Abstract
After exposure to toxicants, degenerating germ cells represents the most common testicular histopathological alteration, regardless of the mechanism of toxicity. Therefore, deciphering the primary toxicant cellular target and mechanism of action can be extremely difficult. However, most testicular toxicants display a cell-specific and a stage-specific pattern of damage, which is the best evidence for identifying the primary cellular target (i.e. germ cell, Sertoli cell, peritubular myoid cell, or Leydig cell). Some toxicant-induced Sertoli cell injury presents with germ cell apoptosis occurring primarily in spermatocytes in rats in stages XI-XIV, I and II. Although some toxicants result in spermatid degeneration and apoptosis, it is still unclear if spermatid apoptosis is a result of Sertoli cell-selective apoptosis or a direct effect of toxicants on spermatids, therefore if this is seen as the earliest change, one cannot infer the mechanism of apoptosis. This review summarizes some of the distinguishing features of Sertoli cell-induced germ cell apoptosis and the associated mechanisms of cell death to provide the toxicologist observing similar cell death, with evidence about a potential mode of action.
Keywords: apoptosis, germ cellsm Sertoli cell, histopathology, spermatocytes, testis
Abbreviations
- 2,5-HD
2,5-hexanedione
- MEHP
mono-(2-ethylhexyl) phthalate
- FasL
Fas Ligand
- DISC
death-inducible complex
- TIMP2
tissue inhibitor of matrix metalloproteinase 2
- sTNFα
soluble form tumor necrosis factor-α
Introduction
Sertoli cells are absolutely necessary for proper germ cell development and viability. Sertoli cells orchestrate the processes of spermatogenesis by supporting and providing nutrition for developing germ cells, compartmentalization of the seminiferous tubule via tight junctions, regulating the release of mature spermatids, secretion of fluid, proteins, energy substrates and several growth factors and the phagocytosis of degenerative germ cells. Although many toxicants directly target Sertoli cells, due to the importance of these Sertoli cell functions for germ cells, oftentimes the only histopathological manifestations that can be observed are alterations in germ cells. These changes include the detachment and sloughing of germ cells from the seminiferous epithelium, failed or delayed maturation of germ cells, incomplete spermiation, and increased germ cell death. This review is focused on the induction of germ cell death via apoptosis as a result of toxicant-induced Sertoli cell injury.
Apoptosis is an active process of cell death characterized by chromatin condensation, fragmentation, and cell disintegration.1 Although the focus of this review is on germ cell apoptosis that occurs in response to toxicant-induced Sertoli cell injury, the reader should recognize that apoptosis of testicular germ cells also occurs under normal physiological conditions and serves as a mechanism to balance the numbers of germ cells to the supportive capacity of the Sertoli cell.2 Therefore, toxicant-induced decreases in the Sertoli cell supportive capacity can result in the increased incidence of germ cell apoptosis in the testis. Germ cell apoptosis can also result after direct physical or chemical-induced injury to the germ cell.3
As an endpoint, germ cell apoptosis is easy to detect and quantify and provides information of potential mechanistic relevance. However, because of the dependence of germ cells on Sertoli cells, deciphering the primary cellular site of action for testicular toxicants is extremely challenging. The goal of this review is to aid the researcher in identifying the histopathological alterations associated specifically with toxicant-induced Sertoli cell injury resulting in the easily observed germ cell apoptosis.
Signature lesion
After exposure to toxicants, degenerating germ cells are the most common testicular histopathological alteration, regardless of the mechanism of toxicity. Therefore, deciphering the primary toxicant cellular target can be extremely difficult. However, most testicular toxicants display a cell-specific and a stage-specific pattern of damage, which is the best evidence for identifying the primary cellular target (i.e., germ cell, Sertoli cell, peritubular myoid cell, or Leydig cell). A hallmark of certain toxicant-induced Sertoli cell injury is the finding that germ cell apoptosis primarily affects spermatocytes in stages XI-XIV, I and II. When germ cells are directly affected, one primarily sees cell death of spermatogonia in stages II-VI.4 Table 1 lists some of the distinguishing features and the associated mechanisms of cell death. It is still unclear if spermatid apoptosis is a result of Sertoli cell selective apoptosis or a direct effect of toxicant on spermatids, therefore one cannot infer the mechanism of apoptosis. Germ cell apoptosis does not always display the morphologic features classically (detailed information below) associated with apoptosis, although the biochemical and molecular characteristics are similar.
Apoptosis
Apoptosis is an active process of cell death characterized by sequential phases of chromatin condensation, fragmentation, and cell disintegration that leads to the orderly destruction and disposal of a cell without a consequent inflammatory response.1 Other forms of cell death that will not be covered in this review include necrosis (passive process, breakdown of cellular structure and function) and autophagy (sequestering of cytoplasmic material within autophagosomes), which are reviewed in.5
Classically, apoptosis is characterized ultrastructurally by cell volume shrinkage, membrane blebbing, chromatin condensation, cytoplasmic vacuolization and breakup of the cell into membrane-bound remnants (apoptotic bodies; Fig. 1).5 Biochemical features of apoptosis include the translocation of phosphatidylserine to the external leaflet of the plasma membrane, the activation of the caspase cascades, and DNA cleavage/fragmentation into a 180–200 basepair ladder. Apoptosis is visualized in situ by terminal deoxynucleotide transferase-mediated deoxy-UTP nick end labeling (TUNEL) and through immunstaining for caspase activation.6-8
Figure 1.
