Abstract
The testicular histology and cytology of spermatogenesis in Graptemys pseudogeographica kohnii were examined using specimens collected between July 1996 and May 2004 from counties in northeastern Arkansas. A histological examination of the testes and germ cell cytology indicates a postnuptial testicular cycle of spermatogenesis and a major fall spermiation event. The majority of the germ cell populations in May and June specimens are represented by resting spermatogonia, type A spermatogonia, type B spermatogonia, pre-leptotene spermatocytes, and numerous Sertoli cell nuclei near the basement membrane. The start of proliferation is evident as spermatogonia in metaphase are present near the basal lamina and many of these germ cells have entered meiosis in June seminiferous tubules. Major spermatogenic events occur in the June and July specimens and result in an increased height of the seminiferous epithelium and increased diameter of the seminiferous tubules. The germ cell population during this time is represented by spermatogonia (type A, B, and resting), hypertrophic cells, large populations of early primary spermatocytes, and early round spermatids. By September, the major germ cell population has progressed past meiosis with abundant round and early elongating spermatids dominating the seminiferous epithelium. October seminiferous epithelia are marked by a decreas in height and mature spermatozoa fill the luminal space. Round and elongating spermatids constitute the largest portion of the germ cell population. Following the spermiation event, the testes enter a period of quiescence that lasts till the next spermatogenic cycle, which begins in the subsequent spring. Based on the cytological development of the seminiferous tubules revealed by our study, Graptemys pseudogeographica kohnii demonstrates a temporal germ cell development strategy similar to other temperate reptiles. A single major generation of germ cells progresses through spermatogenesis each year resulting in a single spermiation event with sperm stored within the epididymis until the next spring mating season.
Keywords: germ cell development, Graptemys pseudogeographica kohnii, seminiferous epithelium, spermatogenesis, testicular cycle, turtle
Introduction
Recent work has characterized germ cell development strategies of several reptiles, including turtles.1 Spermatogenesis is a complex process required of all vertebrate males in which progenitor cells mitotically divide, undergo meiosis, and differentiate into motile spermatozoa with the function of passing on genetic information by means of sexual reproduction.
An understanding of testicular architecture and germ cell arrangement within the seminiferous tubules and testicular epithelia are critical in describing the spermatogenic cycle of reptiles. Two classical germ cell development hypotheses have been described with a possible convergence or reversal resulting in an alternative plesiomorphic germ cell development strategy exhibited by reptiles.2 Temporal germ cell development, as described in anamniotic vertebrates such as fish and amphibians, is characterized by germ cells progressing through spermatogenesis as a single cohort within cysts that line the testis.3 Upon maturation of spermatogenesis, the cysts rupture and release spermatozoa from these cysts into a centralized lumen in a single spermiation event; this event often compromises Sertoli cell integrity, as well as the blood-testis barrier.4
Contrastingly, the germ cell development strategy of amniotes, such as mammals and birds, is characterized by a distinct spatial arrangement of the developing germ cells within the seminiferous epithelium and seminiferous tubules.5 The consistent and predictable distribution from the basal lamina of the seminiferous tubule to the lumen allows germ cells further into development to be associated with early mitotic and meiotic cells.5 In fact, within the amniotic testis, 3 to 5 generations of germ cells are consistently found together within a single cross-sectional view of the seminiferous tubules.5 The spatial germ cell strategy exhibits stages sequentially ordered along the length of the seminiferous tubules, which allows for constant waves of sperm release without damage to the integrity of the Sertoli cells or to the seminiferous epithelium.5
Recently, an additional intermediate type of germ cell development exhibited by reptiles has been described as a plesiomorphic germ cell development strategy resulting from either convergence of the spatial germ cell development or character reversal in modern reptiles to the temporal germ cell strategy.2 Reptiles possess testes structurally typical of that of amniotes including seminiferous tubules, seminiferous epithelium, and a permanent population of germinal cells and Sertoli cells; however, their germ cell development strategy is characterized more closely to that of the anamniotic temporal germ cell development strategy.5 Spermiation occurs along large portions of the seminiferous tubules, and no consistent spatial relationships are observed among the germ cells; this may suggest a decoupling of testicular organization and germ cell development strategy during testicular evolution.
