Abstract
Reduction-oxidation (Redox) reactions are ubiquitous mechanisms for vital activities in all organisms, and they play pivotal roles in the regulation of spermatogenesis as well. Here we focus on 3 redox-involved processes that have drawn much recent attention: the regulation of signal transduction by reactive oxygen species (ROS) such as hydrogen peroxide, oxidative protein folding in the endoplasmic reticulum (ER), and sulfoxidation of protamines during sperm chromatin condensation. The first 2 of these processes are emerging topics in cell biology and are applicable to most living cells, which includes spermatogenic cells. The roles of ROS in signal transduction have been elucidated in the last 2 decades and have received broad attention, most notably from the viewpoint of the proper control of mitotic signals. Redox processes in the ER are important because this is the organelle where secretory and membrane proteins are synthesized and proceed toward their functional structure, so that malfunction of the ER affects not only the involved cells but also the accepting cells of the secreted proteins in multicellular organisms. Sulfoxidation is the third of these processes, and the sulfoxidation of chromatin is a unique process in sperm maturation. During recent sulfoxidase research, GPX4 has emerged as a promising enzyme that plays essential roles in the production of fertile sperm, but the involvement of other redox proteins is also becoming evident. Because the molecules involved in the redox reactions are prone to oxidation, they can be sensitive to oxidative damage, which makes them potential targets for antioxidant therapy.
Keywords: hydrogen peroxide, oxidative protein folding, reactive oxygen species, signal transduction, sulfoxidation
Signature Lesion
The regulation of oxidative processes, and the damage caused by uncontrolled oxidation, appears to affect all cells, from the somatic cells of the testis, through all cell types in spermatogenesis, and into the epididymal sperm. The manifestations of oxidative changes can range from defects in spermatogenesis to reduced sperm motility and altered fertilization. The widespread nature of its involvement in multiple processes in the reproductive system means that no specific signature lesion exists which can alert the pathologist to this as the underlying mechanism.
Introduction
Spermatogenesis occurs in the testis and includes a variety of cellular events, such as proliferation, meiosis, and differentiation. Spermatozoons contain the molecular machinery required for energy production, motility, and fertilization and behave like single-cell organisms during fertilization.1 Thus, various morphological and metabolic changes occur during the spermatogenic process.2 A structural change in chromatin, which involves the replacement of histones with protamines, occurs in the spermatids of the testes. Elevated counts of reactive oxygen species (ROS) are well-known causes of oxidative stress that can deteriorate physiological reactions and cause male infertility.3-5 Ejaculation from the male reproductive organ exposes sperm to a harsh environment, which elevates oxidative stress and damages DNA. Sperm prepare antioxidative systems, which consist of enzymes and low molecular weight antioxidants, to protect against this oxidative insult that may cause damage to DNA. To accomplish the protection of sperm function, certain genes are expressed in a testes- and/or sperm-specific manner.
ROS impair the physiological reactions caused by oxidizing molecules, and the modulatory roles of ROS that modulate the intracellular signal from the receptor tyrosine kinases (RTKs) in ordinary somatic cells are becoming evident.6,7 Many humoral factors are required to support the spermatogenic process, which includes the proliferation of spermatogonia and the differentiation of spermatocytes to sperm. Some receptors for the humoral factors have tyrosine kinase activity and are members of RTKs. Thus, the ROS play seemingly contradictory roles: beneficial roles by regulating phosphorylation signals via controlling phosphatases on one side; and, detrimental roles by oxidatively modifying valuable molecules on the other side. Antioxidant therapy is not always successful at least partly due to this dual nature of ROS.8 Hence, a more precise elucidation of ROS-involved reactions would improve the treatment of male infertility caused by impaired spermatogenesis.
Regarding the cells that secrete humoral factors to support spermatogenesis, such as Sertoli cells and Leydig cells, the endoplasmic reticulum (ER) is the place where nascent proteins are synthesized and subjected to proteolytic cleavage, oxidative folding by disulfide bonds, and the addition of sugar chains. ROS are potential causative agents that trigger the misfolding of proteins and result in the induction of unfolded protein responses (UPR).9 Dysfunction of the UPR system leads to defects in the secretion of the humoral factors that are essential for spermatogenesis. Thus, severe ER stress down-regulates essential humoral factors and may impair sperm morphogenesis.10
During the spermatogenic process, the histones in chromatin are converted to transition proteins and finally to protamines in sperm.1 Sulfoxidation occurs in cysteines, which are low in histones but enriched in the mammalian protamines, and furnish chromatin for resistance against oxidative stress. In addition, some intracellular components, such as spermatogenic cell-specific type 1 hexokinase isozyme in the cytosol, appear to be regulated by the redox state.11
Thus, it is gradually becoming clear that redox reactions contribute to spermatogenesis in multiple ways.1,12 Here we review recent advances in the spermatogenic process from the viewpoint of redox reactions in the cells: the modulation of signals mediated by tryrosine phosphorylation/dephosphorylation, oxidative protein folding in the ER, and sulfoxidation of protamines during sperm maturation. Male infertility is also discussed due to the disruption of this machinery by an elevation in ROS.
Antioxidative Systems and Oxidative Stress-Triggered Injuries in Reproduction
Oxidative stress, as characterized by elevated ROS, is triggered by various pathological conditions such as inflammation, ischemia, and heat stress and is regarded as a major causative factor for male infertility.3-5 (Also see Gregory and Cyr,13 this issue for effects of ROS on epididymal function). Sperm contain a large body of unsaturated fatty acids that are prone to oxidation. Spermatogenic cells with oxidatively damaged DNA undergo elimination by apoptosis via p53-dependent and -independent mechanisms,14 which in excess can result in male infertility.15 In addition, because redox-sensitive proteins are highly reactive to ROS, they are potent targets of ROS under oxidative stress.
There are enzymes that specifically catalyze the detoxification of ROS, so-called antioxidative enzymes, such as superoxide dismutase (SOD) and glutathione peroxidase (GPX). The details of antioxidative enzymes in the male reproductive system have been reviewed elsewhere,16,17 so they are only briefly discussed here in order to address their protective roles in the pathogenesis of the male reproductive system. SOD is an enzyme that converts superoxide radicals to hydrogen peroxide, which stops the harmful radical chain reaction from beginning.18 Cu,ZnSOD, encoded by SOD1, are localized mainly in the cytoplasm and partially in the intermembrane space of mitochondria. While the SOD1-deficient female is infertile, initial studies have found no other defects including those in male fertilizing ability.19,20 SOD1 deficiency indeed causes testicular atrophy and increased vulnerability to heat stress.21 A decrease in sperm numbers and the concomitant elevation in lipid peroxidation products were recently found in aged SOD1-deficient mice compared with wild-type mice,22 although a causal connection with the fertilization ability of the aged male mice was not clarified. SOD2 encodes MnSOD, a mitochondrial isoform, and is inducible under oxidative stress and inflammatory conditions. A deficiency of SOD2 is lethal to an infant after birth.23 Unexpectedly, transgenic male mice that express higher levels of SOD2 are infertile, and the mechanism for this has not been determined.24 Extracellular SOD, encoded by SOD3, is present in high levels in the epididymal fluid25 and is partially localized in the nuclei of the spermatogenic cells.26 Although SOD3 knockout mice show no recognizable phenotypic changes in the male reproductive system,27 transferring SOD3 to the penis has improved erectile function in aged rats.28 Because superoxide reacts quickly with nitric oxide to form peroxinitrite, the increase in SOD3 in blood plasma prolongs the nitric oxide half-life and consequently contributes to an improvement in erectile function. However, excess SOD activities have been negatively correlated with the movement characteristics of human spermatozoa,29 which may be attributed to the removal of superoxide that is essential for sperm movement. Thus, both the source and the microenvironment determine whether the superoxide functions to positively or negatively support reproductive processes.