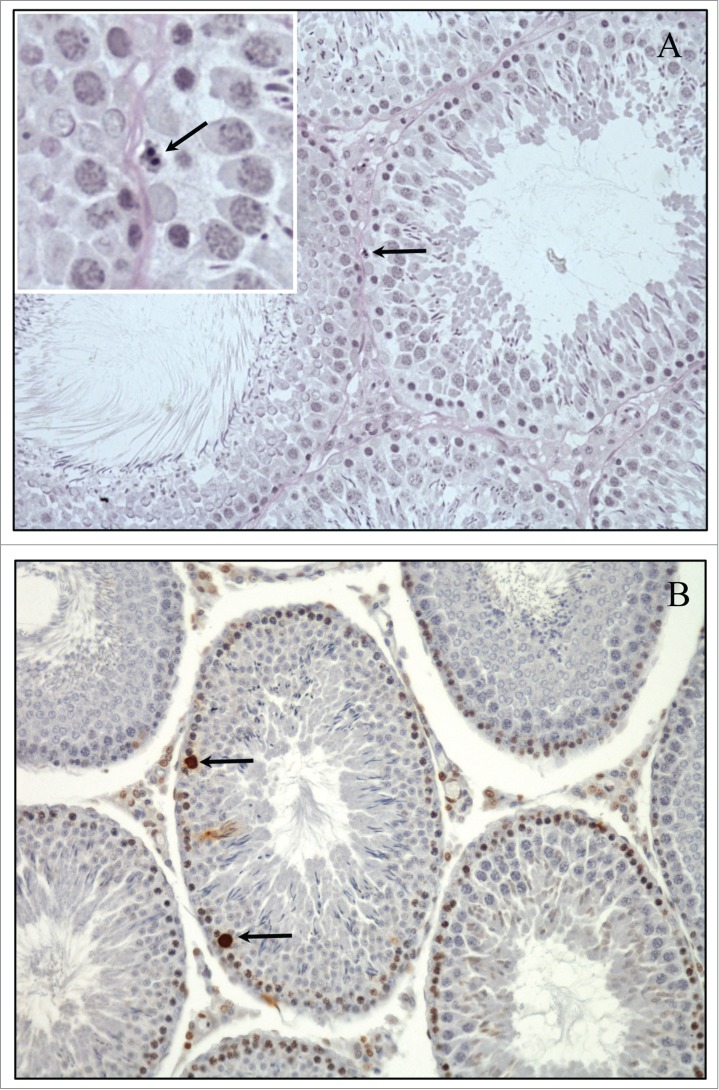
Physiologic Spermatogonial apoptosis in the adult rat testis. (A) Example of spermatogonia undergoing apoptosis (arrow) in rat testis under normal physiological conditions. Insert (magnification 300x) demonstrates the distinct features of DNA fragmentation, blebbing and darkly stained nuclei. The testis was immersion fixed in Bouins, embedded in Glycol methacrylate and 3 μm sections stained with periodic acid-Schiff's reagent and hematoxylin. (B) Example of TUNEL positive cells undergoing apoptosis in the normal adult rat testis (arrows). The testis was immersion fixed in Bouin's, embedded in paraffin and 5 μm sections stained with Apoptag kit (Millipore Cat #S-7001).
While germ cell apoptosis presents with all of the biochemical features of apoptosis, the ultrastructural presentation can be different. For each germ cell subtype apoptosis appears as follows; spermatogonia take the form of typical apoptosis as described above which includes the degeneration of heterchromatin, the margination of chromatin into sharply defined masses, densely stained chromatin along nuclear envelope and nuclear and cytoplasmic shrinkage (Fig. 1).9 Spermatocytes morphologically appear to have some characteristics of necrosis including pyknosis, darkly stained phase-positive prominent chromosomes and an increase in size.9 Apoptotic round spermatids are generally detected by chromatin margination (ring nuclei) and the formation of multinucleated giant cells. Apoptotic elongated spermatids are difficult to detect due to the compact nuclei, although early nuclear changes can be diagnosed by the appearance of clubbing and misshapen heads.9
Sertoli-germ Cell Interdependence
Sertoli cells are known as “nurse cells” due to their importance in supporting germ cells. Sertoli cells extend their cytoplasm around germ cells forming a plastic, always-moving 3-dimensional amoeboid structure, which encases and supports each germ cell. Sertoli cells nurture and maintain this close cellular association throughout the process of spermatogenesis, demonstrating the interdependence of germ cells on the “nurse cells.” Sertoli cells only represent 3% of the population of cells within the adult testis and can only support a limited number of germ cells, reviewed in refs.2,10 It has been estimated that in rodents, each Sertoli cell interacts with approximately 30–50 developing germ cells of various levels of differentiation.11 The Sertoli cell maintains this constant number by directly activating germ cell apoptosis under physiological conditions.2
Although there are multiple reports describing Sertoli cell-induced germ cell apoptosis, there are relatively few reports describing the apoptotic cell death of Sertoli cells themselves in the testis under physiological conditions or after toxicant exposure. However, during embryonic and neonatal development, Sertoli cells are proliferating and have been shown to be sensitive to undergo apoptosis. Apoptotic Sertoli cells have been reported after exposure to x-irradiation, bisphenol A, and alkylphenols in young prepubertal rats or in primary cultures prepared from young rodents.12-14 Thus, the susceptibility of Sertoli cells to undergo apoptosis may differ depending on their developmental/proliferative stage.
