Abstract
Current knowledge on avian spermiogenesis, including strengths and weaknesses, has been reviewed. Information on avian spermiogenesis considerably lags behind that in mammals because of the paucity of reports in birds. Spermiogenesis in passerine birds has received even much less attention than in non-passerine birds. Mechanisms underlying morphogenesis of the acrosome and nucleus, and roles of microtubular assemblies are poorly understood. The proximal centriole found in non-passerine birds, but hitherto considered to be absent in passerine birds, has recently been described in spermatids and mature spermatozoa of 2 passeridan species, including the Masked weaver for which new and detailed spermiogenetic information is provided in this review. A great deal more studies on spermiogenesis, and spermatogenesis generally, in various avian species are required to considerably enhance knowledge of this phenomenon, contribute to comparative spermatology, provide a basis for appropriate applied studies, and contribute to understanding of phylogeny in this vast order of vertebrates.
Keywords: Avian, non-passerine, passerine, seminiferous epithelium, spermiogenesis, testis, ultrastructure
An Overview of Spermiogenesis in Birds
The anatomy of the testis and reproductive tract of birds1 and the kinetics of spermatogenesis have been amply described.2,3 This review is devoted mainly to spermiogenesis in birds, and in particular, in passerine birds in which new information on the phenomenon in the Masked Weaver (Ploceus velatus) is provided. Spermiogenesis is the last phase in the process of spermatogenesis, and it is during this phase that the haploid, round spermatid is transformed into a spermatozoon. Spermiogenesis has been studied extensively in mammals, and most of our knowledge of the developmental and transformational processes in the spermatid is derived from these studies.4-20 The nomenclature used in mammalian spermiogenesis has also been adopted for the phenomenon in birds. However, only a few reports detailing spermiogenesis in birds are to be found in the literature.1,4,5,6,8,9,14,15,21-46 Most of these publications are, understandably, on the more economic domestic species of birds, such as the domestic fowl, turkey, duck and quail,21-25,27,29,30,42,47-51 and even much fewer on other species of birds, including passerines and the Paleognathae.22,33,35,37,41,42,44-46,49,52 However, most of these publications are based upon individual or fragmentary organelle development30,37-40,45,46 rather than the non-fragmentary method of spatiotemporal, in-tandem, development of the early spermatid in the Japanese quail,49 in the Turkey,42 in the House Sparrow,41 in the parrot,45 in the Ostrich,37 in the European nightjar (Caprimulgus europaeus)52 and in the Emu.46
In this review, and as adopted previously,1 both systems involving acrosomal (subdivided into 4 phases of Golgi, cap, acrosome and maturation) as well as nuclear morphogenetic processes, according to Leblond and Clermont4 and Roosen-Runge and Giesel,54 respectively, will be combined, in the so-called “stepwise” changes in spermatid morphogenesis, using spermiogenesis in Turkey,42 as a model. Certain aspects of spermiogenesis in the Japanese quail (this review) and Guinea fowl (Soley and Aire, unpublished observations), will also be included, as found necessary. Jamieson55 remarks that the structure of the spermatozoon of the turkey is typical of the Galloanserae monophyly, and it is also clear that spermatozoa and spermiogenesis in members of this monophyly have been studied the most, among birds. Specific features in spermiogenesis of oscine passerine birds will also be highlighted separately and fully within the limits of current knowledge. An attempt will be made to evaluate the most contentious morphogenetic and structural features between species, with the hope of highlighting areas requiring further research efforts or clarifications, based on current information.
A concise review of the structure of the spermatozoon in both the non-passerine and passerine birds will be presented for proper perspective and understanding of the developmental features of the spermatid, as it evolves into a full-fledged, motile, itinerant cell. The complex structural evolution of the spermatozoon from a round cell, with complete or partial loss of certain organelles, even as new ones are formed, is an interesting and impressive biological phenomenon that has intrigued investigators, over the years. The mature spermatozoon loses more than one hundred times24 or about 97%36 of its volume, while the volume of the nucleus is reduced from 110 cubic micrometres to 2 cubic micrometres24 or by 96%.36 The spermatid also radically changes its shape and evolves a number of morphologically elaborate organelles, including the acrosome, the midpiece and the flagellum,17 and loses a few original organelles or structures. Biochemical changes that are controlled by genes which are active only in spermatids and in the process of spermiogenesis,56 including the elaboration of new and unique structural elements of the spermatozoon, are, indeed, mostly responsible for the visible morphological alterations expressed variably in the spermatid. For critical and proper evaluation of the testis in health or disease, knowledge of spermatogenesis, including spermiogenesis in each species of animal, needs to be known and understood. The morphological changes that take place, including the elaboration or loss of distinctive morphological sperm features, have, also, become important in determining relative positions and associations in the avian phylogenetic tree.55
A Brief Review of the Sperm Structure in Non-Passerine and Passerine Birds
The spermatozoa of non-passerine birds
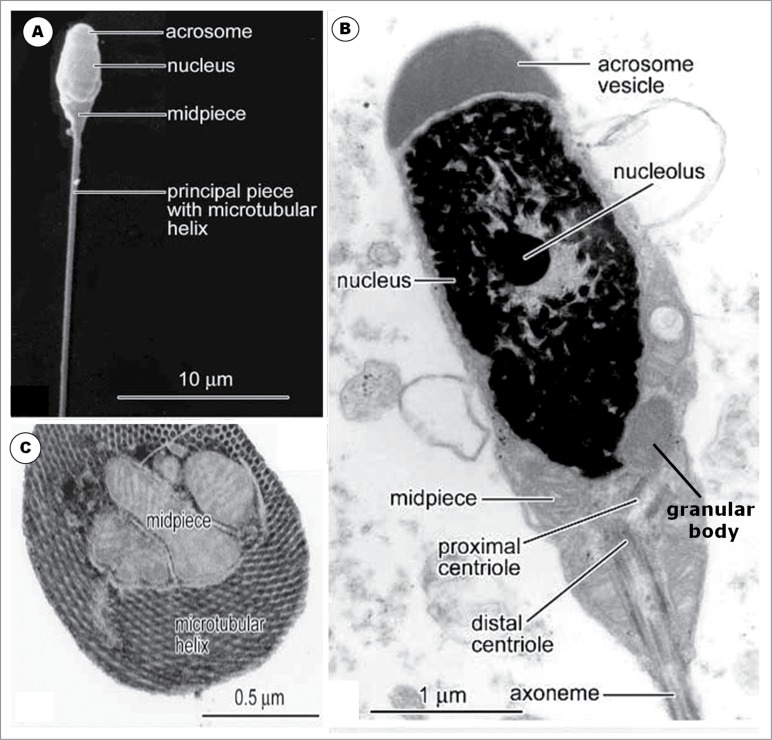
Generally, the spermatozoa of non-passerine birds are of the so-called sauropsid type, as are to be found in reptiles, being filiform, plain-surfaced and elongated.57,58 The nucleus is cylindrically-shaped, long and straight or slightly curved, and it is covered anteriorly by a similarly cylindrical, conical acrosome. The length of the acrosome varies between species,59 and its base covers the most rostral, tapered part of the nucleus, the rostrum, in most species of non-passerine birds. A perforatorium may occupy the hollow of the acrosome, the subacrosomal space, and the endonuclear canal, for varying lengths in non-passerine birds in which they are found (see Asa and Phillips59 for exceptions).
In the neck of the spermatozoon, the proximal and distal centrioles, both of which have the 9 triplet microtubules within a thickened wall of the centriole, are present and lie perpendicular to each other, with a few known exceptions, in the Guinea fowl,60 the Japanese quail55 (and Aire TA, personal observation), and Chinese quail,61 in which they are in-line aligned. The distal centriole is relatively short and retains its position posterior to the base of the nucleus in most non-passerine birds,23,27,53,136, but it may be highly elongated, as in ratites, in which it runs posteriorly, to the end of the mid-piece.38,62
The mid-piece varies widely in length, being short in most non-passerine birds, but long in the dove and pigeon.59 Mitochondria are arranged around the distal centriole, with varying internal configuration.55 The mid-piece ends at the annulus which is a distinct dense ring in the inner part of the cell membrane.
The principal piece of the spermatozoon begins at the annulus, in several birds studied.23,24,27,36,55,62-65 A fibrous sheath encloses the principal piece in some species of non-passerine birds, e.g. the tinamou and rhea,66 ostrich,67 domestic fowl,55 Japanese quail,61 and duck.68
The flagellum is long, although much shorter than in passerine birds. It is the main motile apparatus, and comprises the axoneme comprising the typical 9+2 microtubular configuration. The dense fibers that are peripheral to the 9 doublets of the axoneme are variably displayed between species. They are very well, and better, formed in ratites than in galliforms.59
Passerine birds
Passerine birds belong to the Order Passeriformes which comprises Oscine and Sub-oscine birds. According to Jamieson,55 the ultrastructure of spermatozoa of Sub-oscine birds is very poorly known. However, there are a few, fragmentary reports, including those of Feduccia69 and Asa and Phillips,59 in the literature. The spermatozoa of the oscine group have been better studied ultrastructurally.55,59,70-73 These works will provide the reference points for description, nomenclature and discussion of spermiogenesis in passerine birds. Thus far, only Goés and Dolder41 have given a relatively full account of spermiogenesis in a passerine bird, others being more fragmentary or superficial, in nature. The account on spermiogenesis in the Masked Weaver provided here, attempts to fill a number of gaps in, and consolidate our knowledge of, this phenomenon in passeridan birds. Thus, a comprehensive review of spermiogenesis, incorporating a detailed original account of this process in a passeridan bird, will be the focus of this effort. There are scarcely any publications on spermiogenesis in suboscine birds. This is another area requiring not only knowledge but understanding in order to place, as much as possible, avian spermatogenesis in its proper perspective.
Oscine spermatozoa
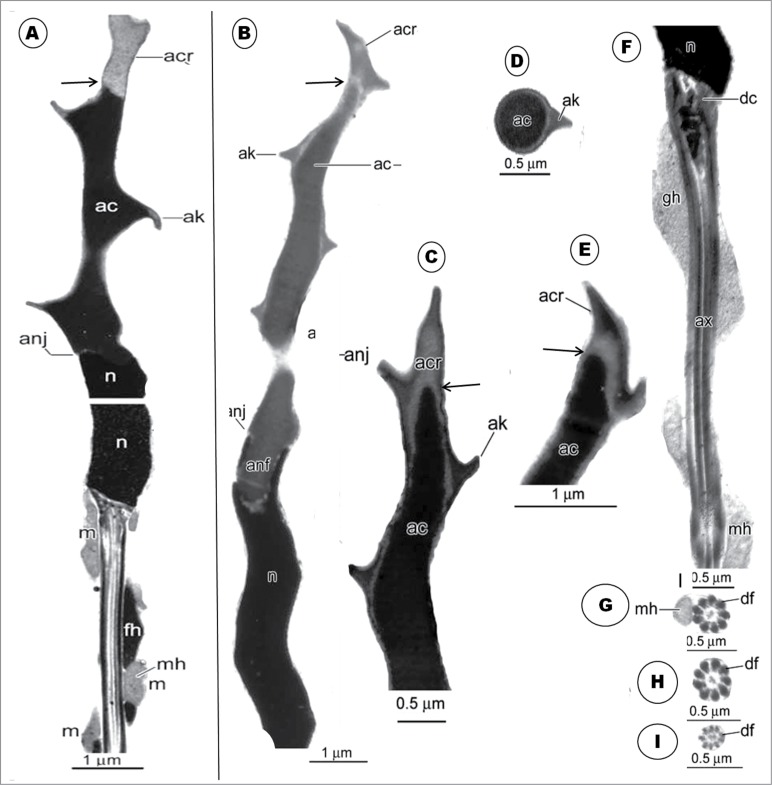
In oscine birds studied, the head region is usually spirally twisted in a helical fashion.59,71,74 The nucleus is also helical in shape, and shorter than that of non-passerine birds.75 The helical acrosome has been reported to be much longer than the nucleus, with an acrosome : nucleus ratio exceeding 1 (see Jamieson55 for details). It is noteworthy that the acrosome in the oscine spermatozoon possesses a lateral membrane evagination or projection, known as the helical membrane.57 Jamieson et al.72 have suggested a different name for this structure, and made a case for the recognition of 2 parts of the oscine acrosome (discussed in detail later on in this section). The neck region has long been observed to contain only one centriole, the distal centriole,22,41,57,72,76 but spermatozoa of some passeridan birds have recently been reported to deviate from this norm, in having both the proximal and distal centrioles.44,74 The midpiece does not display an annulus, and the mitochondria are not arranged as in non-passerine birds, instead they form a single, remarkably long, helical strand, the so-called mitochondrial helix, around the axoneme,72 and runs for varying lengths along the length of the axoneme of the principal piece, depending on the species.55 Within the so-called helical membrane, there is another helical structure, the fibrous helix, which also winds around the axoneme, and usually lies external and close to the mitochondrial helix.9,35,72 A granular structure that winds round the distal centriole, but not present in all passeridan spermatozoa, and situated between the base of the nucleus and the mitochondrial helix, is the granular helix70,72,77 or centriolar adjunct.41 The axoneme is similar to that of the non-passerine spermatozoon in having the 9+2 microtubular configuration, but the outer dense fibers are better developed and more prominent than in non-passerine and sub-oscine birds.57,72
Sub-oscine spermatozoa
Not much is known about the sperm structure of the sub-oscine bird.41 This is due to the paucity of reports, which themselves are very fragmentary. Sub-oscine birds seem to combine structural features which are to be found in oscine as well as non-passerine birds.57 The nuclei are longer than the acrosomes and the midpiece. The mitochondria also exhibit features that are both passerine and non-passerine, such as clustering on one side of the midpiece.57 Some species of sub-oscine birds do not have the typical spiral membrane around the acrosome and nucleus or a helical membrane around the tail segment.78
Spermiogenesis in Non-Passerine Birds
In the testes of sexually mature and active birds, the seminiferous tubule contains a stratified epithelium comprising germ cells at various stages of meiotic division and morphogenesis. The second and last meiotic division of the secondary spermatocyte gives rise to haploid germ cells, the round spermatids. The spermatid subsequently embarks on one of the most complex morphological changes in cell differentiation known to biologists. Differentiating spermatids may be seen at various levels, depending on their phase in spermiogenesis, within the epithelium. The early spermatids are usually round in shape and adluminal in location, and spermatid movements within the seminiferous epithelium are brought about by the Sertoli cells, which are the only somatic, non-germ cells, found in the seminiferous epithelium.
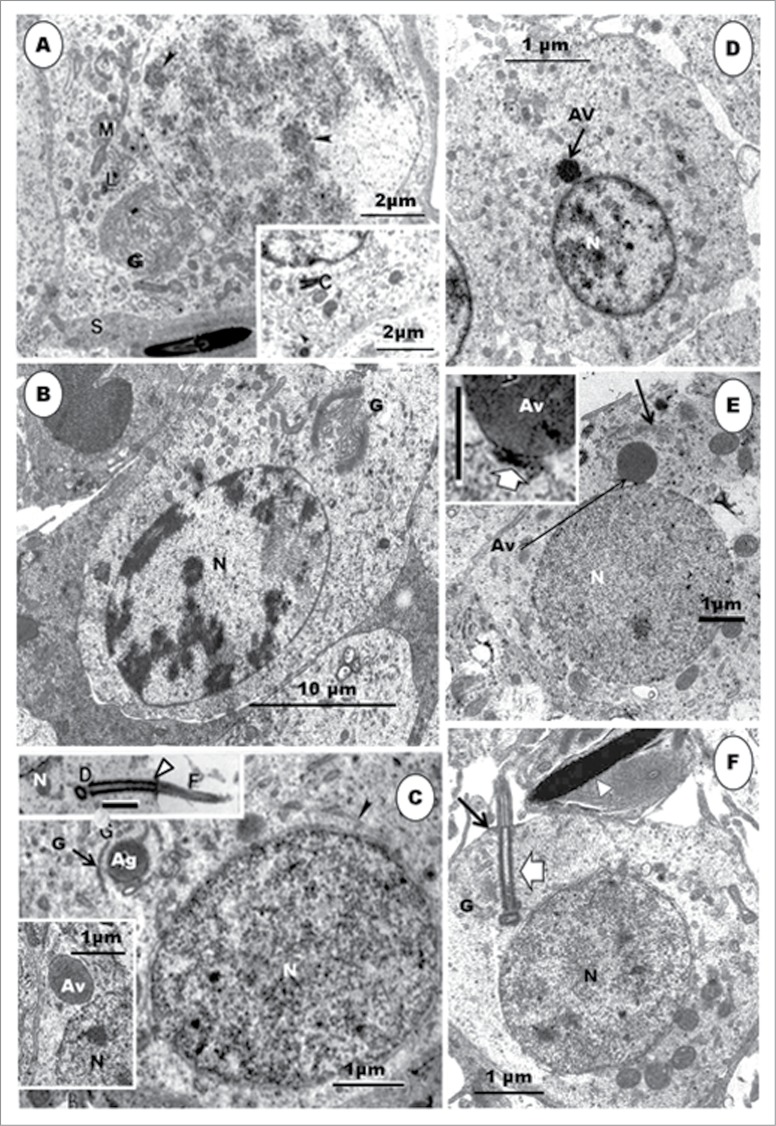
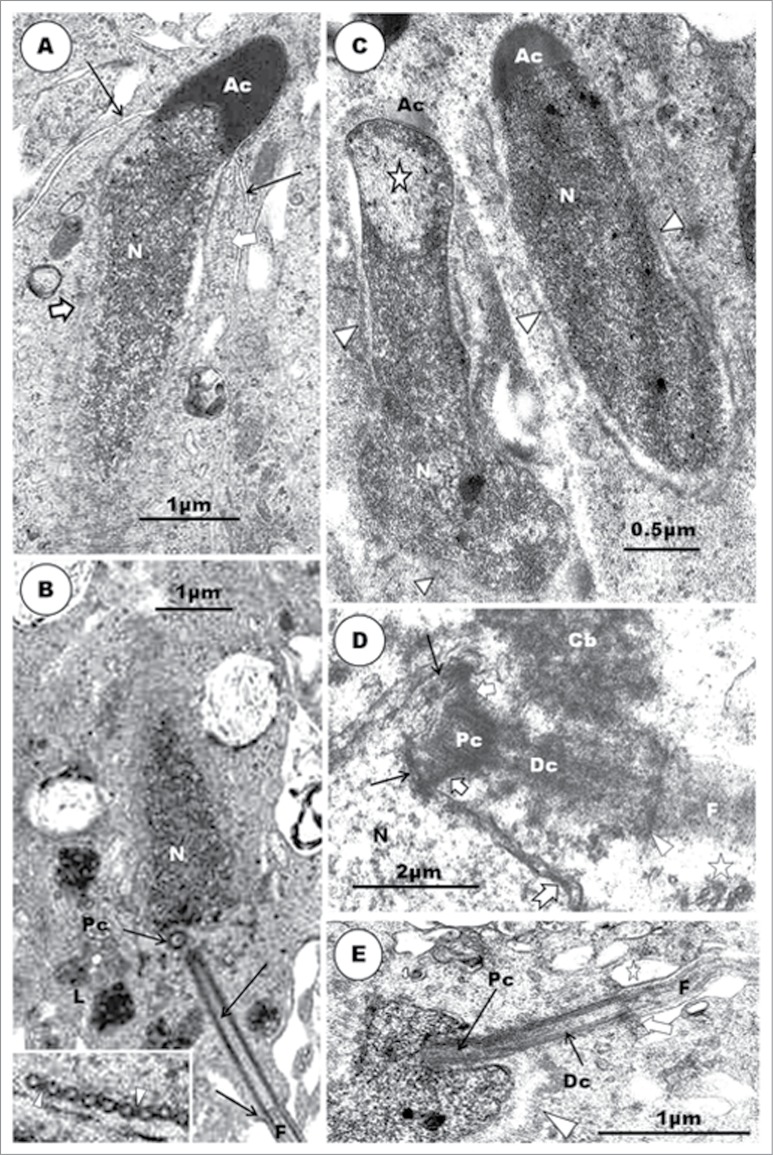
Histologically, the seminiferous tubules which bear the epithelium containing the germ cells and Sertoli cells are surrounded by intertubular tissue (Fig. 1). The latter contains, among others, blood vessels, lymphatics and the Leydig cells. A transverse section of the seminferous epithelium presents a stratified cellular arrangement, and various steps of spermatids are to be found at different levels within the epithelium (Fig. 1B). In addition, there are spermatids (Fig. 1B) at various steps of spermiogenesis, lying quite close together or even mixed, in the same transverse section of the seminiferous tubule. Thus, heterogenous cellular associations are displayed in birds, as in certain primates.79,80-82 This is an additional reason why a step by step description of spermiogenesis is more informative and coherent in birds. Most of the micrographs in the non-passerine section (Figs. 2–11), unless otherwise stated, are taken and/or modified from Aire TA. British Poultry Science 2003;44:674–82, with kind permission of British Poultry Science Ltd.
Figure 1.
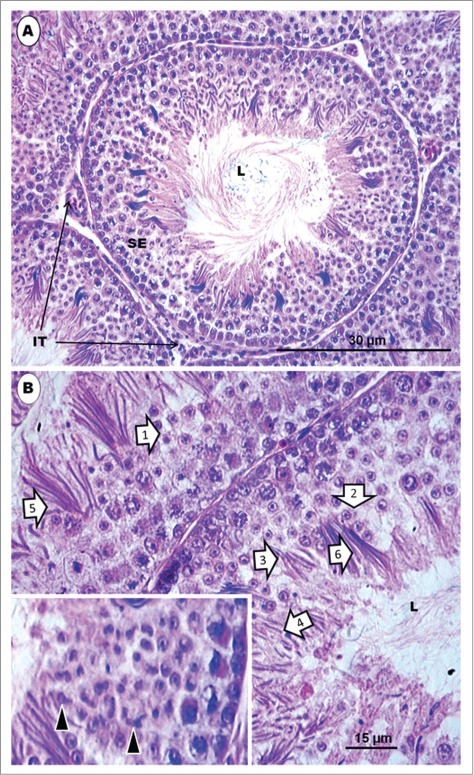
Japanese quail, Coturnix japonica. (A) represents an H&E-stained histological section of a transverse section of a seminiferous tubule surrounded by intertubular tissue in a sexually mature and active testis of the Japanese quail. L = lumen. (B) represents a higher power view of the seminiferous epithelium, which displays several spermatids and other germ cells. The epithelium shows several spermatids [1 to 6] at various steps of spermiogenesis, belonging to various cellular associations, but lying quite close to one another or appearing mixed, in some cases. Inset shows irregularly-shaped elongated spermatids at step 6 of spermiogenesis. L = lumen.
Step 1 spermatid: These round spermatids occur in the seminiferous epithelium along with the step 11 spermatids, occupying the subluminal and luminal border of the epithelium, respectively. The nuclei of the round spermatids are oval, and contain scattered chromatin aggregations in the karyoplasm or adhering to the nuclear membrane (Fig. 2A). The Golgi complex is large during the late phase of this step, and contains a number of proacrosomal granules. The centriolar complex, comprising, typically, the proximal and distal centrioles articulating with each other at right angle, lies free in the cell cytoplasm, between the plasmalemma and nucleus (Figs. 2C, F). In the Japanese quail, the proximal and distal centrioles in step 1 spermatids do not lie at right angle to each other, but, instead, they are in-line aligned, with the posterior end of the distal centriole making contact with the plasmalemma and continuing extracellularly as the fledgling flagellum (Figs. 3B, C). Mitochondria are scattered rather uniformly in the cytoplasm, but are more concentrated the region of the Golgi complex (Figs. 2B, C). A few strands of rough endoplasmic reticulum are scattered throughout the cytoplasm.
Figure 2.
Step 1 spermatid: (A), Step 1 spermatid, along with step 11 spermatid. S, Sertoli cell; clumps of chromatin (arrowheads) in the nucleus of step 1 spermatid; a large Golgi complex (G) and mitochondrial aggregates (M) around it; numerous profiles of SER and a few, small lysosomes (L) occur in the cell. Inset: the centriolar complex (C) in the cytoplasm. (B) shows a round spermatid of the Guinea fowl at step 1, with highly electron-dense scattered heterochromatin, mostly attaching to the inner part of the nuclear membrane. G = Golgi complex; N = nucleus. (C), Step 2 spermatid: the nuclear chromatin is de-condensing. A large proacrosomal granule (Ag) is leaving the Golgi complex. A few profiles of microtubules (arrowheads) have appeared close to the nucleus. Top Inset: shows the diplosome (D), annulus (arrowhead), and the fibrous sheath (F) of the developing tail. The lower Inset shows a free acrosomal vesicle moving close to the nucleus (N). Step 3 spermatid: (D), is a step 3 spermatid of the Japanese quail. Note the movement of the acrosomal vesicle (Av) toward the nucleus (N) whose chromatin, in this species, is still mainly in the heterochromatin phase, unlike that of the turkey (E). The nuclear membrane at the acrosomo-nuclear contact area is thickened (Inset, broad arrow), and the Golgi complex (arrow) is still closely associated with the acrosomal vesicle. (F). During the earlier part of this phase, the centriolar complex (F, broad arrow) lies quite close to the nucleus (N). G, Golgi complex; arrow, annulus; arrowhead, step 11 spermatid.
Figure 3.
Step 4 spermatid. The nuclear chromatin has become granulo-filamentous in appearance. The acrosomal vesicle (Ac) invaginates further into the nucleus at a thickened part of the nuclear envelope (A, broad arrow). (A), inset: The acrosomal vesicle lies close to the cell membrane, and abuts the Sertoli cell (S); arrowhead = transverse sections of scattered groups of microtubules running circularly around the nucleus. (B), (C) and (D) show the centriolar complex at an early phase of this step in the Japanese quail (main B and C), turkey (inset) and Guinea fowl (D). The 2 centrioles are already in-line aligned from the time when the distal centriole (Dc) makes contact with the plasmalemma ((B) (C) and (D); arrowhead) and during subsequent movement of the centriolar complex (D) up to the point at which the proximal centriole (Pc) makes contact with the nucleus. F, flagellum; notched arrow, feint transverse striations of the fibrous sheath.
The step 1 spermatid, as described, here, for the turkey, is generally similar structurally to what has been reported for several other species of birds, such as domestic fowl,23,29 Japanese quail,49 some species of parrots,45 domestic pigeon,32 Guinea fowl (Soley JT and Aire TA, unpublished material), ostrich,67 and emu.46
Step 2 spermatid: The nuclear chromatin begins to de-condense, and appears uniformly distributed in the nucleoplasm. The proacrosomal granules coalesce to form a large, single acrosomal vesicle or granule in the Golgi complex (Fig. 2C). The free, posterior end of the distal centriole of the diplosome or centriolar complex makes contact with the cell cytoplasm, at which junction a poorly defined annulus occurs (Fig. 2C inset; F). The flagellum grows from this junction, projecting into the intercellular space.
Various terms have been used for the precursors of the acrosome. Berruti and Piardi83 have reviewed the development of the mammalian acrosome, beginning with its origin in the Golgi complex. They have adopted the terms, proacrosomal granules/vesicles (within the Golgi complex), as the visible building blocks for the proacrosome, which lies free in the cytoplasm, close to, or approaching, the nucleus. The proacrosome attaches to the nucleus and subsequently forms the acrosome of the spermatid. In this review, the terms, proacrosomal granules or vesicles, acrosomal vesicle and acrosome, that have been used, albeit inconsistently in birds, will be adopted.