The germ cells within the seminiferous epithelium of the reptilian testis progress collectively through the stages of spermatogenesis, and the result is a single sporadic spermiation event during spermatogenically active periods with decreased activity in the non-breeding periods, both events of which are characteristic of the anamniotic testes.3 The mitotic and meiotic cells are restricted to the basement membrane and dividing spermatogonia are always present, except in turtles in the late reproductive season when only resting spermatogonia are typically seen.1 During active spermatogenesis, large units of cells mature together and enter the following stages of spermiogenesis: acrosome development, elongation, and maturation. Deep luminal columns accumulate due to the expansion of the seminiferous epithelium, resulting in the presence of 5 to 6 different spermatids within a column. During the late reproductive season, the testis enters a quiescent period and spermatogenesis slows or ceases in seasonal reptiles.7 The seminiferous tubules during this quiescent period show a remarkably reduced seminiferous epithelial height, and apical sloughing of the Sertoli cell cytoplasm into the lumen to be reabsorbed along the length of the seminiferous tubules.1
Testicular cycles of temperate turtle species can vary in the activity of spermatogenesis within the testis and can exhibit either postnuptial or prenuptial activity.8-11 A postnuptial testicular cycle is characterized by brief periods of testicular enlargement and spermatogenic activity combined with long quiescent periods in which no spermatogenesis occurs. A new cycle of spermatogenic activity does not commence until spring mating has occurred, and this activity is completed prior to winter with sperm being stored in the epididymis.11 The testis of a turtle exhibiting this postnuptial testicular cycle will be active primarily during the spring and summer months with increased testicular size, and then activity will cease in the winter months resulting in decreased testicular size due to quiescence. A prenuptial testicular cycle is characterized by rapid testicular enlargement and spermatogenic activity prior to the mating season in the spring. The rapid proliferation could be due to the large population of type A and B spermatogonia, which have the ability to continually divide. In contrast to a postnuptial testicular cycle, a testis exhibiting a prenuptial cycle does not store sperm in the epididymis but rather sperm remain in the seminiferous tubules until the mating event.12
Among members of the turtle family Emydidae, only 7 of the approximately 95 species have received any attention with respect to their spermatogenic cycles.10 No work describing the early stages in the spermatogenic cycle of any species within the genus Graptemys has been investigated.11 The Mississippi map turtle, Graptemys pseudogeographica kohnii, is a moderately large freshwater emydid turtle that is found primarily along the Mississippi River drainage system from eastern Missouri, central Missouri, and Nebraska to Texas, Louisiana, and Mississippi.13,14 This subspecies is active from late March to mid-October with mating usually occurring in late March to early April.15 In males found in Missouri, individuals as small as 10.3 cm in plastron length contain live sperm, but their testes were relatively small.11 The objectives of the present study were to examine the testicular histology of this species and examine its spermatogenic germ cell cycle and germ cell development strategy.
Materials and Methods
All 21 male Graptemys pseudogeographica kohnii used during the present study had been collected periodically and seasonally over an 8-yr span (1996–2004) from river drainages in northeastern Arkansas. Their testes had been removed, prepared for examination by light microscopy (LM), and then housed in the Herpetological Tissue Collection within the Electron Microscope Facility at Arkansas State University. We employed standard histological techniques to prepare these testes for LM, following either the paraffin embedding method or the plastic embedding method.16,17 These methods have been reviewed and are briefly summarized below.18 Portions of testes were fixed in either 10% neutral buffered formalin—NBF (for paraffin sectioning) for 48 h or 2% glutaraldehyde (GTA) solution buffered with 0.1 M sodium cacodylate at a pH of 7.2 (for plastic sectioning) for 2 h. For postfixation, a 1% osmium tetroxide solution was utilized (buffered as above) for 2 h in GTA-fixed testes. Testes for paraffin embedding were dehydrated in a graded series of increasing ethanol solutions (50–100%), cleared with xylene, and infiltrated and embedded in paraffin wax. Paraffin/tissue blocks were trimmed of excess wax, serially sectioned into ribbons 10 μm thick using a rotary microtome, and affixed to microscope slides using Haupt's adhesive while floating on a 2% NBF solution.
Alternating sets of 4 slides were treated with 4 histological stains: 1) hematoxylin and eosin (H & E) for general cytology; 2) Pollak trichrome stain for connective tissues and mucins; 3) alcian blue 8GX for carboxylated glycosaminoglycans (pH = 2.8), and 4) the periodic-acid Schiff (PAS) procedure for neutral carbohydrates, mucopolysaccharides and other carbohydrate-protein substances.
For LM of plastic-embedded tissues, testes had been dehydrated in a graded series of increasing ethanol solutions (70–100%), placed in a 50/50% acetone/plastic mixture for overnight infiltration, and embedded in Mollenhauer's Epon-Araldite #2.17 For thick sectioning (approximately 1 μm in thickness) and staining, we used glass knives on an LKB Ultrotome (Type 4801A) with Ladd® multiple stain (LMS), respectively. We used a Nikon Eclipse 600 epifluorescent light microscope with a Nikon DXM 1200C digital camera (Nikon Instruments Inc., Melville, NY) for photomicroscopy.
Results
Spermatogenesis
The reptilian testes contain seminiferous tubules that are lined with continuous seminiferous epithelia consisting of Sertoli cells and developing germ cells and are categorized into 3 major morphological phases: proliferative (pre-meiotic), meiotic, and spermiogenic.4,6 The proliferative stage involves the presence of spermatogonia, which will undergo many rapid, incomplete mitotic divisions to result in a large clonal population of germ cells, which allows the testes to produce high numbers of spermatozoa.1 The meiotic phase involves the reductional divisions, segregations, and recombination of genetic material of the spermatocytes. The spermiogenic phase is the final aspect of spermatogenesis where haploid undifferentiated spermatids transform into spermatozoa capable of reaching and fertilizing eggs. The different germ cells within these phases have very specific morphology and are also highly conserved among vertebrates.1
Proliferative Stage
The germ cell morphologies of the proliferative stage include type A and B spermatogonia (Fig. 1; SpA, SpB), as well as resting spermatogonia (Fig. 2D, SpR) described specifically within turtle testes.1 Spermatogonia are restricted to the basal region in close association with the basement membrane of the Sertoli cells. Resting spermatogonia, described only in turtles with postnuptial pattern, lack visible nucleoli but have dark staining chromatin packed tightly within the nucleus. There are no major organelles apparent within the cytoplasm of resting spermatogonia under light microscopy.
Figure 1.