Physiologic Germ Cell Apoptosis
Huckins was the first to identify that germ cells undergo normal physiological loss. He found that “Only 25% of the theoretically possible number of preleptotene spermatocytes are produced from the original population of A1 spermatogonia: most loss is incurred during the maturation of A2 and A3 generations.”15 It was later characterized that the mechanism of germ cell loss was due to apoptosis. The reader should be aware that this manuscript is often misinterpreted to mean that 75% of germ cells undergo apoptosis; an interpretation by a reader that does not understand the expansive potential of the spermatogonial cells of the testis. The loss of early A1-A4 spermatogonia leads to a great reduction in the potential final sperm cell output due to the early limitation in the expansion of this population (Fig. 1). It is now widely recognized that in the normal mature rat testis, every germ cell type can undergo apoptosis and dying cells can be found in all stages of the spermatogenic cycle.16
During testicular development there are 2 peaks of normal physiological germ cell apoptosis that are essential for spermatogenesis. The first period is during the migration of primordial germ cells into the gonads, which is controlled by transforming growth factor β, retinoic acid and possibly estrogens.17 The second is at the beginning of the first round of spermatogenesis.18 This period of increased apoptosis occurs during the first 2–3 weeks after birth in rodents as a single wave affecting spermatocytes, and is critical to the normal development and function of the adult testis (Fig. 2).19,20
Figure 2.
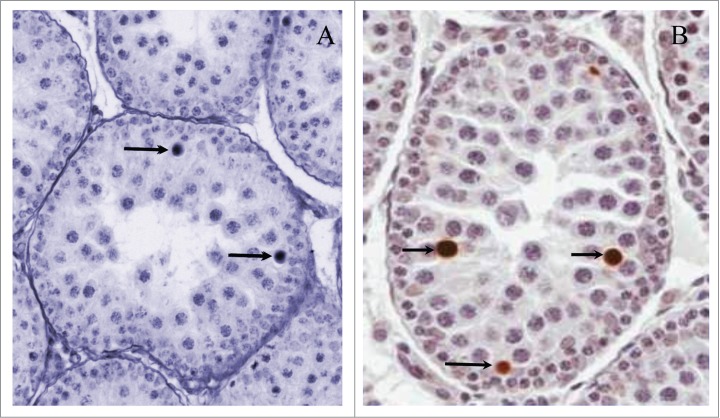
Physiological spermatocyte apoptosis during first wave of spermatogenesis. (A) An example of spermatocyte apoptosis in postnatal day (PND) 28 rats during the first spermatogenic wave (arrows), which appears as darkly stained nuclei with increased size. The testis was immersion fixed in Bouin's, embedded in paraffin and 5 μm sections stained with periodic acid-Schiff's reagent and hematoxylin. (B) Example of TUNEL stained apoptotic spermatocytes during the first spermatogenic wave PND 28 rat testis. The testis was immersion fixed in Bouin's, embedded in paraffin and 5 μm sections stained with Apoptag kit (Millipore Cat #S-7001).
In mature animals under normal physiological conditions there are low levels of spontaneous germ cell apoptosis. Germ cell subtypes found to undergo apoptosis in mature rodents include a small number of type A2–A4 spermatogonia and a more prominent number of spermatocytes and early round spermatids.21,22 For the pathologist, the numbers of apoptotic germ cells observed histologically is very low. This is due to the fact that Sertoli cells rapidly phagocytize apoptotic germ cells,23 thus few apoptotic cells are seen under normal conditions. Sertoli cells express class B of scavenger receptor type I (SR-BI) which functions as a receptor for phosphatidylserine, expressed on apoptotic germ cells.24
Toxicant-induced Apoptosis
An increase in the incidence of germ cell apoptosis in the testis is a commonly reported occurrence after toxicant exposure. The challenge is mechanistically deciphering whether the germ cell apoptosis occurs due to injury of the Sertoli cell or as a consequence of a direct injury to the germ cell. Various types of testicular injuries, including hormonal perturbations25 (see companion manuscript by Weinbauer, this issue), heat exposure,26 Sertoli cell toxicants such as 2,5-hexanedione (2,5-HD)27 and mono-(2-ethylhexyl) phthalate (MEHP),28 and germ cell toxicants as x-irradiation,29 all result in germ cell apoptosis.
Toxicant-injured Sertoli cells have reduced supportive capacity. Thus, the fate of germ cells after toxicant-induced Sertoli cell injury depends on their response to the changing seminiferous tubule environment. A variety of changes including decreased secretion of survival factors, increased apoptotic proteins, or a combination will result in germ cell apoptosis. Toxicants can directly trigger the apoptotic process in germ cells or interfere with the spontaneous apoptosis induced by Sertoli cells that serves an important physiological function.
The subtype of germ cell affected by a toxicant provides the best information for beginning to decipher the primary cellular target and mechanism of action of the toxicant (Table 1). The spermatocyte germ cell subtype primarily undergoes apoptosis after Sertoli cell injury while the spermatogonia are most resistant. On the other hand, spermatogonia are the main cell type affected by direct action on germ cells.4,30 However, unless mechanistic experiments are conducted utilizing molecular biological techniques (immunohistochemistry, in situ hybridization or genetically engineered mice), the exact cellular targets or pathways cannot be deciphered from histopathological evaluation only.