Hydrogen peroxide is produced by a variety of enzymatic and non-enzymatic reactions in the body and is detoxified by several enzymes such as glutathione peroxidase (GPX), catalase, and peroxiredoxin (PRDX). GPX catalyzes the reduction of various peroxides by transferring electrons from glutathione,30 but the roles of each member of the gene family are different and complex.31,32 Peroxiredoxins (PRDXs) catalyze the reductive removal of hydrogen peroxides by using thioredoxin (Trx) instead of glutathione as an electron donor33 and also play multiples roles in redox reactions including ROS signaling.
Redox Proteins and Their Function in Testes
One of the many metabolic processes whereby the redox reaction supports life is during energy production by oxidative phosphorylation in mitochondria. Redox regulation, in a limited sense, can be seen as a reversible reaction of molecules that often involves an oxidative modification and a reduction in cysteine sulfhydryls. While some proteins have redox-sensitive cysteines and are regulated by the redox state of the residues, there are protein families that participate in transferring redox potential from donor molecules to target proteins. Typically, Trx, which is found as a subunit of ribonucleotide reductase, plays pleiotropic roles in redox reactions.34 There are also low molecular weight redox molecules, such as vitamin C and glutathione.35,36 Because reduction on most occasions recovers protein function due to oxidative damage, redox molecules are regarded as potent antioxidants.
As typified in the ribonucleotide reductase reaction, Trx is one of the most pivotal electron donors in a variety of redox reactions.34 A conventional Trx is essential for proliferating cells and, hence, it is fatal to its null mutant mouse.37 There are 3 testis-specific Trxs that are expressed in spermatids: Sptrx-1 (TXNDC2), Sptrx-2 (TXNDC3), and Sptrx-3 (TXNDC8).38 However, a deficiency of the 2 sperm-specific Trxs, TXNDC2 and TXNDC3, in mice has shown elevated oxidative stress, albeit with no impact on spermatogenesis, epididymal sperm maturation, or infertility.39 Sptrx-3 is found in the Golgi apparatus of the spermatocyte/spermatid40 and may function in the ER/Golgi in coordination with PRDX4, as described below. GPX uses glutathione as an electron donor, and PRDX uses Trx as an electron donor. Recently, the role of PRDX in modulating ROS signals has attracted much attention.6,7 Among 6 mammalian PRDX, PRDX4 is an ER-resident protein that appears to play a pivotal role in the redox relay during oxidative protein folding in the ER,41 although sulfhydryls in the nascent proteins are the electron donor. There is a testis-specific variant that lacks the signal peptide required for ER localization but remains in the cytosol via the alternate usage of the promoter/exon 1. As will be discussed further, the testis-specific PRDX4 may play a role in chromatin condensation during spermatogenesis.
Selenium is an essential trace element, and its deficiency produces defective spermatozoa and causes male infertility.32 Selenium is incorporated into selenoproteins and is commonly involved in redox reactions. Selenoprotein P carries 10 selenocysteine residues and is regarded as a selenium transporter in blood plasma. Selenoprotein P transports selenium from the liver to the testis, and selenoprotein P-deficient male mice are infertile.42-44 Selenocysteine (Sec) constitutes the catalytic center of redox proteins in mammals, such as thioredoxin reductase and GPX, so selenium deficiency results in a loss of these enzyme activities. The 4 conventional GPX isoforms (GPX 1–4) contain selenocysteine at the catalytic center. Among the 4 Sec-containing GPXs, GPX4 plays an essential role in spermatogenesis, as described below.
A Sec residue is present at the penultimate position of the carboxy terminus of thioredoxin reductases and forms the catalytic center. Mammals carry 3 types of thioredoxin reductases—one cytosolic, one mitochondrial, and one testis-specific thioredoxin-glutathione reductase (TGR).45 TGR is the fusion enzyme that possesses an N-terminal glutaredoxin domain and the thioredoxin module.46 TGR is induced after puberty, is abundantly present in elongating spermatids at the site of mitochondrial sheath formation, and catalyzes isomerization and the inter protein disulfide bond formation of proteins. Sec incorporation requires a multiprotein complex that includes Sec insertion sequence-binding protein 2 (SECISBP2). Mutation in the SECISBP affects 25 known human selenoproteins and is associated with a multisystem disorder that includes azoospermia.47
ROS Signaling in Response to Extracellular Stimuli
The signaling role of ROS in the proliferation of somatic cells in response to extracellular stimuli has attracted considerable attention during the last 2 decades.6,7 Extracellular stimuli such as growth factors induce the phosphorylation of tyrosine residues on RTK as well as on its substrate proteins. This tyrosine phosphorylation initiates the activation of signal transducing pathways and consequently triggers cellular events that include the proliferation of the cells via gene expression and progression of the cell cycle. Phosphorylation and dephosphorylation are coordinated events, so that controlling the phosphorylation status of the signaling molecules strictly regulates cell growth. Dephosphorylation is catalyzed by phosphatases that are classified into 2 major groups: one reacts with phospho-serine and -threonine, and the other reacts with phospho-tyrosine.48
Phosphatases reactive to phospho-tyrosine residues in RTKs are largely localized underneath plasma membrane and regulate the mitotic signal. The potential role of PTPs is to avoid excess responses to stimuli. The phospho-tyrosine phosphatases (PTPs) have a low pKa sulfhydryl group that functions as a catalytic center. PTP1B is the first identified PTP that is inactivated by hydrogen peroxide under physiological conditions.49 Concomitant with RTK activation, hydrogen peroxide is produced from the superoxide that is generated by NADPH oxidase in the plasma membrane, and it is involved in the sustained activation of the phosphorylation signal (Fig. 1). Similar mechanisms are applicable for other PTP family members48 and for PTEN, which is a phosphatase for phosphatidyl inositid.50 Cysteine sulfhydryl (Cys-SH) at the catalytic center in these phosphatases is oxidatively modified to cysteine sulfenic acid (Cys-SOH).51 Because this process is reversible, the inactivated phosphatase is reductively recovered and ceases the phosphotyrosine-mediated signaling by dephosphorylation. However, under severe oxidative stress, Cys-SOH reacts with other sulfhydryls to form disulfide in PTP. According to this scenario, ROS under oxidative stress will keep stimulating spermatogenic cell proliferation in an uncontrollable manner, which results in tumorigenic proliferation. In fact, PRDX controls the hydrogen peroxide to appropriate levels and performs fine-tuning of the ROS signal,33 although the pathway was omitted from the schematic diagram in Figure 1.
Figure 1.
