Abstract
The blood-epididymis barrier (BEB) is a critical structure for male fertility. It enables the development of a specific luminal environment that allows spermatozoa to acquire both the ability to swim and fertilize an ovum. The presence of tight junctions and specific cellular transporters can regulate the composition of the epididymal lumen to favor proper sperm maturation. The BEB is also at the interface between the immune system and sperm. Not only does the BEB protect maturing spermatozoa from the immune system, it is also influenced by cytokines released during inflammation, which can result in the loss of barrier function. Such a loss is associated with an immune response, decreased sperm functions, and appears to be a contributing factor to post-testicular male infertility. Alterations in the BEB may be responsible for the formation of inflammatory conditions such as sperm granulomas. The present review summarizes current knowledge on the morphological, physiological and pathological components associated with the BEB, the role of immune function on the regulation of the BEB, and how disturbance of these factors can result in inflammatory lesions of the epididymis.
Keywords: ABC transporters, claudins, defensin, granuloma, sperm maturation
Abbreviations
- BEB
blood-epididymis barrier
- Cldns
claudins
- MARVEL
MAL (myelin and lymphocyte associated protein) and related proteins for vesicle trafficking and membrane link
- JAM
Junctional adhesion molecules
- PCBs
Polychlorinated biphenyls
- ZO1
Zona occludens 1
- CDH1
Epithelial-cadherin
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- PDE4
Phosphodiesterase 4
- cAMP
cyclic adenosine monophosphate
- ERKO
Estrogen receptor knock-out
- SED-1
Secreted protein containing EGF repeats and discoidin/F5/8 C domains
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- MMPs
Matrix metalloproteinases
- ALS
Amyotrophic lateral sclerosis
- GST
Glutathione-S-transferase
- MT
Metallothioneins
- ECM
Extracellular matrix
- TGF-β
Transforming growth factor beta
- ERK
Extracellular signal-related kinases
- TLRs
Toll-like receptors
- CD14
Cluster of differentiation 14
- LPS
Lipopolysaccharide
- DEFB126
Beta-Defensin 126
- TNF
Tumor necrosis factor
- PCR
Polymerase chain reaction
- SP-1/-3
Specificity proteins −1 and −3
- CTNN1
Beta-catenin
- COX1
Cyclooxygenase 1
- PGES
Prostaglandin E synthetase
- CFTR
Cystic fibrosis receptor
- ABC
ATP-binding cassette
- DOX
Doxorubicin
- MDR
Multi-drug resistance
Introduction
Sperm maturation in the epididymis represents a critical process in male fertility. During this process, sperm acquire both motility and the ability to fertilize. Sperm maturation is dependent on the composition of the epididymal lumen, which is created, in part, by products secreted from Sertoli cells in the testis which enter the epididymis, by secretion and absorption of ions and proteins by principal cells which line the lumen of the epididymis, and by the blood-epididymis barrier (BEB) which limits the exchange of molecules between blood and lumen (Fig. 1). In addition to creating this environment for sperm maturation, the BEB also provides an immune-privileged, or immune protected, milieu for sperm since they are immunogenic. The BEB is created by tight junctions between adjacent epithelial cells. Tight junctions are comprised of transmembrane proteins that enable the cells to adhere to one another, and intracellular proteins that link the transmembrane proteins to the weight-bearing cytoskeleton of the cell. The tight junctions and selective transport by principal cells allow the epididymis to concentrate organic molecules, such as carnitine and inositol, to levels that are 10- to 100-fold greater than in the blood.1 Three families of transmembrane proteins are implicated in tight junctions: claudins (Cldns), MARVEL proteins, and junctional adhesion molecules (JAM).
Figure 1.
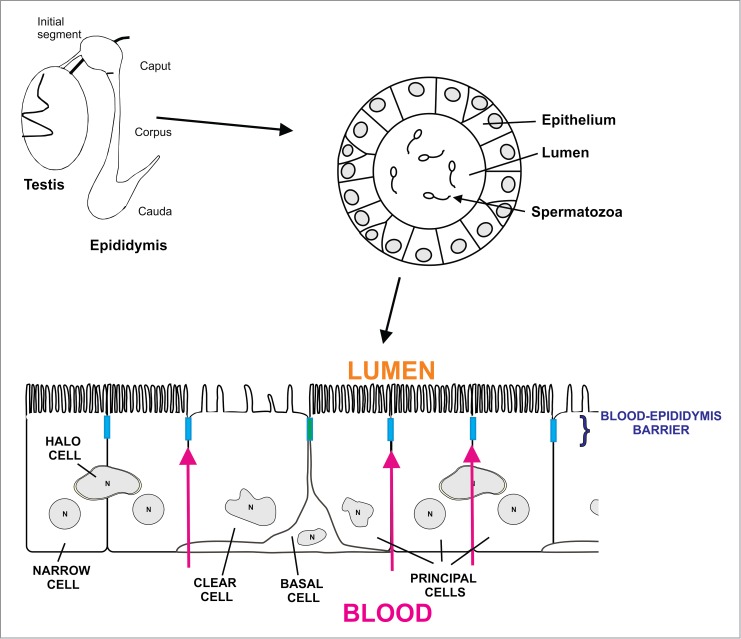
Schematic diagram showing the testis and epididymis including the 4 regions of the epididymis (initial segment, caput, corpus and cauda epididymidis). The epididymis is a single convoluted tubule in which the epithelium regulates the composition of the lumen necessary for sperm to acquire the ability to swim and fertilize. The epithelium of the epididymis contains various cells types, including principal cells, clear cells, basal cells and halo cells. Apical and narrow cells are present exclusively in the initial segment. Apical tight junctions between the cells that line the lumen of the epididymis form the blood-epididymis barrier. The barrier regulates the content of the epididymal lumen by limiting paracellular movement of ions between the blood and the lumen. It also protects maturing spermatozoa from the immune system.
Table 1.
List of ABC transporters detected (mRNA) in the different regions of the epididymis of fertile human patients. Data was analyzed from our submission to GEO (NCBI) access number GSE23812 Data are expressed as mean intensity levels
| ABC Transporter | Caput Epididymidis | Corpus Epididymidis | Cauda Epididymidis |
|---|---|---|---|
| Subfamily A | |||
| ABCA1 | 1.271 | ND | ND |
| ABCA2 | 0.926 | 1.077 | 1.465 |
| ABCA3 | 1.176 | 0.994 | 0.946 |
| ABCA4 | 0.936 | 0.301 | 0.168 |
| ABCA5 | 1.066 | 1.177 | 1.43 |
| ABCA6 | 0.927 | 1.050 | 0.864 |
| ABCA7 | 0.934 | 1.241 | 0.826 |
| ABCA8 | 2.077 | 1.041 | ND |
| ABCA9 | 1.025 | ND | 0.884 |
| ABCA10 | 1.008 | 1.012 | 0.713 |
| ABCA11 | 1.185 | 0.939 | 0.961 |
| ABCA12 | 1.057 | 0.807 | 0.727 |
| ABCA13 | 0.686 | ND | 0.850 |
| Subfamily B | |||
| ABCB1 | 0.0598 | 0.712 | 0.497 |
| ABCB2 | ND | ND | ND |
| ABCB3 | ND | ND | ND |
| ABCB4 | 0.851 | 2.454 | ND |
| ABCB5 | 0.546 | 0.352 | 0.351 |
| ABCB6 | 1.472 | 0.776 | 0.95 |
| ABCB7 | 2.255 | 1.011 | 1.18 |
| ABCB8 | 0.417 | 1.509 | ND |
| ABCB9 | 1.412 | ND | 1.431 |
| ABCB10 | 0.836 | 0.795 | 1.333 |
| ABCB11 | 0.905 | 0.669 | 0.683 |
| Subfamily C | |||
| ABCC1 | 2.201 | ND | 1 |
| ABCC2 | 1.518 | ND | 0.549 |
| ABCC3 | 1.104 | 0.913 | 1.552 |
| ABCC4 | 0.86 | ND | 1.077 |
| ABCC5 | 0.483 | 0.631 | 0.597 |
| ABCC6 | 0.974 | 1.127 | 1.123 |
| ABCC7 | ND | ND | ND |
| ABCC8 | 1.159 | 1.041 | 1.027 |
| ABCC9 | 1.340 | 0.0325 | 0.459 |
| ABCC10 | 1.09 | 0.776 | 0.948 |
| ABCC11 | 0.494 | 5.223 | 6.086 |
| ABCC12 | 1.367 | 1.302 | 1.186 |
| ABCC13 | 0.0114 | ND | 0.964 |
| Subfamily D | |||
| ABCD1 | 1.274 | 1.029 | 0.906 |
| ABCD2 | 2.488 | ND | ND |
| ABCD3 | 1.264 | 1.183 | 1.384 |
| ABCD4 | 1.683 | 1.228 | 1.805 |
| Subfamily E | |||
| ABCE1 | 1.536 | 0.825 | 1.246 |
| Subfamily F | |||
| ABCF1 | 1.079 | 0.855 | 1.277 |
| ABCF2 | 1.418 | 0.655 | 0.78 |
| ABCF3 | 0.931 | 1.105 | 1.086 |
| Subfamily G | |||
| ABCG1 | 0.727 | 0.991 | 1.026 |
| ABCG2 | 1.124 | 0.69 | 0.652 |
| ABCG4 | 3.003 | 0.0782 | 1.348 |
| ABCG5 | 0.975 | ND | 0.92 |
Friend and Gilula2 first characterized the ultrastructure of tight junctions in the epididymis. They noted that epididymal tight junctions were the most elaborate of all the different tissues studied at the time. Subsequent studies demonstrated differences in the ultrastructure of tight junctions along the length of the epididymis.3,4 Agarwal and Hoffer5 reported that the barrier in the rat was impermeable to lanthanum in the proximal region of the epididymis by postnatal day 14, and that by day 21, the entire epididymis was impermeable. Contradictory results by Guan et al.6 reported that the barrier was impermeable by 7 days of age. In the mink, the barrier is impermeable to lanthanum during embryonic development.7 Likewise immunolocalization studies in mice suggest that the lumen of the epididymis is formed during embryonic development and that occludin is localized to the apical region of the epithelium at that time.8 This would support the notion that the BEB begins to form during fetal development and that differences in permeability and ultrastructure may become complete only during postnatal development.
Signature histological lesions of blood-epididymis barrier compromise
Inflammatory lesions (interstitial edema and inflammatory infiltrate) and/or sperm granulomas are the most common histopathological changes that would suggest compromise of the BEB. Epididymal inflammation can represent a primary response to a drug (which may then compromise the BEB), or may be a secondary response to breakdown of the BEB by some other injurious mechanism allowing access of the host immune response to the antigenically foreign sperm (Fig. 2). The BEB, therefore, represents a critical aspect of the interface between the immune system and fertility. As such, changes which alter the structure or function of the BEB inevitably also affect sperm maturation, and impact male fertility and reproductive health.
Figure 2.
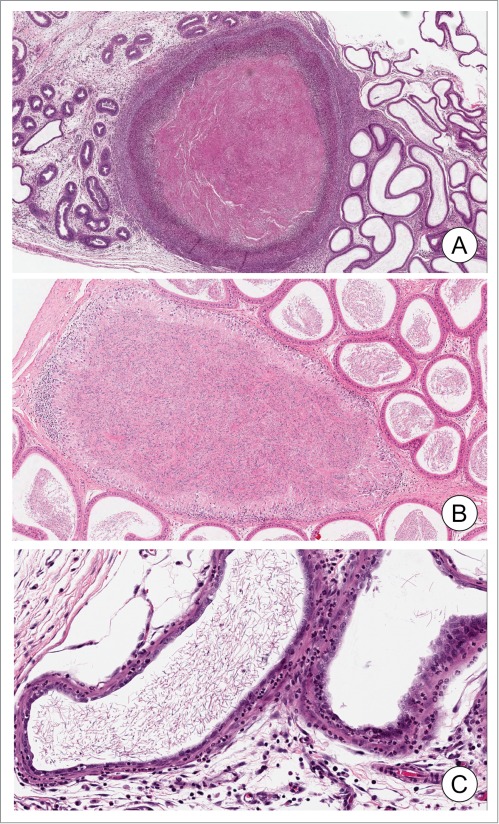
Epididymal granuloma and inflammation. Examples of epididymal granuloma (panels A and B). Note the alterations and degradation of the epithelium around the granuloma containing accumulated sperm. Mixed immune cells can be observed either surrounding (A) or in the periphery of the granuloma (B). Panel B shows an inflammatory response with increased immune cells in the interstitium. Photomicrographs were generously provided by Dr. D. Creasy (Huntingdon Life Sciences).