Acrosomogenesis begins with the formation of proacrosomal granules/vesicles within the Golgi complex. These vesicles that are budded from the trans-Golgi zone (TGN) of the Golgi complex coalesce to form a large acrosomal vesicle that may or may not be enclosed by Golgi cisternae. Generally, the acrosomal vesicle is round or oval in shape, but its content varies between mammals and birds. For example, in mammals, the acrosomal vesicle is a hollow, round structure whose content is an electron-dense granule, the acrosomal granule, which is surrounded by an electron-lucent space,5,6,15,17,84-86 or a clear vesicle/vacuole in Tammar wallaby (Macropus eugenii),17 but in most non-passerine birds, the acrosome vesicle is a membrane-bound vesicle that is filled with a uniformly homogenous, moderately electron-dense material.23,29,32,39,42,45,46,87
There are very few reports on the formation of precursors of the acrosomal vesicle in the Golgi complex, in birds. Proacrosomal granules or vesicles are the first visible evidence of acrosomogenesis in the cell, as described for the turkey, but they are not mentioned or described in the drake,51 European nightjar,88 smooth-billed ani,89 budgerigar,28 turtle dove,26 and emu.46 However, observations in the turkey and passerine birds (vide infra) indicate that the acrosomal precursors in birds are as found in mammals, and to a large extent in reptiles.56 Further studies need to be undertaken in order to augment and consolidate knowledge in this area. However, it is generally agreed that the acrosomal vesicle is an oval or round membrane-bound organelle with a homogenous, moderately electron-dense content, even in the ostrich39 in which the content subsequently disperses peripherally, as acrosomogenesis progresses.
Step 3 spermatid: The nucleus remains spherical in shape, but chromatin condensation has advanced into a finely granular matrix, displaying only a few, small clumps of chromatin in the turkey (Figs. 2E, F), but pronounced scattered clumps in the Japanese quail (Fig. 2D). The Golgi complex becomes inconspicuous, and the acrosomal vesicle lies very close to, or just makes contact with, the nucleus. With contact between the acrosomal vesicle and the nucleus, thickening of the nuclear membrane commences along the site of contact (Fig. 2E inset). The centriolar complex nearly makes contact with the nucleus, close to the developing acrosome and Golgi complex. The developing flagellum is enclosed in a fibrous sheath that demonstrates feint transverse striations in the Japanese quail (Fig. 3B) and guinea fowl (Fig. 4D).
Figure 4.
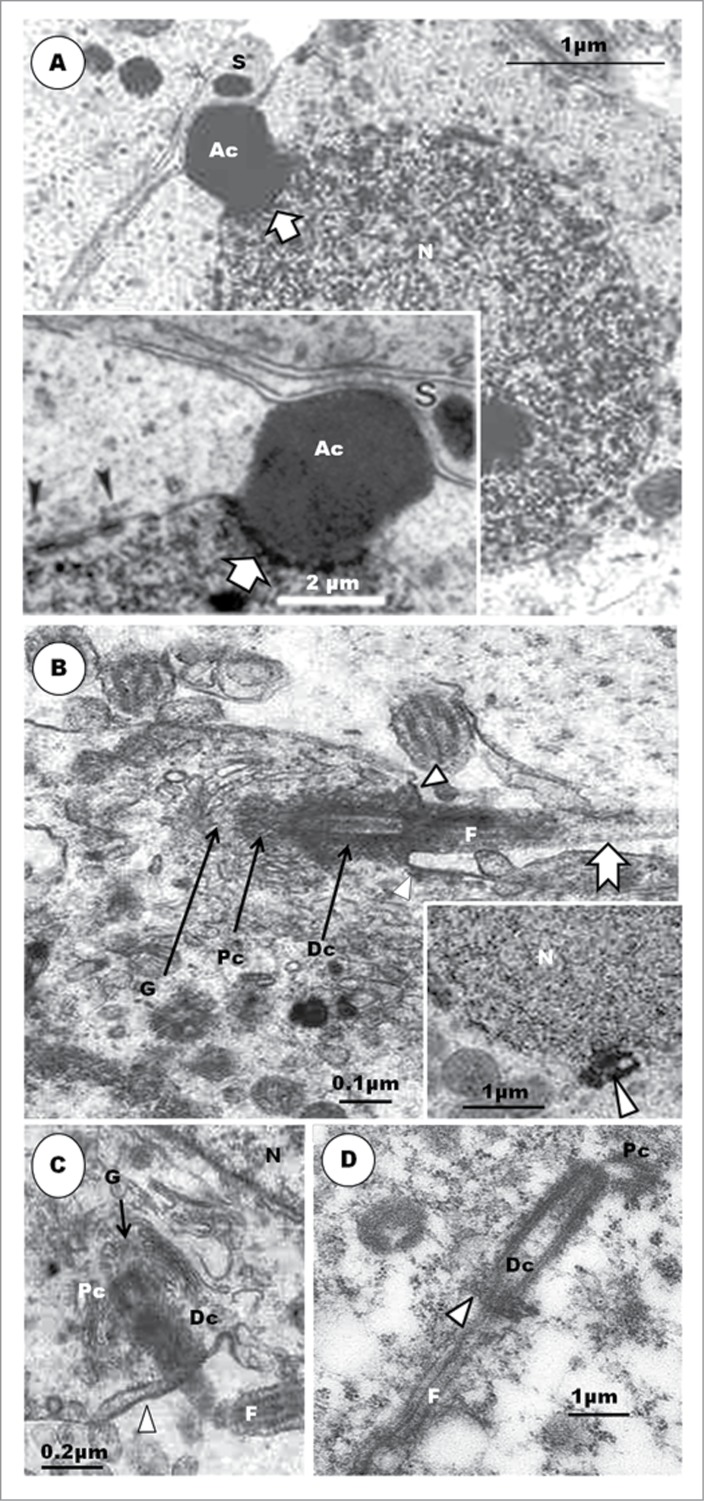
Step 5 spermatid is pear-shaped. Nuclear chromatin is uniformly and finely granulo-filamentous. Acrosomal vesicle (Ac) elongates and is laterally compressed. An obliquely sectioned endonuclear cavity contains the perforatorium (A, arrowhead). Inset shows a longitudinal section of the endonuclear canal (arrowhead), with an invagination from the base of the acrosomal vesicle into it (white arrow). Step 6 spermatid has an elongated nucleus (N); microtubules of developing circular manchette (arrowheads); the acrosome (Ac) lies rostral to most of cell cytoplasm; Arrows = ‘shoulder’ of the nucleus. Inset: the endonuclear canal contains the developing perforatorium. Equivalent spermatids in the Japanese quail exhibit irregular nuclei (C and inset), and even more so in those of the Guinea fowl (D). Note that the shallow implantation fossa lies in a deep vault in the Guinea fowl, unlike in the Japanese quail. Pc, proximal centriole; Dc, distal centriole; F, flagellum; arrowhead, annulus; thick short arrow, electron dense projections from the distal centriole; notched arrow, feint striations on the flagellum.
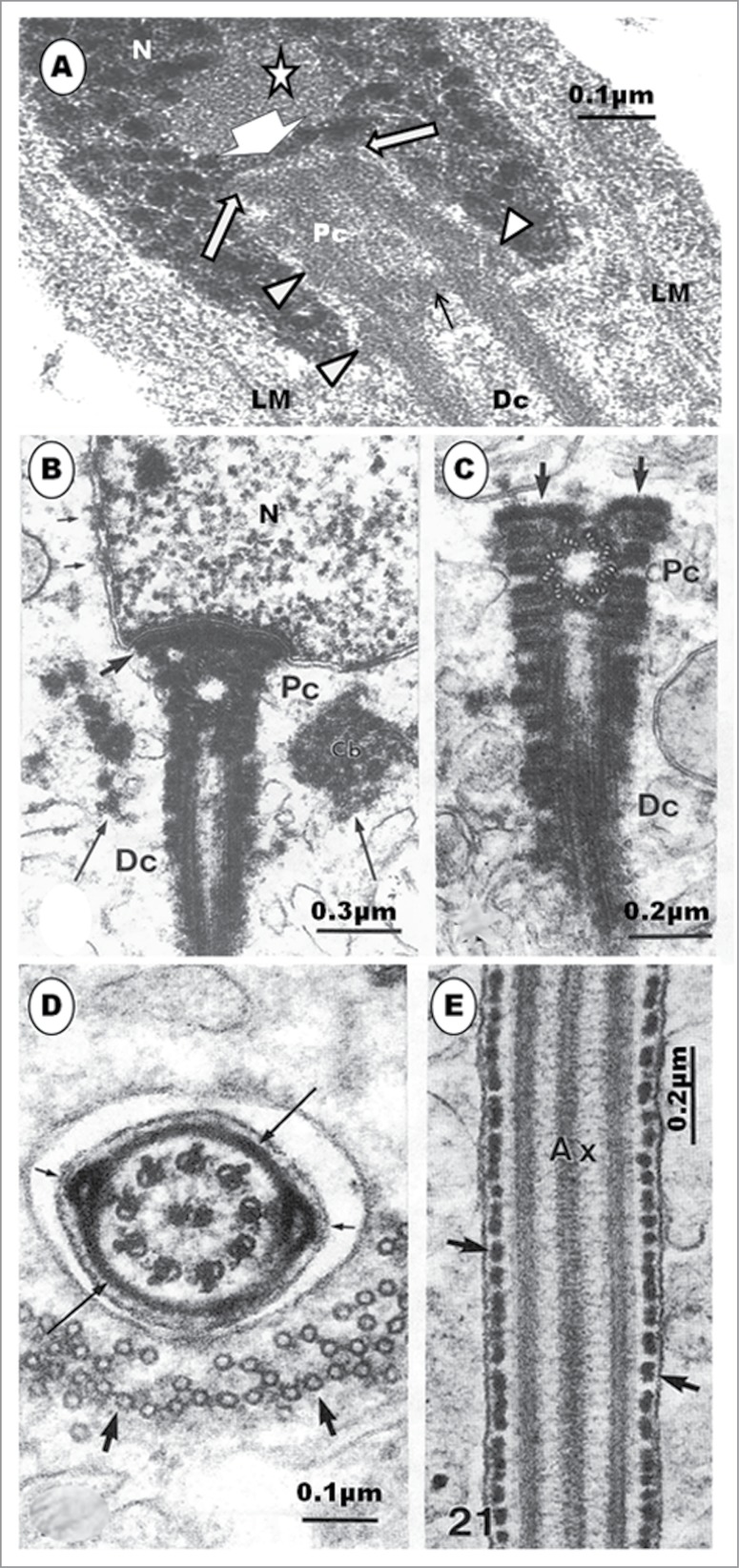
In the Guinea fowl, the centriolar complex, during this step, typically comprises both proximal and distal centrioles arranged perpendicular to each other,60 but in the Japanese quail, both centrioles are in line-aligned in the round spermatid (Fig. 3B), right from the step 1 phase of spermiogenesis. The situation in the quail is therefore a departure from the typical arrangement found in most birds. Whereas subsequent development during the earlier steps of round spermatids in the Guinea fowl shows that the angle of articulation between the proximal and distal centrioles becomes increasingly obtuse (Fig. 3D), that of the Japanese quail remains the same in the round spermatids (Figs. 3B, C) (vide infra). Nagano23 has shown that the 2 centrioles are nearly in line-aligned as the centriolar complex approaches the nucleus, in young, apparently round, spermatids of the domestic fowl, but he has found that this is a transient arrangement because both centrioles articulate with each other, at right angle, subsequently.
Step 4 spermatid: The nucleus remains spherical or oval in shape, and the chromatin continues to condense and become uniformly granulofilamentous (Fig. 3A). The homogeneously dense acrosomal vesicle is no longer round or oval in shape, but becomes slightly elongated and, thus, transforms to become the acrosome (Fig. 3A). The change in shape and indentation of the nucleus marks the beginning of a profound morphogenetic process leading to the formation of the acrosomal complex. Along with this developmental process, the acrosome invaginates a little further into the nucleus, and the nuclear membrane becomes more thickened at the contact site. The nucleus assumes an eccentric shape within the cytoplasm, and the acrosome abuts on the adjacent plasmalemma, and indirectly on the Sertoli cell (Fig. 3A inset). The first evidence of microtubule formation is the presence of transverse sections of microtubules, in small groups, which appear close to the nuclear membrane, especially in the more anterior parts of the nucleus (Fig. 3A inset). The diplosome attaches, by its proximal centriole, obliquely at a shallow indentation or implantation fossa of the nucleus, close to the acrosome. The long axis of the distal centriole remains perpendicular to that of the proximal centriole.
Step 5 spermatid: The nucleus is now pear-shaped, as its elongation process commences, and contains uniform, finely granular chromatin (Fig. 4A). The acrosome elongates further and the central part of the thickened nuclear membrane, at the contact site with the acrosome, invaginates into the nucleoplasm, forming the precursor of the endonuclear canal (Fig. 4A, inset). There is an increased amount of smooth endoplasmic reticulum (SER), sparsely granular endoplasmic reticulum (SGER), as well as lysosomes in the cytoplasm.
The organelle content of the cell, including the increased amount of SER, multivesicular bodies and lysosomes, indicates a cell that is active, probably in building and remodeling. The spermatid displays, for the first time, the primordium of the second component of the acrosomal complex, the perforatorium, as the acrosome elongates further. The invaginated precursor of the endonuclear canal has been reported in the rooster, the Budgerigar (Melopsittacus undulatus), Turkey (Meleagris gallopavo), Japanese quail and Ostrich (Struthio camelus).23,28,30,34,39,42,90 An osmiophilic content of this invagination is the precursor of the perforatorium. However, the major source of confusion and lack of understanding regarding the development of the perforatorium is the exact morphological origin of the perforatorium granule. In the turkey3,42 and Guinea fowl (Fig. 5B) the commencement of the development of the perforatorium is the invagination of the central part of the nuclear membrane into the nucleoplasm, at the acrosomo-nuclear contact surface. This is the primordium of the endonuclear canal. There is considerable agreement on this aspect of this phenomenon among investigators, in the domestic fowl.23,28,30,34,39,42,45 In the turkey, the osmiophilic, dark granule, seen pointing toward the forming endonuclear canal, appears to arise as an evagination of the contact surface of the acrosome, at the acrosomo-nuclear junction, adjacent to the forming endonuclear canal (Fig. 4A), and is regarded as the primordium of the perforatorium rod. A similar evagination of the acrosomal wall occurs in the Guinea fowl (Fig. 5B). What appears as a similar evagination of the acrosomal base, projecting into the endonuclear canal, has been shown in micrographs of spermatids in parrots (Fig. 5C).45 Nagano,23 in the domestic fowl, remained non-committal, as were Baccetti et al.34 and Del Conte,91 in reptiles, when they regarded the precursor of the perforatorium rod to be a granule formed by the interaction between the acrosome and the nucleus, as conjectured in mammals.6 However, Lin and Jones49 consider that the precursor of the perforatorium, in Japanese quail, is an intranuclear granule, probably a consequence of the angle of section of the spermatid. It is hereby advocated that further and precise investigations be carried out in order to clarify this concern in birds.
Figure 5.
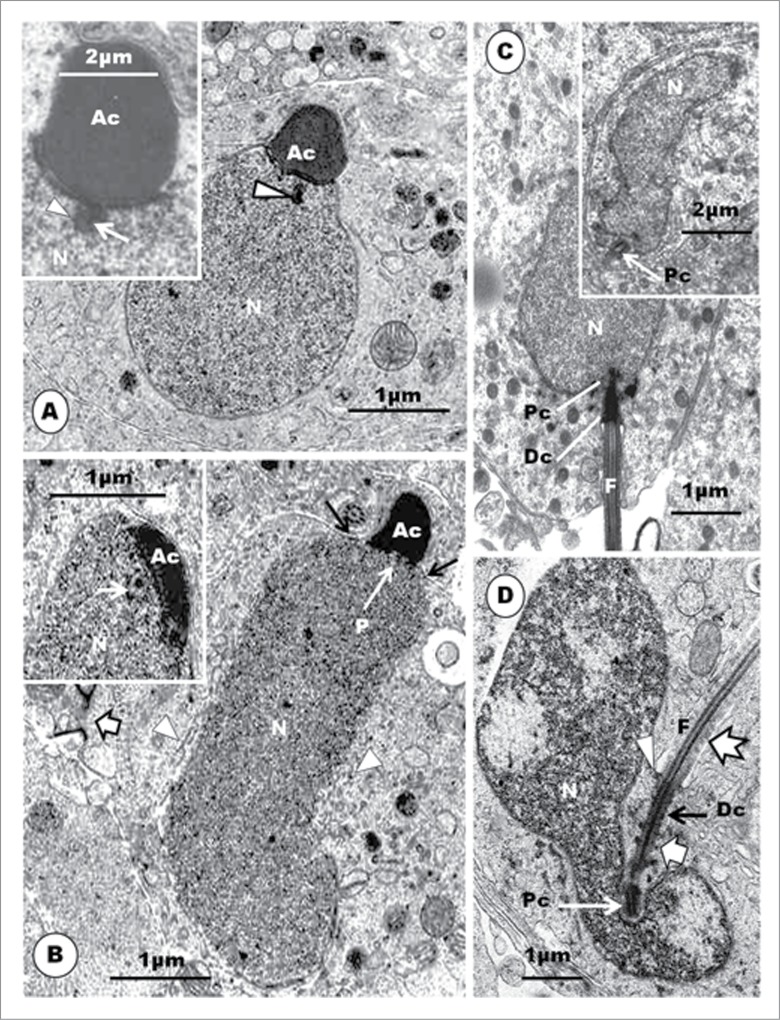
An equivalent of late step 5 spermatid in the Guinea fowl (A) and (B) displays highly electron-dense heterochromatin, concentrated peripherally (A, star), and central electron-lucent areas in the nucleus (N). An irregularly-shaped nucleus is surrounded by scattered clumps or groups of microtubules (block arrow), and the acrosome abuts the plasmalemma as well as the Sertoli cell. The base of the acrosome evaginates (arrowhead) into the endonuclear canal (arrow) (B). In the parrots (C), a similar, but more clearly demonstrated evagination (arrowhead) of the base of the acrosome (Ac) into the endonuclear canal (broad arrow) occurs. The flagellum (F) of an equivalent spermatid at this stage in the Guinea fowl is wavy in outline, and both the proximal (Pc) and distal (Dc) centrioles are in-line aligned and insert into a deep vault at the base of the nucleus (D). In the emu (E) the distal centriole has numerous radiating projections (broad, white arrow). (C) is taken from Lovas E, Filippich LJ, Johnston SD. Spermiogenesis in the Australian cockatiel Nymphicus hollandicus. J Morphol 2012; 273: 1291-1305. (3B). With the kind permission of John Wiley & Sons Ltd.
Step 6 spermatid: The spermatid nucleus displays finely granulofilamentous chromatin, is elongated and slightly wavy in profile (Fig. 4B). The elongating, homogenously dense, acrosome occupies only the central one-third of the anterior surface of the slimming nucleus, and its rostral tip making contact with the spermatid plasmalemma abuts on an adjacent Sertoli cell. Cross-sections of profiles of microtubules of what become the circular manchette (CM) appear patchily or focally along the length of the nucleus, in no regular pattern. The round or oval mitochondria move posteriorly, along with the migrating cell cytoplasm, in which they are randomly scattered. The endonuclear canal is well formed but is narrow, and contains the developing electron-dense perforatorium (Fig. 4B and inset).
Although the step 6 spermatid nucleus is elongated and slightly wavy in profile in the turkey42 as in the rooster,30 that of the Japanese quail49 and personal observations (Figs. 1B and 4C), Guinea fow33 and this review (Figs. 4D and 5A), Ostrich40 and Crested tinamou (Eudromia elegans elegans),66 appears ‘spiral’ or irregular in shape due to differential subnucleolemmal chromatin condensation, and concomitant constriction of the nucleus (Figs. 4D and 5A). Light microscopical observations in the rooster21 and in certain members of the Anseridae92 indicate that the spermatid nucleus, during the elongating phase, is coiled within the cell cytoplasm, apparently because of the fixed volume of cytoplasm. This has not been confirmed in ultrastructural studies.
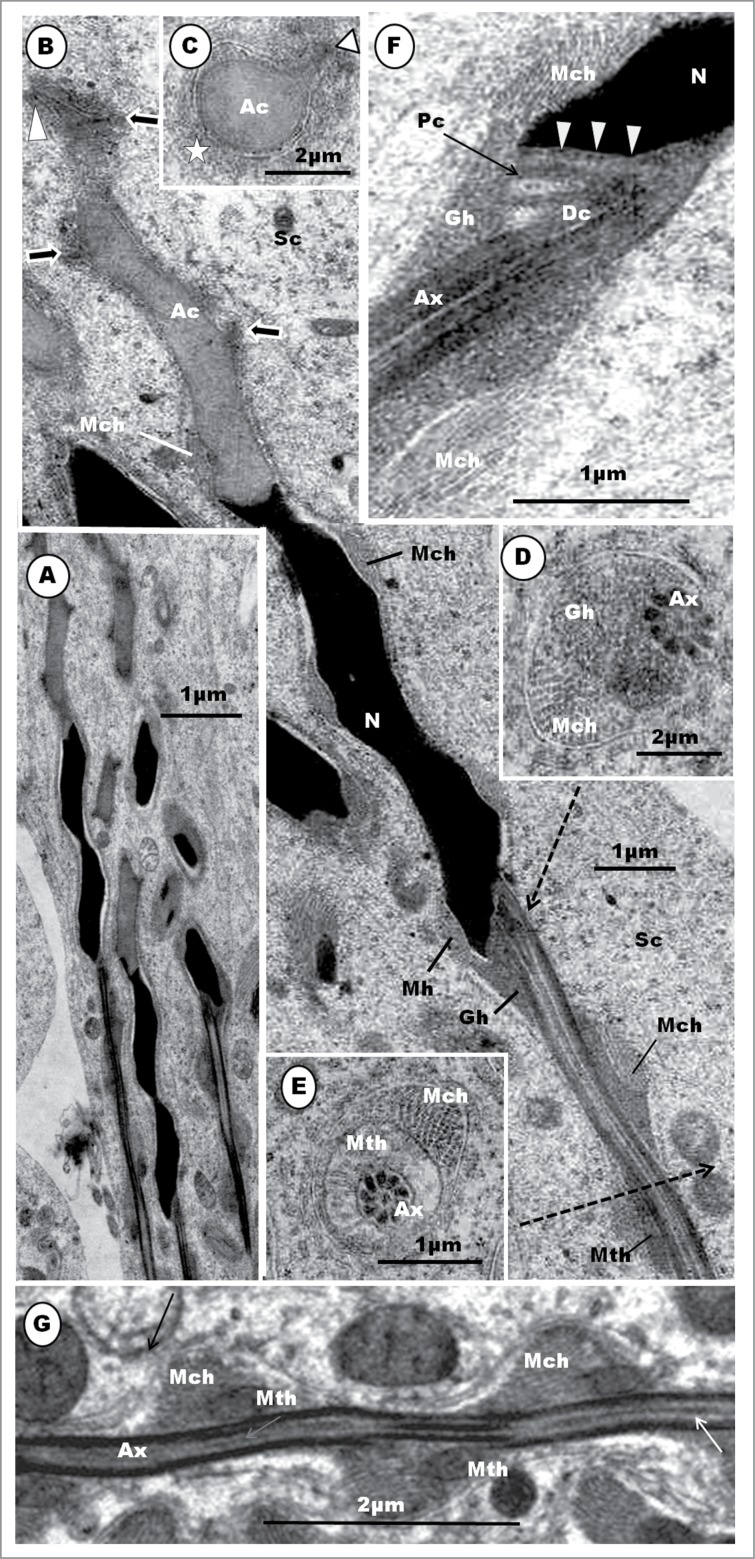
The manchette is a transient microtubular structure which is developed and lost during spermiogenesis in most non-passerine birds, as in reptiles (see Gribbins58). It comprises the circular manchette (CM) and its successor, the longitudinal manchette (LM). It seems to follow a similar developmental pattern in all non-passerine birds investigated by McIntosh and Porter24; Okamura and Nishiyama29; Gunawardana and Scott30; Xia et al.47 in the rooster; Humphreys28 and Lovas et al.45 in parrots, including the Budgerigar and cockatiel; Fawcett et al.9 1971 and Yasuzumi and Yamaguchi32 in pigeons; Phillips and Asa48 in the rhea, Rhea americana albisceus; Lin and Jones49 in Japanese quail, Coturnix japonica; Aire42 2003 in the turkey; Soley40 in the ostrich, and du Plessis46 in the emu. The origin of the CM in various species of birds appears to vary between species. For example, in the domestic fowl,29 Turkey,42 and Japanese quail,49 irregularly scattered groups of a few microtubules are first seen in round spermatids. These groups are more commonly found along the sides of the anterior part of the nucleus. Thereafter, up to step 6 spermatid, the microtubular groupings increase in number, and become more conspicuous, especially around constricted portions of the nucleus, in the Japanese quail,49 turkey,42 Guinea fowl (Soley and Aire, unpublished observations reported in this review), ostrich,40 and emu.46 Lovas et al.,45 in the Australian cockatiel, first observed microtubules, which are probably the precursors of the CM, in spermatids exhibiting a tear-drop shape, i.e. at about step 4 spermatid in the turkey. It is noteworthy that Mattei et al.26 and Gunawardana and Scott30 consider that the CM is formed by a group of microtubules that radiate from satellites of the distal centriole in the turtle dove, Streptopelia rosogrisea and domestic fowl, respectively. This requires confirmation.
With full formation of the CM, it extends from the anterior region of the nucleus, at the acrosomo-nuclear junction, to the region of the centriolar complex.40,42,46 Although the CM is substantially developed in domestic fowl,23 Japanese quail,90 and Turkey,42 it is especially well developed in the Ostrich40 and emu.46 It is poorly formed in Columba sp.9,26 and the Cuckoo (Crotophaga ani),89 and does not develop in the Swift (Apus apus) and the Nightjar (Caprimulgus europaeus) which have only a longitudinal manchette.93,94 The situation in the drake is unclear as Simōes et al.51 describe only the LM in the spermatid of this bird.
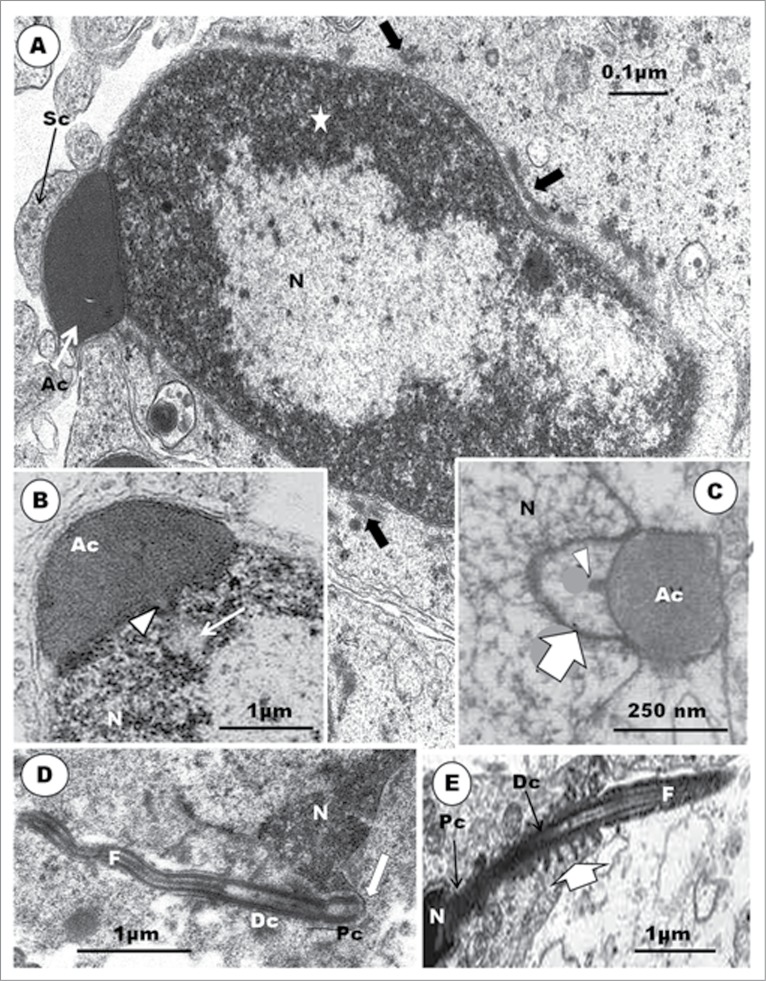
At the equivalent step of spermiogenesis in the Guinea fowl, the centriolar complex inserts into a deep, cylindrical vault (Figs. 4D, 5D). The proximal centriole is contained fully within the vault, while the most anterior part of the distal centriole may project into it, also. No report of any other bird spermatid or spermatozoon reveals an implantation vault or fossa of this configuration or depth. In most spermatids of the Guinea fowl at this level of development, the developing flagellum appears quite wavy in outline (Fig. 5D), and the distal centriole exhibits outward-pointing, electron-dense protrusions (Fig. 4D), as also seen in the emu (Fig. 5E). At a later phase of this step in the Guinea fowl, the nucleus exhibits very dense heterochromatin attaching to the inner nuclear membrane, and leaving an electron-lucent central part. An endonuclear canal begins to form, and a spike-like evagination of the acrosome into this canal occurs (Fig. 5B).