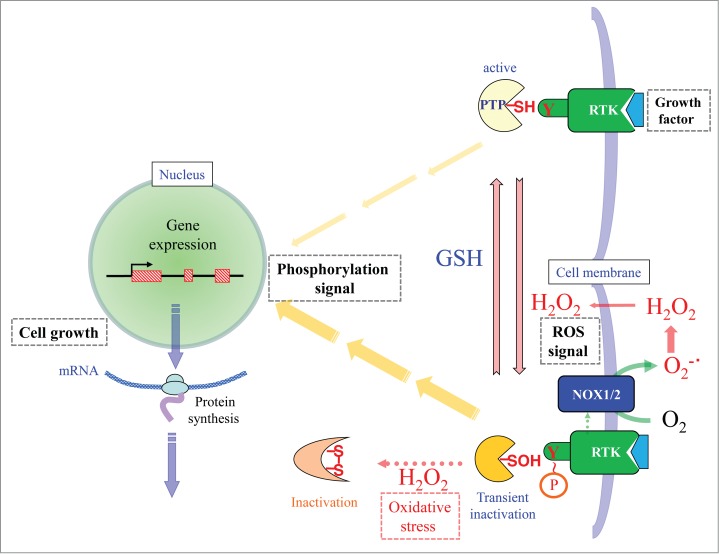
Schematic presentation of the hydrogen peroxide-mediated regulation of phosphorylation signals in response to extracellular stimuli. When a hydrophilic growth factor binds RTK, tyrosine phosphorylation occurs on the receptor. PTP in its active form dephosphorylates RTK, so that only weak phosphorylation signals are transmitted to the nucleus (the upper pathway). When NADPH oxidase (NOX) is activated by ligand binding, superoxide is simultaneously produced and dismutated to hydrogen peroxide. Because catalytic cysteine in PTP is highly sensitive to hydrogen peroxide, the catalytic cysteine sulfhydryl (Cys-SH) is oxidized to cysteine sulfenic acid (Cys-SOH), which causes transient inactivation of PTP. Accordingly, the activated state of RTK with phosphorylated tyrosine is sustained, which transmits mitotic signal for an extended period of time (the lower pathway). Cys-SOH can be reduced back to Cys-SH by reductants such as glutathione (GSH), which ceases the signal transmission. When hydrogen peroxide is produced excessively, PTP is inactivated by intramolecular disulfide bond formation. This mechanism prolongs the phosphorylation signal and hence causes tumorigenic growth of the cells. Terms: Y, tyrosine residue; P, phosphate group; RTK, receptor for tyrosine kinase; PTP; phosphotyrosine phosphatase, NOX; NADPH-dependent oxidase, SH; sulfhydryl, SOH; sulfenic acid.
In the female reproductive system, PTEN deficiency causes sustained activation of the PI3-kinase/Akt system and consequently has led to premature activation of the primordial follicle in mice.52,53 These results suggest the potential involvement of redox regulation in the PI3-kinase/Akt system by controlling PTEN function. However, PTEN is dispensable in mouse spermatogenesis according to at least one study on a conditional knockout mouse.54 Thus, the significance of PTEN-mediated PI3-kinase/Akt regulation in the spermatogenic process is as yet not clearly understood, and must be clarified.
Redox Relay in Oxidative Protein Folding in the ER
Sustentacular cells, such as Sertoli cells and Leydig cells, produce many humoral factors that support spermatogenesis. The receptor molecules for hydrophilic humoral factors are localized in the plasma membrane with the largest portion exposed to the surface of the target cell. A proper alignment of the ligands and receptors is essential for their interaction. Thus, their structures are strictly controlled. The disulfide bond between intra and inter polypeptides is largely involved in maintaining the protein conformation in the extracellular space where the redox state is more oxidized compared with inside the cells. Occasionally the presence of thiol isomerases such as protein disulfide isomerase (PDI) can rearrange the disulfide bonds in the membrane proteins and determine their functional states.55 For example, the disulfide bond in the membrane protein CD4, which is used for the entry of several enveloped viruses, and integrin, which is used by platelets for attachment, are rearranged by the function of PDI family members. Because some surface membrane proteins in sperm, such as Izumo, are essential for fertilization,56 disulfide bond rearranging may also play a role in proper sperm function. Inhibition of potential thiol-disulfide exchanger ERp57 on the sperm cell surface by antibody or PDI inhibitors suppresses gamete fusion,57 which suggests that ERp57 is a potential thiol isomerase in the fertilization process.
Both membrane proteins and secretory proteins, including protein humoral factors, are synthesized and subjected to oxidative protein folding in the ER, after which they move onto either cell membranes or to a secretory pathway (Fig. 2). Endoplasmic reticulum oxidoreductin 1 (ERO1) introduces a disulfide bond in PDI by oxidizing molecular oxygen and releases hydrogen peroxide as a byproduct.58 PDI then introduces disulfide bonds in nascent target proteins. While ERO1 utilizes molecular oxygen, PRDX4 uses hydrogen peroxide to catalyze the formation of disulfide bonds in PDI.59,60 Thus, oxidative protein folding proceeds efficiently via the coordinated function of ERO1 and PRDX4 in mammalian cells. A deficiency of both ERO1 and PRDX4 causes consumption of ascorbic acid, and as a consequence, the development of scurvy when collagen production is decreased.41 On the contrary, NOX4, which is present in the ER membrane and is critical for the regulation of PTP1B during EGF signaling, reduces molecular oxygen to superoxide and results in the production of hydrogen peroxide.61 The resultant hydrogen peroxide may also be used for oxidative protein folding by PRDX4. Although this mechanism has been demonstrated mainly for cell lines under cultural conditions to this point, a similar mechanism could be predicted to be functional not only in Sertoli cells and Leydig cells, but also in spermatogenic cells.
Figure 2.
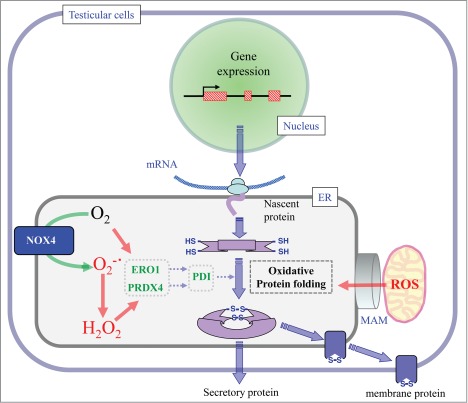
Oxidative folding of secretory and membrane proteins in the ER. Membrane and secretory proteins are produced in rough ER. After cleavage of the signal peptide, nascent proteins undergo oxidative folding in the ER lumen. By using the oxidizing power of oxygen, the ERO1 molecule introduces a disulfide bond in the PDI family proteins and meantime results in the production of hydrogen peroxide. NOX4 that is a NADPH oxidase that is present in the ER membrane converts oxygen molecules to superoxide, which is spontaneously dismutated to hydrogen peroxide. PRDX4 by using the oxidizing power of hydrogen peroxide introduces a disulfide bond in PDI family proteins and meantime results in the production of water. PDI consequently introduces a disulfide bond in the target proteins. ROS may also come from a mitochondria-associated membrane (MAM).