Pathological consequences of BEB disruption
Examples of pathological consequences associated with loss of the BEB are limited in the literature. Hermo et al.9 showed a loss of barrier function in cathepsin A deficient mice. Cathepsin A is a lysosomal caboxypeptidase that facilitates lysosomal targeting and activation of α-neuramididase and protects β-galactosidase and α-neuramididase against proteolytic degradation.10-13 In knockout mice, the membrane localization of Cldns 1, 3, 8 and 10 is either decreased or becomes cytoplasmic in regions of the epididymis. The epididymis of these animals is also permeable to lanthanum nitrate perfusion, indicating a loss of BEB function (Fig 3A, B). This was associated with an increased number of macrophages in the interstitium of the epididymis and abnormal halo cells in the epithelium (Fig. 3C, D).14 Furthermore, the loss of BEB function was associated with an increased percentage of static sperm in the cauda epididymidis, and dramatic changes in sperm motility and velocity parameters. These observations offer basic guides to pathologies resulting from a loss in BEB function and subsequent immune response.
Figure 3.
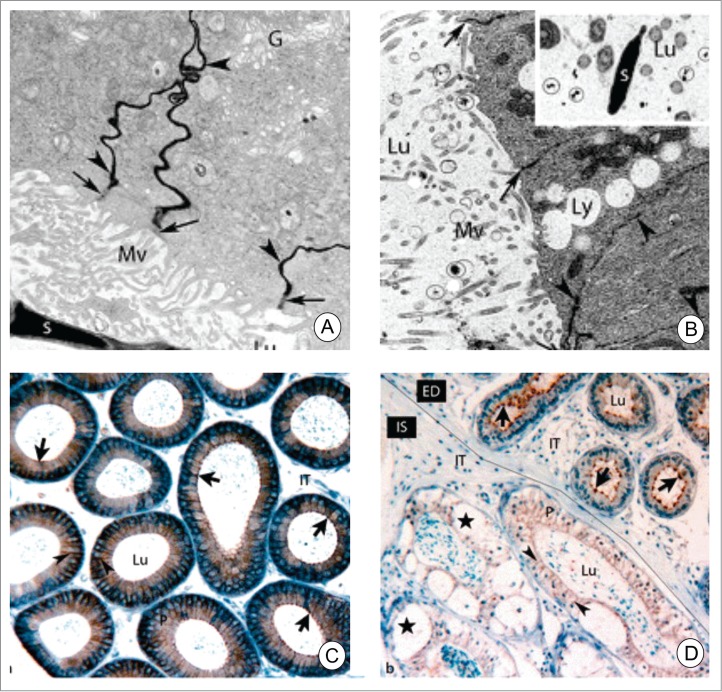
Electron microscope images of adult wild-type (A and B) and deficient in protective protein cathepsin A mice perfused with lanthanum nitrate. In A, lanthanum nitrate percolates through the intercellular spaces (arrowheads) between adjacent principal cells up to the apical tight junctions. In B, lanthanum nitrate permeates the entire intercellular space (arrowheads) including the apical junctional complexes (arrows) indicating a loss of tight junctions. Lanthanum nitrate is also evident in the epididymal lumen (circles, inset of B). In C, paraffin sections of wild-type mice were immunostained for Cldn3. Cldn3 is located in the apical region of the epithelium (arrow). In cathepsin A-knockout mice, the localization of Cldn3 is cytoplasmic. Furthermore, the interstitial area contains numerous macrophages and large vacuoles are present in the epithelium. Reproduced from Figures 1 and 3 of Hermo et al.9
Cai et al.15 reported that a mixture of polychlorinated biphenyls (PCBs; Aroclor 1254) caused increased permeability of epididymal tight junctions to lanthanum perfusion, suggesting that the BEB was compromised. Gene profiling indicated that PCBs altered small G-proteins, and this was associated with a decrease in ZO-1 levels. Unlike the cathepsin A knockout experiments, PCBs did not appear to cause overt changes in the epithelium of the epididymis, nor was there a noticeable increase in macrophages in the interstitium. Whether or not the BEB was fully compromised in this study is difficult to assess but clearly the response appeared to be somewhat attenuated.
Aging studies using Brown Norway rats reported that the BEB was compromised in aging individuals. This was reflected by decreased expression of occludin, ZO1 and the cell adhesion protein E-cadherin (CDH1).16 The loss of barrier function in aging rats was also associated with an increase in number of halo cells (monocytes and lymphocytes) present in the epithelium of the epididymis.17 These observations support the notion that the loss of barrier function in the epididymis is associated with an immune response.
In humans, alterations in components of the BEB are associated with decreased fertility. Studies comparing gene expression in fertile and non-obstructive azoospermic infertile patients indicated that the intracellular targeting of Cldn10 and ZO1 was altered, while the expression of several Cldns was down-regulated in infertile patients (Fig. 4).18 Using cell lines derived from fertile and obstructive azoospermic patients, those from infertile patients were unable to form functional tight junctions.19 Duan et al20 reported the presence of dendritic cells in ejaculates of patients with chronic inflammation of the genital tract. This was inversely related to sperm motility. Furthermore, there was a significant correlation between the presence of dendritic cells and levels of Il-6, Il-17, Il-23, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and neutral α-glucosidase, an epididymal marker. They postulated that the dendritic cells originated from the epididymis, suggesting that the BEB may be compromised in these patients. Shum et al21 have shown that dendritic cells in the epididymis are present in the mouse epithelium and that these are distinct from epididymal basal cells.
Figure 4.
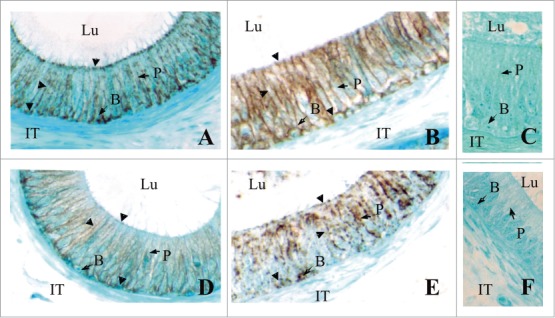
Immunolocalization of CLDN1 (A, B) and CLDN3 (C, D) in the caput epididymides of fertile men (A) and infertile men (B). Negative controls with no primary antibodies are shown (C). While CLDN1 and CLDN3 are localized (arrowheads) to the area of tight junctions between adjacent principal cells in the caput epididymidis of fertile patients, in In infertile patients there is a noticeable increase in cytosolic localization. These results show that the localization of these CLDNS can be negatively regulated in infertile men. Magnification × 640. P, Principal cells; B, basal cells; IT, intertubular space; Lu, lumen. Reproduced from Dubé et al.18.
Interstitial inflammation
Edema and acute interstitial inflammation of the epididymis and/or the efferent ducts are one of the more common drug induced changes seen in the epididymis. Sperm granulomas (a chronic inflammatory response surrounding and sequestering a bolus of extravasated sperm) often develop as a sequel to an earlier interstitial inflammatory response22-25 or following prior epididymal epithelial damage.26 Sperm granulomas and dilatation of testis, efferent ducts, and epididymis have been observed in rats following inhibition of phosphodiesterase 4 (PDE4).22 PDE4 is the main enzyme in mammals that hydrolyzes and thus degrades cAMP. PDE4-selective inhibitors have been reported to increase levels of cAMP in cells, thereby suppressing certain inflammatory processes.27 A variety of PDE4 inhibitors have been reported to cause testicular and epididymal toxicity, although mechanisms by which these occur are unclear.28 Heuser et al.29 reported an interstitial inflammation in the efferent ducts and epididymis following dosing with a PDE4 inhibitor. In a time course study, the acute epididymal inflammation occurred as an early event and at the same time as luminal dilation of the efferent ducts and the ducts of the initial segment of the epididymis. Although the cause of the inflammation is unknown, the authors suggest that the inflammation could be secondary to a physical compromise of the BEB (due to ductular dilation) or more likely due to a direct effect on the vasculature. Epididymal sperm granulomas were also seen, but as a later event.
Administration of L-cysteine to prepubertal rats also produced interstitial edema and acute inflammatory infiltrate as an early change followed by sperm granulomas with longer dosing. In this case the inflammatory changes were considered by the authors to be associated with L-cysteine induced defective development of the caudal epididymal ducts, leading to obstruction of sperm outflow, ductal rupture and resultant sperm granulomas in the corpus and cauda region.23
In man, epididymal inflammation is most commonly caused by bacterial infections, although male urinary tract infections are not common. Sexually-transmitted infections, particularly gonorrhea and Chlamydia, can also cause epididymitis.30-33 Non-infectious causes, which are rare, include reflux of sterile urine via ejaculatory ducts, causing obstruction and consequent inflammation; sarcoidosis; and Behçet disease, a chronic systemic inflammatory disorder characterized by oral and genital ulcerations and widespread vasculitis. More rare causes of epididymitis can be the result of urinary surgeries or vasectomy; drug-induced, as with amiodarone, a heart medication; human brucellosis34; and pinworm (Enterobius vermicularis) infection in the bowel, with concurrent orchido-epididymitis.35
Sperm granulomas
Over 250 articles, dating back to 1964, have reported the incidence of granulomas in various tissues. Granulomas are generally described as a collection of macrophages as well as other substances or cells, and are considered to be inflammatory lesions. They appear to serve essentially as a means by which a tissue or organ attempts to restrict or isolate a systemic immune response, by ‘walling off’ or surrounding the foreign body (or bodies) which may pose an antigenic challenge. When substances perceived as foreign (eg bacteria, cells, fungi, suture fragments, chemicals, sperm, etc) cannot be eliminated, the immune system tries to isolate these by surrounding the foreign bodies with macrophages and connective tissue (Fig. 2).
Sperm or spermatic granulomas have been defined as a lump of extravasated sperm in the epididymis or vas deferens and are reportedly comprised of distintegrating spermatozoa, macrophages, neutrophils, and vacuolated epithelial cells containing cellular debris,29,36,37 and have been widely reported sequelae of vasectomy in both humans and rodents.38-42 In addition to post-vasectomy conditions, spermatic (also called epididymal) granulomas have also been induced in a variety of species, particularly rodents, following treatment with hormones, such as testosterone, and a variety of drugs and chemicals.25,26,29,43,44 Many spermatic granulomas occur in the cauda epididymidis, and have been observed in several types of mice generated with deficiencies in specific genes. For instance, the estrogen receptor-null knockout mice (ERKO)45 and SED1-null mice46 both display disruption of epididymal epithelium integrity, resulting in formation of sperm granulomas.
Although widely considered to be an aspect of immune response, controversy exists in human medicine, as to whether or not spermatic granulomas are a good thing. Some authors report that spermatic granulomas are beneficial in helping to maintain the balance of inner hydrostatic pressure in the male reproductive tract.47,48 Other studies argue against such benefit, and consider granulomas to precede other pathologies, including gangrene and tumors.49,50 Furthermore, evidence is lacking to clearly demonstrate whether or not granulomas arise as a result of a disruption of tight junction proteins of the tissue barrier, namely the BEB, thereby allowing sperm to leak out of the epididymal lumen into the interstitial compartment, and neutrophils, antibodies, and macrophages to leak into the lumen and attack sperm. Several studies have reported that spermatic granulomas may function as a sperm disposal system, as shown by a change in their free radical status51,52 and that there is a link between granulomas, higher protease activity, and superoxide radicals.53
Oxidative stress, inflammation and the epididymis
Reactive oxygen species (ROS) are formed as a result of normal cellular processes in the mitochondria of all cells, and can also act as intermediates in some metal-catalyzed reactions. ROS are known to play a role in numerous inter- and intra-cellular signaling processes, as well as in immune cell responses (also see Fujii and Imai, this issue). There exist several mechanisms to control the level of oxidation within a cell, and to eliminate potentially damaging oxygen-based free radicals. These include anti-oxidant enzymes such as superoxide dismutase (SOD) and catalase, and non-enzymatic molecules, most frequently, glutathione, which has an exposed sulfhydryl group that can readily react with oxidizing free radicals, reducing the glutathione, which can then be regenerated by several cellular reactions.