Step 7 spermatid: The elongating acrosome is as wide as the spermatid nucleus (Figs. 6A, B). The nucleus is slimmer than in step 6, slightly curved, and its tapering rostral end projects into the subacrosomal concavity. The nuclear chromatin condenses to become granulofilamentous, and moderately deeply-stained. The cell cytoplasm extends caudally from the distal border of the acrosome, which now projects into a deep crypt of an adjacent Sertoli cell cytoplasm. The CM is well established as a cylindrical layer or sleeve of microtubules that aligns closely with the external surface of the elongated nucleus, extending distally from the caudal border of the acrosome to the region of the rostral end of the distal centriole. Adjacent microtubules are seen to be linked or joined by short bridges or linkers in the turkey (Fig. 6B inset), as have been observed in the Japanese quail,90 ostrich40 (Fig. 7D), emu46 and rhea.48 These structures probably consolidate and stabilize the sleeve of microtubules in its functional role in the spermatid.95 Mitochondria begin to elongate, and display longitudinal cristae, as they continue to migrate into the cytoplasm, much of which is displaced posteriorly. Both the centriolar complex and the spermatid tail are well formed and in place, as would be found in the mature spermatid or spermatozoon (Fig. 6B). The nuclei of the Guinea fowl spermatid in equivalent step are also elongated, slimmer in profile, and contain granular chromatin. The acrosome forms a cap over the anterior end of the nucleus (Fig.6C).
Figure 6.
In (A), the Step 7 spermatid displays granulo-filamentous nuclear chromatin; the acrosome occupies a deep crypt of the Sertoli cell; black arrows, nuclear hump of cytoplasm; short, white arrows, circular manchette. In (B): L, lysosomes; Arrow, poorly developed annulus; F, flagellum. Inset: microtubules show short linkers (arrowheads) between them. (C): Elongated spermatids of the Guinea fowl; the nuclear chromatin is moderately electron-dense and granular; acrosome (Ac) forms a cap over the slimmer and more regular nucleus; arrowheads, circular manchette; star, electron-lucent parts of the nucleus. (D): Japanese quail; the proximal centriole (Pc) articulates with the distal centriole (Dc) posteriorly, and implants in the nucleus at a shallow fossa by means of dense strands (short, white arrows) arising from the lateral surface of the organelle; black arrows, implantation fossa; arrowhead, plasmalemma; F, flagellum; star, clumps of microtubules; a chromatoid body is present; notched arrow, nuclear membrane pore. (E), Guinea fowl: the proximal and distal centrioles are largely in-line aligned, and the entire proximal, but only the most anterior part of the distal centriole, lies, within the deep articular vault, but the implantation fossa is almost flat. F, flagellum; white arrow, annulus; star, flagellar canal; arrowhead, circular manchette.
Figure 7.
(A) is a Guinea fowl spermatid equivalent to step 8 spermatid of the turkey; the nucleus (N) has large round or rod-like chromatin granules and some clear areas (star); the LM is in place, and the proximal centriole (Pc) lies within the deep implantation vault bearing a nearly flat fossa (broad, squat arrow). The anterior rim of the Pc attaches to the implantation fossa by means of dense struts (white arrows), and amorphous material on the outer surface of the centriole probably attach the organelle to the vault, also. Material of similar density (black arrow) runs across the junction between the Pc and distal centriole (Dc). (B) shows the development of the segmented columns of the Ostrich; Pc, proximal centriole; Dc, distal centriole; Cb, chromatoid body; long arrows, microtubules of circular manchette; thick arrow, capitulum. (C) shows a later stage of development of the segmental columns; electron dense material surrounds the Pc and Dc, and arrows, capitulum. (D) is a later stage of flagellar development showing ribs (long arrows) and longitudinal columns (short arrows); thick arrows, microtubules of the longitudinal manchette in the cytoplasm. (E) is a longitudinal section of the flagellum exhibiting ribs in the form of dense blocks of material (arrows); Ax, axoneme. Figures B to E are from Soley JT. Centriolar development and formation of the flagellum during spermiogenesis in the ostrich (Struthio camelus). J Anat 1994; 185; 301-313. Figs. 16, 17, 20 and 21. With the kind permission of John Wiley & Sons Ltd
The flagellum is enclosed in an amorphous fibrous sheath. Feint transverse lines may be seen in the proximal part of the developing flagellum in the turkey and Guinea fowl (Fig.4D), but they are not ribs, which are found in the fibrous sheath of ratite birds (Fig. 7D, E).38,46,48,66 The ribs and longitudinal columns appear during late spermiogenesis, developing from amorphous material that surrounds the axoneme of the principal piece of ratites.38,46,48
The development of the centriolar complex and flagellum of the spermatozoon is generally similar in mammals7,10,96-98 and non-passerine birds.23,24,26,27,29,30,38,42,48,50 Initially, in all the birds studied, except the quails, Coturnix japonica and probably C. chinensis61 (see step 3, above), the centriolar pair, lying at right angle to each other, is closely associated with the Golgi apparatus, and, initially, lies mid-way between the cell membrane and the nucleus. The diplosome thereafter migrates gradually toward the nucleus, to which it eventually attaches, usually obliquely, in the region of the nucleus destined to become the caudal pole.
The avian proximal and distal centrioles differ in length, and the distal one may be much longer than the proximal centriole, unlike in mammals in which both centrioles, if persistent, are of similar length. Also, the distal centriole persists without much modification in birds, but disintegrates during flagellar formation in mammals.7,11 Thus, in non-passerine birds, the distal centriole forms the foundation upon which the midpiece of the spermatozoon is built. It produces the central pair of the axonemal microtubules at the base of this centriole, determines the length of the midpiece and, according to Phillips and Asa,48 allows the spermatozoon to form a midpiece without moving the annulus, relative to the distal centriole, as occurs in mammals.11 The axonemal microtubules, generally, extend from the proximal centriole posteriorly, into the flagellum, in mammals.7,99,100
The usual pattern in birds is that the proximal and distal centrioles lie perpendicular to each other, in both the spermatid23,31,42,48,49 and mature spermatozoon,27,48,64,101 but those of Guinea fowl initially lie at right angle to each other, and this angle between them gradually becomes obtuse as the centriolar complex inserts in a deep vault in the nucleus of the spermatid.58 Subsequently, the centrioles in the Guinea fowl are nearly in line-aligned, and the lumen of the proximal centriole often has some amorphous moderately electron-dense material in its anterior portion. The walls of both centrioles are thick, and a similar fuzzy amorphous material runs transversely between the proximal and distal centrioles (Fig. 7A). Guineafowl therefore does not lack the proximal centriole, as earlier conjectured by Thurston et al.101 However, this type of centriolar alignment, also found in the quail, is uncommon, and is usually found mainly in invertebrate organisms.102
The attachment of the tail to the nucleus is simpler in birds than in mammals. In the latter, an electron-dense basal plate or capitulum inserts between the proximal centriole and the implantation fossa. In birds, there is no capitulum, although in the ostrich38 and emu,46 a thin layer of dense material occurs, in place of the capitulum (Fig. 7B, C). The implantation fossa is quite shallow in birds, except in the Guinea fowl in which a deep, cylindrical vault houses the entire length of the proximal centriole, as well as the most anterior part of the distal centriole (Figs. 6E and 7A). In the Japanese quail, the proximal centriole articulates with the walls of the shallow implantation fossa by means of strands of dense material extending, mid-way along the length of the centriole, from the abaxial or outer surface, and radiating toward the walls of the shallow implantation fossa (Fig. 6D). On the other hand, although both the proximal and distal centrioles are also in line-aligned in the Guinea fowl (Fig. 6E), as in the quail, it is the anterior rim of the proximal centriole that is attached to the shallow implantation fossa by means of radially arranged dense material (Fig. 7A). In both birds, amorphous material appears between the centrioles and the walls of the implantation vault. These probably assist in attaching the centrioles to the nucleus, or perhaps involved in further development of the centrioles.
Step 8 spermatid: During this step, the diameter of the nucleus further reduces, and the nuclear chromatin begins to form scattered, coarse, round or rod-shaped granules that are immersed in the granulofilamentous matrix of the karyoplasm (Fig. 8A, B, C). An early phase of this step shows, more clearly, a collar of moderately electron-dense aggregation or formation of Sertoli cell material around the free part of the acrosome (Fig. 8A). A chromatoid body is seen close to the centriolar complex in the Turkey (Fig. 8B) and the Guinea fowl (Fig. 8D). The attachment of the centriolar complex to the implantation fossa is by means of radiating strands of dense material arising from the anterior and lateral surfaces of the proximal centriole (Fig. 8C, inset). Other dense, amorphous aggregations, of unknown function, may be seen along the entire length of the centriolar complex. A significant, rarely observed feature of the spermatid during this step is the concurrent occurrence of both the CM and longitudinal manchette (LM). Profiles of the latter lie lateral to those of the former, which appears to be patchy in distribution, at this stage (Fig. 8B). The re-arrangement of the CM to form the LM appears to be very rapid.
Figure 8.
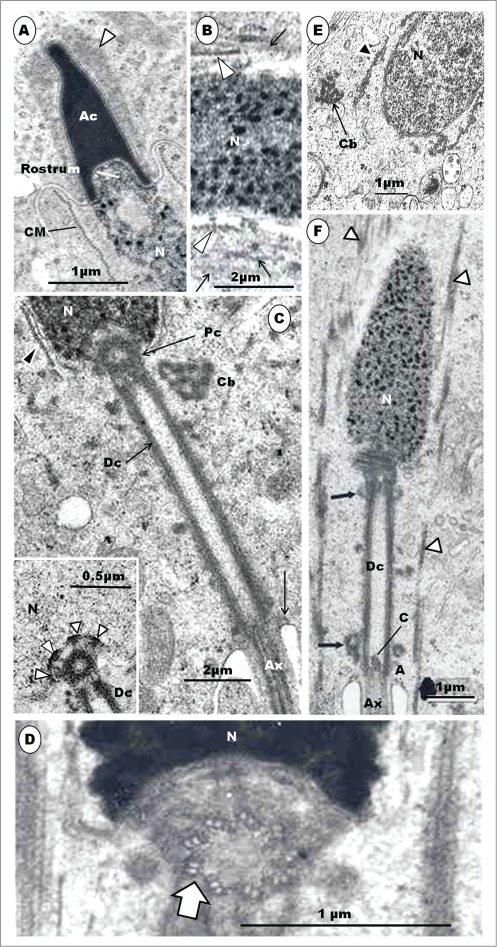
Step 8 spermatid. In (A), the acrosome (Ac) elongates further, and is surrounded by a collar of periacrosomal complexes (arrowhead); the rostrum projects into the subacrosomal space; CM, circular manchette. In (B), both the circular (arrowheads) and longitudinal (arrows) manchette occur concurrently at a later stage of this step of spermiogenesis; the central part of the nucleus is largely devoid of dense granules. In (C), the nuclear chromatin contains dense, coarse, round or rod-shaped granules; arrowhead, circular manchette; cb, chromatoid body; Ax, axoneme; Inset: Radiating strands of dense material arising from the anterior and lateral surfaces of the proximal centriole attach it to the implantation fossa of the nucleus (N) at arrowheads. Dc, distal centriole. (D) displays the triplet fibers of the proximal centriole. N, nucleus. Fig. E shows the caudal half of the Guinea fowl spermatid equivalent to step 8 which displays a chromatoid body and circular manchette (arrowhead). (F) displays the posterior part of step 9 spermatid of the Turkey. The LM is fully established, with the disappearance of the CM. Straight arrows, dense amorphous material; A, annulus; Ax, axoneme surrounded by the amorphous fibrous sheath; Dc, distal centriole; arrowheads, longitudinal manchette; c, ‘basal body’ of the Dc from which the singlet microtubules of the axoneme arise.
The collar of Sertoli cell aggregation around the acrosome has been called the periacrosomal complexes in the musk shrew by Cooper and Bedford,103 and they describe it as comprising bands of microfilaments separated from the Sertoli cell cytoplasm by a largely continuous endoplasmic reticulum. Saita et al.89 have also described such cellular formations in the smooth-billed ani. Apart from attaching the developing spermatid to the Sertoli cell, the periacrosomal complexes may also transmit substances to and from the developing spermatid, especially the developing acrosome. The chromatoid body has been described in male germ cells in several mammals,104-110 but there are still divergent views concerning its origin, precise structure and function. This structure ``is an irregularly shaped, dense mass of fine fibrillar material generally found near the acrosomal vesicle and Golgi complex of early spermatids".107 It is typically reticular in structure in sections. The origin and structure of the chromatoid body has been studied in mammals.104,107,108,111 It is apparently formed by an aggregation of filamentous material that is abundant in spermatocytes. Reports on the chromatoid body in birds are very scanty. Gupta92 has described the presence of this structure in unfixed seminiferous tubules of the drake. The chromatoid body has been demonstrated in spermatids of Rhea by Phillips and Asa,48 while Soley38 and Aire42 have described it in the spermatids of the ostrich and the turkey, respectively. This structure is also present in the Guinea fowl (Fig. 8D), and has been described in reptiles.58 In mammals, the chromatoid body migrates caudally, disperses in the process, and ultimately disappears,107 but its fate in the avian spermatid is not known. Its role in spermiogenesis is also not clearly understood. It may be involved in the development of the connecting piece in mammals107 and in the transportation of ribonucleoproteins to the structures in the neck region of the developing spermatid112 or maturation of the nuclear chromatin of the spermatid.104 The latter view has, however, been faulted by Eddy111 who failed to demonstrate RNA presence in the chromatoid body, nor its involvement in the elaboration of the connecting piece, in various laboratory animals. Other views link this body with the development of the annulus, in mammals.11,86
The presence of a dual set of manchette microtubules (CM and LM) has now been established in most non-passerine birds that have been studied. Although the manchette occurs in mammals, only the LM portion is present.16,113,114 Views on the transition from CM to the LM in the elongated and maturing spermatids of animals have been equivocal. The reorganization of the CM and the establishment of the LM is very brief, and this might be partly responsible for the disagreement on whether or not the CM transforms into the LM, and the mechanism underlying it. However, both sets of manchette microtubules occur concurrently, for apparently a fleeting period only, in the African collared dove (Stretopelia roseogrisea),26 Ostrich,40 Rhea,48 and Turkey42 during which period the microtubules of the CM are probably rearranged to become the LM.42,48 Although Okamura and Nishiyama29 consider the transition to be abrupt in the rooster, an illustration by Gunawardana and Scott,30 in the same species of bird, indicates a concurrent presence of both sets of manchette microtubules during the transitional period, as has been observed in Turkey42 and Rhea.48 This type of transition is not evident in the emu.46
Step 9 spermatid: The LM is fully established, and extends beyond the midpiece and annulus, caudally into the trailing cell cytoplasm (Fig. 8E). Other organelle developments are as in step 8.
Step 10 spermatid: The nucleus elongates further in a gentle curve, and with a reduced diameter compared to the preceding spermatids (Fig. 9A, B). The spermatid is therefore longer and more filiform in shape than in earlier spermatids series. The acrosome is well formed and houses the perforatorium in its subacrosomal space. The coarse nuclear chromatin granules become more electron-dense and more compactly packed together than in spermatid step 9, although the central portion of the spermatid has much fewer chromatin granules (Fig. 9C). Mitochondria continue to elongate and their matrix increases in density.
Figure 9.
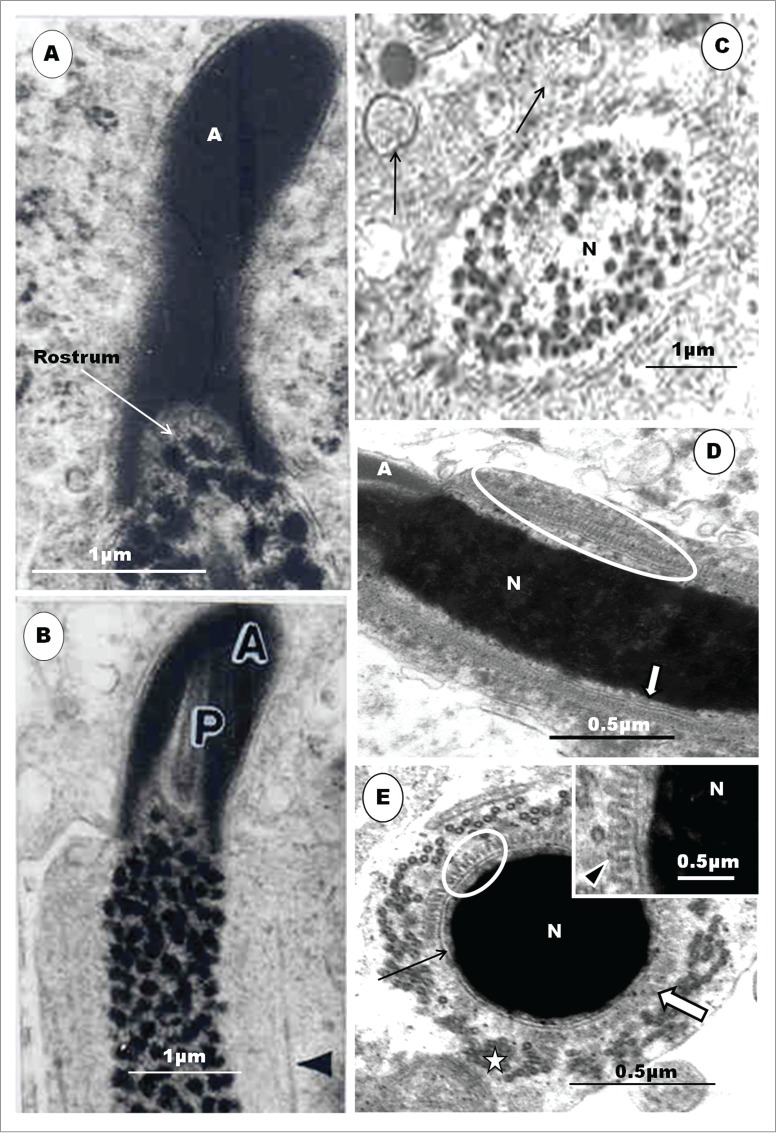
Step 10 spermatid. (A) and (B) – the spermatids have highly elongated, laterally compressed, acrosomes (A); arrowhead, longitudinal manchette. In (C), the central part of the nucleus (N) is devoid of chromatin granules; arrowheads, LM; arrows, numerous multivesicular vesicles and lysosomes. Note the prominence of the projections in the area immediately below the acrosome-nuclear shoulder in a longitudinal section of the spermatid of the emu (D). (E) is a high magnification of the finger-like projections observed in a transverse section of a late stage emu spermatid. Note the prominent longitudinal manchette microtubules (stars), the finger-like projections (encircled) closely associated with the outer nuclear membrane (black arrows) and the condensed chromatin of the nucleus (N). Individual manchette microtubules are closely aligned with, but not attached to, the finger-like projections; White, block arrow, finger-like processes cut tangentially. Inset is a magnified part of the main micrograph showing the finger-like projections (arrowhead). Figures D and E are from du Plessis L, Soley JT. A novel transient structure with phylogenetic implications found in ratite spermatids. BMC Evol Biol 2013; 13:104. Figs. 1A, 2A and 3. With the kind permission of Biomedical Central.
Du Plessis and Soley115 have described, for the first time, in late spermatids of birds, specifically of the emu, ostrich and rhea, a structure comprising regularly-spaced, finger-like projections that are immediately adjacent to the outer nuclear membrane (Fig. 9D, E). This structure runs down the length of the nucleus from the region of the acrosomo-nuclear junction, and confers a cog-wheel appearance on the nucleus. In the ratite, this structure appears when the LM is fully formed, and disappears just before spermiation.115 Its function is not known, but these authors have discussed the significance of this finding, in ratite birds, with regard to phylogeny among birds. Other non-mammalian vertebrates known to display a similar structure are crocodiles117 and certain lizard species.117-126 Du Plessis and Soley115 view this as an additional evidence that reptiles and birds share a common ancestry, as has been previously suggested.122-126
Step 11 spermatid: The nucleus displays large, highly electron-dense and compactly packed chromatin granules (Fig. 10A, B, E). The spermatid is cylindrical, and maintains the gentle curvature of the head. The acrosome is lanceolate and accommodates a perforatorium that extends from the base of the endonuclear canal to close to the apex of the acrosome (Fig. 10B). The short, tapering, anterior end of the nucleus, the rostrum, protrudes, as a permanent feature, into the subacrosomal space. The LM remains fully present during the early period of this step, and it is surrounded distally by elongated mitochondria in the caudal part of the trailing cytoplasm (Fig. 10C). During the latter part of this step, the LM begins to break up patchily. Glycogen aggregations may be seen in the cytoplasm (Fig. 10C). The annulus, although quite small, remains a feature of the spermatid (Fig. 11A).
Figure 10.
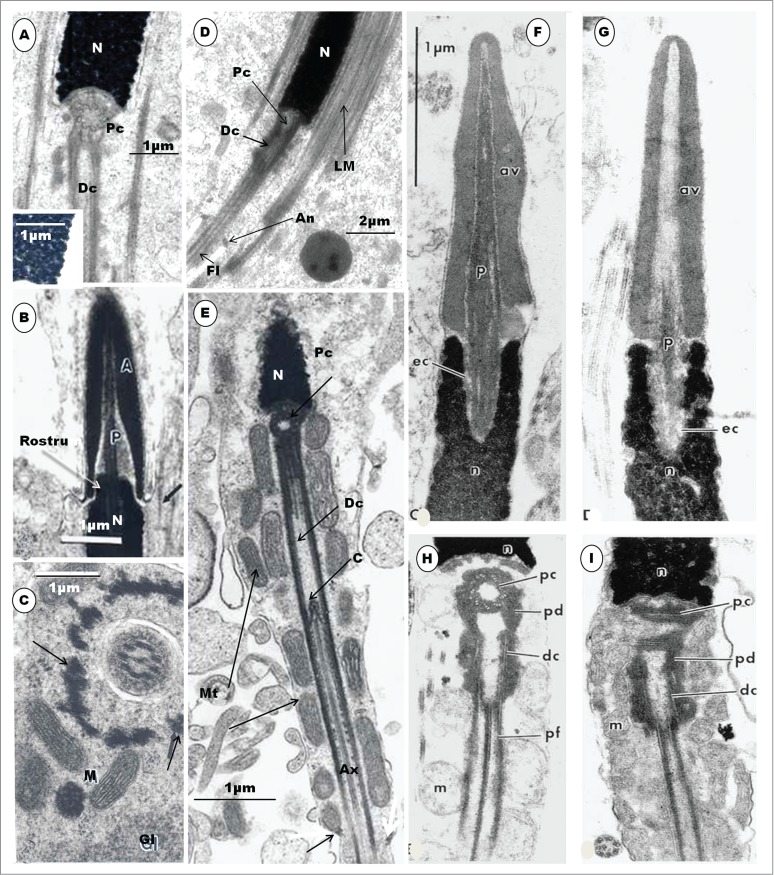
Step 11 spermatid (A), (B) and (C). Chromatin granules are dense and compacted in the nucleus ((A), and inset); the acrosome (A) is lanceolate, P, perforatorium surrounded by fuzzy material in the subacrosomal space; arrow, LM (B). A transverse section of the spermatid showing the principal piece of the spermatid; M, mitochondria surrounding the LM (arrow); G, glycogen granules, arrows, longitudinal manchette (C). (D) is a longitudinal section of the caudal half of a spermatid of the Japanese quail displaying the in-line alignment of both the proximal (Pc) and distal (Dc) centrioles. The longitudinal manchette is in place, but mitochondria have not arranged themselves around the midpiece; An, annulus; Fl, flagellum. In Step 12 spermatid (E), the LM disappears, mitochondria arrange themselves around the midpiece, and a small annulus (arrow) is present, at the end of the midpiece. Ax, axoneme; C, basal body of distal centriole. (F), (G), (H) and (I) are longitudinal sections of parts of the spermatozoa of the parrot, Melopsittacus undulatus. (F) and (G) represent the acrosomal complex and the anterior region of the nucleus, and (H) and (I), the posterior end of the nucleus and part of the midpiece. Note the 9 triplets of the transversely sectioned proximal centriole (H), which lies at right angles to the distal centriole, the latter forming the basal body of the axoneme. pc, proximal centriole, dc, distal centriole, pf, dense peripheral fiber (coarse fiber). (F), (G), (H), and (I) are taken from Jamieson BGM, Koehler L, Todd BJ. Spermatozoal ultrastructure in 3 species of parrots (Aves, Psittaciformes) and its phylogenetic implications. Anat Rec 1995; 241: 461-468. Figs. 1C, D, E, F. With the kind permission of John Wiley & Sons Ltd.
Figure 11.