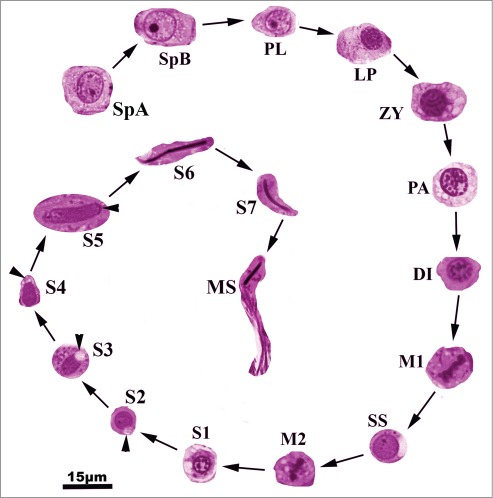
Germ cell types found within the seminiferous epithelium of Graptemys pseudogeographica kohnii. SpA, type A spermatogonium; SpB, type B spermatogonium; PL, pre-leptotene spermatocyte; LP, Leptotene spermatocyte; ZY, zygotene spermatocyte; PA, pachytene spermatocyte; DI, diplotene spermatocyte; M1, metaphase 1; SS, secondary spermatocyte; M2, metaphase II; S1, step 1 spermatid; S2, step 2 spermatid; S3, Step 3 spermatid; S4, step 4 spermatid; S5, step 5 spermatid; S6, step 6 spermatid; S7, step 7 spermatid; MS, mature spermatozoon.
Figure 2.
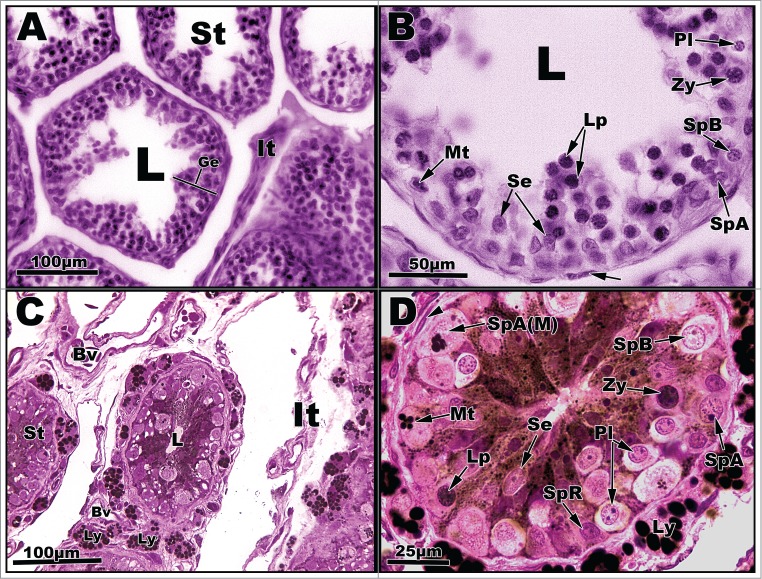
Light micrographs of seminiferous tubules of Graptemys pseudogeographica kohnii from April (A–B) and May (C–D). A. Seminiferous tubules (St) with central one exhibiting seminiferous epithelium (Ge) with an interstitium (It) to its right; L = lumen. B. Segment of seminiferous epithelium reveals several cell types, including Spermatogonia A undergoing mitosis (Mt), type A spermatogonium (SpA), type B spermatogonium (SpB), pre-leptotene spermatocyte (Pl), leptotene spermatocyte (Lp), zygotene spermatocyte (Zy), and Sertoli cells (Se). Arrow points to a fibroblast nucleus; L = lumen. C. Seminiferous tubules (St) surrounded by an interstitium (It), blood vessels (Bv), and individual Leydig cells (Ly) containing lipid droplets. D. Seminiferous tubule circumscribed by Leydig cells (Ly) full of lipid droplets. Germ cell types include a resting spermatogoium (SpR) and spermatogonia A (SpA) undergoing mitosis (Mt) as well as other cells mentioned in B. Paraffin, (A-B); plastic, (C-D).
Type A spermatogonial germ cells are typically found in juxtaposition to the basement membrane of the seminiferous epithelium. They are ovoid in shape with diffuse chromatin, which is absent around the region of the nuclear membrane. A prominent nucleolus (multiple nucleoli in some cases) is present within the nucleus of spermatogonia A. Type A spermatogonia are known as proliferative spermatogonia and provide much of the mitotic power of the seminiferous epithelium extending several layers out into the interior of the seminiferous epithelium. They are the most abundant spermatogonia seen within the reptilian testis. The final proliferative cell morphology is that of the type B spermatogonia. Type B spermatogonia are more round in shape when compared to the ovoid type A spermatogonia and globules of membrane-associated heterochromatin are located with the peripheral nucleoplasm.
Meiotic Stage: Spermatocyte Morphology
The next stage of spermatogenesis, the meiotic stage, is characterized by cells with increasing nuclear size and condensation of chromatin into distinct chromosomes.6 Type B spermatogonia mitotically divide to produce the first cell type in meiosis, pre-leptotene spermatocytes. Prophase I of meiosis is long in reptiles and is characterized by the presence of many different germ cell morphologies: pre-leptotene, leptotene, zygotene, pachytene, and diplotene spermatocytes.