Table 1.
| Extrinsic Pathway | Intrinsic Pathway | |
|---|---|---|
| Mediating Factors | ↑FasL (Sertoli cell) ↑Fas (Germ cell) ↑Caspase-8 | ↑Bax (Germ cell) ↓Bcl2 (Germ cell) ↑Caspase-9 |
| Toxicants | Mono-ethylhexyl Phthalate (MEHP), 2,5,-Hexadione (HD) | Irradiation, 2-Bromopropane, Cyclophosphamide |
| Target Cell | Sertoli cell | Germ cell |
| Germ cell subtype | Spermatocyte>Spermatogonia | Spermatogonia>Spermatocytes |
| Stages | Stages IX-XI (Mouse, MEHP) Stages XI-XIV, I, and II (Rat, MEHP) |
Stages I-VI (Mouse, irradiation) Stages II-VI (Rat, irradiation) |
Cell-Death Pathways
There are believed to be 2 signaling pathways that instigate cellular apoptosis. This occurs through (1) the action of the tumor necrosis factor (TNF) superfamily of ligands binding to their associated receptors (‘extrinsic’ signaling)3,5,7 or (2) through events that result in the release of cytochrome C from the mitochondria (‘intrinsic’ signaling).5,7 Both pathways result in the subsequent activation of the caspase family of cysteinyl aspartate proteinases, which culminates in the characteristic structural, biochemical, and morphological changes of apoptosis.
Extrinsic signaling
Sertoli cells can directly instigate the apoptosis of germ cells through the extrinsic pathway, which involves members of a tumor necrosis factor family of proteins, Fas Ligand (FasL). When the death ligand (FasL) binds to its receptor, Fas, it results in the formation of death-inducible complex (DISC) and activation of caspase-8.5 Histopathologically, this mechanism is primarily characterized by Sertoli cell vacuolization/disruption together with the induction of spermatocyte apoptosis. In rats, the extrinsic pathway is typically characterized by the presence of germ cells undergoing apoptosis in stages XI-XIV, I, and II. When a toxicant injures a Sertoli cell, a paracrine signaling pathway is initiated resulting in the increased expression of FasL on Sertoli cells, which binds to its cognate receptor, Fas, on spermatocytes and consequently instigates the activation of caspases within the cell to lead to their apoptotic elimination. This signaling system serves as a paracrine mechanism, by which Sertoli cells actively regulate the numbers of germ cells they can support.31,32 The 2 most well-characterized Sertoli cell toxicants are MEHP and 2,5-HD. These toxicants induce the upregulation of FasL on Sertoli cells prior to the initiation of apoptosis of Fas-expressing germ cell.31,32 Conversely, direct germ cell toxicants stimulate the increase of Fas on germ cells with no associated FasL increase on Sertoli cells,31 suggesting that Fas is an important regulator of germ cell apoptosis.
The functional participation of the Fas/FasL signaling pathway in mediating the apoptotic removal of germ cells after toxicant-induced Sertoli cell injury is best illustrated in the studies of Richburg et al., using a phthalate exposure model.3 This mechanism was characterized by utilizing the gld mice, which have a point mutation in FasL that prevents it from binding and activating Fas.33 These mice display apparently normal spermatogenesis but when exposed to MEHP, there is a significant protection against the incidence of germ cell apoptosis as compared to their wild-type (C57BL/6J) counterparts.3 This pathway is initially activated by the disruption of tissue inhibitor of matrix metalloproteinase 2 (TIMP2) expression in Sertoli cells by MEHP (Fig. 3).34 This allows for the activation of matrix metalloproteinase 2 (MMP2) in the adluminal space and the consequent production of a soluble form tumor necrosis factor-α (sTNFα), which ultimately induces the increased expression of FasL on Sertoli cells.34-36 Although apoptosis typically does not induce inflammation, recent reports indicate that direct injury to Sertoli cells can change the normal immune privilege environment of the testis (disruption of the blood testis barrier, infiltration of macrophages).37,38 Therefore, Sertoli cell injury can induce a variety of changes in the seminiferous epithelium including the induction of germ cell apoptosis and altered immune environment.
Figure 3.
Induction of the extrinsic pathway of apoptosis by phthalates. When Sertoli cells are injured by mono-ethylhexyl phthalate (MEHP), a signaling cascade is initiated resulting in the upregulation of FasL, which binds the Fas receptor, ultimately resulting in apoptosis of spermatocytes. TIMP2 (tissue inhibitor of matrix metalloproteinase 2), MMP2 (matrix metalloproteinase 2), NFκ-B (nuclear factor kappa-light-chain-enhancer of activated B cells), TNFα (tumor necrosis factor-α).