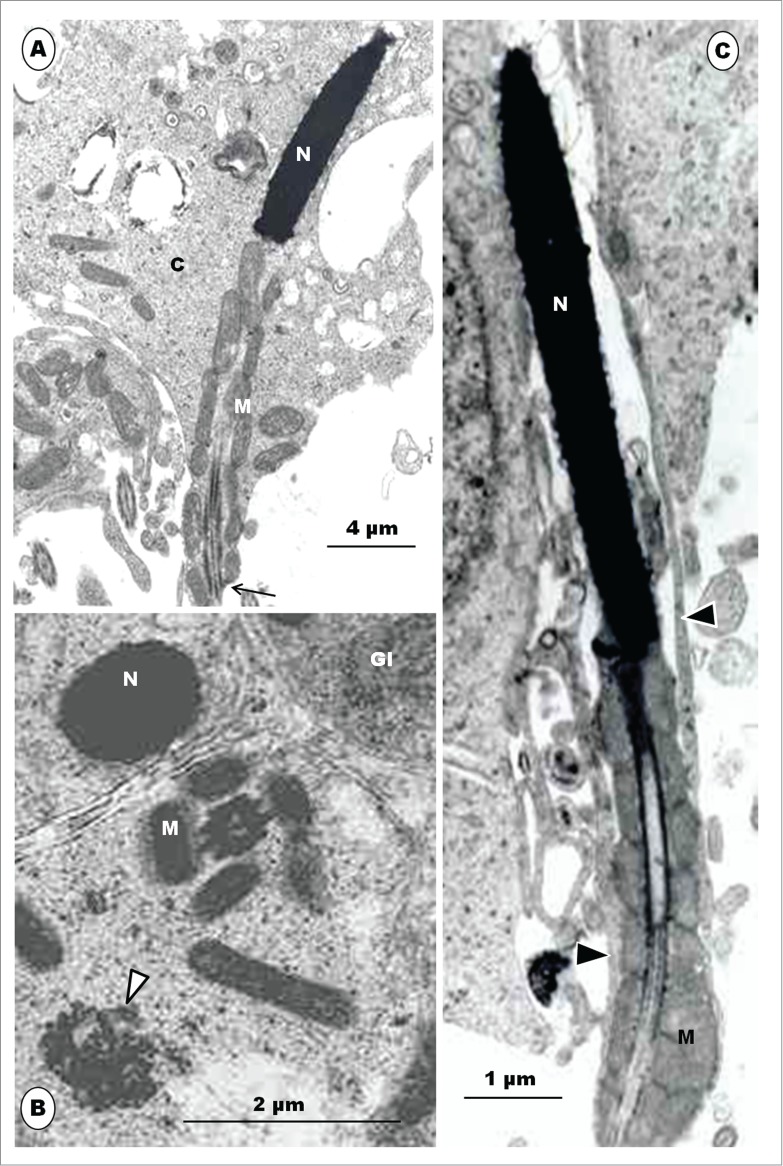
Step 12 spermatids. (A), the mitochondria (M) form a sheath around the midpiece, and the spermatid continues to withdraw from its own redundant, electron-dense cytoplasm (C); N, nucleus of spermatid; arrow, annulus. (B), Transverse sections of step 12 spermatids: the LM disappears and mitochondria (M) then align themselves around the midpiece; glycogen granules (Gl) are still present in the cytoplasm. (C), late phase of step 12, showing a well established mitochondrial sheath. Slips of Sertoli cell cytoplasm (arrowheads) hold on to the spermatid tenuously, as the spermatid is ready for spermiation.
The acrosome and the perforatorium have, together, been regarded as the acrosome complex.127 The perforatorium is a fibrous structure consisting of parallel bundles of filaments127 composed of actin.34,128 The perforatorium has been used by Saita et al.89 to categorize birds into 3 groups: the first group comprises birds that have an elongated spermatozoon and a well developed perforatorium, as in members of the Galloanserae and Psittaciformes (e.g., Nicander and Helstrom,59 Tingari,27 Humphreys,38,70 Jamieson53); the second, has an elongated spermatozoon but has no perforatorium, as in members of the Columbiformes as well as some other species of birds (e.g., Humphreys,70 Fawcett et al.,9 Jamieson53); and the third has a spirally-shaped spermatozoon, but has no perforatorium, as typified by members of the Passeriformes (e.g., Furieri,132,133 Humphreys,70 Jamieson et al.53,72). The structure of the acrosome complex in birds has been described by several authors, in the rooster,64,129,130 turkey,42,101 Guinea fowl,64,101 Mallard drake,68 Rhea,48 Crested tinamou (Eudromia elegans elegans),66 and Ostrich.67,131 However, there are only a few reports on the development of the complex in birds,3 and these are specifically in the rooster,23,27,29,30,34 parrots, including the Budgerigar and cockatiel,28,45 Japanese quail,49,90 Guinea fowl,42 Ostrich,39 and emu.46
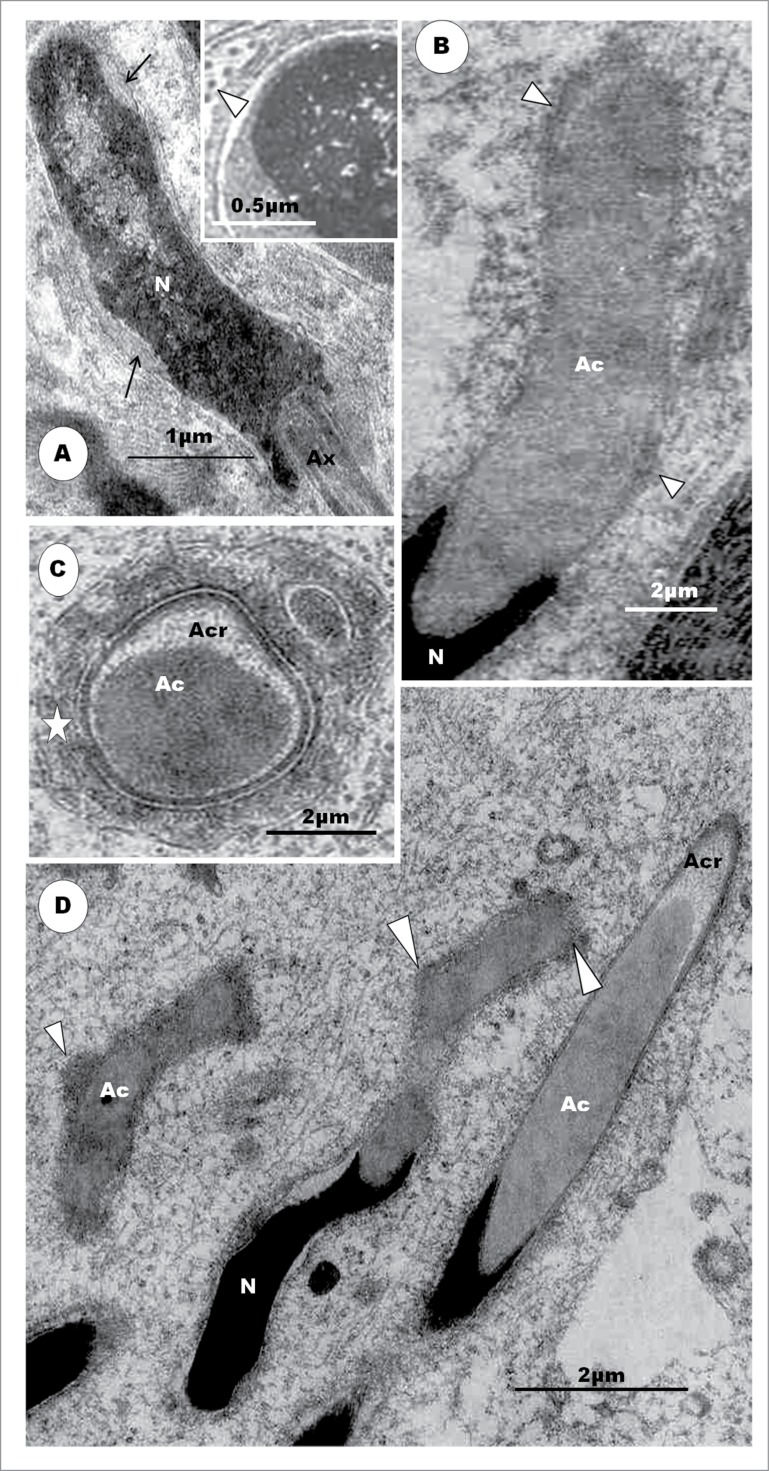
In all reports, a similar process of development of the acrosome complex occurs in non-passerine birds, except the emu which lacks a perforatorium.46 From about Step 6 of spermiogenesis, in the turkey,42 the crater in the nucleus, formed by and lodging the acrosomal granule, flattens out, and the rostral end of the nucleus becomes convex, once again. The caudal part of the elongating acrosome begins the formation of the subacrosomal space. As this space deepens, the developing rostral end of the perforatorium is pulled along or pushes into the space. The perforatorium is thought to assist in elongating and supporting the acrosome, during its development,34 but it is known to project after disruption of the acrosome vesicle in the acrosome reaction of some vertebrates, e.g. Lamprey and Sturgeon (see Jamieson134). The fully developed perforatorium is embedded in the endonuclear canal and projects into the deep and ample subacrosomal space, in most birds, extending to just beneath the rostral end of the acrosome.23, 27,30,34,39,42,53 Jamieson53 describes the main differences in the disposition of the perforatorium between members of the Galliformes and those of the Anseriformes, both of which families belong to the Galloanserae monophyly. The perforatorium is absent in passerine birds (vide infra) and in some non-passerine birds (see Jamieson53).
In most birds, the rostral tip of the nucleus tapers slightly and projects for a short distance into the subacrosomal space, thus forming the intra-acrosomal portion or rostrum, of the nucleus,64,68,129,130 which portion in the tinamou66 and the ostrich39 is extremely long and occupies almost all of the subacrosomal space. In addition, the rostrum in ratites bears an equally extensive endonuclear canal. It is noteworthy that the anterior tip of the nucleus of the Budgerigar,28,135 white-naped Crane,136 cockatiel (Fig. 10F, G), and peach-faced lovebird 45,135 does not project into the subacrosomal space, as in other birds, but makes a direct, en face contact with the caudal rim of the acrosome. In these birds, therefore, the sperm nucleus has no intra-acrosomal portion because the acrosome does not overlap it, and this is probably responsible for the high incidence of decapitated sperm found in the semen of parrots.28 It is also interesting to note that the nucleus of the mature avian spermatid and spermatozoon consists of compact chromatin granules, and not of condensed, homogeneous chromatin, found in insect and mammalian spermatozoa.29,48,101 The chromatin approaches homogeneity in Apus apus43 and attains complete homogeneity, although spaces that are surrounded by the condensed chromatin may occur centrally in most passerine birds studied (vide infra).
The annulus is well developed in members of the Galloanserae monophyly.42,55,68 It is very distinct in the Ostrich38 but ill-defined in the mature Emu.46 In ratite birds, the annulus maintains a fixed position, thus, determining the length of the midpiece in spermatids.38,48,66 This is unlike in mammals11 and the more advanced non-passerine birds23,29,30 in which it migrates posteriorly during spermiogenesis. The annulus is not present in all birds, being absent, for example, in the cockatiel,45,135 budgerigar and lovebird.135 The presence and/or development of the annulus may, therefore, have phylogenetic implications.46
Step 12 spermatid: This step is marked by the disappearance of the LM, allowing the mitochondria to migrate toward, and align themselves around, the midpiece (Fig. 10E). Subsequently, in a later phase of this step (Figs. 10E and 11A), the mitochondria form a helical sheath closely around the midpiece. In the ostrich,38 but not in the emu,46 the mitochondria are connected by ‘inter-mitochondrial cement’. The evolved mature spermatid commences the movement away from most of the redundant cell cytoplasm which still contains glycogen accumulations (Fig. 11A), profiles of the endoplasmic reticulum as well as multivesicular bodies and groupings of discarded LM microtubules (Fig. 11B). The peri-acrosomal complexes disappear, and the mature spermatid attains the luminal surface of the seminiferous tubule, held in place by only slips of Sertoli cell cytoplasm, just prior to spermiation (Fig. 11C). The released spermatozoon possesses no cytoplasmic droplet, as formed in mammals.
The dissolution or disassembly of the LM during the early phase of Step 12 of spermiogenesis in Turkey42 appears to make way for the elongated, dense mitochondria to surround the proximal axoneme as, the mitochondrial sheath, similar to the situation in mammals,8,11 Rhea,48 Ostrich,38 and Emu.46 A departure from this developmental process is in the Japanese quail, in the step 10 spermatid,49 and in the European nightjar, Caprimulgus europaeus88 in which the mitochondrial sheath has already begun to form even when the CM (quail) and the LM (nightjar) are still in place. However, the formation of this sheath is completed before the LM disappears. Okamura and Nishiyama29 in the domestic fowl, and Lovas et al.45 in parrots, have, also, reported the presence of mitochondria on either side of the LM in some spermatids. Interestingly, we have seen spermatids that display fully formed LM, but with no mitochondria passing through this mantle to surround the distal centriole in the Japanese quail (Fig. 10D).
A number of investigators, McIntosh and Porter,24 Okamura and Nishiyama,29 Gunawardana and Scott,30 Lin and Jones49 and Soley40 are of the opinion that the manchette has an important function in nuclear shaping, but Fawcett et al.,9 Asa and Phillips,59 Phillips11,138 are emphatic that the CM plays no role in nuclear shaping in the pigeon spermatid. It is tempting to consider that the CM plays a major role in nuclear-shaping, and this is re-inforced by experimental studies in which the disruption of the structure of the manchette led to deformities in nuclear shapes.138-140 The LM may be involved in the caudal translocation of the cytoplasm and associated organelles during the elongation process of the spermatid, as well as the determination of the final, slender, shape of the nucleus,42 as has been reported for mammals (see Hermo et al.141). The manchette has been closely linked with the processes that are associated with the assembly of the tail components, as well as the development of the nucleus, during spermiogenesis in mammals.142-146
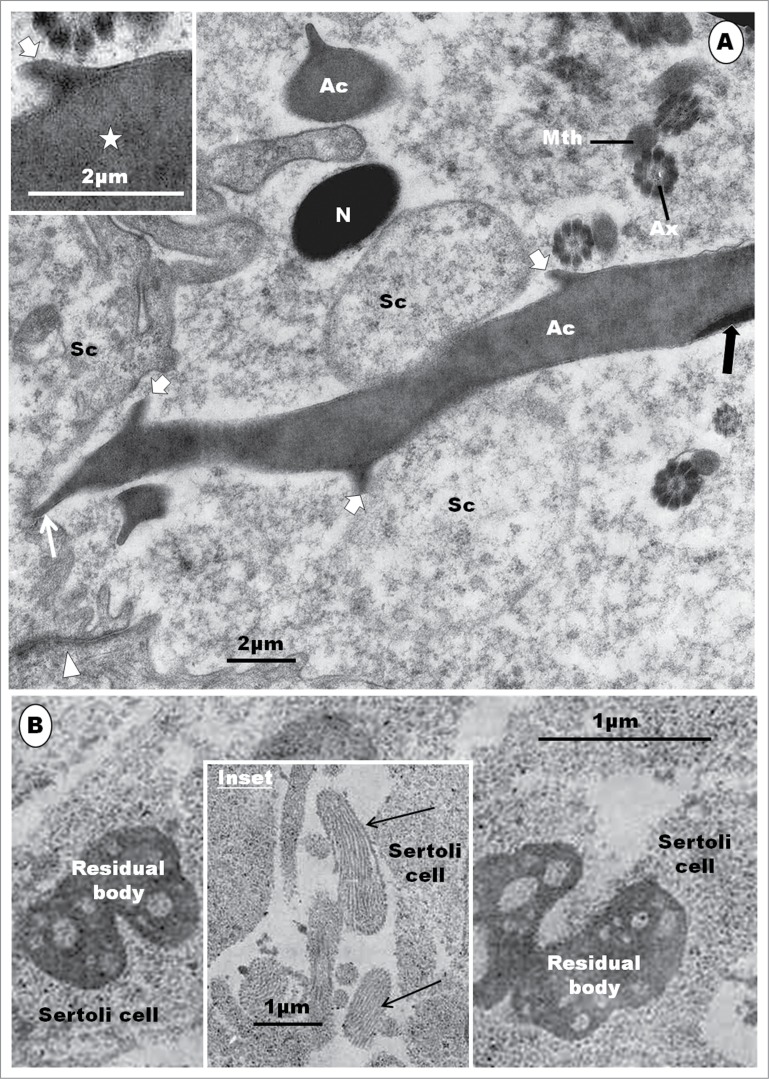
Spermiation has been reported in only 2 birds, the rooster36 and Japanese quail.49 The process is similar in both species of birds, belonging to the Galliformes. During the process of spermiation, the spermatid cytoplasm tends to condense and become more electron-dense relative to the cytoplasm of other germ cells, as well as the Sertoli cells.39 Residual bodies, light-staining in birds, but highly condensed in mammals, form late in spermiogenesis, near the time of sperm release.36 They are phagocytised by Sertoli cells.36,49 The tubulobulbar complex has not been observed in birds. Further studies on spermiation, involving other orders of birds are necessary for a complete picture of this phenomenon in this large class of animals. The cytoplasmic droplet, found in spermiated mammalian spermatozoa, is absent in most birds, except in the spermatozoa of the ostrich37,147 in which it normally occurs. In the emu,46 a thread-like appendage of the spermatozoon, close to the base of the nucleus, has been reported, but it may be a remnant of the residual cytoplasm.
Spermiogenesis in Passerine Birds
Introduction
As already indicated, studies on spermatogenesis in birds, compared to mammals, are relatively few. Yet, there are much fewer reports on this process in passerine than in non-passerine birds9,22,33-35,41,44,77 In this section of the review, spermiogenesis in the Masked Weaver (Ploceus velatus), in which species, some novel unpublished observations on passerine spermiogenesis have been made, will be used as a type passeridan bird for the discussion of this phenomenon in passerine birds.
Spermiogenesis in the Masked Weaver (Ploceus Velatus)
The seminiferous epithelium of the Masked Weaver (MW) is typically avian in organization, in displaying heterogeneous cellular associations (Fig. 12). Several spermatids at various steps of spermiogenesis may be found close together, even in one small microscopic field of view (Fig. 12B). The seminiferous epithelium of passerine birds is therefore much more heterogeneous and mixed than in non-passerine birds as well as in man and other primates.79-82 However, the description of spermiogenesis in this species will be provided in a methodical, step-wise and orderly manner, from the earliest spermatid to the mature spermatozoon that is ready for spermiation. There are some organelles which bear several different names in the literature. The terms and names adopted in this review will be well argued for.
Figure 12.
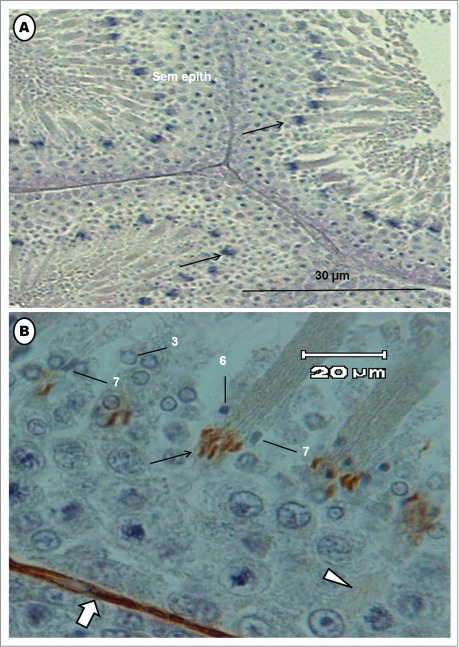
Photomicrographs of the testis of the Masked Weaver. (A) shows transverse sections of seminiferous tubules; arrows = bundles of elongated spermatids. (B) is a higher power view of part of the seminiferous epithelium immunostained for actin microfilaments. Several steps of spermiogenesis are mixed, in the typical avian heterogeneous cellular associations. Numbers 3, 6 and 7 are spermatids at various steps of spermiogenesis. Bundles of immunostained germ cells (arrow) are elongated spermatids whose microfilaments are stained.
Step-wise description of spermiogenesis in the Masked Weaver
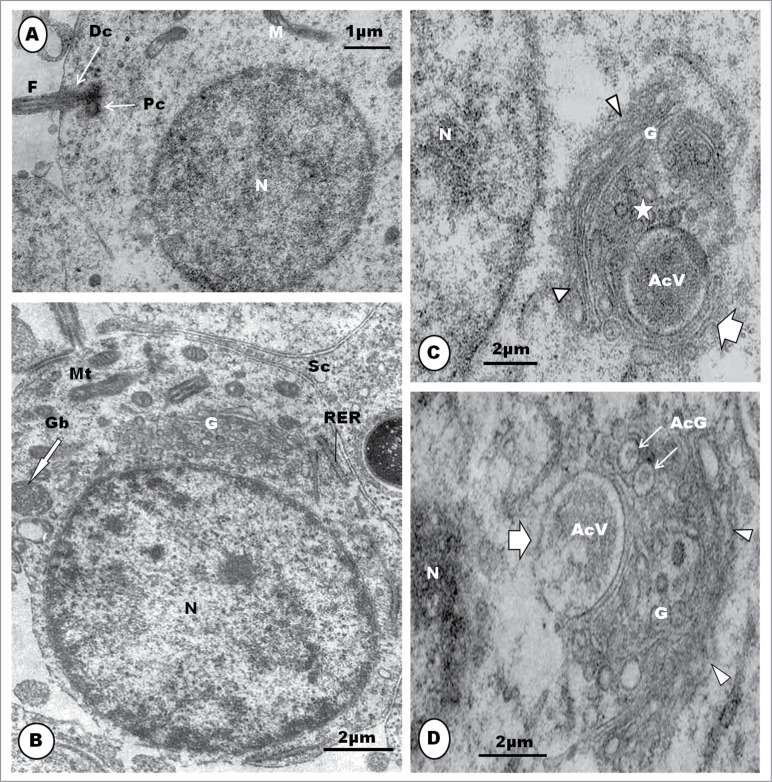
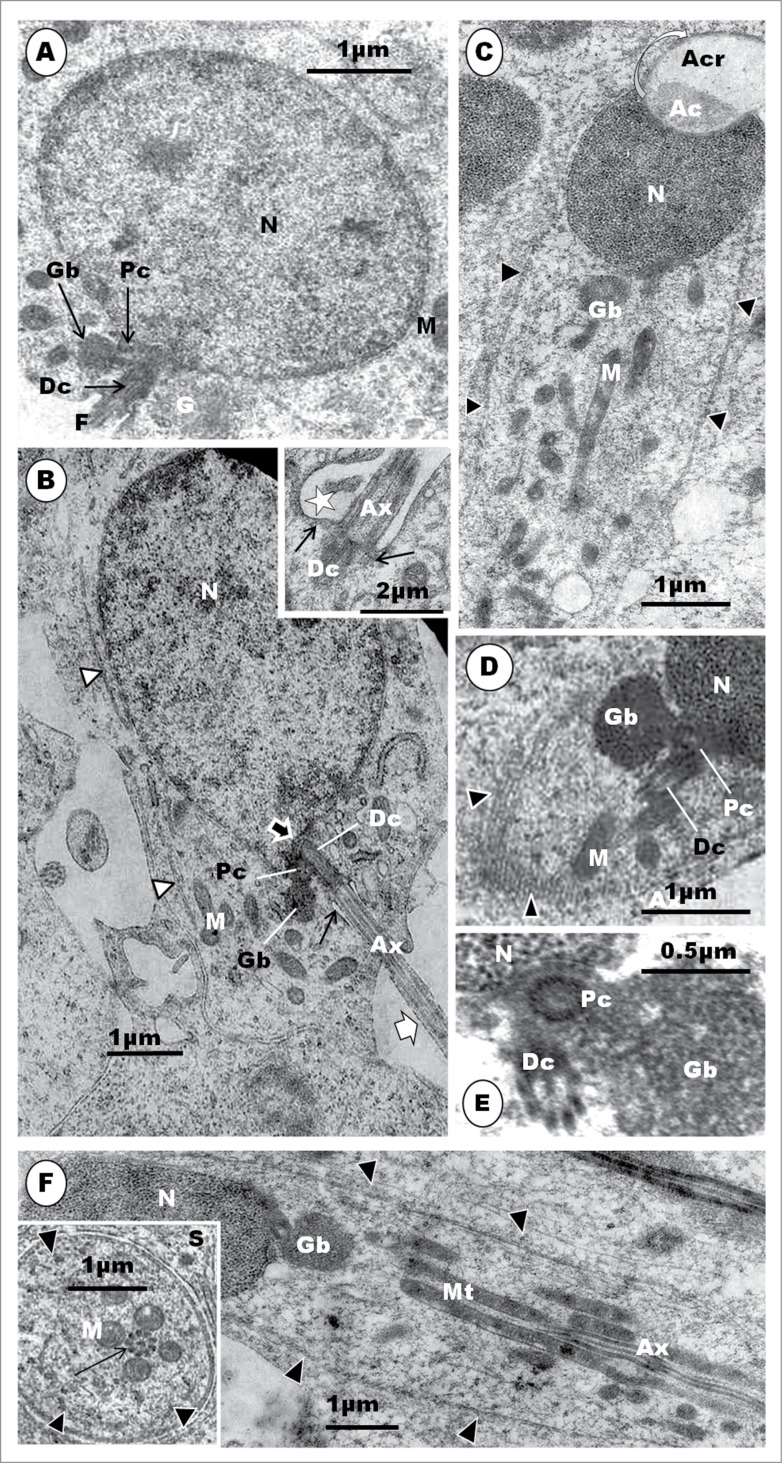
Step 1: This spermatid is the result of the last meiotic division of the primary spermatocyte, and it contains a round or oval nucleus which is uniformly filled with clumps of granulofilamentous chromatin (Fig. 13A). The Golgi complex is prominent, and consists of several stacks of saccules (Fig. 13B). The mitochondria are moderately elongated or oval in shape, and are uniformly dispersed in the cytoplasm (Fig. 13B). The centriolar complex exhibits both proximal and distal centrioles arranged perpendicular to each other, and situated peripherally in the cytoplasm (Fig. 13C). The distal centriole makes contact with the cell membrane, and is subsequently continued extracellularly by a flagellum (Fig. 13A). A round to oval or irregular retiform and granular structure is seen to lie close to the nucleus and the Golgi complex. This is regarded to be the granular body (GB) [vide infra] (Fig. 13B). Toward the end of this step, the nucleoplasm displays scattered electron-dense clumps of heterochromatin mostly attached to the inner aspect of the nuclear membrane (Fig. 13B). Only a few strands of the rough endoplasmic reticulum (RER) are to be found in the cytoplasm.
Figure 13.
The step 1 spermatid is round or oval in shape, and the nucleus (N) contains granulofilamentous chromatin; M, moderately elongated mitochondria; the centriolar complex lies closer to the plasmalemma than to the nucleus, and comprises both proximal (Pc) and distal (Dc) centrioles arranged perpendicular to each other, and followed extra-cellularly by the flagellum (F) (A), and (B) shows a moderately large Golgi complex (G), an oval, granular or retiform structure, the granular body (Gb) lies close to the nucleus and Golgi complex. A few strands of rough endoplasmic reticulum (RER) also occur. (C): in early step 2, the Golgi complex (G) becomes large, and shows a number of well formed proacrosomal granules (star) in the TGN that is directed away from the nucleus. One large, bipartite, proacrosomal vesicle (AcV) is peripherally situated, and surrounded by 2 or more cisternae (broad white arrow); arrowheads, cis side of Golgi complex. In (D), the Golgi complex rotates, with the TGN toward the nucleus (N); several proacrosomal granules (AcG) exhibiting a bipartite nature. The large proacrosomal vesicle (AcV), now devoid of the outer parts of surrounding cisternae (broad white arrow), orientates and moves toward the nucleus; arrowheads, cis face of the Golgi complex.
The granular body is first observed in the Masked Weaver in step 1 spermatid, as a small, round, granular structure lying close to the nucleus and Golgi complex. The granular body first observed in step 2 spermatids of the sparrow, Passer domesticus,41 but Sotelo and Trujillo-Cenóz22 in the same species, and Tripepi and Perrotta77 in the Cirl bunting (Emberiza cirlus) describe this structure, for the first time, during the beginning of the elongation phase and formation of the mid-piece. Tripepi and Perrotta77 call it the granular body while Sotelo and Trujillo-Cenóz22 call it the juxtanuclear body, and Góes and Dolder,41 the centriolar adjunct. Neither Kondo et al.35 in the swift nor Fawcett et al.9 in the Zebra finch (see Jamieson55) have described this structure. But the chromatoid body and a few other structures are juxtanuclear, and therefore the term, juxtanuclear body, is not specifically descriptive. In mammals, subsequent to flagellar development, the centriolar adjunct107 originates as a fine filamentous material that gathers around the free or distal end of the proximal centriole. It elongates further, but lacks the characteristic structure of the proximal centriole, neither is it granular. Furthermore, the centriolar adjunct is a transient organelle, lasting for only a few days in the mammal.107 The term ‘centriolar adjunct’ is therefore not appropriate for the organelle found in passerine birds. For consistency and avoidance of confusion, the apt and descriptive terms, ‘granular body’ and its successor, ‘granular helix’, are adopted in this work. It is noteworthy that the centriolar complex comprises both the proximal and distal centrioles, lying perpendicular to each other.