ROS are known to be produced, likely by spermatozoa themselves, as part of the normal process of sperm capacitation.54,55 Circulating leukocytes within the male reproductive tract also produce ROS, generally in response to stress, including radiation, chemicals, and infectious agents, among others.56
It has been demonstrated that excessive levels of ROS, resulting from leukocyte production, can negatively impact fertility and lead to inflammation, although the precise mechanism(s) by which this is achieved is/are unclear. The assumption is that the leukocytes infiltrate the BEB in response to infection or foreign agents, but it is not clear if the stress (infection, chemical, etc) leads to disruption of the barrier, permitting infiltration of leukocytes, or if some other mechanism is involved. For instance, accumulation of free radicals can disrupt the blood-brain barrier by activating matrix metalloproteinases (MMPs), which leads to the degradation of tight junction proteins and increased brain-blood barrier permeability.57 Such conditions could presumably arise in the epididymis, allowing for tight junction protein degradation and subsequent disruption, and increased permeability. This could, in turn, permit infiltration of leukocytes and consequently, increased ROS, as well as increases in other aspects of immune response.
Several studies have reported that basal cells express a number of genes that are implicated in the protection against free oxygen radical species. Cu-Zn SOD (SOD1) was one of the first markers of epididymal basal cells identified.58 SOD genes play an essential role in maintaining the balance of ROS. In tissues such as the brain, the loss of functional SOD1 has been associated with impair-ment of the tight junctions of the blood-brain barrier observed in patients with neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS).59-61 Another gene implicated in basal cells is glutathione-S-transferase (GST), which can protect against ROS by donating an electron to stabilize free oxygen radicals and peroxides.62 Metallothioneins (MT) are a family of proteins (MT1, MT2, and MT3) associated with protection from heavy metal toxicity.63-65 The promoters of MT1 and MT2 have metal response elements which can be induced by divalent cations (eg. Cd, Cu, and Zn), resulting in increased MT synthesis. MT can bind metals ions, thereby reducing their bioavailability, and hence their toxicity. In addition to their role in heavy metal toxicity, MTs can scavenge ROS, and thus reduce their toxicity.66-68 While several studies have reported that sperm are particularly sensitive to the effects of high levels of ROS,69-72 whether or not the presence of various ROS defense mechanisms in basal cells is sufficient to decrease ROS levels in the lumen of the epididymis remains to be demonstrated. A more likely function of the ROS defense mechanisms is to protect the epithelial cells that line the lumen of the epididymis and the BEB. Clearly, however, basal cells play an important function in detoxification. While the structure of these cells may be altered by anti-androgens,73 and hence by certain endocrine disruptors, careful analysis of cell numbers and their projections is needed to assess basal cell health and function when toxic effects on the epididymis are suspected.
Inflammation, matrix metalloproteinases and the blood-epididymis barrier
MMPs are a family of zinc-dependent endopeptidase enzymes involved in tissue matrix degradation and remodelling. They play a role in several normal physiological processes, such as embryonic development, menstrual cycle, and tissue damage repair, and are also associated with diseases such as arthritis and cancers.74 They are often associated with cancer progression, since they can break down tissue barriers and permit metastatic spread (NIH/NCBI).
Wilson et al.75 demonstrated that MMP7, also known as matrilysin, which is involved in normal tissue remodelling, is expressed in the initial segment and caput epididymal epithelium, suggestive of a role in maturation of sperm during epididymal transit, as well as a role in fluid regulation. It is likely that these functions are achieved by MMPs via their regulation of some signaling pathways, via enzyme cascades and by generating some cytokines, growth factors, and adhesion molecules (NIH). Curiously, a recent study by Stammler et al76 reported that the permeability of the BEB could be modulated by the TGF-β group of cytokines. Using an in vitro murine epididymal cell line, they showed that addition of TGFβ-1,-2, or -3 to the cell medium was sufficient to reduce Cldn1 protein levels, and significantly increase paracellular permeability, with TGFβ3 having the greatest effect. It is not, therefore, inconceivable that BEB permeability, and by extension, retention of tightness of the BEB, is regulated by various cytokines. Cytokines such as interleukin-4 and -13 have been shown to alter expression of some of the junction proteins in other tissues77 and increase sinonasal epithelial barrier permeability. It has been shown that in vivo, the extracellular matrix (ECM) of the stroma of the rat or monkey epididymis contains TGFβ1 in all regions of the epididymis, and TGFβ3 mainly in the corpus epididymidis, and both exert significant effects on paracellular permeability.78-80 Similar effects of TGFβ-3 have been observed on the blood-testis barrier.81,82 Stammler et al.76 suggested that the role of TGFβs, under certain conditions such as infection or inflammation, could be to permit loosening of the BEB for dendritic cell migration into the epididymal epithelium. Dendritic cells, derived from monocytes, are specialized antigen-presenting cells and play a role in regulation of the immune system. These dendritic cells have recently been reported to be present in the murine epididymis,83 although their role has not yet been elucidated.
Prostaglandins, such as prostaglandin E2 (PGE2), are known to mediate inflammation and to enhance migration of dendritic cells.28 Paradoxically, PGE2 can also suppress cytokine production by dendritic cells.84,85 Dendritic cells have been identified in the peritubular region of the epididymis and have extensive and lateral projections that reach in the epithelium of the epididymis.86
The ratio of T cells vs dendritic cells appears to be important in determining effects of PGE2 on immune system.28 By extension, this interplay may be, at least in part, responsible for segregating immune responses to infectious agents versus chemical toxicants, for example.
In contrast, it has also been shown that the tight junction protein Cldn1, can regulate Notch-signaling via its regulation of MMP-9 and p-ERK signaling in murine intestinal epithelium.87 The result is a regulation of epithelial proliferation and therefore a role in epithelial homeostasis. Given the presence of multiple Cldns, particularly Cldn1, in both human and rodent epididymis, it is possible that the Cldns play an important role not only in the establishment and maintenance of the tight junctions of the BEB, but also in regulation of signaling and of epithelial homeostasis.
Role of toll-like receptors and defensins in epididymal inflammation and immune protection
The occurrence of spermatic granulomas as a result of bacterial challenge has been examined in numerous studies, and indeed, the role of innate immune receptors, including the family of Toll-Like Receptors or TLRs, has been investigated in several species and cell lines.88 TLRs function as receptors for specific ligands present on the cell surface or within the cell. To date, 11 TLRs have been identified, and binding of TLRs to their respective ligands activates a variety of cell signaling pathways.89,90 The role and regulation of TLRs in the epididymis, as well as the role of glucocorticoids in immunity, have been extensively reviewed elsewhere.91 Crosstalk between TLRs and other innate immunity receptors has also been reviewed elsewhere.92 The role of TLRs and their receptors has not yet been elucidated in the male reproductive tract, although several studies have examined expression of various TLRs and ligands in male reproductive cells and organs of humans and rodents.93-101
It has been shown, in both humans and mice, that the epididymis constitutively expresses multiple TLR proteins, inducing TLR2 and TLR4.93,102-104 Additionally, CD14, a 54 kDa protein, part of the LPS-receptor complex, has also been found in seminal plasma and sperm membranes, suggesting a role in the epididymal innate immune response.105 LPS-induced epididymitis has been reported to impair sperm motility, by disruption of epididymal defensin expression.106
Defensins are part of the innate immunity of the epididymis and the epididymal epithelium has been shown to express a variety of defensins.107,108 Defensins are small cationic antimicrobial peptides that, in mammals, can be separated into 2 subfamilies: α- and β-defensins.109 They can bind to the negatively charged extracellular surface of the microbial membrane, where they generate pores that increase the permeability of the membrane, thereby killing the microbe.110 β-defensins have been shown to exert anti-microbial, as well as anti-fungal and anti-viral properties.111 These proteins can be actively secreted into the epididymal lumen, where they not only protect sperm from microbial infections but also bind to the sperm and play a role in sperm maturation and the acquisition of motility.112-116 Zhou et al115 first reported that Bin1b, an epididymis-specific β-defensin found in the rat, can induce Ca2+ uptake by the sperm, resulting in increased progressive motility of immature spermatozoa.
βeta-Defensin 126 (DEFB126; also known as epididymal secretory protein 13.2) was first identified in epididymis of Macaque monkeys.117 Immunostaining indicated that the protein covered the entire surface of spermatozoa.113,118,119 It has been shown to be part of the sperm glycocalyx that encapsulates the sperm and is thought to be important for stabilizing the sperm plasma membrane.120 There are several proteins that are secreted by the principal cells of the epididymis, but only a few are integrated into the sperm plasma membrane.121 Studies have suggested that DEFB126 is implicated in protecting sperm from the immune system, capacitation, penetration into the cervical mucus, and binding to the epithelium of the oviduct.119,122-124
Tollner et al.119 showed that DEFB126 disassociates when sperm are capacitated and if the protein is added back to the sperm, zona pellucida binding is inhibited. Alterations in pH appear to be responsible for removing DEFB126 from the sperm following capacitation.116 Dubé et al125 showed, using both microarray and real-time PCR, that the levels of DEFB126 mRNA are 4-fold higher in the caput as compared to the corpus and cauda epididymidis. Furthermore, DEFB126 was also shown to be expressed at lower levels in the caput epididymidis of patients with non-obstructive azoospermia.18 Tollner et al.126 also showed that a dinucleotide deletion in the human DEFB126 was associated with reduced fertility.
Role of proteins in the composition of the BEB
Cldns are tetraspan transmembrane proteins linked to the cytoskeleton via the zona occludens (ZO) proteins. In mammals, there are 27 different Cldns that can polymerize with other Cldns and/or occludin (a Marvel protein) in both homotypic and heterotypic fashion to form tight junctions.127-131 They are not only necessary for the barrier function of the epithelium, but can form ion selective pores in the tight junction to allow paracellular transport between adjacent cells. The selectivity of such pores is dependent on specific Cldns.128
Occludin, tricellulin, and MarvelD3 are members of the tight junction associated MARVEL protein family which are characterized as having a tetra-spanning domain known as MARVEL (MAL and related proteins for vesicle trafficking and membrane link).132,133 While these proteins are expressed in a variety of tissues134 there is little information on their interactions. Raleigh et al.133 investigated the relationship between these 3 proteins in an intestinal cell line (Caco-2) and concluded that they display some overlap in localization and function, yet this overlap appears to be non-redundant. Previous studies have suggested that any one of these proteins is not critical or essential for tight junction formation. For example, Saitou et al.135 showed that while occludin-knockout mice displayed growth retardation, testicular atrophy, and other effects, there were no defects in epidermal, renal, respiratory, or intestinal barrier function. However, Raleigh et al.133 concluded from their studies that although there is overlap among these 3 proteins, no compensation by the other family members occurs following knockdown of either tricellulin, occludin, or marvelD3, which may result in effects on tight junction assembly. Interestingly, tumor necrosis factor (TNF)-treatment of intestinal Caco-2 cells showed that protein expression of marvelD3 and tricellulin were increased, but that of occludin did not change.133 Occludin was the first tight junction protein identified in the epididymis, and the same study reported that tight junctions were heterogeneous, and that occludin was not expressed or was expressed at low levels in the initial segment of the epididymis.136 This led to studies which showed that Cldn1 was localized to epididymal tight junctions in all epididymal regions, and that the localization of the protein was regulated by androgens, but only in the initial segment, indicating that Cldns may be regulated differently in the different regions of the epididymis (Fig 5).137 Studies by Guan et al138 as well as by Gregory and Cyr139 demonstrated that the presence of multiple Cldns in the epididymis, and that the localization of Cldns varied as a function of postnatal development, being localized primarily to the cytoplasm in young animals, and becoming progressively localized to the plasma membrane as a function of age (Fig 6). While the mechanism responsible for this is unclear, DeBellefeuille et al140 showed that during development, ZO-1 associates with β-catenin (CTNN1) in the adherens junction prior to the formation of tight junctions, suggesting that this association was necessary for the initial assembly of the tight junctions. These studies point to the fact that pathological examination of the BEB barrier necessitates immunolocalization studies as the localization of tight junction proteins may be mistargeted under certain conditions. Certainly the conclusion from developmental studies indicates that post-transcriptional modifications in cellular targeting associated with the formation, or completion of the BEB, are as important as the transcription regulation of the genes. Furthermore, the effects of androgens on Cldn1 localization indicate that anti-androgens may affect, if not the entire function of the barrier, certainly its permeability, which may result in consequences for sperm maturation.