The important role of the Fas/FasL system in triggering germ cell apoptosis has been further characterized by the use of FasL gene deficient, gld (point mutation in FasL) and lpr (nonfunctional Fas receptor) mice. A variety of papers utilize these gene deficient mice in exposures to toxicants and injuries including ischemia–reperfusion of the testis,39 ionizing radiation,40 and exposure to 2,5-hexanedione,32 diethylstilbesterol,41 and dinitrobenzene42 to elucidate the role of this system in germ cell apoptosis. Increased expression (protein or mRNA) of Fas/FasL has also been implicated in the mechanism of germ cell apoptosis after exposure to microcystins,43 Lindane,44 Lipopolysaccharide,45 lead46 and ethanol exposure in wild-type rodents.47
Intrinsic signaling
The intrinsic apoptotic pathway is critical for mediating germ cell apoptosis. However, the involvement of the intrinsic pathway in mediating Sertoli cell-induced germ cell apoptosis is minimal. Although, it is recognized that constant Sertoli cell support is required to prevent germ cell apoptosis through paracrine signaling via the pro-survival stem cell factor/c-kit.48 If germ cells are injured or damaged by a toxicant, spermatogonia undergoing apoptosis can be detected as well as Sertoli cell vacuoles within their basal cytoplasm. If the intrinsic pathway is induced, apoptotic germ cell loss in stages VII to VIII and II-VI in the mouse will be detected. Example of direct intrinsic germ cell apoptosis include hormone deprivation (see companion manuscript by Weinbauer, this issue), gamma irradiation, 2-bromopropane induced germ cell apoptosis.4,49
Evidence for the functional participation of regulators of the intrinsic signaling system in spermatogenesis comes from several transgenic and gene knockout mice (reviewed in 3). From these mouse studies, it is clear that the overexpression or inhibition of either proapoptotic or antiapoptotic family members results in the loss of germ cells by apoptosis. This pathway has not yet been shown to be involved in the induction of germ cell apoptosis of animals exposed to Sertoli cell toxicants,50 which supports the involvement of the intrinsic pathway in response to direct injury to germ cells. Intrinsic germ cell apoptosis is primarily attributed to the induction of Bax/Bcl2, cytochrome c, and the activation of caspase-9.5 The downregulation of anti-apoptotic protein Bcl-2 and upregulation of pro-apoptotic protein bax results in primary apoptosis of spermatogonia. Within any cell there is a balance between the inhibitors of apoptosis Bcl-2, Bcl-xL, Bcl-w and the activators of apoptosis Bax, Bik, Bak, Bad, Bid, and Bcl-xs.51 This pathway occurs autonomously within the germ cell and does not depend on the participation of factors from other cells.
Upstream activators of apoptotic pathways
Apoptosis is the end results of injury to the testis. However, the initial mechanism inducing apoptosis is not always definitive. The primary signals that induce the extrinsic and/or intrinsic pathways resulting in the activation of the caspase cascade and ultimately apoptosis are varied. The testicular toxicant, Methoxyacetic-acid induces spermatocyte apoptosis, Sertoli cell vacuolization, and multinucleated giant cells through a calcium dependent mechanism.52,53 Bulsufan, a germ cell toxicant initially causes the loss of c-kit/stem cell factor signaling before spermatogonia apoptosis occurs through the intrinsic pathway.54 Thus, the subtype of the apoptotic germ cell provides evidence for the cell death pathway but not the mechanism of induction.
Conclusions and perspectives
The testis is a complex organ with multiple cell types, all of which are coordinated to produce spermatozoa. During spermatogenesis, germ cell apoptosis is a normal occurrence, which increases when Sertoli cells are injured and can no longer support their normal complement of germ cells. Germ cell apoptosis can also be induced through direct injury to the germ cells. The interdependence of all the cells of the seminiferous epithelium makes it especially challenging to decipher the primary cellular site of action of a particular toxicant. The subtype of germ cell undergoing apoptosis provides the best evidence into the cellular target (spermatocyte associated with Sertoli cell injury or spermatogonia associate with direct effect; Table 1). This interdependence is not only within the seminiferous epithelium, as it has been recently shown that immune cells can be activated due to toxic effects on the Sertoli cell, and ultimately influence germ cell survival due to an extragonadal influence.55 While it is true that any toxicant that injures Sertoli cells can influence the normal testicular environment and alter germ cell survival, it is possible to distinguish different mechanisms of cell death in the testis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Dr. Kim Boekelheide and Linnea Anderson for providing the histological image used in Table 1 of this manuscript and Drs. Bob Chapin and Diane Creasy for critical reading of the manuscript prior to submission.
Funding
Supported, in part, by grants from the National Institutes of Health (ES016591 & ES007784 to J.H.R) and The University of Texas at Austin's Center for Molecular and Cellular Toxicology.