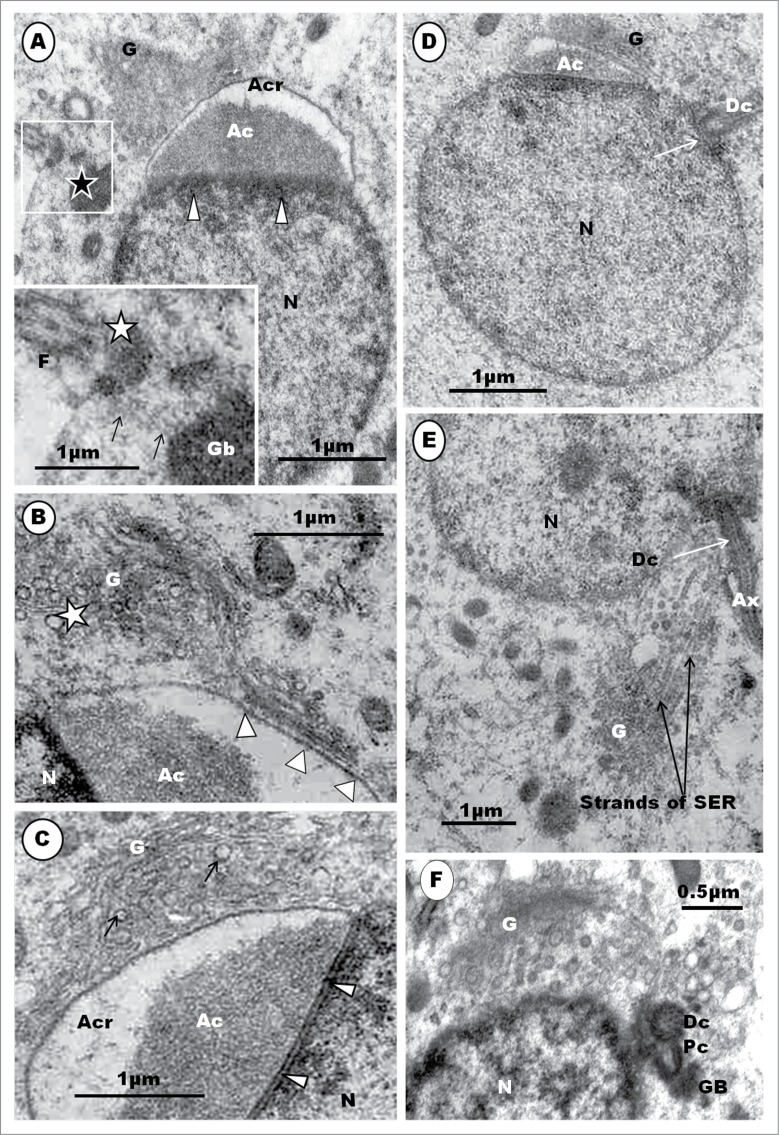
Step 2: The major morphogenetic feature during this step is to be found in the Golgi complex whose cis- surface is directed toward the nucleus. The organelle displays proacrosomal granules of varying sizes, but invariably, exhibits one large vesicle, the presumptive proacrosomal vesicle that lies fully within the substance of the Golgi complex (Fig. 13C). This vesicle exhibits 2 parts, a fledgling acrosomal crest and an acrosomal core [see following discussion]. The Golgi complex subsequently rotates to expose its trans-Golgi network zone (TGN) toward the nucleus of the cell. The proacrosomal vesicle is, now, not covered by Golgi cisternae on the protruding surface by which this vesicle subsequently makes contact with the nucleus of the cell (Fig. 13D). Several, small granules are still to be seen in the Golgi complex (Figs. 13B and C).
The major spermiogenetic feature in step 2 involves the Golgi complex and acrosome development. In the Golgi complex of the Masked Weaver, the precursors of the acrosome begin as proacrosomal granules, several of which coalesce to form one, large proacrosomal vesicle. Both the proacrosomal granules and vesicle are of similar configuration. It is interesting that the large proacrosomal vesicle, during the earlier stage of its formation in the TGN region of the Golgi complex, is turned away from the nucleus, but the Golgi complex subsequently rotates the vesicle toward the nucleus, immediately prior to its nuclear attachment. At this stage, the proacrosomal vesicle already exhibits 2 different components, a dense core surrounded by a clear halo and the vesicular wall. This has been referred to by Jamieson et al.72 and Jamieson55 as the bipartite nature of the acrosome of the passeridan bird. The nomenclature for parts of the developing and mature passerine acrosome (acrosome crest for the outer sleeve, and acrosome core for the inner dense material), adopted in this study and review, is as suggested by Jamieson et al.72 In their reports, Baccetti et al.,34 Fawcett et al.9 and Góes and Dolder41 have not described the formation of the acrosomal vesicles in the Golgi complex. It is noteworthy that a free acrosomal vesicle in the cytoplasm, as found in the spermatids of most non-passerine birds,23, 29,30,39,42,49 does not appear to occur in the Masked Weaver. Instead, the acrosomal vesicle remains a protruding component of the TGN component of the Golgi complex with which it is associated throughout the first 3 steps of spermiogenesis. This indicates that the role of the Golgi complex in acrosomal development extends and continues well beyond the mere formation of the acrosomal vesicle.
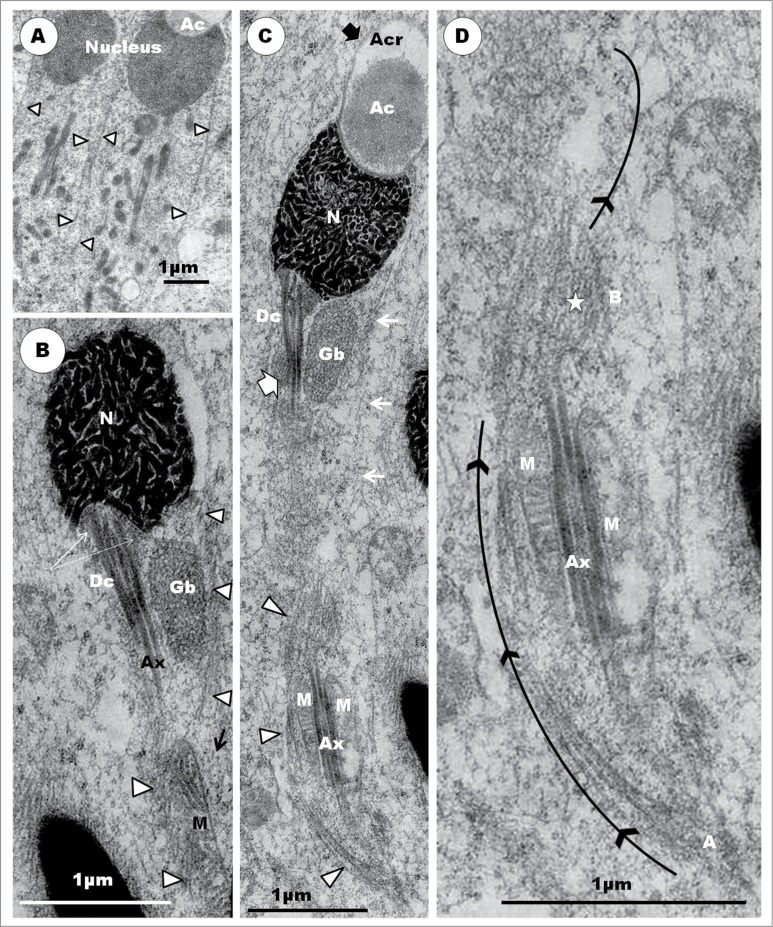
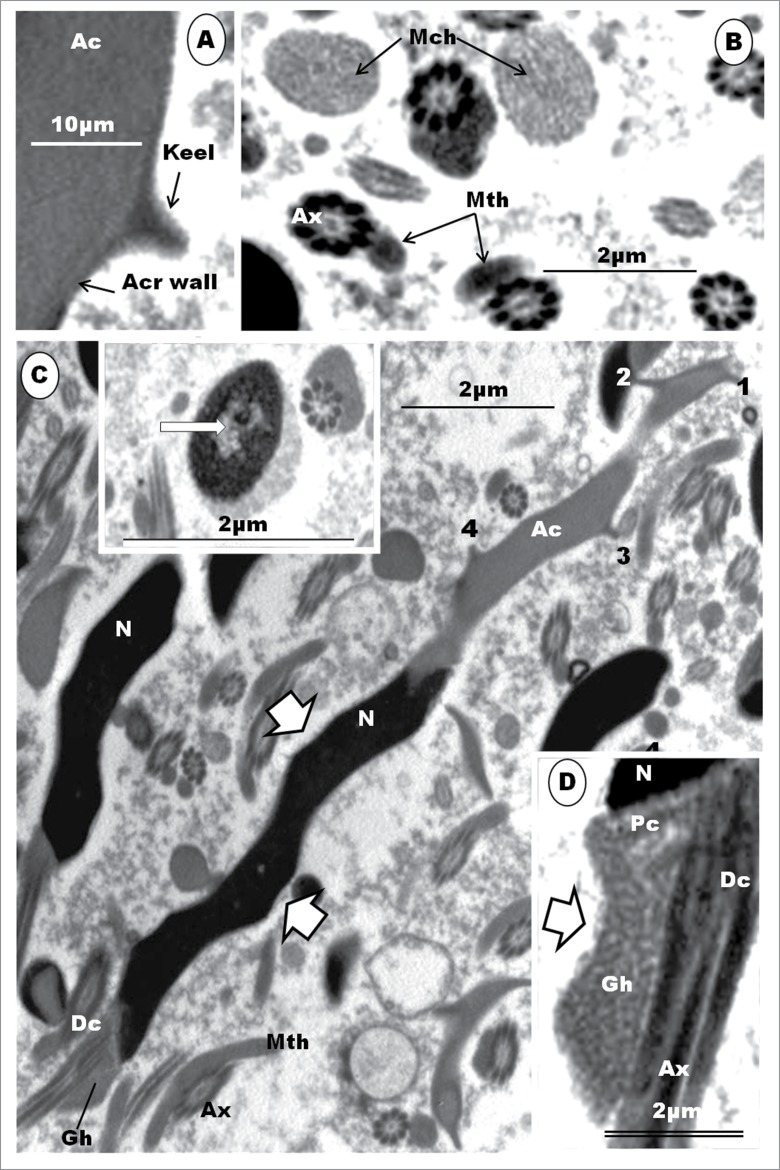
Step 3: The acrosomal vesicle becomes triangular or conical in shape, with the flat base attaching to a correspondingly flat surface of the nucleus (Fig. 13A, B, C, D). The acrosomal vesicle is still closely related to the Golgi complex to which it is attached. A stalk of cisternae of the Golgi complex, comprising an inner, regularly straight, cisterna connects this organelle with the convex, anterior surface of the acrosome vesicle by means of regularly-spaced, short, pedicels (Fig. 14B). The free part of the Golgi complex continues to bud off several vesicles from the TGN surface that lies adjacent to the acrosome vesicle. The mound of finely granular content (the acrosomal core) of the acrosome, occupies the base of this vesicle, by which it attaches to the nucleus (Fig. 14B, C). The acrosomal core continues to be separated from the acrosomal crest portion of the vesicle by a clear part of the crest which displays varying size and shape. The nucleoplasm continues to display clumps of heterochromatin, which, however, are now mostly displaced peripherally toward the nuclear membrane, and especially along the attachment junction with the acrosomal vesicle (Fig. 14A, B, C). The acrosomal vesicle and nuclear membrane are separated by a uniform clear, thin, electron-lucent zone (Fig.14B, C). Mitochondria remain mostly short, oval-shaped, organelles scattered uniformly throughout the cytoplasm. Toward the end of this step, the nuclear chromatin is finely granular and homogenously dispersed within the nucleoplasm (Fig.14D). The centriolar complex finally reaches, and attaches to, the nucleus at a circular, shallow, concave fossa (Fig. 14D, E), and it is connected by means of strands of smooth endoplasmic reticulum (Fig. 14E) with the Golgi complex which, along with the centriolar complex, migrated away from the region of the acrosome.
Figure 14.
(A). Step 3, continued. The triangularly shaped acrosomal vesicle comprising the acrosome crest (Acr) and acrosome core (Ac) components, attaches to a flattened part of the nucleus (N). Arrowheads, aggregated heterochromatin attaching to nuclear membrane at the junction between the acrosome and nucleus. The Golgi complex (G) is closely related to the acrosome vesicle (A), (B), (C).The inset is an enlarged part of (A) (box) displays strands of SER (arrows) attaching the granular body (Gb) to the centriolar complex (star). F, flagellum. In (B), note the attachment of a saccule of the Golgi complex by means of regularly arranged pedicel-like strands to a flattened or depressed part of the acrosome crest wall (arrowheads); star, proacrosomal granules. (C) displays a large Golgi complex still closely associated with the acrosome vesicle, and the uniform, translucent junction between the acrosome crest base and the nucleus (arrowheads); arrows, proacrosomal granules. The nucleus generally displays finely granular chromatin, and the implantation fossa (arrow) for the centriolar complex is shallow and circular (D). (E) shows strands of SER connecting the Golgi complex with the region of the distal centriole (Dc). (F) shows an enlarged articulation and contact between the proximal and distal centrioles, and the granular body.
The acrosomal vesicle makes a broad contact with the nucleus, at a straight junction, in step 3 of spermiogenesis in the Masked Weaver, but in step 2 in the House sparrow.41 It is generally agreed that the base of the acrosomal vesicle flattens out on the surface of the nucleus, and displays the bipartite nature, except in the swift34 in which it is homogeneous. The vesicle is invariably triangular or pyramidal in shape in the Masked Weaver, but round in the swift,34 and irregularly oval in the finch35 and the Cirl bunting, Emberiza cirlus.77 The acrosome does not form a cone-shaped cap over the anterior end of the nucleus as it does in ratite birds.39,42,66,67 A few dense granules occur in the core of the acrosomal vesicle at this stage of its development in the sparrow,41 but these have not been seen in the Masked Weaver, or reported in other passerine birds. A noteworthy feature is the intimate contact between the acrosomal vesicle and the Golgi complex, during this early phase of acrosomogenesis. The TGN surface of the Golgi complex in the Masked Weaver is adjacent to the free convex acrosomal surface in all spermatids during this step of spermiogenesis. The obvious, numerous granules and vesicles budded off the TGN of the Golgi complex indicates that this organelle continues to be associated with acrosomal growth. The attachment of the Golgi complex to the growing acrosome is a fortuitous observation. The Golgi complex subsequently detaches from the acrosome, and along with the centriolar complex and granular body, moves to the caudal pole of the nucleus (vide infra). The prolonged association between the developing acrosome and Golgi complex probably suggests that the latter continues, for an unknown period of time, to play an important role in the development of the former, even after its attachment to the nucleus. The molecular mechanisms regulating acrosomogenesis are still unknown,83 but it appears that the numerous, small vesicles or granules being budded off in the trans-Golgi network (TGN) region continue to be added to the developing acrosome. Further growth of the acrosome, thereafter, has been attributed, in part, to extra-Golgi pathways.150,151 The close relationship between the centriolar complex and the Golgi complex is evidenced by strands of SER which connect them, at this time. It would appear that the Golgi complex is involved in a number of processes that culminate in visibly demonstrated structures or features in both the spermatid as well as in the mature spermatozoon.
Step 4: At the beginning of this step, the centriolar complex migrates away from the region of the acrosome to its definitive nuclear posterior pole, opposite the acrosomal vesicle (Fig. 15A). In doing so, the centriolar complex pulls along with it the granular body and the Golgi complex which, at this time, seems to have numerous, small vesicles but no proacrosomal granules. The contact between the Golgi complex and the centriolar complex is by means of strands of SER (Fig. 14E). At this time of spermatid morphogenesis, its granular body moves closer to the free end of the proximal centriole, with which it makes contact subsequently. The other end of the proximal centriole articulates at an acute angle, of about 45°, with the anterior pole of the distal centriole (Fig. 14F; Fig. 15A). The spatial and perhaps functional relationship between the Golgi complex and granular body with the centriolar complex is noteworthy. It is not inconceivable that the Golgi complex contributes to the formation of both the centriolar complex and granular body during further development of these organelles. It is not exactly certain when the Golgi complex moves away from these other organelles, but the complex progressively gets smaller in size than it appears during the early periods of acrosomogenesis. A novel observation made during spermiogenesis of the Masked Weaver44 is the retention of the proximal centriole, and its articulation with the anterior end of the distal centriole, as well as its attachment by its free end to the granular body. In the House Sparrow, the proximal centriole is seen only in the early, round spermatids, at about step 2 of Góes and Dolder,41 but Sotelo and Trujillo-Cénoz22 report that this centriole is lost during the course of spermatogenesis.
Figure 15.
(A), Step 4 spermatid. The centriolar (Pc = proximal, Dc = distal centrioles) and Golgi (G) complexes as well as the granular body (Gb) together migrate to the posterior pole of the nucleus to which the centriolar complex attaches. The proximal centriole (Pc) attaches by its free end to the granular body and distal end to the distal centriole (Dc). Pc, proximal centriole; Dc, distal centriole. (B) displays a step 5 spermatid which has commenced the elongation process. Mitochondria (M) translocate into the posteriorly migrating cytoplasm, and the microtubular sleeve runs patchily from the anterior part of the nucleus to a little beyond its posterior end (arrowheads). F, flagellum; M, mitochondrion; Gb, granular body; back arrow, implantation fossa; Ax, flagellum. White thick arrow shows feint transverse striations of the amorphous sheath; long black arrow, flagellar canal; arrowheads, microtubular sleeve around the nucleus (N). Inset: Dc, distal centriole; arrows, lack of an annulus; star, flagellar canal; Ax, axoneme. (C) shows step 6 spermatid displaying a goblet-shaped nucleus (N), and, relative to the acrosome core of the acrosome, an enlarged acrosome crest (Acr) is beginning to twist on its own axis. The microtubular sleeve (arrowheads) forms a cylinder around the nucleus and most of the other organelles, well into the trailing cytoplasm. M, mitochondria; Gb, granular body. (D) exhibits the helical nature (arrowheads) of the microtubular sleeve. Pc, proximal centriole; Dc, distal centriole; Gb, granular body; M, mitochondria. (E) shows the relationship between the distal centriole (Dc), granular body (Gb), proximal centriole (Pc) and the nucleus to which the proximal centriole attaches at a shallow implantation fossa. (F) is a step 7 spermatid with a further elongated nucleus, and highly elongated mitochondria aligned along the long axis of, and surrounding the, axoneme (Ax) within the microtubular sleeve (arrowheads). The inset is a transverse section of the elongated mid-piece of the spermatid, and elongated mitochondria surround the axoneme. Arrowheads, microtubular sleeve; arrow, axoneme; S, Sertoli cell.
Step 5: The acrosomal vesicle enlarges, elongates slightly, and indents the nucleus more deeply. The bipartite nature of this developing organelle is maintained. The nucleus is generally euchromatic, with a few, fuzzy, scattered, chromatin clumps, and it begins the elongation phase, with a pointed posterior end (Fig. 15B). The cytoplasm also begins to migrate or translocate posteriorly. Mitochondria aggregate and move into the caudally migrating cytoplasm. For the first time, a patchy line or an assemblage of a sleeve of microtubules in transverse section, appear in the sub-cytoplasmic membrane and is seen to run from the anterior pole to the level of the caudal pole of the nucleus (Fig. 15B). With the caudal migration of the cytoplasm, a flagellar canal is formed. The flagellum, at close observation at this stage, exhibits very feint, rib-like striations, but has well defined centrally-placed double singlet microtubules, as well as peripherally situated 9 sets of microtubules (Fig. 15B). The annulus is only faintly developed. Other organelles and structures present in the cytoplasm, at this time, include, but are not limited, to strands of RER and multivesicular bodies.
The Masked weaver does not display a distinct annulus in its spermatids or spermatozoa, although Kondo et al.35 and Góes and Dolder41 consider that there is an annulus in the spermatids of the Common finch (Lochura striata var. domestica) and house sparrow, respectively, this structure is not convincingly demonstrated in any of these birds. Passerine birds, in this regard, are different from most non-passerine birds which normally exhibit a distinct annulus. Thus, the turkey, chicken, guinea fowl,42,64 mallard duck,28,68 and members of the ratitae38,46,48,59,131 demonstrate a distinct annulus, but passerine birds are similar to some members of the Psittaciformes45,135 in lacking this structure or showing a poorly developed one.
Passerine birds, as also members of the Psittaciformes,45,135 do not have a fibrous sheath which is usually displayed distinctly in most non-passerine birds, such as members of palaeognaths, e.g., ostrich and emu38,46,48,66 and Galloanserae.23,42,49,53,64 An interesting observation made during the elongation phase in step 5 of the turkey spermatid, is the concurrent lengthening of the nucleus along its antero-posterior axis, the caudal migration of the cytoplasm, caudal migration of the mitochondria along with the cytoplasm, the formation of the flagellar canal and the appearance of perinuclear microtubules. Thus, a number of morphogenetic and transformational processes commence concurrently during this step of spermiogenesis.
Step 6: The acrosomal vesicle elongates further, and continues to indent the nucleus rather deeply (Fig. 15C). Its composition is similar to that in step 5, but the clear space between the 2 parts increases relatively in size, and the acrosome core generally appears to twist out of line with the crest portion. The nucleus also elongates along the axis of both poles, and appears goblet-shaped. It is filled with compact fine granules that render this organelle more electron-dense in consistency than during the previous step. A cylindrical sleeve of microtubules, formed just deep to the cell membrane, runs from the region of the acrosomo-nuclear junction to close to the caudal end of the cytoplasm (Fig. 15C). The caudal pole of the nucleus displays similar organelles and structures, such as the proximal centriole, granular body and distal centriole, as are found in step 5 spermatids (Figs. 15D, E). A transverse section of both the proximal and distal centrioles shows that the former is hollow and displays the usual 9 sets of triplet microtubules (Fig. 15E). Organelle content of the posteriorly-trailing cytoplasm appears more obvious and is enclosed in the microtubular sleeve or girdle. Mitochondria are larger, but are still scattered within the cytoplasm (Fig. 15C).
The spermatid exhibits a goblet-shaped nucleus with characteristically finely granular chromatin, and its cytoplasm is situated mostly posterior to the nucleus. This step is similar to Góes and Dolder's41 step 3 in the house sparrow. The microtubular sleeve is closer to the cell membrane than in non-passerine birds (compare with Figs. 6A, C and Fig. 15B, C, F). An important morphological feature of this step is the full development of microtubules that are arranged in a single assemblage that runs as a helical sleeve antero-posteriorly, from the region of the acrosomo-nuclear junction into the caudally migrating cytoplasm. This is akin to the CM of non-passerine birds, although the microtubules are transversely, and not helically, oriented in the non-passerine birds. The CM of non-passerine birds does not extend beyond the posterior pole of the nucleus. Also noteworthy is that, unlike in passerine birds, neither the CM nor the succeeding LM of the non-passerine bird encloses the organelles, including mitochondria. At this stage in the Masked Weaver, the nucleus has not shown any evidence of twisting along its long axis, although Kondo et al.,35 in the Common finch, consider that a cluster of microtubules has begun to twist the nucleus spirally in corresponding spermatids. As in the Masked Weaver, Góes and Dolder41 believe that the microtubules twist helically around, but do not twist, the nucleus and the developing flagellum.
The canal of the distal centriole contains a linearly oriented but irregular electron-dense material that appears to be continuous with the central singlet microtubules of the axoneme, as seen in the Zebra finch9 and in spermatozoa of the southern anteater-chat and social weaver.72
Step 7: The nucleus of this spermatid elongates further, but its chromatin remains finely granular and compact (Fig. 15F). The sleeve of helical microtubules is pronounced and extends caudally toward the end of the cytoplasm that has moved posteriorly, enclosing a highly elongated mid-piece. The long axes of the highly elongated mitochondria are now aligned with the long axis of the cell, and toward the end of this step, the mitochondria join end-to-end, in a remarkable manner, to form, usually, 4 long strands that run parallel to the axoneme of the elongated mid-piece (Fig. 15F inset).
The main differences between the step 6 and step 7 spermatids are in the elongation of the narrow nucleus, from goblet shape, and the remarkable fusion and elongation of mitochondria in step 7. The acrosome continues to indent the anterior pole of the nucleus in the Masked Weaver (this study) as in the house sparrow41 but not in the Zebra finch9 and the Common finch35 in which a shallow junction occurs between it and the nucleus. The phenomenon of mitochondrial fusion to form long strands during spermiogenesis has also been documented in a number of passerine birds.9,35,41
Step 8: The acrosome elongates further, and both the crest and core portions twist a little out of line or synchrony (Fig. 16C). The nucleus also commences a subtle spiralization along the same axis as the acrosome. The convex surfaces of these acrosomal twists are the presumptive keels in the making. The nucleus shortens from the previous step, and becomes dumb-bell shaped (Fig. 16B, C). Its chromatin condenses into coarse, strongly electron-dense, rod-like strands that are arranged variably within the nucleoplasm. The proximal and distal centrioles are surrounded by amorphous, moderately electron-dense material, as they insert at the shallow, concave implantation fossa (Fig. 16B, C). The distal centriole displays linearly oriented but variably-shaped or irregular electron-dense strands of material in its lumen (Fig. 16B, C). The latter appears to continue, caudally, with the singlets of the axoneme of the flagellum. On one side of the distal centriole, the granular body elongates further and vertically between the base of the nucleus and the beginning of the elongated strands of mitochondria. The granular body, as the mitochondrial strand, has not yet formed a helix. The helical sleeve of microtubules (Fig. 16A) begins to transform into a loose bundle of microtubules that runs helically around the axoneme and the long strands of mitochondria (Fig. 16C, D). The sleeve of helical microtubules of the spermatid is usually cylindrical in shape (Fig. 16A), but during its morphological transformation, the microtubules are seen to move, remarkably, toward the axoneme (Figs. 16B, C), as they begin the formation of the helical microtubular bundle, albeit only a loose bundle at this stage (Figs. 16C, D). The long strands of mitochondria continue to run parallel to the axoneme, and have not yet joined together to form a single winding strand.
Figure 16.
(A) shows spermatids of step 7 displaying a well formed and complete microtubular sleeve (arrowheads) that extends nearly throughout the length of the spermatid. (B) and (C) show step 8 spermatids whose acrosomes twist a little out of line with the nuclei, and begin to exhibit convex surfaces which ultimately form the keels (thick arrow). Note the variable arrangement of the highly thickened strands of chromatin within the dumb-bell-shaped nuclei and the amorphous material (B), white broad arrow) which surrounds the distal centriole (Dc) as it attaches to the nucleus. The lumen of the distal centriole contains electron-dense linear but slightly irregular strands (white arrow), from which the singlet microtubules of the axoneme arise. In (B) and (C), the microtubular sleeve has moved toward the nucleus (white arrowheads in (B), and black arrows in (C)) [compare with Figs. 14F and 15A], and commences a rearrangement to form a loose bundle of microtubules (C) which is the precursor of the microtubular helix. (D) is an enlarged part of the lower half of (C), showing the profile of the microtubular helix in formation (curved black arrow), and transected roughly transversely at A and B (star). At point A, the bundle runs over the axoneme (Ax), but under it at point B. Note that the long strands of mitochondria (M) lie on all sides of the axoneme, as in Fig. F inset, and have, thus, not yet formed the mitochondrial helix. The granular body (GB) is elongated, but remains on only one side of the distal centriole (Dc) (B), (C).