In the processes involving either protein secretion from cells or membrane localization, a significant portion of nascent proteins is misfolded in the ER, and the accumulation induces the unfolded protein responses (UPR) in the cells.9 UPR is a self-protective mechanism whereby cells attempt to rescue the normal function of ER. However, when the misfolded proteins are too much to handle under extreme situations, cell death is induced. Both extrinsic causes such as toxicants and intrinsic causes such as ischemia and heat induce UPR and may ultimately induce spermatogenic cell death. For example, human chorionic gonadotropin (hCG) triggers ER stress that results in the induction of apoptosis in Leydig cells.62 Cadmium, which is a potent testicular toxicant, also triggers ER stress and induces germ cell apoptosis in the testes.63 Although the data are still phenomenological and requires further elucidation, dysfunction of the machinery for oxidative protein folding in the ER must surely be involved.
In addition to its role in oxidative protein folding, a potential role of the testis-specific variant of PRDX4 has been proposed.64,65 In fact, a testis-specific variant mRNA is expressed in only a certain stage of spermatogenesis, notably spermatid.65 Deletion of the conventional promoter/exon1 of the PRDX4 gene results in no expression in ordinary cells66 but retains expression of the testis-specific PRDX4 variant in reduced levels in spermatogenic cells.65 This causes delays in the sexual maturation of male mice and makes testicular cells vulnerable to heat stress.66 Since the amino-terminal signal peptide in the conventional PRDX4 is substituted for a hydrophilic one in the testicular PRDX4, the testis-specific PRDX4 protein cannot localize in the ER lumen, but remains in the cytosol. Meanwhile, the domain required for catalysis remains in the testis-specific variant, which may assign the testis-specific PRDX4 to the oxidative protein folding in the cytosol and/or in the nucleus.
Sulfoxidation During Sperm Maturation in the Epididymis
Spermatogenic cells undergo meiosis and experience tremendous morphological changes. In somatic cells, basic protein histones constitute chromatin and stabilize DNA. Histone modifications such as methylation and acetylation induce structural changes that enable transcriptional factors to bind DNA, which consequently activates the transcription of genes by an epigenetic mechanism. During the spermatogenic process, histones are replaced by transition proteins and ultimately by protamines (Fig. 3). While the transition proteins also play significant roles during spermatogenesis,67,68 protamines are prerequisite for sperm function as well as structure.69,70 Both lysine and arginine are rich in histones, but next to arginine, cysteine is predominant in the protamines of rodents and humans.1 Regarding the primary structure, histones are conserved among different species while protamine is highly variable among species. It is postulated that the divergence may contribute to the avoidance of fertilization with other species.71 Sulfoxidation occurs in the cysteines of the protamines and furnishes chromatin with resistance against oxidative stress, which is accomplished in the epididymis.1 Disulfide bond formation that occurs on cysteine sulfhydryls in sperm protamines is only partial in the testes, and may be performed by a testis-specific PRDX4 protein.
Figure 3.
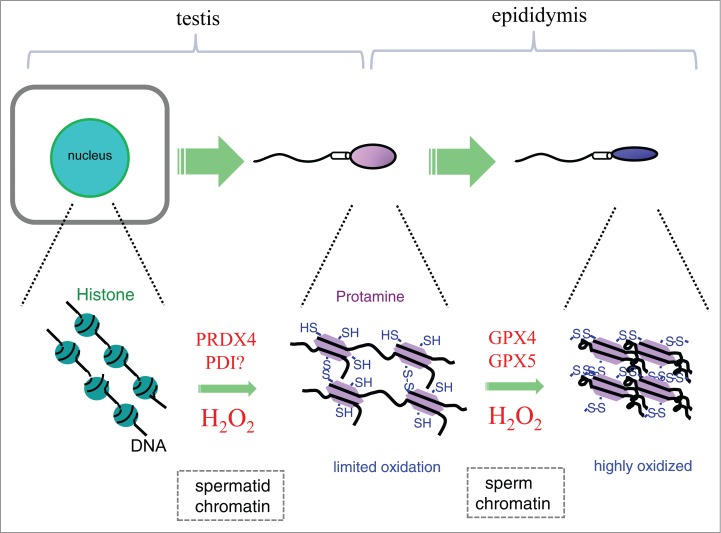
Conversion of the chromatin structure during spermatocyte differentiation to sperm, and the redox state of chromatin during sperm maturation. During spermiogenesis, histones in chromatin are replaced by protamines that are rich in Cys residues. A testis-specific PRDX4 may be involved in the introduction of the disulfide bond in protamine to a limited extent. The maturation of sperm is accomplished in the epididymis by sulfoxidation that involves GPX4 and/or GPX5.
The majority of the sulfoxidation in protamines occurs in the epididymis where the maturation of sperm proceeds. Phospholipid hydroperoxide glutathione peroxidase encoded by GPX4 specifically detoxifies phospholipid hydroperoxides, and plays the most important role in the testes among the members of the GPX family.30 GPX4 protein constitutes about 50% of the capsule material of the helix of mitochondria in the midpiece of sperm.72 Male infertility appears to be correlated with a genetic defect in GPX4,73,74 although results obtained by an analysis of the linkage between male infertility and genetic variations of GPX4 in men are inconclusive.75
GPX4-null knockout mice show premature embryonic death in uteri, but the direct cause of the death is unclear.76,77 Three GPX4 variants, mitochondrial, cytosolic, and nucleolar GPX4, are generated from one gene by alternative transcription.76,78 The disruption of mitochondrial GPX4 causes male infertility.79 Severe structural abnormalities are observed in the midpiece of sperm that is constructed by mitochondria. Higher protein thiol contents in the sperm compared to wild-type sperm support the role of mitochondrial GPX4 as a sulfoxidase. Because the deficiency of mitochondrial GPX4 does not affect germinal and somatic cells, it appears that the embryonic lethality is caused by a deficiency in the cytosolic GPX4.
The spermatozoa of mice lacking both the sperm nuclear GPX4 and the epididymal glutathione peroxidase 5 activities display structural abnormalities in the sperm nucleus that include delayed and defective nuclear compaction, nuclear instability and DNA damage.80 Sperm from nuclear GPX4-knockout mice are more prone to decondense than those from wild-type mice during epididymal maturation, implying that nuclear GPX4 is required for a correct sperm chromatin compaction.81 Thus, the nuclear GPX4 appears to function as a protein thiol peroxidase in the epididymis82 and contributes to sperm chromatin stability.80,83
All spermatocyte-specific GPX4 knockout male mice are infertile, and isolated epididymal GPX4-null spermatozoa cannot fertilize oocytes in vitro. Significant reductions in forward motility and in mitochondrial membrane potential have been observed. Structural abnormalities such as a hairpin-like flagella bend at the midpiece and swelling of the mitochondria in the spermatozoa are also evident (Fig 4A). In the testis, GPX4 null spermatozoa have extended flagellum. In contrast, caput GPX4-null spermatozoa display an abnormal flagellar bending in the posterior midpiece of the flagellum, while cauda GPX4 null spermatozoa are sharply bent at the mid-piece-principal piece junction and exhibit a hairpin flagellar configuration (Fig 4B). These results indicate that the formation of the flagellar in spermatozoa matures via a crosslinking of mitochondrial capsule protein and GPX4 as a sulfoxidase of mitochondrial GPX4 in the epididymis (Fig 4C). On the other hand, the mitochondrial membrane potential of cauda GPX4 null spermatozoa is normally maintained, however a loss of the mitochondrial membrane potential of cauda GPX4 null spermatozoa quickly occurs after incubation in vitro, indicating that mitochondrial GPX4 also function as antioxidant enzymes in order to maintain the mitochondrial membrane potential.84
Figure 4.