Figure 5.
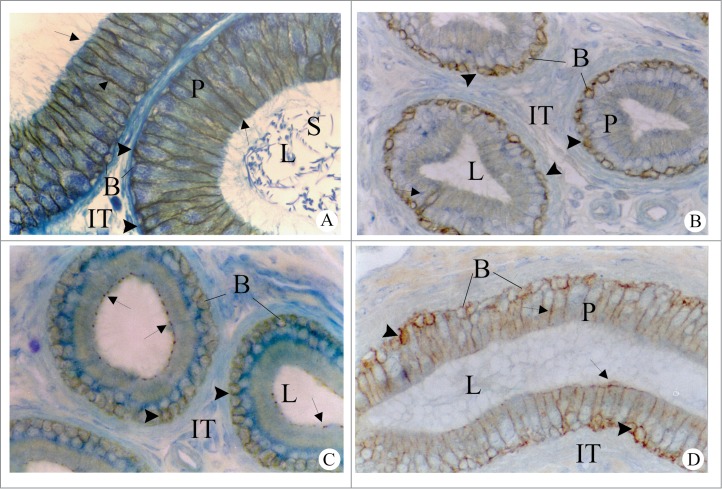
Regulation of Cldn1 in the initial segment (A–C) of the adult rat epididymis. In intact adult rats (A), Cldn1 is immunolocalized to the lateral plasma membranes between neighboring principal cells (arrows) and between principal and basal cells (arrowheads). In adult rats, 14 days after orchidectomy (B), immunoreactive Cldn1 is associated at the interface between basal and principal cells (arrowheads) and not between the lateral plasma membranes of adjacent principal cells. In orchidectomized rats given testosterone replacement (C), immunoreactive Cldn1 is observed between the lateral plasma membranes at apical sites in areas of tight junctions (arrows), but not at more distal sites of these membranes; reaction, however, is maintained between basal and principal cells (arrowheads). P, Principal cells; B, basal cells; IT, intertubular space; L, lumen. Magnification, ×640. Reproduced from Gregory et al.,137.
Figure 6.
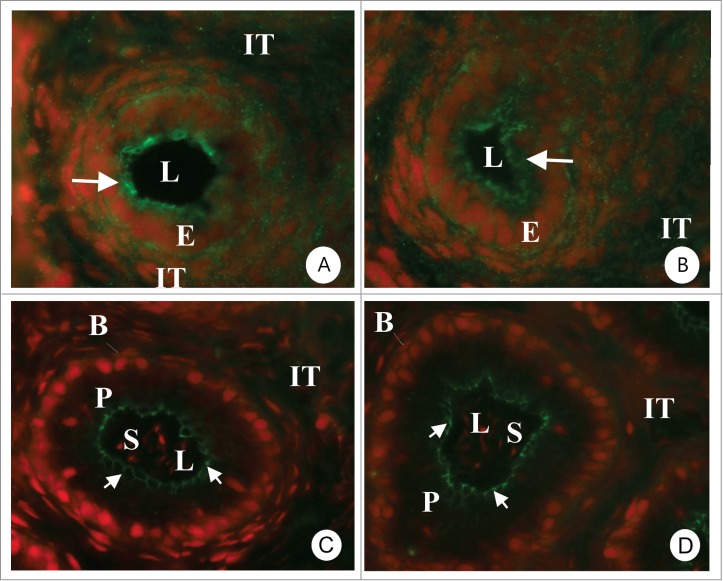
Immunocytochemical localization of Cldn-3 (A, C) and Cldn-4 (B, D) in the epididymis of 14 (A, B)- and 42 (C, D) day old rats. At 14 days there is an important cytoplasmic reaction (arrows) suggesting that Cldns have not yet completely localized to the area of the tight junction. In 42 day old rats the reaction in all regions of the epididymis is present exclusively in the area of tight junction and there is no cytoplasmic staining. The photomicrographs are from the initial segment region. Magnification 640×. E = epithelial cells, P = principal cells, IT = intertubular space, L = lumen. Reproduced from Gregory et al.,139.
The complex regulation of the formation and maintenance of the tight junctions of the BEB remains to be completely elucidated, and involves both transcriptional and post-transcriptional regulation of Cldns.9,141,142 Dufresne and Cyr142 showed that the Cldn1 promoter is regulated by specificity proteins (SP)-1 and -3 in the epididymis. Furthermore, studies have shown that p63, expressed in basal cells of the epididymis, can bind to the SP1/SP3 consensus sequence of the DNA of the Cldn1 promoter in skin.143 Given that p63 as well as Cldn1 have been shown to be expressed in basal cells, it is likely that p63 may regulate the expression of Cldn1 in basal cells. Micro RNAs have also been shown to regulate Cldn10 in the epididymis. Belleannee et al.144 showed that there is a negative correlation between miRNA 145 and Cldn10 in the human epididymis. It is therefore likely that miRNAs are implicated in the regulation of other Cldns.
Role of basal cells in the BEB: protector of the barrier?
In pseudo-stratified epithelia, such as the epididymis, it has generally been accepted that basal cells are restricted to the basal compartment of the epithelium. These cells have thin projections that interact with projections from adjacent basal cells to form a basket-like structure at the base of the epithelium.145 These projections can extend apically between principal cells and reach the epididymal lumen.73 Cldn1 was shown to be expressed in basal cells,141 as well as along the lateral margins of the plasma membrane of principal cells. Shum et al.73 suggested that expression of Cldns by basal cells allowed these cells to bind to the Cldns of the tight junctional complex of the BEB to cross the barrier to reach the lumen. It has been suggested that lumenal basal cell projections act as sensors of angiotensin II, and that these cells regulate acidification of the lumen via the production of nitrous oxide, which act on epididymal clear cells to stimulate proton secretion.146
Basal cells also express elevated levels of cyclooxygenase 1 (COX1) and prostaglandin E synthetase (PGES), which are implicated in prostaglandin synthesis. The expression of these enzymes increases along the epididymis.147 Increased prostaglandin synthesis is thought to regulate functions of the principal cells. For example, PGE2 has been shown to be an important regulator of the cystic fibrosis receptor (CFTR) in principal cells.148
It is well-known that reactive oxygen species (ROS) and other oxidative stress agents can alter or negatively affect one or multiple aspects of spermatogenesis.149 In addition, multiple diseases, including chronic inflammatory diseases and bacterial infections, can also impact and compromise the functioning of Sertoli cells in the testis, and various components of the BEB.
Access of drugs across the BEB
Many factors can affect an individual's response to toxic or therapeutic substances. Toxicokinetics/pharmacokinetics examines time-dependent processes regulating the interaction between substances and living organisms. This encompasses 4 fundamental principles of toxicology: absorption, distribution, bio-transformation, and excretion. Dose and physico-chemical characteristics of the substance, route and duration of exposure, and inter-individual variability are all important factors which can influence the kinetic profile of a given substance. Any compound is capable of eliciting a response if given at sufficiently high doses. Chemical lipophilicity and routes of exposure will also greatly affect how a substance is both absorbed and distributed throughout the body. As a general rule, lipid-soluble compounds are more readily absorbed through the skin, the gut and the lungs, and are more easily distributed between internal compartments. Other substances, while innocuous in their original form, can be bio-activated by xenobiotic metabolizing enzymes in the liver to become more toxic. Cellular barriers, such as the BEB, regulate the paracellular movement of molecules, including toxic substances, across blood-tissue barriers thereby affecting the distribution, absorption and transfer of substances. As such these structures play an important role in drug distribution.
Cellular transporters and the BEB
A functional aspect of cellular barriers is the ability to regulate the movement of materials across the barrier to maintain ideal conditions within immuno-privileged environments while protecting cells within these sites. As discussed above, one of the functions of Cldns in the tight junctions is to regulate and in some case, restrict, the paracellular movement of ions between cells. To regulate larger molecules including organic toxicants, transport systems have been shown to be associated with different barrier systems in the body. ATP-binding cassette (ABC) transporters are a large family of membrane-embedded transport proteins implicated in multidrug resistance.150-152 There are 49 ABC transporters in humans that can be subdivided into 7 families. They actively transport various molecules including drugs, lipids, metabolites, and ions.150-152 They can act as drug/toxicant efflux proteins and have been shown to be important in the detoxification of a diverse array of xenobiotics by excreting potentially harmful substances. ABC transporters are expressed in a tissue-specific manner and have been associated with various blood-tissue barriers.153 They represent one of the physiological components of blood-tissue barriers. Gene expression profiling in the human epididymis has shown that there are 46 different ABC transporters in the epididymis, and that the transcript levels of these transporters varies between the different segments of the epididymis (Table 1). While the functionality of these transporters needs to be established, it is noteworthy that so many members of the ABC transporter proteins are present in the epididymis, and for many, their distribution is segment-specific suggesting that they are specifically regulated along the epididymis.
Studies in the rat have identified ABCB1a/b transcript and protein throughout the different epididymal segments.154 ABCB1 was localized to apical region of the epithelial cells lining the lumen of the epididymis, as well to the epididymal spermatozoa of the corpus and cauda epididymidis.154 Protein levels were significantly higher in both corpus and cauda epididymidis relative to the initial segment and caput. Furthermore, efflux activity and protein levels could be stimulated in rat epididymal cells, RCE,155 exposed to either doxorubicin or nonylphenol154 thus showing that the transporter was functional. Likewise, functional MDR assays have suggested that ABCC1 may also play a role in epididymal barrier defense, although this transporter was not induced by either DOX or nonylphenol treatment.154
Another member of the ABC transporter family is the cystic fibrosis transductance regulator (CFTR/ABCC7). CFTR has been shown to be essential for male fertility.148,156 Some studies have shown that mutations in the CFTR can cause male infertility resulting from the malformation of the epididymis and congenital absence of vas deferens in humans.157,158 Furthermore, there appears to be a correlation between infertility and CFTR mutations in men with normal reproductive tract development.159,160 Animal model studies have reported an association between CFTR mutations and developmental absence of vas deferens in the ferret and porcine animal models.161-163 CFTR-knockout mice do not exhibit congenital malformations of the epididymis or absence of vas deferens, yet the animals are subfertile.164,165
Conclusions
In conclusion, the morphological and physiological components of the BEB represent essential aspects of male fertility. Disruption or loss of specific BEB components or disturbances to the epididymal epithelium which compromise integrity and functioning of the BEB can elicit immune responses, and have been associated with decreased fertility in humans. Although disruption or loss of the BEB can result in an immune response, the barrier itself is clearly also subject to regulation by the immune system, especially in cases of inflammation. There have been limited reports of pathologies resulting directly from loss of BEB function; perhaps the most obvious reason for this resides in the complexity of the BEB and the large number of proteins that comprise the junctions of the BEB. We are now beginning to better understand the roles of the various protein components of the BEB, and the loss of BEB function may not necessarily result in full loss of the barrier but rather in its physiological functions, whether these are associated with the tight junctions, cellular transporters, or components of epididymal immune functions, such as the defensins. Clearly, assessing pathological effects on the epididymis will require multiple approaches to fully understand how epididymal function may be impacted by environmental toxicants, drugs or infection and the consequences for male fertility.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We wish to thank Dr. Dianne Creasy (Huntington Life Sciences) for her generous contribution of granuloma photomicrographs. J. Dufresne (INRS) is thanked for her assistance.