The beginning of angulations of the acrosome, and subsequent formation of the keels from them, has also been noted by other investigators in the Zebra finch,9 Common finch,35 house sparrow,41 and the Wren, Troglodytes troglodytes.71 It has been observed, in all reports, that the acrosomal angulations, at this stage, are convex, broad and smooth.
The nuclear, mitochondrial and microtubular changes seem to be quite rapid between steps 7 and 8 spermatids. A noteworthy feature of step 8 spermatid is the shortening of the nucleus. This is probably due to the condensation and transformation of the fine chromatin granules into thick irregularly-shaped and arranged strands of chromatin which are very evident in the nucleoplasm. The dumb-bell shape of the nucleus is probably and partly due to the further incursion made by the acrosome into the nuclear concavity at the proximal pole of the nucleus. This step of spermiogenesis is not illustrated or described in the common finch,35 house sparrow41 or in cirl bunting, Emberiza cirlus.77
Also noteworthy is the transformation of the sleeve of helically arranged microtubules, formed in step 5, into helical microtubular bundles, during this step of spermiogenesis. It is reasonable to expect that the microtubular sleeve would play a role in the developing spermatid, and that role appears to be that of a forerunner for the bundle of microtubular helix. This transformation from the sleeve to the bundle also appears to be quite rapid, and therefore the observation made during this process in the Masked Weaver, in which aspects or certain stages of the formation of the bundle was observed (Fig. 16C, D), is a fortuitous one. There are no previous reports on the rearrangement of the microtubular sleeve to form the microtubular bundle/helix or on the possible origin of this organelle in passerine birds. Sotelo and Trujillo-Cenóz22 think that the microtubular helix arises from a certain unnamed content of the cell, but which could possibly be the equivalent of the sleeve of helical microtubules found in young spermatids of the Masked Weaver. However, this process of formation of the microtubular helix, as described for the Masked weaver, is reminiscent of the rapid rearrangement or transformation of the circular manchette to form the longitudinal manchette in non-passerine birds, such as the turkey,42 African colored dove (Streptopellia roseogrisa),26 Rhea,48 and ostrich.40
Along with the strands of mitochondria and the forming microtubular helix, the granular body also begins to elongate prior to formation of the granular helix. This organelle will be discussed further, at the end of step 12 of spermiogenesis in the Masked Weaver. However, Fawcett et al.9 consider that both the microtubules and mitochondria develop into helices concurrently, although conceding that the microtubular helix provides the basis for the mitochondria to assume a helical course around the axoneme, with the same pitch as the microtubular bundle. Góes and Dolder41 are non-committal on the relative spatiotemporal development of these 2 helical organelles. Kondo et al.,35 in the common finch, observe that the microtubular helix develops prior to the mitochondrial helix, as has been observed in this study in the Masked Weaver. It is therefore not unreasonable to assume that the microtubular helix prescribes and describes the path for the mitochondrial helix as has been suggested by Fawcett et al.9
Step 9: The developing acrosome is considerably elongated (Fig. 17A) and surrounded by moderately electron-dense periacrosomal complexes (Fig. 17A, C, D) elaborated by the Sertoli cell whose cytoplasm contains an abundance of longitudinally arranged microtubules (Fig. 17A). The rostral part of the acrosomal crest is still much separated from the acrosomal core (Fig. 17A). The acrosome indents the anterior part of the nucleus more deeply, and, thus, forming a deep, circular concavity. The entire spermatid is greatly elongated, and its long axis forms a gentle curvature (Fig. 17A). A bundle of microtubules, the fibrous helix or microtubular helix, lies medial to the cell membrane, makes contact with, and straddles the junction between, the acrosome and the nucleus (Fig. 17A). The anterior end of this bundle forms a slight lateral projection, away from the acrosomal surface (Fig.17A, F, G). The microtubular helix begins at this projection, and runs caudally. Its nuclear portion ends at the caudal pole of the nucleus. At this time, the microtubular helix, although making a close contact with the nucleus, indents the latter only rather slightly (Fig. 17A, B).
Figure 17.
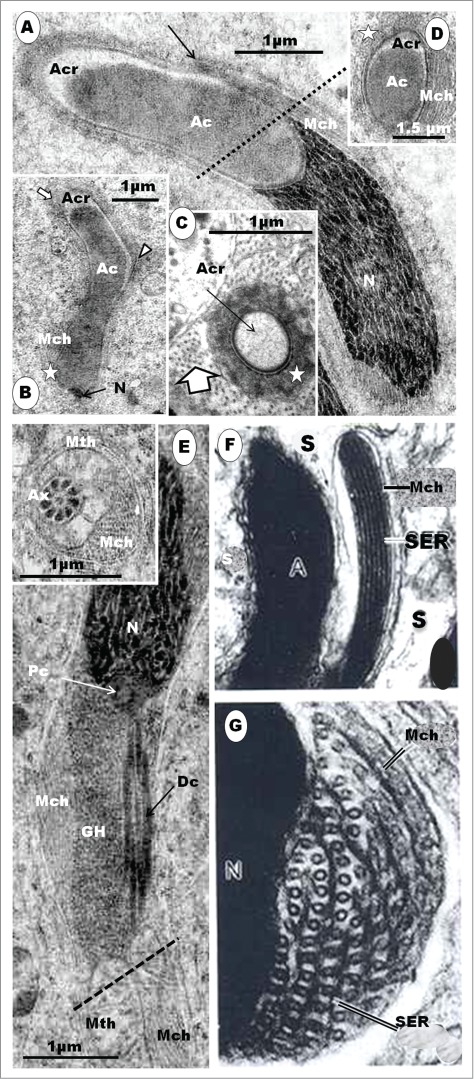
In (A), the step 9 spermatid displays an elongated acrosome exhibiting both the acrosomal crest (Acr) and core (Ac) components; Mch, microtubular helix. The acrosomal crest (Acr) is surrounded by an abundance of Sertoli cell microtubules (Fig. C, large arrow) as well as periacrosomal complexes (star in (C), (D)), and it indents the anterior end of the nucleus deeply (A). The angulations of the acrosomal crest which become the keels (A), (B) are forming. The nuclear chromatin forms deeply electron-dense, long, and helically oriented strands (A), (E). The microtubular helix (Mch) is fully developed, and its origin, just rostral to the acrosomo-nuclear junction, forms a slight anterior projection (arrow in (A)). This projection, as seen in the domestic lovebird, Lonchura striata (Kondo et al., 1988), is enlarged in (F), (G): an oblique section through the posterior region of the acrosome (A), showing the Sertoli cell process (arrow) protruding between the acrosome and the microtubular bundle (Mth), and a further enlarged section of the microtubule bundle (Mch) lying on the ridge of the nucleus (G). It displays alternating layers of microtubules and flattened cisternae of endoplasmic reticulum (SER). The mitochondria (E and inset) join to form a common strand that winds around the axoneme helically (Mth). At its origin, the mitochondrial helix (Mth) wraps itself around the axoneme (Ax) (inset: cut transversely at broken line in E). The granular body (GB) is still linearly elongated, on one side of the distal centriole (Dc), from the level of the proximal centriole (Pc) to the origin of the mitochondrial helix (E), and is deep to the microtubular helix (Mth). The granular body (Gb) remains linear and not helical (E). Figures F and G are taken and slightly modified from Kondo T, Hasegawa K, Uchida T. Formaton of the microtubule bundle and helical shaping of the spermatid in the common finch Lonchura-Striata-Var-Domestica. J Ultrastruct Molec Struct Res 1988; 98: 158-168. Figs.19 and 20. With the kind permission of Elsevier.
The nucleus becomes highly elongated with a reduced diameter, and its chromatin forms very dense filamentous strands of heterochromatin, running slightly helically along the long axis of this organelle (Fig. 17A, E). Posteriorly, the mid-piece attaches to the nucleus by means of the distal centriole, at the level of the last nuclear portion of the microtubular helix. The flagellum begins with the distal centriole, and may become gently wavy because of the microtubular helix which impinges on it. The granular body persists in a linear form, elongates further and occupies the space between the caudal pole of the nucleus and the beginning of the mitochondrial helix which has become fully formed (Fig. 17E). The microtubular helix winds laterally over the granular body, and, thus, indenting the latter, slightly (Fig. 17B). The tail is slimmer and both the microtubular as well as mitochondrial helices wind round the axoneme. The entire spermatid, at the end of this step, becomes quite slim, and the long granulofilamentous strands of heterochromatin become thicker, denser and run a slight helical course. At this phase, the microtubular helix indents the nucleus more markedly.
During the lifespan of the step 9 spermatid, the organelles and structures that are characteristic of the mature spermatozoon are well established, although require further refinements in later steps. The periacrosomal complexes around the developing acrosome as well as the rapid and profound elongation of both the acrosome and nucleus are noteworthy features. At this time, the microtubular helix has formed, and there is a concurrent abundance of microtubules in the Sertoli cell cytoplasm, a situation similar to that described in the Alpine swift (Apus melba) and Crested tinamou.55 Thus, the rapid progression of the nucleus and acrosome into the slender, attenuated, profile seen in the definitive spermatozoon begins during this step of spermiogenesis. Similar observations have been made in the Zebra finch,9 common finch,35 and house sparrow.41 The ‘step’ 4b spermatid of Góes and Dolder41 in the house sparrow is equivalent to step 9 of the Masked Weaver, showing dense filamentous strands of chromatin arranged helically along the long axis of the nucleus. It is clear that Góes and Dolder41 have combined steps 8 and 9 of the Masked Weaver, as they have done with certain other steps in their description of spermiogenesis in the house sparrow. In the Masked Weaver, during this step (9), the microtubular bundle or helix has developed fully, commencing at the acrosomo-nuclear junction where it straddles both the most posterior part of the acrosome and anterior part of the nucleus. It, thus, makes a flattened impression on them. This configuration has been described previously in the spermatids of the Zebra finch,9 common finch35 and house sparrow.41 The transformation of the single, long mitochondrial strand into a helix that winds around the axoneme throughout the length of the principal piece of the tail has previously been reported during spermiogenesis in the Zebra finch,9 common finch,35 house sparrow41 and the Wren, Troglodytes troglodytes,77 and has been described in the mature spermatozoa of several passerine birds (see Jamieson).55 At this spatiotemporal period, the granular body, although further elongated, has not yet formed a helix, unlike in the house sparrow41 and the Wren77 in which the organelle has already become helical in the spermatid that appears to be equivalent to step 9 in the Masked Weaver.
Step 10: The acrosome is assuming a more distinct helical or spiral shape, and the primordia of the keels become more obvious (Figs. 18B, C, D). Evidence of the presence of 3 keels, in longitudinal sections of the acrosome, appears alternately as convex hillocks or angulations surrounded by a condensation of periacrosomal complexes (Fig. 18B, D). The moderately electron-dense acrosomal core has nearly filled up the entire acrosome crest, leaving only a small crescentic electron-lucent part of the acrosomal crest (Figs. 18B, C, D). The caudal end of the acrosome occupies a deep concavity at the anterior end of the nucleus (Fig. 18B, D).
Figure 18.
Step 10 spermatids. The nucleus (N) assumes a helical shape, with indentations on the ridges by the microtubular helix (A, arrows), and the chromatin forms round granules that fill the nucleoplasm except at various foci where there are large or scattered, small, clear spaces (A, inset). The acrosome assumes a more helical shape, and displays convexities or protrusions which are the precursors of the keels (arrowheads in (B), (D)). Note that the acrosomal core (Ac) nearly fills completely the acrosomal crest (Acr) ((B), (D), and that microtubules in Sertoli cell cytoplasm (A, inset, arrowhead) as well as periacrosomal complexes remain evident (C), star). N, nucleus.
The nucleus also attains a more spiral or helical shape, and displays oval to round electron-dense chromatin granules (Fig. 18A, B). The microtubular helix continues caudally, from the acrosomo-nuclear junction, in a spiral manner, thus, occurring in longitudinal sections of the nucleus as bundles that indent convex surfaces of the nucleus, alternately (Fig. 18A, D). Five of such configurations occur, with the last at the most caudal part of the nucleus, at the level of the attachment of the tail/flagellum.
Further lengthening and angulations of the acrosome accompanies further nuclear chromatin condensation. At this time, the chromatin forms moderately large, round or oval granules that compactly fill the nucleus. The step 10 spermatid appears to last for a short period only in the spermatogenetic process. Fig. 18A and inset (T.S. section) show the round chromatin granules appropriately. The acrosomal core has not completely filled the acrosomal crest during this step. Similar observations on the differentiation of the acrosome have been made in the Zebra finch,9 common finch,35 house sparrow,41 and Wren.77 Besides, the angulations formed by the acrosomal crest are fuzzy, and are surrounded by moderately-dense periacrosomal complexes. Other than these, all other organelles have attained their definitive configurations, such that the elongated, linear, granular body of step 9 spermatid becomes helical in shape, winding round the midpiece, between the base of the nucleus and the commencement of the mitochondrial helix which has become fully established.
Step11: The acrosome has acquired the definitive spiral shape, with 3 gyres, but the 3 alternating acrosomal crest protrusions or keels, representing the 3 gyres, and a terminal spike or spur are still in the formative stage, requiring refinement and greater definition. Periacrosomal complexes continue to surround the developing keels, which maintain a fuzzy outline (Figs. 19A, B, C). At this step of spermiogenesis, the acrosomal core, which is of medium electron-density, fills the acrosomal crest completely (Fig. 19B, C). The nucleus assumes its definitive helical shape, and its chromatin has condensed nearly fully and homogenously. In longitudinal sections of spermatids at this level of spermiogenesis, 5 alternating convexities of the nucleus are seen, and the microtubular helix makes obvious impressions or indentations on them (Figs. 19A, B, F). The anterior end of the nucleus exhibits a concavity of varying depth and regularity, depending on the angle of section. Both the anterior and posterior poles of the nucleus make contact with the microtubular helix. The proximal centriole remains in position, articulating with the distal centriole at an acute angle, and in association with the granular helix (Fig. 18f). The microtubular helix (MH) makes contact with, and indents, the granular helix (GH) (Fig. 19D, F). In transverse sections of the spermatid, the microtubular helix is seen to lie external to the granular helix which, in turn, makes contact with, and winds around, the axoneme, for only a short distance, between the base of the nucleus and the mitochondrial helix (Figs. 19D, F). Most of the length, including the acrosome and nucleus, of the spermatid is fully embedded in the Sertoli cell. The midpiece is considerably elongated, and the axoneme appears slightly wavy due to the pressure of both the mitochondrial and microtubular helices (Fig. 19G).
Figure 19.
Step 11 spermatid. (A) is a survey micrograph of spermatids at step 11. The spermatid is fully embedded in Sertoli cells (Sc). The acrosome assumes a definitive shape, and the core (Ac) fills the crest portion, completely and homogenously. The keels (B), arrows; (C), arrowhead) are still fuzzy in outline and the acrosome is enclosed in periacrosomal complexes (C, star); Arrowhead in (B), terminal spur of the acrosome. The nucleus (N) is homogenously strongly electron-dense, and the microtubular helix (Mch) makes contact with it, at the ridges. The granular (Gh) and mitochondrial (Mth) helices are in place (A). A section of the spermatid tail along the line of the upper broken arrow (D) shows the relationship between the axoneme (Ax), and granular (Gh) and microtubular (Mch) helices. A section of the tail, along the line of the lower broken arrow (E) shows the relationship between axoneme, microtubular (MH) and mitochondrial (Mth) helices. (F) displays the posterior end of the nucleus and the anterior part of the midpiece. The shallow implantation fossa is obliquely slanted, and the proximal centriole (Pc) articulates with the distal centriole (Dc), anteriorly, and with the granular helix (Gh), posteriorly; the anterior surface of the proximal centriole is also attached to the base of the nucleus by short, wedge-shaped strands (arrowheads). (G) is part of the mid-piece of the tail, and demonstrates the wavy nature of the axoneme, and its relationship with the mitochondrial helix (Mth); the white arrow indicates the singlet microtubules of the axoneme.
This is the last step of spermiogenesis prior to full maturity of the spermatid, and its release, as a spermatozoon, from the seminiferous epithelium. The equivalent of this step is illustrated in Figures 19- 23 in the account on aspects of spermiogenesis in the common finch,35 although Fawcett et al.9 and Góes and Dolder41 do not identify this step in the Zebra finch and house sparrow, respectively. The fuzzy, periacrosomal complexes are much less dense in this step than in steps 9 and 10. The keels of the developing acrosome are indistinct, but are fully recognizable at this level of spermiogenesis. All other organelles are present as they are found in mature, released spermatozoa, except, of course, the microtubular helix whose presence or absence in mature spermatids, or in spermatozoa that are already free in the lumen of the seminiferous tubule or excurrent duct, appears to vary between species (vide infra). Although similar developmental features are to be found in birds that have been studied, Step 11 spermatid has, however, been specifically described in the Masked Weaver, but not in the House Sparrow,41 Zebra9,35 or various species of passerine birds that have been studied by Tripepi and Perrotta.77 During this step of spermiogenesis, only the acrosome is further refined, structurally.
Figure 23.
Ultrastructure of the spermatozoon of the Eurasian bullfinch, Pyrrhula pyrrhula. (A) is a scanning electron micrograph of the sperm head and anterior region of the flagellum, showing its spermatid-like appearance. Note the cap-like acrosome differing from the elongate helical acrosome of other passerines. (B): Corresponding longitudinal section (LS) by transmission electron microscopy (TEM) of the spermatozoon showing retention of the proximal centriole which is lost in most passerine sperm. (C): Transverse section (TS) of the midpiece showing a well-developed microtubular helix surrounding the mid-piece of the spermatozoon. All figures taken from: Birkhead TR, Giusti F, Immler S, Jamieson BGM. Ultrastructure of the unusual spermatozoon of the Eurasian bullfinch (Pyrrhula pyrrhula). Acta Zoologica (Stockholm) 2007; 88: 119–128. Figs. 2A and B, and Fig. 4A. With the kind permission of John Wiley & Sons Ltd.
Step 12: The spermatid is mature. The acrosome is fully formed, and the keels of the gyres are now sharply demarcated and project laterally from the main body of the acrosome (Fig. 20: main figure). In both transverse and longitudinal sections, the keels which develop from the acrosome crest appear solid, with no apparent contribution to its formation from the acrosome core (Fig. 20: main figure and top inset). The spermatid, during the process of spermiation, begins to move away from the core of the Sertoli cell which still attaches tenuously to the germ cell by means of slips or lateral extensions of cytoplasm. In several of these spermatids the microtubular helix has been shed, and found free and in the midst of residual bodies in the seminiferous epithelium (Fig. 20B and inset). A number of mature spermatids or spermatozoa retain the microtubular helix, but lose it along the length of the excurrent ducts of the testis, especially in the caudal part, and, in particular, in the seminal glomus where some of the microtubular bundles can be seen to float freely in the seminal fluid (Fig. 21B). Both proximal and distal centrioles are present, and continue to be aligned to each other at about 45° (Fig. 21D). The proximal centriole maintains its close association with the granular helix, which also retains its granular form. The acrosome in this spermatid, as in the free spermatozoon, remains unified in constitution, with no spatial separation between the acrosomal crest and core.
Figure 20.
Step 12 spermatid. The acrosomal end of the step 12 spermatid displays a well formed acrosome, and the spermatid is disengaging from the Sertoli cell (Sc), parts of which embrace the acrosome tenuously (A). The keels (white, short arrows) and terminal spur (white arrow) of the acrosome are sharp and distinct. Upper inset shows a high power view of the acrosomal wall: the core of the acrosome (star) is homogenously moderately electron-dense, and the keel (white arrow) is more electron-dense, and has no contribution from the core. Block arrow shows part of the nucleus. (B) exhibits residual bodies in the Sertoli cell, and the inset demonstrates the microtubular helices discarded between Sertoli cell cytoplasmic extensions, in the seminiferous epithelium.
Figure 21.
Parts of spermatozoa within the lumen of the ductus deferens (A), (B), (C), (D). (B) exhibits transverse sections of the axoneme (Ax), and mitochondrial helix (Mth) attached to the axoneme, and loose microtubular bundles (Mch) free in the seminal fluid, after being discarded by the spermatozoon. Several sections of spermatozoa, including a full spermatozoon obliquely represented in (C) display various parts, including the acrosome (Ac) and its numbered keels (2 to 4) and apical spur (1), nucleus (N), the centriolar complex (Dc) and granular helix (GH). The broad white arrow shows indentations of the ridges of the nucleus made by the microtubular helix; inset – clear areas in the central part of the nucleus (broad arrow). (A) is a high power view of part of the sperm acrosome which shows that the homogenous core is distinct and does not contribute to the formation of the more electron-dense keel. (D) is an enlarged part of the distal end of the nucleus, showing the proximal centriole (Pc) articulating with both the distal centriole (Dc) and the granular helix (Gh). Ax, axoneme.
General discussion on features of passerine spermiogenesis and spermatozoa
The mature spermatid, at the time of spermiation, is a highly elongated cell, and its relatively long acrosome, along with the flagellar elements, becomes spiral or helical in shape. The keels of the acrosome are, therefore, sharply demarcated from the Sertoli cell cytoplasm, which, in turn, loses the periacrosomal complexes. Only slips of Sertoli cell cytoplasm hold onto the spermatid, tenuously. The retention or loss of the microtubular helix is variable in the different species studied. Sotelo and Trujillo-Cenóz22 consider that the helix is retained in spermatozoa of the domestic sparrow, but it is said to disappear as the spermatid matures in the Zebra finch9 or while the mature spermatid is still embedded in the Sertoli in the common finch.35 However, Góes and Dolder41 have found spermiated spermatozoa which have retained the microtubular helix in the house sparrow, as has been observed in the Masked Weaver. Indeed, mature spermatozoa in the seminal glomus of the Masked Weaver have been seen to discard the microtubular helix that is found to float in the seminal fluid. Humphreys70 has made similar observations in the canary. Mature spermatozoa, still retaining the microtubular helix, are numerous in the proximal parts of the excurrent ducts of the testis, but not in the seminal glomus in the Masked Weaver (this report) or canary.72 The loss of the microtubular helix in passeridan birds therefore commences during spermiation in some, and is completed in other, spermatozoa within the excurrent duct system.
The main structural features of the spermatozoon of the Masked Weaver are generally similar to those that have been described for various species of passeridan birds. For example, the keeled, helical acrosome of the Masked Weaver is about as long as the nucleus (acrosome : nucleus ratio of 0.90 - 0.98), unlike in most suboscine and Corvida as well as certain passerida.72 The perforatorium and endonuclear canal are absent in passeridan birds, but are distinct morphological features of most non-passerine birds.26-28,39,49,55 However, Jamieson et al.72 suggest that the deep concavity at the anterior pole of the nucleus (anterior fossa) might be the homolog of the endonuclear canal that is well formed in several non-passerine birds. Although the base of the acrosome, comprising the acrosome core which is bounded externally by the acrosome crest, inserts into the nuclear fossa, there are, as at now, no other developmental or structural features to indicate that the base of the acrosome could be homologous to the perforatorium. The bipartite nature of the developing acrosome72 appears to be the norm in passerine birds, right from the development of the acrosomal vesicle (or even the proacrosome granule) in the Golgi complex. A clear separation between the crest and core components of the acrosome is evident until step 11 spermatid when the homogenous, electron-dense core segment completely fills the crest segment, no longer leaving any electron-lucent space between them. The keels are solid projections and are entirely products of the acrosomal crest. The core component of the acrosome is drawn out toward the keel in M. formicivora (Fig. 22)72 but not in Fringilla coelebs,133 Philetarius socius (Fig. 22)72 or in the Masked Weaver (this study). The acrosome core in the acrosomal vesicle in the step 4-spermatid is pyramidal in shape, surrounded on all sides except its attached base, by a clear area that is enveloped by the acrosomal crest. But as the crest (in step 5 spermatid) commences the process of elongation, so does the core. However, the crest elongates further than the core because the clear part of the crest increases in area and volume, relative to the core. It appears that the crest lays down the framework for acrosomal shape and, that at the completion of the helical shape of the acrosome, the core enlarges and fills up the crest. Thus, the clear halo that is first seen to separate the acrosomal core from the crest in both proacrosomal granules and acrosomal vesicles disappears. In the mature spermatozoon of the Masked Weaver, the core is therefore closely invested by the crest throughout the length and breadth of the acrosome.
Figure 22.
(A): Electron micrographs of a spermatozoa of Myrmecocichla formicivora. Longitudinal section of the spermatozoon: note that the acrosome (ac) surmounts the nucleus (n). The acrosome is bipartite, consisting of a long, electron-dense acrosome core (ac), which bears a prominent keel, and an outer, spirally angular acrosome crest (acr). The acrosome core fits into an oblique fossa at the tip of the nucleus (at the level of the acrosome-nucleus junction, anj). The lower half of the spermatozoon is a LS of the base of the nucleus and adjacent centriolar complex, midpiece and anterior axoneme. Note 2 components spiralled around the axoneme: a very elongate mitochondrion (mh) that proximally forms a continuous ring and a strongly electron-dense component here termed the fibrous helix (fh). Dense fibers are also seen encircling the axoneme (ax). acr, acrosome crest; ac, acrosome core; ak, acrosome keel; anj, acrosome–nucleus junction; ax, axoneme; dc, distal (only) centriole; df, dense fiber; dr, dense ring around centriole; fh, fibrous helix; m, mitochondrion; mh, mitochondrial helix; n, nucleus. Arrow shows the anterior extent of the acrosome core within the acrosome crest. (B) to (I), Philetairus socius. (B) Longitudinal section (LS) of acrosome and anterior nucleus, (C) LS acrosome crest and anterior acrosome core. (D) Transverse section (TS) of acrosome core through the helical keel, (E) Detail of acrosome crest and anterior acrosome core. (F) LS base of nucleus, and centriolar and anterior axonemal region surrounded by the granular helix preceding the mitochondrial helix, (G) TS axoneme and mitochondrial helix, showing 9 dense fibers associated with the axonemal doublets, (H) TS axoneme posterior to the mitochondrial helix, (I) TS posterior region of axoneme with reduced dense fibers . acr, acrosome crest; ac, acrosome core; ak, acrosome keel; anj, acrosomo-nuclear junction; ax, axoneme; dc, distal centriole; df, dense fiber; gh, granular helix; mh, mitochondrial helix; n, nucleus. All figures taken from: Jamieson BGM, Hodgson A, Spottiswoode CN. Ultrastructure of the spermatozoon of Myrmecocichla formicivora (Vieillot, 1881) and Philetairus socius (Latham, 1790) (Aves; Passeriformes), with a new interpretation of the passeridan acrosome. Acta Zoologica (Stockholm) 2006; 87: 297–304. Parts of Figs. 1 and 2. With the kind permission of John Wiley & Sons Ltd.
In the spermatozoon of the Southern anteater-chat (M. formicivora), the crest portion of the acrosome extends anteriorly, well beyond the core portion (Fig. 22). But in the social weaver (P. socius), the structure of the mature acrosome (Fig. 22) is closer to that of the Masked Weaver, although clear or electron-lucent areas may be seen between the crest and the core portions, in certain sections of the spermatozoa of the social weaver, possibly due to tangential angles of section.72 An obvious asymmetrical electron-lucent fossa receives the anterior tip of the acrosomal core in the social weaver. This fossa is absent in the Masked Weaver because the core fits fully and snugly into the crest, although it does not protrude into the solid keels formed by the crest portion of the acrosome. A notable departure from this general structure of passerine acrosome is to be found in the Eurasian bullfinch (Pyrrhula pyrrhula) in which this organelle (Fig. 23) is a thickly, concavo-convex, cap-like, structure overlying the blunt anterior part of the nucleus.73 This configuration is normally seen in acrosomal vesicles of early round spermatids of birds, especially in non-passerine birds (vide supra). Besides, the acrosome is, as is the nucleus, non-helical in the Eurasian bullfinch.73 A great deal more studies are obviously needed to expose the variety of acrosomal shapes and configurations that there may be among passerine birds.