Pre-leptotene spermatocytes (Fig. 1, PL) are morphologically similar to type B spermatogonial cells, but are distinguishable due to their much smaller size and location away from the basement membrane.1 Pre-leptotene spermatocytes, along with step 1 spermatids, are the smallest of the developing germ cells. Pre-leptotene cells are characterized by the presence of a nucleus with a well-defined, dark staining nucleoli.6 Leptotene cells (Fig. 1, LP) have threads of chromatin that represent condensing unpaired chromosomes. In the zygotene stage of meiosis (Fig. 1, ZY), however, the chromosomes become partially paired homologs that form into tetrads.6 The zygotene spermatocytes are slightly larger than the leptotene stage and also possess tetrads with thicker chromosomal fibers.1
Pachytene spermatocytes (Fig. 1, PA) are considerably larger than zygotene spermatocytes and are characterized by fully paired homologous chromosomes with thick fibers separated within the nucleus by open nucleoplasm.4 Unlike mammalian pachytene spermatocytes, reptilian pachytene spermatocytes do not possess the prominent sex vesicle at the inner portion of the nuclear envelope. Numerous synaptonemal complexes form in the pachytene stage, which aid in crossing over events within the chiasmata. Pachytene spermatocytes are important for genetic recombination and genetic diversity within species of vertebrates.1 Diplotene spermatocytes (Fig. 1, DI) are commonly found within clusters in the seminiferous epithelium accompanied by metaphase I cells, secondary spermatocytes, and metaphase II cells.6 Diplotene spermatocytes are characterized by condensed chromosomal fibers arranged in a circle beneath a degenerating nuclear membrane resulting in a spoke-like pattern.1
Metaphase I cells (Fig. 1, M1) are characterized by chromosomal aggregation along the metaphase plate.1 Meiosis I results in haploid secondary spermatocytes, which exhibit a clearly visible nuclear membrane and chromatin fibers dispersed throughout the nucleoplasm. Other structures found within secondary spermatocytes (Fig. 1, SS) include the presence of a prominent amorphic inclusion within the cytoplasm, known as a nuage, and vacuolated mitochondria in proper thick section. Found in close proximity to metaphase I cell, metaphase II (Fig. 1, M2) cells are characterized by chromosomal aggregation along the metaphase plate.6 Metaphase I and metaphase II cells are distinguishable based on size and amount of chromatin present. Metaphase II cells are half the size of metaphase I cells and possess half the amount of chromatin.5
Spermiogenesis
The transformation process of secondary spermatocytes into mature spermatozoa can be separated into 7 steps (Fig. 1, S1–S7) in the Mississippi map Turtle. Step 1 spermatids are small in size, comparable to that of pre-leptotene spermatocytes, with a well-defined nuclear membrane but no definable acrosomal vesicle.1 The spherical nucleus is centrally located with at least one chromatin body.2 Step 2 spermatids are characterized with a well-defined acrosomal vesicle. Step 2 spermatids have chromatin dispersed throughout the nucleoplasm.2 Step 3 spermatids are distinguishable due to the presence of a large, towering acrosome that extends away from the apex of the nucleus.4 The acrosome of step 4 spermatids envelops the nucleus and flattens it. The nucleus of step 4 spermatids also starts to elongate and represent the transition between the round and elongating spermatids. Step 5–7 spermatids represent the developmental stages of elongation. The acrosome at this stage is deeply embedded into the nuclear fossa and is tubular in shape. An elongation event begins as the nucleus is displaced caudally, causing the acrosome vesicle to collapse onto itself as it is pushed against the cell membrane.6 The nuclei of step 5 and 6 spermatids are longer than they are wide. The chromatin is fully condensed in step 6 and 7 spermatids, which have undergone considerable nuclear condensation and cytoplasmic elimination. These spermatids possess intensely stained nuclei and a nuclear implantation fossa in which the flagellum inserts at the base of the nucleus.3,6 Step 7 spermatids are released into the lumen of the seminiferous tubules as spermatozoa.4
Histological Chronology of the Seminiferous Epithelium
The majority of the germ cells develop within the tubules of the testis as a single cohort and undergo spermatogenesis, spermiogenesis, and a single spermiation event, which are characteristic of a temporal germ cell development strategy. Germ cell development occurs between mid-April to mid-October in the Mississippi map Turtle testis. Recrudescence takes place after spring mating in late March. Spermatogenesis then progresses through summer and early fall with spermiation ensuing in mid-to-late October. Upon the completion of spermiation, the testes enter a quiescent period that is characterized by a regression of the seminiferous epithelium.
In mid-to-late April after a brief recrudescent period, the seminiferous epithelium enters a proliferative phase, which is marked by increased mitotic division of spermatogonia A to produce a large clonal population of developing germ cells (Figs. 2A, B). The primary cell types present within the seminiferous tubules in the sample tissues consisted of resting spermatogonia, type A spermatogonia, and type B spermatogonia. The resting spermatogonia, type A spermatogonia, and type B spermatogonia cells are situated along the basement membrane in close association with Sertoli cells, which also line the basement membrane (Figs. 2B, D).
The seminiferous epithelium continues to progress into the meiotic stages of spermatogenesis from April into May with the presence of pre-leptotene spermatocytes (Figs. 2B, C; Pl). The major cell types within the seminiferous tubules of May specimens consist of type A spermatogonia, type B spermatogonia, and pre-leptotene spermatocytes (Figs. 2C–D). Additionally, a few large hypertrophic cells and resting spermatogonia are found within the tubules (Fig. 2D) at this time. The Sertoli cells along the basement membrane are accompanied by numerous lipid droplets that occupied the luminal space (Fig. 2D). In addition, Leydig cells filled with lipid droplets surround the seminiferous tubules (Fig. 2D).
Major spermatogenic activity in June/July results in increased height of seminiferous epithelia into the luminal spaces (Figs. 3, A–D). The major population of cells within the tubules during this time consist of type A and B spermatogonia, pre-leptotene spermatocytes, and readily recognizable phases of primary spermatocytes, such as zygotene, pachytene (Fig. 3B). Leptotene cells are difficult to distinguish from zygotene cells using light microscopy, and the diplotene phase (Fig. 3D, Lp) is very brief in reptiles and, thus, infrequently observed.
Figure 3.