Funding
This work was supported by CIHR (grant 84576).
References
- 1. Hinton BT, Howards SS. Permeability characteristics of the epithelium in the rat caput epididymidis. J Reprod Fertil. 1981; 63:95-9; PMID:7277337; http://dx.doi.org/ 10.1530/jrf.0.0630095 [DOI] [PubMed] [Google Scholar]
- 2. Friend DS, Gilula NB. Variations in tight and gap junctions in mammalian tissues. J Cell Biol. 1972; 53:758-76; PMID:4337577; http://dx.doi.org/ 10.1083/jcb.53.3.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suzuki F, Nagano T. Regional differentiation of cell junctions in the excurrent duct epithelium of the rat testis as revealed by freeze-fracture. Anat Rec 1978; 191:503-19; PMID:697060; http://dx.doi.org/ 10.1002/ar.1091910409 [DOI] [PubMed] [Google Scholar]
- 4. Cyr DG, Robaire B, Hermo L. Structure and turnover of junctional complexes between principal cells of the rat epididymis. Microsc Res Tech 1995; 30:54-66; PMID:7711320; http://dx.doi.org/ 10.1002/jemt.1070300105 [DOI] [PubMed] [Google Scholar]
- 5. Agarwal A, Hoffer AP. Ultrastructural studies on the development of the blood-epididymis barrier in immature rats. J Androl 1989; 10:425-31; PMID:2621151 [DOI] [PubMed] [Google Scholar]
- 6. Guan X, Inai T, Shibata Y. Segment-specific expression of tight junction proteins, claudin-2 and -10, in the rat epididymal epithelium. Arch Histol Cytol 2005; 68:213-25; PMID:16276027; http://dx.doi.org/ 10.1679/aohc.68.213 [DOI] [PubMed] [Google Scholar]
- 7. Pelletier RM. Blood barriers of the epididymis and vas deferens act asynchronously with the blood barrier of the testis in the mink (Mustela vison). Microsc Res Tech 1994; 27:333-49; PMID:8186451; http://dx.doi.org/ 10.1002/jemt.1070270408 [DOI] [PubMed] [Google Scholar]
- 8. Cyr DG, Hermo L, Egenberger N, Mertineit C, Trasler JM, Laird DW. Cellular immunolocalization of occludin during embryonic and postnatal development of the mouse testis and epididymis. Endocrinology 1999; 140:3815-25; PMID:10433243 [DOI] [PubMed] [Google Scholar]
- 9. Hermo L, Korah N, Gregory M, Liu LY, Cyr DG, D’Azzo A, Smith CE. Structural alterations of epididymal epithelial cells in cathepsin A-deficient mice affect the blood-epididymal barrier and lead to altered sperm motility. J Androl 2007; 28:784-97; PMID:17522420; http://dx.doi.org/ 10.2164/jandrol.107.002980 [DOI] [PubMed] [Google Scholar]
- 10. D’Azzo A, Hoogeveen A, Reuser AJ, Robinson D, Galjaard H. Molecular defect in combined β-galactosidase and neuraminidase deficiency in man. Proc Natl Acad Sci U S A 1982; 79:4535-9; http://dx.doi.org/ 10.1073/pnas.79.15.4535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galjart NJ, Morreau H, Willemsen R, Gillemans N, Bonten EJ, D’Azzo A. Human lysosomal protective protein has cathepsin A-like activity distinct from its protective function. J Biol Chem 1991; 266:14754-62; PMID:1907282 [PubMed] [Google Scholar]
- 12. Zhou XY, Morreau H, Rottier R, Davis D, Bonten E, Gillemans N, Wenger D, Grosveld FG, Doherty P, Suzuki K, et al. Mouse model for the lysosomal disorder galactosialidosis and correction of the phenotype with overexpressing erythroid precursor cells. Genes Dev 1995; 9:2623-34; PMID:7590240; http://dx.doi.org/ 10.1101/gad.9.21.2623 [DOI] [PubMed] [Google Scholar]
- 13. van der Spoel A, Bonten E, d’Azzo A. Transport of human lysosomal neuraminidase to mature lysosomes requires protective protein/cathepsin A. EMBO J 1998; 17:1588-97; PMID:9501080; http://dx.doi.org/ 10.1093/emboj/17.6.1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Korah N, Smith CE, D’Azzo A, Mui J, Hermo L. Characterization of cell- and region-specific abnormalities in the epididymis of cathepsin A deficient mice. Mol Reprod.Dev 2003; 66:358-73; http://dx.doi.org/ 10.1002/mrd.10359 [DOI] [PubMed] [Google Scholar]
- 15. Cai J, Wang C, Huang L, Chen M, Zuo Z. A novel effect of polychlorinated biphenyls: impairment of the tight junctions in the mouse epididymis. Toxicol Sci 2013; 134:382-90. [DOI] [PubMed] [Google Scholar]
- 16. Levy S, Robaire B. Segment-specific changes with age in the expression of junctional proteins and the permeability of the blood-epididymis barrier in rats. Biol Reprod 1999; 60:1392-401; http://dx.doi.org/ 10.1095/biolreprod60.6.1392 [DOI] [PubMed] [Google Scholar]
- 17. Serre V, Robaire B. Distribution of immune cells in the epididymis of the aging Brown Norway rat is segment-specific and related to the luminal content. Biol Reprod 1999; 61:705-14; http://dx.doi.org/ 10.1095/biolreprod61.3.705 [DOI] [PubMed] [Google Scholar]
- 18. Dube E, Hermo L, Chan PT, Cyr DG. Alterations in gene expression in the caput epididymides of nonobstructive azoospermic men. Biol Reprod 2008; 78:342-51; http://dx.doi.org/ 10.1095/biolreprod.107.062760 [DOI] [PubMed] [Google Scholar]
- 19. Dube E, Hermo L, Chan PT, Cyr DG. Alterations in the human blood-epididymis barrier in obstructive azoospermia and the development of novel epididymal cell lines from infertile men. Biol Reprod 2010; 83:584-96; http://dx.doi.org/ 10.1095/biolreprod.110.084459 [DOI] [PubMed] [Google Scholar]
- 20. Duan YG, Zhang Q, Liu Y, Mou L, Li G, Gui Y, Cai Z. Dendritic cells in semen of infertile men:association with sperm quality and inflammatory status of the epididymis. Fertil Steril 2014; 101:70-7; http://dx.doi.org/ 10.1016/j.fertnstert.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 21. Shum WW, Smith TB, Cortez-Retamozo V, Grigoryeva LS, Roy JW, Hill E, Pittet MJ, Breton S, Da Silva N, et al. Epithelial basal cells are distinct from dendritic cells and macrophages in the mouse epididymis. Biol Reprod 2014; 90:90; http://dx.doi.org/ 10.1095/biolreprod.113.116681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heuser A, Mecklenburg L, Ockert D, Kohler M, Kemkowski J. Selective inhibition of PDE4 in Wistar rats can lead to dilatation in testis, efferent ducts, and epididymis and subsequent formation of sperm granulomas. Toxicol Pathol 2013; 41:615-27. [DOI] [PubMed] [Google Scholar]
- 23. Sawamoto O, Kurisu K, Kuwamura M, Kotani T, Yamate J. Relationship of interstitial edema with L-cysteine-induced sperm granulomas in the pubertal rat epididymis. Exp Toxicol Pathol 2003; 55:121-7; http://dx.doi.org/ 10.1078/0940-2993-00316 [DOI] [PubMed] [Google Scholar]
- 24. Sawamoto O, Yamate J, Kuwamura M, Kotani T, Kurisu K. Macrophage populations in L-cysteine-induced rat sperm granulomas. J Comp Pathol 2003; 129:308-12; http://dx.doi.org/ 10.1016/S0021-9975(03)00043-4 [DOI] [PubMed] [Google Scholar]
- 25. Sawamoto O, Yamate J, Kuwamura M, Kotani T, Kurisu K. Development of sperm granulomas in the epididymides of L-cysteine-treated rats. Toxicol Pathol 2003; 31:281-9. [DOI] [PubMed] [Google Scholar]
- 26. Chapin RE, White RD, Morgan KT, Bus JS. Studies of lesions induced in the testis and epididymis of F-344 rats by inhaled methyl chloride. Toxicol Appl Pharmacol 1984; 76:328-43; http://dx.doi.org/ 10.1016/0041-008X(84)90014-0 [DOI] [PubMed] [Google Scholar]
- 27. Pages L, Gavalda A, Lehner MD. PDE4 inhibitors: a review of current developments (2. Expert Opin Ther Pat 2009; 19:1501-19. [DOI] [PubMed] [Google Scholar]
- 28. Shimabukuro-Vornhagen A, Liebig TM, Koslowsky T, Theurich S, von Bergwelt-Baildon MS. The ratio between dendritic cells and T cells determines whether prostaglandin E2 has a stimulatory or inhibitory effect. Cell Immunol. 2013; 281:62-7; PMID:23454682; http://dx.doi.org/ 10.1016/j.cellimm.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 29. Heuser A, Mecklenburg L, Ockert D, Kohler M, Kemkowski J. Selective inhibition of PDE4 in Wistar rats can lead to dilatation in testis, efferent ducts, and epididymis and subsequent formation of sperm granulomas. Toxicol Pathol. 2013; 41:615-27. [DOI] [PubMed] [Google Scholar]
- 30. Chan PT, Schlegel PN. Inflammatory conditions of the male excurrent ductal system. Part II. J Androl 2002; 23:461-9; PMID:12065447 [PubMed] [Google Scholar]
- 31. Chan PT, Schlegel PN. Inflammatory conditions of the male excurrent ductal system. Part I. J Androl 2002; 23:453-60; PMID:12065446 [PubMed] [Google Scholar]
- 32. Trojian TH, Lishnak TS, Heiman D. Epididymitis and orchitis: an overview. Am Fam Physician 2009; 79:583-7; PMID:19378875 [PubMed] [Google Scholar]
- 33. Lee YS, Lee KS. Chlamydia and male lower urinary tract diseases. Korean J Urol 2013; 54:73-7; PMID:23550267; http://dx.doi.org/ 10.4111/kju.2013.54.2.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dean AS, Crump L, Greter H, Hattendorf J, Schelling E, Zinsstag J. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS Negl Trop Dis 2012; 6:e1929; http://dx.doi.org/ 10.1371/journal.pntd.0001929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sinikumpu JJ, Serlo W. Persistent scrotal pain and suspected orchido-epididymitis of a young boy during pinworm (Enterobius vermicularis) infection in the bowel. Acta Paediatr 2011; 100:e89-e90; PMID:21272069; http://dx.doi.org/ 10.1111/j.1651-2227.2011.02177.x [DOI] [PubMed] [Google Scholar]
- 36. Flickinger CJ, Yarbro ES, Howards SS, Herr JC, Caloras D, Gallien TN, Spell DR. The incidence of spermatic granulomas and their relation to testis weight after vasectomy and vasovasostomy in Lewis rats. J Androl 1986; 7:285-91; PMID:3490465 [DOI] [PubMed] [Google Scholar]
- 37. Belker AM, Konnak JW, Sharlip ID, Thomas AJ, Jr. Intraoperative observations during vasovasostomy in 334 patients. J Urol 1983; 129:524-7; PMID:6834537 [DOI] [PubMed] [Google Scholar]
- 38. Adams CE, Wald M. Risks and complications of vasectomy. Urol Clin North Am 2009; 36:331-6. [DOI] [PubMed] [Google Scholar]
- 39. McGinn JS, Sim I, Bennett NK, McDonald SW. Observations on multiple sperm granulomas in the rat epididymis following vasectomy. Clin Anat 2000; 13:185-94; PMID:10797625; http://dx.doi.org/ 10.1002/(SICI)1098-2353(2000)13:3%3c185::AID-CA5%3e3.0.CO;2-0 [DOI] [PubMed] [Google Scholar]
- 40. Flickinger CJ, Herr JC, Baran ML, Howards SS. Testicular development and the formation of spermatic granulomas of the epididymis after obstruction of the vas deferens in immature rats. J Urol 1995; 154:1539-44; PMID:7658586; http://dx.doi.org/ 10.1016/S0022-5347(01)66924-6 [DOI] [PubMed] [Google Scholar]