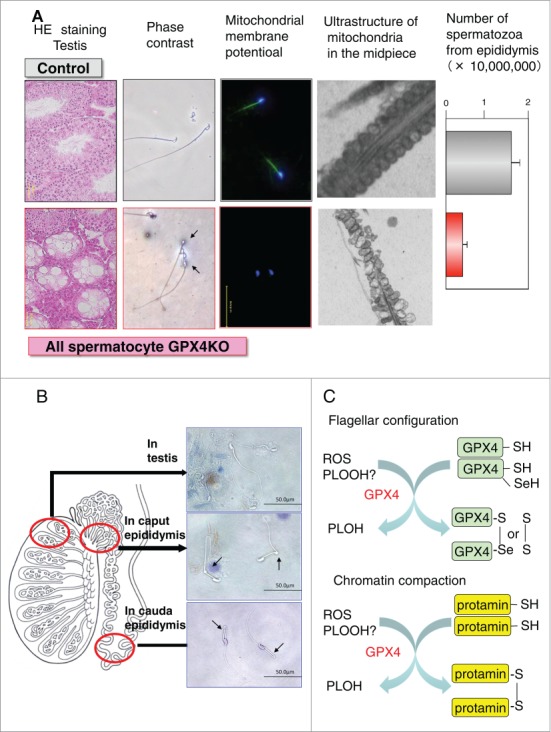
Phenotype of all spermatocyte GPX4 null mice and the functions of GPX4 as a sulfoxidase (A) All the spermatocyte-specific GPX4 knockout male mice show male infertility with 2 significant conspicuous phenotypes: first, a decreased number of spermatozoa in the epididymis caused by the depletion of spermatogenic cells in seminiferous tubules and second, a loss of the forward motility of spermatozoa due to a hairpin-like bending of the tail in the distal midpiece region and/or mitochondrial dysfunction including mitochondrial membrane potential and ultrastructure. (B) The developmental progression of defects in the flagellar structure in spermatozoa of all spermatocyte GPx4 null mice during maturation in epididymis. (C) GPX4 functions as a sulfoxidase in the epididymis to mature flagellar configuration and chromatin compaction. Terms: ROS, reactive oxygen species, PLOOH, phospholipid hydroperoxide, PLOH, hydroxy phospholipid.
All spermatocyte-specific GPX4 knockout male mice have a decreased number of spermatozoa by the depletion of spermatogenic cells in the seminiferous tubules, but no decrease in the number of spermatozoa have been observed in mitochondrial GPX4 knockout mice, indicating that a defect in spermatogenesis is caused by a deficiency in the cytosolic GPX4 (Fig 4A). Human infertile patients with GPX4-null spermatozoa are classified as suffering from oligoasthenospermatozoa.73
Thus, the depletion of both cytosolic and mitochondrial GPX4 in spermatocytes severely affects sperm generation, structure and function, which may explain male infertility in mice and humans.3 Collectively, these data are consistent with the notion that GPX4 functions as a sulfoxidase in the epididymis that promotes the maturation of flagellar configuration and chromatin compaction (Fig 4C). However, ambiguity remains regarding the source of the oxidation power. When GPX4 functions as thiol oxidase toward protamines in a similar manner to PRDX4 in the ER, it is unclear what supplies the oxidation power to GPX4. Structural studies to elucidate the molecular mechanisms for cross-linking the protamine by GPX4 are needed.
The final sulfoxidation occurs in the epididymis in a highly regulated manner, and both GPX4 and GPX5 appear to be involved. When redox regulation occurs to the phosphorylation signal from RTK, the ROS comes from the plasma membrane NOX (Fig. 1). In the case of oxidative protein folding in the ER, the ROS comes from the ER membrane NOX4, the ERO1, or the mitochondria via MAM (Fig. 2). However, corresponding oxidases or ROS sources have not been clearly revealed. Thus, an important issue regarding the source of ROS remains unclear in the chromatin condensation process. Also, PDI-like protein that may relay the oxidation potential from PRDX4 in the testes or from GPX4/GPX5 in the epididymis to the protamines is missing. Because PRDX also behaves as a molecular chaperone,85 instead of a sulfoxidase, PRDX4 may function as a molecular chaperone in the replacement of histones with protamines during spermatogenesis. Because tyrosine phosphorylation is elevated in sperm tail proteins during maturation in the epididymis,86 ROS produced in the epididymis is involved not only in sulfoxidation in protamines but also in modulating the phosphorylation signal in response to extracellular stimuli, as discussed above.
Perspectives
Redox reactions have various involvements in the reproductive process; sperm are formed via marked morphological changes, and behave as single organisms. Spermatogenesis consists of multiple complex processes, and, hence, is regulated by a variety of redox systems that are so complex we have not yet gained a full understanding of them. The subjects covered in this manuscript contain recent topics that are commonly observed in conventional somatic cells, ROS signaling and oxidative protein folding in the ER, as well as unique phenomenon to the spermatogenic process, such as the sulfoxidation of protamines during sperm maturation. While an application of the findings from other biological systems provides us clues to unveil the spermatogenic processes, we must continue our efforts toward clarification. Because the molecules involved in the redox reactions possess cysteine sulfhydryl that is highly sensitive to ROS, the proteins involved in the redox reactions are also potent targets of oxidative stress. Thus, this approach would lead to a better understanding of the pathogenesis of male infertility that is caused by oxidative stress, which could advance antioxidant therapy in a rational manner.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work is partly supported by a grant for Research on Child Health and Development from the Ministry of Health, Labor and Welfare, Japan, and by the YU-COE program from Yamagata University.