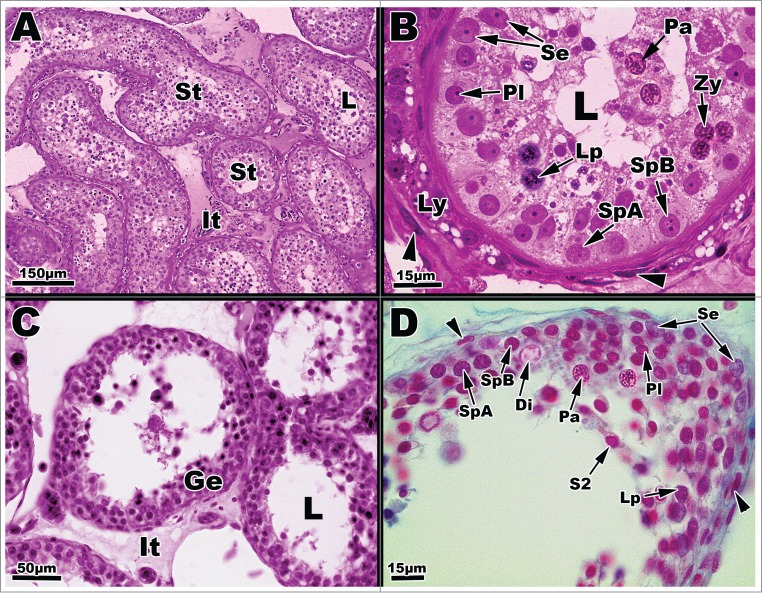
Light micrographs of seminiferous tubules of Graptemys pseudogeographica kohnii from in June (A–B) and July (C–D). (A) Seminiferous tubules (St) surrounded by interstitial cells (It); L = lumen. (B) A cross section of June seminiferous tubule reveals several cell types, including a type A spermatogonium (SpA), type B spermatogonium (SpB), pre-leptotene spermatocyte (Pl), leptotene spermatocyte (Lp), zygotene spermatocyte (Zy), pachytene spermatocyte (Pa), and Sertoli cells (Se); Leydig cells (Ly) are mostly void of lipid. Arrow points to a fibroblast nucleus; L = lumen. (C) July Seminiferous tubules lined with seminiferous epithelia (Ge) surrounded by the interstitium (It); L = lumen. (D) July seminiferous tubule exhibiting a variety of germ cell types including type A spermatogonium (SpA), type B spermatogonium (SpB), pre-leptotene spermatocyte (Pl), leptotene spermatocyte (Lp), pachytene spermatocyte (Pa), diplotene spermatocyte (Di), Sertoli cells (Se), and a step 2 spermatid (S2). Arrowheads point the nucleus of fibroblast cells. Paraffin, (C-D); plastic (A-B).
Throughout early July to late August, the seminiferous epithelium greatly increases in height and cells move through the meiotic phase into early spermiogenic events with the appearance of undifferentiated haploid spermatids (Figs. 3C and D). The majority of the cell population during this time consists of late stage primary spermatocytes. The presence of a few spermatids (Step 1 and 2) characterize the transition from the meiotic phase to the spermiogenic phase of spermatogenesis (Fig. 3D, S2). Type A and B spermatogonia are present in close association to the basement membrane (Fig. 3D).
The spermatogonial and meiotic phase cell populations are relatively exhausted by October with type A and B spermatogonia present within the tubules (Figs. 4C, D). Most of the germ cells represented within the tubules consist of haploid spermatids (Steps 1–7) and mature spermatozoa. Step 5–7 spermatids contribute most to the germ cell population in October (Fig. 4D). The lumina of the seminiferous tubules are filled with mature spermatozoa in early October and void of most spermatozoa in some October tubules (not shown) following a major spermiation event in which sperm will then move to the epididymis. Many cell types dominate the lumen of the seminiferous tubules as they enlarge in September. Few sperrmatogonial cells (resting and Type A) are present at the basement membrane, but the majority of the cell population of the seminiferous epithelium consist of secondary spermatocytes, haploid spermatids (Steps 2–7), and mature spermatozoa (Figs. 4A–B). Sertoli cell nuclei are also present along the basement membrane.
Figure 4.
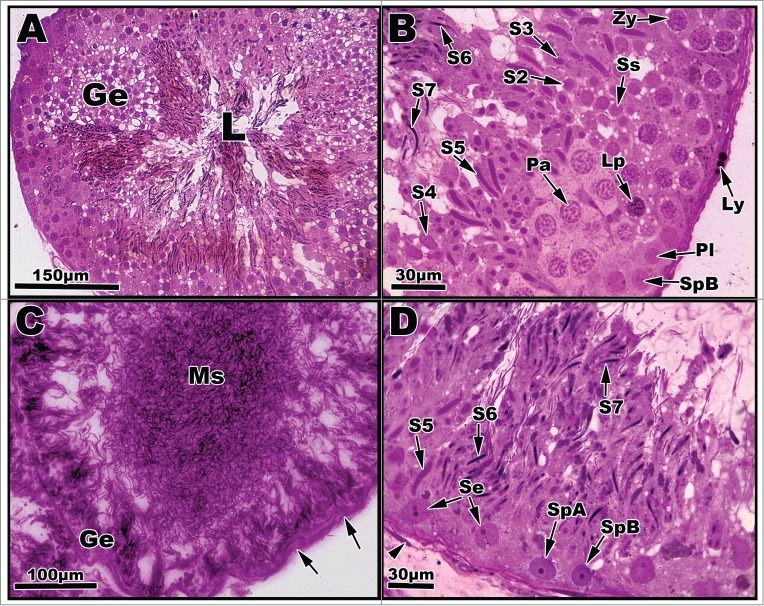
Light micrographs of seminiferous tubules of Graptemys pseudogeographica kohnii from September (A–B) and October (C–D). (A) Greatly enlarged seminiferous epithelium (Ge) dominated transforming spermatids; lumen (L) filled with mature sperm. (B) Segment of seminiferous epithelium reveals a type B spermatogonium (SpB), primary spermatocytes in various phases (Pl, Lp, Zy, Pa) previously mentioned in Figs. 2–3, a secondary spermatocyte (Ss), and morphological steps of spermatid development (S2-S7). A Leydig cell with lipid droplets (Ly) can be seen adhering to the basement membrane. (C) The height of seminiferous epithelium (Ge) is greatly reduced with concomitant increase of mature sperm (Ms) occupying the lumen; arrows point to nucleus of fibroblast cells. (D) Segment of seminiferous epithelium exhibiting a variety of germ cell types including type A and B spermatogonia and Sertoli cells (Se) along with spermatid steps 5–7. Arrowhead points to the nucleus of a fibroblast cell. Paraffin, (C); plastic, (A, B, and D).