- 41. Flickinger CJ, Howards SS, Herr JC. Effects of vasectomy on the epididymis. Microsc Res Tech 1995; 30:82-100; http://dx.doi.org/ 10.1002/jemt.1070300107 [DOI] [PubMed] [Google Scholar]
- 42. Flickinger CJ, Baran ML, Howards SS, Herr JC. Sperm autoantigens recognized by autoantibodies in developing rats following prepubertal obstruction of the vas deferens. J Androl 1996; 17:433-42; PMID:8889707 [PubMed] [Google Scholar]
- 43. Sadek MI, Sada E, Toossi Z, Schwander SK, Rich EA. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am J Respir Cell Mol Biol 1998; 19:513-21; PMID:9730880; http://dx.doi.org/ 10.1165/ajrcmb.19.3.2815 [DOI] [PubMed] [Google Scholar]
- 44. Tani Y, Foster PM, Sills RC, Chan PC, Peddada SD, Nyska A. Epididymal sperm granuloma induced by chronic administration of 2-methylimidazole in B6C3F1 mice. Toxicol Pathol 2005; 33:313-9; PMID:15814360; http://dx.doi.org/ 10.1080/01926230590922866 [DOI] [PubMed] [Google Scholar]
- 45. Hess RA, Bunick D, Lubahn DB, Zhou Q, Bouma J. Morphologic changes in efferent ductules and epididymis in estrogen receptor-α knockout mice. J Androl 2000; 21:107-21; PMID:10670526 [PubMed] [Google Scholar]
- 46. Raymond AS, Elder B, Ensslin M, Shur BD. Loss of SED1/MFG-E8 results in altered luminal physiology in the epididymis. Mol Reprod Dev 2010; 77:550-63; PMID:20422713; http://dx.doi.org/ 10.1002/mrd.21189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bedford JM. Adaptations of the male reproductive tract and the fate of spermatozoa following vasectomy in the rabbit, rhesus monkey, hamster and rat. Biol Reprod 1976; 14:118-42; PMID:1260083; http://dx.doi.org/ 10.1095/biolreprod14.2.118 [DOI] [PubMed] [Google Scholar]
- 48. Silber SJ. Sperm granuloma and reversibility of vasectomy. Lancet 1977; 2:588-9; PMID:71402; http://dx.doi.org/ 10.1016/S0140-6736(77)91432-5 [DOI] [PubMed] [Google Scholar]
- 49. Miller RJ, Killian GJ, Vasilenko P, III. Effects of long- and short-term vasectomy on structural and functional parameters of the rat. J Androl 1984; 5:381-8. [DOI] [PubMed] [Google Scholar]
- 50. Viddeleer AC, Nijeholt GA. Lethal Fournier's gangrene following vasectomy. J Urol 1992; 147:1613-4. [DOI] [PubMed] [Google Scholar]
- 51. Chatterjee S, Laloraya M, Kumar GP. Free radical bombing of spermatozoa in spermatic granuloma:an attempt to prevent autoimmune switch-on. Biochem Biophys Res Commun 1994; 201:472-7; http://dx.doi.org/ 10.1006/bbrc.1994.1725 [DOI] [PubMed] [Google Scholar]
- 52. Chatterjee S, Laloraya M, Kumar PG. Free radical-induced liquefaction of ejaculated human semen:a new dimension in semen biochemistry. Arch.Androl 1997; 38:107-11; PMID:9049031; http://dx.doi.org/ 10.3109/01485019708987887 [DOI] [PubMed] [Google Scholar]
- 53. Chatterjee S, Rahman MM, Laloraya M, Kumar GP. Sperm disposal system in spermatic granuloma:a link with superoxide radicals. Int J Androl 2001; 24:278-83; PMID:11554985; http://dx.doi.org/ 10.1046/j.1365-2605.2001.00298.x [DOI] [PubMed] [Google Scholar]
- 54. Sikka SC. Relative impact of oxidative stress on male reproductive function. Curr Med Chem 2001; 8:851-62; PMID:11375755; http://dx.doi.org/ 10.2174/0929867013373039 [DOI] [PubMed] [Google Scholar]
- 55. Ford WC. Reactive oxygen species and sperm. Hum Fertil (Camb) 2001; 4:77-8; PMID:11591259; http://dx.doi.org/ 10.1080/1464727012000199321 [DOI] [PubMed] [Google Scholar]
- 56. Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res 2009; 129:357-67. [PubMed] [Google Scholar]
- 57. Gu Y, Dee CM, Shen J. Interaction of free radicals, matrix metalloproteinases and caveolin-1 impacts blood-brain barrier permeability. Front Biosci (Schol Ed) 2011; 3:1216-31; PMID:21622267; http://dx.doi.org/ 10.2741/222 [DOI] [PubMed] [Google Scholar]
- 58. Nonogaki T, Noda Y, Narimoto K, Shiotani M, Mori T, Matsuda T, Yoshida O. Localization of CuZn-superoxide dismutase in the human male genital organs. Hum Reprod 1992; 7:81-5. [DOI] [PubMed] [Google Scholar]
- 59. Pickles S, Vande Velde C. Misfolded SOD1 and ALS: zeroing in on mitochondria. Amyotroph Lateral Scler 2012; 13:333-40; http://dx.doi.org/ 10.3109/17482968.2012.648645 [DOI] [PubMed] [Google Scholar]
- 60. Tan W, Pasinelli P, Trotti D. Role of mitochondria in mutant SOD1 linked amyotrophic lateral sclerosis. Biochim Biophys Acta 2014; 1842:1295-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Garbuzova-Davis S, Sanberg PR. Blood-CNS Barrier Impairment in ALS patients vs. an animal model. Front Cell Neurosci. 2014; 8:21; PMID:24550780; http://dx.doi.org/ 10.3389/fncel.2014.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Veri JP, Hermo L, Robaire B. Immunocytochemical localization of the Yf subunit of glutathione S-transferase P shows regional variation in the staining of epithelial cells of the testis, efferent ducts, and epididymis of the male rat. J Androl 1993; 14:23-44; PMID:8473235 [PubMed] [Google Scholar]
- 63. Namdarghanbari M, Wobig W, Krezoski S, Tabatabai NM, Petering DH. Mammalian metallothionein in toxicology, cancer, and cancer chemotherapy. J Biol Inorg Chem 2011; 16:1087-101; http://dx.doi.org/ 10.1007/s00775-011-0823-6 [DOI] [PubMed] [Google Scholar]
- 64. Gunther V, Lindert U, Schaffner W. The taste of heavy metals: gene regulation by MTF-1. Biochim Biophys Acta 2012; 1823:1416-25; PMID:22289350; http://dx.doi.org/ 10.1016/j.bbamcr.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 65. Sears ME. Chelation:harnessing and enhancing heavy metal detoxification–a review. ScientificWorldJournal 2013; 2013:219840; PMID:23690738; http://dx.doi.org/ 10.1155/2013/219840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, et al. Cadmium stress:an oxidative challenge. Biometals 2010; 23:927-40; PMID:20361350; http://dx.doi.org/ 10.1007/s10534-010-9329-x [DOI] [PubMed] [Google Scholar]
- 67. Chiaverini N, De LM. Protective effect of metallothionein on oxidative stress-induced DNA damage. Free Radic Res 2010; 44:605-13. [DOI] [PubMed] [Google Scholar]
- 68. Namdarghanbari M, Wobig W, Krezoski S, Tabatabai NM, Petering DH. Mammalian metallothionein in toxicology, cancer, and cancer chemotherapy. J Biol Inorg Chem 2011; 16:1087-101; http://dx.doi.org/ 10.1007/s00775-011-0823-6 [DOI] [PubMed] [Google Scholar]
- 69. De LE, Gagnon C. Human sperm hyperactivation and capacitation as parts of an oxidative process. Free Radic Biol Med 1993; 14:157-66. [DOI] [PubMed] [Google Scholar]
- 70. Aitken RJ, Paterson M, Fisher H, Buckingham DW, van DM. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci 1995; 108 (Pt 5):2017-25. [DOI] [PubMed] [Google Scholar]
- 71. Aitken RJ. Possible redox regulation of sperm motility activation. J Androl 2000; 21:491-6; PMID:10901434 [PubMed] [Google Scholar]
- 72. Griveau JF, Renard P, Le LD. Superoxide anion production by human spermatozoa as a part of the ionophore-induced acrosome reaction process. Int J Androl 1995; 18:67-74; PMID:7665212; http://dx.doi.org/ 10.1111/j.1365-2605.1995.tb00388.x [DOI] [PubMed] [Google Scholar]
- 73. Shum WW, Da SN, McKee M, Smith PJ, Brown D, Breton S. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell 2008; 135:1108-17; PMID:19070580; http://dx.doi.org/ 10.1016/j.cell.2008.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wilson CL, Matrisian LM. Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol 1996; 28:123-36; http://dx.doi.org/ 10.1016/1357-2725(95)00121-2 [DOI] [PubMed] [Google Scholar]
- 75. Wilson CL, Heppner KJ, Rudolph LA, Matrisian LM. The metalloproteinase matrilysin is preferentially expressed by epithelial cells in a tissue-restricted pattern in the mouse. Mol Biol Cell 1995; 6:851-69; PMID:7579699; http://dx.doi.org/ 10.1091/mbc.6.7.851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stammler A, Muller D, Tabuchi Y, Konrad L, Middendorff R. TGFbetas modulate permeability of the blood-epididymis barrier in an in vitro model. PLoS One 2013; 8:e80611; http://dx.doi.org/ 10.1371/journal.pone.0080611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wise SK, Laury AM, Katz EH, Den Beste KA, Parkos CA, Nusrat A. Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. Int Forum Allergy Rhinol 2014; 4:361-70; http://dx.doi.org/ 10.1002/alr.21298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Desai KV, Flanders KC, Kondaiah P. Expression of transforming growth factor-β isoforms in the rat male accessory sex organs and epididymis. Cell Tissue Res 1998; 294:271-7; PMID:9799443; http://dx.doi.org/ 10.1007/s004410051177 [DOI] [PubMed] [Google Scholar]
- 79. Bomgardner D, Wehrenberg U, Rune GM. TGF-β could be involved in paracrine actions in the epididymis of the marmoset monkey (Callithrix jacchus). J Androl 1999; 20:375-83; PMID:10386817 [PubMed] [Google Scholar]
- 80. Henderson NA, Cooke GM, Robaire B. Region-specific expression of androgen and growth factor pathway genes in the rat epididymis and the effects of dual 5alpha-reductase inhibition. J Endocrinol 2006; 190:779-91; http://dx.doi.org/ 10.1677/joe.1.06862 [DOI] [PubMed] [Google Scholar]
- 81. Lui WY, Lee WM, Cheng CY. Transforming growth factor beta3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod 2003; 68:1597-612; http://dx.doi.org/ 10.1095/biolreprod.102.011387 [DOI] [PubMed] [Google Scholar]
- 82. Lui WY, Lee WM, Cheng CY. Transforming growth factor-beta3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology 2001; 142:1865-77; PMID:11316752 [DOI] [PubMed] [Google Scholar]
- 83. Da SN, Cortez-Retamozo V, Reinecker HC, Wildgruber M, Hill E, Brown D, Swirski FK, Pittet MJ, Breton S. A dense network of dendritic cells populates the murine epididymis. Reproduction 2011; 141:653-63; PMID:21310816; http://dx.doi.org/ 10.1530/REP-10-0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shiraishi N, Nomura T, Tanizaki H, Nakajima S, Narumiya S, Miyachi Y, Tokura Y, Kabashima K. Prostaglandin E2-EP3 axis in fine-tuning excessive skin inflammation by restricting dendritic cell functions. PLoS One 2013; 8:e69599; http://dx.doi.org/ 10.1371/journal.pone.0069599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shiraishi H, Yoshida H, Saeki K, Miura Y, Watanabe S, Ishizaki T, Hashimoto M, Takaesu G, Kobayashi T, Yoshimura A. Prostaglandin E2 is a major soluble factor produced by stromal cells for preventing inflammatory cytokine production from dendritic cells. Int Immunol 2008; 20:1219-29; PMID:18640970; http://dx.doi.org/ 10.1093/intimm/dxn078 [DOI] [PubMed] [Google Scholar]