References
- 1. Kanippayoor RL, Alpern JH, Moehring AJ. Protamines and spermatogenesis in Drosophila and Homo sapiens: a comparative analysis. Spermatogenesis 2013; 3(2):e24376; PMID:23885304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rathke C, Baarends WM, Awe S, Renkawitz-Pohl R. Chromatin dynamics during spermiogenesis. Biochim Biophys Acta 2013; S1874-9399(13):00138-7; PMID:24091090; http://dx.doi.org/ 10.1016/j.bbagrm.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 3. Aitken RJ, Baker MA. Oxidative stress, sperm survival and fertility control. Mol Cell Endocrinol 2006; 250: 66-9; PMID:16412557 [DOI] [PubMed] [Google Scholar]
- 4. Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol 2008; 59: 2-11; PMID:18154591 [DOI] [PubMed] [Google Scholar]
- 5. Tremellen K. Oxidative stress and male infertility–a clinical perspective. Hum Reprod Update 2008; 14: 243-58; PMID:18281241; http://dx.doi.org/ 10.1093/humupd/dmn004 [DOI] [PubMed] [Google Scholar]
- 6. Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006; 312:1882-1883; PMID:16809515 [DOI] [PubMed] [Google Scholar]
- 7. Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 2011; 194:7-15; PMID:21832045; http://dx.doi.org/ 10.1074/jbc.R111.271999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kefer JC, Agarwal A, Sabanegh E. Role of antioxidants in the treatment of male infertility. Int J Urol 2009; 16: 449-57; PMID:19383039; http://dx.doi.org/ 10.1111/j.1442-2042.2009.02280.x [DOI] [PubMed] [Google Scholar]
- 9. Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 2011; 334(6059):1081-6; PMID:22116877; http://dx.doi.org/ 10.1126/science.1209038 [DOI] [PubMed] [Google Scholar]
- 10. Kim JH, Park SJ, Kim TS, Park HJ, Park J, Kim BK, Kim GR, Kim JM, Huang SM, Chae JI, et al. Testicular hyperthermia induces unfolded protein response signaling activation in spermatocyte. Biochem Biophys Res Commun 2013; 434(4):861-6; PMID:23611781; http://dx.doi.org/ 10.1016/j.bbrc.2013.04.032 [DOI] [PubMed] [Google Scholar]
- 11. Nakamura N, Miranda-Vizuete A, Miki K, Mori C, Eddy EM. Cleavage of disulfide bonds in mouse spermatogenic cell-specific type 1 hexokinase isozyme is associated with increased hexokinase activity and initiation of sperm motility. Biol Reprod 2008; 79:537-45; PMID:18509164; http://dx.doi.org/ 10.1095/biolreprod.108.067561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujii J, Tsunoda S. Redox regulation of spermatogenic process and fertilization. Asian J Androl 2011; 13:420-3; PMID:21460861; http://dx.doi.org/ 10.1038/aja.2011.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gregory and Cyr, in this issue of the Journal. [Google Scholar]
- 14. Yin Y, DeWolf WC, Morgentaler A. Experimental cryptorchidism induces testicular germ cell apoptosis by p53-dependent and-independent pathways in mice. Biol Reprod 1998; 58:492-6; PMID:9475406 [DOI] [PubMed] [Google Scholar]
- 15. Garrido N, Meseguer M, Simon C, Pellicer A, Remohi J. Pro-oxidative and anti-oxidative imbalance in human semen and its relation with male fertility. Asian J Androl 2004; 6:59-65; PMID:15064836 [PubMed] [Google Scholar]
- 16. Bauche F, Fouchard MH, Jegou B. Antioxidant system in rat testicular cells. FEBS Lett 1994; 349:392-6; PMID:8050602 [DOI] [PubMed] [Google Scholar]
- 17. Fujii J, Iuchi Y, Matsuki S, Ishii T. Cooperative function of antioxidant and redox systems against oxidative stress in male reproductive tissues. Asian J Androl 2003; 5:231-42; PMID:12937808 [PubMed] [Google Scholar]
- 18. Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem 1995; 64:97-112; PMID: 7574505 [DOI] [PubMed] [Google Scholar]
- 19. Ho YS, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J Biol Chem 1998; 273:7765-9; PMID:9516486 [DOI] [PubMed] [Google Scholar]
- 20. Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology 1998; 139: 4008-11; PMID:9724058 [DOI] [PubMed] [Google Scholar]
- 21. Ishii T, Matsuki S, Iuchi Y, Okada F, Toyosaki S, Ikeda Y, Fujii J. Accelerated impairment of spermatogenic cells in SOD1-knockout mice under heat stress. Free Radic Res 2005; 39:697-705; PMID:16036348 [DOI] [PubMed] [Google Scholar]
- 22. Tsunoda S, Kawano N, Miyado K, Kimura N, Fujii J. Impaired fertilizing ability of superoxide dismutase 1-deficient mouse sperm during in vitro fertilization. Biol Reprod 2012; 87(5):121; PMID:22933517; http://dx.doi.org/ 10.1095/biolreprod.112.102129 [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet 1995; 11:376-81; PMID:7493016 [DOI] [PubMed] [Google Scholar]
- 24. Raineri I, Carlson EJ, Gacayan R, Carra S, Oberley TD, Huang TT, Epstein CJ. Strain-dependent high-level expression of a transgene for manganese superoxide dismutase is associated with growth retardation and decreased fertility. Free Radic Biol Med 2001; 31:1018-30; PMID:11595386 [DOI] [PubMed] [Google Scholar]
- 25. Mruk DD, Silvestrini B, Mo MY, Cheng CY. Antioxidant superoxide dismutase - a review: its function, regulation in the testis, and role in male fertility. Contraception 2002; 65:305-11; PMID:12020784 [DOI] [PubMed] [Google Scholar]
- 26. Ookawara T, Kizaki T, Takayama E, Imazeki N, Matsubara O, Ikeda Y, Suzuki K, Li Ji L, Tadakuma T, et al. Nuclear translocation of extracellular superoxide dismutase. Biochem Biophys Res Commun 2002; 296:54-61; PMID:12147226 [DOI] [PubMed] [Google Scholar]
- 27. Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci USA 1995; 92:6264-8; PMID:7603981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bivalacqua TJ, Armstrong JS, Biggerstaff J, Abdel-Mageed AB, Kadowitz PJ, Hellstrom WJ, Champion HC. Gene transfer of extracellular SOD to the penis reduces O2−* and improves erectile function in aged rats. Am J Physiol Heart Circ Physiol 2003; 284:H1408-21; PMID:12505874 [DOI] [PubMed] [Google Scholar]
- 29. Aitken RJ, Fisher H. Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioessays 1994; 16:259-67; PMID:8031303 [DOI] [PubMed] [Google Scholar]
- 30. Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med 2003 Jan 15; 34(2):145-69; PMID:12521597 [DOI] [PubMed] [Google Scholar]
- 31. Drevet JR. The antioxidant glutathione peroxidase family and spermatozoa: a complex story. Mol Cell Endocrinol 2006; 250:70-9; PMID:16427183 [DOI] [PubMed] [Google Scholar]
- 32. Flohé L. Selenium in mammalian spermiogenesis. Biol Chem 2007; 388:987-95; PMID:17937612 [DOI] [PubMed] [Google Scholar]
- 33. Rhee SG, Woo HA, Kil IS, Bae SH. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem 2012; 287(7):4403-10; PMID:22147704; http://dx.doi.org/ 10.1074/jbc.R111.283432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Masutani H, Yodoi J. Thioredoxin. Overview. Methods Enzymol 2002; 347:279-86; PMID:11898417 [DOI] [PubMed] [Google Scholar]
- 35. Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J 2007; 274(1):1-22; PMID:17222174 [DOI] [PubMed] [Google Scholar]