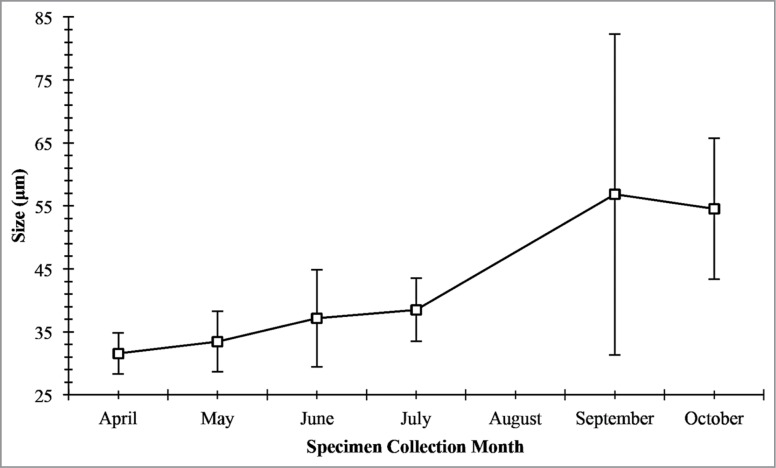
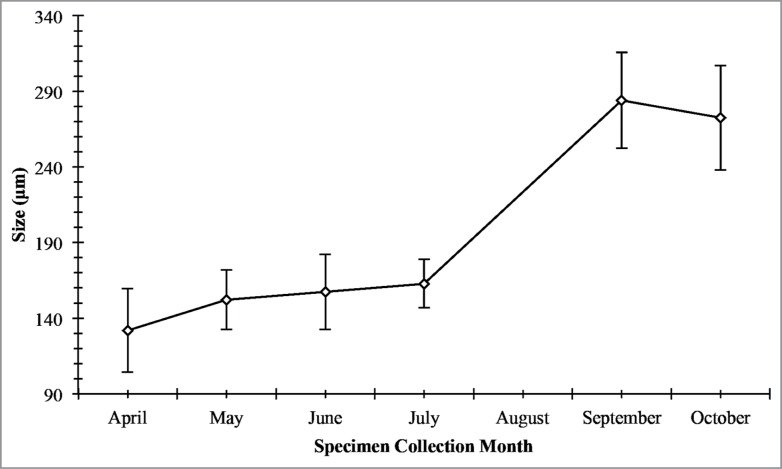
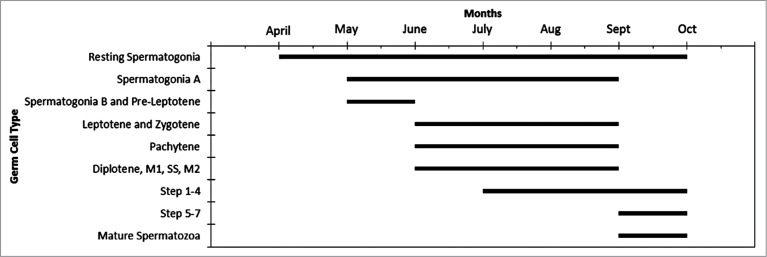
Seminiferous epithelium height (Fig. 5) is shown to be directly associated with the increase in seminiferous tubules diameter (Fig. 6). The seminiferous epithelium increases slightly throughout April, May, June, and July, with the largest increase between July in September. This large increase in height suggests that the main portion of spermatogenic activity occurs between July and September. The decrease in October suggests that spermatogenesis is finishing, and this is evident by the appearance of residual spermiogenic cells in close association to the basement membrane (Fig. 7). The spermatogenic cycle (Fig. 7) shows that the majority of the germ cells progress through spermatogenesis as a population even though there is considerable overlap during the peak of spermatogenesis in the late summer/fall.
Figure 5.
Mean seminiferous epithelium height (in μm) during the annual reproductive cycle in Graptemys pseudogeographica kohnii in northeast Arkansas. Vertical bars = one standard deviation.
Figure 6.
Mean seminiferous tubule diameter (in μm) during the annual reproductive cycle in Graptemys pseudogeographica kohnii in northeast Arkansas. Vertical bars = one standard deviation.
Figure 7.
Time and appearance of germ cell distribution within the seminiferous epithelium of Graptemys pseudogeographica kohnii. M1, Metaphase 1; SS, Secondary spermatocyte; M2, metaphase 2; Step 1–4 and Step 5–7, spermatids steps 1–8.