References
- 1. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British J Cancer 1972; 26:239-57; PMID:4561027; http://dx.doi.org/ 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Print CG, Loveland KL. Germ cell suicide: new insights into apoptosis during spermatogenesis. BioEssays 2000; 22:423-30; PMID:10797482; http://dx.doi.org/ 10.1002/(SICI)1521-1878(200005)22:5%3c423::AID-BIES4%3e3.0.CO;2-0 [DOI] [PubMed] [Google Scholar]
- 3. Richburg JH. The relevance of spontaneous- and chemically-induced alterations in testicular germ cell apoptosis to toxicology. Toxicol Lett 2000; 112-113:79-86; PMID:10720715 [DOI] [PubMed] [Google Scholar]
- 4. Boekelheide K. Mechanisms of toxic damage to spermatogenesis. J Natl Cancer Inst Monogr 2005:6-8; PMID:15784812; http://dx.doi.org/ 10.1093/jncimonographs/lgi006 [DOI] [PubMed] [Google Scholar]
- 5. Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol Sci 2011; 119:3-19; PMID:20829425; http://dx.doi.org/ 10.1093/toxsci/kfq268 [DOI] [PubMed] [Google Scholar]
- 6. Shukla KK, Mahdi AA, Rajender S. Apoptosis, spermatogenesis and male infertility. Front Biosci 2012; 4:746-54; http://dx.doi.org/ 10.2741/E415 [DOI] [PubMed] [Google Scholar]
- 7. Shaha C, Tripathi R, Mishra DP. Male germ cell apoptosis: regulation and biology. Philos Trans R Soc London Series B, Biol Sci 2010; 365:1501-15; PMID:20403866; http://dx.doi.org/ 10.1098/rstb.2009.0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wlodkowic D, Telford W, Skommer J, Darzynkiewicz Z. Apoptosis and beyond: cytometry in studies of programmed cell death. Methods Cell Biol 2011; 103:55-98; PMID:21722800; http://dx.doi.org/ 10.1016/B978-0-12-385493-3.00004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henriksen K, Parvinen M. Stage-specific apoptosis of male germ cells in the rat: mechanisms of cell death studied by supravital squash preparations. Tissue Cell 1998; 30:692-701; PMID:10036792; http://dx.doi.org/ 10.1016/S0040-8166(98)80088-8 [DOI] [PubMed] [Google Scholar]
- 10. De Franca LR, Hess RA, Cooke PS, Russell LD. Neonatal hypothyroidism causes delayed Sertoli cell maturation in rats treated with propylthiouracil: evidence that the Sertoli cell controls testis growth. Anatom Record 1995; 242:57-69; PMID:7604982; http://dx.doi.org/ 10.1002/ar.1092420108 [DOI] [PubMed] [Google Scholar]
- 11. Weber JE, Russell LD, Wong V, Peterson RN. Three-dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli–Sertoli and Sertoli–germ-cell relationships. Am J Anat 1983; 167:163-79; PMID:6613902; http://dx.doi.org/ 10.1002/aja.1001670203 [DOI] [PubMed] [Google Scholar]
- 12. Qian J, Bian Q, Cui L, Chen J, Song L, Wang X. Octylphenol induces apoptosis in cultured rat Sertoli cells. Toxicol Lett 2006; 166:178-86; PMID:16893618; http://dx.doi.org/ 10.1016/j.toxlet.2006.06.646 [DOI] [PubMed] [Google Scholar]
- 13. Iida H, Maehara K, Doiguchi M, Mori T, Yamada F. Bisphenol A-induced apoptosis of cultured rat Sertoli cells. Reprod Toxicol (Elmsford, NY 2003; 17:457-64; PMID:12849858; http://dx.doi.org/ 10.1016/S0890-6238(03)00034-0 [DOI] [PubMed] [Google Scholar]
- 14. Allan DJ, Gobe GC, Harmon BV. Sertoli cell death by apoptosis in the immature rat testis following x-irradiation. Scann Microscopy 1988; 2:503-12; PMID:3368774 [PubMed] [Google Scholar]
- 15. Huckins C. The morphology and kinetics of spermatogonial degeneration in normal adult rats: an analysis using a simplified classification of the germinal epithelium. Anat Record 1978; 190:905-26; PMID:637327; http://dx.doi.org/ 10.1002/ar.1091900410 [DOI] [PubMed] [Google Scholar]
- 16. Blanco-Rodriguez J. A matter of death and life: the significance of germ cell death during spermatogenesis. Int J Androl 1998; 21:236-48; PMID:9805237; http://dx.doi.org/ 10.1046/j.1365-2605.1998.00133.x [DOI] [PubMed] [Google Scholar]
- 17. Culty M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res Part C, Embryo today : reviews 2009; 87:1-26; PMID:19306346; http://dx.doi.org/ 10.1002/bdrc.20142 [DOI] [PubMed] [Google Scholar]
- 18. Wang RA, Nakane PK, Koji T. Autonomous cell death of mouse male germ cells during fetal and postnatal period. Biol Reprod 1998; 58:1250-6; PMID:9603260; http://dx.doi.org/ 10.1095/biolreprod58.5.1250 [DOI] [PubMed] [Google Scholar]
- 19. Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J 1997; 16:2262-70; PMID:9171341; http://dx.doi.org/ 10.1093/emboj/16.9.2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bartke A. Apoptosis of male germ cells, a generalized or a cell type-specific phenomenon? Endocrinology 1995; 136:3-4; PMID:7828545; http://dx.doi.org/ 10.1210/endo.136.1.7828545 [DOI] [PubMed] [Google Scholar]