Most of the length of the developing acrosome is not invested by the helical singlet microtubular sleeve or girdle that surrounds the nucleus and other organelles in the elongating spermatid. Only the most posterior part of the acrosome is consistently in contact with the microtubular helix, but much after the commencement of twisting of the acrosome in a helical manner. It is not known what mechanism brings about this configuration, but both the core and crest portions of the developing acrosome concurrently undergo the helical twists in the early- to mid- phase spermatids, before the core portion completely fills the crest part in steps 10 to 12. In spermatids, the developing acrosome is surrounded intimately by Sertoli cell periacrosomal complexes. Cooper and Bedford103 have described this formation in the musk shrew. The function of these complexes, is not clearly understood, but it is probably supportive and developmental because Tanii et al.150 and West and Willison151 have implicated extra-Golgi pathways in acrosome development, which process appears to continue until the spermatozoon is realeased. The complexes may also help to anchor the acrosome during the spiralization of the posterior portions of the spermatid. It is inconceivable that Sertoli cells do not play a role in this morphogenetic process, along with mechanisms that are intrinsic to the acrosome. The Sertoli cell cytoplasm is replete with microtubules that run along the long axis of the developing spermatid (see Fig. 17C), and could be involved in the helical twisting of the acrosome. It is, also, not unreasonable to speculate that the helical rotation of the nucleus similarly twists the acrosome along the common long axis of both parts of the cell which are closely connected, at the acrosomo-nuclear junction.
The nucleus is roughly cylindrical and helically-shaped, and its chromatin is homogenous and deeply electron-dense in most passeridan birds, except the Eurasian bullfinch, in which the chromatin is non-condensed and forms ‘a loose assemblage of small bundles or fascicles around a chromatin-less central region’.73 This pattern of chromatin disposition and configuration is a normal feature of a mid-phase spermatid, such as step 8 spermatids in the Masked Weaver. Although helically-shaped, as is the acrosome, the nucleus is not keeled in the passeridan birds studied, thus far. A morphogical feature of the nucleus in the spermatozoon is the persistence, even after the microtubular helix has been discarded, of the shallow impressions made on the convex surface of this organelle.9 In the Eurasian bullfinch, the nucleus is quite short and ellipsoidal in shape73 even though the microtubular helix develops in this passeridan bird.
Several organelles are to be found lying posterior to the nucleus. These include the centriolar complex, the granular helix (preceded by the globular granular body found in early spermatid differentiation), loosely aggregated mitochondria (in steps 5, 6 and 7 spermatids) and the mitochondrial helix in steps 9-12 spermatids as well as in mature spermatozoa.
Until recently,44,73 it was generally agreed that the proximal centriole was absent in spermatids and spermatozoa of the oscine clade of passerine birds.55,72 Nicander77 considers that passerine birds normally possess only one modified centriole. Humphreys70 would not say which centriole, proximal or distal, formed the long mid-piece of the flagellum of the house sparrow, and Asa and Phillips59 were silent on the proximal centriole. Recent reports on the ultrastructure of the spermatozoa of 2 passerine birds, Myrmecocichla formicivora (the Southern ant-eater chat) and Philetairus socius (the Social weaver) (Fig. 22), have failed to demonstrate the presence of the proximal centriole.72 However, Birkhead et al.73 are the first to demonstrate the proximal centriole (Fig. 23) in the spermatozoon of a passerine bird, the Eurasian bullfinch (Pyrrhula pyrrhula). The observation of the proximal centriole in both spermatids at all levels of development as well as in mature spermatozoa of the Masked Weaver (Ploceus velatus)44 is novel in a passeridan bird, and along with its demonstration in the spermatozoon of another passerine bird, the Eurasian bullfinch,73 it is strongly advocated that spermiogenesis and sperm structure be studied in many more species of passeridan birds in order to determine the status of the proximal centriole as an organelle in the vast number of passerine birds, constituting more than half of about 10,000 species of birds.55
In the Masked Weaver, the centriolar complex, and, in particular, the proximal centriole is seen to be closely associated or connected with the granular body or helix in both spermatids and spermatozoa, respectively. In spermatids, the granular body is globular in shape (steps1 to 7), but it progressively elongates and forms a short helix around the midpiece during the late developmental phases (steps 8 to 12) of spermatid morphogenesis. Tripepi and Perrotta,77 in their study of 13 species of passerine birds, observe that oscine spermatids, and probably spermatozoa, are of 2 types, depending on whether or not the granular body is present. In their brief report, focus is mainly on the granular body, whose morphogenesis is generally in accord with that of the Masked Weaver. However, although the granular body is present even in the step 1 spermatid of the Masked Weaver, Tripepi and Perrotta77 note the presence of this organelle, for the first time, during the commencement of the elongation phase and formation of the midpiece of several passerine birds, which phase is equivalent to our steps 5 and 6 in the Masked Weaver. Although the acrosome : nucleus ratio in the Masked Weaver is between 0.90 and 0.98, this bird possesses the granular body, in dissonance with the observations and postulates by Tripepi and Perrotta77 that those birds that have an acrosome : nucleus ratio of >1 (and spermatozoal length of >100μm) demonstrate the granular body which is absent in those birds with a ratio of <1 (and spermatozoal length of <100μm). In 8, except the white-eyed vireo (Vireo griseus), out of 9 passerine birds, Asa and Phillips59 observe that an unnamed electron-dense material surrounds the neck of the spermatozoon, immediately anterior to the mitochondrial helix. Humphreys70 also describes such an organelle in the spermatozoon of the canary (Serinus canaria), and Koehler75 observes a spiral granular mass in the anterior region of the midpiece of some passerine birds. In a recent report, Jamieson et al.72 have described the granular helix (Fig. 22) in the spermatozoon of the social weaver (Ploceus socius), but not in the spermatozoon of the southern anteater-chat (Myrmecocichla formicivora). Although the relationship between the granular body and the neck region and midpiece of the spermatozoon in the Eurasian bullfinch (Fig. 23) is not stated,73 nevertheless, it is present in a globular form, in which form it is usually present in early spermatids, unlike the helical form found in late phase spermatids as well as spermatozoa in the Masked Weaver (this report). The presence of the granular body or helix has been recognized in spermatids or spermatozoa of several other species of passerine birds,55,75 and house sparrow.22,41,70 The significance and function of this organelle is unknown, since sperm function is not known to be impaired in spermatozoa that lack it. The significance of the close relationship between the granular body and the centriolar complex, especially the proximal centriole, is also not known. But it has been observed that in early spermatids (steps 3 and 4) strands of smooth endoplasmic reticulum (SER) attach the granular body to the centriolar complex, and, from spermatid step 4 onwards, it is directly connected with the free end of the proximal centriole. It may be involved in the development of the centriolar complex, but may not be necessary for sperm function, even when present. Its main function, at this time, may be involvement in the determination of avian phylogeny, with special regard to passerine birds.
The formation of the microtubular helix commences in step 8 when the microtubular sleeve rapidly rearranges, to form bundles of microtubules, which are initially loose but subsequently form thick, compact bundles known as the fibrous helix by Jamieson et al. (2006). Contrary to the view that the microtubular helix is a permanent cell organelle that is involved in sperm motility,22 it is generally regarded as a transient feature of the passerine spermatozoon, it being discarded either during the process of spermiation or as the spermatozoon traverses the excurrent ducts.9,35,72,76,79 This organelle has been called by several names: tubuli of undulating membrane (Sotelo and Trujillo-Cenóz 1958), microtubule bundle.9,35,41,72 Fawcett et al.9,55 use the term microtubular helix, which term has been adopted in the study of spermiogenesis in the Masked Weaver and in this review, for the sake of consistency, since the single strand of spermatid mitochondria forms the mitochondrial helix.
The microtubular helix has been implicated in the helical re-structuring of the nucleus35.41 contrary to the strong views held by Fawcett et al.9 In the Masked Weaver, evidence of helical twisting of the nucleus is observed first in step 8 spermatid during which spatiotemporal period the helical sleeve of singlet microtubules begins to rearrange to form a loose bundle of helically arranged microtubules. This phenomenon is similar to that in some non-passerine birds, in which the CM rearranges to form the LM.26,40,42,48 Both passerine and non-passerine birds therefore have 2 sets of microtubular assemblage, albeit variably configured in both orders. Major differences in the presence of these organelles between the 2 orders, are the helical form in the passerine but longitudinal form in the non-passerine, and, of course, the persistence of the microtubular helix in the passerine until the mature sperm is formed. However, it is tempting to consider that the disposition of the helical sleeve (akin to the CM of non-passerine birds) and its successor, the microtubular helix (analogous to the LM of non-passerine), have, together, brought about the helical shape of the nucleus. However, this cannot be said for the helical nature of the acrosome which lacks a microtubular girdle during spermiogenesis. Pressures imposed upon the nucleus, along its long axis, by the possible rotation of microtubules of the helical sleeve and subsequently, the microtubular helix, may be responsible for its helical shape. Both types of microtubules need not be attached to the nucleus in order for their helical contractile activities to effectively twist the nucleus, and perhaps, the acrosome, along the long axis of the nucleus to which the latter is attached anteriorly. Direct pressure of the helical sleeve, and, in particular, of the microtubular helix on the spermatid nucleus, is not a plausible proposition, as has been amply argued against by Fawcett et al.9 The microtubular helix, for convincing and full effect, would best be situated in the grooves (concavities) rather than the ridges (convexities) of the spermatid nucleus in which they are situated in spermatozoa of all species studied, except the Eurasian bullfinch. Although the spermatozoon of the latter bird (see Fig. 23) possesses a microtubular helix, its nucleus is neither helical nor noticeably twisted.73 This appears to lend much support to the argument by Fawcett et al.9 that helical twisting of both the acrosome and nucleus of the spermatid in the Zebra finch, and most probably, also, in other passeridan birds, may be dependent on mechanisms that are intrinsic to the organelle rather than on extrinsic, microtubular activity only.
A third helical organelle, in addition to the granular and microtubular helices, found in passerine spermatids is the mitochondrial helix. Mitochondria in spermatids of the Masked Weaver are involved in 4 sets of movements and structural transformations. First, they translocate into the caudally migrating cytoplasm of the spermatid, from step 5, and secondly, they translocate, as a group, by moving anteriorly once again toward the caudal pole of the nucleus in step 7 spermatids. The third and most profound transformation is the elongation and end-to-end fusion of mitochondria, usually forming 4 parallel columns around the axoneme. The fourth phase is the formation of a single, highly elongated, strand of mitochondria, winding helically around the axoneme of spermatids from step 9. Although Fawcett et al.9 consider that the microtubular helix and mitochondrial helix develop concurrently it has been observed that the former becomes a helix/develops earlier than the latter in the Masked Weaver. We agree, however, with Fawcett et al.9 that the microtubular helix seems to have plotted and prescribed the same path for the mitochondrial helix to follow, so that both of them run together, at the same pitch. This is quite possible and plausible in spite of the fact that the mitochondrial helix is deep to the microtubular helix, even during spermiogenesis. There are a few discrepancies regarding mitochondrial morphogenesis during spermiogenesis, as referred to, above. However, in mature spermatids and spermatozoa, both the granular helix and mitochondrial helix are interposed between the axoneme and the microtubular helix in the Masked Weaver, as has been illustrated and described in the Zebra finch,9,55 Common finch,35 House sparrow,22,41 the Wren,77 and the Eurasian bullfinch (Fig. 23).73 The passeridan, M. formicivora, is the notable exception in which the microtubular helix intervenes between the axoneme and the mitochondrial helix.72 The loss of the microtubular helix, by an unknown mechanism, in mature spermatozoa prior to spermiation or while the spermatozoa are in the excurrent ducts of the testis, has been addressed, above.
Conclusions
This review on avian spermiogenesis sought to expose the state of available knowledge of this phenomenon in birds. Consequently, it is shown clearly that there is a profound dearth of knowledge of spermatogenesis, and with special regard to spermiogenesis, in birds which constitute more than 50% of all vertebrates55. Several hiatuses occur in the current, very limited, knowledge base, and therefore, with regard to birds at large, knowledge has not yielded to understanding in several particulars of avian spermiogenesis. This is not surprising because birds of the tropical and sub-tropical parts of the world have hardly been studied at all, as succinctly put by Deviche et al.,152 who observe that ‘large groups of birds, in particular those inhabiting tropical and equatorial regions, where most of the global diversity in avian species is present, remain essentially unstudied’. There appear to be no studies at all in all aspects of spermatogenesis in sub-oscine birds although such studies in oscine birds are, at best, remarkably and ridiculously few and fragmentary.
There are several aspects and processes in avian spermiogenesis that require basic information. Thus, there are controversies due to lack of clarity on morphogenetic processes, and variable interpretation of observations. Attention to, and further analyses of, acrosomogenesis, nuclear morphogenesis, microtubular assembly ‘systems’ and flagellar development are particularly needed. The recent finding of the proximal centriole in 2 passeridan birds,44,73 and the unusual and peculiar nuclear shape and composition in the Eurasian bullfinch73 call for focused analyses of spermiogenesis and sperm structure in a much wider range of species in not only passerine but also in non-passerine birds.
Jamieson et al.72 encapsulate the thinking and anxiety of ornithologists, with regard to spermiogenetic studies in birds, when they observe that because ‘molecular analyses have produced widely conflicting avian phylogenies, morphological characters assume special significance when testing the validity of different phylogenetic hypotheses’.
There is an increase in reproductive toxicological studies, involving environmental and other toxicants, in birds, and these investigations which are considered to be essential in animals such as birds that have unfettered access to a variety of habitats across most boundaries, are dependent upon adequate and unassailable information and data.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The co-operation and assistance of the Library of the St. George's University is gratefully acknowledged. My gratitude to the University of Ibadan, Nigeria and University of Pretoria, South Africa, where most of the work cited in the review was done.
References
- 1. Aire TA. Anatomy of the Male Reproductive Tract and organs. In: Jamieson BGM. (ed.), Reproductive Biology and Phylogeny of Birds, Vol. 1, Phylogeny, morphology, hormones and fertilization, New Hampshire: Science Publishers, Inc., 03479, USA; Plymouth, UK 2007; 37-114. [Google Scholar]
- 2. Jones RC, Lin M. Spermatogenesis in birds. In Milligan SR. (ed.), Oxford Reviews of Reproductive Biology. Oxford: Oxford University Pre, 1993; 15:233-64. [PubMed] [Google Scholar]
- 3. Aire TA. Spermatogenesis and Testicular Cycles. In: Jamieson BGM. (ed.), Reproductive Biology and Phylogeny of Birds, Vol. 1, Phylogeny, morphology, hormones and fertilization. New Hampshire 03479, USA; Plymouth, UK: Science Publishers, Inc, 2007; 279-348. [Google Scholar]
- 4. Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci 1952; 55:548-73; PMID:13139144; http://dx.doi.org/ 10.1111/j.1749-6632.1952.tb26576.x [DOI] [PubMed] [Google Scholar]
- 5. Leblond CP, Clermont Y. Spermiogenesis of rat, mouse, hamster and guinea pig as revealed by the “Periodic acid-fuchsin sulfurous acid” technique. Am J Anat 1952; 90:167-215; PMID:14923625; http://dx.doi.org/ 10.1002/aja.1000900202 [DOI] [PubMed] [Google Scholar]
- 6. Clermont Y, Leblond CP. Spermiogenesis in man, monkey, ram and other mammals as shown by the periodic acid Schiff technique. Am J Anat 1955; 96:229-50; PMID:14376352; http://dx.doi.org/ 10.1002/aja.1000960203 [DOI] [PubMed] [Google Scholar]
- 7. Fawcett DW, Phillips DM. The fine structure and development of the mammalian spermatozoon. Anat Rec 1969; 165:153-84; PMID:5387815; http://dx.doi.org/ 10.1002/ar.1091650204 [DOI] [PubMed] [Google Scholar]
- 8. Courot M, Hochereau-de Reviers M-T, Ortavant R. Spermatogenesis. In Johnson AD, Gomes WR, Vandemark NL. (eds). The Testis. Vol. I New York: Academic Press, 1970; 339-432. [Google Scholar]
- 9. Fawcett DW, Anderson WA, Phillips DM. Morphogenetic factors influencing the shape of the sperm head. Dev Biol 1971; 26:220-51; PMID:5168310; http://dx.doi.org/ 10.1016/0012-1606(71)90124-2 [DOI] [PubMed] [Google Scholar]
- 10. Yasuzumi G, Shiraiwa S, Yamamoto H. Spermatogenesis in animals as revealed by electron microscopy. Z Zellforsch Mikrosck Anat 1972; 125:497-505; PMID:4551791; http://dx.doi.org/ 10.1007/BF00306656 [DOI] [PubMed] [Google Scholar]
- 11. Phillips DM. Spermiogenesis. New York: Academic Press, 1974. [Google Scholar]
- 12. Holstein AF. Ultrastructural observations on the differentiation of spermatids in man. Andrologia 1976; 8:157-65; PMID:962174; http://dx.doi.org/ 10.1111/j.1439-0272.1976.tb02126.x [DOI] [PubMed] [Google Scholar]
- 13. Setchell BP. Spermatogenesis. In Finn CA. (ed.), The Mammalian Testis. Reproductive Biology Handbooks. London: Paul Elek Books Ltd., 1978; 181-232. [Google Scholar]
- 14. Ekstedt E, Söderquist L, Plöen L. Fine structure of spermatogenesis and Sertoli cells (Epitheliocytus sustentans) in the bull. Anat Histol Embryol 1986; 15:23-48; PMID:2939773; http://dx.doi.org/ 10.1111/j.1439-0264.1986.tb00529.x [DOI] [PubMed] [Google Scholar]
- 15. Plöen L, Courtens J-C. Comparative aspects of mammalian spermiogenesis. Scan Elect Microsc 1986; II:639-52; PMID:3099374 [PubMed] [Google Scholar]
- 16. Barth AD, Oko RJ. Abnormal morphology of bovine spermatozoa. Ames: Iowa State University Press, 1989; [Google Scholar]
- 17. Lin M, Harman A, Rodger JC. Spermiogenesis and spermiation in a marsupial, the tammar wallaby (Macropus eugenii). J Anat 1997; 190:377-95; PMID:9147224; http://dx.doi.org/ 10.1046/j.1469-7580.1997.19030377.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin M, Jones RC. Spermiogenesis and spermiation in a monotreme mammal, the platypus, Ornithorhynchus anatinus. J Anat 2000; 196:217-32; PMID:10739018; http://dx.doi.org/ 10.1046/j.1469-7580.2000.19620217.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lloyd S, Carrick F, Hall L. Unique features of spermiogenesis in the Musky Rat-kangaroo: reflection of a basal lineage or a distinct fertilization process? J Anat 2008; 212:257-74; PMID:18304206; http://dx.doi.org/ 10.1111/j.1469-7580.2008.00861.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma RK, Bhardwaj JK, Ahlawat N. Ultrastructural developments during spermiogenesis in goat (Capra hircus). Internl J Integr Biol 2009; 6:112-14 [Google Scholar]
- 21. Zlotnik I. The cytoplasmic components of germ-cells during spermatogenesis in the domestic fowl. Quart J Morphol Sci 1947; 88:353-65 [Google Scholar]
- 22. Sotelo JR, Trujillo-Cenóz O, Electron microscope study of the kinetic apparatus in animal sperm cells. Z Zellforsch Bd. 1958; 48(S):565-601; http://dx.doi.org/ 10.1007/BF00342732 [DOI] [PubMed] [Google Scholar]
- 23. Nagano T. Observations on the fine structure of developing spermatid in the domestic chicken. J Cell Biol 1962; 14:193-205; PMID:14477885; http://dx.doi.org/ 10.1083/jcb.14.2.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McIntosh JR, Porter KR. Microtubules in the spermatids of the domestic fowl. J Cell Biol 1967; 35:153-73; PMID:6061713; http://dx.doi.org/ 10.1083/jcb.35.1.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamamoto S, Tamate H, Itikawa O. Morphological studies on the sexual maturation in the male Japanese quail (Coturnix coturnix japonica). II. The germ cell types and cellular associations during spermatogenesis. Tohoku J Agric Res 1967; 18:27-37; [Google Scholar]
- 26. Mattei C, Mattei X, Manfredi J-L. Electron microscope study of the spermatogenesis of Streptopelia roseogrisea. J Submicr Cytol 1972; 4:57-73 [Google Scholar]
- 27. Tingari MD. Observations on the fine structure of spermatozoa in the testis and excurrent ducts of the male fowl, Gallus domesticus. J Reprod Fertil 1973; 34:255-65; PMID:4741694; http://dx.doi.org/ 10.1530/jrf.0.0340255 [DOI] [PubMed] [Google Scholar]
- 28. Humphreys PN. The differentiation of the acrosome in the spermatid of the budgerigar (Melopsittacus undulatus). Cell Tiss Res 1975; 156:411-16; http://dx.doi.org/ 10.1007/BF00225369 [DOI] [PubMed] [Google Scholar]
- 29. Okamura F, Nishiyama H. The early development of the tail and the differentiation of the shape of the nucleus of the spermatid of the domestic fowl, Gallus domesticus. Cell Tiss Res 1976; 169:345-59; http://dx.doi.org/ 10.1007/BF00219607 [DOI] [PubMed] [Google Scholar]
- 30. Gunawardana VK, Scott MGAD. Ultrastructural studies on the differentiation of spermatids in the domestic fowl. J Anat 1977; 124:741-55; PMID:604341 [PMC free article] [PubMed] [Google Scholar]
- 31. Marchand C-R, Gomot L, de Reviers M. Étude par autoradiographie et maaquage á la thymidine tritiee de la duree de la spermatogonèse du canard de barbarie (Cairina moschata L.). C R Soc Biol 1977; 21:927-31; PMID:145305 [PubMed] [Google Scholar]
- 32. Yasuzumi F, Yamaguchi S. Some aspects of Spermiogenesis in the domestic pigeon. Okijamas Fol Anat Jap 1977; 54:139-74; PMID:917459; http://dx.doi.org/ 10.2535/ofaj1936.54.2-3_139 [DOI] [PubMed] [Google Scholar]
- 33. Aire TA, Olowo-okorun MO, Ayeni JS. The seminiferous epithelium in the guinea fowl (Numida meleagris). Cell Tiss Res 1980; 205:319-25; PMID:7357577; http://dx.doi.org/ 10.1007/BF00234690 [DOI] [PubMed] [Google Scholar]
- 34. Baccetti B, Bigliardi E, Burrini AG. The morphogenesis of vertebrate perforatorium. J Ultrastr Res 1980; 71:272-87; PMID:6893210; http://dx.doi.org/ 10.1016/S0022-5320(80)90079-9 [DOI] [PubMed] [Google Scholar]
- 35. Kondo T, Hasegawa K, Uchida TA. Formation of the microtubule bundle and helical shaping of the spermatid in the common finch, Lonchura striata var. domestica. J Ultrastr Res 1988; 98:158-68; PMID:3373069; http://dx.doi.org/ 10.1016/S0889-1605(88)80908-X [DOI] [PubMed] [Google Scholar]
- 36. Sprando RL, Russel LD. Spermiogenesis in the red-eared turtle (Pseudemys scripta) and the domestic fowl (Gallus domesticus): a study of cytoplasmic events including cell volume changes and cytoplasmic elimination. J Morphol 1988; 198:95-118; PMID:3199450; http://dx.doi.org/ 10.1002/jmor.1051980110 [DOI] [PubMed] [Google Scholar]
- 37. Soley JT. A histological study of spermatogenesis in the ostrich (Struthio camelus). Ph.D. Thesis, Pretoria: University of Pretoria, 1992; 187. [Google Scholar]
- 38. Soley JT. Centriole development and formation of the flagellum during spermiogenesis in the ostrich (Struthio camelus). J Anat 1994; 185:301-13; PMID:7961137 [PMC free article] [PubMed] [Google Scholar]
- 39. Soley JT. Differentiation of the acrosomal complex in the ostrich (Struthio camelus) spermatids. J Morphol 1996; 227:101-11; http://dx.doi.org/ 10.1002/(SICI)1097-4687(199601)227 [DOI] [PubMed] [Google Scholar]