Discussion
The testes of Graptemys pseudogeographica kohnii are composed of seminiferous tubules lined continuously with seminiferous epithelia composed of developing germ cells and Sertoli cells, which is characteristic of an amniotic testis. These developing germ cells mature as a single cohort and are released into the lumen of the seminiferous tubules in a single major spermiation event. The testicular structure is consistent with other studies.2,3,6,9 After the spermiation event in late fall, the sperm are assumed to be stored in the epididymis over the winter months until the spring mating event in late March to early April.8 This postnuptial testicular cycle is consistent with studies of other temperate reptiles, particularly turtles and snakes that are seasonal in reproductive activity.19
Seminiferous tubule diameter increased continuously throughout April, May, June, and July, with a large increase between July and September. This steady increase has been observed in other emydid turtles as well as other turtle species, such as kinosternids.20-26 The rapid increase in diameter is due to the rapid proliferation of developing germ cells within the seminiferous epithelium, which causes the tubule to distend.27 In October, the seminiferous tubule diameters decreased. The decrease in diameter in October is due to mature sperm being released into the lumen and the loss of the seminiferous epithelium and storage of sperm into the epididymis, which results in a reduction of seminiferous tubule diameter. Spring mating uses spermatozoa produced in the previous season and stored in the epididymis. This is consistent with other reptilian studies to date.9,22
The germ cell cytology revealed several features that were important in determining the testicular cycle of Graptemys pseudogeographica kohnii. Non-dividing, resting spermatogonial cells were found to be present from April to October. While these cells were not metabolically active, it can be theorized that these cells are present in cases of reproductive stress and to replenish other spermatogonial populations in times of need. The appearance of resting spermatogonia in Graptemys p. kohnii is consistent with a similar study involving Trachemys scripta.8 We hypothesize that these resting cells are stem cells that are resistant to stress and damage and are present to be utilized in the early spring after hibernation in order to provide a resilient population of stem cells capable of jump starting spermatogenesis. More rough endoplasmic reticulum and larger Golgi complexes are found in type B spermatogonia than those in type A spermatogonia.1 Similarly to type A spermatogonia, type B spermatogonial cells incompletely divide mitotically to sustain the population of spermatogonial cells; these cells also divide to enter into prophase I of meiosis.6 The incomplete mitotic divisions result in cytoplasmic bridges between developing germ cells, which allow for coordinated communication that controls simultaneous development of germ cell cohorts within the reptilian testis.1 The major function for type A and B spermatogonia is contingent on the need for high numbers of clonal germ cells that enter the phases of spermatogenesis seasonally. The rapid divisions of the spermatogonia fulfill the need for the large population of spermatocytes needed to produce adequate numbers of functional gametes. Developing germ cells appeared to be aggregated in bundles, especially evident in September and October testes, which implies that cytoplasmic bridges of communication may exist between groups of developing germ cells, although this cannot be ascertained via light microscopy. Many consecutive steps of spermatids are found within the seminiferous epithelium at one time. The appearance of several steps of spermatids suggests that the elongation phase of spermiogenesis may be longer than the production of round spermatids through spermatogenesis. The late stage elongated spermatids (steps 5, 6, 7) were interspersed with early stage round spermatids. The differentiation of steps 3 and step 4 spermatids was hard to distinguish due to poor staining properties of the acrosomal vesicle.
The rapid proliferation of the seminiferous epithelium between April and October and subsequent maturation and spermiation events in Graptemys pseudogeographica kohnii occur over a relatively short period of time. Following the spermiation event in October, sperm are presumably stored in the epididymis over the winter months, and the testes enter a quiescence period. Upon the spring mating event in late March to early April, sperm are released from the epididymis and the testes enter a recrudescence period. This recrudescence period is necessary for initiation of another testicular cycle. The relevance of this postnuptial testicular cycle could be due to an asynchronous female reproductive cycle, although this has not been confirmed in this species of turtle and would be an interesting question to test in future reproductive studies.