- 86. Da SN, Cortez-Retamozo V, Reinecker HC, Wildgruber M, Hill E, Brown D, Swirski FK, Pittet MJ, Breton S. A dense network of dendritic cells populates the murine epididymis. Reproduction 2011; 141:653-63; PMID:21310816; http://dx.doi.org/ 10.1530/REP-10-0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pope JL, Bhat AA, Sharma A, Ahmad R, Krishnan M, Washington MK, Beauchamp RD, Singh AB, Dhawan P. Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut 2014; 63:622-34; PMID:23766441; http://dx.doi.org/ 10.1136/gutjnl-2012-304241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Muhlbauer M, Cheely AW, Yenugu S, Jobin C. Regulation and functional impact of lipopolysaccharide induced Nod2 gene expression in the murine epididymal epithelial cell line PC1. Immunology 2008; 124:256-64; PMID:18284470; http://dx.doi.org/ 10.1111/j.1365-2567.2007.02763.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Reuven EM, Fink A, Shai Y. Regulation of innate immune responses by transmembrane interactions:Lessons from the TLR family. Biochim Biophys Acta 2014; 1838:1586-93. [DOI] [PubMed] [Google Scholar]
- 90. Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 2004; 430:257-63; PMID:15241424; http://dx.doi.org/ 10.1038/nature02761 [DOI] [PubMed] [Google Scholar]
- 91. Silva EJ, Queiroz DB, Rodrigues A, Honda L, Avellar MC. Innate immunity and glucocorticoids: potential regulatory mechanisms in epididymal biology. J Androl 2011; 32:614-24. [DOI] [PubMed] [Google Scholar]
- 92. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011; 34:637-50; PMID:21616434; http://dx.doi.org/ 10.1016/j.immuni.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 93. Malm J, Nordahl EA, Bjartell A, Sorensen OE, Frohm B, Dentener MA, et al. Lipopolysaccharide-binding protein is produced in the epididymis and associated with spermatozoa and prostasomes. J Reprod Immunol 2005; 66:33-43; PMID:15949560; http://dx.doi.org/ 10.1016/j.jri.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 94. Gatti G, Rivero V, Motrich RD, Maccioni M. Prostate epithelial cells can act as early sensors of infection by up-regulating TLR4 expression and proinflammatory mediators upon LPS stimulation. J Leukoc Biol 2006; 79:989-98; PMID:16522744; http://dx.doi.org/ 10.1189/jlb.1005597 [DOI] [PubMed] [Google Scholar]
- 95. Quintar AA, Roth FD, De Paul AL, Aoki A, Maldonado CA. Toll-like receptor 4 in rat prostate: modulation by testosterone and acute bacterial infection in epithelial and stromal cells. Biol Reprod 2006; 75:664-72; PMID:16870940; http://dx.doi.org/ 10.1095/biolreprod.106.053967 [DOI] [PubMed] [Google Scholar]
- 96. Girling JE, Hedger MP. Toll-like receptors in the gonads and reproductive tract: emerging roles in reproductive physiology and pathology. Immunol Cell Biol 2007; 85:481-9. [DOI] [PubMed] [Google Scholar]
- 97. Palladino MA, Johnson TA, Gupta R, Chapman JL, Ojha P. Members of the Toll-like receptor family of innate immunity pattern-recognition receptors are abundant in the male rat reproductive tract. Biol Reprod 2007; 76:958-64; PMID:17314314; http://dx.doi.org/ 10.1095/biolreprod.106.059410 [DOI] [PubMed] [Google Scholar]
- 98. Palladino MA, Savarese MA, Chapman JL, Dughi MK, Plaska D. Localization of Toll-like receptors on epididymal epithelial cells and spermatozoa. Am J Reprod Immunol 2008; 60:541-55; PMID:19032616; http://dx.doi.org/ 10.1111/j.1600-0897.2008.00654.x [DOI] [PubMed] [Google Scholar]
- 99. Bhushan S, Schuppe HC, Tchatalbachev S, Fijak M, Weidner W, Chakraborty T, Meinhardt A. Testicular innate immune defense against bacteria. Mol Cell Endocrinol 2009; 306:37-44; PMID:19010387; http://dx.doi.org/ 10.1016/j.mce.2008.10.017 [DOI] [PubMed] [Google Scholar]
- 100. Rodrigues A, Queiroz DB, Honda L, Silva EJ, Hall SH, Avellar MC. Activation of toll-like receptor 4 (TLR4) by in vivo and in vitro exposure of rat epididymis to lipopolysaccharide from Escherichia Coli. Biol Reprod 2008; 79:1135-47; PMID:18703421; http://dx.doi.org/ 10.1095/biolreprod.108.069930 [DOI] [PubMed] [Google Scholar]
- 101. Zhao YT, Guo JH, Wu ZL, Xiong Y, Zhou WL. Innate immune responses of epididymal epithelial cells to Staphylococcus aureus infection. Immunol Lett 2008; 119:84-90. [DOI] [PubMed] [Google Scholar]
- 102. Albiger B, Dahlberg S, Henriques-Normark B, Normark S. Role of the innate immune system in host defence against bacterial infections: focus on the Toll-like receptors. J Intern Med 2007; 261:511-28; PMID:17547708; http://dx.doi.org/ 10.1111/j.1365-2796.2007.01821.x [DOI] [PubMed] [Google Scholar]
- 103. Dann SM, Eckmann L. Innate immune defenses in the intestinal tract. Curr Opin Gastroenterol 2007; 23:115-20; http://dx.doi.org/ 10.1097/MOG.0b013e32803cadf4 [DOI] [PubMed] [Google Scholar]
- 104. Heidemann J, Domschke W, Kucharzik T, Maaser C. Intestinal microvascular endothelium and innate immunity in inflammatory bowel disease: a second line of defense? Infect Immun 2006; 74:5425-32; PMID:16988217; http://dx.doi.org/ 10.1128/IAI.00248-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Harris G, Kuolee R, Chen W. Role of Toll-like receptors in health and diseases of gastrointestinal tract. World J Gastroenterol 2006; 12:2149-60; PMID:16610014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cao D, Li Y, Yang R, Wang Y, Zhou Y, Diao H, et al. Lipopolysaccharide-induced epididymitis disrupts epididymal β-defensin expression and inhibits sperm motility in rats. Biol Reprod 2010; 83:1064-70; PMID:20826730; http://dx.doi.org/ 10.1095/biolreprod.109.082180 [DOI] [PubMed] [Google Scholar]
- 107. Voss E, Wehkamp J, Wehkamp K, Stange EF, Schroder JM, Harder J. NOD2/CARD15 mediates induction of the antimicrobial peptide human β-defensin-2. J Biol Chem 2006; 281:2005-11; PMID:16319062; http://dx.doi.org/ 10.1074/jbc.M511044200 [DOI] [PubMed] [Google Scholar]
- 108. Com E, Bourgeon F, Evrard B, Ganz T, Colleu D, Jegou B, et al. Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol Reprod. 2003; 68:95-104; PMID:12493700; http://dx.doi.org/ 10.1095/biolreprod.102.005389 [DOI] [PubMed] [Google Scholar]
- 109. Yamaguchi Y, Ouchi Y. Antimicrobial peptide defensin:identification of novel isoforms and the characterization of their physiological roles and their significance in the pathogenesis of diseases. Proc Jpn Acad Ser B Phys Biol Sci 2012; 88:152-66; PMID:22498979; http://dx.doi.org/ 10.2183/pjab.88.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J. Human β-defensins. Cell Mol Life Sci 2006; 63:1294-313; PMID:16710608; http://dx.doi.org/ 10.1007/s00018-005-5540-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kluver E, Adermann K, Schulz A. Synthesis and structure-activity relationship of β-defensins, multi-functional peptides of the immune system. J Pept Sci 2006; 12:243-57; PMID:16491514; http://dx.doi.org/ 10.1002/psc.749 [DOI] [PubMed] [Google Scholar]
- 112. Li P, Chan HC, He B, So SC, Chung YW, Shang Q, Zhang YD, Zhang Y. An antimicrobial peptide gene found in the male reproductive system of rats. Science 2001; 291:1783-5; PMID:11230693; http://dx.doi.org/ 10.1126/science.1056545 [DOI] [PubMed] [Google Scholar]
- 113. Yudin AI, Tollner TL, Li MW, Treece CA, Overstreet JW, Cherr GN. ESP13.2, a member of the β-defensin family, is a macaque sperm surface-coating protein involved in the capacitation process. Biol Reprod 2003; 69:1118-28; PMID:12773404; http://dx.doi.org/ 10.1095/biolreprod.103.016105 [DOI] [PubMed] [Google Scholar]
- 114. Zanich A, Pascall JC, Jones R. Secreted epididymal glycoprotein 2D6 that binds to the sperm's plasma membrane is a member of the β-defensin superfamily of pore-forming glycopeptides. Biol Reprod 2003; 69:1831-42; PMID:12890730; http://dx.doi.org/ 10.1095/biolreprod.103.018606 [DOI] [PubMed] [Google Scholar]
- 115. Zhou CX, Zhang YL, Xiao L, Zheng M, Leung KM, Chan MY, Lo PS, Tsang LL, Wong HY, Ho LS, et al. An epididymis-specific β-defensin is important for the initiation of sperm maturation. Nat Cell Biol 2004; 6:458-64; PMID:15122269; http://dx.doi.org/ 10.1038/ncb1127 [DOI] [PubMed] [Google Scholar]
- 116. Tollner TL, Bevins CL, Cherr GN. Multifunctional glycoprotein DEFB126–a curious story of defensin-clad spermatozoa. Nat Rev Urol 2012; 9:365-75; PMID:22710670; http://dx.doi.org/ 10.1038/nrurol.2012.109 [DOI] [PubMed] [Google Scholar]
- 117. Perry AC, Jones R, Hall L. The monkey ESP14.6 mRNA, a novel transcript expressed at high levels in the epididymis. Gene 1995; 153:291-2; PMID:7875608; http://dx.doi.org/ 10.1016/0378-1119(94)00739-F [DOI] [PubMed] [Google Scholar]
- 118. Yudin AI, Treece CA, Tollner TL, Overstreet JW, Cherr GN. The carbohydrate structure of DEFB126, the major component of the cynomolgus Macaque sperm plasma membrane glycocalyx. J Membr Biol 2005; 207:119-29; PMID:16550483; http://dx.doi.org/ 10.1007/s00232-005-0806-z [DOI] [PubMed] [Google Scholar]
- 119. Tollner TL, Yudin AI, Treece CA, Overstreet JW, Cherr GN. Macaque sperm release ESP13.2 and PSP94 during capacitation: the absence of ESP13.2 is linked to sperm-zona recognition and binding. Mol Reprod Dev 2004; 69:325-37; PMID:15349845; http://dx.doi.org/ 10.1002/mrd.20132 [DOI] [PubMed] [Google Scholar]
- 120. Schroter S, Osterhoff C, McArdle W, Ivell R. The glycocalyx of the sperm surface. Hum Reprod Update 1999; 5:302-13; PMID:10465522; http://dx.doi.org/ 10.1093/humupd/5.4.302 [DOI] [PubMed] [Google Scholar]
- 121. Dacheux JL, Gatti JL, Dacheux F. Contribution of epididymal secretory proteins for spermatozoa maturation. Microsc Res Tech 2003; 61:7-17; PMID:12672118; http://dx.doi.org/ 10.1002/jemt.10312 [DOI] [PubMed] [Google Scholar]
- 122. Yudin AI, Generao SE, Tollner TL, Treece CA, Overstreet JW, Cherr GN. Beta-defensin 126 on the cell surface protects sperm from immunorecognition and binding of anti-sperm antibodies. Biol Reprod 2005; 73:1243-52; PMID:16079310; http://dx.doi.org/ 10.1095/biolreprod.105.042432 [DOI] [PubMed] [Google Scholar]