- 40. Soley JT. Nuclear morphogenesis and the role of the manchette during spermiogenesis in the ostrich (Struthio camelus). J Anat 1997; 190:563-76; PMID:9183679; http://dx.doi.org/ 10.1046/j.1469-7580.1997.19040563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Góes RM, Dolder H. Cytological steps during spermiogenesis in the house sparrow (Passer domesticus, Linnaeus). Tiss Cell 2002; 34:273-82; PMID:12176310; http://dx.doi.org/ 10.1016/S0040-8166(02)00017-4 [DOI] [PubMed] [Google Scholar]
- 42. Aire TA. Ultrastructural study of spermiogenesis in the turkey, Meleagris gallopavo. Brit Poult Sci 2003; 44:674-82; PMID:14965087; http://dx.doi.org/ 10.1080/00071660310001643651 [DOI] [PubMed] [Google Scholar]
- 43. Jamieson BMG, Tripepi R. Ultrastructure of the spermatozoon of Apus apus (Linnaeus 1758), the common swift (Aves: Apodiformes; Apodidae), with phylogenetic implications. Acta Zool 2005; 86:239-44; http://dx.doi.org/ 10.1111/j.1463-6395.2005.00204.x [DOI] [Google Scholar]
- 44. Aire TA, Ozegbe PC. Components and development of the centriolar complex during and beyond spermiogenesis in a passerine bird, the Masked Weaver (Ploceus velatus). Tiss Cell 2012; 44:63-67; PMID:22129754; http://dx.doi.org/ 10.1016/j.tice.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 45. Lovas EM, Filippich LJ, Johnston SD. Spermiogenesis in the Australian cockatiel Nymphicus hollandicus. J Morphol 2012; 273:1291-1305; PMID:22821829; http://dx.doi.org/ 10.1002/jmor.20060 [DOI] [PubMed] [Google Scholar]
- 46. du Plessis L. The morphology and development of normal and abnormal spermatozoa in emu, Dromaius novaehollandiae. Ph.D. thesis, University of Pretoria, Pretoria, South Africa, 2012; pp 172. [Google Scholar]
- 47. Xia L, Clermont Y, Lalli M, Buckland RB. Evolution of the endoplasmic reticulum during spermiogenesis of the rooster: an electron microscopic study. Am J Anat 1986; 177:301-12; PMID:3799487; http://dx.doi.org/ 10.1002/aja.1001770303 [DOI] [PubMed] [Google Scholar]
- 48. Phillips DM, Asa CS. Development of spermatozoa in the rhea. Anat Rec 1989; 223:276-82; PMID:2923278; http://dx.doi.org/ 10.1002/ar.1092230306 [DOI] [PubMed] [Google Scholar]
- 49. Lin M, Jones RC. Spermiogenesis and spermiation in the Japanese quail (Coturnix coturnix japonica). J Anat 1993; 183:525-35; PMID:8300429 [PMC free article] [PubMed] [Google Scholar]
- 50. Maretta M. Formation and role of the manchette microtubules in the poultry spermatids. Acta Vet Brno 1995; 64:23-29; http://dx.doi.org/ 10.2754/avb199564010023 [DOI] [Google Scholar]
- 51. Simões K, Orsi AM, Viegas KAS. Ultrastructural characteristics of spermiogenesis in the domestic duck (Anas platyrhynchos). Anat Histol Embryol 2005; 34:307-11; PMID:16159372; http://dx.doi.org/ 10.1111/j.1439-0264.2005.00620.x [DOI] [PubMed] [Google Scholar]
- 52. Tripepi S, Jamieson BGM, Brunelli E. Ultrastructure of the spermatid of Caprimulgus europaeus Linnaeus 1758, the European nightjar (Aves, Caprimulgidae), with phylogenetic implications. J Morph 2006; 267:1157-64; PMID:16804921; http://dx.doi.org/ 10.1002/jmor.10466 [DOI] [PubMed] [Google Scholar]
- 53. Humphreys PN. Brief observations on the semen and spermatozoa of certain passerine and non-passerine birds. J Reprod Fertil 1972; 29:327-36; PMID:4113685; http://dx.doi.org/ 10.1530/jrf.0.0290327 [DOI] [PubMed] [Google Scholar]
- 54. Roosen-Runge EC, Giesel LO. Quantitative studies on spermatogenesis in the albino rat. Am J Anat 1950; 87:1-30; PMID:14771006; http://dx.doi.org/ 10.1002/aja.1000870102 [DOI] [PubMed] [Google Scholar]
- 55. Jamieson BGM. Avian spermatozoa: structure and phylogeny. In: Jamieson BMG. (ed.) Reproductive Biology and Phylogeny of Birds Vol. 6A. Jersey: Science Publishers, 2007; 349-512. [Google Scholar]
- 56. Oko RJ. Developmental expression and possible role of perinuclear theca proteins of mammalian spermatozoa. In Seventh International Symposium on Spermatology: Plenary Papers. Reprod Fertil Dev 1995; 7:119-40; PMID:7569050; http://dx.doi.org/ 10.1071/RD9950777 [DOI] [PubMed] [Google Scholar]
- 57. Vieira GHC, Colli GR, Bao SN. The ultrastructure of the spermatozoon of the lizard Iguana iguana (Reptilia, Squamata, Iguanidae) and the variability of sperm morphology among iguanian lizards. J Anat 2000; 204:451-64; PMID:15198687; http://dx.doi.org/ 10.1111/j.0021-8782.2004.00300.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gribbins KM. Reptilian spermatogenesis. A histological and ultrastructural perspective. Spermatogenesis 2011; 1:250-69; PMID:22319673; http://dx.doi.org/ 10.4161/spmg.1.3.18092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Asa CS, Phillips DM. Ultrastructure of avian spermatozoa. In: Mohari H. (ed.) New Horizons in Sperm Cell Research. Tokyo: Japan Scientific Society Press, 1987; 365-73. [Google Scholar]
- 60. Aire TA, Soley JT. The guinea fowl centriolar complex: a morphological deviation for a non-passerine bird. Proc Microsc Soc South Africa 2003; 33:75 [Google Scholar]
- 61. Wooley DM. The structure of the spermatozoon of the Japanese quail, Coturnix coturnix L. var japonica. Acta Zool (Copenhagen) 1995; 76:45-50; http://dx.doi.org/ 10.1111/j.1463-6395.1995.tb00981.x [DOI] [Google Scholar]
- 62. Nicander L, Helstrom B. Increased thickness of the inner mitochondrial membrane during sperm maturation in the domestic rooster. Exptl Cell Res 1967; 48:622-24; http://dx.doi.org/ 10.1016/0014-4827(67)90329-1 [DOI] [Google Scholar]
- 63. Lake PE, Smith W, Young D. The ultrastructure of the ejaculated fowl spermatozoa. Q J Exptl Physiol 1968; 53:356-66; PMID:5188427 [DOI] [PubMed] [Google Scholar]
- 64. Thurston RJ, Hess RA. Ultrastructure of spermatozoa from domesticated birds; comparative study of turkey, chicken and guinea fowl. Scann Microsc 1987; 1:1829-1938; PMID:3433064 [PubMed] [Google Scholar]
- 65. Jamieson BGM. Spermatozoal phylogeny of the Vertebrata. In Gagnon C. (ed.) The male gamete. From basic science to clinical applications. Cache Vienna: River Press, USA; 1999; 303-31. [Google Scholar]
- 66. Asa CS, Phillips DM, Stover J. Ultrastructure of spermatozoa of the crested tinamou. J Ultrastr Mol Struct Res 1986; 94:170-5; http://dx.doi.org/ 10.1016/0889-1605(86)90063-7 [DOI] [Google Scholar]
- 67. Soley JT. Ultrastructure of ostrich (Struthio camelus) spermatozoa: I. transmission electron microscopy. Onderstepoort J Vet Res 1993; 60:119-130; PMID:8332322 [PubMed] [Google Scholar]
- 68. Maretta M. The ultrastructure of the spermatozoon of the drake. II. Tail. Acta Vet Acad Sci Hung 1975; 25:53-60; PMID:1181910 [PubMed] [Google Scholar]
- 69. Feduccia A. Comments on the phylogeny of perching birds. Proc Biol Soc Wash 1979; 92:689-96; [Google Scholar]
- 70. Humphreys PN. Brief observations on the semen and spermatozoa of certain passerine and non-passerine birds. J Reprod Fert 1972; 29:327-36; PMID:4113685; http://dx.doi.org/ 10.1530/jrf.0.0290327 [DOI] [PubMed] [Google Scholar]
- 71. Henley C, Feduccia A, Costello DP. Oscine spermatozoa: a light and electron-microscopy study. Condor 1978; 80, 41-48; http://dx.doi.org/ 10.2307/1367789 [DOI] [Google Scholar]
- 72. Jamieson BMG, Hodgson A, Spottiswoode CN. Ultrastructure of the spermatozoon of Myrmecocichla formicivora (Vieillot, 1881) and Philetarius socius (Latham, 1790) (Aves; Passeriformes), with a new interpretation of the passeridan acrosome. Acta Zool 2006; 87:297-304; http://dx.doi.org/ 10.1111/j.1463-6395.2006.00242.x [DOI] [Google Scholar]
- 73. Birkhead TR, Giusti F, Immler S, Jamieson BGM. Ultrastructure of the unusual spermatozoon of the Eurasian bullfinch (Pyrrhula pyrrhula). Acta Zool. (Stockholm) 2007; 88, 119-128; http://dx.doi.org/ 10.1111/j.1463-6395.2007.00259.x [DOI] [Google Scholar]
- 74. Birkhead TR, Immler S, Pellatt EJ, Freckleton R. Unusual sperm morphology in the Northern bullfinch (Pyrrhula pyrrhula). Auk 2006; 383-92; http://dx.doi.org/ 10.1642/0004-8038(2006)123 [DOI] [Google Scholar]
- 75. Koehler LD. Diversity of avian spermatozoa ultrastructure with emphasis on the members of the order Passeriformes. Mémoires du Muséum National d’Histoire Naturelle, Paris 1995; 166, 437-44 [Google Scholar]
- 76. Nicander L. Comparative studies on the fine structure of vertebrate spermatozoa. In Baccetti B. (ed.), Comparative Spermatology. New York: Academic Press, 1970; 47-55. [Google Scholar]
- 77. Tripepi S, Perotta E. Spermiogenesis and Sperm of Passerine Birds. In: Bacetti B. New York: Raven Press, 1991; 1021-23. [Google Scholar]
- 78. McFarlane RW. The taxonomic significance of avian sperm. In Sibley GC. (ed.), Proceedings of the XIII International Ornithological Congress Ithaca, New York: American Ornithologists’ Union, 1963; 91-102. [Google Scholar]
- 79. Chowdhury AK, Marshall G. Irregular pattern of spermatogenesis in the baboon (Papio anubis) and its possible mechanism. In Steinberger AE, Steinberger E. (eds.), Testicular Development, Structure and Function. New York: Raven Press, 1980; 129-37. [Google Scholar]
- 80. Schulze W. Evidence of a wave of spermatogenesis in human testis. Andrologia 1982; 14:200-7; PMID:7103139; http://dx.doi.org/ 10.1111/j.1439-0272.1982.tb03124.x [DOI] [PubMed] [Google Scholar]
- 81. Schulze W, Riemer M, Rehder U, Höhne K -H. Computer-aided three dimensional reconstructions of the arrangement of primary spermatocytes in human seminiferous tubules. Cell Tiss Res 1986; 244:1-8; http://dx.doi.org/ 10.1007/BF00218375 [DOI] [PubMed] [Google Scholar]
- 82. Dietrich T, Schulze W, Riemer M. Untersuchung zur gliederung des keimepithels beim javaneraffen (Macaca cynomolgus) mittels digitaler bildverarbeitung. Urologe, Ausgabe A. Zeitschrift Klim Prakt Urol 1986; 25:179-86; PMID:3739108 [PubMed] [Google Scholar]
- 83. Berruti G, Paiardi C. Acrosome biogenesis. Spermatogenesis. 2011; 1:95-98; PMID:22319656; http://dx.doi.org/ 10.4161/spmg.1.2.16820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Burgos MH, Fawcett DW. An electron microscope study of spermatid differentiation in the toad, Bufo arenarum Hensel. J Biophys Biochem Cytol 1955; 2:223-40; PMID:13331956; http://dx.doi.org/ 10.1083/jcb.2.3.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Burgos MH, Vitale-Calpe R, Aoki A. Fine structure of the testis and its functional significance. In Johnson AD, Gomes WR, Vandermark NL. (eds.), The Testis. Development, Anatomy and Physiology. Vol. 1 London UK: Academic Press, 1970; 552-649. [Google Scholar]
- 86. Kristić RV. Illustrated Encyclopedia of Human Histology. New York: Springer-Verlag, 1984. [Google Scholar]
- 87. de Reviers M. Le dévelopment testiculaire chez le coq. II. morphologie de l’ épithélium et établissement de la spermatogenése. Ann Biol Anim Biochim Biophys 1971; 11:531-46; http://dx.doi.org/ 10.1051/rnd:19710402 [DOI] [Google Scholar]
- 88. Tripepi S, Jamieson BGM, Brunelli E. Ultrastructure of the spermatid of Caprimulgus europaeus Linnaeus 1758, the European nightjar (Aves; Caprimulgidae). J Morph 2006; 267, 1157-64; PMID:16804921; http://dx.doi.org/ 10.1002/jmor.10466 [DOI] [PubMed] [Google Scholar]
- 89. Saita A, Tripepi S, Longo OM. Comparative observations on spermiogenesis. II. Nuclear shaping in the absence of a microtubular manchette in the spermatids of the bird Crotophaga ani, (Cuculiformes). Boll Zool 1982; 49:115-23; http://dx.doi.org/ 10.1080/11250008209439379 [DOI] [Google Scholar]
- 90. Saita A, Tripepi S, Longo OM. Osservazioni coparative sulla spermiogenesi. I. modificazioni ultrastrutturali nella spermiogenesi di Coturnix coturnix L. Accad Naz Lincei 1980; 69:209-22; [Google Scholar]
- 91. Del Conte E. The subacrosomal granule and its evolution during spermiogenesis in a lizard. Observations about the acrosomal fringe and the spermatid-Sertoli cell relationship. Cell Tiss Res 1976; 171:483-98; PMID:975226 [DOI] [PubMed] [Google Scholar]
- 92. Gupta BL. Spermatogenesis of the Domestic Duck with Observation on the Living Material Under the Phase-Contrast Microscope. Research Bulletin of the Panjab University; 1955; 77:131-40. [Google Scholar]
- 93. Tripepi S, Tavolaro P, Rossi F. The evolution of microtubular organization during spermiogenesis in birds. In Ghiara G. (ed), Selected Symposia and Monographs U. Z. I, 4, Symp Evol Terrest Vert. Modena: Mucchi, 1991; 631-36. [Google Scholar]
- 94. Jamieson BGM, Tripepi R. Ultrastructure of the spermatozoon of Apus apus (Linnaeus 1758), the common swift (Aves: apodiformes; Apodidae). Acta Zool 86:239-44; http://dx.doi.org/ 10.1111/j.1463-6395.2005.00204.x [DOI] [Google Scholar]
- 95. MacKinnon EA, Abraham PJ, Svatek A. Long link induction between the microtubules of the manchette in intermediate stages of spermiogenesis. Z Zellforsch Mikrosk Anat 1973; 136:447; PMID:4685832; http://dx.doi.org/ 10.1007/BF00307363 [DOI] [PubMed] [Google Scholar]
- 96. Irons MJ, Clermont Y. Formation of the outer dense fibers during spermiogenesis in the rat. Anat Rec 1982; 202:463-71; PMID:7200337; http://dx.doi.org/ 10.1002/ar.1092020405 [DOI] [PubMed] [Google Scholar]
- 97. Irons MJ, Clermont Y. Kinetics of fibrous sheath formation in the rat spermatid. Am J Anat 1982; 165:121-30; PMID:6890760; http://dx.doi.org/ 10.1002/aja.1001650204 [DOI] [PubMed] [Google Scholar]
- 98. Clermont Y, Oko R, Hermo L. Immunocytochemical localization of proteins utilized in the formation of outer dense fibers and fibrous sheath in rat spermatids: an electron microscope study. Anat Rec 1990; 227:447-57; PMID:2393097; http://dx.doi.org/ 10.1002/ar.1092270408 [DOI] [PubMed] [Google Scholar]
- 99. Gordon M. The distal centriole in guinea pig spermiogenesis. J Ultrastr Res 1972; 39:364-88; PMID:4112748; http://dx.doi.org/ 10.1016/S0022-5320(72)90029-9 [DOI] [PubMed] [Google Scholar]
- 100. Fawcett DW. The mammalian spermatozoon. Dev Biol 1975; 44:394-436; PMID:805734; http://dx.doi.org/ 10.1016/0012-1606(75)90411-X [DOI] [PubMed] [Google Scholar]
- 101. Thurston RJ, Hess RA, Hughes BL, Froman DP. Ultrastructure of the guinea fowl (Numida meleagris) spermatozoon. Poult Sci 1982; 61:1738-43; PMID:7134129; http://dx.doi.org/ 10.3382/ps.0611738 [DOI] [PubMed] [Google Scholar]
- 102. Afzelius BA. Sperm structure in relation to phylogeny in the lower Metazoa. In Fawcett DW, Bedford JM. (eds.), The Spermatozoon: Maturation, Motility, Surface Properties and Comparative Aspects. Urban and; Baltimore: Schwarzenberg, 1979; 243-51. [Google Scholar]
- 103. Cooper GW, Bedford JM. Asymmetry of spermiation and sperm surface charge patterns over the giant acrosome in the musk shrew Suncus murinus. J Cell Biol 1976; 69:415-28; PMID:1262397; http://dx.doi.org/ 10.1083/jcb.69.2.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sud BN. The ‘chromatoid body’ in spermatogenesis. Quart J Microsc Sci 1961; 102:273-92; [Google Scholar]
- 105. Fawcett DW, Leak LV, Heideger PM. Electron-microscopic observation on the structural components of the blood-testis barrier. J Reprod Fert, Supplement 1970; 10:105-22; PMID:4951168 [PubMed] [Google Scholar]
- 106. Susi FR, Clermont Y. Fine structural modifications of the rat chromatoid body during spermiogenesis. Am J Anat 1970; 129:177-92; PMID:4097414; http://dx.doi.org/ 10.1002/aja.1001290205 [DOI] [PubMed] [Google Scholar]
- 107. Fawcett DW. Observations on cell differentiation and organelle continuity in spermatogenesis. Proc Int symp Genetics of the Spermatozoon 1971; 37-68; [Google Scholar]
- 108. Bawa SR. Comparative studies on the origin of the chromatoid body. In Duckett JG. Racey RA. (eds.), The Biology of the Male Gamete. London: Academic Press, 1975; 275-78. [Google Scholar]
- 109. Söderstrom K-O. Formation of chromatoid body during rat spermatogenesis. Z Mikrosk-anat Forsch 1978; 92:417-30; PMID:751333 [PubMed] [Google Scholar]
- 110. Thorne-Tjömsland G, Clermont Y, Hermo L. Contribution of the Golgi apparatus components to the formation of the acrosomic system and chromatoid body in the rat spermatids. Anat Rec 1988; 221:591-98; PMID:2843065; http://dx.doi.org/ 10.1002/ar.1092210205 [DOI] [PubMed] [Google Scholar]
- 111. Eddy EM. Cytochemical observations on the chromatoid body of the male germ cell. Biol Reprod 1970; 2:114-20; PMID:4106273; http://dx.doi.org/ 10.1095/biolreprod2.1.114 [DOI] [PubMed] [Google Scholar]
- 112. Paniagua L, Nistal M, Amat P, Rodriguez MC. Ultrastructural observations on nucleoli and related structures during human spermatogenesis. Anat Embryol 1986; 174:301-6; PMID:3766986; http://dx.doi.org/ 10.1007/BF00698780 [DOI] [PubMed] [Google Scholar]
- 113. Ploën L. Scheme of rabbit spermateliosis based upon electron microscopic observations. Z Mikrosk-anat Forsch 1971; 11:553-64; PMID:5574598 [DOI] [PubMed] [Google Scholar]
- 114. Holstein AF, Roosen-Runge EC. Atlas of Human Spermatogenesis. Berlin: Grosse Verlag, 1981. [Google Scholar]
- 115. du Plessis L, Soley JT. A novel transient structure with phylogenetic implications found in ratite spermatids. BMC Evol Biol 2013; 13:104; PMID:23705947; http://dx.doi.org/ 10.1186/1471-2148-13-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Saita A, Comazzi M, Perrotta E: Electron microscope study of spermiogenesis in Caiman crocodylus L. Ital J Zool 1987; 4:307-18; [Google Scholar]
- 117. Da Cruz-Landim C, Da Cruz-Höfling MA. Electron microscope study of lizard spermiogenesis in Tropidurus torquatus (Lacertilia). Caryology 1977; 30:151-62; http://dx.doi.org/ 10.1080/00087114.1977.10796688 [DOI] [Google Scholar]
- 118. Da Cruz-Landim MA, Da Cruz-Landim C. The fine structure of nuclei during spermiogenesis in the lizard Tropidurus torquatus (Lacertilia). Cytologia 1978; 43:61-68; http://dx.doi.org/ 10.1508/cytologia.43.61 [DOI] [Google Scholar]
- 119. Butler RD, Gabri MS. Structure and development of the sperm head in the lizard Podarcis (=Lacerta) taurica. J Ultrastr Res 1984; 88:261-74; http://dx.doi.org/ 10.1016/S0022-5320(84)90124-2 [DOI] [Google Scholar]
- 120. Vieira GHC, Wiederhecker HC, Colli GR, Bao SN. Spermiogenesis and testicular cycle of the lizard Tropidurus torquatus (Squamata, Tropiduridae) in the Cerrado of central Brazil. Amphibia-Reptilia 2001; 22:217-23; http://dx.doi.org/ 10.1163/15685380152030445 [DOI] [Google Scholar]
- 121. Ferreira A, Dolder H. Sperm ultrastructure and spermiogenensis in the lizard, Tropidurus itambere. Biol Cell 2003; 353-62; PMID:15002752 [PubMed] [Google Scholar]
- 122. Janke A, Arnason U. The complete mitochondrial genome of Alligator mississippiensis and the separation between recent Archosauria (birds and crocodiles). Mol Biol Evol 1997; 14:1266-72; PMID:9402737; http://dx.doi.org/ 10.1093/oxfordjournals.molbev.a025736 [DOI] [PubMed] [Google Scholar]
- 123. Walker AD. New light on the origin of birds and crocodiles. Nature 1972; 237:257-66; http://dx.doi.org/ 10.1038/237257a0 [DOI] [Google Scholar]
- 124. Whetstone KN, Martin LD: New look at the origin of birds and crocodile. Nature 1979; 279:234-35; PMID:440432; http://dx.doi.org/ 10.1038/279234a0 [DOI] [PubMed] [Google Scholar]
- 125. Rest JS, Ast JC, Austin CC, Waddell PJ, Tibbetts EA, Hay JM, Mindell DP. Molecular systematics of primary reptilian lineages and the tuatara mitochondrial genome. Mol Phylogenet Evol 2003; 29:289-97; PMID:13678684; http://dx.doi.org/ 10.1016/S1055-7903(03)00108-8 [DOI] [PubMed] [Google Scholar]
- 126. Fraser RDB, Parry DAD. The structural basis of the filament-matrix texture in the avian/reptilian group of hard β-keratins. J Struct Biol 2011; 173:391-405; PMID:20869443; http://dx.doi.org/ 10.1016/j.jsb.2010.09.020 [DOI] [PubMed] [Google Scholar]
- 127. Baccetti B. The evolution of the acrosomal complex. In Fawcett DW, Bedford JM. (eds.), The Spermatozoon. Baltimore-Munich: Urban and Schwarzenberg, 1979; 305-29. [Google Scholar]
- 128. Campanella CG, Gabbiani G, Baccetti B, Burrini AG, Pallini V. Actin and myosin in the vertebrate acrosome region. J Submicrosc Cytol 1979; 11:53-71; [Google Scholar]
- 129. Lake PE, Smith W, Young D. The ultrastructure of the ejaculated fowl spermatozoon. Quart J Exptl Physiol 1968; 53:356-66; PMID:5188427 [DOI] [PubMed] [Google Scholar]
- 130. Bakst MR, Howarth B, Jr. The head, neck and midpiece of the cock spermatozoa examined with the transmission of electron microscope. Biol Reprod 1975; 12:632-40; PMID:1220830; http://dx.doi.org/ 10.1095/biolreprod12.5.632 [DOI] [PubMed] [Google Scholar]
- 131. Baccetti B, Burrini AG, Falchetti E. Spermatozoa and relationships in paleognath birds. Biol Cell (Paris) 1991; 71:209-16; PMID:1912945; http://dx.doi.org/ 10.1016/0248-4900(91)90067-W [DOI] [PubMed] [Google Scholar]
- 132. Furieri P. Caratteri ultrastrutturali di spermi flagellati di Anfibi e Ucelli. Studio al microscopio elettronico. Arch Zool Ital 1961; 46:123-47; [Google Scholar]
- 133. Furieri P. Prime osservazioni al microscopio elettronico sull’ultrastruttura degli spermatozoi di Fringilla coelebs L. Boll Soc Ital Biol Sper 1962; 38:29-31; PMID:13895938 [PubMed] [Google Scholar]
- 134. Jamieson BGM. Fish Evolution and Systematics: Evidence from Spermatozoa. Cambridge, U K: Cambridge University Press, 1991; 319. [Google Scholar]
- 135. Jamieson BGM, Koehler L, Todd BJ. Spermatozoal ultrastructure in three species of parrots (Aves, Psittaciformes) and its implications. Anat Rec 1995; 241:461-68; PMID:7604961; http://dx.doi.org/ 10.1002/ar.1092410404 [DOI] [PubMed] [Google Scholar]
- 136. Phillips DM, Asa CS, Stover J. Ultrastructure of spermatozoa if the White-naped crane. J Submicroscop Cytol 1987; 19:489-94; PMID:3302284 [PubMed] [Google Scholar]
- 137. Phillips DM. Development of spermatozoa in the woolly opossum with special reference to the shaping of the sperm head. J Ultrastruct Res 1989; 33:369-80; PMID:5494322; http://dx.doi.org/ 10.1016/S0022-5320(70)90029-8 [DOI] [PubMed] [Google Scholar]
- 138. Phillips DM. Nuclear shaping during spermiogenesis in the ship scorpion. J Ultrastruct Res 1976; 54:397-405; PMID:943568; http://dx.doi.org/ 10.1016/S0022-5320(76)80025-1 [DOI] [PubMed] [Google Scholar]
- 139. Myles DG, Hepler PK. Shaping of the sperm nucleus in Marsilea: distinction between factors responsible for shape generation and shape determination. Dev Biol 1982; 90:238-52; PMID:7200435; http://dx.doi.org/ 10.1016/0012-1606(82)90373-6 [DOI] [PubMed] [Google Scholar]
- 140. Russel LD, Russel JA, MacGregor GR, Meistrich ML. Linkage of manchette microtubules to the nuclear envelope and observations of the role of the manchette in nuclear shaping during spermiogenesis in rodents. Am J Anat 1991; 192:97-120; PMID:1759685; http://dx.doi.org/ 10.1002/aja.1001920202 [DOI] [PubMed] [Google Scholar]
- 141. Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 3: developmental changes in spermatid flagellum and cytoplasmic droplet and interaction of sperm with the zona pellucida and egg plasma membrane. Mirosc. Res Tech 2010; 73:320-63; PMID:19941287; http://dx.doi.org/ 10.1002/jemt.20783 [DOI] [PubMed] [Google Scholar]
- 142. Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 1985; 42:39-50; PMID:3926325; http://dx.doi.org/ 10.1016/S0092-8674(85)80099-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Cole A, Meistrich ML, Cherry LM, Trostle-Weige PK. Nuclear and manchette development in spermatids of normal and azh/azh mutant mice. Biol Reprod 1988; 38:385-401; PMID:3282554; http://dx.doi.org/ 10.1095/biolreprod38.2.385 [DOI] [PubMed] [Google Scholar]
- 144. Kierszenbaum AL. Intramanchette transport (IMT): managing the making of the spermatid head, centrosome, and tail. Mol Reprod Dev 2002; 63:1-4; PMID:12211054; http://dx.doi.org/ 10.1002/mrd.10179 [DOI] [PubMed] [Google Scholar]
- 145. Kierszenbaum AL, Gil M, Rivkin E, Tres LL. Ran, a GTP-binding protein involved in nucleocytoplasmic transport and microtubule nucleation, relocates from the manchette to the centrosome region during rat spermiogenesis. Mol Reprod Dev 2002; 63:131-40; PMID:12211070; http://dx.doi.org/ 10.1002/mrd.10164 [DOI] [PubMed] [Google Scholar]
- 146. Kierszenbaum AL, Tres LL. The acrosome-acroplaxone-manchette complex and the shaping of the spermatid head. Arch Histol Cytol 2004; 67:271-84; PMID:15700535; http://dx.doi.org/ 10.1679/aohc.67.271 [DOI] [PubMed] [Google Scholar]
- 147. Aire TA, Soley JT. The surface features of the epithelial lining of the epididymis of the ostrich. Anat Histol Embryol 2000; 29:119-26; PMID:10932389; http://dx.doi.org/ 10.1046/j.1439-0264.2000.00247.x [DOI] [PubMed] [Google Scholar]
- 148. Roosen-Runge EC. Kinetics of spermatogenesis in mammals. Annals New York Acad Sci 1952; 55:574-84; PMID:13139145; http://dx.doi.org/ 10.1111/j.1749-6632.1952.tb26577.x [DOI] [PubMed] [Google Scholar]
- 149. Clermont Y. The cycle of the seminiferous epithelium in man. Am J Anat 1963; 112:35-51; PMID:14021715; http://dx.doi.org/ 10.1002/aja.1001120103 [DOI] [PubMed] [Google Scholar]
- 150. Tanii I, Toshimori K, Akari S, Oura C. Extra-Golgi pathway of an acrosomal antigen during spermiogenesis in the rat. Cell Tiss Res 1992; 270:451-57; PMID:1486599; http://dx.doi.org/ 10.1007/BF00645046 [DOI] [PubMed] [Google Scholar]
- 151. West AP, Willison KR. Brefeldin A and mannose 6-phosphate regulation of acrosomic related vesicular trafficking. Eur J Cell Biol 1996; 70:315-21; PMID:8864659 [PubMed] [Google Scholar]
- 152. Deviche P, Hurley LL, Fokidis HB. Hormones and reproduction of vertebrates. In Avian testicular structure, function, and regulation, Vol. 4d, Birds. Elsevier Inc, 2011; 27-70. [Google Scholar]