The germ cell development strategy exhibited by Graptemys pseudogeographica kohnii is consistent with other reptilian studies.1,3,6,9 Graptemys p. kohnii possess a germ cell development strategy similar to that of anamniotes within a structurally amniotic testis. It has been suggested that this could represent a common intermediate germ cell development strategy between that of the cystic testes of anamniotes and the tubule-like testes of mammals and birds; however, in order to provide further evidence for this assertion, continued spermatogenic studies within reptiles must be performed.1 The distribution of this turtle and its semi-aquatic life history might allow for this species to be a significant histopathological model for the studies of aquatic pesticides and their effects on spermatogenesis within an amniotic testis. The straight forward spermatogenic cycle and the ease of germ cell type identification and the development of the majority of the germ cell population through the phases of spermatogenesis should allow for one to trace abnormal germ cell distribution and/or the absence of specific cell types in experimental turtles treated with pesticides.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Gribbins KM. Reptilian spermatogenesis: a histological and ultrastructural perspective. Spermatogenesis 2011; 1:1-20; http://dx.doi.org/ 10.4161/spmg.1.3.18092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rheubert JL., Poldemann EH, Eckstut ME, Collier MH, Sever DM, Gribbins KM. Temporal germ cell development strategy during mixed spermatogenesis within the male Mediterranean Gecko, Hemidactylus turcicus. Copeia 2009; 4:791-98; http://dx.doi.org/ 10.1643/CG-09-034 [DOI] [Google Scholar]
- 3. Pudney J. Spermatogenesis in nonmammalian vertebrates. Microsc Res Tech 1995; 32:459-97; PMID:8605396; http://dx.doi.org/ 10.1002/jemt.1070320602 [DOI] [PubMed] [Google Scholar]
- 4. Gribbins KM, Happ CS, Sever DM. Ultrastructure of the reproductive system of the Black Swamp Snake (Seminatrix pygaea). V. The temporal germ cell development strategy of the testis. Acta Zool 2005; 86:223-30; http://dx.doi.org/ 10.1111/j.1463-6395.2005.00201.x [DOI] [Google Scholar]
- 5. Russel LD, Hikim SAP, Ettlin RA, Clegg ED. Histological and histopathological evaluation of the testis. Clearwater Fl: Cache River Press; 1990. [Google Scholar]
- 6. Gribbins KM, Rheubert JL, Anzalone M, Siegel DS, Sever DM. The ultrastructure of spermiogenesis in the Western Cottonmouth, Agkistrodon piscivorus. J Morph 2008; 268:181-92; PMID:17154286; http://dx.doi.org/ 10.1002/jmor.10505 [DOI] [PubMed] [Google Scholar]
- 7. Licht P Reptiles. In: Lamming GE, Marshall's Physiology of Reproduction, Vol. 1 (Reproductive Cycles of Vertebrates. Churchill Livingston, Edinburgh; 1984. 1:206-82. [Google Scholar]
- 8. Mahmoud IY, Cyrus RV. The testicular cycle of the Common Snapping Turtle, Chelydra serpentina, in Wisconsin. Herpetologica 1992; 2:193-01. [Google Scholar]
- 9. Gribbins KM. 2003 The cytological evaluation of spermatogenesis and ultrastructure of the junctional complexes within the germinal epithelium of reptiles. Doctoral Thesis. The University of Cincinnati, Cincinnati, OH 2003. [DOI] [PubMed] [Google Scholar]
- 10. Glesenkamp LL, Zug GR, Mitchell JC. Reproductive cycle of male snapping turtles (Chelydra serpentina) in southeastern Virginia. Chel Cons Biol 2003; 3:697-700. [Google Scholar]
- 11. Miller JD, Dinkelacker SA. Reproductive structures and strategies of turtles. In: Wyneken J, Godfrey MH, and Bells V, Eds Biology of Turtles. Boca Raton, FL; CRC Press; 2008; 225-78. [Google Scholar]
- 12. Saint Girons H. Reproductive cycles of male snakes and their relationships with climate and female reproductive cycles. Herpetologica 1982; 1:5-16. [Google Scholar]
- 13. Vogt RC. Graptemys pseudogeographica (Gray). Cat Amer Amph Rept 1995; 604:1-6. [Google Scholar]
- 14. Ernst CH, Lovich JE. Turtles of the United States and Canada. 2nd ed. Baltimore, MD; The John Hopkins University Press; 2009. [Google Scholar]
- 15. Johnson TR. The amphibians and reptiles of Missouri. 2nd ed Jefferson City, MO; Missouri Dept Conserv; 1987. [Google Scholar]
- 16. Presnell JK, Schreibman MP. Humason's Animal Tissue Techniques. Fifth Ed Baltimore, MD; The Johns Hopkins University Press; 1997. [Google Scholar]
- 17. Dawes CJ. Introduction to Biological Electron Microscopy: Theory and Techniques. Burlington, VT; Ladd Research Industries, Inc; 1988. [Google Scholar]
- 18. Plummer MV, Trauth SE. The structure of Rathke's glands in the softshell turtles Apalone mutica and A. spinifera. Herpet Cons Biol 2009; 2:207-20. [Google Scholar]
- 19. Dubois WJ, Pudney J, Callard IP. The annual testicular cycle in the turtle, Chrysema picta: a histochemical and electron microscopic study. Gen Comp Endocrin 1988; 71:191-204; http://dx.doi.org/ 10.1016/0016-6480(88)90248-1 [DOI] [PubMed] [Google Scholar]
- 20. Gribbins KM, Rheubert JL. The testis, spermatogenesis, and the mature speramtooza. In: Aldridge RD. and Sever DM, Reproductive biology and phylogeny of Snakes. Enfield, NH; Science Publishers; 2011; 183-264. [Google Scholar]
- 21. Lofts B, Boswell C. Seasonal changes in the distribution of the testis lipids of the Caspian terrapin Clemmys caspica. Proc Zool Soc Lond 1961; 136;581-92; http://dx.doi.org/ 10.1111/j.1469-7998.1961.tb05893.x [DOI] [Google Scholar]
- 22. Moll EO, Legler JM. The life history of a Neotropical slider turtle, Pseudemys scripta (Schoepff) in Panama. Bull Los Angeles Co Mus 1971; 11:1-102. [Google Scholar]
- 23. Gibbons JW. Reproductive potential, activity, and cycles in the painted turtle, Chrysemys picta. Ecology 1968; 49:400-9; http://dx.doi.org/ 10.2307/1934106 [DOI] [Google Scholar]
- 24. Ernst CH. Sexual cycles and maturity of the turtle Chrysemys picta. Biol Bull 1971; 140:191-200; http://dx.doi.org/ 10.2307/1540068 [DOI] [Google Scholar]
- 25. Legler JM. Natural history of the ornate box turtle, Terrapene ornata ornata Agassiz. Univ Kans Mus Nat Hist Publs 1960; 11:527-669. [Google Scholar]
- 26. Mahmoud I, Klicka J. Seasonal gonadal changes in kinosternid turtles. J Herpetol 1972; 6:183-9; http://dx.doi.org/ 10.2307/1562769. [DOI] [Google Scholar]
- 27. Risley PL. Seasonal changes in the testes of the musk turtle, Sternotherus odoratus L. J Morphol 1938; 63:3-1-317. [Google Scholar]