- 123. Tollner TL, Yudin AI, Treece CA, Overstreet JW, Cherr GN. Macaque sperm coating protein DEFB126 facilitates sperm penetration of cervical mucus. Hum Reprod 2008; 23:2523-34; PMID:18658160; http://dx.doi.org/ 10.1093/humrep/den276 [DOI] [PubMed] [Google Scholar]
- 124. Tollner TL, Yudin AI, Tarantal AF, Treece CA, Overstreet JW, Cherr GN. Beta-defensin 126 on the surface of macaque sperm mediates attachment of sperm to oviductal epithelia. Biol Reprod 2008; 78:400-12; PMID:18003946; http://dx.doi.org/ 10.1095/biolreprod.107.064071 [DOI] [PubMed] [Google Scholar]
- 125. Dube E, Chan PT, Hermo L, Cyr DG. Gene expression profiling and its relevance to the blood-epididymal barrier in the human epididymis. Biol Reprod. 2007; 76:1034-44; PMID:17287494; http://dx.doi.org/ 10.1095/biolreprod.106.059246 [DOI] [PubMed] [Google Scholar]
- 126. Tollner TL, Venners SA, Hollox EJ, Yudin AI, Liu X, Tang G, et al. A common mutation in the defensin DEFB126 causes impaired sperm function and subfertility. Sci Transl Med 2011; 3:92ra65; http://dx.doi.org/ 10.1126/scitranslmed.3002289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol 2006; 68:403-29; PMID:16460278; http://dx.doi.org/ 10.1146/annurev.physiol.68.040104.131404 [DOI] [PubMed] [Google Scholar]
- 128. Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb. Perspect Biol 2009; 1:a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K, et al. Predicted expansion of the claudin multigene family. FEBS Lett 2011; 585:606-12; PMID:21276448; http://dx.doi.org/ 10.1016/j.febslet.2011.01.028 [DOI] [PubMed] [Google Scholar]
- 130. Dube E, Cyr DG. The blood-epididymis barrier and human male fertility. Adv Exp Med Biol 2012; 763:218-36; PMID:23397627 [DOI] [PubMed] [Google Scholar]
- 131. Gunzel D, Fromm M. Claudins and other tight junction proteins. Compr Physiol 2012; 2:1819-52; PMID:23723025 [DOI] [PubMed] [Google Scholar]
- 132. Sanchez-Pulido L, Martin-Belmonte F, Valencia A, Alonso MA. MARVEL: a conserved domain involved in membrane apposition events. Trends Biochem Sci 2002; 27:599-601; PMID:12468223; http://dx.doi.org/ 10.1016/S0968-0004(02)02229-6 [DOI] [PubMed] [Google Scholar]
- 133. Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell 2010; 21:1200-13; PMID:20164257; http://dx.doi.org/ 10.1091/mbc.E09-08-0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 2005; 171:939-45; PMID:16365161; http://dx.doi.org/ 10.1083/jcb.200510043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 2000; 11:4131-42; PMID:11102513; http://dx.doi.org/ 10.1091/mbc.11.12.4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Cyr DG, Hermo L, Egenberger N, Mertineit C, Trasler JM, Laird DW. Cellular immunolocalization of occludin during embryonic and postnatal development of the mouse testis and epididymis. Endocrinology 1999; 140:3815-25; PMID:10433243 [DOI] [PubMed] [Google Scholar]
- 137. Gregory M, Dufresne J, Hermo L, Cyr D. Claudin-1 is not restricted to tight junctions in the rat epididymis. Endocrinology 2001; 142:854-63; PMID:11159859 [DOI] [PubMed] [Google Scholar]
- 138. Guan X, Inai T, Shibata Y. Segment-specific expression of tight junction proteins, claudin-2 and -10, in the rat epididymal epithelium. Arch Histol Cytol 2005; 68:213-25; PMID:16276027; http://dx.doi.org/ 10.1679/aohc.68.213 [DOI] [PubMed] [Google Scholar]
- 139. Gregory M, Cyr DG. Identification of multiple claudins in the rat epididymis. Mol Reprod Dev 2006; 73:580-8; PMID:16489631; http://dx.doi.org/ 10.1002/mrd.20467 [DOI] [PubMed] [Google Scholar]
- 140. DeBellefeuille S, Hermo L, Gregory M, Dufresne J, Cyr DG. Catenins in the rat epididymis: their expression and regulation in adulthood and during postnatal development. Endocrinology 2003; 144:5040-9; PMID:12960056; http://dx.doi.org/ 10.1210/en.2002-0139 [DOI] [PubMed] [Google Scholar]
- 141. Gregory M, Dufresne J, Hermo L, Cyr D. Claudin-1 is not restricted to tight junctions in the rat epididymis. Endocrinology 2001; 142:854-63; PMID:11159859 [DOI] [PubMed] [Google Scholar]
- 142. Dufresne J, Cyr DG. Activation of an SP binding site is crucial for the expression of claudin 1 in rat epididymal principal cells. Biol Reprod 2007; 76:825-32; PMID:17251524; http://dx.doi.org/ 10.1095/biolreprod.106.057430 [DOI] [PubMed] [Google Scholar]
- 143. Lopardo T, Lo IN, Marinari B, Giustizieri ML, Cyr DG, Merlo G, Crosti F, Costanzo A, Guerrini L. Claudin-1 is a p63 target gene with a crucial role in epithelial development. PLoS.One. 2008; 3:e2715; http://dx.doi.org/ 10.1371/journal.pone.0002715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Belleannee C, Calvo E, Thimon V, Cyr DG, Legare C, Garneau L, Sullivan R. Role of microRNAs in controlling gene expression in different segments of the human epididymis. PLoS One 2012; 7:e34996; PMID:22511979; http://dx.doi.org/ 10.1371/journal.pone.0034996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Veri JP, Hermo L, Robaire B. Immunocytochemical localization of glutathione S-transferase Yo subunit in the rat testis and epididymis. J Androl 1994; 15:415-34; PMID:7860422 [PubMed] [Google Scholar]
- 146. Shum WW, Da SN, Brown D, Breton S. Regulation of luminal acidification in the male reproductive tract via cell-cell crosstalk. J Exp Biol 2009; 212:1753-61; PMID:19448084; http://dx.doi.org/ 10.1242/jeb.027284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wong PY, Chan HC, Leung PS, Chung YW, Wong YL, Lee WM, Ng V, Dun NJ. Regulation of anion secretion by cyclo-oxygenase and prostanoids in cultured epididymal epithelia from the rat. J Physiol 1999; 514 (Pt 3):809-20; PMID:9882752; http://dx.doi.org/ 10.1111/j.1469-7793.1999.809ad.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wong PY. CFTR gene and male fertility. Mol Hum Reprod 1998; 4:107-10; PMID:9542966; http://dx.doi.org/ 10.1093/molehr/4.2.107 [DOI] [PubMed] [Google Scholar]
- 149. Abd-Allah AR, Helal GK, Al-Yahya AA, Aleisa AM, Al-Rejaie SS, Al-Bakheet SA. Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L-carnitine. Oxid.Med.Cell Longev. 2009; 2:73-81; http://dx.doi.org/ 10.4161/oxim.2.2.8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Leslie EM, Deeley RG, Cole SP. Toxicological relevance of the multidrug resistance protein 1, MRP1 (ABCC1) and related transporters. Toxicology 2001; 167:3-23; PMID:11557126; http://dx.doi.org/ 10.1016/S0300-483X(01)00454-1 [DOI] [PubMed] [Google Scholar]
- 151. Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol.Rev. 2010; 62:1-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Seeger MA, van Veen HW. Molecular basis of multidrug transport by ABC transporters. Biochim Biophys Acta 2009; 1794:725-37; PMID:19135557; http://dx.doi.org/ 10.1016/j.bbapap.2008.12.004 [DOI] [PubMed] [Google Scholar]
- 153. Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A 1987; 84:7735-8; PMID:2444983; http://dx.doi.org/ 10.1073/pnas.84.21.7735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Jones SR, Cyr DG. Regulation and characterization of the ATP-binding cassette transporter-B1 in the epididymis and epididymal spermatozoa of the rat. Toxicol.Sci. 2011; 119:369-79. [DOI] [PubMed] [Google Scholar]
- 155. Dufresne J, St-Pierre N, Viger RS, Hermo L, Cyr DG. Characterization of a novel rat epididymal cell line to study epididymal function. Endocrinology 2005; 146:4710-20; PMID:16099865; http://dx.doi.org/ 10.1210/en.2004-1634 [DOI] [PubMed] [Google Scholar]
- 156. Chan HC, Ko WH, Zhao W, Fu WO, Wong PY. Evidence for independent Cl- and. Exp Physiol 1996; 81:515-24; PMID:8737084 [DOI] [PubMed] [Google Scholar]
- 157. Radpour R, Gourabi H, Dizaj AV, Holzgreve W, Zhong XY. Genetic investigations of CFTR mutations in congenital absence of vas deferens, uterus, and vagina as a cause of infertility. J Androl 2008; 29:506-13; PMID:18567645; http://dx.doi.org/ 10.2164/jandrol.108.005074 [DOI] [PubMed] [Google Scholar]
- 158. Claustres M. Molecular pathology of the CFTR locus in male infertility. Reprod Biomed Online 2005; 10:14-41; PMID:15705292; http://dx.doi.org/ 10.1016/S1472-6483(10)60801-2 [DOI] [PubMed] [Google Scholar]
- 159. van d V, Messer L, van d V, Jeyendran RS, Ober C. Cystic fibrosis mutation screening in healthy men with reduced sperm quality. Hum Reprod 1996; 11:513-7; PMID:8671256; http://dx.doi.org/ 10.1093/HUMREP/11.3.513 [DOI] [PubMed] [Google Scholar]
- 160. Schulz S, Jakubiczka S, Kropf S, Nickel I, Muschke P, Kleinstein J. Increased frequency of cystic fibrosis transmembrane conductance regulator gene mutations in infertile males. Fertil Steril 2006; 85:135-8; PMID:16412743; http://dx.doi.org/ 10.1016/j.fertnstert.2005.07.1282 [DOI] [PubMed] [Google Scholar]
- 161. Cuppens H, Cassiman JJ. CFTR mutations and polymorphisms in male infertility. Int J Androl 2004; 27:251-6; PMID:15379964; http://dx.doi.org/ 10.1111/j.1365-2605.2004.00485.x [DOI] [PubMed] [Google Scholar]
- 162. Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, Joo NS, Zhang Y, Zhou W, Yi Y, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest 2010; 120:3149-60; PMID:20739752; http://dx.doi.org/ 10.1172/JCI43052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Pierucci-Alves F, Akoyev V, Stewart JC, III, Wang LH, Janardhan KS, Schultz BD. Swine models of cystic fibrosis reveal male reproductive tract phenotype at birth. Biol Reprod. 2011; 85:442-51; PMID:21593481; http://dx.doi.org/ 10.1095/biolreprod.111.090860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Reynaert I, Van Der Schueren B, Degeest G, Manin M, Cuppens H, Scholte B, Cassiman JJ. Morphological changes in the vas deferens and expression of the cystic fibrosis transmembrane conductance regulator (CFTR) in control, deltaF508 and knock-out CFTR mice during postnatal life. Mol Reprod Dev 2000; 55:125-35; PMID:10618651; http://dx.doi.org/ 10.1002/(SICI)1098-2795(200002)55:2%3c125::AID-MRD1%3e3.0.CO;2-Q [DOI] [PubMed] [Google Scholar]
- 165. Xu WM, Shi QX, Chen WY, Zhou CX, Ni Y, Rowlands DK, Yi Liu G, Zhu H, Ma ZG, Wang XF, et al. Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc Natl Acad Sci U S A 2007; 104:9816-21; PMID:17519339; http://dx.doi.org/ 10.1073/pnas.0609253104 [DOI] [PMC free article] [PubMed] [Google Scholar]