- 36. Fujii J, Ito JI, Zhang X, Kurahashi T. Unveiling the roles of the glutathione redox system in vivo by analyzing genetically modified mice. J Clin Biochem Nutr 2011 Sep; 49(2):70-8; PMID:21980221; http://dx.doi.org/ 10.3164/jcbn.10-138SR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matsui M, Oshima M, Oshima H, Takaku K, Maruyama T, Yodoi J, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol 1996; 178:179-85; PMID:8812119 [DOI] [PubMed] [Google Scholar]
- 38. Miranda-Vizuete A, Sadek CM, Jiménez A, Krause WJ, Sutovsky P, Oko R. The mammalian testis-specific thioredoxin system. Antioxid Redox Signal 2004; 6:25-40; PMID:14713334 [DOI] [PubMed] [Google Scholar]
- 39. Smith TB, Baker MA, Connaughton HS, Habenicht U, Aitken RJ. Functional deletion of Txndc2 and Txndc3 increases the susceptibility of spermatozoa to age-related oxidative stress. Free Radic Biol Med 2013; 65:872-81; PMID:23707457; http://dx.doi.org/ 10.1016/j.freeradbiomed.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 40. Jiménez A, Zu W, Rawe VY, Pelto-Huikko M, Flickinger CJ, Sutovsky P, Gustafsson JA, Oko R, Miranda-Vizuete A. Spermatocyte/spermatid-specific thioredoxin-3, a novel Golgi apparatus-associated thioredoxin, is a specific marker of aberrant spermatogenesis. J Biol Chem 2004; 279:34971-82; PMID:15181017 [DOI] [PubMed] [Google Scholar]
- 41. Zito E, Hansen HG, Yeo GS, Fujii J, Ron D. Endoplasmic reticulum thiol oxidase deficiency leads to ascorbic acid depletion and noncanonical scurvy in mice. Mol Cell 2012; 48(1):39-51; PMID:22981861; http://dx.doi.org/ 10.1016/j.molcel.2012.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olson GE, Winfrey VP, Nagdas SK, Hill KE, Burk RF. Selenoprotein P is required for mouse sperm development. Biol Reprod 2005; 73:201-11; PMID:12606380 [DOI] [PubMed] [Google Scholar]
- 43. Renko K, Werner M, Renner-Müller I, Cooper TG, Yeung CH, Hollenbach B, Scharpf M, Köhrle J, Schomburg L, et al. Hepatic selenoprotein P (SePP) expression restores selenium transport and prevents infertility and motor-incoordination in Sepp-knockout mice. Biochem J 2008; 409:741-9; PMID:17961124 [DOI] [PubMed] [Google Scholar]
- 44. Hill KE, Zhou J, Austin LM, Motley AK, Ham AJ, Olson GE, Atkins JF, Gesteland RF, Burk RF. The selenium-rich C-terminal domain of mouse selenoprotein P is necessary for the supply of selenium to brain and testis but not for the maintenance of whole body selenium. J Biol Chem 2007; 282:10972-80; PMID:17311913 [DOI] [PubMed] [Google Scholar]
- 45. Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem Biophys Res Commun 2010; 396(1):120-4; PMID:20494123; http://dx.doi.org/ 10.1016/j.bbrc.2010.03.083 [DOI] [PubMed] [Google Scholar]
- 46. Su D, Novoselov SV, Sun QA, Moustafa ME, Zhou Y, Oko R, Hatfield DL, Gladyshev VN. Mammalian selenoprotein thioredoxin-glutathione reductase. Roles in disulfide bond formation and sperm maturation. J Biol Chem 2005; 280:26491-8; PMID:15901730 [DOI] [PubMed] [Google Scholar]
- 47. Schoenmakers E, Agostini M, Mitchell C, Schoenmakers N, Papp L, Rajanayagam O, Padidela R, Ceron-Gutierrez L, Doffinger R, Prevosto C, et al. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J Clin Invest 2010; 120(12):4220-35; PMID:21084748; http://dx.doi.org/ 10.1172/JCI43653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Böhmer F, Szedlacsek S, Tabernero L, Ostman A, den Hertog J. Protein tyrosine phosphatase structure-function relationships in regulation and pathogenesis. FEBS J 2013; 280(2):413-31; PMID:22682070 [DOI] [PubMed] [Google Scholar]
- 49. Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem 1998; 273:15366-72; PMID:9624118 [DOI] [PubMed] [Google Scholar]
- 50. Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J 2003; 22:5501-10; PMID:14532122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cho SH, Lee CH, Ahn Y, Kim H, Kim H, Ahn CY, Yang KS, Lee SR. Redox regulation of PTEN and protein tyrosine phosphatases in H2O2 mediated cell signaling. FEBS Lett 2004; 560:7-13; PMID:15017976 [DOI] [PubMed] [Google Scholar]
- 52. Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008; 319:611-3; PMID:18239123; http://dx.doi.org/ 10.1126/science.1152257 [DOI] [PubMed] [Google Scholar]
- 53. John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol 2008; 321:197-204; PMID:18601916; http://dx.doi.org/ 10.1016/j.ydbio.2008.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang Y, Mao X, Boyce T, Zhu GZ. Dispensable role of PTEN in mouse spermatogenesis. Cell Biol Int 2011; 35(9):905-8; PMID:21524277; http://dx.doi.org/ 10.1042/CBI20110161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stegmann M, Metcalfe C, Barclay AN. Immunoregulation through membrane proteins modified by reducing conditions induced by immune reactions. Eur J Immunol 2013; 43(1):15-21; PMID:23233323; http://dx.doi.org/ 10.1002/eji.201242849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ikawa M, Inoue N, Benham AM, Okabe M. Fertilization: a sperm's journey to and interaction with the oocyte. J Clin Invest 2010; 120(4):984-94; PMID:20364096; http://dx.doi.org/ 10.1172/JCI41585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ellerman DA, Myles DG, Primakoff P. A role for sperm surface protein disulfide isomerase activity in gamete fusion: evidence for the participation of ERp57. Dev Cell 2006. Jun; 10(6):831-7; PMID:1674048421495850 [DOI] [PubMed] [Google Scholar]
- 58. Braakman I, Bulleid NJ. Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem 2011; 80:71-99; PMID:21495850; http://dx.doi.org/ 10.1146/annurev-biochem-062209-093836 [DOI] [PubMed] [Google Scholar]
- 59. Zito E, Melo EP, Yang Y, Wahlander Å, Neubert TA, Ron D. Oxidative protein folding by an endoplasmic reticulum-localized peroxiredoxin. Mol Cell 2010; 40(5):787-97; PMID:21145486; http://dx.doi.org/ 10.1016/j.molcel.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sato Y, Kojima R, Okumura M, Hagiwara M, Masui S, Maegawa K, Saiki M, Horibe T, Suzuki M, Inaba K. Synergistic cooperation of PDI family members in peroxiredoxin 4-driven oxidative protein folding. Sci Rep 2013; 3:2456; PMID:23949117; http://dx.doi.org/ 10.1038/srep02456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF Jr. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 2008; 181(7):1129-39; PMID:18573911; http://dx.doi.org/ 10.1083/jcb.200709049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Park SJ, Kim TS, Park CK, Lee SH, Kim JM, Lee KS, Lee IK, Park JW, Lawson MA, Lee DS. hCG-induced endoplasmic reticulum stress triggers apoptosis and reduces steroidogenic enzyme expression through activating transcription factor 6 in Leydig cells of the testis. J Mol Endocrinol 2013; 50(2):151-66; PMID:23256993; http://dx.doi.org/ 10.1530/JME-12-0195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ji YL, Wang Z, Wang H, Zhang C, Zhang Y, Zhao M, Chen YH, Meng XH, Xu DX. Ascorbic acid protects against cadmium-induced endoplasmic reticulum stress and germ cell apoptosis in testes. Reprod Toxicol 2012 Nov; 34(3):357-63; PMID:22569276; http://dx.doi.org/ 10.1016/j.reprotox.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 64. Sasagawa I, Matsuki S, Suzuki Y, Iuchi Y, Tohya K, Kimura M, Nakada T, Fujii J. Possible involvement of the membrane-bound form of peroxiredoxin 4 in acrosome formation during spermiogenesis of rats. Eur J Biochem 2001; 268:3053-61; PMID:11358524 [DOI] [PubMed] [Google Scholar]