- 21. Blanco-Rodriguez J, Martinez-Garcia C. Induction of apoptotic cell death in the seminiferous tubule of the adult rat testis: assessment of the germ cell types that exhibit the ability to enter apoptosis after hormone suppression by oestradiol treatment. Int J Androl 1996; 19:237-47; PMID:8940662; http://dx.doi.org/ 10.1111/j.1365-2605.1996.tb00468.x [DOI] [PubMed] [Google Scholar]
- 22. Blanco-Rodriguez J, Martinez-Garcia C. Spontaneous germ cell death in the testis of the adult rat takes the form of apoptosis: re-evaluation of cell types that exhibit the ability to die during spermatogenesis. Cell Prolif 1996; 29:13-31; PMID:8603107; http://dx.doi.org/ 10.1111/j.1365-2184.1996.tb00091.x [DOI] [PubMed] [Google Scholar]
- 23. Elliott MR, Zheng S, Park D, Woodson RI, Reardon MA, Juncadella IJ, Kinchen JM, Zhang J, Lysiak JJ, Ravichandran KS. Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature 2010; 467:333-7; PMID:20844538; http://dx.doi.org/ 10.1038/nature09356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xiong W, Wang H, Wu H, Chen Y, Han D. Apoptotic spermatogenic cells can be energy sources for Sertoli cells. Reproduction 2009; 137:469-79; PMID:19074501; http://dx.doi.org/ 10.1530/REP-08-0343 [DOI] [PubMed] [Google Scholar]
- 25. Nandi S, Banerjee PP, Zirkin BR. Germ cell apoptosis in the testes of Sprague Dawley rats following testosterone withdrawal by ethane 1,2-dimethanesulfonate administration: relationship to Fas? Biol Reprod 1999; 61:70-5; PMID:10377033; http://dx.doi.org/ 10.1095/biolreprod61.1.70 [DOI] [PubMed] [Google Scholar]
- 26. Allan D, Harmon B, Kerr J. Cell death in spermatogenesis. In: Potten C, ed. Perspectives on Mammalian Cell Death. London: Oxford University Press, 1987:229-58. [Google Scholar]
- 27. Blanchard KT, Allard EK, Boekelheide K. Fate of germ cells in 2,5-hexanedione-induced testicular injury. I. Apoptosis is the mechanism of germ cell death. Toxicol Appl Pharmacol 1996; 137:141-8; PMID:8661338; http://dx.doi.org/ 10.1006/taap.1996.0066 [DOI] [PubMed] [Google Scholar]
- 28. Richburg JH, Boekelheide K. Mono-(2-ethylhexyl) phthalate rapidly alters both Sertoli cell vimentin filaments and germ cell apoptosis in young rat testes. Toxicol Appl Pharmacol 1996; 137:42-50; PMID:8607140; http://dx.doi.org/ 10.1006/taap.1996.0055 [DOI] [PubMed] [Google Scholar]
- 29. Hasegawa M, Wilson G, Russell LD, Meistrich ML. Radiation-induced cell death in the mouse testis: relationship to apoptosis. Radiation Res 1997; 147:457-67; PMID:9092926; http://dx.doi.org/ 10.2307/3579503 [DOI] [PubMed] [Google Scholar]
- 30. Hales BF, Robaire B. The Male Germ cell as a Target for Toxicants In: McQueen CA, ed. Comprehensive Toxicology Kidlington, United Kingdom: Elsevier Ltd; 2010:115-29. [Google Scholar]
- 31. Lee J, Richburg JH, Shipp EB, Meistrich ML, Boekelheide K. The Fas system, a regulator of testicular germ cell apoptosis, is differentially up-regulated in Sertoli cell versus germ cell injury of the testis. Endocrinology 1999; 140:852-8; PMID:9927315 [DOI] [PubMed] [Google Scholar]
- 32. Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology 1997; 138:2081-8; PMID:9112408 [DOI] [PubMed] [Google Scholar]
- 33. Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 1994; 76:969-76; PMID:7511063; http://dx.doi.org/ 10.1016/0092-8674(94)90375-1 [DOI] [PubMed] [Google Scholar]
- 34. Yao PL, Lin YC, Richburg JH. Transcriptional suppression of Sertoli cell Timp2 in rodents following mono-(2-ethylhexyl) phthalate exposure is regulated by CEBPA and MYC. Biol Reprod 2011; 85:1203-15; PMID:21832167; http://dx.doi.org/ 10.1095/biolreprod.111.093484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yao PL, Lin YC, Richburg JH. TNF α-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol Reprod 2009; 80:581-9; PMID:19038859; http://dx.doi.org/ 10.1095/biolreprod.108.073122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yao PL, Lin YC, Sawhney P, Richburg JH. Transcriptional regulation of FasL expression and participation of sTNF-α in response to sertoli cell injury. J Biol Chem 2007; 282:5420-31; PMID:17192273; http://dx.doi.org/ 10.1074/jbc.M609068200 [DOI] [PubMed] [Google Scholar]
- 37. Yao PL, Lin YC, Richburg JH. Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biol Reprod 2010; 82:516-27; PMID:19828778; http://dx.doi.org/ 10.1095/biolreprod.109.080374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murphy CJ, Stermer AR, Richburg JH. Age- and Species-Dependent Infiltration of Macrophages into the Testis of Rats and Mice Exposed to Mono-(2-Ethyl- hexyl) Phthalate (MEHP). Biol Reprod 2014; 91:1-11; PMID:24876407; http://dx.doi.org/ 10.1095/biolreprod113.115527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koji T, Hishikawa Y, Ando H, Nakanishi Y, Kobayashi N. Expression of Fas and Fas ligand in normal and ischemia-reperfusion testes: involvement of the Fas system in the induction of germ cell apoptosis in the damaged mouse testis. Biol Reprod 2001; 64:946-54; PMID:11207212; http://dx.doi.org/ 10.1095/biolreprod64.3.946 [DOI] [PubMed] [Google Scholar]
- 40. Embree-Ku M, Venturini D, Boekelheide K. Fas is involved in the p53-dependent apoptotic response to ionizing radiation in mouse testis. Biol Reprod 2002; 66:1456-61; PMID:11967210; http://dx.doi.org/ 10.1095/biolreprod66.5.1456 [DOI] [PubMed] [Google Scholar]
- 41. Nair R, Shaha C. Diethylstilbestrol induces rat spermatogenic cell apoptosis in vivo through increased expression of spermatogenic cell Fas/FasL system. J Biol Chem 2003; 278:6470-81; PMID:12477725; http://dx.doi.org/ 10.1074/jbc.M209319200 [DOI] [PubMed] [Google Scholar]
- 42. Richburg JH, Nanez A. Fas- or FasL-deficient mice display an increased sensitivity to nitrobenzene-induced testicular germ cell apoptosis. Toxicol Lett 2003; 139:1-10; PMID:12595153; http://dx.doi.org/ 10.1016/S0378-4274(02)00419-8 [DOI] [PubMed] [Google Scholar]
- 43. Xiong Q, Xie P, Li H, Hao L, Li G, Qiu T, Liu Y. Involvement of Fas/FasL system in apoptotic signaling in testicular germ cells of male Wistar rats injected i.v. with microcystins. Toxicon 2009; 54:1-7; PMID:19562849 [DOI] [PubMed] [Google Scholar]
- 44. Saradha B, Vaithinathan S, Mathur PP. Lindane induces testicular apoptosis in adult Wistar rats through the involvement of Fas-FasL and mitochondria-dependent pathways. Toxicology 2009; 255:131-9; PMID:19038305; http://dx.doi.org/ 10.1016/j.tox.2008.10.016 [DOI] [PubMed] [Google Scholar]
- 45. Kajihara T, Okagaki R, Ishihara O. LPS-induced transient testicular dysfunction accompanied by apoptosis of testicular germ cells in mice. Med Mol Morphol 2006; 39:203-8; PMID:17187183; http://dx.doi.org/ 10.1007/s00795-006-0334-7 [DOI] [PubMed] [Google Scholar]
- 46. Dong S, Liang D, An N, Jia L, Shan Y, Chen C, Sun K, Niu F, Li H, Fu S. The role of MAPK and FAS death receptor pathways in testicular germ cell apoptosis induced by lead. Acta Biochimica et Biophysica Sinica 2009; 41:800-7; PMID:19727529; http://dx.doi.org/ 10.1093/abbs/gmp069 [DOI] [PubMed] [Google Scholar]
- 47. Eid NA, Shibata MA, Ito Y, Kusakabe K, Hammad H, Otsuki Y. Involvement of Fas system and active caspases in apoptotic signalling in testicular germ cells of ethanol-treated rats. Int J Androl 2002; 25:159-67; PMID:12031044; http://dx.doi.org/ 10.1046/j.1365-2605.2002.00341.x [DOI] [PubMed] [Google Scholar]
- 48. Rossi P, Sette C, Dolci S, Geremia R. Role of c-kit in mammalian spermatogenesis. J Endocrinol Invest 2000; 23:609-15; PMID:11079457; http://dx.doi.org/ 10.1007/BF03343784 [DOI] [PubMed] [Google Scholar]
- 49. Yu X, Kubota H, Wang R, Saegusa J, Ogawa Y, Ichihara G, Takeuchi Y, Hisanaga N. Involvement of Bcl-2 family genes and Fas signaling system in primary and secondary male germ cell apoptosis induced by 2-bromopropane in rat. Toxicol Appl Pharmacol 2001; 174:35-48; PMID:11437647; http://dx.doi.org/ 10.1006/taap.2001.9187 [DOI] [PubMed] [Google Scholar]
- 50. Giammona CJ, Sawhney P, Chandrasekaran Y, Richburg JH. Death receptor response in rodent testis after mono-(2-ethylhexyl) phthalate exposure. Toxicol Appl Pharmacol 2002; 185:119-27; PMID:12490136; http://dx.doi.org/ 10.1006/taap.2002.9536 [DOI] [PubMed] [Google Scholar]
- 51. Blanco-Rodriguez J, Martinez-Garcia C. Apoptosis pattern elicited by several apoptogenic agents on the seminiferous epithelium of the adult rat testis. J Androl 1998; 19:487-97; PMID:9733152 [PubMed] [Google Scholar]
- 52. Li LH, Wine RN, Miller DS, Reece JM, Smith M, Chapin RE. Protection against methoxyacetic-acid-induced spermatocyte apoptosis with calcium channel blockers in cultured rat seminiferous tubules: possible mechanisms. Toxicol Appl Pharmacol 1997; 144:105-19; PMID:9169075; http://dx.doi.org/ 10.1006/taap.1997.8129 [DOI] [PubMed] [Google Scholar]
- 53. Barone F, Aguanno S, D’Alessio A, D’Agostino A. Sertoli cell modulates MAA-induced apoptosis of germ cells throughout voltage-operated calcium channels. FASEB J 2004; 18:353-4; PMID:14656996 [DOI] [PubMed] [Google Scholar]
- 54. Choi YJ, Ok DW, Kwon DN, Chung JI, Kim HC, Yeo SM, Kim T, Seo HG, Kim JH. Murine male germ cell apoptosis induced by busulfan treatment correlates with loss of c-kit-expression in a Fas/FasL- and p53-independent manner. FEBS Lett 2004; 575:41-51; PMID:15388331; http://dx.doi.org/ 10.1016/j.febslet.2004.08.034 [DOI] [PubMed] [Google Scholar]
- 55. Foster PM, Earl Gray L. Toxic Responses of the Reproductive System In: Klaassen CD, ed. The Basic Science of Poisons. New York, NY: McGraw-Hill; 2013:861-906. [Google Scholar]