- 65. Yim SH, Kim YJ, Oh SY, Fujii J, Zhang Y, Gladyshev VN, Rhee SG. Identification and characterization of an alternatively transcribed form of peroxiredoxin IV that is specifically expressed in spermatids of the postpubertal mouse testis. J Biol Chem 2011; 286:39002-12; http://dx.doi.org/ 10.1074/jbc.M111.257220; PMID:21835919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Iuchi Y, Okada F, Tsunoda S, Kibe N, Shirasawa N, Ikawa M, Okabe M, Ikeda Y, Fujii J. Peroxiredoxin 4 knockout results in elevated spermatogenic cell death via oxidative stress. Biochem J 2009; 419:149-58; PMID:19105792; http://dx.doi.org/ 10.1042/BJ20081526 [DOI] [PubMed] [Google Scholar]
- 67. Yu YE, Zhang Y, Unni E, Shirley CR, Deng JM, Russell LD, Weil MM, Behringer RR, Meistrich ML. Abnormal spermatogenesis and reduced fertility in transition nuclear protein 1-deficient mice. Proc Natl Acad Sci U S A 2000; 97(9):4683-8; PMID:107815189834 1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shirley CR, Hayashi S, Mounsey S, Yanagimachi R, Meistrich ML. Abnormalities and reduced reproductive potential of sperm from Tnp1- and Tnp2-null double mutant mice. Biol Reprod 2004; 71(4):1220-9; PMID:15189834 [DOI] [PubMed] [Google Scholar]
- 69. Cho C, Willis WD, Goulding EH, Jung-Ha H, Choi YC, Hecht NB, Eddy EM. Haploinsufficiency of protamine-1 or -2 causes infertility in mice. Nat Genet 2001; 28(1):82-6; PMID:11326282 [DOI] [PubMed] [Google Scholar]
- 70. Cho C, Jung-Ha H, Willis WD, Goulding EH, Stein P, Xu Z, Schultz RM, Hecht NB, Eddy EM. Protamine 2 deficiency leads to sperm DNA damage and embryo death in mice. Biol Reprod 2003; 69(1):211-7; PMID:12620939 [DOI] [PubMed] [Google Scholar]
- 71. Wyckoff GJ, Wang W, Wu CI. Rapid evolution of male reproductive genes in the descent of man. Nature 2000; 403:304-9; PMID:10659848 [DOI] [PubMed] [Google Scholar]
- 72. Ursini F, Heim S, Kiess M, Maiorino M, Roveri A, Wissing J, Flohé L. Dual function of the selenoprotein PHGPx during sperm maturation. Science 1999; 285:1393-6; PMID:10464096 [DOI] [PubMed] [Google Scholar]
- 73. Imai H, Suzuki K, Ishizaka K, Ichinose S, Oshima H, Okayasu I, Emoto K, Umeda M, Nakagawa Y. Failure of the expression of phospholipid hydroperoxide glutathione peroxidase in the spermatozoa of human infertile males. Biol Reprod 2001; 64:674-83; PMID:11159372 [DOI] [PubMed] [Google Scholar]
- 74. Foresta C, Flohé L, Garolla A, Roveri A, Ursini F, Maiorino M. Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol Reprod 2002; 67:967-71; PMID:12193409 [DOI] [PubMed] [Google Scholar]
- 75. Maiorino M, Bosello V, Ursini F, Foresta C, Garolla A, Scapin M, Sztajer H, Flohe L. Genetic variations of gpx-4 and male infertility in humans. Biol Reprod 2003; 68:1134-41; PMID:12606444 [DOI] [PubMed] [Google Scholar]
- 76. Imai H, Hirao F, Sakamoto T, Sekine K, Mizukura Y, Saito M, Kitamoto T, Hayasaka M, Hanaoka K, et al. Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem Biophys Res Commun 2003; 305:278-86; PMID:12745070 [DOI] [PubMed] [Google Scholar]
- 77. Yant LJ, Ran Q, Rao L, Van Remmen H, Shibatani T, Belter JG, Motta L, Richardson A, Prolla TA. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med 2003; 34:496-502; PMID:12566075 [DOI] [PubMed] [Google Scholar]
- 78. Imai H. New strategy of functional analysis of PHGPx knockout mice model using transgenic rescue method and Cre-LoxP system. J Clin Biochem Nutr 2010; 46(1):1-13; PMID:20104259; http://dx.doi.org/ 10.3164/jcbn.09-94R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schneider M, Förster H, Boersma A, Seiler A, Wehnes H, Sinowatz F, Neumüller C, Deutsch MJ, Walch A, Hrabé de Angelis M, Wurst W, Ursini F, Roveri A, Maleszewski M, Maiorino M, Conrad M. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J 2009; 23(9):3233-42; PMID:19417079; http://dx.doi.org/ 10.1096/fj.09-132795 [DOI] [PubMed] [Google Scholar]
- 80. Noblanc A, Peltier M, Damon-Soubeyrand C, Kerchkove N, Chabory E, Vernet P, Saez F, Cadet R, Janny L, Pons-Rejraji H, Conrad M, Drevet JR, Kocer A. Epididymis response partly compensates for spermatozoa oxidative defects in snGPx4 and GPx5 double mutant mice. PLoS One 2012; 7(6):e38565; PMID:22719900; http://dx.doi.org/ 10.1371/journal.pone.0038565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Puglisi R, Maccari I, Pipolo S, Conrad M, Mangia F, Boitani C. The nuclear form of glutathione peroxidase 4 is associated with sperm nuclear matrix and is required for proper paternal chromatin decondensation at fertilization. J Cell Physiol 2012; 227(4):1420-7; PMID:21618532; http://dx.doi.org/ 10.1002/jcp.22857 [DOI] [PubMed] [Google Scholar]
- 82. Pfeifer H, Conrad M, Roethlein D, Kyriakopoulos A, Brielmeier M, Bornkamm GW, Behne D. Identification of a specific sperm nuclei selenoenzyme necessary for protamine thiol cross-linking during sperm maturation. FASEB J 2001; 15(7):1236-8; PMID:11344099 [PubMed] [Google Scholar]
- 83. Conrad M, Moreno SG, Sinowatz F, Ursini F, Kölle S, Roveri A, Brielmeier M, Wurst W, Maiorino M, Bornkamm GW. The nuclear form of phospholipid hydroperoxide glutathione peroxidase is a protein thiol peroxidase contributing to sperm chromatin stability. Mol Cell Biol 2005; 25(17):7637-44; PMID:16107710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Imai H, Hakkaku N, Iwamoto R, Suzuki J, Suzuki T, Tajima Y, Konishi K, Minami S, Ichinose S, Ishizaka K, et al. Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J Biol Chem 2009; 284(47):32522-32; PMID:19783653; http://dx.doi.org/ 10.1074/jbc.M109.016139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chae HZ, Oubrahim H, Park JW, Rhee SG, Chock PB. Protein glutathionylation in the regulation of peroxiredoxins: a family of thiol-specific peroxidases that function as antioxidants, molecular chaperones, and signal modulators. Antioxid Redox Signal 2012; 16(6):506-23; PMID:22114845; http://dx.doi.org/ 10.1089/ars.2011.4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Seligman J, Zipser Y, Kosower NS. Tyrosine phosphorylation, thiol status, and protein tyrosine phosphatase in rat epididymal spermatozoa. Biol Reprod 2004; 71(3):1009-15; PMID:15151929 [DOI] [PubMed] [Google Scholar]


